Abstract
We report the effect of DNA hydration level on damage yields induced by soft X-rays and photo-emitted low energy electrons (LEEs) in thin films of plasmid DNA irradiated in N2 at atmospheric pressure under different humidity levels. Contrary to a dilute solution of DNA, the number of H2O molecules per nucleotide (Γ) in these films can be varied from Γ=2.5 to ~33, where Γ≤20 corresponds to layers of hydration and Γ=33 to an additional bulk-like water layer. Our results indicate that DNA damage induced by LEEs does not increase significantly until the second hydration shell is formed. However, this damage increases dramatically as DNA coverage approaches bulk-like hydration conditions. A number of phenomena are invoked to account for these behaviors including: dissociative electron transfer from water-interface electron traps to DNA bases, quenching of dissociative electron attachment to DNA and quenching of dissociative electronically excited states of H2O in contact with DNA.
Keywords: DNA damage, strand breaks, humidity level, solvation, interface electron
The discovery that low energy electrons (LEEs, E<30 eV) can directly damage the DNA molecule with considerable efficiency1,2,3 has established that almost all of the secondary electrons (SEs) produced by ionizing radiation can be lethal to the genome.4 As a result, the new concepts that were developed during the studies of the mechanisms of action of LEEs1,5 are starting to be applied to the development of new anti-cancer drugs6,7,8 and the modification of clinical protocols.9,10,11,12 Whereas the mechanisms of the direct action of LEEs on biomolecules are now fairly well understood,4,5 the indirect effect of these electrons in irradiated cells remains unknown, owing essentially to the experimental difficulties associated with the production and observation of LEEs in aqueous media. Direct-type damage results from radiation energy being deposited directly into the DNA, whereas indirect damage occurs when the species created by the interaction of the primary radiation and SEs within the molecular environment surrounding the DNA (e.g., salts, proteins, oxygen and water) react with the molecule.13,14 SEs with energies below 30 eV (i.e., LEEs) can attach to DNA components and thus form transient negative ions (TNIs) of DNA subunits (a base, sugar or phosphate group). In this manner, LEEs induce direct damage to DNA, such as single and double strand breaks (SSBs and DSBs) principally via the decay of TNIs into dissociating electronically excited states and dissociative electron attachment (DEA).4,15 Since cells contain 70–80% water,16 LEEs also react with water molecules near DNA in the cell nucleus and create reactive species to produce indirect damage.
To estimate the relative contribution of the indirect and direct modes of damage of LEEs in cells, experiments were undertaken with thin films of short single DNA strands (i.e., oligonucleotides) embedded into biomolecular environments under ultrahigh vacuum (UHV). The oligonucleotides were embedded into multilayer films of amorphous ice to simulate the water molecules surrounding cellular DNA.17,18,19 The presence of water around DNA was found to modify the TNI manifold, the corresponding decay channels and thus the SSB and DSB yield functions.20,21 More recently, theoretical studies indicated that the solvation of DNA molecules (i.e., immersion in an environment of polar molecules such as water) could significantly increase their ability to capture electrons with energies near zero or lower, via the modification of adiabatic electron affinity of solvated DNA bases in bulk water.22,23
Owing to recent advances in LEE techniques,24 we present in this work, the first results of a study on the indirect effect of LEEs with liquid water condensed on and within plasmid DNA. The experiments are performed at standard ambient temperature and pressure (SATP) in a N2 atmosphere under different humidity levels up to solvation. We report the yields of DNA strand breaks induced by soft X-rays. The damage yields for DNA deposited on glass are due to X-rays, whereas those arising from DNA on tantalum are due to the interaction of both X-rays and photo-emitted LEEs from the metal. Varying the hydration level of DNA, up to a bulk-like water environment, allows us to shift progressively from the direct to indirect effect of LEEs.
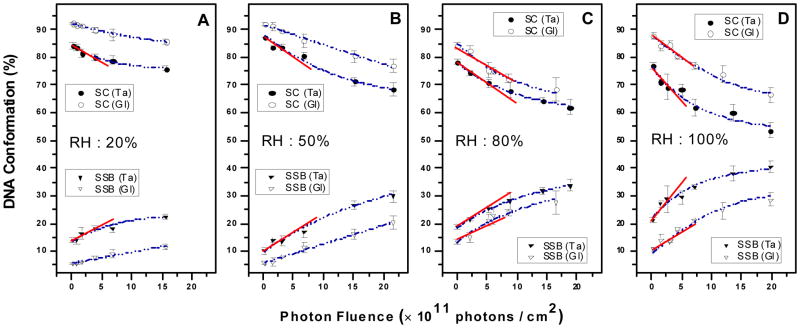
Figure 1 shows the loss of SC and gain in circular form DNA with increasing photon fluence for irradiated DNA samples deposited on glass and tantalum (Ta) substrates under relative humidity (RH) levels of 20%, 50%, 80% and 100%. The data are fitted with an exponential function. As explained elsewhere,24 the gradients of the exposure-response curves at zero fluence provide the yields (Y) of loss of SC form and DNA strand breaks in percentage loss or gain, respectively, per incident photon/cm2. Such values are given in Table 1 for samples deposited onto glass (YGl) and Ta (YTa) at various RH. They were obtained by linear regression exclusively at low fluence. Enhancement factors (EFs) in Table 1 are calculated by dividing the yield obtained with the films deposited on Ta by that measured with films on glass. In other words, EF=1+YLEE/YGl corresponds to the effectiveness of adding photo-emitted LEEs in inducing damage to DNA, where YLEE=YTa−YGl is the yield of DNA damage induced by LEEs.
Figure 1.
Exposure-response curves for five monolayer films of plasmid DNA irradiated by 1.5 keV X-rays under four different hydration levels (RH:%) in a N2 atmosphere at SATP for films deposited on glass and Ta. Each point corresponds to the mean value of the yields from three samples, prepared under identical conditions; the error bars denote the standard deviation of the three experiments. The solid lines exhibit the linear behavior at low fluence and the dash lines exhibit the fit to an exponential function.
Table 1.
Percentage yield per 1012 photons.cm−2 and G values for the loss of SC and induction of SSBs and DSBs in films of plasmid DNA deposited on Ta and glass substrates in a N2 atmosphere, under various hydration levels (RH: %). G values are given in the units of nmol of molecules damaged per joule of absorbed energy. EFs are calculated by dividing the yield obtained with the films deposited on Ta by that measured with films on glass. DSBs were not detected below 50% RH.
| Humidity | DNA Form | YGl | YTa | EF = YTa/YGl | GX (nmol/J) | GLEE (nmol/J) | |
|---|---|---|---|---|---|---|---|
| Dry DNA | RH 0.0% Γ = 2.5 |
SC | 3.6 ± 0.6 | 4.9 ± 0.7 | 1.4 ± 0.2 | 98 ± 20 | 260 ± 50 |
| SSB | 3.3 ± 0.3 | 4.5 ± 0.4 | 1.4 ± 0.2 | 93 ± 19 | 248 ± 65 | ||
|
| |||||||
| First Hydration Layer | RH 20% |
SC | 4.6 ± 0.5 | 5.8 ± 1 | 1.3 ± 0.3 | 126 ± 25 | 247 ± 64 |
| Γ = 5 ± 1 | SSB | 3.9 ± 0.3 | 5.0 ± 1 | 1.3 ± 0.3 | 107 ± 21 | 226 ± 59 | |
|
| |||||||
| RH 50% Γ = 10 ± 1 |
SC | 7.0 ± 0.4 | 8.5 ± 1.6 | 1.2 ± 0.2 | 191 ± 38 | 309 ± 80 | |
| SSB | 6.5 ± 0.4 | 7.5 ± 1 | 1.1 ± 0.2 | 178 ± 36 | 206 ± 54 | ||
| DSB | Non-detected | 0.1 ± 0.01 | - | - | 21 ± 5 | ||
|
| |||||||
| Second Hydration Layer | RH 80% Γ = 20 ± 1 |
SC | 9.0 ± 1.7 | 11.0 ± 1.4 | 1.2 ± 0.3 | 246 ± 49 | 412 ± 107 |
| SSB | 8.7 ± 1.7 | 10.0 ± 0.7 | 1.1 ± 0.2 | 238 ± 48 | 268 ± 70 | ||
| DSB | 0.3 ± 0.02 | 0.4 ± 0.01 | 1.3 ± 0.1 | 8 ± 2 | 21 ± 5 | ||
|
| |||||||
| Bulk Water | RH 100% Γ = 33± 1 |
SC | 10.0 ± 1 | 19 ± 5 | 2.0 ± 0.5 | 274 ± 55 | 1852 ± 482 |
| SSB | 9.0 ± 1 | 18 ± 5 | 2.0 ± 0.5 | 246 ± 49 | 1852 ± 482 | ||
| DSB | 0.3 ± 0.02 | 0.4 ± 0.04 | 1.3 ± 0.2 | 8 ± 2 | 21 ± 5 | ||
The formation of DSBs is not detectable at low humidity, while in higher humidity, as shown in Figure 2, it depends linearly on the photon fluence. The yields of DSBs are given for 50%, 80% and 100% RH in Table 1, as well as the Γ-values corresponding to each RH with hydration shells of DNA and our previous results obtained with the same apparatus under N2 at 0.0% RH.24 The 0.0% RH level corresponds to 2.5 water molecules per nucleotide (Γ=2.5), which are tightly bound water molecules attached to the phosphate group and cannot be removed by vacuum desiccation.25 RH levels of 20%, 50%, 80% and 100%, correspond to Γ=5±1, 10±1, 20±1 and 33±1, respectively. The primary hydration shell in direct contact with the DNA is formed below 50%. At 80% RH, 9–10 water molecules per base are added to DNA. They are in contact with the primary layer and form the more loosely bound second layer of hydration. The additional layer of water molecules at 100% RH (Γ=33) is not bound and resembles bulk water.26
Figure 2.
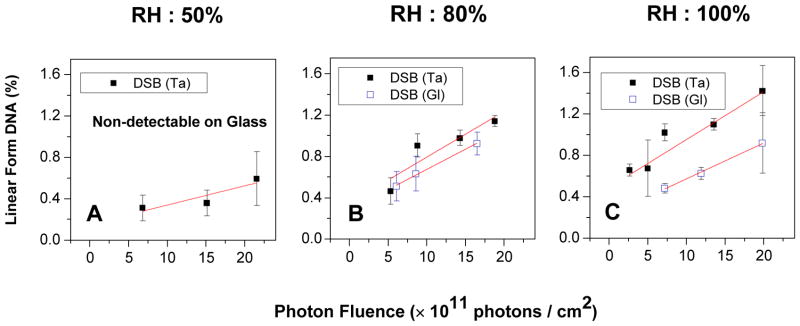
Exposure-response curves for the formation of linear DNA (DSB) in five monolayer films of plasmid DNA irradiated by 1.5 keV X-rays under three different hydration levels (RH:%) in a N2 atmosphere at SATP for films deposited on glass and Ta. Each point corresponds to the mean value of the yields from three samples, prepared under identical conditions; the error bars denote the standard deviation of the three experiments.
The higher yields YTa compared to YGl for dry DNA show that SEs emitted from the Ta surface can cause considerable DNA damage via the direct effect; i.e., via mechanisms which have been extensively discussed in previous articles.4,24,27 The yields of damage induced by X-rays alone (YGI) and LEEs with X-rays (YTa) increase constantly with the RH level. For X-rays, SSB yields saturate around 80% RH and DSBs increase at 80% and above. On the other hand, hydration does not increase DSBs induced simultaneously by both LEEs and X-rays beyond RH = 80%, but a strong rise in SSBs occurs at 100%.
The damage induced by X-rays and LEEs can be expressed independently by calculating the G values. Such values are generally given in nanomole of molecules formed (or destroyed) per joule of energy absorbed from a radiation source. Details of the procedure to derive G values for thin films are presented in the Supporting Information.28 The G values for loss of SC as well as the formation of circular and linear DNA by X-rays (GX) and LEEs (GLEE) are listed on the right of Table 1. From comparison of YGl and YTa at 0.0% and 100% RH in Table 1, we find that the contribution of the indirect effect due to added water is 64% on the glass substrate, while for samples deposited on Ta it is 74%. These behaviors are reflected in GX and GLEE, which both increase with Γ. On the other hand, within experimental errors, the EFs remain the same from 0% to 80% RH, indicating that the ratio of the damage induced by LEEs to that caused by X-rays remains fairly constant up to 80% RH. These G values are consistent with many studies mentioned in the introduction, which have shown that the waters of hydration considerably affect radiation damage to DNA. Although no data is available on GLEE, GX values can be compared with those from group of Sevilla,29,30 who obtained 133 nmol/J at Γ=2.5, 145 nmol/J at Γ=5.7, 236 nmol/J at Γ=14, 300 nmol/J at Γ=18.1 and 261 nmol/J at Γ=22.5, in good agreement with the present results. As mentioned in the works of Cai et al.31 and Brun et al.,32 G values for LEE impact on 5-ML films of DNA should be considered highly reliable, because essentially all LEEs lose their energy in such films. Present and previously-deduced24,31,32 G values indicate that LEEs are more efficient than X-ray photons to induce DNA damage. In other words, when the same amount of energy is deposited in DNA by photons and low-energy SE, the latter produce more damage.
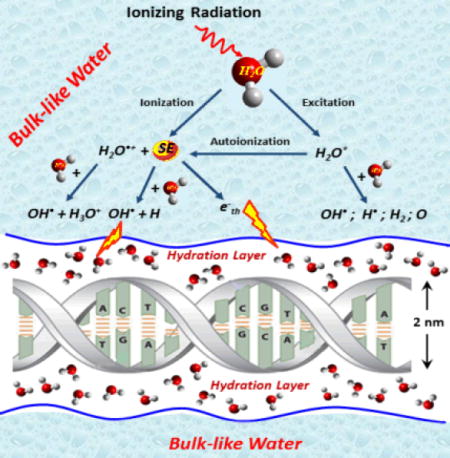
The initial ionization of water molecules by high-energy radiation, , results in the formation of a water cation and usually one SE. This reaction within the primary hydration layer, rapidly transfers electrons and holes to DNA; i.e., SE(e−) + DNA → DNA•− and H2O•+ + DNA → DNA•+ + H2O. Since H2O•+ is a strong acid, it is also involved in another competing reaction, i.e., H2O•+ + H2O → H3O+ + OH•. H2O+ may migrate quickly over distances of a few molecular diameters by resonant electron transfer with a succession of neighboring water molecules.33 Consequently, we can consider the net ionization reaction to be responsible for the production of an OH• and a solvated electron ( ). Moreover, electronically excited states H2O* can also be produced directly by either the primary fast charged particles or SEs and can dissociate into H and OH radicals.5 Although the cross sections for these particles to form H2O* are relatively small, multiple scattering of these increases considerably the probability of water dissociation via this channel. The contribution of SEs in the production of OH• is smaller than that produced via the ionization of water, but far from being negligible.5 It is well-recognized,16,33 that reactions of the OH• radicals, created from water of hydration, increase the yields of SSBs and DSBs in DNA samples.
LEEs constitute another major cell ionization product34,35 that can react with water molecules surrounding DNA in the cell to further ionize H2O or create H−, H, OH radicals and solvated electrons. Electrons of energy below 15 eV can be captured by a water molecule near DNA to form a TNI (i.e., a core-excited Feshbach resonance), which decays into the DEA or electronic excitation channels.1 DEA and dissociative electronically excited states result principally in the formation of OH• and along with H− from dissociation of the 2B1 state of H2O− and OH• along with H• from dissiociation of the 3,1B1 states of H2O* both located in the 7–11 eV region. Smaller contributions arise from the 2A1 and 2B2 anionic states, which are formed near 9 and 11 eV, respectively, and direct excitation of the 3,1B1 states. Above 10 eV, ionization begins and progressively dominates other energy-loss processes.
For Γ>22, our data shows that damage considerably increases at 100% RH, for samples deposited on Ta compared to that produced on glass substrates. Hence, LEEs induce much more damage to DNA when bulk water surrounds the molecule and play a significant role in the indirect effect. This is evidenced in the EFs and G values listed and defined in Table 1. Beyond 80% RH, the EFs for the loss of SC and formation of SSBs increase from 1.2±0.3 and 1.1±0.2 to 2±0.5, respectively, when a bulk-like layer of water is added to the second hydration shell. This behavior is reflected in the substantial rise in GLEE within the Γ=20 to 33 range compared to GX, which does not rise appreciably in this range. With the addition of the water layers GLEE is expected to increase owing to the formation of H, H− and OH radicals. According to the GLEE values in Table 1, the previously-mentioned processes creating these species would be much less efficient below Γ=20. This behavior may result from quenching of DEA of DNA subunits by their interaction with H2O molecules. This type of quenching has been reported previously for smaller molecules in presence of H2O.36,37 It is also possible that the probability to excite the 3,1B1 states of H2O is reduced near DNA. Cho et al.38 reported a considerable reduction of the magnitude of excitation of the 3,1B states induced by electrons of energies below 12 eV, when water was condensed on thymine.38 If these processes are not quenched in the bulk layer, owing to the lack of contact between H2O and DNA, a more significant contribution to the production of OH and H radicals is expected. It could contribute to the substantial rise in formation of SSBs from 80% to 100% RH, induced by the indirect effect of LEEs. However, only 30% of LEEs produced at the Ta surface in our experiments have energies higher than 7 eV.24 We therefore suspect another contribution to the damage, involving lower energy electrons, to be responsible for the high YTa and GLEE values in the presence of bulk water. This contribution could arise from species produced during LEE solvation.
In the liquid phase, water molecules are collectively capable of trapping electrons, a phenomenon which leads to solvation; i.e., a LEE stabilizes in a deep trap by interaction with the dipole moments of surrounding water molecules.5 Before the electron becomes fully solvated, randomly arranged ensembles of water molecules form much shallower electron traps, lying slightly below the conduction band edge. Taking the vacuum level as the zero electron energy reference, the conduction band edge lies around −1.5 eV in liquid water. Electron transfer can occur between these short-lived quasi-bound electron states of the shallow traps. When such states occur at the interface between water and another medium, they are referred to as surface bond electrons.22 At 100% RH, similar electron states could exist between the hydrated DNA surface and the bulk-like water layer at energies close to that of the band edge of bulk water. In such shallow traps, the electron wavefunction is expected to be relatively extended compared to that in deep traps, thus promoting overlap between the wavefunctions of transient anion states of subunits of DNA and that of the interface electron. This overlap should increase the probability of electron transfer to DNA subunits. As clearly shown with CH3Cl, O2 and H2O adsorbed on a n-hexane amorphous solid surface,39 an electron temporary captured into an extended surface state can couple and transfer to a transient anionic state of the adsorbate, so as to increase electron stabilization on the adsorbate molecule, or its DEA cross section, by orders of magnitude. It has also been shown that modification of polarization at the surface between the DNA molecule and the surrounding water medium results in an adiabatic electron affinity in the range −1 to −2 eV for the bases,23 which matches the expected energy of about 1.6 eV of interface quasi-bound electrons.14 These similar energies should favor the transfer of interface electrons to the bases and formation of TNIs at these sites.22,30 Accordingly, in the vicinity of DNA, when a SE has reached sufficiently low energy to enter an interface trap, it should be scavenged in regions of large electron affinities such as those around nucleobases. As shown experimentally and theoretically,27 TNIs of the DNA bases have a strong tendency to transfer the extra electron to the phosphate group, where it resides in the usually unfilled σ* orbital of the C-O bond. The potential energy surface of that state being dissociative, the C-O bond ruptures causing a SSB. This process could therefore contribute to the high yields of SSB found at 100% RH.
In conclusion, we find that LEEs contribute considerably to indirect DNA damage. This contribution does not substantially arise from water molecules in contact with DNA, but mostly from highly reactive species created within the subsequent bulk-like water layer. The huge increase in DNA damage with addition of bulk like water may be essentially due to two mechanisms: 1) a rise in the amount of the species H−, OH• and H• in bulk water and 2) the slowing-down of SEs to energies close to that of the conduction band edge of amorphous water, followed by their transient capture into shallow traps at the interface between the hydration water layers of DNA and bulk water. Owing to modification of the adiabatic electron affinities of the DNA bases by surrounding water molecules, electrons in these shallow traps would rapidly transfer of to the bases of solvated DNA to form transient anions. These anions may dissociate or transfer the excess electron to the DNA backbone to cause a strand break. Thus, the present results provide experimental evidence in favor of the interface-electron transfer mechanism of DNA damage in irradiated water similar to that proposed by Abel and co-workers.14,22
Experimental Methods
Bacterial pGEM-3Zf(−) plasmid DNA was prepared as a dry thin film of five monolayer thickness deposited onto clean Ta and glass substrates, by a method previously described in details.24 The films were kept at < 0.1% RH in a vacuum chamber by filling and flushing with dry N2. They were humidified by exposure to water vapor under N2 atmospheric pressure at RH level of 20%, 50%, 80% and 100%, corresponding to Γ=5±1, 10±1, 20±1 and 33±1, respectively. The humidity was monitored by a hygrometer sensor (Fisher Scientific) and the temperature during the irradiation was 23 ± 1 °C. According to fundamental thermodynamics principles,40 we can roughly estimate the velocity with which a water molecule is moving in a DNA thin film at room temperature. In a time of about picosecond, water molecules diffuse homogeneously in the entire volume of our dry DNA films. Afterwards, our films were exposed to soft X-rays (effective energy 1.5 keV) of varying fluence. In samples deposited on Ta, X-rays produce energetic photoelectrons and Auger electrons inside the metal. These electrons lose energy inside the metal and generate at the film-metal interface an energy distribution of emitted LEEs peaking at 1.4 eV with an average energy of 5.85 eV.24 Whereas DNA damage for films deposited on glass is due to X-rays, that for films deposited on the Ta substrate arises from the interaction of both X-rays and LEEs.24
Irradiated samples were dissolved from the substrates in TE buffer and the fractions of various DNA forms induced by irradiation were determined, by quantifying with ImageQuant software (Molecular Dynamics), the intensity of the bands obtained from agarose gel electrophoresis. The relative proportion of each configuration was expressed as a percentage (%) of the total amount of SC form of DNA in each sample. Undamaged DNA in SC plasmid DNA converts into a nicked circular form by induction of a SSB, while induction of a DSB within both the SC and circular forms changes the plasmid into the linear configuration.
Supplementary Material
Acknowledgments
This work was funded by the Canadian Institutes of Health Research (CIHR). The authors would like to thank P. Cloutier for technical support, Dr. A. D. Bass and M. Michaud for helpful comments and S. Sahbani and M. Rezaee for preparation of plasmid DNA. A. G. S. and G. G. acknowledge partial support from the Spanish Ministerio de Economiay Competitividad (Project FIS2009-10245) and the EU COST program (Action MP1002 Nano-IBCT).
Footnotes
Supporting Information Available: G values for Al Kα X-rays of 1.5 keV and LEEs are calculated from the yields obtained from the slopes of a linear-least-squares fits of respective exposure curves (Figures 1). It is commonly given in SI units as the number of moles of product per Joule of radiation energy present in each primary particles (commonly expressed as μmol/J or nmol/J). This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Boudaïffa B, Cloutier P, Hunting DJ, Huels MA, Sanche L. Resonant Formation of DNA Strand Breaks by Low-energy (3 to 20 eV) Electrons. Science. 2000;287:1658–1660. doi: 10.1126/science.287.5458.1658. [DOI] [PubMed] [Google Scholar]
- 2.Sanche L. In: In Radiation Damage in Biomolecular Systems. Garcia Gomez-Tejedor G, Fuss MC, editors. Springer Sciences; Dordrecht, Netherland: 2012. [Google Scholar]
- 3.Michael BD, O’Neill P. A Sting in the Tail of Electron Tracks. Science. 2000;287:1603–1604. doi: 10.1126/science.287.5458.1603. [DOI] [PubMed] [Google Scholar]
- 4.Sanche L. In: In Radical and Radical Ion Reactivity in Nucleic Acid Chemistry. Greenberg MM, editor. John Wiley and Sons; New Jersey, NJ: 2009. references therein. [Google Scholar]
- 5.Alizadeh E, Sanche L. Precursors of Solvated Electrons in Radiobiological Physics and Chemistry. Chem Rev. 2012;112:5578–5602. doi: 10.1021/cr300063r. [DOI] [PubMed] [Google Scholar]
- 6.Seiwert TY, Salama JK, Vokes EE. The Concurrent Chemoradiation Paradigm - General Principles. Nat Clin Prac Oncol. 2007;4:86–100. doi: 10.1038/ncponc0714. [DOI] [PubMed] [Google Scholar]
- 7.Chithrani DB, Jelveh S, Jalali F, van Prooijen M, Allen C, Bristow RG, Hill RP, Jaffray DA. Gold Nanoparticles as Radiation Sensitizers in Cancer Therapy. Radiat Res. 2010;173:719–728. doi: 10.1667/RR1984.1. [DOI] [PubMed] [Google Scholar]
- 8.Jain S, Hirst DG, O’Sullivan JM. Gold Nanoparticles as Novel Agents for Cancer Therapy. Br J Radiol. 2012;85:101–113. doi: 10.1259/bjr/59448833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng Y, Hunting DJ, Ayotte P, Sanche L. Role of Secondary Low-energy Electrons in the Concomitant Chemoradiation Therapy of Cancer. Phys Rev Lett. 2008;100:198101. doi: 10.1103/PhysRevLett.100.198101. [DOI] [PubMed] [Google Scholar]
- 10.Kong T, Zeng J, Wang X, Yang X, Yang J, McQuarrie S, McEwan A, Roa W, Chen J, Xing JZ. Enhancement of Radiation Cytotoxicity in Breast-cancer Cells by Localized Attachment of Gold Nanoparticles. Small. 2008;4:1537–1543. doi: 10.1002/smll.200700794. [DOI] [PubMed] [Google Scholar]
- 11.Charest G, Paquette B, Sanche L, Fortin D, Mathieu D. Glioblastoma Ttreatment: Bypassing the Toxicity of Platinum Compounds by Using Liposomal Formulation and Increasing Treatment Efficiency with Concomitant Radiotherapy. Int J Radiat Oncol Biol Phys. 2012;84:244–249. doi: 10.1016/j.ijrobp.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chattopadhyay N, Cai Z, Kwon YL, Lechtman E, Pignol JP, Reilly RM. Molecularly Targeted Gold Nanoparticles Enhance the Radiation Response of Breast Cancer Cells and Tumor Xenografts to X-radiation. Breast Cancer Res Treat. 2012 doi: 10.1007/s10549-012-2338-4. [DOI] [PubMed] [Google Scholar]
- 13.O’Neil P. In Radiation Chemistry: Present Status and Future Trends. Elsevier Science; Amsterdam: 2001. [Google Scholar]
- 14.Siefermann KR, Lin Y, Lugovoy E, Link O, Faubel M, Buck U, Winter B, Abel B. Binding Energies, Lifetimes and Implications of Bulk and Interface Solvated Electrons in Water. Nature Chemistry. 2010;2:274–279. doi: 10.1038/nchem.580. [DOI] [PubMed] [Google Scholar]
- 15.Huels MA, Boudaïffa B, Cloutier P, Hunting DJ, Sanche L. Single, Double, and Multiple Double Strand Breaks Induced in DNA by 3–100 eV Electrons. J Am Chem Soc. 2003;1252:4467–4477. doi: 10.1021/ja029527x. [DOI] [PubMed] [Google Scholar]
- 16.Sonntag C. von Free-Radical-Induced DNA Damage and its Repair. Springer-Verlag; Beling Heidelberg: 2006. [Google Scholar]
- 17.Simpson WC, Orlando TM, Parenteau L, Nagesha K, Sanche L. Dissociative Electron Attachment in Nanoscale Ice Films: Thickness and Charge Trapping Effects. J Chem Phys. 1998;108:5027–5034. [Google Scholar]
- 18.Michaud M, Cloutier P, Sanche L. Low-energy-loss Spectroscopy of Amorphous Ice: Electronic Excitations. Phys Rev A. 1991;44:5624–5627. doi: 10.1103/physreva.44.5624. [DOI] [PubMed] [Google Scholar]
- 19.Smith RS, Petrik NG, Kimmel GA, Kay BD. Thermal and Nonthermal Physiochemical Processes in Nanoscale Films of Amorphous Solid Water. Acc Chem Res. 2012;45:33–42. doi: 10.1021/ar200070w. [DOI] [PubMed] [Google Scholar]
- 20.Ptasińska S, Sanche L. Dissociative Electron Attachment to Hydrated Single DNA Strands. Phys Rev E. 2007;75:030915. doi: 10.1103/PhysRevE.75.031915. [DOI] [PubMed] [Google Scholar]
- 21.Grieves GA, McLain JL, Orlando TM. In: In Charged Particle and Photon Interactions with Matter, Recent Advances, Applications, and Interfaces. Hatano Y, Katsumura Y, Mozumder A, editors. CRC Press; Boca Raton, FL: 2011. [Google Scholar]
- 22.Siefermann KR, Abel B. The Hydrated Electron: A Seemingly Familiar Chemical and Biological Transient. Angew Chem Int Edit. 2011;50:5264–5272. doi: 10.1002/anie.201006521. [DOI] [PubMed] [Google Scholar]
- 23.Smyth M, Kohanoff J. Excess Electron Localization in Solvated DNA Bases. Phys Rev Lett. 2011;106:238108. doi: 10.1103/PhysRevLett.106.238108. [DOI] [PubMed] [Google Scholar]
- 24.Alizadeh E, Cloutier P, Hunting DJ, Sanche L. Soft X-ray and Low Energy Electron- induced Damage to DNA under N2 and O2 Atmospheres. J Phys Chem B. 2011;115:4523–4531. doi: 10.1021/jp200947g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swarts SG, Sevilla MD, Becker D, Tokar CJ, Wheeler KT. Radiation-induced DNA Damage as a Function of Hydration I. Release of Unaltered Bases. Radiat Res. 1992;129:333–344. [PubMed] [Google Scholar]
- 26.Jeffrey G, Saenger W. Hydration Bonding in Biological Structures. Springer-Verlag; New York, NY: 1991. [Google Scholar]
- 27.Caron LG, Sanche L. In: In Low-energy Electron Scattering from Molecules, Biomolecules and Surfaces. Čársky P, Čurík R, editors. CRC Press (Taylor and Francis Group); Boca Raton, FL: 2012. [Google Scholar]
- 28.Alizadeh E, Sanche L. Measurements of G-values for DNA Damage Induced by Low-energy Electrons. J Phys Chem B. 2011;115:14852–14858. doi: 10.1021/jp207922n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.La Vere T, Becker D, Sevilla MD. Yields of •OH in Gamma-irradiated DNA as a function of DNA Hydration: Hole Transfer in Competition with •OH Formation. Radiat Res. 1996;145:673–680. [PubMed] [Google Scholar]
- 30.Wang W, Becker D, Sevilla MD. The Influence of Hydration on the Absolute Yields of Primary Ionic Free Radicals in γ-irradiated DNA at 77 K: I. Total Radical Yields. Radiat Res. 1993;135:146–154. [PubMed] [Google Scholar]
- 31.Cai Z, Cloutier P, Hunting DJ, Sanche L. Comparison between X-ray Photon and Secondary Electron Damage to DNA in Vacuum. J Phys Chem B. 2005;109:4796–4800. doi: 10.1021/jp0459458. [DOI] [PubMed] [Google Scholar]
- 32.Brun E, Cloutier P, Sicard-Reselli C, Fromm M, Sanche L. Damage Induced to DNA by Low-Energy (0–30 eV) Electrons under Vacuum and Atmospheric Conditions. J Phys Chem B. 2009;113:10008–10013. doi: 10.1021/jp902540k. [DOI] [PubMed] [Google Scholar]
- 33.De Waele V, Lampre I, Mostafavi M. In: In Charged Particle and Photon Interactions with Matter, Recent Advances, Applications, and Interfaces. Hatano Y, Katsumura Y, Mozumder A, editors. CRC Press; Boca Raton, FL: 2011. [Google Scholar]
- 34.Pimblott SM, LaVerne JA. Production of Low-energy Electrons by Ionizing Radiation. Radiat Phys Chem. 2007;76:1244–1247. [Google Scholar]
- 35.Mucke M, Braune M, Barth S, Förstel M, Lischke T, Ulrich V, Arion T, Becker U, Bradshaw A, Hergenhahn U. A Hitherto Unrecognized Source of Low-energy Electron in Water. Nature Physics. 2010;6:143–146. [Google Scholar]
- 36.Lu QB, Sanche L. Enhancements in Dissociative Electron Attachment to CF4, Chlorofluorocarbons and Hydrochlorofluorocarbons Adsorbed on H2O Ice. J Chem Phys. 2004;120:2434–2438. doi: 10.1063/1.1637335. [DOI] [PubMed] [Google Scholar]
- 37.Azria R, Le Coat Y, Lachgar M, Tronc M, Parenteau L, Sanche L. Morphology Effects in Anion Electron-stimulated Desorption. Surface Science. 2000;451:91–96. [Google Scholar]
- 38.Cho W, Michaud M, Sanche L. Vibrational and Electronic Excitations of H2O on Thymine Films Induced by Low-energy Electrons. J Chem Phys. 2004;121:11289. doi: 10.1063/1.1814057. [DOI] [PubMed] [Google Scholar]
- 39.Nagesha K, Sanche L. Effects of Band Structure on Electron Attachment to Adsorbed Molecules: Cross Section Enhancements via Coupling to Image States. Phys Rev Lett. 1998;81:5892–5895. [Google Scholar]
- 40.Crummett WP, Western AB. University Physics: Models and Applications. Wm. C. Brown Publishers; 1994. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.