Abstract
Arterial hypertension, a condition characterized by sustained elevated blood pressure, is associated with pathological cardiac remodeling (i.e. cardiac hypertrophy and fibrosis) and is a major risk factor for cardiac failure. These processes can be triggered by excess vasoconstrictive agonists, which induce metalloproteinase-dependent shedding of growth factors to transactivate growth factor receptors and initiate disease signaling. Here, we review emerging evidence that agonist-activated metalloproteinases exhibit different expression patterns and mutual transcriptional regulation during the development of hypertension and cardiac remodeling.
Introduction
Cardiovascular diseases remain the primary cause of human morbidity and mortality in the developed world. The pathogenesis of hypertension and pathological cardiac remodeling, two major forms of cardiovascular disease, can be multi-factorial [1]. Common causative factors of hypertension and cardiac remodeling include vasoconstrictive Gq protein-coupled receptor (GqPCR) agonists such as catecholamines, endothelins (ETs) and angiotensin II (Ang II). Downstream of these agonists, matrix metalloproteinases (MMPs) and ‘a disintegrin and metalloproteinases’ (ADAMs) are activated to enhance vascular tone and promote cardiac remodeling. MMPs and ADAMs can cleave and modulate the biological activity of vasoactive peptides (e.g. big ET and adrenomedullin), pro-inflammatory mediators (e.g. tumor necrosis factor-α), growth factors (e.g. epidermal growth factor receptor (EGFR) ligands: heparin-binding EGF (HB-EGF), transforming growth factor-α (TGF-α)), cytokines (e.g. transforming growth factor-β (TGF-β)), cell surface receptors (e.g. β2-adrenergic receptor, vascular endothelial growth factor receptor-2 (VEGFR-2), insulin receptor) and extracellular matrix components [2–10]. We review here emerging evidence that MMPs and ADAMs are key mediators that might regulate one another (e.g. at the transcriptional level) to promote the development of hypertension and cardiac remodeling.
Metalloproteinases as signaling mediators in agonist-induced hypertension and cardiac remodeling
Pathological cardiac remodeling is a clinically significant complication in hypertensive disorders referring to the development of hypertrophy [11–13] and fibrosis [13,14]. The precise pathogenesis of hypertension and hypertensive cardiac remodeling in the general population remains difficult to delineate. Diverse conditions such as stress, diabetes, obesity, life style as well as environmental and genetic factors predispose to hypertension and cardiac remodeling, at least in part, through the excessive production of vasoconstrictive GqPCR agonists. These agonists bind to cognate receptors to activate phospholipase C. One of the actions of phospholipase C leads to the cell membrane assembly of the NADPH oxidase complex, which next generates reactive oxygen species (ROS) that activate multiple MMPs and ADAMs [15,16].
Metalloproteinase regulation
Metalloproteinase activity is regulated by multiple mechanisms. Proenzymes are activated in response to cleavage of the propeptide by serine proteinases (e.g. plasmin and thrombin) and metalloproteinases (e.g. membrane type1-MMP (MT1-MMP)) [17–20]. Alternatively, proenzymes can be activated without cleavage of the propeptide by reaction with ROS [21] that disrupt a bond involving a cysteine residue and the catalytic Zn2+ found in the active site [22]. Metalloproteinases are further controlled by regulatory proteins (e.g. tissue inhibitors of metalloproteinases and α-2-macroglobulin), post-translational modifications (e.g. phosphorylation) [22–25] and transcriptional regulation. At the transcriptional level, agonists (e.g. Ang II), growth factors (e.g. EGF) and some cytokines (e.g. TGF-β) induce mitogen-activated protein kinase (MAPK) pathways and downstream transcription of metalloproteinases by activating transcription factor-2 and activating protein-1 [26,27].
Signaling downstream of metalloproteinases
Metalloproteinase-shed ligands, such as HB-EGF and TGF-α, bind the EGFR to propagate agonist signaling via the MAPK and PI3K pathways. A key feature of this GqPCR agonist → metalloproteinase → EGFR pathway is that metalloproteinases provide a common link between multiple GqPCRs and the EGFR [3]. Although discovered in the context of cancer research, the EGFR transactivation pathway has been increasingly implicated in cardiovascular conditions including hypertension and cardiac remodeling, where vasoconstrictive GqPCR agonists are upregulated.
Downstream of the EGFR, signaling through MAPK and PI3K pathways leads to transcription and translation of immediate-early genes and the re-expression of fetal genes (e.g. α-skeletal actin, β-myosin heavy chain) to promote cellular growth of cardiomyocytes and thus the development of cardiac hypertrophy [28]. In addition, extracellular matrix components (e.g. fibronectin-1 and collagen types I and III) are transcribed and synthesized, which is crucial to the development of cardiac fibrosis [28,29].
MMP-dependent signaling through the MAPK and PI3K pathways also increases the production of ROS and ATP in mitochondria. Blockade of the latter pathways induces relaxation of arteries pre-constricted with an adrenergic agonist (phenylephrine) but not with KCl (which induces receptor-independent vasoconstriction) [30]. Therefore, modulation of mitochondrial function and thereby cellular redox and metabolic states is a plausible mechanism by which metalloproteinases could regulate vascular contractility.
These signaling events merge to modulate vascular tone as well as the transcription of pro-hypertrophic, pro-fibrotic and metalloproteinase genes (Fig. 1).
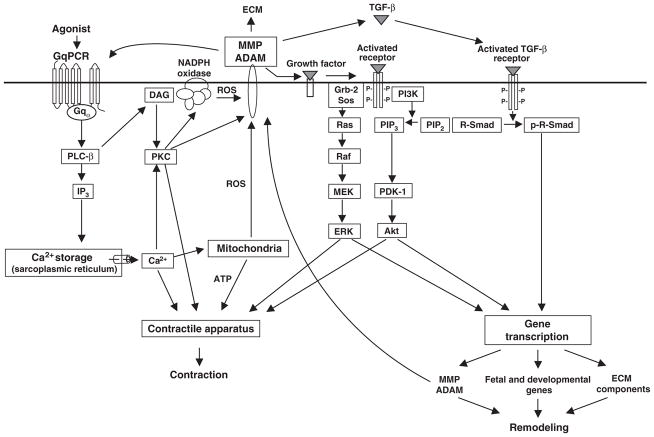
Figure 1.
Postulated signaling pathways involving metalloproteinases in agonist-induced hypertension and cardiac disease. Agonist activation of constitutively expressed metalloproteinases induces transcription of metalloproteinase genes as well as pro-hypertrophic and pro-fibrotic genes. This is a candidate model to explain the observed overexpression and mutual regulation among metalloproteinases, from the MMP and ADAM families, during the course of hypertension and remodeling. ECM: extracellular matrix; GqPCR: Gq protein-coupled receptor. PLC-β: phospholipase C isoform β. IP3: inositol 1,4,5-triphosphate. DAG: 1,2-diacylglycerol. PKC: protein kinase C. ROS: reactive oxygen species. MMP: matrix metalloproteinase. ADAM: a disintegrin and metalloproteinase. Grb-2: growth factor receptor-bound protein 2. Sos: son of sevenless. MEK: mitogen-activated protein kinase kinase. ERK: extracellular signal-regulated kinases. PI3K: phosphatidylinositol 3-kinase. PIP2: phosphatidylinositol (4,5)-bisphosphate. PIP3: phosphatidylinositol (3,4,5)-bisphosphate. PDK-1: phosphoinositide-dependent protein kinase 1. TGF-β: transformation growth factor β. R-Smad: receptor-regulated Smad.
Roles of metalloproteinases in disease development
In the context of agonist-induced cardiovascular and renal disease, MMP-7, ADAM-12 and ADAM-17 (which is often termed tumor necrosis factor converting enzyme – TACE) act as sheddases of EGFR ligands. MMP-7 seems be involved in the mediation of both agonist-induced vascular tone and cardiac remodeling. The involvement of MMP-7 in vascular tone regulation is supported by studies of small mesenteric arteries from MMP-7 knock-out and knock-down (i.e. siRNA) mouse models that display resistance to Ang II and norepinephrine-induced acute hypertension as well as cardiac hypertrophy and fibrosis [31]. Furthermore, pharmacological inhibition of ADAM-12 blocks shedding of HB-EGF thus attenuating cardiac hypertrophy, but not hypertension [4]. Whether and if so, how ADAM-12 influences the development of cardiovascular fibrosis remains unclear. During our studies, we observed increased levels of ADAM-12 in hearts that exhibit both hypertrophy and fibrosis. Recent research using hepatic stellate cells, Rhabdomyosarcoma and C2C12 cells, has shown that ADAM-12, independent of its metalloproteinase activity, enhances TGF-β/TβR/Smad signaling by interacting with TGF-β receptors (TβR) II. These findings suggest a mechanism for ADAM-12 in cardiac fibrosis [32,33]. In addition, TGF-β can induce the expression of ADAM-12, which is mediated by TGF-β’s ability to promote the degradation of SnoN, a negative regulator of TGF-β/Smad signaling [34,35].
The shedding activity of ADAM-17 has been found to mediate the progression of Ang II-induced chronic renal disease, where increased shedding of TGF-α activates the EGFR. EGFR transactivation in the kidneys, renal lesions and fibrosis induced by long-term (over 28 days) Ang II infusion in mice are prevented in both TGF-α knock-out models and by pharmacological inhibition of ADAM-17 [6].
Metalloproteinases can impact cardiovascular disease development through cleavage of substrates other than EGFR ligands. In a model of myocardial infarction, MMP-7 mediates left ventricular remodeling by cleaving a gap junction protein, connexin-43. When the MMP-7 gene is deleted, connexin-43 cleavage decreases and an improvement is observed in myocardial conduction patterns and survival after myocardial infarction [36]. In the spontaneously hypertensive rat vasculature, MMP-2 cleaves vascular VEGFR-2 in cardiac microvessels to mediate endothelial apoptosis and capillary rarefaction which contribute to the development of hypertension [10]. MMP-2 and MMP-9 have also been implicated in cleavage of big ET at a Gly-Leu bond of big ET (ET[1–38]) to form a smaller peptide (ET[1–32]), which activates the endothelial ETB/NO vasodilatory pathway and could thus mediate the vascular effects of the pregnancy hormone, relaxin [37]. In addition MMP-2, but not MMP-9, has been proposed to cleave the vasodilator peptide adrenomedullin, which adds yet another pathway by which MMP-2 could regulate systolic blood pressure [38].
In many conditions including hypercholesterolemia, obesity, hyperglycemia, insulin resistance and hypertension, vascular endothelial dysfunction is common and is associated with oxidative stress, a potent activator of metalloproteinases [39]. Sites of vascular lesions are also triggers of inflammation through the recruitment of platelets and leukocytes. Platelet–leukocyte aggregation is essential for leukocyte recruitment and infiltration, two processes accompanied by the secretion of MMPs (e.g. MMP-2) [40]. Local metalloproteinases catalyze leukocyte infiltration into the subendothelial matrix, at least in part, by disrupting intercellular junctions through the cleavage of vascular endothelial cadherin by activated leukocyte-derived metalloproteinases (e.g. ADAM-10) [41]. These processes set the stage for inflammation, vascular damage, atherosclerotic plaque formation and atherosclerotic plaque rupture owing to high local activity of multiple metalloproteinases (e.g. MMP-1, MMP-2, ADAM-10 and ADAM-17) [40].
Differential expression and mutual regulation of metalloproteinases in hypertension and cardiac remodeling
Multiple diverse metalloproteinases are involved in disease development, which leads us to ask: are they all expressed at the same time and competing for substrates? Do they regulate one another and if so, how? These topical research questions could be answered, at least in part, by recent findings suggesting that metalloproteinases may regulate one another during the course of disease progression.
Metalloproteinase regulation by other metalloproteinases
In a mouse model of Ang II-induced hypertension and cardiac remodeling, early activation of MMP-7 and ADAM-17/TACE appears to be necessary for subsequent upregulation of MMP-2 and ADAM-12 transcription. Indeed, administration of MMP-7 siRNA, TACE siRNA or both siRNAs simultaneously prevents the upregulation of MMP-2 by Ang II in terms of both mRNA levels and enzymatic activity, which leads to an attenuation of the severity of Ang II-induced hypertension. However, an upregulation of MMP-2 expression in mice with Ang II-induced hypertension may have negligible effects on the development of cardiac hypertrophy and fibrosis induced by short-term (over 14 days) Ang II infusion [42]. Gene knock-out, siRNA and pharmacological inhibition of MMP-7 in mice, prevent Ang II-induced ADAM-12 overexpression as well as cardiac hypertrophy [31]. Indeed, Ang II induces an upregulation of ADAM-12 that mediates hypertrophic processes in the heart [4]. Conceivably agonist activation of constitutively expressed metalloproteinases such as MMP-7, MMP-2, ADAM-12 and ADAM-17 induces transcription of metalloproteinases, such as MMP-2 and ADAM-12, through EGFR transactivation and MAPK signaling. The resulting feed-forward loop allows constitutively expressed metalloproteinases to mediate de novo production of metalloproteinases (Fig. 1).
There is evidence that MMP-7 proteolytically activates MMP-8 (along with MMP-1, MMP-2 and MMP-9) but not MMP-13 [43]. Therefore, MMP-8 may be an in vivo substrate of MMP-7. Pro-MMP-8 accumulation in the absence of MMP-7 leads to decreased pro-MMP-13 levels, perhaps to maintain baseline collagenolytic levels. The interaction between MMP-8 and MMP-13 does not lead to changes in mouse left ventricle dimensions, which suggests that these MMPs play redundant roles. The design of selective MMP inhibitors, therefore, must take into consideration changes in parallel MMP types if there is potential for such compensatory mechanisms [43]. The compensatory mechanism may be created by mutual regulation of metalloproteinases at the transcriptional level and may lead to differences in upregulation and specific roles for metalloproteinases mediating various stages of a disease.
Differential metalloproteinase expression leads to varying physiological roles in hypertension and cardiovascular diseases
MMP-7 is ubiquitously expressed, albeit in small quantities, in various systems including the immune system, vasculature and heart. These features may help MMP-7 act as a signaling mediator downstream of many agonists. However, its role might be limited to strict time windows, such as the early stages of agonist signaling. Indeed, following the administration of Ang II to mice, vascular (aortic) MMP-7 (but not MMP-2) may be acutely activated (within one hour). Lack of acute detectable activation of pro-MMP-2 (72 kDa) to yield MMP-2 (62 kDa) indicates that basal MMP-2 may be engaged in agonist-induced processes [31]. Studies show that broad-spectrum blockade of MMPs (using doxycycline) as well as a biphenylsulfonamido-hydroxamate inhibitor selective for MMP-2 (MMP-2 inhibitor III, Calbiochem) relaxes phenylephrine pre-constricted small mesenteric arteries [42]. Therefore, agonist-induced vasoconstriction may depend at least in part on MMP-7 acute activation as well as on MMP-2 basal activity.
Emerging data further suggest that different MMPs and ADAMs might contribute to agonist signaling in different time windows. For instance, several days after Ang II infusion begins through minipumps, cardiac MMP-7 and MMP-9 mRNA levels are found to decrease [31]. By contrast, the mRNA levels of MMP-2 and ADAM-12 are strongly upregulated [31]. MMP-2 activity is elevated in both heart and arteries. These processes probably amplify as well as sustain agonist-induced signaling over time.
Interestingly, studies using MMP-9 knock-out mouse models have shown that MMP-9 may play a key role in the early stages of hypertensive vascular disease. The onset (ten days) of Ang II-induced hypertension was found to be accompanied by increased MMP-9 activity in conductance vessels which is associated with a beneficial role early on by preserving vessel compliance and alleviating blood pressure increase. Conversely, the absence of MMP-9 activity resulted in vessel stiffness and increased pulse pressure [44].
In the setting of established hypertension, the regulation of MMP promoter activity is likely to depend on both mechanical and hormonal stimuli. Recent work suggests that vascular (aortic) metalloproteinase promoters may be differently responsive to single and collective mechanical (wall tension, up to 100 mmHg) and hormonal (Ang II) stimuli [45]. Increased tension was found to enhance MT1-MMP promoter activity, but further addition of Ang II did not have an additional effect on MT1-MMP promoter activity. Elevated tension plus Ang II administration had an additive effect on MMP-2 promoter activation, while MMP-9 promoter activity decreased. Therefore, exposure to a biological stimulus such as Ang II in the presence of high vessel-wall tension can modulate MMP promoter activation.
Differential metalloproteinase expression leads to varying physiological roles in a variety of diseases and conditions
Differential upregulation of MMP-2 and MMP-9 may have both pathogenic and protective effects during the development and progression of Alport syndrome [46], a progressive hereditary kidney disease (which leads to glomeruli damage and kidney failure) [47]. Preservation of the glomerular basement membrane and the integrity of the extracellular matrix by inhibiting MMP-2 and MMP-9 before the onset of proteinuria (high amount of protein in urine) leads to significant disease protection, but if this window of opportunity is lost, MMP-inhibition in later stages of Alport disease causes accelerated glomerular and interstitial fibrosis. In addition, the expression patterns of MMP-2, MMP-3 and MMP-9 in the kidney glomerulus, seem to be linked in a compensatory manner, as was shown in genetic knock-out mouse models [46]. Similarly, differential actions and expression profiles of MMP-2 and MMP-9 have been implicated in platelet aggregation [48] as well as in the response to injury in the carotid artery [49].
As expected, complex interactions characterize the biology of metalloproteinases in cardiovascular as well as non-cardiovascular settings including nervous and immune systems. In the setting of neuropathic pain, a condition of constant pain in the absence of a stimulus resulting from damage to the nervous system [50], MMP-9 is both necessary and sufficient for producing the neuropathic pain syndrome whereas MMP-2 expression is necessary to maintain neuropathic pain [51]. Even though MMP-9 is active in the early stages with MMP-2 activity increasing at later time points, both MMP-9 and MMP-2 act through cleavage of a cytokine, interleukin-1β. Similarly, MMP-2 and MMP-9 play complex and multiphasic roles after acute stroke and brain damage. Although these MMPs mediate neurovascular injury, they have also been involved in neuroplasticity and stroke recovery. As in other models that involve MMPs, spatial and temporal regulation remains to be defined so as to allow targeting of acute MMPs to ameliorate neurovascular pathophysiology without interfering with brain tissue repair [52].
Emerging evidence links immune system responses induced by pathogens to MMP gene expression. For instance, acute Pseudomonas aeruginosa pulmonary infection results in the induction of both MMP-7 and MMP-10 [53]. Analysis of gene expression changes in P. aeruginosa-infected tracheal epithelial cell cultures identified 2091 MMP-7-dependent and 1628 MMP-10-dependent genes that were differentially expressed. MMPs control distinct gene expression programs (as shown through key node analysis) involved in proliferation, cell death, immune responses and signal transduction, among other host defense processes. Because MMP-7 functions to promote inflammation, and MMP-10 acts to restrain inflammation it appears that MMPs could play unique roles in different stages of epithelial responses to Pseudomonas infection [53]. These latter data from a non-cardiovascular disease model further suggest that the expression and activity of single metalloproteinases can have profound effects on the expression of many other genes.
Conclusion
Metalloproteinases are emerging as key mediators upstream of the EGFR and downstream of multiple GqPCRs implicated in causation of cardiovascular and non-cardiovascular disorders. Therefore, metalloproteinases represent a transduction point for multiple signaling systems and a molecular communication network that governs a great variety of physiological processes [15,54]. Two features make the GqPCR agonist → metalloproteinase → EGFR transactivation mechanism attractive from a therapeutic point of view are: (1) growth factor receptor transactivation is significant only when levels of the agonist are exceedingly high and (2) growth factor receptor transactivation seems to be a common response to multiple pro-hypertensive agonists. Therefore, targeting the transactivation pathway has therapeutic potential in conditions caused by multiple or unknown agonists; that is in conditions with complex or unknown etiology.
Research using experimental animal models of agonist-induced and spontaneous hypertension suggests that MMPs and ADAMs might regulate one another through transcriptional mechanisms to mediate pro-hypertensive, pro-hypertrophic and pro-fibrotic responses to GqPCR agonists [31,55,56]. Therefore, different MMPs and ADAMs probably contribute to disease development within different time windows through overlapping mechanisms and substrates.
From a clinical translation point of view, future studies on the mutual regulation of MMPs and ADAMs should establish the need for timely inhibition of specific, versus multiple metalloproteinases, for effective treatment of hypertension and the associated disorders. Although it remains unknown whether metalloproteinases are networked in the same fashion in different organs and models of disease, it appears that similarities exist that may be used to predict interactions in other metalloproteinase-mediated diseases.
Acknowledgments
This work was supported by Canadian Institutes Health Research (CIHR) and Natural Sciences and Engineering Research Council of Canada (NSERC) grants to CF-P and by an NSERC Alexander Graham Bell Canada Graduate Scholarship (to A-MB), an Alberta Innovates Health Solutions doctoral studentship (to XW), a University of Alberta Faculty of Medicine and Dentistry Motyl Graduate Studentship (to JO).
Footnotes
Conflict of interest
None.
References
- 1.Lifton RP, et al. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Patron C, et al. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ Res. 1999;85:906–911. doi: 10.1161/01.res.85.10.906. [DOI] [PubMed] [Google Scholar]
- 3.Prenzel N, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 4.Asakura M, et al. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat Med. 2002;8:35–40. doi: 10.1038/nm0102-35. [DOI] [PubMed] [Google Scholar]
- 5.Hao L, et al. Agonist-induced activation of matrix metalloproteinase-7 promotes vasoconstriction through the epidermal growth factor-receptor pathway. Circ Res. 2004;94:68–76. doi: 10.1161/01.RES.0000109413.57726.91. [DOI] [PubMed] [Google Scholar]
- 6.Lautrette A, et al. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med. 2005;11:867–874. doi: 10.1038/nm1275. [DOI] [PubMed] [Google Scholar]
- 7.Ohtsu H, et al. ADAM17 mediates epidermal growth factor receptor transactivation and vascular smooth muscle cell hypertrophy induced by angiotensin II. Arterioscler Thromb Vasc Biol. 2006;26:e133–e137. doi: 10.1161/01.ATV.0000236203.90331.d0. [DOI] [PubMed] [Google Scholar]
- 8.Schmid-Schonbein GW. Matrix metalloproteinases activities in hypertension. Emerging opportunities. Hypertension. 2011;57:24–25. doi: 10.1161/HYPERTENSIONAHA.110.162032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigues SF, et al. Matrix metalloproteinases cleave the beta2-adrenergic receptor in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;299:H25–H35. doi: 10.1152/ajpheart.00620.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran ED, et al. Enhanced matrix metalloproteinase activity in the spontaneously hypertensive rat: VEGFR-2 cleavage. Endothelial apoptosis, and capillary rarefaction. J Vasc Res. 2010;47:423–431. doi: 10.1159/000281582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berk BC. Vascular smooth muscle growth: autocrine growth mechanisms. Physiol Rev. 2001;81:999–1030. doi: 10.1152/physrev.2001.81.3.999. [DOI] [PubMed] [Google Scholar]
- 12.Frohlich ED. Left ventricular hypertrophy: dissociation of structural and functional effects by therapy. Adv Exp Med Biol. 1991;308:175–190. doi: 10.1007/978-1-4684-6015-5_14. [DOI] [PubMed] [Google Scholar]
- 13.Berk BC, et al. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568–575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38 (Pt 2):581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 15.Zwick E, et al. The EGF receptor as central transducer of heterologous signalling systems. Trends Pharmacol Sci. 1999;20:408–412. doi: 10.1016/s0165-6147(99)01373-5. [DOI] [PubMed] [Google Scholar]
- 16.Grote K, et al. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ Res. 2003;92:e80–e86. doi: 10.1161/01.RES.0000077044.60138.7C. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Patron C, et al. Rapid release of matrix metalloproteinase (MMP)-2 by thrombin in the rat aorta: modulation by protein tyrosine kinase/phosphatase. Thromb Haemost. 1999;82:1353–1357. [PubMed] [Google Scholar]
- 18.Imai K, et al. Matrix metalloproteinase 7 (matrilysin) from human rectal carcinoma cells. Activation of the precursor, interaction with other matrix metalloproteinases and enzymic properties. J Biol Chem. 1995;270:6691–6697. doi: 10.1074/jbc.270.12.6691. [DOI] [PubMed] [Google Scholar]
- 19.Deryugina EI, et al. MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res. 2001;263:209–223. doi: 10.1006/excr.2000.5118. [DOI] [PubMed] [Google Scholar]
- 20.Chakraborti S, et al. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- 21.Weiss SJ, et al. Oxidative autoactivation of latent collagenase by human neutrophils. Science. 1985;227:747–749. doi: 10.1126/science.2982211. [DOI] [PubMed] [Google Scholar]
- 22.Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci USA. 1990;87:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoyhtya M, et al. Immunohistochemical localization of matrix metalloproteinase 2 and its specific inhibitor TIMP-2 in neoplastic tissues with monoclonal antibodies. Int J Cancer. 1994;56:500–505. doi: 10.1002/ijc.2910560408. [DOI] [PubMed] [Google Scholar]
- 24.Corcoran ML, et al. MMP-2: expression, activation and inhibition. Enzyme Protein. 1996;49:7–19. doi: 10.1159/000468613. [DOI] [PubMed] [Google Scholar]
- 25.Sariahmetoglu M, et al. Regulation of matrix metalloproteinase-2 (MMP-2) activity by phosphorylation. FASEB J. 2007;21:2486–2495. doi: 10.1096/fj.06-7938com. [DOI] [PubMed] [Google Scholar]
- 26.Kim ES, et al. TGF-beta-induced transcriptional activation of MMP-2 is mediated by activating transcription factor (ATF)2 in human breast epithelial cells. Cancer Lett. 2007;252:147–156. doi: 10.1016/j.canlet.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 27.Bergman MR, et al. A functional activating protein 1 (AP-1) site regulates matrix metalloproteinase 2 (MMP-2) transcription by cardiac cells through interactions with JunB–Fra1 and JunB–FosB heterodimers. Biochem J. 2003;369 (Pt 3):485–496. doi: 10.1042/BJ20020707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molkentin JD, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKinsey TA, Olson EN. Toward transcriptional therapies for the failing heart: chemical screens to modulate genes. J Clin Invest. 2005;115:538–546. doi: 10.1172/JCI24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao L, et al. Vascular responses to alpha1-adrenergic receptors in small rat mesenteric arteries depend on mitochondrial reactive oxygen species. Arterioscler Thromb Vasc Biol. 2006;26:819–825. doi: 10.1161/01.ATV.0000204344.90301.7c. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, et al. Matrix metalloproteinase-7 and ADAM-12 (a disintegrin and metalloproteinase-12) define a signaling axis in agonist-induced hypertension and cardiac hypertrophy. Circulation. 2009;119:2480–2489. doi: 10.1161/CIRCULATIONAHA.108.835488. [DOI] [PubMed] [Google Scholar]
- 32.Gruel J, et al. In silico investigation of ADAM12 effect on TGF-beta receptors trafficking. BMC Res Notes. 2009;2:193. doi: 10.1186/1756-0500-2-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atfi A, et al. The disintegrin and metalloproteinase ADAM12 contributes to TGF-beta signaling through interaction with the type II receptor. J Cell Biol. 2007;178:201–208. doi: 10.1083/jcb.200612046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solomon E, et al. The role of SnoN in transforming growth factor beta1-induced expression of metalloprotease-disintegrin ADAM12. J Biol Chem. 2010;285:21969–21977. doi: 10.1074/jbc.M110.133314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Pabic H, et al. Involvement of the serine/threonine p70S6 kinase in TGF-beta1-induced ADAM12 expression in cultured human hepatic stellate cells. J Hepatol. 2005;43:1038–1044. doi: 10.1016/j.jhep.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 36.Lindsey ML, et al. Matrix metalloproteinase-7 affects connexin-43 levels, electrical conduction, and survival after myocardial infarction. Circulation. 2006;113:2919–2928. doi: 10.1161/CIRCULATIONAHA.106.612960. [DOI] [PubMed] [Google Scholar]
- 37.Jeyabalan A, et al. Essential role for vascular gelatinase activity in relaxin-induced renal vasodilation, hyperfiltration, and reduced myogenic reactivity of small arteries. Circ Res. 2003;93:1249–1257. doi: 10.1161/01.RES.0000104086.43830.6C. [DOI] [PubMed] [Google Scholar]
- 38.Overall CM. Dilating the degradome: matrix metalloproteinase 2 (MMP-2) cuts to the heart of the matter. Biochem J. 2004;383 (Pt 3):e5–e7. doi: 10.1042/BJ20041433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;54:24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- 40.Santos-Martinez MJ, et al. Role of metalloproteinases in platelet function. Thromb Res. 2008;121:535–542. doi: 10.1016/j.thromres.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Schulz B, et al. ADAM10 regulates endothelial permeability and T-Cell transmigration by proteolysis of vascular endothelial cadherin. Circ Res. 2008;102:1192–1201. doi: 10.1161/CIRCRESAHA.107.169805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Odenbach J, et al. MMP-2 mediates angiotensin II-induced hypertension under the transcriptional control of MMP-7 and TACE. Hypertension. 2011;57:123–130. doi: 10.1161/HYPERTENSIONAHA.110.159525. [DOI] [PubMed] [Google Scholar]
- 43.Dozier S, et al. Matrix metalloproteinase (MMP)-7 activates MMP-8 but not MMP-13. Med Chem. 2006;2:523–526. doi: 10.2174/157340606778250261. [DOI] [PubMed] [Google Scholar]
- 44.Flamant M, et al. Role of matrix metalloproteinases in early hypertensive vascular remodeling. Hypertension. 2007;50:212–218. doi: 10.1161/HYPERTENSIONAHA.107.089631. [DOI] [PubMed] [Google Scholar]
- 45.Ruddy JM, et al. Differential effects of mechanical and biological stimuli on matrix metalloproteinase promoter activation in the thoracic aorta. Circulation. 2009;120 (Suppl):S262–S268. doi: 10.1161/CIRCULATIONAHA.108.843581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeisberg M, et al. Stage-specific action of matrix metalloproteinases influences progressive hereditary kidney disease. PLoS Med. 2006;3:e100. doi: 10.1371/journal.pmed.0030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kashtan CE. Familial hematuria due to type IV collagen mutations: Alport syndrome and thin basement membrane nephropathy. Curr Opin Pediatr. 2004;16:177–181. doi: 10.1097/00008480-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 48.Fernandez-Patron C, et al. Differential regulation of platelet aggregation by matrix metalloproteinases-9 and -2. Thromb Haemost. 1999;82:1730–1735. [PubMed] [Google Scholar]
- 49.Zempo N, et al. Matrix metalloproteinases of vascular wall cells are increased in balloon-injured rat carotid artery. J Vasc Surg. 1994;20:209–217. doi: 10.1016/0741-5214(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 50.Treede RD, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 51.Kawasaki Y, et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14:331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosell A, Lo EH. Multiphasic roles for matrix metalloproteinases after stroke. Curr Opin Pharmacol. 2008;8:82–89. doi: 10.1016/j.coph.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Kassim SY, et al. Individual matrix metalloproteinases control distinct transcriptional responses in airway epithelial cells infected with Pseudomonas aeruginosa. Infect Immun. 2007;75:5640–5650. doi: 10.1128/IAI.00799-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernandez-Patron C. Therapeutic potential of the epidermal growth factor receptor transactivation in hypertension: a convergent signaling pathway of vascular tone, oxidative stress, and hypertrophic growth downstream of vasoactive G-protein-coupled receptors? Can J Physiol Pharmacol. 2007;85:97–104. doi: 10.1139/y06-097. [DOI] [PubMed] [Google Scholar]
- 55.Wang X, et al. Tumor necrosis factor-alpha-converting enzyme is a key regulator of agonist-induced cardiac hypertrophy and fibrosis. Hypertension. 2009;54:575–582. doi: 10.1161/HYPERTENSIONAHA.108.127670. [DOI] [PubMed] [Google Scholar]
- 56.Zamilpa R, et al. Tumor necrosis factor-alpha-converting enzyme roles in hypertension-induced hypertrophy: look both ways when crossing the street. Hypertension. 2009;54:471–472. doi: 10.1161/HYPERTENSIONAHA.109.135848. [DOI] [PMC free article] [PubMed] [Google Scholar]