Abstract
Background
Meningococcal serogroup C (MenC) specific antibody levels decline rapidly after a single primary MenC conjugate (MenCC) vaccination in preschool children. A second MenCC vaccination during (pre)adolescence might attain longer lasting individual and herd protection. We aimed to establish an appropriate age for a (pre)adolescent MenCC booster vaccination.
Methods
A phase-IV trial with healthy 10-year-olds (n = 91), 12-year-olds (n = 91) and 15-year-olds (n = 86) who were primed with a MenCC vaccine nine years earlier. All participants received a booster vaccination with the same vaccine. Serum bactericidal antibody assay titers (SBA, using baby rabbit complement), MenC-polysaccharide (MenC-PS) specific IgG, IgG subclass and avidity and tetanus-specific IgG levels were measured prior to (T0) and 1 month (T1) and 1 year (T2) after the booster. An SBA titer ≥8 was the correlate of protection.
Results
258 (96.3%) participants completed all three study visits. At T0, 19% of the 10-year-olds still had an SBA titer ≥8, compared to 34% of the 12-year-olds (P = 0.057) and 45% of the 15-year-olds (P<0.001). All participants developed high SBA titers (GMTs>30,000 in all age groups) and MenC-PS specific IgG levels at T1. IgG levels mainly consisted of IgG1, but the contribution of IgG2 increased with age. At T2, 100% of participants still had an SBA titer ≥8, but the 15-year-olds showed the highest protective antibody levels and the lowest decay.
Conclusion
Nine years after primary MenCC vaccination adolescents develop high protective antibody levels in response to a booster and are still sufficiently protected one year later. Our results suggest that persistence of individual - and herd - protection increases with the age at which an adolescent booster is administered.
Trial Registration
EU Clinical Trials Database 2011-000375-13 Dutch Trial Register NTR3521
Introduction
Invasive meningococcal disease (IMD) is a severe illness, with a mortality rate of ∼10% and serious sequelae in a substantial number of survivors. In Europe serogroup B and C are responsible for the majority of disease [1]. A single Meningococcal Serogroup C conjugated (MenCC) vaccination was implemented into the Dutch National Immunization Programme (NIP) in 2002 for all children aged 14 months. In addition, a large catch-up campaign was conducted in 2002 during which all children between 1 and 19 years of age were invited to receive a MenCC vaccination. Total vaccine coverage of the catch-up campaign was 94% [2] whereas the current vaccine coverage for MenC in the NIP is >95% [3]. After 2002, MenC disease has almost completely disappeared. Thus far only three cases of vaccine failure have occurred, of which two had an underlying immune deficiency, and only a few cases of MenC disease occur yearly among unvaccinated individuals [4]. This successful elimination is likely due to the large-scale catch-up campaign that included adolescents [5], [6]. Teenagers and young adults have the highest nasopharyngeal meningococcal carriage levels and are considered the main source of spread [7]. As the MenCC vaccine not only protects against disease but also reduces nasopharyngeal carriage levels [8], large-scale vaccination of adolescents was very effective in preventing dissemination of MenC in the population [9].
In the past decade it became clear that MenC specific antibody levels wane rapidly in infants, even when primed with multiple doses [10], as well as in toddlers primed or boosted with a single dose [11]–[14]. The rapid decrease in quantitative antibody levels is associated with a fast reduction in the proportion of children with a serum bactericidal antibody (SBA) level – or protective antibody level - above the internationally accepted correlate of protection (≥8, when using baby rabbit complement). Different studies showed that the immunological memory response to MenC and the subsequent rise in protective antibody levels develop only after several days [15]–[18], whereas the meningococcus can invade the bloodstream and cause (fatal) disease within hours upon acquisition. Protective antibody levels above the correlate of protection are therefore crucial to maintain protection against MenC disease. Hence, priming young children with single or multiple MenCC vaccinations is not sufficient to maintain long term individual immunity. An additional MenCC vaccination at an older age may be required, especially since adolescents are also at particular risk for IMD. Primary vaccination in older children has led to higher and longer lasting protective antibody levels than in preschool children [12], [18]–[20]. Since adolescents are also considered as the main source of transmission, an additional vaccination prior to or during adolescence will prolong individual immunity but might also maintain the herd protection that has been achieved.
Studies investigating the response to a booster MenCC vaccination in adolescents showed promising results [19], [21] with good persistence of antibody levels in the following years [19], [20], [22]. However, these studies had relatively short intervals between the primary and booster vaccinations. To our knowledge, no studies exist that investigated the effect of an adolescent MenCC booster vaccination more than seven years after priming. To establish what would be an appropriate age for a (pre)adolescent booster, we recruited three age groups of 10-, 12- and 15-year-olds respectively who had received a primary MenCC vaccination nine years earlier. We investigated possible differences with age in MenC-specific SBA, IgG, IgG subclass and avidity levels at baseline and one month and one year after a booster MenCC vaccination.
Methods
Ethics Statement
This study was approved by the local ethics committee Verenigde Commissies Mensgebonden Onderzoek (VCMO, Nieuwegein, The Netherlands). The protocol for this trial and supporting TREND checklist are available as supporting information; see Protocol S1 and Checklist S1.
Study design and participants
This study was a phase IV, single center, open-label study. In September 2011, an invitation letter was sent to parents of all children from Nieuwegein, Houten and Zeist (in the surrounding area of Utrecht, The Netherlands) within the targeted age range of 9.5–10.5 years, 11.5–12.5 years and 14.5–15.5 years (n = 4667). Inclusion criteria were good general health and a vaccination history according to the Dutch NIP, including one MenCC vaccination approximately nine years earlier (either during the catch-up campaign in 2002 or as part of the NIP) and the DT-IPV booster at 9 years [23]. Exclusion criteria were: severe acute illness/fever/use of antibiotics within 14 days prior to enrolment, disease/medical treatment that could interfere with results, allergy to vaccine component, serious adverse event after previous vaccination, previous meningococcal disease, multiple MenC vaccinations in immunization history, other vaccination within one month prior to enrolment, use of plasma products in the previous 6 months and pregnancy. Individual immunization history was verified by checking personal vaccination cards or from centralized immunization records. Written informed consent was obtained from both parents and participants aged ≥12 years prior to enrolment. The study was registered at the EU Clinical Trials database (EudraCT number: 2011-000375-13). Due to a communication error, this study was registered at the Dutch Trial register (www.trialregister.nl; NTR3521) after start of recruitment. The authors confirm that all ongoing and related trials for this intervention are registered.
Clinical procedures
At the beginning of the study, participants received one vaccination with the MenC-polysaccharide conjugated to tetanus toxoid vaccine (MenC-TT, Baxter). Vaccinations were administered by intramuscular injection into the deltoid muscle, using a 23 gauge 0.6×25 mm needle. Blood samples (5 mL) were taken prior to the MenC-TT booster vaccination (T0) and 1 month (T1) and 1 year (T2) afterwards. Vaccinations and blood collections were performed by trained and authorized nurses at local study sites in Nieuwegein, Zeist and Houten. Blood samples were transported to the laboratory where sera were separated and stored at −20°C for serological analyses.
Serological analyses
The level of MenC-specific protective antibodies was determined using the serum bactericidal antibody assay (SBA) with baby rabbit complement (Pelfreez, Rogers, AR) and target strain C11. SBA titers were expressed as the reciprocal of the final serum dilution yielding ≥50% killing at 60 minutes [24] with a titer of ≥8 as correlate of protection [25], [26].
MenC-polysaccharide (MenC-PS) specific IgG, IgG subclass, avidity and tetanus toxoid (TT) specific IgG levels were quantified using the fluorescent-bead-based multiplex immunoassay (MIA) as previously described [16], [27]–[29].
Primary and secondary objectives
The primary objective was to determine potential differences between the age groups in SBA titers and proportion of participants with an SBA titer ≥8 and ≥128 prior to (T0) and 1 month (T1) and 1 year (T2) after the MenC-TT booster vaccination.
Secondary objectives included assessment of differences between groups in: 1) levels of serum MenC-PS specific IgG at T0; 2) persistence of vaccine induced MenC-PS specific IgG levels between T1 and T2; 3) MenC-PS specific IgG subclass and avidity levels at T0, T1 and T2; 4) serum IgG levels against tetanus toxoid (TT), the carrier protein for MenC polysaccharide in the conjugate vaccine.
Statistical analysis
The study was powered to detect a twofold difference in SBA geometric mean titers (GMTs) between two of the age groups with and estimated SD of the log-transformed titer of 1.27 [19].
Proportion and 95% confidence intervals (95%CIs) of participants with an SBA titer ≥8 or ≥128 were calculated using the Agresti-Coull method [30]. Differences between groups in gender and proportion of participants with an SBA titer ≥8 and ≥128 were determined with a Chi-squared test.
GMTs of SBA, geometric mean concentrations (GMCs) of MenC-PS specific IgG, IgG subclass and TT-specific IgG, mean avidity and corresponding 95%CIs were calculated. Normal distribution of (log-transformed) values was checked prior to each analysis. Differences between groups in log-transformed titers and concentrations at the different time points were determined with linear regression analyses, adjusting for titers and concentrations at T0. Differences in mean avidity and GMC ratios were determined with paired and independent sample t-tests, respectively. Correlation between TT-specific IgG GMC at T0 and the difference between T0 and T2 was determined using Pearson correlation test. All p-values were adjusted for three comparisons with Bonferroni correction. A p-value ≤0.05 after correction was considered as statistically significant. Data were analyzed using Excel 2010 software (Microsoft Office) and SPSS statistics 19 (IBM).
Results
Study population
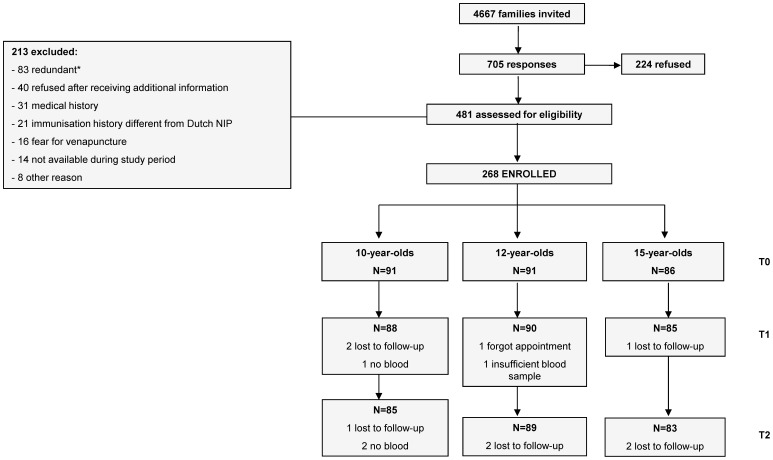
In October 2011, 268 participants were enrolled and received the MenC-TT booster vaccination: a group with 10-year-olds (n = 91), a group with 12-year-olds (n = 91) and a group with 15-year-olds (n = 86). Blood samples of the 1 month (T1) and 1 year (T2) follow-up were collected in November 2011 and October 2012, respectively. 258 participants (96.3%) completed all three study visits (Figure 1). All participants were treated according to the protocol; analyses were performed on all available data.
Figure 1. Flow-chart Recruitment.
Flow chart for recruitment, enrolment and loss to follow-up. Participants were recruited in September 2011 from the surrounding area of Utrecht, The Netherlands. At the beginning of the study (T0) all participants received one booster vaccination with the Meningococcal serogroup C polysaccharide conjugated to tetanus toxoid vaccine (MenC-TT, Baxter); T1 and T2 indicate 1 month and 1 year after the MenC-TT booster respectively. *83 potential participants were excluded because the enrolment target of the study had been achieved.
Baseline characteristics are outlined in Table 1. There was no difference in gender between the groups. The 10-year-olds were primed at the age of 14 months according to the Dutch NIP whereas the 12- and 15-year-olds were primed during the catch-up campaign in 2002. There was a slight difference in interval since primary vaccination between the groups with the shortest interval for the 10-year-olds (8.7 years) and the longest for the 12-year-olds (9.3 years).
Table 1. Baseline Characteristics Study Population.
| 10-year-olds | 12-year-olds | 15-year-olds | |
| No. of enrolled participants | 91 | 91 | 86 |
| Mean age at enrolment (T0); years (±SD) | 9.9 (0.3) | 12.0 (0.3) | 15.0 (0.3) |
| Female; No. (%) | 53 (58) | 44 (48) | 41 (48) |
| Mean age at MenCC vaccine priminga; years (±SD) | 1.2 (0.1) | 2.7 (0.3) | 5.8 (0.4) |
| Mean interval since primary MenCC vaccination and T0b; years (±SD) | 8.7 (0.3) | 9.3 (0.1) | 9.2 (0.2) |
a. All participants were primed with one vaccination with the Meningococcal serogroup C polysaccharide conjugated to tetanus toxoid vaccine (MenC-TT, Baxter) and received a booster with the same vaccine at the beginning of the study (T0). Individual immunization histories were verified by checking personal vaccination cards or from centralized immunization records.
b. Intervals slightly differed between groups (separate t-tests; P<0.001). Further testing showed no relation between interval duration and antibody levels (data not shown). Analyses were therefore not adjusted for this interval.
Primary objective
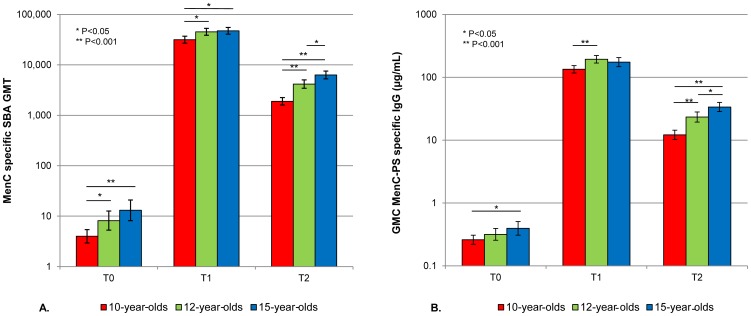
At T0, nine years after the primary vaccination and prior to the MenC-TT booster vaccination, 17 (19%) of the 10-year-olds still had an SBA titer ≥8, compared to 31 (34%) of the 12-year-olds (P = 0.057) and 39 (45%) of the 15-year-olds (P<0.001; Table 2). In addition, 6 (7%) of the 10-year-olds had an SBA titer ≥128, compared to 16 (18%) of the 12-year olds (P = 0.069) and 23 (27%) of the 15-year olds (P<0.001; Table 2). Overall SBA GMTs were low, though values were slightly lower among the 10-year-olds compared to the 12-year-olds (P = 0.006) and the 15-year-olds (P<0.001; Figure 2a).
Table 2. Geometric Mean Titers (GMTs) of Meningococcal Serogroup C (MenC) Specific Serum Bactericidal Antibody (SBA) and Proportion of Participants with an SBA titer ≥8 and ≥128 prior to (T0) and 1 Month (T1) and 1 Year (T2) after the MenC Conjugate Booster.
| Age at T0 | P-value difference between groups* | ||||||
| 10 years | 12 years | 15 years | 10 vs.12 | 10 vs. 15 | 12 vs. 15 | ||
| T0 | GMT (95%CI) | 4.0 (2.9–5.4) | 8.2 (5.3–12.6) | 13.1 (8.1–21.0) | 0.006 | <0.001 | 0.459 |
| SBA ≥8: proportion | 17/91 | 31/91 | 39/86 | ||||
| SBA ≥8: % (95%CI) | 19 (12–28) | 34 (25–45) | 45 (35–56) | 0.057 | <0.001 | 0.375 | |
| SBA ≥128: proportion | 6/91 | 16/91 | 23/91 | ||||
| SBA ≥128: % (95%CI) | 7 (3–14) | 18 (11–27) | 27 (18–37) | 0.069 | <0.001 | 0.426 | |
| T1 | GMT (95%CI) | 31,564 (26,899–37,038) | 45,175 (38,608–52,859) | 47,289 (40,422–55,322) | 0.021 | 0.003 | 1.000 |
| SBA ≥8: proportion | 88/88 | 89/89 | 85/85 | ||||
| SBA ≥8: % (95%CI) | 100 (95–100) | 100 (95–100) | 100 (95–100) | 1.000 | 1.000 | 1.000 | |
| SBA ≥128: proportion | 88/88 | 89/89 | 85/85 | ||||
| SBA ≥128: % (95%CI) | 100 (95–100) | 100 (95–100) | 100 (95–100) | 1.000 | 1.000 | 1.000 | |
| T2 | GMT (95%CI) | 1,987 (1,602–2,247) | 4,165 (3,444–5,038) | 6,292 (5,272–7,509) | <0.001 | <0.001 | 0.021 |
| SBA ≥8: proportion | 85/85 | 89/89 | 83/83 | ||||
| SBA ≥8: % (95%CI) | 100 (95–100) | 100 (95–100) | 100 (95–100) | 1.000 | 1.000 | 1.000 | |
| SBA ≥128: proportion | 85/85 | 89/89 | 83/83 | ||||
| SBA ≥128: % (95%CI) | 100 (95–100) | 100 (95–100) | 100 (95–100) | 1.000 | 1.000 | 1.000 | |
NOTE: Differences between groups in SBA GMTs at T0 were determined using the Mann-Whitney U test. Differences between groups in SBA GMTs at T1 and T2 were determined with linear regression analyses, adjusting for titers at T0. An SBA titer ≥8 was considered as international correlate of protection. Differences between groups in proportion of participants with an SBA titer ≥8 and ≥128 was determined with χ2-tests.
* P-values were adjusted for three comparisons with Bonferroni correction. Extensive results of the crude and adjusted linear regression analyses are outlined in supplementary table S1.
Figure 2. Meningococcal Serogroup C (MenC) Specific Geometric Mean Titers (GMTs) of Serum Bactericidal Antibody (SBA) and Geometric Mean Concentrations (GMCs) of MenC Polysaccharide (MenC-PS) specific Immunoglobulin G (IgG).
MenC-specific GMTs of SBA (a) and GMCs of MenC-PS specific IgG (b) of different age groups prior to (T0) and 1 month (T1) and 1 year (T2) after the MenC conjugate booster.
At T1, one month after the MenC-TT booster vaccination, SBA GMTs had strongly increased to >30,000 in all age groups (Table 2, Figure 2a). SBA GMT in the 10-year-olds was lower compared to the 12-year-olds (P = 0.021) and the 15-year-olds (P = 0.003). All participants had an SBA titer ≥8 and ≥128 (Table 2).
At T2, one year after the MenC-TT booster vaccination, SBA GMTs had declined 16-fold in the 10-year-olds, 11-fold in the 12-year-olds and 8-fold in the 15-year-olds. SBA GMTs differed significantly between all groups (Figure 2a). Still, all participants had an SBA titer ≥8 and ≥128 (Table 2).
Secondary objectives
MenC-PS specific IgG concentrations
Prior to the booster, the MenC-PS specific IgG GMCs were <0.5 µg/mL in all age groups, though slightly lower in the 10-year-olds compared to the 15-year-olds (P = 0.018; Table 3). In coherence with the SBA titers, GMCs of MenC-PS specific IgG had increased considerably at T1 in all age groups and decreased one year later. The level of decrease in IgG between T1 and T2 differed between all age groups and was the highest among the 10-year-olds and the lowest among the 15-year-olds (Table 3, Figure 2b).
Table 3. Geometric Mean Concentrations (GMCs) of Meningococcal Serogroup C Polysaccharide (MenC-PS) Specific Immunoglobulin G (IgG) prior to (T0) and 1 Month (T1) and 1 Year (T2) after the MenC Conjugate Booster.
| Age at T0 | P-value difference between groups* | ||||||
| 10 years | 12 years | 15 years | 10 vs. 12 | 10 vs. 15 | 12 vs. 15 | ||
| T0 | No. analyzed | 91 | 91 | 86 | |||
| GMC MenC-PS specific IgG µg/mL (95%CI) | 0.26 (0.22–0.31) | 0.32 (0.26–0.39) | 0.40 (0.31–0.51) | 0.444 | 0.018 | 0.579 | |
| T1 | No. analyzed | 88 | 90 | 85 | |||
| GMC MenC-PS specific IgG µg/mL (95%CI) | 134 (117.0–153.4) | 193.6 (168.2–222.3) | 174.3 (147.5–206.0) | <0.001 | 0.063 | 1.000 | |
| T2 | No. analyzed | 85 | 89 | 83 | |||
| GMC MenC-PS specific IgG µg/mL (95%CI) | 12.2 (10.2–14.4) | 23.3 (19.3–28.0) | 33.7 (28.4–39.9) | <0.001 | <0.001 | 0.033 | |
| T1/T0 | GMC ratio (95%CI) | 511 (417–627) | 607 (469–787) | 439 (322–598) | 0.924 | 1.000 | 0.345 |
| T1/T2 | GMC ratio (95%CI) | 11.0 (9.5–12.8) | 8.3 (7.1–9.8) | 5.2 (4.5–6.2) | 0.036 | <0.001 | <0.001 |
NOTE: Differences between age groups in GMC of MenC-PS specific IgG were determined with linear regression analyses, adjusting for concentrations at T0. GMC ratios indicate the level of increase (T1/T0) or decrease (T1/T2) between time points. Differences in GMC ratios between groups were determined with independent sample t-tests.
* P-values were adjusted for three comparisons with Bonferroni correction. Extensive results of the crude and adjusted linear regression analyses are outlined in supplementary table S1.
MenC-PS specific IgG subclass concentrations
GMCs of MenC-PS specific IgG1 at T0 were equal in all age groups. The 10-year-olds showed a higher IgG1/IgG2 subclass ratio compared to the 12-year-olds (P = 0.027) and the 15-year-olds (P<0.001), which was due to a lower level of IgG2 compared to the other two groups. At T1 and T2, total MenC-PS specific IgG levels in all age groups mainly consisted of IgG1 subclass. The contribution of IgG2 remained the lowest in the 10-year-olds (Table 4).
Table 4. Geometric Mean Concentrations (GMCs) of Meningococcal Serogroup C Polysaccharide (MenC-PS) Specific Immunoglobulin G (IgG) Subclass and Subclass Ratio.
| Age at T0 | P-value difference between groups* | ||||||
| 10 years | 12 years | 15 years | 10 vs. 12 | 10 vs. 15 | 12 vs. 15 | ||
| T0 | No. analyzed | 91 | 91 | 86 | |||
| GMC IgG1 µg/mL (95%CI) | 0.34 (0.29–0.41) | 0.34 (0.27–0.44) | 0.34 (0.27–0.43) | 1.000 | 1.000 | 1.000 | |
| GMC IgG2 µg/mL (95%CI) | 0.09 (0.08–0.11)a | 0.13 (0.11–0.17)b | 0.16 (0.12–0.21) | 0.024 | 0.003 | 1.000 | |
| IgG1/IgG2 | 3.98 (3.23–4.91)a | 2.71 (2.23–3.30)b | 2.17 (1.68–2.79) | 0.027 | <0.001 | 0.522 | |
| T1 | No. analyzed | 88 | 90 | 85 | |||
| GMC IgG1 µg/mL (95%CI) | 158.6 (138.2–181.9) | 213.4 (183.4–248.3) | 168.7 (139.5–203.9) | 0.015 | 1.000 | 0.174 | |
| GMC IgG2 µg/mL (95%CI) | 14.0 (11.1–17.7) | 28.0 (23.3–33.6) | 30.5 (23.9–38.9) | 0.003 | <0.001 | 1.000 | |
| IgG1/IgG2 | 11.30 (9.44–13.51) | 7.59 (6.40–9.00) | 5.54 (4.31–7.10) | 0.255 | 0.054 | 0.276 | |
| T2 | No. analyzed | 85 | 89 | 83 | |||
| GMC IgG1 µg/mL (95%CI) | 13.21 (11.32–15.42) | 22.22 (18.51–26.68) | 26.29 (22.39–30.88) | <0.001 | <0.001 | 0.531 | |
| GMC IgG2 µg/mL (95%CI) | 1.28 (1.02–1.59) | 3.23 (2.54–4.12) | 5.32 (3.99–7.10) | <0.001 | <0.001 | 0.09 | |
| IgG1/IgG2 | 10.36 (8.36–12.84) | 6.87 (5.61–8.42) | 4.94 (3.73–6.55) | 0.969 | 0.156 | 0.297 | |
NOTE: Differences between age groups in GMC of MenC-PS specific IgG subclass and subclass ratio were determined with linear regression analyses, adjusting for concentrations and ratios at T0.
* P-values were adjusted for three comparisons with Bonferroni correction. Extensive results of the crude and adjusted linear regression analyses are outlined in supplementary table S1.
a. Concentration IgG2 too low to measure in nine participants; GMC IgG2 and subclass ratio presented from 82 participants.
b. Concentration IgG2 too low to measure in five participants; GMC IgG2 and subclass ratio presented from 86 participants.
Avidity of MenC-PS specific IgG
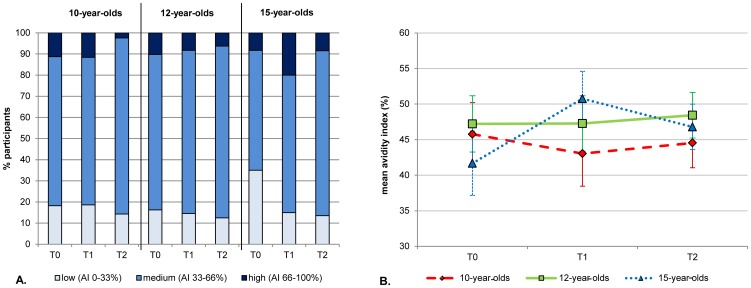
Prior to the booster, mean avidity indices of MenC-PS specific IgG were similar between the three age groups (46%, 47% and 42% respectively). After the booster, avidity indices in the 10- and 12-year-olds remained at the same level. In contrast, the mean avidity index in the 15-year-olds increased to 51% at T1 and (P = 0.009) and subsequently decreased to 47% at T2 (P = 0.039; Figure 3).
Figure 3. Meningococcal Serogroup C Polysaccharide Specific Immunoglobulin G (IgG) Avidity Indices (AI).
Avidity indices divided in low, intermediate and high (a) and mean avidity indices (b) per age group prior to (T0) and 1 month (T1) and 1 year (T2) after the MenC conjugate booster. Only serum samples from participants with an IgG concentration of ≥0.25 µg/ml at T0 were included in the analyses (44 10-year-olds, 49 12-year-olds and 60 15-year-olds). Serum samples were diluted to a concentration of 25 ng/ml. Avidity index = (amount of IgG still bound after treatment with 0.5 M NH4SCN/amount of IgG with PBS) x 100.
Tetanus toxoid specific IgG concentrations
At T0, the GMC of TT-specific IgG was highest in the 10-year-olds and lowest in the 15-year-olds. One month after the MenC-TT booster, TT-specific IgG GMCs had increased in all age groups. The 10-year-olds showed the highest absolute concentration of antibody, but the level of increase was highest in the 15-year-olds. At T2, TT-specific IgG GMCs had decreased and remained highest in the 10-year-olds (Table 5). Overall, there was a negative correlation between the TT-specific IgG concentration at T0 and the difference in concentration between T0 and T2 (R = −0.694, P<0.001). A number of participants with a high concentration at T0, mostly 10-year-olds, showed a lower concentration at T2 and some even at T1.
Table 5. Geometric Mean Concentrations (GMCs) of Tetanus Toxoid (TT) Specific Immunoglobulin G (IgG) prior to (T0) and 1 Month (T1) and 1 Year (T2) after the Meningococcal Serogroup C Tetanus Toxoid Conjugate Booster.
| Age at T0 | P-value difference between groups* | ||||||
| 10 years | 12 years | 15 years | 10 vs. 12 | 10 vs. 15 | 12 vs. 15 | ||
| T0 | No. analyzed | 91 | 91 | 86 | |||
| GMC TT-specific IgG µg/mL (95%CI) | 5.1 (4.3–6.0) | 1.6 (1.3–1.9) | 0.7 (0.6–0.8) | <0.001 | <0.001 | <0.001 | |
| T1 | No. analyzed | 88 | 90 | 85 | |||
| GMC TT-specific IgG µg/mL (95%CI) | 7.4 (6.5–8.5) | 4.4 (3.4–5.1) | 2.9 (2.4–3.5) | 0.069 | 0.213 | 1.000 | |
| T2 | No. analyzed | 85 | 89 | 83 | |||
| GMC TT-specific IgG µg/mL (95%CI) | 3.6 (3.1–4.2) | 1.9 (1.6–2.2) | 1.1 (1.0–1.4) | 0.006 | 0.153 | 0.186 | |
| T1/T0 | GMC ratio (95%CI) | 1.49 (1.34–1.64) | 2.75 (2.42–3.13) | 4.26 (3.59–5.05) | <0.001 | <0.001 | <0.001 |
| T1/T2 | GMC ratio (95%CI) | 2.03 (1.89–2.18) | 2.34 (2.14–2.55) | 2.50 (2.27–2.75) | 0.048 | 0.001 | 0.921 |
NOTE: Differences between age groups in GMC of TT-specific IgG were determined with linear regression analyses, adjusting for concentrations at T0. GMC ratios indicate the level of increase (T1/T0) or decrease (T1/T2) between time points. Differences in GMC ratios between groups were determined with independent sample t-tests.
* P-values were adjusted for three comparisons with Bonferroni correction. Extensive results of the crude and adjusted linear regression analyses are outlined in supplementary table S1.
Discussion
To our knowledge, this is the first study that investigated the immunological effect of an adolescent MenCC booster vaccination nine years after a single priming dose. Prior to the booster, the majority of participants had insufficient MenC-specific protective antibody levels. All participants developed high MenC-specific antibody levels one month after the booster. In addition, all participants were adequately protected one year later with 100% of the SBA titers ≥128. Of importance, the oldest age group (15-year-olds) maintained the highest antibody levels one year after the booster and showed the lowest level of antibody decrease.
The age of priming of the 10-, 12- and 15-year-olds in this study were 14 months, 2.8 years and 5.8 respectively. A Dutch serosurveillance study performed five years after the implementation of the MenCC vaccination in 2002, found that the percentage of children primed at these same ages with an SBA titer ≥8 were 47%, 39% and 50% respectively [12], [31]. At the beginning of our study and nine years after priming, the percentage of children with an SBA titer ≥8 had decreased to 19%, 34% and 45%, respectively. Clearly, the persistence of protective SBA titers after MenC primary vaccination is lower in children primed at 14 months compared to children primed at an older age. This corresponds with the established age-dependent increase in levels and persistence of MenC-specific antibody after primary vaccination [12], [20], [21]. A recent study from the UK reported protective SBA titers in only 15% of children primed ten years earlier with a single dose at age 1–4 years [32]. These findings clearly indicate that a single MenC conjugate vaccination at 14 months, as recommended in the Dutch NIP, is not sufficient to protect against MenC carriage and invasive disease during adolescence.
One month after the MenCC booster vaccination, all participants had developed very high antibody levels, despite differences in age at priming. Antibody levels in all age groups were higher than in comparable studies [18], [19]. An important difference with these studies is that all participants in our study were primed and boosted with a MenC-TT conjugate vaccine and not with the MenC-CRM197 conjugate vaccine. It has been shown that MenC-TT vaccines induce higher antibody responses after primary and booster vaccination and better persistence of antibody levels than MenC-CRM197 vaccines [11], [13], [33]–[35]. MenC-specific antibody levels at T1 were slightly lower in the 10-year-olds compared to the other two age groups. However, no difference between the age groups was found in the level of antibody increase one month following the booster (T1/T0 ratio). This suggests that the mechanism underlying the age-dependent differences in antibody levels after primary vaccination has limited influence on the antibody response to a booster when administered nine years later.
One year after the booster, the 15-year-olds showed the highest antibody levels and the lowest decay. Primary MenC conjugate vaccination during (pre)adolescence has been described to lead to long term persistence of protective SBA titers in >90% of individuals [12], [20]. A recent study from the UK reported even better persistence of SBA titers up to seven years after a booster dose given at the age of 13–15 years [20]. Of note, this booster was administered three years after the primary vaccination and the majority of participants still had protective SBA titers prior to the booster. Nevertheless, SBA-titers at one month and one year after the booster in this UK-study [22] were similar to our results. These findings suggest that a MenC booster administered at the age of 15 years could lead to persistence of protective antibody levels throughout young adulthood. It might even increase the transmission of antibodies from pregnant women to their babies, thereby prolonging protection of young infants and abrogating the need for infant priming. On the other hand, schoolchildren and younger adolescents might remain unprotected for a number of years if the booster is administered at an older age. However, since meningococcal carriage levels peak at approximately 19 years [7], a booster dose at 15 years is likely to prevent most transmission and thereby secure the herd protection. Interestingly, a recent model-based evaluation from Canada predicted that priming infants at 12 months and boosting adolescents at 15 years with a quadrivalent meningococcal conjugate vaccine against serogroups A, C, W and Y will be most effective at reducing incidence of meningococcal disease [36]. These results complement our findings and recommendation.
The high antibody levels after the booster were mostly caused by a rise in IgG1, indicating a T-cell dependent (TD) response. Another feature of TD responses is avidity maturation. Besides a small increase in avidity in the 15-year-olds one month after the booster, no clear increase in avidity was found in any of the groups one year after the booster. This suggests that maximal avidity has been achieved after primary vaccination. Data on avidity development after a (MenC) conjugate booster are scarce. Thus far, it has been shown that avidity increases with time following a primary MenC conjugate vaccination in infants and toddlers [35], [37], [38] and further increases one month after a polysaccharide or a conjugate booster have been described [35], [37], [39]. In contrast, a study in young adults showed high avidity one month after priming (regardless of the vaccine type) with no increase over time, nor - in the case of priming with polysaccharide vaccine - after a booster dose of conjugate vaccine. [40]. Hence, the absence of avidity increase in our study might be an age-related rather than a booster-related phenomenon. Interestingly, we recently reported decreasing avidity indices with increasing age in the cohort primed five years earlier during the catch-up campaign [38]. This reduction in avidity was related to increasing levels of MenC-specific IgG2 with age. In the current study, we also found increasing levels of IgG2 with age, moderate levels of avidity of MenC-specific antibodies overall and a higher proportion of 15-year-olds with low avidity prior to the booster. Our current results therefore underline the suggestion that the immune response to a conjugate vaccine at ages above infancy is not entirely T-cell dependent but also shows increasing features of a T-cell independent response with age [38].
The Dutch NIP offers children a final DT-IPV booster at the age of 9 years. Consequently, TT-specific IgG levels at T0 were highest in the 10-year-olds. After the MenC-TT booster, TT-specific GMCs increased in all age groups. However, a number of participants with a high concentration at T0 – mostly 10-year-olds - showed only a small increase in concentration at T1 and T2. Some 10-year-olds even showed a decrease. It is possible that the newly administered tetanus toxoid was cleared by the high levels of pre-existing antibodies in these individuals. Although the TT-specific GMCs remained far above the international protective threshold of 0.1 IU/mL, this extra MenC-TT booster was not very beneficial for these individuals in terms of tetanus immunity. Therefore, postponing an adolescent MenC-TT booster up to a few years after the last routine tetanus booster seems more beneficial in the long term with regards to protection against tetanus.
This study has important strengths, such as the longitudinal study design, adequate sample size and small loss to follow-up. Furthermore, all participants were primed and boosted with a single dose of the same vaccine. Finally, the 10-year-olds represented children primed according to the current NIP. Limitations of this study were the short follow-up period and that additional demographic information other than age and sex was not collected. Furthermore, participants were primed at different ages. To what extent the latter has influenced the antibody response to the booster is unclear. High antibody levels in all participants at T1 and the absence of significant differences between the age groups in the level of antibody increase in the first month after the booster, suggests that the influence of the age at priming on the memory response was minor. However, we cannot be certain that we would observe similar antibody responses in 15-year-olds primed at 14 months. The proportion of 10-year-olds in this study, all primed at 14 months, with an SBA titer <8 at T0 was >80%. Therefore, it is to be expected that virtually all individuals primed at 14 months will have an SBA titer<8 by the time they are 15. To anticipate for this, we performed a sub analysis including only participants with an SBA titer <8 (i.e. not protected) at T0. We found similar antibody levels following the booster and similar differences between the age groups in antibody decay as when total groups were analyzed (data not shown).
To conclude, nine years after their primary MenCC vaccination, adolescents develop considerably high antibody levels in response to a MenCC booster vaccination and are still sufficiently protected one year later. The oldest age group in this study maintained the highest (protective) antibody levels one year after the booster and showed the lowest level of antibody decrease. This suggests that persistence of individual - and indirect herd - protection increases with the age at which an adolescent MenCC booster is administered.
Supporting Information
Results of crude and adjusted linear regression analyses for differences between age groups in Meningococcal serogroup C specific SBA geometric mean titers (GMTs) and geometric mean concentrations (GMCs) of IgG, IgG1, IgG2, IgG1/IgG2 ratio and tetanus toxoid (TT)-specific IgG.
(DOCX)
Study protocol as approved by the local ethics committee Verenigde Commissies Mensgebonden Onderzoek (VCMO, Nieuwegein, The Netherlands).
(PDF)
TREND checklist for standardized reporting of this non-randomized controlled trial.
(XLSX)
Acknowledgments
We thank all the individuals that participated in this study and their parents. We also thank the nurses who performed the vaccinations and blood collections and Dr. Tom Wolfs from the UMC Utrecht for being the independent doctor for this study. Furthermore, from the RIVM we would like to thank Jan van de Kassteele for performing the power calculation, Petra Oomen for providing the list of eligible children and assistance with retrieving individual vaccination histories of participants, Nelleke Bakker for assistance during the recruitment of participants and Pieter van Gageldonk for measurement of tetanus toxoid specific IgG levels.
Funding Statement
This study was funded by the Dutch Ministry of Health. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Baxter kindly provided the vaccines.
References
- 1. Jafri RZ, Ali A, Messonnier NE, Tevi-Benissan C, Durrheim D, et al. (2013) Global epidemiology of invasive meningococcal disease. Popul Health Metr 11: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neppelenbroek SE, de Vries M, de Greeff SC, Timen A (2003) “Da's goed gedaan?” Report of the Dutch national vaccination campaign against meningococcal serogroup C disease in 2002. Utrecht: Municipal and Regional Health Service (GGD), The Netherlands. [Google Scholar]
- 3.van Lier EA, Oomen PJ, Mulder M, Conyn-van Spaendonck MAE, Drijfhout IH, et al. (2013) Vaccinatiegraad Rijksvaccinatieprogramma Nederland: Verslagjaar 2013 Rijksinstituut voor Volksgezondheid en Milieu (RIVM), Bilthoven, Nederland.
- 4.van 't Klooster T, de Melker H (2013) The National Immunisation Programme in the Netherlands: Developments in 2012. Institute of Public Health and the Environment (RIVM), Bilthoven, The Netherlands. 201001002/2012.
- 5. Maiden MC, Ibarz-Pavon AB, Urwin R, Gray SJ, Andrews NJ, et al. (2008) Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis 197: 737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Trotter CL, Gay NJ, Edmunds WJ (2005) Dynamic models of meningococcal carriage, disease, and the impact of serogroup C conjugate vaccination. Am J Epidemiol 162: 89–100. [DOI] [PubMed] [Google Scholar]
- 7. Christensen H, May M, Bowen L, Hickman M, Trotter CL (2010) Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis 10: 853–861. [DOI] [PubMed] [Google Scholar]
- 8. Maiden MC, Stuart JM (2002) Group UKMC (2002) Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. Lancet 359: 1829–1831. [DOI] [PubMed] [Google Scholar]
- 9. Trotter CL, Maiden MC (2009) Meningococcal vaccines and herd immunity: lessons learned from serogroup C conjugate vaccination programs. Expert Rev Vaccines 8: 851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trotter CL, Andrews NJ, Kaczmarski EB, Miller E, Ramsay ME (2004) Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet 364: 365–367. [DOI] [PubMed] [Google Scholar]
- 11. Borrow R, Andrews N, Findlow H, Waight P, Southern J, et al. (2010) Kinetics of antibody persistence following administration of a combination meningococcal serogroup C and haemophilus influenzae type b conjugate vaccine in healthy infants in the United Kingdom primed with a monovalent meningococcal serogroup C vaccine. Clin Vaccine Immunol 17: 154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Voer RM, Mollema L, Schepp RM, de Greeff SC, van Gageldonk PG, et al. (2010) Immunity against Neisseria meningitidis serogroup C in the Dutch population before and after introduction of the meningococcal c conjugate vaccine. PLoS One 5: e12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khatami A, Snape MD, John T, Westcar S, Klinger C, et al. (2011) Persistence of immunity following a booster dose of Haemophilus influenzae type B-Meningococcal serogroup C glycoconjugate vaccine: follow-up of a randomized controlled trial. Pediatr Infect Dis J 30: 197–202. [DOI] [PubMed] [Google Scholar]
- 14. Snape MD, Kelly DF, Green B, Moxon ER, Borrow R, et al. (2005) Lack of serum bactericidal activity in preschool children two years after a single dose of serogroup C meningococcal polysaccharide-protein conjugate vaccine. Pediatr Infect Dis J 24: 128–131. [DOI] [PubMed] [Google Scholar]
- 15. Blanchard Rohner G, Snape MD, Kelly DF, John T, Morant A, et al. (2008) The magnitude of the antibody and memory B cell responses during priming with a protein-polysaccharide conjugate vaccine in human infants is associated with the persistence of antibody and the intensity of booster response. J Immunol 180: 2165–2173. [DOI] [PubMed] [Google Scholar]
- 16. de Voer RM, van der Klis FR, Engels CW, Schepp RM, van de Kassteele J, et al. (2009) Kinetics of antibody responses after primary immunization with meningococcal serogroup C conjugate vaccine or secondary immunization with either conjugate or polysaccharide vaccine in adults. Vaccine 27: 6974–6982. [DOI] [PubMed] [Google Scholar]
- 17. Kelly DF, Snape MD, Perrett KP, Clutterbuck EA, Lewis S, et al. (2009) Plasma and memory B-cell kinetics in infants following a primary schedule of CRM 197-conjugated serogroup C meningococcal polysaccharide vaccine. Immunology 127: 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Snape MD, Kelly DF, Salt P, Green S, Snowden C, et al. (2006) Serogroup C meningococcal glycoconjugate vaccine in adolescents: persistence of bactericidal antibodies and kinetics of the immune response to a booster vaccine more than 3 years after immunization. Clin Infect Dis 43: 1387–1394. [DOI] [PubMed] [Google Scholar]
- 19. Perrett KP, Winter AP, Kibwana E, Jin C, John TM, et al. (2010) Antibody persistence after serogroup C meningococcal conjugate immunization of United Kingdom primary-school children in 1999–2000 and response to a booster: a phase 4 clinical trial. Clin Infect Dis 50: 1601–1610. [DOI] [PubMed] [Google Scholar]
- 20. de Whalley PC, Snape MD, Plested E, Thompson B, Nuthall E, et al. (2013) Long-term seroprotection after an adolescent booster meningococcal serogroup C vaccination. Arch Dis Child 98: 686–691. [DOI] [PubMed] [Google Scholar]
- 21. Snape MD, Kelly DF, Lewis S, Banner C, Kibwana L, et al. (2008) Seroprotection against serogroup C meningococcal disease in adolescents in the United Kingdom: observational study. BMJ 336: 1487–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Whalley PC, Snape MD, Kelly DF, Banner C, Lewis S, et al. (2011) Persistence of serum bactericidal antibody one year after a booster dose of either a glycoconjugate or a plain polysaccharide vaccine against serogroup C Neisseria meningitidis given to adolescents previously immunized with a glycoconjugate vaccine. Pediatr Infect Dis J 30: e203–208. [DOI] [PubMed] [Google Scholar]
- 23.European Centre for Disease Prevention and Control, Vaccine Scheduler (Acces date: January 6, 2014) available from: http://vaccine-schedule.ecdc.europa.eu/Pages/Scheduler.aspx.
- 24. Maslanka SE, Gheesling LL, Libutti DE, Donaldson KB, Harakeh HS, et al. (1997) Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. The Multilaboratory Study Group. Clin Diagn Lab Immunol 4: 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andrews N, Borrow R, Miller E (2003) Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin Diagn Lab Immunol 10: 780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Borrow R, Balmer P, Miller E (2005) Meningococcal surrogates of protection—serum bactericidal antibody activity. Vaccine 23: 2222–2227. [DOI] [PubMed] [Google Scholar]
- 27. de Voer RM, van der Klis FR, Engels CW, Rijkers GT, Sanders EA, et al. (2008) Development of a fluorescent-bead-based multiplex immunoassay to determine immunoglobulin G subclass responses to Neisseria meningitidis serogroup A and C polysaccharides. Clin Vaccine Immunol 15: 1188–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lal G, Balmer P, Joseph H, Dawson M, Borrow R (2004) Development and evaluation of a tetraplex flow cytometric assay for quantitation of serum antibodies to Neisseria meningitidis serogroups A, C, Y, and W-135. Clin Diagn Lab Immunol 11: 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Gageldonk PG, van Schaijk FG, van der Klis FR, Berbers GA (2008) Development and validation of a multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J Immunol Methods 335: 79–89. [DOI] [PubMed] [Google Scholar]
- 30. Newcombe RG (1998) Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 17: 857–872. [DOI] [PubMed] [Google Scholar]
- 31. van der Klis FR, Mollema L, Berbers GA, de Melker HE, Coutinho RA (2009) Second national serum bank for population-based seroprevalence studies in the Netherlands. Neth J Med 67: 301–308. [PubMed] [Google Scholar]
- 32. Khatami A, Peters A, Robinson H, Williams N, Thompson A, et al. (2011) Maintenance of immune response throughout childhood following serogroup C meningococcal conjugate vaccination in early childhood. Clin Vaccine Immunol 18: 2038–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Diez-Domingo J, Cantarino MV, Torrenti JM, Sansano MI, Rosich AJ, et al. (2010) A randomized, multicenter, open-label clinical trial to assess the immunogenicity of a meningococcal C vaccine booster dose administered to children aged 14 to 18 months. Pediatr Infect Dis J 29: 148–152. [DOI] [PubMed] [Google Scholar]
- 34. Findlow H, Borrow R, Andrews N, Waight P, Sheasby E, et al. (2012) Immunogenicity of a single dose of meningococcal group C conjugate vaccine given at 3 months of age to healthy infants in the United kingdom. Pediatr Infect Dis J 31: 616–622. [DOI] [PubMed] [Google Scholar]
- 35. Richmond P, Borrow R, Goldblatt D, Findlow J, Martin S, et al. (2001) Ability of 3 different meningococcal C conjugate vaccines to induce immunologic memory after a single dose in UK toddlers. J Infect Dis 183: 160–163. [DOI] [PubMed] [Google Scholar]
- 36.Vickers DM, Anonychuk AM, De Wals P, Demarteau N, Bauch CT (2013) Evaluation of serogroup C and ACWY meningococcal vaccine programs: Projected impact on disease burden according to a stochastic two-strain dynamic model. Vaccine. [DOI] [PubMed]
- 37. Borrow R, Goldblatt D, Finn A, Southern J, Ashton L, et al. (2003) Immunogenicity of, and immunologic memory to, a reduced primary schedule of meningococcal C-tetanus toxoid conjugate vaccine in infants in the United kingdom. Infect Immun 71: 5549–5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Voer RM, van der Klis FR, Schepp RM, Rijkers GT, Sanders EA, et al. (2011) Age-related immunity to meningococcal serogroup C vaccination: an increase in the persistence of IgG2 correlates with a decrease in the avidity of IgG. PLoS One 6: e23497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harris SL, Tsao H, Ashton L, Goldblatt D, Fernsten P (2007) Avidity of the immunoglobulin G response to a Neisseria meningitidis group C polysaccharide conjugate vaccine as measured by inhibition and chaotropic enzyme-linked immunosorbent assays. Clin Vaccine Immunol 14: 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goldblatt D, Borrow R, Miller E (2002) Natural and vaccine-induced immunity and immunologic memory to Neisseria meningitidis serogroup C in young adults. J Infect Dis 185: 397–400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of crude and adjusted linear regression analyses for differences between age groups in Meningococcal serogroup C specific SBA geometric mean titers (GMTs) and geometric mean concentrations (GMCs) of IgG, IgG1, IgG2, IgG1/IgG2 ratio and tetanus toxoid (TT)-specific IgG.
(DOCX)
Study protocol as approved by the local ethics committee Verenigde Commissies Mensgebonden Onderzoek (VCMO, Nieuwegein, The Netherlands).
(PDF)
TREND checklist for standardized reporting of this non-randomized controlled trial.
(XLSX)