Abstract
Planar cell polarity (PCP) is the uniform orientation and alignment of a group of cells orthogonal to the apical–basal axis within a tissue. Originally described in insects, it is now known that PCP is required for many processes in vertebrates, including directional cell movement, polarized cell division, ciliary orientation, neural tube closure, heart development and lung branching. In this review, we outline the evidence implicating PCP in kidney development and disease focusing initially on the function of PCP in ureteric bud branching and elongation. We then describe how defects in PCP may lead to polycystic kidney disease and discuss a newly identified role for PCP in the kidney filtration barrier.
Keywords: cell alignment, glomerular filtration barrier, kidney development, polycystic kidney disease, ureteric bud branching
WHAT IS PLANAR CELL POLARITY?
For tissues and organs to function correctly, co-ordinated behaviour between groups of cells is required. Planar cell polarity (PCP) is the uniform orientation and alignment of a group of epithelial cells orthogonal to the apical–basal axis within a tissue [1]. PCP was originally described in the hairs and bristles on the wings of Drosophilia but there has been an explosion of recent work showing that PCP is also important in vertebrates. It is now known that PCP is required for vertebrate processes including directional cell movement [2], polarized cell division [3], ciliary orientation [4], neural tube closure [5], heart development [6] and lung branching [7]. In this review, we describe the importance of PCP in kidney development and disease focusing initially on the function of PCP in tubular formation and cystic disease and then discussing a potential role for PCP in the kidney glomerulus.
COMPONENTS OF THE PCP PATHWAY
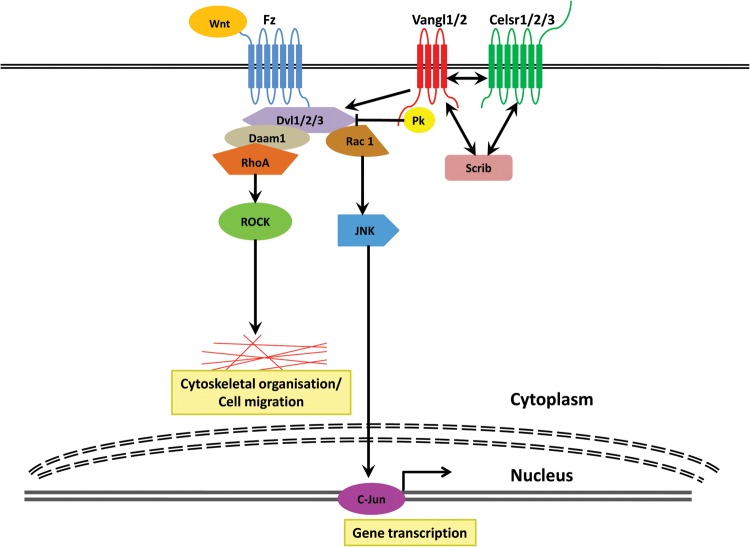
The process of PCP is largely controlled by two groups of proteins referred to as the ‘core’ planar polarity pathway and the Fat/Dachsous (Ds) system [1, 8]. Genetic studies in mice have revealed that both pathways affect much more than the planar organization of cells and are in fact better thought of as signalling pathways allowing cells to make directed changes in the cytoskeleton that are co-ordinated both locally and globally. It is still not fully understood whether the core PCP proteins and Fat/Ds system function as a single linear pathway or two parallel ones [9]. The core set of PCP proteins (Figure 1) mediate the local polarity of groups of cells and consists of the transmembrane proteins Vangl1 and Vangl2 (homologues of Drosophila Van Gogh), Celsr1, -2 and -3 (homologues of Drosophila Flamingo) and the Wnt receptors, Frizzled (Fz) [1, 8]. Other components of the PCP core are the cytoplasmic proteins Dishevelled (Dvl1, -2 and -3) and Prickle (Pk1 and Pk2) [1, 8]. When activated, for example by the actions of Wnt ligands most often associated with non-canonical pathways such as Wnt5a, Wnt7b, Wnt9b and Wnt11, the components of the PCP core form complexes that are asymmetrically distributed at either the proximal or distal cell membrane. Some of the components of the ‘core’ PCP pathway (Fz and Dvl) are also involved in the canonical (β-catenin) Wnt signalling cascade, which regulates cell proliferation and differentiation [10]. The role of canonical Wnt signalling in kidney development and disease has been reviewed recently [11, 12]; hence, we will focus on the less well-studied PCP pathway in this article.
FIGURE 1:
The ‘core’ PCP proteins. The core set of PCP proteins consists of the transmembrane proteins Vangl1, Vangl2, Celsr1, -2 and -3 and the Wnt receptors, Frizzled (Fz) and the cytoplasmic proteins Dishevelled (Dvl1, -2 and -3) and Prickle (Pk1 and Pk2). The core PCP proteins exert their effects via members of the Rho GTPase family, c-Jun kinase and dishevelled-associated activator of morphogenesis (Daam1), which regulates the polarization of the cytoskeleton. Other genes have also been implicated in PCP, for example the cytoplasmic protein Scribble which genetically interacts with the core PCP protein Vangl2.
The role of the Fat/Ds system in PCP is well established in flies but less so in vertebrates. The Fat/Ds system includes the atypical cadherins Fat 1–4 (paralogs of Drosophila Fat) and Dchs1-2 (orthologues of Drosophila Ds) that preferentially bind to each other at the cell surface [8]. Unlike the core PCP proteins, which are asymmetrically distributed, Fat and Ds are a receptor–ligand signalling complex which is modulated by four-jointed (Fj), a Golgi-associated kinase, that phosphorylates and thus alters the binding affinity of Fat and Ds for each other [13]. Fj is expressed in a graded pattern in many tissues, resulting in graded activity of Fat and Ds and the suggestion that this system may regulate global polarity [8]. The core PCP and Fat/Ds systems exert their effects via downstream effectors such as the intracellular proteins fuzzy, inturned and fritz [8] and signalling molecules including members of the Rho GTPase family (RhoA, Rac1 and Cdc42), c-Jun kinase and dishevelled associated activator of morphogenesis (Daam1). Other genes have also been implicated in PCP, for example the cytoplasmic protein Scribble which genetically interacts with the core PCP protein Vangl2 [14, 15]; mutations in Scribble lead to classical PCP phenotypes including neural tube defects [15] and impaired lung branching [14].
PCP PROTEINS REGULATE TUBULAR BRANCHING AND ELONGATION IN THE DEVELOPING KIDNEY
The formation of the mammalian kidney is initiated around the fifth week of human gestation and embryonic day 11 (E11) in mice when the ureteric bud branches out from the mesonephric duct and invades the surrounding metanephric mesenchyme [16, 17]. The ureteric bud then undergoes branching morphogenesis to form a highly complex, tree-like system of tubes that make up the renal collecting duct system of the mature kidney [16, 17]. The metanephric mesenchyme contains a self-renewing, pluripotent progenitor cell population that proliferates and covers every new ureteric bud. After each round of branching, a subpopulation of mesenchymal cells undergo mesenchyme-to-epithelium transition forming a primitive epithelial structure known as the renal vesicle [16, 17]. The renal vesicle will undergo extensive morphogenesis to form the future nephron consisting of glomeruli and proximal tubules. Tubular branching involves several processes in which PCP signalling has been implicated including cytoskeletal re-organization, directed cell movement and oriented cell division, which ultimately establish tubular diameter, length and shape [16]. The tubules then elongate through another process involving PCP called convergent extension which has been extensively studied in Drosphilia [18]. Analogous to the visceral endoderm of vertebrates [19], the kidney cells become organized in groups (rosettes) during the process of convergent extension. These groups of cells then elongate mediolaterally and move directionally to intercalate with neighbouring cells [20]. This change in shape and movement results in convergence of the cells towards the midline and elongation of the tissue along the antero-posterior axis.
In the kidney, many of the core PCP components are expressed in developing tubular epithelia, although whether they are asymmetrically localized is less well understood. In situ hybridization showed Celsr1-3 transcripts localized to the ureteric bud in mice at E14 [21]. Later in development, at E18.5, Celsr1 protein is expressed in collecting duct stalks and proximal tubules [22]. Vangl2 is also expressed in the ureteric bud [23] and to gain insights into the role of this molecule in kidney development we examined ureteric bud branching in metanephroi obtained from mice with a spontaneous homozygous mutation in Vangl2 (Vangl2Lp/Lp; Looptail) [22]. By E13.5 Vangl2Lp/Lp mice contained significantly fewer ureteric branch tips than wild-type littermates [22]. E13.5 and E18.5 Vangl2Lp/Lp kidneys were also smaller than wild-type littermates [22], especially in their antero-posterior axis with a lower length/width ratio. Knock-out mice of other PCP genes (Dchs1, Fat4 and double mutants for both Fz4 and Fz8) also have impaired ureteric bud branching compared with wild-type littermates and smaller kidneys at birth [24, 25]. In addition, mice with mutations in Wnt ligands thought to signal through the PCP pathway (Wnt7b, Wnt9b and Wnt11) also exhibit ureteric bud branching defects [26–28]. Wnt9b also plays a role in tubular elongation, with mice lacking this gene specifically in their tubules demonstrating reduced complexity of rosettes [20]. The link between PCP and tubular elongation was further strengthened through studies inhibiting PCP signalling during tubular elongation in Xenopus [20]; this disrupted rosette topology and was accompanied by a reduction in nephron elongation and tubular diameter [20]. Finally, a recent study in Xenopus showed that morpholino knock-down of Daam1 led to reduced tubular branching in pronephric proximal segments; Daam1 knock-down had no effect on proliferation and apoptosis suggesting the phenotype was due to changes in differentiation and morphogenesis [29]. Knock-down of WGEF, a Rho-GEF that associates with Daam1 also altered tubular morphogenesis [29] in Xenopus. Furthermore, zebrafish with Daam1 knock-down exhibited phenotypes with defective convergence-extension and convoluted tubules [29].
What could be the potential mechanisms by which PCP genes alter ureteric bud branching? The most likely explanation is that the phenotype seen in Looptail mice is due to an intrinsic defect in ureteric bud epithelia which express Vangl2. This could be tested by deleting Vangl2 specifically in the ureteric bud tips. Making parallels with the role of Vangl2 in lung branching [7], it could be speculated that the kidneys of Vangl2Lp/Lp mice have deficiencies in the actin cytoskeleton impairing cell motility and subsequently causing abnormal ureteric bud branching. Recent studies have shown that Looptail mice do not only have changes in Vangl2, but also other PCP genes, namely Vangl1 and Pk2 [30], which may contribute to the phenotypes observed in these mice. Therefore, future studies need to use Cre-loxP technology to pin point the precise functions of specific PCP genes in tubular branching. Fz4 and Fz8 also are expressed in the developing ureteric buds as early as E11.5 and subsequently in tubular epithelia [25]. In contrast, the effect of Dchs1 and Fat4 on ureteric bud branching is likely to be indirect as the expression of these genes was found to be expressed in mesenchyme rather than epithelium [24]. However, to-date the molecular mechanisms underlying the defects in ureteric bud branching seen in Dchs1 and Fat4 mutant mice are currently unknown.
Defective ureteric bud branching can subsequently lead to reduced nephron numbers, as fewer ureteric bud tips are available to induce mesenchymal cells to differentiate into nephrons [31]. As homozygous Looptail mice die at birth, we examined heterozygous Vangl2Lp/+mice at 4 weeks of age and demonstrated that they had a modest but significant reduction in nephron numbers compared with wild-type littermates [22]. This may have important clinical implications as prior studies have shown that low nephron numbers per kidney correlate with systemic hypertension in Caucasian subjects [32]. A deficiency in nephron numbers can also lead to microalbuminuria in mice [33]; however we did not observe changes in urinary albumin in Vangl2Lp/+ 4-week-old mice [22]. It would be of interest for further studies to be performed examining nephron numbers ideally using detailed stereology or injection of cationized ferritin [34] and determining the possible associations with albuminuria and blood pressure in older Vangl2Lp/+ mice and other PCP mutants. If a link between PCP and glomerular number is firmly established then mutations in PCP genes may be a potential explanation for the large degree of variability in glomerular numbers seen in the human population with normotensive individuals having between 1 and 2 × 106 glomeruli per kidney [32] and for chronic kidney disease currently attributed to ‘small kidneys’ undergoing hyperfiltration [35].
DEFECTIVE PCP AND POLYCYSTIC KIDNEY DISEASE
The logical extension from the finding that PCP regulates tubular branching and elongation is that defects in PCP proteins may occur in situations where tubular shape is altered. Indeed, several studies have implicated changes in PCP processes in polycystic kidney diseases (PKD); conditions where multiple fluid-filled tubules expand causing a loss of normal kidney structure leading to morbidity, renal failure and death from before birth through adulthood [36]. Autosomal dominant (AD)PKD is common, affecting 1 in 800 people and is caused by mutations in the polycystin (PKD1 and PKD2) genes [36]. Cysts can occur early in life with ADPKD but mostly it is a disease of middle to later ages. In contrast, autosomal recessive (AR)PKD (1:20 000 births) is characterized by the formation of multiple cysts often during the second half of gestation which can cause problems at birth or during childhood; here the mutated gene is PKHD1, coding for fibrocystin [37]. PKD is often referred to as a ciliopathy because PKD1, PKD2 and PKHD1 localize to the primary cilia/basal body [37, 38], a microtubular structure which extends from apical membranes into tubular lumina and acts as a flow sensor [39].
Fischer et al. [40] demonstrated that PCP processes may be involved in PKD by observing that distortions in mitotic orientations occurred in pck rats, a model of ARPKD. It was proposed that the defect in mitotic orientations led to a failure to regulate tubular size, leading to dilatation and cyst formation. In support of this finding, mice that lack Pkd1 in their distal tubule segments also have defects in orientated cell division in kidney tubules [41]. Critical to the process of mitotic cell division is the primary cilium, and loss of the ciliary proteins Ift88 and inversin leads to defects in mitotic spindle orientation and thus defective orientation of cell division [42, 43]. Whether the disruption of orientated cell division actually drives the pathophysiology of PKD is unclear. Some studies have indicated that changes in orientated cell division occur prior to tubular dilation and cyst formation [40, 41]. In contrast, other investigations using Pkhd1del4/del4 mice which have a mutation in Pkhd1; but do not subsequently develop cysts demonstrated that these mice still lost orientated cell division in their renal tubules [44]. The same group showed that following kidney-selective inhibition of either Pkd1 or Pkd2 the orientated division prior to cyst formation was normal and only lost once tubular dilation began [44]. The discrepancies between these studies could be due to the different animal models used or time points and methods used to evaluate orientated cell division.
The importance of PCP signalling in cystic disease was highlighted by the finding that inversin acts as a molecular switch leading to the dampening of canonical Wnt signalling and the promotion of PCP signalling [45]. This alteration in signalling is critical for normal kidney development [45] and patients with mutations in inversin develop nephronophthisis type II, an autosomal recessive cystic kidney disease [46]. Changes in PCP protein expression also occur in PKD animal models with the cystic kidneys of Pkd1 mice containing upregulated levels of Frizzled-3 and its downstream effector Cdc42 [41]. Furthermore, some animal models with disrupted PCP proteins develop PKD. Fat4 mutant mice develop cystic kidneys with abnormal orientated cell division and tubular elongation [47]; the phenotype of these animals was also exaggerated in Fat4 mutants with a haploinsufficiency of Vangl2. Loss of Wnt9b in mice also leads to cyst formation [28] with the mutant tubules having defective convergent extension [20, 28]. Finally, zebrafish with morpholino knock-down of Pk1 develop renal cysts and basal body disorganization [48]. However, disruption of other PCP proteins may not lead to cyst formation; for example in fetal Vangl2Lp/Lp or 1-month old Vangl2Lp/+ mice [22]. This is a striking observation as the Looptail mutant effect encompasses not only Vangl2, but also the function of other PCP proteins including Vangl1 and Pk2 [30]. PCP proteins have also been shown to play a direct role in cilia function with Vangl2 [49], Fat4 [47] and Fuzzy [50] all localized to the cilia basal body. Vangl2 can control the orientation of motile primary cilia [4, 51], and zebrafish with Vangl2 knock-down have defects in mitotic spindle orientation and fewer ciliated cells [52]. Fuzzy is involved in the assembly of primary cilium by controlling the localization of the key cilia regulator, Rab8 [50]. The effect of PCP genes on cilia may provide an explanation as to why Vangl2Lp/+ fail to develop cysts; cilia number or orientation were not evaluated in these studies [22] and subtle changes in cilia function may result in cystic kidneys at later time points.
PCP IN PODOCYTE BIOLOGY
Podocytes are specialized epithelia which, together with the glomerular basement membrane and glycocalyx on adjacent endothelia, constitute the size and charge-selective filtration barrier in the mammalian kidney [53]. During glomerular development, podocytes undergo dramatic changes in cell morphology. Initially, they are columnar cells connected laterally by tight junctions, whilst in their differentiated state they become highly branched and polarized with foot processes interdigitating to form a junctional complex called the slit diaphragm [54], which retains circulating macromolecules such as albumin. The branched morphology is essential for podocyte function within the glomerulus and if this is disrupted it leads to foot process effacement and proteinuria [55]. The development and maintenance of podocyte structure involves cytoskeletal reorganization [55] leading to the idea that PCP signalling might play a role in maintaining podocyte architecture.
Several studies have shown that podocytes express PCP genes. In culture, proliferating and differentiating conditionally immortalized mouse podocytes have been shown to express transcripts for the PCP genes Celsr1, Dvl2, Mpk1, Mpk2, Scrib, Vangl1 and Vangl2 [22, 56] DAAM1, DVL2, PK1 and VANGL2 transcripts have also been detected in cultured human podocytes [56]. In vivo, Vangl2 is expressed on the basal-lateral plasma membrane of cuboidal epithelial cells in comma- and S-shaped bodies and is later localized to the basal aspect of the podocyte when foot processes form at the capillary loop stage [57]. Vangl2 is then down-regulated in later development where mature podocytes express high levels of the slit diaphragm protein nephrin [57]. Using detailed immunogold techniques, Scrib has been specifically localized to the cell–cell contacts of immature podocytes in new-born rats whilst in adult animals it is localized to the basolateral side of the podocyte foot process [58]. Celsr1 has also been detected in S-shaped bodies and podocytes of E18.5 kidneys [22].
To begin to investigate the functional role of PCP genes in glomeruli we examined E18.5 kidneys obtained from Vangl2Lp/Lp mice and their wild-type littermates [22]. Glomeruli from Vangl2Lp/Lp fetuses were smaller containing fewer proliferating cells and less prominent capillary loops than wild-type littermates [22]. How Vangl2 may alter glomerular morphology is unclear. A possibility is that Vangl2 plays a role in a mitotic orientation in podocytes; however, to date there is no evidence that mitotic orientation or convergent extension occurs in podocyte development. Furthermore, the presence of primary cilia on podocytes is controversial with only one study showing that developing, but not mature rat podocytes are ciliated [59]. Some clues into the possible role of PCP proteins in podocytes have been obtained from studies using cultured cells and these experiments have indicated that PCP proteins may play a role in podocyte cytoskeletal organization. Babayeva et al. [56] activated PCP signalling by incubating podocytes with conditioned media from cells overexpressing Wnt5a protein. This led to a rearrangement in the distribution of Daam1 from a diffuse pattern in mock-stimulated podocytes to expression in aggregates along stress fibres in Wnt5a-stimulated cells [56]. Wnt5a exposure also led to changes in podocyte morphology with more stress fibres in each cell [56] and increased nephrin endocytosis mediated by clathrin and β-arrestin [57], a manoeuvre which can lead to impairment of the glomerular slit diaphragm [60]. Loss of Vangl2 also has effects on podocyte biology: siRNA depletion of Vangl2 reduces the number of cell projections, decreased actin stress fibres and impaired cell motility [56]. Reduction of Vangl2 also increased nephrin levels at the cell surface [57]. In Vangl2Lp/Lp mice [22] we observed changes in the pattern of actin staining as assessed by phalloidin staining, but no alternations in nephrin; however, endocytosis or phosphorylation of this molecule was not examined. An alternative explanation for the glomerular phenotypes seen in Vangl2Lp/Lp mice could be that PCP proteins affect other cells within the glomerulus. Recent studies have indicated that PCP may alter angiogenesis and endothelial cell proliferation [61] and this is an area that warrants investigation in the kidney glomerulus.
Hartleben et al. [58] generated podocyte-specific Scrib knock-out mice using Cre-loxP technology and examined the glomerular ultrastructure using electron microscopy. Unlike the Vangl2Lp/Lp mice they observed no abnormalities in podocyte morphology, neither were there changes in albumin excretion in mice up to 12 months of age [58]. In addition, the Scrib mouse mutant Circletail which is embryonically lethal by E18.5 also displayed no changes in podocyte morphology when examined by electron microscopy [58]. The simplest explanation for the lack of phenotype in mice with a deficiency in Scrib is that not all of the PCP components are critical for glomerular function. Therefore, further site-specific knock-out studies are required for the PCP proteins Vangl2 and Celsr1 to establish whether they have a specific role in glomerular biology. Another important consideration may be genetic background: the podocyte-specific Scrib mice were bred to a C57BL/6 [58] background which has been shown to be resistant to renal damage [33] and hence may mask any subtle kidney phenotypes.
FUTURE PERSPECTIVES
The above findings suggest that PCP signalling may play important roles in renal development and disease (Figure 2), but there are still many questions which remain unanswered. In the developing kidney, it is unclear what mechanisms link defects in PCP proteins to abnormal ureteric bud branching and glomerular maturation. This needs to be addressed using renal site-specific knock-outs followed by detailed examination of PCP functions including the cell cytoskeleton, migration, ciliary function and mitotic orientation. A significant amount of work has now been done on several PCP genes such as Vangl2 and Fat4, but many others in the pathway remain unexplored; these studies will considerably enhance our understanding of PCP in the kidney. The clinical importance of PCP warrants investigation in greater detail. First, defects in kidney development lead to congenital anomalies of the kidney and urinary tract (CAKUT) [17] and it would be of interest to examine whether mutations of PCP genes occur in these patients. A potentially interesting group of patients to examine could be those with neural tube defects who are born with CAKUT [62]. Given the fact that Vangl2Lp/Lp mice have defects in both the neural tube and kidney it could be speculated that mutations in VANGL2 or other PCP genes may account for the phenotypes seen in these patients. Secondly, as PCP genes appear to alter podocyte morphology which is a key event in the progression of glomerular disease [55], it could be postulated that PCP proteins could be modulated in these conditions. Therefore, it would be of interest to examine PCP proteins in animal models of glomerular disease and human biopsy samples. Further studies could also investigate whether glomerular disease progression could be altered in mice lacking PCP genes. Finally, another area of study may be other situations where tubular shape is altered for example the dilatation that occurs in acute tubular necrosis [63]. A study by Li et al. [64] examined the role of processes in which PCP signalling has been implicated in dilated tubules caused by urinary tract obstruction in mice. They demonstrated that orientated cell division is disrupted in urinary tract obstruction which was accompanied by altered Fz3 expression [64]. Interestingly, once the tubules had recovered, orientated cell division was returned to normal raising the possibility that manipulation of PCP signalling may repair damaged tubules. Therefore, a future challenge could be to determine if modulation of PCP signalling can restore tubular damage in renal disease; this may be achieved through modification of Wnt pathways [56].
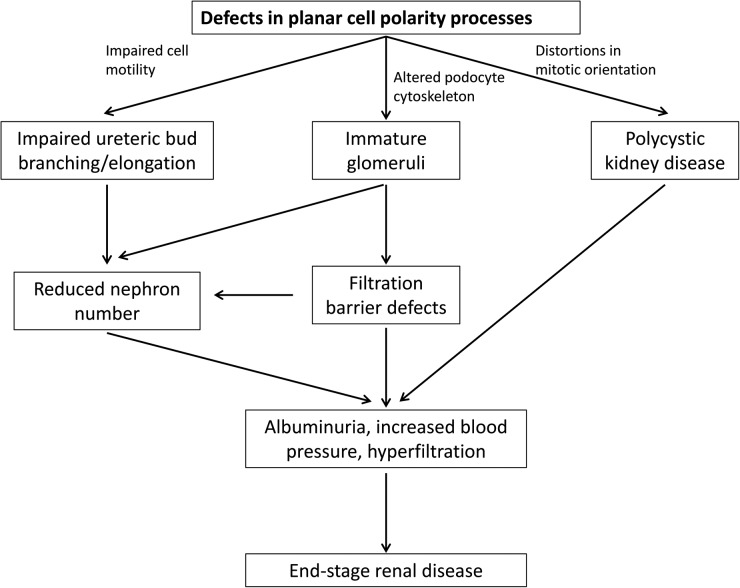
FIGURE 2:
How could defects in PCP processes lead to end-stage renal disease? Defects in PCP processes lead to impaired branching and elongation of the ureteric bud and the formation of immature glomeruli. These changes could subsequently lead to reduced nephron number leading to albuminuria, increased blood pressure and hyperfiltration subsequently causing end-stage renal disease. In addition, distortions in mitotic orientation may directly cause PKD, the leading inherited cause of end-stage renal disease.
CONFLICT OF INTEREST STATEMENT
The work presented in this paper has not been published previously. The authors have nothing to declare.
ACKNOWLEDGEMENTS
We thank Professor Adrian Woolf (University of Manchester) and Dr Paul Winyard (UCL Institute of Child Health) for helpful discussions regarding this work and Thanushiyan Poobalasingam (Imperial College London) for the preparation of Figure 1. E.P. is funded by a Wellcome Trust Postdoctoral Training Fellowship for MB/PhD graduates. D.A.L. is funded by Kidney Research UK Senior Non-Clinical Fellowship and MRC New Investigator Award. A.J.C.'s research is funded by grants from the Wellcome Trust (087525) and MRC (G0801124).
REFERENCES
- 1.Wallingford JB. Planar cell polarity and the developmental control of cell behaviour in vertebrate embryos. Annu Rev Cell Dev Biol. 2012;28:627–653. doi: 10.1146/annurev-cellbio-092910-154208. [DOI] [PubMed] [Google Scholar]
- 2.Williams BB, Cantrell VA, Mundell NA, et al. VANGL2 regulates membrane trafficking of MMP14 to control cell polarity and migration. J Cell Sci. 2012;125:2141–2147. doi: 10.1242/jcs.097964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devenport D, Oristian D, Heller E, et al. Mitotic internalization of planar cell polarity proteins preserves tissue polarity. Nat Cell Biol. 2011;13:893–902. doi: 10.1038/ncb2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borovina A, Superina S, Voskas D, et al. Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nat Cell Biol. 2010;12:407–412. doi: 10.1038/ncb2042. [DOI] [PubMed] [Google Scholar]
- 5.Ybot-Gonzalez P, Savery D, Gerrelli D, et al. Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development. 2007;134:789–799. doi: 10.1242/dev.000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips HM, Murdoch JN, Chaudhry B, et al. Vangl2 acts via RhoA signaling to regulate polarized cell movements during development of the proximal outflow tract. Circ Res. 2005;96:292–299. doi: 10.1161/01.RES.0000154912.08695.88. [DOI] [PubMed] [Google Scholar]
- 7.Yates LL, Schnatwinkel C, Murdoch JN, et al. The PCP genes Celsr1 and Vangl2 are required for normal lung branching morphogenesis. Hum Mol Genet. 2010;19:2251–2267. doi: 10.1093/hmg/ddq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray RS, Roszko I, Solnica-Krezel L. Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev Cell. 2011;21:120–133. doi: 10.1016/j.devcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawrence PA, Casal J. The mechanisms of planar cell polarity, growth and the Hippo pathway: some known unknowns. Dev Biol. 2013;377:1–8. doi: 10.1016/j.ydbio.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lancaster MA, Gleeson JG. Cystic kidney disease: the role of Wnt signaling. Trends Mol Med. 2010;16:349–360. doi: 10.1016/j.molmed.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawakami T, Ren S, Duffield JS. Wnt signalling in kidney diseases: dual roles in renal injury and repair. J Pathol. 2013;229:221–231. doi: 10.1002/path.4121. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt-Ott KM, Barasch J. Wnt/beta-catenin signaling in nephron progenitors and their epithelial progeny. Kidney Int. 2008;74:1004–1008. doi: 10.1038/ki.2008.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brittle AL, Repiso A, Casal J, et al. Four-jointed modulates growth and planar polarity by reducing the affinity of Dachsous for Fat. Curr Biol. 2010;20:803–810. doi: 10.1016/j.cub.2010.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yates LL, Schnatwinkel C, Hazelwood L, et al. Scribble is required for normal epithelial cell-cell contacts and lumen morphogenesis in the mammalian lung. Dev Biol. 2013;373:267–280. doi: 10.1016/j.ydbio.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murdoch JN, Henderson DJ, Doudney K, et al. Disruption of scribble (Scrb1) causes severe neural tube defects in the circletail mouse. Hum Mol Genet. 2003;12:87–98. doi: 10.1093/hmg/ddg014. [DOI] [PubMed] [Google Scholar]
- 16.Constantini F, Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell. 2010;18:698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renkema KY, Winyard PJ, Skovorodkin IN, et al. Novel perspectives for investigating congenital anomalies of the kidney and urinary tract (CAKUT) Nephrol Dial Transplant. 2011;26:3843–3851. doi: 10.1093/ndt/gfr655. [DOI] [PubMed] [Google Scholar]
- 18.Denholm B. Shaping up for action. The path to physiological maturation in the renal tubules of Drosophilia. Organogenesis. 2013;9:40–54. doi: 10.4161/org.24107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trichas G, Smith AM, White N, et al. Multi-cellular rosettes in the mouse visceral endoderm facilitate the ordered migration of anterior visceral endoderm cells. PLoS Biol. 2012;10:e1001256. doi: 10.1371/journal.pbio.1001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lienkamp SS, Liu K, Karner CM, et al. Vertebrate kidney tubules elongate using a planar cell polarity-dependent, rosette-based mechanism of convergent extension. Nat Genet. 2012;44:1382–1387. doi: 10.1038/ng.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shima Y, Copeland NG, Gilbert DJ, et al. Differential expression of the seven-pass transmembrane cadherin genes Celsr1–3 and distribution of the Celsr2 protein during mouse development. Dev Dyn. 2002;223:321–332. doi: 10.1002/dvdy.10054. [DOI] [PubMed] [Google Scholar]
- 22.Yates LL, Papakrivopoulou J, Long DA, et al. The planar cell polarity gene Vangl2 is required for mammalian kidney-branching morphogenesis and glomerular maturation. Hum Mol Genet. 2010;19:4663–4676. doi: 10.1093/hmg/ddq397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torban E, Wang HHJ, Patenaude AM, et al. Tissue, cellular and sub-cellular localization of the Vangl2 protein during embryonic development: effect of the Lp mutation. Gene Expr Patterns. 2007;7:346–354. doi: 10.1016/j.modgep.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Mao Y, Mulvaney J, Zakaria S, et al. Characterization of a Dchs1 mutant mouse reveals requirements for Dchs1-Fat4 signaling during mammalian development. Development. 2011;138:947–957. doi: 10.1242/dev.057166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye X, Wang Y, Rattner A, Nathans J. Genetic mosaic analysis reveals a major role for frizzled 4 and frizzled 8 in controlling ureteric growth in the developing kidney. Development. 2011;138:1161–1172. doi: 10.1242/dev.057620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majumdar A, Vainio S, Kispert A, et al. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development. 2003;130:3175–3185. doi: 10.1242/dev.00520. [DOI] [PubMed] [Google Scholar]
- 27.Yu J, Carroll TJ, Rajagopal J, et al. A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development. 2009;136:161–171. doi: 10.1242/dev.022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karner CM, Chirumamilla R, Aoki S, et al. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet. 2009;41:793–799. doi: 10.1038/ng.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller RK, Canny SG, Jang CW, et al. Pronephric tubulogenesis requires Daam1-mediated planar cell polarity. J Am Soc Nephrol. 2011;22:1654–1664. doi: 10.1681/ASN.2010101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin H, Copley CO, Goodrich LV, et al. Comparison of phenotypes between different vangl2 mutants demonstrates dominant effects of the Looptail mutation during hair cell development. Plos One. 2012;7:e31988. doi: 10.1371/journal.pone.0031988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dziarmaga A, Eccles M, Goodyer P. Suppression of ureteric bud apoptosis rescues nephron endowment and adult renal function in Pax2 mutant mice. J Am Soc Nephrol. 2006;17:1568–1575. doi: 10.1681/ASN.2005101074. [DOI] [PubMed] [Google Scholar]
- 32.Keller G, Zimmer G, Mall G, et al. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348:101–108. doi: 10.1056/NEJMoa020549. [DOI] [PubMed] [Google Scholar]
- 33.Long DA, Kolatsi-Joannou M, Price KL, et al. Albuminuria is associated with too few glomeruli and too much testosterone. Kidney Int. 2013;83:1118–1129. doi: 10.1038/ki.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heilmann M, Neudecker S, Wolf I, et al. Quantification of glomerular number and size distribution in normal rat kidneys using magnetic resonance imaging. Nephrol Dial Transplant. 2012;27:100–107. doi: 10.1093/ndt/gfr273. [DOI] [PubMed] [Google Scholar]
- 35.Helal I, Fick-Brosnahan GM, Reed-Gitomer B, et al. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol. 2012;8:293–300. doi: 10.1038/nrneph.2012.19. [DOI] [PubMed] [Google Scholar]
- 36.Grantham JJ, Mulamalla S, Swenson-Fields KI. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2011;7:556–566. doi: 10.1038/nrneph.2011.109. [DOI] [PubMed] [Google Scholar]
- 37.Ward CJ, Yuan D, Masyuk TV, et al. Cellular and subcellular localization of the ARPKD protein; fibrocystin is expressed on primary cilia. Hum Mol Genet. 2003;12:2703–2710. doi: 10.1093/hmg/ddg274. [DOI] [PubMed] [Google Scholar]
- 38.Nauli SM, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 39.Kotsis F, Boehlke C, Kuehn EW. The ciliary flow sensor and polycystic kidney disease. Nephrol Dial Transplant. 2013;28:518–526. doi: 10.1093/ndt/gfs524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer E, Legue E, Doyen A, et al. Defective planar cell polarity in polycystic kidney disease. Nat Genet. 2006;38:21–23. doi: 10.1038/ng1701. [DOI] [PubMed] [Google Scholar]
- 41.Luyten A, Su X, Gondela S, et al. Aberrant regulation of planar cell polarity in polycystic kidney disease. J Am Soc Nephrol. 2010;21:1521–1532. doi: 10.1681/ASN.2010010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delaval B, Bright A, Lawson ND, et al. The cilia protein IFT88 is required for spindle orientation in mitosis. Nat Cell Biol. 2011;13:461–468. doi: 10.1038/ncb2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Werner ME, Ward HH, Philips CL, et al. Inversin modulates the cortical actin network during mitosis. Am J Physiol Cell Physiol. 2013;305:C36–C47. doi: 10.1152/ajpcell.00279.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishio S, Tian X, Gallagher AR, et al. Loss of oriented cell division does not initiate cyst formation. J Am Soc Nephrol. 2010;21:295–302. doi: 10.1681/ASN.2009060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simons M, Gloy J, Ganner A, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Otto EA, Schermer B, Obara T, et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet. 2003;34:413–420. doi: 10.1038/ng1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saburi S, Hester I, Fischer E, et al. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet. 2008;40:1010–1015. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- 48.Cao Y, Park A, Sun Z. Intraflagellar transport proteins are essential for cilia formation and for planar cell polarity. J Am Soc Nephrol. 2010;21:1326–1333. doi: 10.1681/ASN.2009091001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross AJ, May-Simera H, Eichers ER, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 50.Zilber Y, Babayeva S, Seo JH, et al. The PCP effector Fuzzy controls cilial assembly and signaling by recruiting Rab8 and Dishevelled to the primary cilium. Mol Biol Cell. 2013;24:555–565. doi: 10.1091/mbc.E12-06-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guirao B, Meunier A, Mortaud S, et al. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat Cell Biol. 2010;12:341–350. doi: 10.1038/ncb2040. [DOI] [PubMed] [Google Scholar]
- 52.Bubenshchikova E, Ichimura K, Fukuyo Y, et al. Wtip and Vangl2 are required for mitotic spindle orientation and cloaca morphogenesis. Biol Open. 2012;1:588–596. doi: 10.1242/bio.20121016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brinkkoetter PT, Ising C, Benzing T. The role of the podocyte in albumin filtration. Nat Rev Nephrol. 2013;9:328–336. doi: 10.1038/nrneph.2013.78. [DOI] [PubMed] [Google Scholar]
- 54.Quaggin SE, Kreidberg JA. Development of the renal glomerulus: good neighbors and good fences. Development. 2008;135:609–620. doi: 10.1242/dev.001081. [DOI] [PubMed] [Google Scholar]
- 55.Kriz W, Shirato I, Nagata M, et al. The podocyte's response to stress: the enigma of foot process effacement. Am J Physiol Renal Physiol. 2013;304:F333–F347. doi: 10.1152/ajprenal.00478.2012. [DOI] [PubMed] [Google Scholar]
- 56.Babayeva S, Zilber Y, Torban E. Planar cell polarity pathway regulates actin rearrangement, cell shape, motility and nephrin distribution in podocytes. Am J Physiol Renal Physiol. 2011;300:F549–F560. doi: 10.1152/ajprenal.00566.2009. [DOI] [PubMed] [Google Scholar]
- 57.Babayeva S, Rocque B, Aoudjit L, et al. Planar cell polarity regulates nephrin endocytosis in developing podocytes. J Biol Chem. 2013;288:24035–24048. doi: 10.1074/jbc.M113.452904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hartleben B, Widmeier E, Wanner N, et al. Role of the polarity protein Scribble for podocyte differentiation and maintenance. PLoS One. 2012;7:e36705. doi: 10.1371/journal.pone.0036705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ichimura K, Kurihara H, Sakai T. Primary cilia disappear in rat podocytes during glomerular development. Cell Tissue Res. 2010;341:197–209. doi: 10.1007/s00441-010-0983-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quack I, Rump LC, Gerke P. Beta-arrestin2 mediates nephrin endocytosis and impairs slit diaphragm integrity. Proc Natl Acad Sci USA. 2006;103:14110–14115. doi: 10.1073/pnas.0602587103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Descamps B, Sewduth R, Ferreira Tojais N, et al. Frizzled 4 regulates arterial network organisation through noncaninical Wnt/planar cell polarity signaling. Circ Res. 2012;110:47–58. doi: 10.1161/CIRCRESAHA.111.250936. [DOI] [PubMed] [Google Scholar]
- 62.Stoll C, Dott B, Alembik Y, Roth MP. Associated malformations among infants with neural tube defects. Am J Med Genet A. 2011;155A:565–568. doi: 10.1002/ajmg.a.33886. [DOI] [PubMed] [Google Scholar]
- 63.Kolatsi-Joannou M, Price KL, Winyard PJ, et al. Modified citrus pectin reduces galectin-3 expression and disease severity in experimental acute kidney injury. PLoS One. 2011;6:e18683. doi: 10.1371/journal.pone.0018683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li L, Zepeda-Orozco D, Patel V, et al. Aberrant planar cell polarity induced by urinary tract obstruction. Am J Physiol Renal Physiol. 2009;297:F1526–F1533. doi: 10.1152/ajprenal.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]