Abstract
Background
Multiple studies have shown cigarette smoking to be a risk factor for chronic kidney disease. However, it is unknown whether smoking similarly increases the risk for progression of membranous nephropathy.
Methods
This study used the Nagoya Nephrotic Syndrome Cohort Study (N-NSCS), including 171 patients with idiopathic membranous nephropathy (IMN) from 10 nephrology centers in Japan. The dose-response relationships between cigarette smoking and the outcomes were assessed by using multivariate Cox proportional hazards models adjusted for clinically relevant factors. The primary outcome was a 30% decline in the estimated glomerular filtration rate (eGFR). The secondary outcome was first complete remission (CR) of proteinuria.
Results
During the observation period (median, 37 months; interquartile range, 16–71 months), 37 (21.6%) patients developed a 30% decline in eGFR and 2 (1.2%) progressed to ESRD. CR occurred in 103 (60.2%) patients. Multivariate Cox proportional hazards models revealed current smoking (adjusted hazard ratio [HR], 7.81 [95% confidence interval (CI), 3.17–19.7]), female sex (adjusted HR, 3.58 [95% CI, 1.87–8.00]), older age (adjusted HR, 1.71 [95% CI, 1.13–2.62] per 10 years), the number of cigarettes smoked daily (adjusted HR, 1.61 [95% CI, 1.23–2.09] per 10 cigarettes daily), and cumulative smoking of ≥40 pack-years (adjusted HR, 5.56 [95% CI, 2.17–14.6]) to be associated with a 30% decline in eGFR. However, smoking was not associated with CR.
Conclusion
Smoking is a significant and dose-dependent risk factor for IMN progression. All patients with IMN who smoke should be encouraged to quit.
Introduction
Membranous nephropathy (MN) is a very common cause of nephrotic syndrome in adults [1], [2]. Spontaneous remission occurs in 30–50% of patients, whereas another 30–50% experience progressive renal failure [3], [4]. Previously identified clinical predictors of poor renal survival include older age, male sex, elevated serum creatinine level at the time of diagnosis, and the severity of proteinuria at the time of disease onset and during follow-up [5]–[9]. However, most of these cannot be modified, and the long-term outcome is not unfavorable regardless: the 10-year renal survival ranges from 60 to 80% [10], [11]. The optimal management strategy for MN remains unclear.
It has recently become apparent that cigarette smoking, in addition to promoting cardiovascular disease (CVD), is an important independent renal risk factor [12]–[21]. However, these studies included patients with heterogeneous causes of CKD, such as diabetes, nephrosclerosis, and other diseases. Therefore, it was uncertain whether all kidney diseases were equally exacerbated by cigarette smoking. Elisabeth et al. reported in a nationwide population-based case-control study that the relationship between cigarette smoking and kidney impairment varied with the underlying kidney disease [22].
Yamamoto et al. reported in a large-scale retrospective cohort study that cigarette smoking was a key, dose-dependent prognostic factor in patients with IgA nephropathy [23]. The link between smoking and nephrotic syndrome has been investigated in only one case-control study of 80 patients with MN [24]. Although that study showed no relationship between smoking and MN, it was limited by the omission of two potentially significant independent variables, baseline proteinuria and kidney function at the time of study entry.
This study aimed to determine whether a history of smoking is an independent risk factor for the progression of MN and whether such risk is dose-dependent. This multicenter observational cohort study was organized as part of the Nagoya Nephrotic Syndrome Cohort Study (N-NSCS) based at 10 major nephrology centers in Nagoya, Japan.
Subjects and Methods
Study Population and Data Source
This cohort study included patients aged >18 years who had been diagnosed with membranous nephropathy (MN) on the basis of kidney biopsy between January 2003 and December 2012 at Nagoya University, Chubu Rosai Hospital, Japanese Red Cross Nagoya Daiichi Hospital, Tsushima City Hospital, Kasugai Municipal Hospital, Nagoya Kyoritsu Hospital, Anjo Kosei Hospital, Ichinomiya Municipal Hospital, Handa City Hospital, or Tosei General Hospital. Of the 272 such patients, we excluded patients with conditions generally considered to cause secondary MN, such as exposure to drugs associated with MN, diabetes mellitus, systemic lupus erythematosus, malignancy, or any other systemic disease known to be associated with secondary MN [25].
After exclusion of an additional 7 (3.9%) patients with missing data related to smoking status, 171 (62.9%) patients with idiopathic membranous nephropathy (IMN) were enrolled and followed up until September 2013. Clinically relevant factors at the baseline of those excluded (n = 7) and enrolled (n = 171) were not significantly different.
Our study was conducted by using linkable anonymous data set. No informed consent was obtained. The study protocol and consent procedure were approved by the ethics committees of Nagoya University, Chubu Rosai Hospital, Japanese Red Cross Nagoya Daiichi Hospital, Tsushima City Hospital, Kasugai Municipal Hospital, Nagoya Kyoritsu Hospital, Anjo Kosei Hospital, Ichinomiya Municipal Hospital, Handa City Hospital, and Tosei General Hospital Approval number:2012–0268.
Data Collection
Data pertaining to the patients' clinical characteristics at baseline were collected retrospectively from their medical records. The time of kidney biopsy was used as the baseline if the patient had not received immunosuppressive therapy or had received immunosuppressive therapy only after kidney biopsy. In patients who had received immunosuppressive therapy before kidney biopsy, the time of the initiation of immunosuppressive treatment was used as the baseline. The clinical characteristics included age, sex, body mass index, systolic and diastolic blood pressure, serum total cholesterol level, serum creatinine level, glomerular filtration rate (GFR; estimated using the equation recently generated by the Japanese Society of Nephrology: eGFR [mL/min/1.73 m2] = 194×Scr−1.094×Age−0.287×0.739 [if female] [26]), serum albumin level, 24-hour urinary protein excretion or urinary protein-to-creatinine ratio, smoking status, use of any antihypertensive drugs, and initial use of corticosteroids and/or other immunosuppressive agents. Urinary protein excretion and eGFR data were collected from the medical record at each checkup in 1 to 3 month intervals.
We obtained detailed information about each patient's smoking status at the time of kidney biopsy, including the amount smoked (cigarettes/day), starting and stopping dates, and changes in use over time, from the medical records. The relationships between smoking and outcome measures were then studied for the following variables: (1) smoking status (all patients): never smoked, ex-smoker, or current smoker; (2) number of cigarettes smoked daily (current and ex-smokers); and (3) cumulative quantity smoked (current and ex-smokers): 0, 1–20, 21–39, or ≥40 pack-years (PY; 1 PY = 1 pack of 20 cigarettes/day ×1 year). Because we were most interested in whether a history of smoking was a risk factor for the progression of IMN, current smokers and ex-smokers were combined into a single group (current/ex-smokers).
The antihypertensive drugs used in this cohort were angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARB), calcium channel blockers, β-blockers, and thiazides. The drugs used for therapeutic intervention, including the prescription of ACE inhibitors/ARB and corticosteroids or other immunosuppressive agents within 6 months of kidney biopsy, were also examined.
Nephrotic syndrome was defined as urinary protein excretion of ≥3.5 g/day (or a urinary protein/creatinine ratio of ≥3.5) and a serum albumin level of <3.0 mg/dL.
Complete remission (CR) of proteinuria was defined as urinary protein excretion of <0.3 g/day, a urinary protein/creatinine ratio of <0.3, and/or a negative/trace result for urinary protein on a dipstick test.
Partial remission (PR) of proteinuria was defined as urinary protein excretion of <3.5 g/day and a urinary protein/creatinine ratio of <3.5.
Relapse was defined as urinary protein excretion of ≥1.0 g/day, a urinary protein/creatinine ratio of ≥1.0, or a urinary protein dipstick result of ≥2+ on at least 2 occasions after achievement of CR.
The rate of decline in eGFR per year (mL/min per 1.73 m2/year) was determined by plotting the eGFR against the observation time.
The anonymous data set is available in the Table S4.
Outcomes
The primary outcome was a 30% decline in eGFR before end-stage renal disease (ESRD). The secondary outcome was first CR.
The serum creatinine level was measured as required for each patient. Patients were followed up until September 2013 and censored at the time of death (if before ESRD or CR) or as of the last serum creatinine measurement before September 2013.
Statistical Analysis
Differences in clinical characteristics between those who had never smoked and current-/ex-smokers were compared by using the Wilcoxon rank-sum test or Fisher's exact test. To identify predictors independently associated with each outcome, potential covariates were examined by using the log-rank test and/or univariate and multivariate Cox proportional hazards models. The proportional hazards assumption for covariates was tested by using scaled Schoenfeld residuals. For continuous variables, the Wilcoxon rank-sum test was performed to assess the significance of inter-group differences. Categorical variables were expressed as percentages and compared by using Fisher's exact test. The rate of survival without a 30% decline in eGFR and the cumulative probability of achieving a first CR were calculated by using the Kaplan-Meier method and the log-rank test. The trend in each outcome with respect to the cumulative quantity smoked was examined statistically by scoring never smoking as 0 and current-/ex-smokers with 1–20, 21–39, and ≥40 pack-years' exposure as 1, 2, and 3, respectively; the resulting scores were then included in the regression model. The level of statistical significance was set at P<0.05. All statistical analyses were performed by using JMP version 10.0.0 (SAS Institute, Cary, NC, USA; www.jmp.com) and STATA version 13.0 (STATA Corp, www.stata.com).
Results
Study participants and clinical characteristics
The present study included 171 patients with IMN, of whom 108 (63.2%) had never smoked, 28 (16.4%) were ex-smokers, and 35 (20.5%) were current smokers. The clinical characteristics of the two groups (those who had never smoked and current/ex-smokers) are summarized in Table 1.
Table 1. Clinical Characteristics of the 171 Patients with IMN.
| Never smoked | Smokers (Current/Ex-) | P value | |
| Number | 108 | 63 (35/28) | |
| Baseline characteristics | |||
| Age (years) | 66 (59–73) | 63 (53–68) | 0.012 |
| Male [n (%)] | 63 (58.3) | 55 (87.3) | <0.001 |
| Body mass index (kg/m2) | 22.9 (21.1–24.7) | 23.5 (22.3–26.2) | 0.046 |
| Systolic blood pressure (mmHg) | 130 (120–145) | 132 (121–143) | 0.873 |
| Diastolic blood pressure (mmHg) | 76 (70–84) | 79 (72–86) | 0.156 |
| Serum creatinine (mg/dL) | 0.78 (0.66–1.00) | 0.80 (0.71–1.00) | 0.171 |
| eGFR (mL/min/1.73 m2) | 76 (58–92) | 76 (63–86) | 0.639 |
| Serum albumin (g/dL) | 2.6 (2.1–3.2) | 2.4 (2.0–3.2) | 0.518 |
| Urinary protein (g/day) | 3.9 (2.4–6.1) | 4.8 (3.2–8.4) | 0.025 |
| Urinary protein >3.5 (g/day) [n (%)] | 68 (63.0) | 46 (73.0) | 0.239 |
| Total cholesterol (mg/dL) | 283 (238–375) | 295 (247–368) | 0.617 |
| Use of antihypertensive drugs [n (%)] | 33 (30.6) | 25 (39.7) | 0.244 |
| Smoking status | |||
| Number of cigarettes smoked daily | NA | 20 (20–30) | |
| Pack-years | NA | 40 (24–49) | |
| Therapeutic interventions within 6 months after kidney biopsy | |||
| ACE inhibitor or ARB therapy [n (%)] | 65 (60.2) | 48 (76.2) | 0.044 |
| Immunosuppressive treatment | 0.413 | ||
| No immunosuppressive agent | 58 (53.7) | 28 (44.4) | |
| Prednisolone [n (%)] | 19 (17.6) | 11 (17.5) | |
| Prednisolone + cyclosporine [n (%)] | 31 (28.7) | 24 (38.1) | |
| Outcomes | |||
| 30% reduction in eGFR [n (%)] | 16 (14.8) | 21 (33.3) | 0.007 |
| 50% reduction in eGFR [n (%)] | 6 (5.6) | 11 (17.5) | 0.017 |
| Decline in eGFR (mL/min per 1.73 m2 per year) | 2.42 (−1.44 to 6.91) | 4.01 (0.51–8.78) | 0.046 |
| ESRD [n (%)] | 1 (0.9) | 1 (1.6) | 1.000 |
| Death [n (%)] | 8 (7.4) | 3 (4.8) | 0.748 |
| Remission | |||
| Complete remission [n (%)] | 63 (58.3) | 40 (63.5) | 0.522 |
| Partial remission [n (%)] | 96 (88.9) | 54 (85.7) | 0.631 |
| Relapse [n (%)] | 14 (16.1) | 12 (26.7) | 0.170 |
| Observation period (months) | 36 (15–71) | 39 (18–74) | 0.375 |
Median (interquartile range), Conversion factors for units: SCr in mg/dL to µmol/L, ×88.4; eGFR (mL/min/1.73 m2) = 194×Scr−1.094×Age−0.287×0.739 (if female), total cholesterol in mg/dL to mmol/L, ×0.02586.
Abbreviations: IMN, idiopathic membranous nephropathy; eGFR, estimated glomerular filtration rate; ACE inhibitor/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; SCr, serum creatinine; ESRD, end-stage renal disease; NA, not applicable.
The two groups differed significantly in age, proportion of males, body mass index, urinary protein excretion, and rate of therapeutic intervention with an ACE inhibitor or ARB within 6 months of kidney biopsy. At baseline, 114 patients (66.7%) had nephrotic syndrome as previously defined. The median follow-up period of those who had never-smoked, ex-smokers, and current-smokers was 36 (15–71), 33 (16–63), and 46 (18–87) months, respectively (P = 0.288).
We obtained the number of cigarettes smoked daily for 62 (98.4%) of the current/ex-smokers (1–10 cigarettes for 10 [16.1%], 11–20 cigarettes for 32 [51.6%], 21–30 cigarettes for 9 [14.5%], and 31–50 cigarettes for 11 [17.7%] patients) and the cumulative smoking dose for 60 (95.2%) of the current-/ex-smokers (≤20 pack-years for 11 [18.3%], 21–39 pack-years for 15 [25.0%], and ≥40 pack-years for 34 [56.7%] patients).
Treatment during the observation period
An ACE inhibitor or ARB was newly prescribed within 6 months of kidney biopsy in 113 (66.1%) patients and was used by 156 (91.2%) by the end of follow-up. The enrolled patients were divided according to the treatment within 6 months of kidney biopsy into three groups: (1) the prednisolone group, comprising 30 patients (17.5%) who received prednisolone alone; (2) the cyclosporine group, comprising 55 patients (32.1%) who received prednisolone and cyclosporine; and (3) the supportive therapy group, comprising 86 patients (50.3%) who received neither prednisolone nor other immunosuppressive drugs. One patient in the cyclosporine group (0.6%) also received mizoribine. Most of the patients in the prednisolone group were prescribed prednisolone starting at 0.8–1.0 mg/kg/day orally and tapered according to the response to therapy, whereas most in the cyclosporine group started with cyclosporine at 1 to 2 mg/kg and prednisolone at 0.4–0.6 mg/kg. The cyclosporine dosage was adjusted to achieve the target whole-blood trough level, and the prednisolone dose was tapered according to the response to therapy. The time from renal biopsy to the initiation of immunosuppressive therapy was 0.7 months (interquartile range, 0.3–3.3 months). The duration of prednisolone use was 11 months (interquartile range, 8–25 months) in the prednisolone group and 14 months (interquartile range, 9–17 months) in the cyclosporine group.
Outcome data
The median observation time for the entire cohort was 37 months (interquartile range, 15–72 months). In total, 37 (21.6%) patients developed a 30% decline in eGFR before ESRD, 17 (9.9%) developed a 50% decline in eGFR before ESRD, and 2 (1.2%) progressed to ESRD.
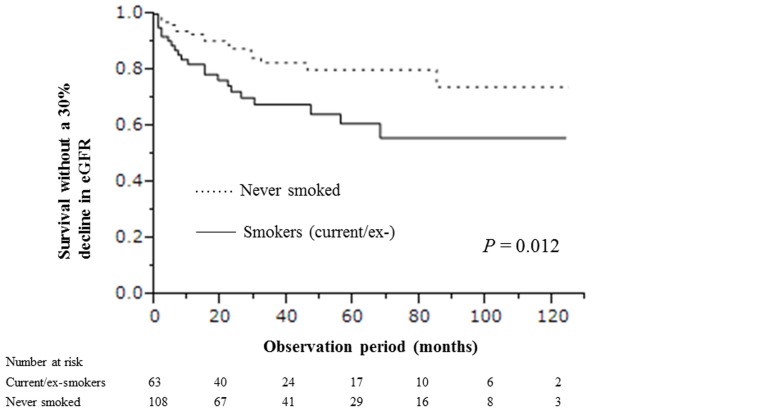
The rates of avoiding a 30% decline in eGFR for 1, 5, and 10 years were 0.82 (95% confidence interval [CI], 0.71–0.90), 0.61 (95% CI, 0.46–0.74), and 0.56 (95% CI, 0.40–0.71), respectively, in current-/ex-smokers and 0.93 (95% CI, 0.86–0.97), 0.80 (95% CI, 0.69–0.88), and 0.74 (95% CI, 0.57–0.86) in those who had never smoked; therefore, current/ex-smokers were at higher risk for developing a 30% decline in eGFR (P = 0.012; Fig. 1).
Figure 1. Time to 30% decline in eGFR in current/ex-smokers and those who never smoked.
The eGFR decreased significantly faster in current/ex-smokers than in those who had never smoked (4.01 mL/min per 1.73 m2 per year [interquartile range, 0.51–8.78 mL/min per 1.73 m2 per year] versus 2.42 mL/min per 1.73 m2 per year [interquartile range, −1.44 to 6.91 mL/min per 1.73 m2 per year]).
The eGFR did not improve by the last follow-up visit in any of the 37 patients who experienced a 30% decline in eGFR.
Meanwhile, 103 (60.2%) patients achieved CR of proteinuria after a median follow-up of 14 months (interquartile range, 6–25 months). The cumulative probabilities of achieving CR within 1, 5, and 10 years were 0.31 (95% CI, 0.20–0.44), 0.77 (95% CI, 0.62–0.87), and 0.81 (95% CI, 0.65–0.90) in current-/ex-smokers and 0.43 (95% CI, 0.33–0.54), 0.80 (95% CI, 0.67–0.88), and 0.81 (95% CI, 0.65–0.92) in those who had never smoked. Therefore, smoking was not associated with the achievement of CR (P = 0.570).
Among the patients who achieved a first remission, 26 (25.2%) relapsed at least once.
No patient progressed to ESRD before developing a 30% decline in eGFR.
Eleven (6.4%) patients died during follow-up, one due to infection, and one each to acute subdural hematoma, traffic accident, sudden death, and intestinal bleeding. Malignancy occurred in 5 (2.9%) patients; the diagnoses were esophageal cancer, stomach cancer, colon cancer, prostate cancer, and malignant lymphoma in one patient each.
Predictors of a 30% decline in eGFR
Univariate Cox proportional hazards models revealed that older age, female sex, initial use of cyclosporine combination therapy, and current smoking were significantly associated with the primary outcome (Table 2). Adjustment for clinically relevant factors revealed current smoking (adjusted hazard ratio [HR], 7.81 [95% CI, 3.17–19.7], P<0.001), female sex (adjusted HR, 3.58 [95% CI, 1.87–8.00], P = 0.002), older age (adjusted HR, 1.71 [95% CI, 1.13–2.62], per 10 years, P = 0.010), and use of cyclosporine in combination with prednisolone (adjusted HR, 2.67 [95% CI, 1.08–6.59], P = 0.034) to be significant predictors of the primary outcome.
Table 2. Predictors of a 30% decline in eGFR.
| Univariate model | Multivariate model | |||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (per 10 years) | 1.47 (1.07–2.07) | 0.018 | 1.71 (1.13–2.62) | 0.010 |
| Male (versus female) | 0.43 (0.22–0.83) | 0.012 | 0.28 (0.13–0.63) | 0.002 |
| Systolic blood pressure (per 10 mmHg) | 1.06 (0.90–1.23) | 0.455 | 1.07 (0.86–1.32) | 0.546 |
| Diastolic blood pressure (per 10 mmHg) | 1.20 (0.94–1.53) | 0.142 | 1.08 (0.77–1.52) | 0.660 |
| Serum albumin (per 1.0 g/dL) | 0.80 (0.51–1.24) | 0.317 | 1.32 (0.73–2.36) | 0.359 |
| Serum creatinine (per 1.0 mg/dL) | 0.67 (0.18–1.98) | 0.501 | 0.90 (0.19–3.13) | 0.885 |
| Urinary protein excretion (per 1.0 g/day) | 1.03 (0.94–1.12) | 0.519 | 1.02 (0.91–1.13) | 0.673 |
| Therapeutic interventions within 6 months after kidney biopsy | ||||
| ACE inhibitor or ARB therapy | 0.72 (0.26–2.99) | 0.598 | 0.90 (0.29–3.96) | 0.874 |
| Immunosuppressive treatment | ||||
| No immunosuppressive agent | Reference | Reference | ||
| Prednisolone | 0.84 (0.24–2.33) | 0.758 | 1.30 (0.33–4.21) | 0.687 |
| Prednisolone + cyclosporine | 2.18 (1.09–4.42) | 0.027 | 2.67 (1.08–6.59) | 0.034 |
| Smokers (Current/Ex-) | 2.24 (1.17–4.35) | 0.015 | 4.00 (1.87–8.79) | <0.001 |
| Ex-smokers | 1.68 (0.64–3.93) | 0.273 | 2.23 (0.79–5.79) | 0.127 |
| Current smokers | 2.68 (1.29–5.52) | 0.009 | 7.81 (3.17–19.7) | <0.001 |
HR, hazard ratio; CI, confidence interval.
Data are the HR, 95% CI, and P value from Cox proportional hazard regression analyses.
“Never smoked” was used as the reference category.
Adjusted for baseline characteristics (age, sex, systolic/diastolic pressure, serum creatinine level, urinary protein, use of ACE inhibitor or ARB within 6 months after kidney biopsy, and immunosuppressive therapy within 6 months after kidney biopsy).
Abbreviations: IMN, idiopathic membranous nephropathy; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
No interactive effect on the primary outcome was detected between use of cyclosporine and smoking (adjusted HR, 1.16 [95% CI, 0.61–2.24], P = 0.644).
To confirm the robustness of our results, we similarly assessed predictors of a 50% decline in eGFR and found that current smoking (adjusted HR, 9.85 [95% CI, 2.68–41.0], P<0.001) and female sex (adjusted HR, 4.20 [95% CI, 1.19–15.3], P = 0.027) were also significant predictors of this outcome (Table S1).
Dose-dependent association between smoking and a 30% decline in eGFR
Table 3 shows that the total smoking dose had a strong independent effect on the risk of renal progression. Both univariate and multivariate Cox proportional hazards models showed a significant association between the number of cigarettes per day and the primary outcome (adjusted HR, 1.61 [95% CI, 1.23–2.09], P<0.001, multivariate model 1). Cumulative smoking of ≥40 pack-years was also significantly associated with the primary outcome (adjusted HR, 5.56 [95% CI, 2.17–14.6], P<0.001, multivariate model 2). These results suggested that the risk of renal progression increased almost linearly with the number of cigarettes smoked.
Table 3. Influence of smoking dose on the risk of a 30% decline in eGFR.
| Univariate model | Multivariate model | |||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Model 1 | ||||
| No. of cigarettes (/10/d) | 1.32 (1.05–1.63) | 0.021 | 1.61 (1.23–2.09) | <0.001 |
| Model 2 | ||||
| 1–20 pack-years | 1.07 (0.17–3.75) | 0.933 | 1.36 (0.20–5.94) | 0.715 |
| 21–39 pack-years | 1.64 (0.47–4.49) | 0.399 | 3.51 (0.87–12.5) | 0.076 |
| ≥40 pack-years | 2.61 (1.21–5.48) | 0.016 | 5.56 (2.17–14.6) | <0.001 |
| Test for trend | 0.015 | <0.001 | ||
HR, hazard ratio; CI, confidence interval.
Data are the HR, 95% CI, and P value from Cox proportional hazard regression analyses.
“Never smoked” was used as the reference category. Models 1 and 2 are based on data from 168 patients because the number of cigarettes was missing for 1 current and 2 ex-smokers. Adjusted for baseline characteristics (age, sex, systolic/diastolic pressure, serum creatinine level, urinary protein, use of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker within 6 months after kidney biopsy, and immunosuppressive therapy within 6 months after kidney biopsy).
Predictors of first complete remission
Univariate Cox proportional hazards models revealed a lower serum creatinine level, initial use of prednisolone monotherapy, and initial use of cyclosporine in combination with prednisolone to be significantly associated with first CR of proteinuria (Table S2). After adjustment for clinically relevant factors, the initial use of prednisolone monotherapy (adjusted HR, 2.15 [95% CI, 1.17–3.84], P = 0.015) and cyclosporine in combination with prednisolone (adjusted HR, 2.90 [95% CI, 1.68–5.00], P<0.001) remained significant predictors of first CR of proteinuria. Neither a history of smoking (Table S2) nor the total smoking dose (Table S3) was associated with CR.
Discussion
We found that smoking increased the risk of renal dysfunction in Japanese patients with IMN in a dose-dependent manner, although former smoking did not appear to be a risk factor. These results highlight the clinical importance of smoking cessation in the treatment of patients with IMN.
Several cohort studies in the general population have suggested a dose-dependent association between smoking and CKD. The Cardiovascular Health Study identified smoking as a risk factor related to CKD progression in 4142 U.S. participants aged >65 years but did not include proteinuria as an independent variable [12]. The NHANRS II study followed 9082 middle-aged (49.3±13.3 years) U.S. participants for 13.2 years and showed a higher adjusted risk of end-stage kidney disease among those smoking >20 cigarettes per day. Baggio et al. studied 2981 elderly subjects from Italy and found smoking ≥21 cigarettes per day to be a very strong risk factor for pathological loss of kidney function [17]. Furthermore, the HUNT II study of 65,589 Norwegian participants revealed that the risk increased significantly as the cumulative amount of smoking (pack-years) increased; however, the risk significantly decreased as the years elapsed since smoking cessation increased among male ex-smokers aged <70 years [27]. Yamagata reported in a 10-year follow-up study of 123,764 Japanese participants aged ≥40 years who received community-based annual examinations that current smoking increased the risk of developing proteinuria, but that study did not evaluate the dose-dependency of the relationship between smoking and CKD [21]. These reports collectively identify smoking as a risk factor for the progression of CKD in the general population. Our present results were consistent with this literature, particularly the HUNT II study, in which current/ex-smokers reporting larger cumulative numbers of cigarettes smoked were at significantly higher risk of renal progression than were those who had never smoked.
Only a few studies of primary kidney disease have found a dose-dependent association between cigarette smoking and progression of primary glomerulonephritis. Yamamoto et al. identified cigarette smoking as a key dose-dependent prognostic factor for IgA nephropathy in a multivariate model. There has been only one case-control study addressing the relationship between smoking and nephrotic syndrome, which revealed no relationship between smoking and kidney progression in patients with MN [24]. However, that study failed to include baseline proteinuria and kidney function at study entry as independent variables. In contrast, we included those variables and identified smoking as an important dose-dependent prognostic factor in patients with IMN after adjustment for relevant confounders.
The present study identified immunosuppressive therapy as both a risk factor for renal progression (although urinary protein excretion was greater in such patients than in those who initially received supportive therapy) and the most significant independent predictor of achievement of CR. These results may, however, reflect indication bias, for which the present retrospective cohort study did not control. Furthermore, we should consider that unmeasured factors associated with treatment may not be included in the model.
Combination therapy with cyclosporine and prednisolone was frequently used for treatment of IMN in our cohort. Cattran et al. demonstrated that cyclosporine significantly decreased the rate of renal progression and reduced proteinuria in patients with IMN at high risk for progression and persistent nephrotic-range proteinuria [28], [29]. Alexopoulos et al. also found that cyclosporine with or without corticosteroids effectively induced remission in the majority of nephrotic patients with IMN and well-preserved renal function [30]. Based on these findings, we often use cyclosporine in combination with corticosteroids for first-line treatment of patients with IMN. Although the 2012 Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guideline for IMN recommends a cytotoxic agent (cyclophosphamide) for patients at high risk of progression [31], no patient in our study was treated with cyclophosphamide. This should be considered when interpreting our results.
Interestingly, our study identified female sex as an independent risk factor after adjustment for relevant clinical factors. Previous studies had shown male sex to be associated with a higher risk of developing ESRD [5]–[9]. In contrast, female sex has generally been associated with a relatively benign course [32]. However, these studies did not include smoking status as an independent variable, which may have caused overestimation of the relationship between male sex and risk of renal progression. Furthermore, Mina Yu reported in a cross-sectional study that menopause was an independent risk factor for CKD because of the accompanying loss of possibly cardioprotective estradiol production [33]. Previous studies of membranous nephropathy have generally enrolled younger patients than our study, suggesting that our study may have included a relatively large number of postmenopausal patients. Therefore, membranous nephropathy may lead to more rapid decline in GFR in postmenopausal women, who are already in an unfavorable hormonal state (i.e., estrogen deficient) with respect to the kidney. The generalizability of our results should be confirmed in another cohort study.
Although the exact mechanisms of the nephrotoxic effects of smoking are poorly understood, we can present some potential explanations of the relationship between smoking and CKD onset and progression. First, nephrotic syndrome increases oxidative stress by impairing antioxidant pathways [34]. The addition of smoking-induced oxidative stress to nephrotic syndrome-associated oxidative stress may accelerate renal progression [35]–[38]. Second, ex vivo studies have suggested that nicotine could promote mesangial cell proliferation and increase production of critical molecules involved in extracellular matrix production [39], [40]. Third, smoking (or nicotine) could increase the plasma endothelin level [41], which has been shown to correlate with effective renal plasma flow in smokers [42]. Furthermore, Alves et al. reported that smoking aggravates the cyclosporine-induced impairment of GFR and that smoking in combination with cyclosporine induced periglomerular structural lesions and oxidative stress in a rat model of cyclosporine nephrotoxicity [43]. However, our results showed no interactive effect on renal function between use of cyclosporine and smoking. The external validity of the result should be confirmed in another study.
It is also possible that smoking might relate to medication adherence in some other way. A cross-sectional study showed that current smokers were less compliant with medication use than nonsmokers [44]. Therefore, we should consider the possibility of medication adherence in smokers. Moreover, we are aware that MN can itself have extra-renal associations and these should be considered for their possible relationship with smoking.
Our study has some limitations that must be discussed. First, underreporting of smoking and changes in smoking habits during the follow-up period can cause misclassification and tend to overestimate the effect of smoking on renal progression. Second, we did not address the relationship between smoking cessation and renal progression because our sample size was insufficient to evaluate it. Third, the patients in this study may not be representative of patients with IMN in other countries: different consequences of smoking have been reported in different populations [45]. We therefore advise caution when interpreting and generalizing our results.
Allowing for these methodological issues, our study has several advantages: it is one of the largest multicenter adult MN cohorts in Japan ever reported and is also, to the best of our knowledge, the first description of a relationship between smoking and renal dysfunction in patients with IMN.
In conclusion, our retrospective cohort study in patients with IMN showed cigarette smoking to be a key, dose-dependent predictor of IMN progression. All patients with IMN who smoke should be encouraged to quit.
Supporting Information
Predictors of a 50% decline in eGFR.
(DOC)
Predictors of first CR.
(DOCX)
Influence of smoking dose on first CR.
(DOCX)
The anonymous data set of 171 patients with IMN.
(XLSX)
Acknowledgments
We are grateful for the time and efforts of the nephrologists who supported the present study: Dr. Shizunori Ichida, Dr. Hideaki Shimizu, Dr. Junichiro Yamamoto, Dr. Tomohiko Naruse, Dr. Hirofumi Tamai, Dr. Kei Kurata, Dr. Hirotake Kasuga, Dr. Arimasa Shirasaki, and Dr. Makoto Mizutani.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the manuscript.
Funding Statement
This study was supported by a Grant-in-Aid for Progressive Renal Diseases Research, Research on Rare and Intractable Disease, from the Ministry of Health, Labour, and Welfare of Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Fervenza FC, Sethi S, Specks U (2008) Idiopathic membranous nephropathy: diagnosis and treatment. Clin J Am Soc Nephrol 3: 905–919. [DOI] [PubMed] [Google Scholar]
- 2. Cattran DC (2001) Idiopathic membranous nephropathy. Kidney Int 59: 1983–1994. [DOI] [PubMed] [Google Scholar]
- 3. van den Brand JAJG, Hofstra JM, Wetzels JFM (2011) Low-molecular-weight proteins as prognostic markers in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 6: 2846–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. du Buf-Vereijken PW, Branten AJ, Wetzels JF (2005) Idiopathic membranous nephropathy: outline and rationale of a treatment strategy. Am J Kidney Dis 46: 1012–1029. [DOI] [PubMed] [Google Scholar]
- 5. Shiiki H, Saito T, Nishitani Y, Mitarai T (2004) Research Group on Progressive Renal Diseases in Japan, (2004) et al. Prognosis and risk factors for idiopathic membranous nephropathy with nephrotic syndrome in Japan. Kidney Int 65: 1400–1407. [DOI] [PubMed] [Google Scholar]
- 6. Marx BE, Marx M (1999) Prediction in idiopathic membranous nephropathy. Kidney Int 56: 666–673. [DOI] [PubMed] [Google Scholar]
- 7. Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC (2004) Idiopathic membranous nephropathy: definition and relevance of a partial remission. Kidney Int 66: 1199–1205. [DOI] [PubMed] [Google Scholar]
- 8. Cattran DC, Pei Y, Greenwood CM, Ponticelli C, Passerini P, et al. (1997) Validation of a predictive model of idiopathic membranous nephropathy: its clinical and research implications. Kidney Int 51: 901–907. [DOI] [PubMed] [Google Scholar]
- 9. Pei Y, Cattran D, Greenwood C (1992) Predicting chronic renal insufficiency in idiopathic membranous glomerulonephritis. Kidney Int 42: 960–966. [DOI] [PubMed] [Google Scholar]
- 10. Noel LH, Zanetti M, Droz D, Barbanel C (1979) Long-term prognosis of idiopathic membranous glomerulonephritis. Study of 116 untreated patients. Am J Med 66: 82–90. [DOI] [PubMed] [Google Scholar]
- 11. Zucchelli P, Ponticelli C, Cagnoli L, Passerini P (1987) Long-term outcome of idiopathic membranous nephropathy with nephrotic syndrome. Nephrol Dial Transplant 2: 73–78. [PubMed] [Google Scholar]
- 12. Bleyer AJ, Shemanski LR, Burke GL, Hansen KJ, Appel RG (2000) Tobacco, hypertension, and vascular disease: risk factors for renal functional decline in an older population. Kidney Int 57(5): 2072–2079. [DOI] [PubMed] [Google Scholar]
- 13. Tozawa M, Iseki K, Iseki C, Oshiro S, Ikemiya Y, et al. (2002) Influence of smoking and obesity on the development of proteinuria. Kidney Int 62: 956–962. [DOI] [PubMed] [Google Scholar]
- 14. Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, et al. (2003) Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol 14(11): 2934–2941. [DOI] [PubMed] [Google Scholar]
- 15. Stengel B, Tarver-Carr ME, Powe NR, Eberhardt MS, Brancati FL (2003) Lifestyle factors, obesity and the risk of chronic kidney disease. Epidemiology 14(4): 479–487. [DOI] [PubMed] [Google Scholar]
- 16. Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, et al. (2004) Predictors of new-onset kidney disease in a community-based population. JAMA 29(7): 844–850. [DOI] [PubMed] [Google Scholar]
- 17. Baggio B, Budakovic A, Perissinotto E, Maggi S, Cantaro S, et al. (2005) Atherosclerotic risk factors and renal function in the elderly: the role of hyperfibrinogenaemia and smoking. Results from the Italian Longitudinal Study on Ageing (ILSA). Nephrol Dial Transplant 20(1): 114–123. [DOI] [PubMed] [Google Scholar]
- 18. Hallan S, de Mutsert R, Carlsen S, Dekker FW, Aasarod K, et al. (2006) Obesity, smoking, and physical inactivity as risk factors for CKD: are men more vulnerable? Am J Kidney Dis 47(3): 396–405. [DOI] [PubMed] [Google Scholar]
- 19. Ishani A, Grandits GA, Grimm RH, Svendsen KH, Collins AJ, et al. (2006) Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol 17(5): 1444–1452. [DOI] [PubMed] [Google Scholar]
- 20. Shankar A, Klein R, Klein BE (2006) The association among smoking, heavy drinking, and chronic kidney disease. Am J Epidemiol 164(3): 263–271. [DOI] [PubMed] [Google Scholar]
- 21. Yamagata K, Ishida K, Sairenchi T, Takahashi H, Ohba S, et al. (2007) Risk factors for chronic kidney disease in a community-based population: a 10-year follow-up study. Kidney Int 71(2): 159–166. [DOI] [PubMed] [Google Scholar]
- 22. Ejerblad E, Fored CM, Lindblad P, Fryzek J, Dickman PW, et al. (2004) Association between smoking and chronic renal failure in a nationwide population-based case-control study. J Am Soc Nephrol 15(8): 2178–2185. [DOI] [PubMed] [Google Scholar]
- 23. Yamamoto R, Nagasawa Y, Shoji T, Iwatani H, Hamano T, et al. (2010) Cigarette smoking and progression of IgA nephropathy. Am J Kidney Dis 56(2): 313–324. [DOI] [PubMed] [Google Scholar]
- 24. Stengel B, Couchoud C, Cénée S, Hémon D (2000) Age, blood pressure and smoking effects on chronic renal failure in primary glomerular nephropathies. Kidney Int 57(6): 2519–2526. [DOI] [PubMed] [Google Scholar]
- 25. Hofstra JM, Wetzels JF (2012) Management of patients with membranous nephropathy. Nephrol Dial Transplant 27: 6–9. [DOI] [PubMed] [Google Scholar]
- 26. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, et al. (2009) Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 27. Hallan SI, Orth SR (2011) Smoking is a risk factor in the progression to kidney failure. Kidney Int 80: 516–523. [DOI] [PubMed] [Google Scholar]
- 28. Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, et al. (2001) North America Nephrotic Syndrome Study Group (2001) Cyclosporine in patients with steroid-resistant membranous nephropathy: a randomized trial. Kidney Int 59: 1484–1490. [DOI] [PubMed] [Google Scholar]
- 29. Cattran DC, Greenwood C, Ritchie S, Bernstein K, Churchill DN, et al. (1995) A controlled trial of cyclosporine in patients with progressive membranous nephropathy. Canadian Glomerulonephritis Study Group. Kidney Int 47: 1130–1135. [DOI] [PubMed] [Google Scholar]
- 30. Alexopoulos E, Papagianni A, Tsamelashvili M, Leontsini M, Memmos D (2006) Induction and long-term treatment with cyclosporine in membranous nephropathy with the nephrotic syndrome. Nephrol Dial Transplant 21: 3127–3132. [DOI] [PubMed] [Google Scholar]
- 31.KDIGO Working Group (2012) KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2: 186–197.
- 32. Reichert LJ, Koene RA, Wetzels JF (1998) Prognostic factors in idiopathic membranous nephropathy. Am J Kidney Dis 31(1): 1–11. [DOI] [PubMed] [Google Scholar]
- 33. Yu M, Ryu D-R, Kim S-J, Choi K-B, Kang D-H (2010) Clinical implication of metabolic syndrome on chronic kidney disease depends on gender and menopausal status: results from the Korean National Health and Nutrition Examination Survey. Nephrol Dial Transplant 25: 469–477. [DOI] [PubMed] [Google Scholar]
- 34. Soyoral YU, Aslan M, Emre H, Begenik H, Erdur FM, et al. (2011) Serum paraoxonase activity and oxidative stress in patients with adult nephrotic syndrome. Atherosclerosis 218(1): 243–246. [DOI] [PubMed] [Google Scholar]
- 35. Nagasawa Y, Yamamoto R, Rakugi H, Isaka Y (2012) Cigarette smoking and chronic kidney diseases. Hypertens Res 35(3): 261–265. [DOI] [PubMed] [Google Scholar]
- 36. Amaral S, Oliveira PJ, Ramalho-Santos J (2008) Diabetes and the impairment of reproductive function: possible role of mitochondria and reactive oxygen species. Curr Diabetes Rev 4(1): 46–54. [DOI] [PubMed] [Google Scholar]
- 37. Maiese K, Chong ZZ, Shang YC (2007) Mechanistic insights into diabetes mellitus and oxidative stress. Curr Med Chem 14(16): 1729–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fatehi-Hassanabad Z, Chan CB, Furman BL (2010) Reactive oxygen species and endothelial function in diabetes. Eur J Pharmacol 636(1–3): 8–17. [DOI] [PubMed] [Google Scholar]
- 39. Hua P, Feng W, Ji S, Raij L, Jaimes EA (2010) Nicotine worsens the severity of nephropathy in diabetic mice: implications for the progression of kidney disease in smokers. Am J Physiol Renal Physiol 299: F732–F739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jaimes EA, Tian RX, Raij L (2007) Nicotine: the link between cigarette smoking and the progression of renal injury? Am J Physiol Heart Circ Physiol 292: H76–H82. [DOI] [PubMed] [Google Scholar]
- 41. Haak T, Jungmann E, Raab C, Usadel KH (1994) Elevated endothelin-1 levels after cigarette smoking. Metabolism 43(3): 267–269. [DOI] [PubMed] [Google Scholar]
- 42. Gambaro G, Verlato F, Budakovic A (1998) Renal impairment in chronic cigarette smokers. J Am Soc Nephrol 9(4): 562–567. [DOI] [PubMed] [Google Scholar]
- 43. Alves SA, Carlos CP, Mendes GEF, Oliveira SM, Luz MAM, et al. (2012) Previous exposure to cigarette smoke aggravates experimental cyclosporine-induced nephrotoxicity. Am J Nephrol 36: 334–341. [DOI] [PubMed] [Google Scholar]
- 44. Sherman BW, Lynch WD (2014) The association of smoking with medical treatment adherence in the workforce of a large employer. Patient Prefer Adherence (8): 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sellers EM (1998) Pharmacogenetics and ethnoracial differences in smoking. JAMA 280: 179–180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Predictors of a 50% decline in eGFR.
(DOC)
Predictors of first CR.
(DOCX)
Influence of smoking dose on first CR.
(DOCX)
The anonymous data set of 171 patients with IMN.
(XLSX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the manuscript.