Abstract
Hypertensive cardiac disease remains a major cause of death worldwide because its typically complex etiology renders current treatments ineffective. Primary causative factors include environmental stressors, genetic predisposition and metabolic morbidities such as obesity and diabetes. These factors all trigger a systemic pathological production of agonists of G-protein-coupled receptors (GPCRs). When produced in excess, GPCR agonists transactivate many metalloproteinases, which relay agonist signaling. Here we review evidence supporting a global therapeutic concept for treatment of hypertensive cardiac disease with complex or unknown etiology by targeting common mediators of multiple GPCRs such as metalloproteinases and their downstream effectors.
Introduction
Hypertensive cardiac disease remains a major cause of death worldwide and is characterized by the co-occurrence of sustained hypertension, cardiac hypertrophy and cardiac fibrosis [1]. Despite advances in the identification of triggers of disease, the treatment of cardiovascular disorders remains empirical and ineffective due to the complex and often unknown etiology of hypertensive cardiac disease in the general population [2,3].
Among the primary causative factors of hypertensive cardiac disease are life style, stress, environmental factors and genetic predisposition as well as metabolic morbidities such as diabetes and obesity [1–3].
Here we propose that these factors contribute to the development of hypertensive cardiac disease by activating common effector mechanisms, which involve agonists of G-protein-coupled receptors (GPCRs). GPCRs signal, at least in part, through extracellular matrix metalloproteinases (MMPs) and ‘a disintegrin and metalloproteinases’ (ADAMs).
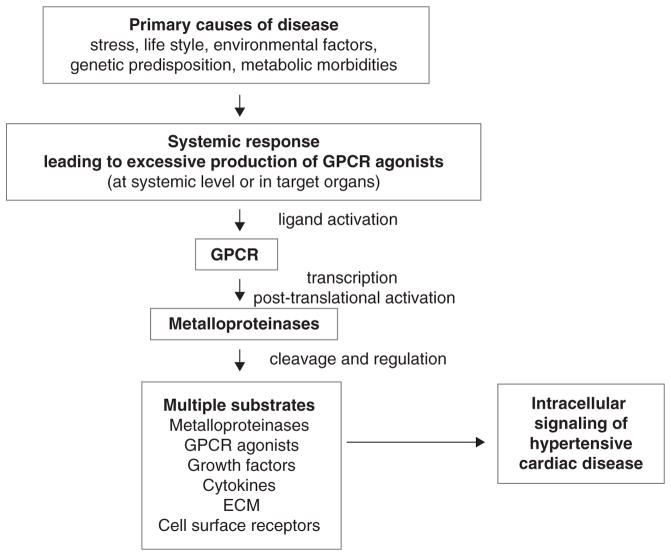
MMPs and ADAMs, which modulate vascular tone and transcription and translation of prohypertrophy and profibrosis genes downstream of multiple GPCRs, are candidate therapeutic targets to treat hypertensive cardiac disease with complex or unknown etiology (Fig. 1).
Figure 1.
Hypertensive cardiac disease is a consequence of diverse conditions and morbidities. Conditions such as chronic stress, environmental factors, genetic predisposition and metabolic morbidities trigger responses systemically and locally (on target cardiovascular organs) which lead to excessive signaling by GPCR agonists. This translates into unchecked proteolytic activity of metalloproteinases that regulate many substrates including growth factors, cytokines and cell surface receptors. Uncontrolled cleavage of these substrates contributes to the development of hypertensive cardiac disease.
We review how MMPs and ADAMs are activated, what substrates they cleave and what functions they might serve in models of agonist-induced hypertensive cardiac disease.
Comorbidities of hypertensive cardiac disease
The prevalence of chronic, noncommunicable diseases is increasing dramatically worldwide with close to 20 million people dying every year from cardiovascular disease associated with hypertension, diabetes, or obesity [1–3]. The association of metabolic disorders such as diabetes type I, insulin resistance (diabetes type II) and obesity with hypertension and cardiac problems is higher than would be the case by chance. The presence of multiple comorbidities makes the treatment of any single morbidity very difficult [1,4].
Over 60% of hypertensive individuals are obese, which has been explained through the chronic activation of the sympathetic nervous system by adipocyte-derived hormones such as leptin, which promotes energy expenditure but also systemic vasoconstriction and pathological cardiovascular remodeling [3].
Obesity predisposes patients to insulin resistance and diabetes type II with about 90% of diabetes type II being attributable to excess weight. In the USA, up to 75% of the adult patients with diabetes have hypertension and renovascular disease [5,6]. The chronic activation of the renin–angiotensin system results in sympathetic activation further impairing cardiovascular and renal functions. The co-occurrence of obesity and diabetic complications makes treatment of hypertension and the associated damage to target organs such as the kidneys and heart very challenging and often ineffective.
Although it remains unclear how diabetes predisposes to hypertension, it is clear that diabetes triggers a systemic hormonal response involving multiple agonists. Indeed, anti-hypertensive drugs targeting signaling by one or more GPCRs such as angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers and β-blockers are prescribed to treat and prevent cardiovascular complications including microvascular complications of retinopathy and progression of nephropathy in diabetic patients.
Similar to diabetes [5] and obesity [7], chronic stress (e.g. due to job strain, social environment or emotional distress) [6,8–12] and genetic predisposition [2,13] can cause various degrees of hypertension and cardiac disease. The major mechanism of disease by chronic stress is sympathetic nervous system activation, which results in high systemic or local (on target organs) production of stress hormones (e.g. catecholamines). The detrimental cardiovascular effects of high catecholamine levels are well established in humans as well as many disease models including rodents. Genetic predisposition plays a major role in disease development and, therefore, in the responses to antihypertensive treatments [2,5,13,14]. This has been well studied in North America where multiple ethnic groups coexist and are at risk of cardiovascular disease associated with metabolic syndrome. For instance, the incidence of hypertension, mortality from hypertensive heart disease, stroke and hypertensive renal disease are higher in African Americans. By contrast, Hispanic Americans have a lower overall risk for hypertension but are at a greater risk for hypertension due to diabetes and dyslipidemia. Although poorly understood, when compared to European Americans and other ethnic minorities, African Americans respond less favorably to β-blockers and ACE inhibitors whereas Asian American respond to calcium antagonists better than to ACE inhibitors and equally well to diuretics and β-blockers [14]. These facts indicate that identifying and pharmacologically manipulating common mediators of disease are very important for effective treatment of disease when the exact etiology is complex or unknown, as can be the case for individuals with various genetic predispositions.
Metalloproteinases: key and common mediators of multiple GPCRs in models of hypertensive cardiac disease
As discussed earlier, hypertensive cardiac disease can have a very complex etiology which poses a significant challenge for physicians. Current pharmacologic interventions are designed to interfere with specific causative factors (e.g. when pathological levels of angiotensin II are suspected, inhibitors of angiotensin II-converting enzyme or angiotensin II receptor blockers are prescribed to limit either angiotensin II generation or angiotensin II binding to its cellular receptors). However, when the causative agonists are unknown, current pharmacological interventions may not be effective if the causative factors are not targeted by the drug.
As a result, current treatment of patients with hypertensive cardiac disease remains highly empirical and requires the use of multiple drugs. Each drug is prescribed to afford complete or at least partial blood pressure reduction; however, often the blood pressure reduction target is not met [2,15]. The use of multiple drugs can result in undesired side-effects and elevated treatment costs to patients. Therefore, we believe that the identification of common effector mechanisms of multiple GPCR agonists could suggest more robust and general therapeutic approaches applicable to treat diseases with complex or unknown etiology [16].
Zinc-dependent endopeptidases (i.e. metalloproteinases) from the MMP and ADAM families are emerging as common mediators downstream of multiple GPCRs. The following section will cover how MMPs and ADAMs are activated, what substrates they cleave and what roles they play in models of agonist-induced hypertensive cardiac disease.
GPCR agonist-induced transactivation of growth depends on metalloproteinases
We and others have observed that multiple GPCR agonists transactivate growth factor receptors such as the epidermal growth factor receptor (EGFR) to enhance vascular tone and promote prohypertrophic and profibrotic processes in the cardiovascular system [16].
Transactivation of the EGFR can explain how and why agonists of vasoactive G-protein-coupled receptors (GPCRs) promote cancer cell growth and proliferation of cancer cells [17]. In arteries, EGFR transactivation is also mediated, at least in part, by metalloproteinases, such as MMP-2 and MMP-7, which are overexpressed or activated downstream of GPCRs including adrenoceptors, endothelin-1 and angiotensin II receptors. Angiotensin II and catecholamines rapidly activate MMP-7 which next sheds EGFR ligands (such as HB-EGF) to trigger intracellular signaling through the EGFR → mitogen-activated protein kinase (MAPK) cascade as well as the PI3K → AKT axis. This metalloproteinase-mediated transactivation mechanism appears to be a common response to multiple agonists irrespective of their chemical structure, enzymatic generators or site of production.
Activation of metalloproteinases by GPCR agonists
In response to high levels of GPCR agonists, some metalloproteinases are rapidly activated through post-translational mechanisms, which in turn trigger transcription of genes whose overexpression promotes cell proliferation, hypertrophy and profibrotic processes. Agonist-induced metalloproteinase activation can result from either proteolytic or nonproteolytic mechanisms.
Proteolytic activation of metalloproteinases
In the classical sense, the activation of metalloproteinases requires separation of the catalytic zinc ion from a cysteine residue (PKVCGY) in the metalloproteinase N-terminal prodomain [18]. Some metalloproteinases activate other metalloproteinases by cleaving their prodomain. This is the case for membrane-type MMPs which act in combination with tissue inhibitor of metalloproteinases (TIMP)-2 to activate pro-MMP-2. In addition, MMP-3 can directly cleave and activate pro-MMP-1, pro-MMP-7, pro-MMP-8 pro-MMP-9 and pro-MMP-13. Similarly, MMP-7 activates pro-MMP-1, pro-MMP-2, pro-MMP-8 and pro-MMP-9, whereas MMP-10 activates pro-MMP-7, pro-MMP-8 and pro-MMP-9.
Nonproteolytic mechanisms
Metalloproteinase activation can result from oxidative stress [19,20], post-translational modifications such as phosphorylation [21] and protein–protein interactions [22,23].
Oxidative stress
Agonist-induced Ca2+ signaling is intimately linked to MMP and ADAM activation, at least in part, by enabling the assembly of NADPH oxidase complex at the plasma membrane where it becomes active. Fully assembled, active NADPH oxidase generates superoxide anion which directly activates the catalytic cysteine switch in metalloproteinases. This may be a major mechanism of metalloproteinase activation in agonist-induced oxidative stress models [24]. For instance, when angiotensin II binds to its GPCR (AT1R), it sparks a Ca2+ signal that is followed by a burst in reactive oxygen species (ROS) which activates ADAM-17/TACE [25].
Protein kinases
Protein kinase C (PKC) may play a key role in metalloproteinase activation. PKC is allosterically activated by diacylglycerol (DAG) which is made by GPCR agonist-activated phospholipase C. Both DAG and its phorbol ester analogs trigger ADAM-12 activity and subsequent HB-EGF shedding in cardiomyocytes [26,27]. Active ADAM-12 is a major mediator of cardiac hypertrophy induced by angiotensin II, phenylephrine and pressure overload [28]. Cardiac PKC may modulate intracellular MMP-2 activity by phosphorylation at multiple sites including Ser and Thr residues. Indeed, mammalian MMP-2 contains 29 potential phosphorylation sites and mass spectrometry reveals that at least five of these sites are phosphorylated [21]. The kinase activity of extracellular signal-regulated kinase, ERK, leads to phosphorylation of the Thr735 of ADAM-17/TACE rendering ADAM-17 able to shed tumor necrosis factor (TNF)-α [29]. ADAM-17 activation can thus be pro-inflammatory, prohypertrophic and profibrotic.
Protein–protein interactions
A recently discovered mechanism of MMP and ADAM activation independent of proteolytic cleavage, oxidative stress, or kinase activity involves metalloproteinase interaction with protein interaction modules in other proteins. Binding of Src homology 3 (SH3) domains in PACSIN-3 and eve-1 to proline-rich domains of ADAM-12 enhances ADAM-12 proteolytic activity [30,31]. PKC-ε domains C1 and C2 bind to ADAM-12 enabling ADAM-12 translocation to the cell membrane and subsequent activation [32]. Protein–protein nonproteolytic interactions could explain the activation of ADAM-9, -12 and -17 in models where proteolytic activation and oxidative stress play a little or no role in activating MMPs such as GPCR agonist stimulation at subpressor doses.
Metalloproteinases regulate vasoactive peptides, contractile proteins, growth factors and cytokines as well as cell surface receptors
For many years, the cardiovascular effects of GPCR agonists were interpreted as a direct consequence of Ca2+ release from intracellular stores and Ca2+ uptake from the extracellular milieu. These two events are triggered rapidly following the allosteric binding of Gαq to phospholipase C which releases a Ca2+ channel agonist, inositol 1,4,5-trisphosphate (IP3), from membrane-anchored phosphoinositols. Induction of Ca2+ signaling by agonists can successfully explain how agonists evoke contractile responses in the cardiovascular system. However, to explain the maintenance of contraction over prolonged periods of agonist stimulation or the mitogenic effects induced by agonists, it is necessary to consider additional pathways such as the transactivation of growth factor receptor signaling by agonist-activated metalloproteinases.
Originally thought to degrade only components of the extracellular matrix (ECM) during the long-term process of tissue remodeling, MMP and ADAM family members also cleave and regulate many non-ECM substrates in an acute fashion. Excessive GPCR signaling thus translates into pathological metalloproteinase cleavage of ECM and non-ECM substrates. This section will focus on two classes of non-ECM substrates of metalloproteinases: ligands of receptors and cell surface receptors.
Cleavage of receptor ligands
The cleavage of receptor ligands can explain many acute actions of metalloproteinases and those of their pharmacological inhibitors such as modulation of vascular contractile responses. For instance, MMP-2 and MMP-9 can acutely enhance vascular tone through the cleavage of vasoactive peptides such as adrenomedullin, calcitonin gene-related peptide and big endothelin-1 [16,33–35]. Another non-ECM acute action of MMPs and ADAMs is shedding of membrane-anchored ligands of receptors such as epidermal growth factor (EGF) and platelet-derived growth factor (PDGF) to transactivate intracellular signaling of growth via the MAPK cascade. Thus, metalloproteinases modulate MAPK signaling to help maintain agonist-induced vascular tone and signaling of hypertrophic growth [36].
Acute cleavage of non-ECM substrates can affect immunity. MMP-2 cleaves monocyte chemoattractant peptide, MCP-3, turning it into a chemokine antagonist [37]. Agonist-activation of MMP-2 may thus dampen inflammation in hypertensive cardiac disease.
The remodeling of the contractile machinery of the cell is yet another novel action of MMPs. Recent work suggests that MMP-2 cleaves myofilament proteins such as titin and myosin light chain in models of ischemia–reperfusion in isolated perfused working hearts [24]. Unchecked cleavage of contractile components by metalloproteinases may be deleterious for cardiac muscle function.
Some metalloproteinases share substrates [38]. For instance, activation of TNF-α as well as shedding of HB-EGF can follow activation of either MMP-7 or ADAM-17/TACE. As such, these metalloproteinases have redundant roles with regards to inflammation and cardiac hypertrophy.
Cell surface receptors as substrates of metalloproteinases
This novel paradigm can help explain some features of the co-occurrence of hypertension and other morbidities [39]. MMPs have been found to cleave and inactivate vascular endothelial growth factor receptor (VEGFR)-2 to enable endothelial apoptosis and capillary rarefaction – a feature of malignant hypertension in humans and rodent models of disease. MMP cleavage of β2-adrenoceptor may impair vascular smooth muscle relaxation and increase peripheral resistance in spontaneously hypertensive rats. MMP-mediated degradation of the insulin receptor in the same model provides a novel explanation for why insulin resistance sometimes accompanies hypertensive disorders. Pathologically high levels of GPCR agonists in these models may trigger the observed MMP- and ADAM-mediated destruction of cell surface receptors.
GPCR signaling through MMPs and ADAMs: a clock for disease development?
The working hypothesis that has served as foundation for this review states that hypertensive cardiac disease can develop as a common pathological response to high levels of multiple GPCR agonists. MMPs and ADAMs are mediators of multiple GPCRs. However, it remains unclear whether metalloproteinases are central to cardiovascular regulation all the time or only in some stages of disease development.
Ongoing research from our laboratory is directed at answering this question. We have identified MMP-2, MMP-7, ADAM-12 and ADAM-17/TACE as mediators of angiotensin II-induced hypertensive cardiac disease. We have also found that gene expression of these metalloproteinases changes throughout disease development [16,36,40–42]. Moreover, some MMPs and ADAMs (such as MMP-7 and ADAM-17) appear to regulate others (such as MMP-2 and ADAM-12). In addition to the transient expression of individual MMPs and ADAMs, we observe the emergence of a novel specialization of functions: MMP-7 and MMP-2 may regulate vascular tone whereas ADAM-17 and ADAM-12 may signal cardiac hypertrophy and fibrosis under transcriptional control of MMP-7 and ADAM-17. This functional specialization is determined by temporal differences in expression and hierarchical relationships among MMPs and ADAMs [16,36,40–42].
Although we expect some MMPs and ADAMs to contribute to disease through overlapping mechanisms, their unique substrates and different time windows of expression will probably dominate their unique functions. The emerging functional specialization of metalloproteinases suggests new ways to manipulate and normalize specific processes such as signaling of vascular contractile responses, hypertrophy and fibrosis.
From a clinical translation point of view, studies are warranted to establish the impact of timely inhibition of specific metalloproteinases for effective treatment of hypertension and the associated damage of target organs such as the heart. As hypertension often goes undetected for years, currently it is unclear how timely inhibition of metalloproteinases can be achieved for the treatment of hypertension. In addition to timing, other areas of potential intervention include metalloproteinase activation mechanisms (such as oxidative stress), metalloproteinase-specific activities or expression, metalloproteinase substrates and the mechanisms elicited by these substrates after cleavage.
Conclusions
Metalloproteinases have emerged as key and common mediators of multiple GPCR agonists, which makes them candidate therapeutic targets for treatment of hypertensive cardiac disease when the etiology is complex or unknown. This type of global therapeutic concept would have potential for treatment of hypertensive cardiac disease in metabolic syndrome patients and perhaps also for treatment of populations where ethnic diversity might result in significant differences in the responses to current antihypertensives.
Acknowledgments
This work has been funded by a graduate studentship award from Alberta Innovates Health Solutions (to XW) and the Queen Elizabeth II and 75th anniversary graduate studentships from Faculty of Medicine and Dentistry University of Alberta (to AMB) and operating grants from the Canadian Institutes of Health Research and Natural Sciences and Engineering Research Council of Canada (to CF-P).
References
- 1.Kearney PM, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Lifton RP, et al. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 3.Biaggioni I. Should we target the sympathetic nervous system in the treatment of obesity-associated hypertension? Hypertension. 2008;51:168–171. doi: 10.1161/HYPERTENSIONAHA.107.090514. [DOI] [PubMed] [Google Scholar]
- 4.Jones DW, Hall JE. World Hypertension Day 2007. Hypertension. 2007;49:939–940. doi: 10.1161/HYPERTENSIONAHA.107.088740. [DOI] [PubMed] [Google Scholar]
- 5.Appel LJ, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363:918–929. doi: 10.1056/NEJMoa0910975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith SR. Metabolic syndrome targets. Curr Drug Targets CNS Neurol Disord. 2004;3:431–439. doi: 10.2174/1568007043337030. [DOI] [PubMed] [Google Scholar]
- 7.Esler M, et al. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48:787–796. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman D, et al. Early-life stress and the development of obesity and insulin resistance in juvenile bonnet macaques. Diabetes. 2007;56:1382–1386. doi: 10.2337/db06-1409. [DOI] [PubMed] [Google Scholar]
- 9.Marroquin OC, et al. Metabolic syndrome modifies the cardiovascular risk associated with angiographic coronary artery disease in women: a report from the Women’s Ischemia Syndrome Evaluation. Circulation. 2004;109:714–721. doi: 10.1161/01.CIR.0000115517.26897.A7. [DOI] [PubMed] [Google Scholar]
- 10.Feihl F, et al. Hypertension and microvascular remodelling. Cardiovasc Res. 2008;78:274–285. doi: 10.1093/cvr/cvn022. [DOI] [PubMed] [Google Scholar]
- 11.Ye S, et al. Oxidative stress mediates the stimulation of sympathetic nerve activity in the phenol renal injury model of hypertension. Hypertension. 2006;48:309–315. doi: 10.1161/01.HYP.0000231307.69761.2e. [DOI] [PubMed] [Google Scholar]
- 12.Koomans HA, et al. Sympathetic hyperactivity in chronic renal failure: a wake-up call. J Am Soc Nephrol. 2004;15:524–537. doi: 10.1097/01.asn.0000113320.57127.b9. [DOI] [PubMed] [Google Scholar]
- 13.Jamerson K, DeQuattro V. The impact of ethnicity on response to antihypertensive therapy. Am J Med. 1996;101:22S–32S. doi: 10.1016/s0002-9343(96)00265-3. [DOI] [PubMed] [Google Scholar]
- 14.Wright JT, Jr, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 15.Ban K, et al. Cardiovascular consequences of drugs used for the treatment of diabetes: potential promise of incretin-based therapies. J Am Soc Hypertens. 2009;3:245–259. doi: 10.1016/j.jash.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Patron C. Therapeutic potential of the epidermal growth factor receptor transactivation in hypertension: a convergent signaling pathway of vascular tone, oxidative stress, and hypertrophic growth downstream of vasoactive G-protein-coupled receptors? Can J Physiol Pharmacol. 2007;85:97–104. doi: 10.1139/y06-097. [DOI] [PubMed] [Google Scholar]
- 17.Zwick E, et al. The EGF receptor as central transducer of heterologous signalling systems. Trends Pharmacol Sci. 1999;20:408–412. doi: 10.1016/s0165-6147(99)01373-5. [DOI] [PubMed] [Google Scholar]
- 18.Welch AR, et al. Understanding the P1′ specificity of the matrix metalloproteinases: effect of S1′ pocket mutations in matrilysin and stromelysin-1. Biochemistry. 1996;35:10103–10109. doi: 10.1021/bi9601969. [DOI] [PubMed] [Google Scholar]
- 19.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- 20.Galis ZS, et al. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sariahmetoglu M, et al. Regulation of matrix metalloproteinase-2 (MMP-2) activity by phosphorylation. FASEB J. 2007;21:2486–2495. doi: 10.1096/fj.06-7938com. [DOI] [PubMed] [Google Scholar]
- 22.Kawaguchi N, et al. ADAM12 induces actin cytoskeleton and extracellular matrix reorganization during early adipocyte differentiation by regulating beta1 integrin function. J Cell Sci. 2003;116:3893–3904. doi: 10.1242/jcs.00699. [DOI] [PubMed] [Google Scholar]
- 23.Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16:558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulz R. Intracellular targets of matrix metalloproteinase-2 in cardiac disease: rationale and therapeutic approaches. Annu Rev Pharmacol Toxicol. 2007;47:211–242. doi: 10.1146/annurev.pharmtox.47.120505.105230. [DOI] [PubMed] [Google Scholar]
- 25.Myers TJ, et al. Mitochondrial reactive oxygen species mediate GPCR-induced TACE/ADAM17-dependent transforming growth factor-alpha shedding. Mol Biol Cell. 2009;20:5236–5249. doi: 10.1091/mbc.E08-12-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemjabbar-Alaoui H, et al. TACE/ADAM-17 phosphorylation by PKC-epsilon mediates premalignant changes in tobacco smoke-exposed lung cells. PLoS One. 2011;6:e17489. doi: 10.1371/journal.pone.0017489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jolly-Tornetta C, Wolf BA. Protein kinase C regulation of intracellular and cell surface amyloid precursor protein (APP) cleavage in CHO695 cells. Biochemistry. 2000;39:15282–15290. doi: 10.1021/bi001723y. [DOI] [PubMed] [Google Scholar]
- 28.Asakura M, et al. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat Med. 2002;8:35–40. doi: 10.1038/nm0102-35. [DOI] [PubMed] [Google Scholar]
- 29.Zheng Y, et al. Evaluation of the contribution of different ADAMs to tumor necrosis factor alpha (TNFalpha) shedding and of the function of the TNFalpha ectodomain in ensuring selective stimulated shedding by the TNFalpha convertase (TACE/ADAM17) J Biol Chem. 2004;279:42898–42906. doi: 10.1074/jbc.M403193200. [DOI] [PubMed] [Google Scholar]
- 30.Peschon JJ, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 31.Loechel F, et al. Human ADAM 12 (meltrin alpha) is an active metalloprotease. J Biol Chem. 1998;273:16993–16997. doi: 10.1074/jbc.273.27.16993. [DOI] [PubMed] [Google Scholar]
- 32.Sundberg C, et al. Regulation of ADAM12 cell-surface expression by protein kinase C epsilon. J Biol Chem. 2004;279:51601–51611. doi: 10.1074/jbc.M403753200. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Patron C, et al. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ Res. 1999;85:906–911. doi: 10.1161/01.res.85.10.906. [DOI] [PubMed] [Google Scholar]
- 34.Jeyabalan A, et al. Vascular matrix metalloproteinase-9 mediates the inhibition of myogenic reactivity in small arteries isolated from rats after short-term administration of relaxin. Endocrinology. 2007;148:189–197. doi: 10.1210/en.2006-0989. [DOI] [PubMed] [Google Scholar]
- 35.Jeyabalan A, et al. Essential role for vascular gelatinase activity in relaxin-induced renal vasodilation, hyperfiltration, and reduced myogenic reactivity of small arteries. Circ Res. 2003;93:1249–1257. doi: 10.1161/01.RES.0000104086.43830.6C. [DOI] [PubMed] [Google Scholar]
- 36.Hao L, et al. Agonist-induced activation of matrix metalloproteinase-7 promotes vasoconstriction through the epidermal growth factor-receptor pathway. Circ Res. 2004;94:68–76. doi: 10.1161/01.RES.0000109413.57726.91. [DOI] [PubMed] [Google Scholar]
- 37.McQuibban GA, et al. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000;289:1202–1206. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- 38.Morrison CJ, et al. Matrix metalloproteinase proteomics: substrates, targets, and therapy. Curr Opin Cell Biol. 2009;21:645–653. doi: 10.1016/j.ceb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 39.DeLano FA, Schmid-Schonbein GW. Proteinase activity and receptor cleavage: mechanism for insulin resistance in the spontaneously hypertensive rat. Hypertension. 2008;52:415–423. doi: 10.1161/HYPERTENSIONAHA.107.104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Odenbach J, et al. MMP-2 mediates angiotensin II-induced hypertension under the transcriptional control of MMP-7 and TACE. Hypertension. 2011;57:123–130. doi: 10.1161/HYPERTENSIONAHA.110.159525. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, et al. Tumor necrosis factor-alpha-converting enzyme is a key regulator of agonist-induced cardiac hypertrophy and fibrosis. Hypertension. 2009;54:575–582. doi: 10.1161/HYPERTENSIONAHA.108.127670. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, et al. Matrix metalloproteinase-7 and ADAM-12 (a disintegrin and metalloproteinase-12) define a signaling axis in agonist-induced hypertension and cardiac hypertrophy. Circulation. 2009;119:2480–2489. doi: 10.1161/CIRCULATIONAHA.108.835488. [DOI] [PubMed] [Google Scholar]