Abstract
Background
Many authors have claimed that snakebite risk is associated with human population density, human activities, and snake behavior. Here we analyzed whether environmental suitability of vipers can be used as an indicator of snakebite risk. We tested several hypotheses to explain snakebite incidence, through the construction of models incorporating both environmental suitability and socioeconomic variables in Veracruz, Mexico.
Methodology/Principal Findings
Ecological niche modeling (ENM) was used to estimate potential geographic and ecological distributions of nine viper species' in Veracruz. We calculated the distance to the species' niche centroid (DNC); this distance may be associated with a prediction of abundance. We found significant inverse relationships between snakebites and DNCs of common vipers (Crotalus simus and Bothrops asper), explaining respectively 15% and almost 35% of variation in snakebite incidence. Additionally, DNCs for these two vipers, in combination with marginalization of human populations, accounted for 76% of variation in incidence.
Conclusions/Significance
Our results suggest that niche modeling and niche-centroid distance approaches can be used to mapping distributions of environmental suitability for venomous snakes; combining this ecological information with socioeconomic factors may help with inferring potential risk areas for snakebites, since hospital data are often biased (especially when incidences are low).
Introduction
Only a small percentage (10–15%) of ca. 3000 known species' of snakes is venomous, and thus potentially dangerous to humans [1]. However, in the tropics, snakebites are a significant cause of human mortality and morbidity, with important impacts on human health, as well as to economy through treatment-related expenses and loss of productivity [2]. Recent estimates suggest that at least 421,000 bites and 20,000 deaths occur worldwide from snakebite annually, possibly ranging as high as 1,841,000 bites and 94,000 deaths [3]. The most affected regions in the world are sub-Saharan Africa, Southeast Asia, and Latin America [3]–[5].
Despite the scale of effects on human populations, snakebites has not received much attention from national and international health authorities, and has now been categorized as a “neglected tropical disease” [6]. Diverse authors have studied the problem [3], [7]–[10]; however, most of these studies involve hospital records, and the representativeness of this information has been questioned [7]–[10]. According to Chippaux [8], prospective enquiries in randomly selected localities is preferable, but this procedure would be long and expensive. Hansson et al. [9] developed an index of potential underreported cases of snakebites using environmental, socioeconomics and health-care related variables, a valuable contribution towards a better understanding of snakebite incidence, but other aspects of the phenomenon may enrich the view, such as the specific identity, geographic distribution and abundance of venomous snakes involved in the incidents.
Ecological niche modeling (ENM) has been used widely in recent years to map potential geographic distribution of species', with reliable results [11]. Nevertheless, capacity of these models to inform about the distribution patterns of abundance is, in the best case, limited [12], [13]. Recently, however, a new method based on the distance to niche centroid (DNC) was proposed, that may offer a better understanding of how abundance is structured within the margins of species' distributions [14]. This method seeks a relationship between distance to the niche centroid and abundance; therefore, it can used as a measure of environmental suitability (ES), wherein conditions close to the niche centroid represent higher suitability, and therefore potentially higher abundance [14], [15].
The principal goal of this paper was to evaluate the utility of ENM and DNC approaches in combination with socioeconomic variables to infer potential risk areas of snakebites in the state of Veracruz, Mexico. We first modeled the geographic and ecological distribution of each venomous snake species' occurring in the region; then DNCs were calculated for each species based on the ENMs. We analyzed relationships between reported incidences of snakebite and DNC values. Finally, we built several models relating DNC of snakes and socioeconomic variables to snakebite incidence. We selected the state of Veracruz based on the next criteria: (1) it has the second highest snakebite rate in Mexico, with approximately 15% of the country's total fatal accidents per year [16]; (2) arguably the most dangerous snake in Latin America (Bothrops asper) is widely distributed in the state [17], [18]; and (3) three authors of this paper have been working and living in this region for many years, and hence know well the area and the snakes occurring there.
Methods
Study area
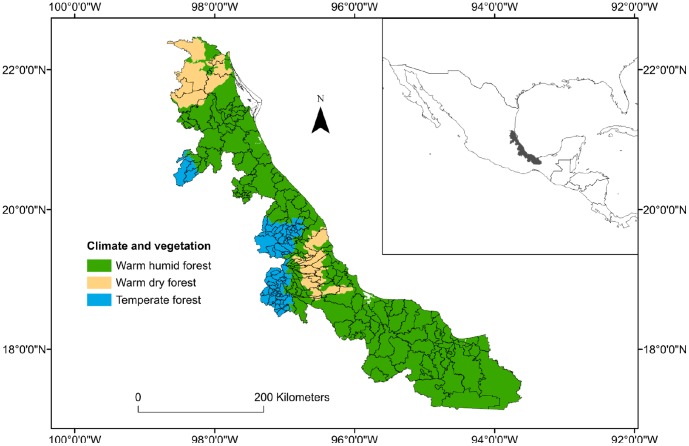
The state of Veracruz is located on Mexico's Gulf coastal fringe extending 745 km north to south, covering 72,420 km2 (3.7% of the total area of the country) (Figure 1). About 80% of the area has been transformed by expansion of the agricultural frontier and human settlements [19]. The state includes a long coastal plain and a complex mountain system including parts of the Eje Volcánico Transmexicano and the Sierra Madre Oriental [20]; elevations vary from sea level to more than 5000 m [21], [22].
Figure 1. Climate, vegetation and topography of study area (Veracruz, Mexico).
Black lines represent municipalities.
Study species
Twenty-one species' of venomous snakes are present in Veracruz: 16 vipers and 5 coral snakes [17]. However, we discarded coral snakes for consideration since the potential danger that they present is minimal [23]. We also eliminated rare species' as the probability of encounter of these snakes with humans is low [17], and because presence records for these species' are scarce, which affects performance of ENMs (i.e., [24]–[26]). Therefore, we selected as study species' only vipers with six or more presence records in the state: Atropoides nummifer, Bothrops asper, Crotalus atrox, C. intermedius, C. molossus, C. ravus, C. simus, C. totonacus, and C. triseriatus.
Ecological Niche Modeling
Occurrence data for the entire geographic range of snake species' were gathered from three sources: 1. Unpublished personal records (these were occasional observations obtained from work activities in wildlife monitoring. No special permits were required because we did not handle the snakes in any way), 2. Specialized literature [17], [27], [28], and 3. Online available information accessible through the Global Biodiversity Information Facility (GBIF; http://www.gbif.org), the World Information Network on Biodiversity (REMIB: www.conabio.gob.mx/remib/doctos/remib_esp.html) and HerpNet (http://www.herpnet.org). Occurrences lacking latitude-longitude coordinates were georeferenced with gazetteers and Google Earth (http://earth.google.es/). Because presence-only data may present sampling bias and spatial autocorrelation that negatively impact model performance [29], we overlaid a 0.05° resolution reticule over the study region and randomly removed duplicates, leaving a single occurrence per grid cell [30]. As well, we removed doubtful and ambiguous occurrences.
Environmental layers regarding climate and topography were used to generate the ENMs (Table S1); climate variables were obtained from the WorldClim database (http://www.worldclim.org) [31] and topographic information was derived from the SRTM elevation model (http://srtm.csi.cgiar.org). All environmental data were standardized to geographic coordinates (Datum WGS-84) at a spatial resolution of 30”. We screened for collinearity by examining pairwise correlations between variables for each species. When a pair had a Pearson product-moment correlation coefficient >0.7, one of the two variables was removed [32]. For each viper, the extent of the layers varied according to the limits of biogeographic provinces [33] containing all its occurrences [34].
Maps of potential distributions for each species were obtained using desktop GARP [35], an evolutionary, computing algorithm that has been tested extensively for predictions of the geographic distributions of species' [36]–[39]. We developed 100 replicate models for each species based on bootstrapped subsamples of available occurrence data. Following Anderson et al. [40] we retained the 10 best models as these having the lowest omission error and lowest departure from the median area predicted suitable. For each species these models were summed in ArcGIS 10 to produce a consensus map [41]. Finally, consensus maps were transformed to produce binary maps using the minimum presence value (MPV) as a threshold criteria, namely the highest raw suitability value at which all input occurrence points were included in the presence area.
In all cases, 80% of presence records were used in model calibration and the remaining 20% were used for model evaluation. We evaluated model performance using a partial ROC approach following Peterson et al. [42], a modification of the area under the curve (AUC), and receiver operating characteristic (ROC) approach [43]. This method avoids some disadvantages of the traditional ROC method [44], and is implemented in a stand-alone software [45].
To characterize ecological niches of vipers and calculate DNC values, we followed Yañez-Arenas et al. [15] and Martínez-Meyer et al. [14]. In brief, we extracted values of environmental variables for all pixels where the species' was predicted present according to the binary potential distribution maps. We standardized each dimension by subtracting the mean and dividing by the standard deviation, producing a standard normal variable (i.e., mean = 0, variance = 1). In this way, the multidimensional niche centroid is the point where values of all variables is 0, and calculations of DNCs become simple decompositions of Euclidean distances. Finally, using the “Zonal Statistics as Table” option in ArcGIS 10 [41], we obtained the mean distance to the niche centroid in each municipality of Veracruz based in a data set of municipalities [46]. Distances were calculated for each municipality because snakebite reports and the socioeconomic variables described in the next section were developed at this level.
Snakebite incidence and socioeconomic variables
Snakebite reports for each municipality in the period 2003-2012 were obtained through the Sistema Único Automatizado para la Vigilancia Epidemiológica (SUAVE; Secretaría de Salud del Estado de Veracruz). In order to reduce variability caused by unequal population size in municipalities, we estimated the smoothed snakebite incidence (expressed per 100,000 inhabitants over this ten year period) using the tool for automated spatial Bayesian smoothing of incidence rates available in SIGEpi v 1.4 [47]. Smoothed incidence was linked to the dataset of municipalities. Separately, we obtained socioeconomic information from INEGI [46] including fields summarizing human population density, an index of marginalization, and percentage of population without health insurance, all of which have previously been considered as associated with snakebites [48], [49].
Generalized additive models (GAMs) with quasi-Poisson family responses were used to evaluate relationships between smoothed snakebite incidence and predictor variables [50]. We first built univariate models and then tested all possible combinations (multivariate models with interactions) of significant variables (P<0.05). All estimated parameter effects for each hypothesis were evaluated by comparing generalized cross-validation (GCV) scores [51]. GAM analyses were performed via the mgcv library [52] in R 2.8.1 [53].
Results
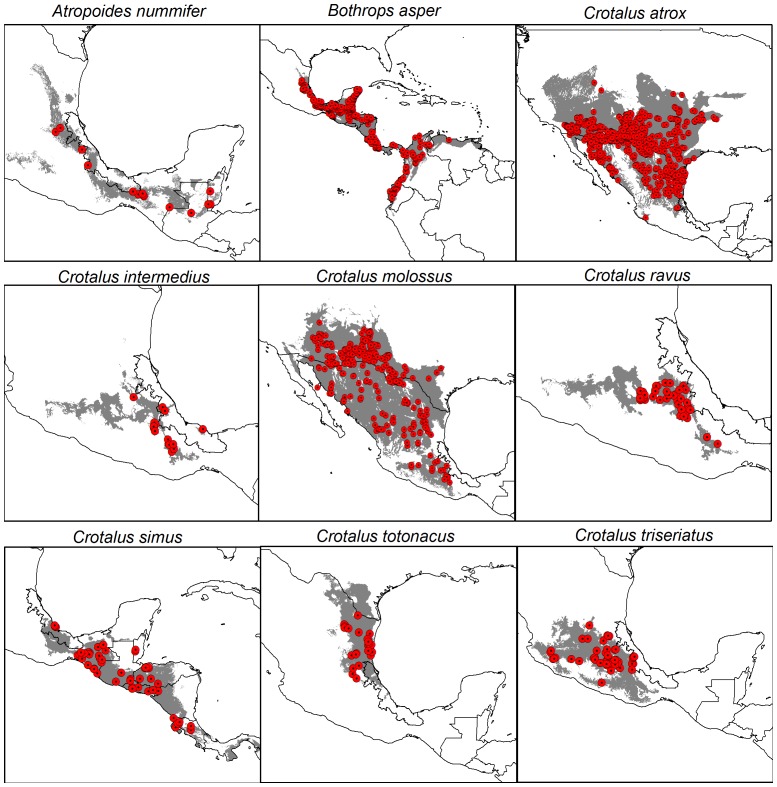
We first obtained 2,408 occurrences for viper species' distributed in Veracruz. However, after depuration and filtering there were 1,665 spatially unique localities (Figure 2, Dataset S1). Records were not distributed equally among species': almost 90% belonged to three species (B. asper, C. atrox and C. molossus). Whereas for A. nummifer and C. intermedius, we could only gather 14 and 15 presences (Table 1).
Figure 2. Occurrences and potential distributions of the vipers commonly distributed in Veracruz, Mexico.
Table 1. Viper occurrence data (N) and Partial ROC analyses results (Mean Ratio = MR, standard deviation = SD, significance = P).
| Partial ROC | ||||
| Species | N | MR | SD | P |
| A. nummifer | 14 | 1.977 | 0.008 | <0.001*** |
| B. asper | 273 | 1.406 | 0.029 | <0.001*** |
| C. atrox | 851 | 1.262 | 0.025 | <0.001*** |
| C. intermedius | 15 | 1.449 | 0.078 | <0.001*** |
| C. molossus | 336 | 1.146 | 0.018 | <0.001*** |
| C. ravus | 41 | 1.340 | 0.095 | <0.001*** |
| C. simus | 53 | 1.660 | 0.035 | <0.001*** |
| C. totonacus | 24 | 1.964 | 0.004 | <0.001*** |
| C. triseratus | 58 | 1.938 | 0.029 | <0.001*** |
| Total | 1665 | - | - | |
*** = <0.001, ** = <0.01, * = <0.05.
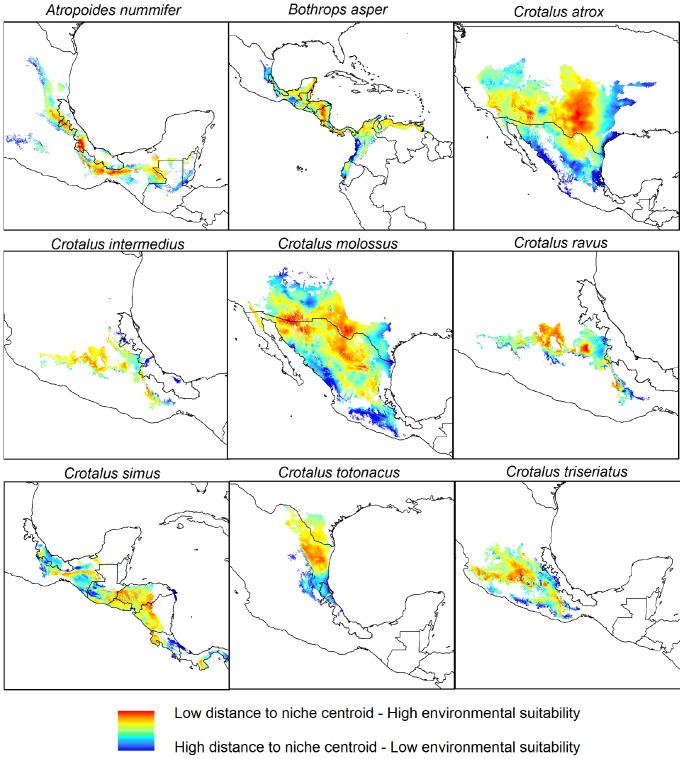
Models developed for each species corresponded generally well with knowledge of species' distributions (Figure 2) and their environmental preferences (Figure 3) [16]. Partial ROC analysis exhibited high average AUC ratios and low standard deviations for all models; according to this test, all were significantly better than random (Table 1).
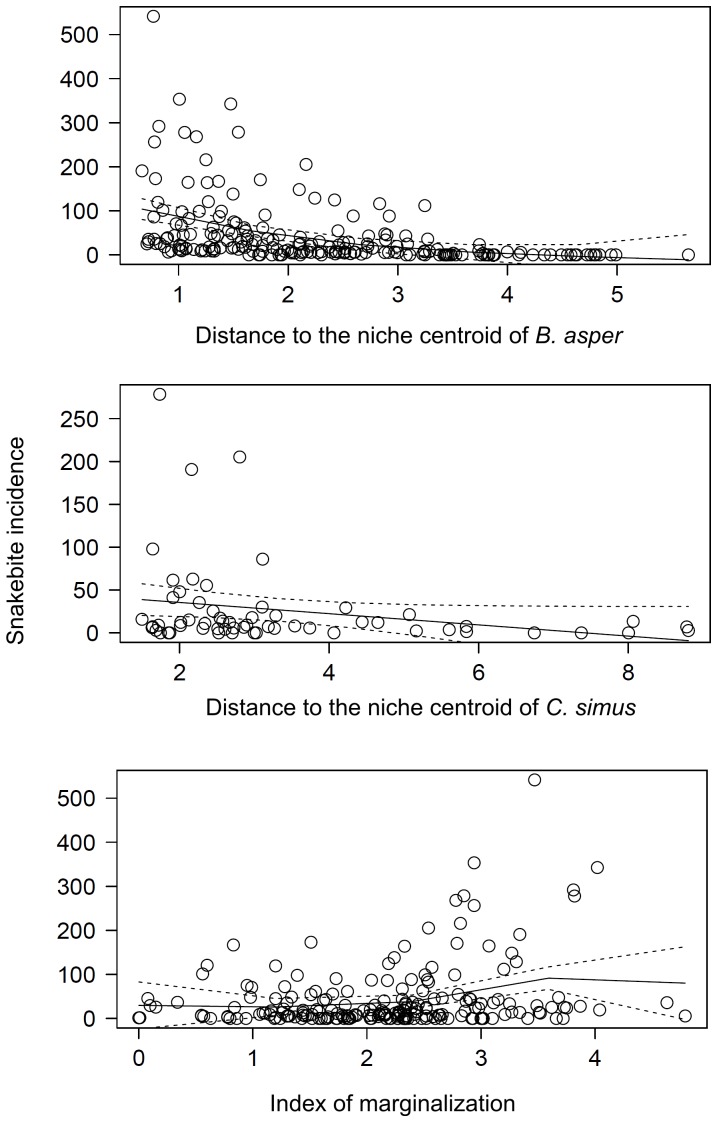
Figure 3. Geographic representation of the distance to niche centroid of the vipers.
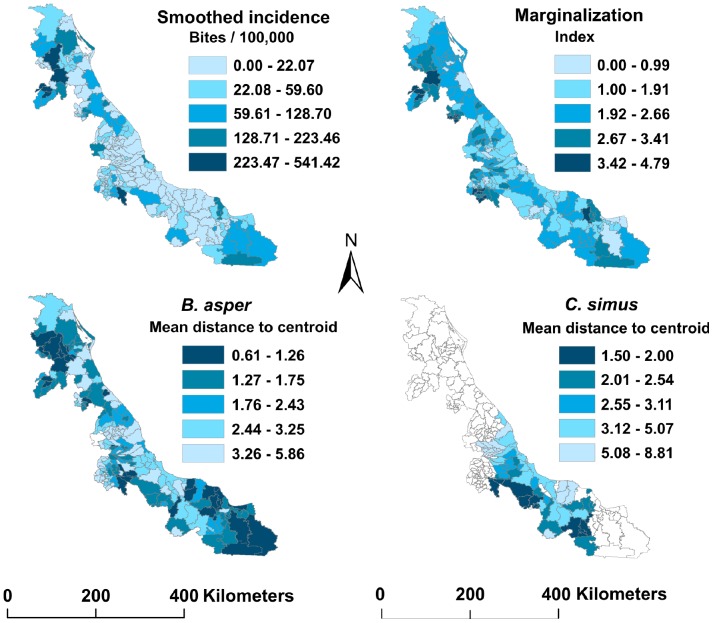
A total of 3,765 snakebite cases were reported for Veracruz during 2003–2012, corresponding to an average incidence of 4.93 (±1.30) snakebites per 100,000 inhabitants per year. Municipalities with the highest smoothed incidence corresponded to the northern and southern regions of Veracruz, whereas the central-east region presented the lowest incidence (Figure 4).
Figure 4. Distribution per municipality of smoothed snakebite incidence (2003–2012), marginalization and distances to niche centroid of Crotalus simus and Bothrops asper.
White municipalities have no data.
Univariate generalized additive models showed that DNC of most vipers were negatively correlated with snakebite incidence (all but C. totonacus and A. nummifer). However, these relationships were significant only for B. asper and C. simus. The former explained almost 35% of model deviance and the latter 15%. Marginalization of human populations was positive correlated with snakebites, and explained an additional 17% of deviance (Table 2, Figure 5).
Table 2. Univariate generalized additive models relating snakebite incidence and explanatory variables.
| Variable | p value | R2 (adj) | DE (%) |
| DNC of B. asper | <0.001*** | −0.191 | 34.9 |
| Index of marginalization | <0.001*** | 0.101 | 17.5 |
| DNC of C. simus | 0.014* | −0.074 | 15.1 |
| DNC of C. atrox | 0.063 | −0.057 | 14.3 |
| DNC of C. intermedius | 0.052 | −0.036 | 13.6 |
| DNC of C. triseriatus | 0.141 | −0.025 | 11.4 |
| DNC of C. ravus | 0.606 | −0.024 | 9.3 |
| DNC of C. molossus | 0.494 | −0.017 | 9.1 |
| Population density (ind/km) | 0.302 | 0.005 | 3.4 |
| DNC of C. totonacus | 0.330 | 0.009 | 2.7 |
| Population without health insurance (%) | −0.702 | −0.008 | 1.3 |
| DNC of A. nummifer | 0.677 | 0.007 | 0.1 |
R2 (adj) = adjusted coefficient of determination, DE (%) = percentage of deviance explained.
*** = <0.001, ** = <0.01, * = <0.05.
Figure 5. Relationships between snakebite incidence and distances to niche centroid of Crotalus simus, Bothrops asper and the marginalization index (INEGI 2010).
In multivariate models, comparisons of candidate models revealed that DNC for B. asper was a very important parameter explaining snakebite incidence, being included in all of the top five best models. The best-fit model explained 76% of deviance and is based on the DNCs of B. asper and C. simus and marginalization (Table 3).
Table 3. Multivariate generalized additive models relating snakebite incidence and explanatory variables.
| Model | R2(adj) | DE (%) | GCV |
| 1. (csim, marg)+(basp, marg) | 0.695 | 76.0% | 24.893 |
| 2. csim+basp+marg | 0.678 | 71.7% | 25.335 |
| 3. csim+basp | 0.265 | 46.1% | 37.797 |
| 4. basp+marg | 0.406 | 52.6% | 41.97 |
| 5. (basp, marg) | 0.388 | 53.0% | 42.614 |
R2 (adj) = adjusted coefficient of determination, DE (%) = percentage of deviance explained, GCV = generalized cross validation score. Model parameter keys: basp = DNC of Bothrops asper, csim = DNC of Crotalus simus, marg = marginalization.
Discussion
Recent developments in the field of ENM and broad availability of rich global environmental data sets have augmented ability to predict distributions of species' related to transmission of diseases [54]–[57]. However, until now, the problem of snakebite has never been addressed. Our results demonstrate that this can be done by mapping potential environmental suitability of vipers through the DNC approach [14], [15].
We found significant inverse relationships between snakebite incidence and DNCs for B. asper and C. simus. These species' are the main cause of incidents in the region [58], and our results suggest that, when sufficient occurrence information is available, ENM and DNC approaches, offer an alternative approach to understanding snakebite incidence risk. B. asper is probably the most dangerous snake in Latin America, because of its broad distribution, size, habits and aggressiveness; also, according to Sasa and Vázquez [18], this viper is well adapted to environments affected by small-scale agriculture, making snake-human encounters frequent during agricultural activities in fields and close to rural dwellings. C. simus is not widely distributed in Latin America, but it is quite common in Veracruz and is frequently found in areas of livestock and crops, increasing rates of encounter with humans [17].
For the remaining species', we found no significant relationships between DNC and snakebite incidence. Habitat preferences could be responsible for this lack of relationship, although certainly other factors enter the picture as well (behavior, demography, distribution patterns, among others). Crotalus molossus, C. triseriatus, C. ravus and C. intermedius inhabit diverse vegetation types, but principally desert and pine-oak forest [17]; more open vegetation than tropical forests, making it easier to detect the presence of the vipers. C. totonacus and C. atrox also occur in tropical deciduous forest, but the former is a very uncommon snake and the latter prefers other ecosystems (deserts, mesquite grasslands, scrublands and pine-oak forest) [17]. The probability of encounters between humans and A. nummifer is low, because this species inhabits mainly primary well-preserved forests, where anthropogenic activities are scarce [17].
Examination of the shape of the relationships between DNC and snakebite incidence suggest that DNC indicates the upper limit of snakebite incidence rates, rather than the average (Figure 5). An important observation is that several municipalities presented high environmental suitability, but incidences that were nil or very low. That is, according to the abundance-DNC relationship hypothesis, when DNC is low, the species is expected to be abundant; however, diverse factors may affect this relationship, such as microclimate, biotic interactions and dispersal limitations, which may depress abundance in otherwise suitable areas [59]. On the other hand, hospital data may be biased, as has been noted elsewhere in the world [60], [61], especially when incidences are low. Snakebite incidence is frequently underreported owing to lack of effective health infrastructure in marginalized rural communities, and because many cases are not reported because patients either prefer traditional medicine or die before reaching hospitals [62]–[65]. The contrasts between hospital reports and community data suggest that most snakebite victims turn first to traditional healers and only go to hospitals when poisoning is severe and traditional treatments inadequate [48]. Conversely, in some areas adjacent to the big cities the experience and reputation of urban health centers can attract some patients which does not reflect actual local incidence rates. Our use of smoothed incidences to attenuate variability caused by unequal population size in municipalities should help in this regard, however there are many drawbacks in case notification rates that encourage develop of alternative approaches to inferring potential snakebite risk, such as the DNC method; environmental suitability can complement socioeconomic and health-related factors to complete the picture of the phenomenon (see below).
Human population has been considered as inversely correlated with snakebite incidence [48], [66], [67]. This inverse relationship may be explained by both, reduction of snake populations in human populated areas, and changes in human condition and occupation [68], [69]. Activities in rural areas such as agriculture, grazing and fishing significantly increase risk of snake encounter [7], [8]. In Veracruz, we did not observe significant relationships between human population density and snakebite incidence (Table 2). This may be due to the scale of our analysis, the characteristics of human populations, or the presence and abundance of the vipers. For instance, some of the municipalities with lowest human densities in the state are also arid areas, where B. asper and C. simus are absent or uncommon. Another important social factor is poverty: at global scales, Harrison et al. [49] demonstrated that socioeconomic indicators of poverty correlate with snakebite-induced mortality. We observed a positive correlation between snakebite incidence and marginalization of municipalities in Veracruz, would could reflect a frequent association between the latter and increased manual agricultural activities (Figure 5, Table 2). Regarding health related factors, we did not observe relationships between snakebite incidence rates and percentage of population without health insurance (Table 2). One possible explanation for this result is because municipalities with 100% of inhabitants without health insurance may also lack proper systems of snakebite detection. As such, zero cases in a municipality could be real, or may reflect failures in data collection.
Each of these factors interacts with others, as was demonstrated in this study via the multivariate generalized additive models; consequently, it is important to take all into account for a better understanding of the epidemiological problem that snakebites present. The ENM-DNC method approach, in combination with socioeconomic variables, could help in this task by mapping potential distributions and environmental suitability for dangerous snakes, identifying areas of greater potential risk in which to focus educational and medical remediation in the form of supplies and facilities. These analyses should also be applied elsewhere in the world to evaluate the generality of our findings. A further corroboration of our models would be direct population density estimates for key viper species in areas of differing DNC (although this task is much complicated by the secretive nature of many of the species), and detailed assessment of snakebite incidence through household surveys.
Supporting Information
Variables used in distribution models. Please note that temperature data are in °C * 10, units used for the precipitation data is mm (millimeters), altitude is expressed in masl (meters above sea level) and slope in percentage (%).
(DOCX)
Sources for occurrence data of vipers.
(DOCX)
Databases with the occurrences for all species' modeled. Codes for each database: Atropoides nummifer = r_a_num, Bothrops asper = r_b_asper, Crotalus atrox = r_c_atr, C. intermedius = r_c_int, C. molossus = r_c_mol, C. ravus = r_c_rav, C. simus = r_c_sim, C. totonacus = r_c_tot, C. triseriatus = r_c_tri.
(RAR)
Cases of snakebites in Veracruz (2003–2012). Raw information obtained through the Sistema Único Automatizado para la Vigilancia Epidemiológica (SUAVE; Secretaría de Salud del Estado de Veracruz).
(RAR)
Acknowledgments
We thank CONACyT for supporting the postdoctoral stay of Carlos Yañez-Arenas at the University of Kansas, Jorge M. Lobo, Karla Rodríguez Medina, Rocío Arenas and Arturo Yañez for comments on the preliminary idea, biocollections institutions for occurrences of vipers (Table S2) and the Secretaría de Salud del Estado de Veracruz for providing snakebite data (Dataset S2).
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the Supporting Information files.
Funding Statement
CONACYT support the postdoctoral stay of Carlos Yañez-Arenas at the University of Kansas. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Warrell D (2010) Snake bite. The Lancet 375: 77–88. [DOI] [PubMed] [Google Scholar]
- 2. Faiz MA, Hossain MR, Younus A, Das EB, Karim JC (1995) A hospital based survey of snake bite in Chittagong Medical College. J Bangladesh Coll Phys Surg 13: 3–8. [Google Scholar]
- 3. Kasturiratne A, Wickremasinghe AR, de Silva N, Gunawardena NK, Pathmeswaran A, et al. (2008) The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med 5: 1591–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gutiérrez JM, Williams D, Fan HW, Warrell DA (2010) Snakebite envenoming from a global perspective: towards an integrated approach. Toxicon 56: 1223–1235. [DOI] [PubMed] [Google Scholar]
- 5. White J (2000) Bites and stings from venomous animals: a global overview. Ther Drug Monit 22: 65–68. [DOI] [PubMed] [Google Scholar]
- 6.WHO (2009) Neglected tropical diseases: snakebite. Available: http://www.who.int/neglected_diseases/diseases/snakebites/en/index.html [accessed on-line August 2013].
- 7. Alirol E, Sharma SK, Bawaskar HS, Kuch U, Chappuis F (2010) Snake bite in South Asia: a review. PLoS Negl Trop Dis 4: e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chippaux JP (2012) Epidemiology of snakebites in Europe: a systematic review of the literature. Toxicon 59: 86–99. [DOI] [PubMed] [Google Scholar]
- 9. Hansson E, Cuadra S, Oudin A, de Jong K, Stroh E, et al. (2010) Mapping snakebite epidemiology in Nicaragua-pitfalls and possible solutions. PLoS Negl Trop Dis 4: e896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rahman R, Faiz MA, Selim S, Rahman B, Basher A, et al. (2010) Annual incidence of snake bite in rural Bangladesh. PLoS Negl Trop Dis 4: e860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterson AT, Soberón J, Pearson RG, Anderson RP, Martínez-Meyer E, et al. (2011) Ecological niches and geographic distributions. Princeton: Princeton University Press.
- 12. Tôrres NM, De Marco P, Santos T, Silveira L, de Almeida Jácomo AT, et al. (2012) Can species distribution modelling provide estimates of population densities? A case study with jaguars in the Neotropics. Divers Distrib 18: 615–627. [Google Scholar]
- 13. VanDerWal J, Shoo LP, Johnson CN, Williams SE (2009) Abundance and the environmental niche: environmental suitability estimated from niche models predicts the upper limit of local abundance. Am Nat 174: 282–291. [DOI] [PubMed] [Google Scholar]
- 14. Martínez-Meyer E, Díaz-Porras DF, Peterson AT, Yañez-Arenas C (2013) Ecological niche structure and rangewide abundance patterns of species. Biol Lett 9(1): 20120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yañez-Arenas C, Martínez-Meyer E, Mandujano S, Rojas-Soto O (2012) Modelling geographic patterns of population density of the white-tailed deer in central Mexico by implementing ecological niche theory. Oikos 121: 2081–2089. [Google Scholar]
- 16.Guzmán GS (1990) Mordeduras de serpientes venenosas en Veracruz. I Reunión de Herpetología Villahermosa, Tabasco México.
- 17.Campbell JA, Lamar WW (2004) The venomous reptiles of the Western Hemisphere. Ithaca: Cornell University Press. [Google Scholar]
- 18. Sasa M, Vázquez S (2003) Snakebite envenomation in Costa Rica: a revision of incidence in the decade 1990–2000. Toxicon 41: 19–22. [DOI] [PubMed] [Google Scholar]
- 19. Chiappy CJ, Gama L, Soto M, Geissert D, Chávez J (2002) Regionalización paisajística del Estado de Veracruz, México. Universidad y Ciencia 18: 87–113. [Google Scholar]
- 20.Morrone JJ (2001) Biogeografía de América Latina y el Caribe. Zaragoza: M&T-Manuales & Tesis SEA.
- 21.Flores Villela O, Gerez P (1994) Biodiversidad y conservación en México: vertebrados, vegetación y uso del suelo. México, D. F.: CONABIO-UNAM.
- 22.Soto M, García E (1989) Atlas climático del estado de Veracruz. Xalapa: Instituto de Ecología, A. C.
- 23. Tay-Zavala J, Díaz-Sánchez JG, Sánchez-Vega JT, Ruíz-Sánchez D, Castillo L (2002) Serpientes y reptiles de importancia médica en México. Rev Fac Med Univ Nac Auton Mex 45: 212–219. [Google Scholar]
- 24. Hernández PA, Graham CH, Master LL, Albert DL (2006) The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 29: 773–785. [Google Scholar]
- 25. Stockwell D, Peterson AT (2002) Effects of sample size on accuracy of species distribution models. Ecol Modell 148: 1–13. [Google Scholar]
- 26. Wisz MS, Hijmans RJ, Li J, Peterson AT, Graham CH, et al. (2008) Effects of sample size on the performance of species distribution models. Divers Distrib 14: 763–773. [Google Scholar]
- 27.Lee JC (1996) The amphibians and reptiles of the Yucatan Peninsula. New York: Cornell University Press. [Google Scholar]
- 28.Pérez-Higareda G, Smith H (1991) Ofidiofauna de Veracruz: análisis taxonómico y zoogeográfico. México, D. F.: Instituto de Biología, UNAM. [Google Scholar]
- 29. Phillips SJ, Dudík M, Elith J, Graham CH, Lehmann A, et al. (2009) Sample selection bias and presence-only distribution models: implications for background and pseudo-absence data. Ecol Appl 19: 181–197. [DOI] [PubMed] [Google Scholar]
- 30. Soberón J, Nakamura M (2009) Niches and distributional areas: concepts, methods, and assumptions. Proc Natl Acad Sci USA 106: 19644–19650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25: 1965–1978. [Google Scholar]
- 32. Gormley AM, Forsyth DM, Griffioen P, Lindeman M, Ramsey DSL, et al. (2011) Using presence only and presence–absence data to estimate the current and potential distributions of established invasive species. J Appl Ecol 48: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CONABIO (1997) Provincias biogeográficas de México. Escala 1:4 000 000. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, México, D. F. http://www.conabio.gob.mx/informacion/gis/ [accessed on-line August 2013].
- 34. Barve N, Barve V, Jiménez-Valverde A, Lira-Noriega A, Maher SP, et al. (2011) The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol Modell 222: 1810–1819. [Google Scholar]
- 35. Stockwell D, Peters D (1999) The GARP modelling system: problems and solutions to automated spatial prediction. Int J Geogr Inf Sci 13: 143–158. [Google Scholar]
- 36. López-Arévalo HF, Gallina S, Landgrave R, Martínez-Meyer E, Muñoz-Villers LE (2011) Local knowledge and species distribution models' contribution towards mammalian conservation. Biol Conserv 144: 1451–1463. [Google Scholar]
- 37. Martínez-Meyer E, Peterson AT, Servín JI, Kiff LF (2007) Ecological niche modelling and prioritizing areas for species reintroductions. Oryx 40: 411–418. [Google Scholar]
- 38. Peterson AT, Martínez-Campos C, Nakazawa Y, Martínez-Meyer E (2005) Time-specific ecological niche modeling predicts spatial dynamics of vector insects and human dengue cases. Trans R Soc Trop Med Hyg 99: 647–655. [DOI] [PubMed] [Google Scholar]
- 39. Raxworthy CJ, Martínez-Meyer E, Horning N, Nussbaum RA, Schneider GE, et al. (2003) Predicting distributions of known and unknown reptile species in Madagascar. Nature 426: 837–841. [DOI] [PubMed] [Google Scholar]
- 40. Anderson RP, Lew D, Peterson AT (2003) Evaluating predictive models of species' distributions: criteria for selecting optimal models. Ecol Modell 162: 211–232. [Google Scholar]
- 41.ESRI (2011) ArcMap 10. Environmental Systems Research Institute, Inc, USA. [Google Scholar]
- 42. Peterson AT, Papeş M, Soberón J (2008) Rethinking receiver operating characteristic analysis applications in ecological niche modelling. Ecol Modell 213: 63–72. [Google Scholar]
- 43. Hanley JA, McNeil BJ (1983) A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148: 839–843. [DOI] [PubMed] [Google Scholar]
- 44. Lobo JM, Jiménez-Valverde A, Real R (2007) AUC: a misleading measure of the performance of predictive distribution models. Glob Ecol Biogeogr 17: 145–151. [Google Scholar]
- 45.Barve N (2008) Tool for Partial-ROC. (Biodiversity Institute, Lawrence, KS), ver 1.0.
- 46.INEGI (2010) Instituto Nacional de Estadística y Geografía. http://www.inegi.org.mx/inegi/default.aspx [accessed on-line August 2013].
- 47. Leynaud GC, Reati GJ (2009) Identifying areas of high risk for ophidism in Cordoba, Argentina, using SIGEpi software. Rev Panam Salud Publica 26: 64–69. [DOI] [PubMed] [Google Scholar]
- 48. Chippaux JP (2011) Estimate of the burden of snakebites in sub-Saharan Africa: a meta-analytic approach. Toxicon 57: 586–599. [DOI] [PubMed] [Google Scholar]
- 49. Harrison RA, Hargreaves A, Wagstaff SC, Faragher B, Lalloo DG (2009) Snake envenoming: a disease of poverty. PLoS Negl Trop Dis 3: e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hastie T, Tibshirani R (1990) Generalized additive models. London: Chapman & Hall.
- 51.Wood SN (2006) Generalized additive models: an introduction with R. Boca Raton: CRC Press.
- 52.Wood SN (2010) mgcv: mixed GAM Computation Vehicle with GCV/AIC/REML smoothness estimation - R package version 1.6-2. http://www.r-project.org/cran.
- 53.R Development Core Team (2012) R: a language and environment for statistical computing Version 2.15.1. http://cran.R-project.org. R Foundation for Statistical Computing, Vienna.
- 54. Mak S, Morshed M, Henry B (2010) Ecological niche modeling of Lyme disease in British Columbia, Canada. J Med Entomol 47: 99–105. [DOI] [PubMed] [Google Scholar]
- 55. Miller RH, Masuoka P, Klein TA, Kim HC, Somer T, et al. (2012) Ecological niche modeling to estimate the distribution of Japanese encephalitis virus in Asia. PLoS Negl Trop Dis 6: e1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Peterson AT (2006) Ecologic niche modeling and spatial patterns of disease transmission. Emerg Infect Dis 12: 1822–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Peterson AT, Sánchez-Cordero V, Beard CB, Ramsey JM (2002) Ecologic niche modeling and potential reservoirs for Chagas disease, Mexico. Emerg Infect Dis 8: 662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Luna-Bauza E, Martínez-Ponce G, Salazar-Hernández AC (2004) Mordeduras por serpiente. Panorama epidemiológico de la zona de Córdoba, Veracruz. Rev Fac Med Univ Nac Auton Mex 47: 149–153. [Google Scholar]
- 59. Soberón J, Peterson AT (2005) Interpretation of models of fundamental ecological niches and species' distributional areas. Biodiversity Informatics 2: 1–10. [Google Scholar]
- 60. Coombs MD, Dunachie SJ, Brooker S, Haynes J, Church J, et al. (1997) Snake bites in Kenya: a preliminary survey of four areas. Trans R Soc Trop Med Hyg 91: 319–321. [DOI] [PubMed] [Google Scholar]
- 61. Fayomi B, Massougbodji A, Chobli M (2002) Données épidémiologiques sur les cas de morsures de serpent déclarés au Bénin de 1994 à 2000. Bull Soc Pathol Exot 95: 178–180. [PubMed] [Google Scholar]
- 62. Baldé MC, Dieng B, Inapogui AP, Barry AO, Bah H, et al. (2002) Problématique des envenimations en Guinée. Bull Soc Pathol Exot 95: 157–159. [PubMed] [Google Scholar]
- 63.Chippaux JP (2002) The treatment of snake bites: analysis of requirements and assessment of therapeutic efficacy in tropical Africa. In: Ménez A, editor. Perspectives in molecular toxinology. Chichester: John Wiley, Sons, Ltd. pp. 457–472. [Google Scholar]
- 64. Newman WJ, Moran NF, Theakston RDG, Warrell DA, Wilkinson D (1997) Traditional treatments for snake bite in a rural African community. Ann Trop Med Parasitol 91: 967–969. [DOI] [PubMed] [Google Scholar]
- 65. Snow RW, Bronzan R, Roques T, Nyamawi C, Murphy S, et al. (1994) The prevalence and morbidity of snake bite and treatment-seeking behaviour among a rural Kenyan population. Ann Trop Med Parasitol 88: 665–671. [DOI] [PubMed] [Google Scholar]
- 66. Chippaux JP, Diallo A (2002) Evaluation de l'incidence des morsures de serpent en zone de Sahel Senegalais, l'exemple de Niakhar. Bull Soc Pathol Exot 95: 151–153. [PubMed] [Google Scholar]
- 67. Pugh RNH, Theakston RDG (1980) Incidence and mortality of snake bite in savanna Nigeria. The Lancet 316: 1181–1183. [DOI] [PubMed] [Google Scholar]
- 68. Chippaux JP, Bressy C (1981) L'endémie ophidienne des plantations de Côte-d'Ivoire. Bull Soc Pathol Exot 74: 458–467. [PubMed] [Google Scholar]
- 69. Stock RP, Massougbodji A, Alagón A, Chippaux JP (2007) Bringing antivenoms to sub-Saharan Africa. Nat Biotechnol 25: 173–177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Variables used in distribution models. Please note that temperature data are in °C * 10, units used for the precipitation data is mm (millimeters), altitude is expressed in masl (meters above sea level) and slope in percentage (%).
(DOCX)
Sources for occurrence data of vipers.
(DOCX)
Databases with the occurrences for all species' modeled. Codes for each database: Atropoides nummifer = r_a_num, Bothrops asper = r_b_asper, Crotalus atrox = r_c_atr, C. intermedius = r_c_int, C. molossus = r_c_mol, C. ravus = r_c_rav, C. simus = r_c_sim, C. totonacus = r_c_tot, C. triseriatus = r_c_tri.
(RAR)
Cases of snakebites in Veracruz (2003–2012). Raw information obtained through the Sistema Único Automatizado para la Vigilancia Epidemiológica (SUAVE; Secretaría de Salud del Estado de Veracruz).
(RAR)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the Supporting Information files.