Abstract
This study examined change in patient and caregiver ratings of patient quality of life (QOL) over one year in individuals with dementia living in rural and remote settings. The sample was selected from non-institutionalized patients who were assessed at an interprofessional memory clinic. Measures of QOL, cognitive function, depression, and functional ability were completed by the patient. Caregivers completed measures of patient QOL and behavior, and their own burden and distress. At baseline (clinic day) 119 patients and family caregivers were assessed. Thirty-two families had complete data at clinic day and one-year follow-up. There was no significant change in either patient or caregiver-rated QOL over one year. Significant predictors of patient self-rated QOL were patient symptoms of depressed mood and functional ability at clinic day, and symptoms of depressed mood and clinic day QOL at one year. Significant predictors of caregiver-rated patient QOL were caregiver burden, patient functional ability, and symptom severity at clinic day, and caregiver burden at one year. Patient and caregiver ratings of patient QOL were moderately associated, but neither patients nor their caregivers reported a significant change in patient QOL. Changes in QOL over time remain a unique individual experience that cannot be entirely predicted by analytical models.
Keywords: Dementia, Quality of Life, Rural
Introduction
Dementia refers to the progressive decline of cognitive, social, and physical functioning due to damage or disease of the brain beyond that of the normal aging process (World Health Organization, 2008). A recent report by the Alzheimer Society of Canada (2010) indicates that the incidence of dementia in those over age 65 will more than double in the next 30 years, increasing to over 257,000 new cases per year. By 2038 it is estimated that the number of Canadians with dementia will more than double to 1.1 million. The total economic burden is expected to increase from approximately $15 billion in 2008 to $153 billion by 2038.
Presently, there is no cure for dementia and the objectives of anti-dementia therapies are to improve two outcomes, one of which is quality of life (QOL) (Whitehouse & Rabins, 1992). Whitehouse and Rabins (1992) suggest that QOL is not only a measurement of the benefits of care, but the “central goal of our professional activity” during clinical and research efforts. They suggest that the second outcome should be a more traditional indicator such as improved cognitive performance, psychopathology, or functional status (Albert et al., 2001; Whitehouse & Rabins, 1992).
Prior studies have raised concerns about the ability of persons with dementia to report their own QOL due to the inability to reliably complete self-report questionnaires (Albert et al., 2001). Nevertheless, recent studies involving persons with early stage dementia have found that patient-reported QOL may be as valid as proxy reports (Brod, Stewart, Sands, & Walton, 1999; Trigg, Jones, & Skevington, 2007). Brod et al. (1999) conclude that the gold standard of QOL reporting is by direct patient assessment and not by proxy reporting. Cahill et al. (2004) have shown that patients can competently participate in QOL research if disease-specific scales are used. Ready and Ott (2008) indicate that patients with milder dementia and in preclinical stages of memory loss are better able to participate in formal assessments of QOL. Due to the subjective nature of QOL, patients with dementia should be allowed to participate, whenever possible, in rating their own quality of life (Whitehouse, Patterson, & Sami, 2003). Important insight into the perspective of individuals with dementia can be gained by obtaining self-reported QOL and this insight can be used to improve quality of care and QOL overall (Moyle, Mcallister, Venturato, & Adams, 2007).
Cognitive impairments and decreased insight of patients do not justify ignoring the patients’ perspectives on their own QOL issues (Ready & Ott, 2008; Trigg et al., 2007). Patients with poor insight into their cognitive and functional decline have provided self-report QOL data that were less reliable than data provided by patients with intact insight (Ready, Ott, & Grace, 2006). However, even patients with better insight or less cognitive impairment had poor patient-caregiver agreement with respect to QOL reports, indicating that patients and caregivers provide two unique perspectives on patient QOL (Ready et al., 2006). The debate remains as to whose perspective should be used to guide decisions about patient care. The use of proxy ratings of patient QOL needs to be revisited if we are to provide patient-centered treatments. A caregiver is in a very difficult position in that what may seem like the best decision for the patient may not coincide with the wishes of the patient. Furthermore, proxy ratings by a caregiver can be influenced by the patient’s mood status, neuropsychiatric symptoms (Hoe, Katona, Orrell, & Livingston, 2007), and their own caregiver burden (Sands, Ferreira, Stewart, Brod, & Yaffe, 2004) and are not necessarily as reliable as the patient’s rating. To best determine a patient’s quality of life it is reasonable to both query the patient directly and to obtain a proxy’s perspective (Brod et al, 1999; Rabins & Black, 2007).
Definition of Quality of Life
QOL is a multi-dimensional concept that encompasses many personal characteristics such as culture, ethnicity, geographic location, and religion (Rabins & Black, 2007), along with physical, mental, and spiritual health (Whitehouse & Rabins, 1992). The concept of QOL has been defined and applied in various ways over the course of the last twenty years (Hendry & Douglas, 2003). A limitation to the use of QOL is that it is different for each individual in different situations and thus cannot be readily encompassed in an objective measure (Hendry & McVittie, 2004). A critical component of QOL is an individual’s subjective or self-assessed well-being (Bond, 1999; Whitehouse & Rabins, 1992). Regardless of how long a caregiver has known the patient, the patient’s QOL remains unique to him or her and cannot be conclusively assessed by a proxy reporter, presumably because proxy ratings “filter a subjective measure through the opinion of another person” (Schölzel-Dorenbos et al., 2007, p. 517). Even with supporting evidence that self-rated QOL of dementia patients is a valid source of information, very few longitudinal QOL studies have used patient subjective information. For example, Missotten et al. (2007) and Lyketsos et al. (2003) both used proxy ratings of patient QOL in their assessments of changes in QOL over time.
Previous studies of QOL of people with dementia have focused predominantly on individuals living in long-term care (Lyketsos et al., 2003) or assisted living facilities (Edelman, Fulton, Kuhn, & Chang, 2005) in urban or semi-urban areas. There is little published research involving non-institutionalized, community-dwelling persons with dementia in rural settings, and it is unknown whether the QOL of patients with dementia in a rural setting will resemble the QOL of patients in an urban environment. In addition, many of the previous longitudinal investigations (Albert et al., 2001; Lyketsos et al., 2003) involved patients with relatively severe dementia. Missotten et al. (2007) concluded that previous studies have used different QOL measurements, different follow-up periods, and patients in diverse care contexts. These varying factors challenge our understanding of change or stability of QOL for patients at different stages of dementia.
The results of the longitudinal studies of QOL among people with dementia are inconsistent. Selwood, Thorgrimsen, and Orrell (2005) found that there was no significant change in Quality of Life-Alzheimer’s Disease (QOL-AD), Dementia Quality of Life Scale (DQOL), or EuroQOL-5 Domain (EQ-5D) scores over a one-year period. Lyketsos et al. (2003) found that Alzheimer Disease Related Quality of Life (ADRQL) scores declined slightly over a two-year interval. In contrast, Missotten et al. (2007) did not find any significant differences between ADRQL scores at baseline and two-year follow-up, although within the first year the ADRQL scores increased slightly and then decreased to levels similar to baseline scores by the second year.
In summary, there is limited literature about longitudinal change in QOL of patients with dementia, especially regarding a rural setting, and the findings of the published studies are inconsistent. Differences such as who reported the QOL (patient or proxy), the stage of the disease, the residential status of the patient (special care facility or family home), and the time lapse to follow-up indicate the need for further studies in this area.
Study Goals
This study had three main goals: (1) to compare patient and caregiver ratings of patient QOL at baseline and one-year follow-up; (2) to examine the change in patient and caregiver ratings of patient QOL over a one-year period, and; (3) to determine the predictors of patient QOL both at baseline and at one-year follow-up.
Methods
Overview
The research reported here is part of a larger study evaluating strategies to improve the care of persons with dementia in rural and remote communities in Saskatchewan, Canada. The main project is the development and evaluation of a one-stop interprofessional Rural and Remote Memory Clinic (RRMC) aimed at improving access to diagnosis and management of early stage dementia for individuals living in rural and remote areas greater than 100 km from a tertiary care centre. The patients were referred to the RRMC by their family physician. The design and methodology of the RRMC are further explained elsewhere (Morgan et al., 2009). In brief, all participants underwent a pre-clinic assessment via telehealth videoconference; a subsequent one-day clinic assessment (baseline) in a tertiary care centre by a neurologist, neuropsychology team, geriatrician, neuroradiologist, and physical therapist; and then follow-up assessments conducted by the neurologist at 6 weeks, 12 weeks, 6 months, one year, and then yearly. The follow-up assessments at 6 weeks, 12 weeks and 6 months were performed alternately via telehealth videoconferencing and in-person appointments (McEachern, Kirk, Morgan, Crossley, & Henry, 2008; Morgan et al., in press). The one-year follow-up was conducted in-person by a neurologist, neuropsychology team, and physical therapist. The patient’s main caregiver, usually a family member, was also interviewed at clinic day and at each follow-up assessment. At clinic day the caregiver completed self-report measures of burden, distress, and health, as well as functional and behavioural ratings of the patient. Patients were followed until admission to long term care, unless ongoing follow-up was requested by the family. Two new patients, in addition to pre-clinic and follow-up patients, are assessed in the RRMC per week. For the remainder of this paper, “follow up” will refer to the assessment at one year.
Participants
Participants in this study were 119 non-institutionalized patients living in rural and remote areas in the mid-western Canadian province of Saskatchewan. Patients included in this study were diagnosed at clinic day (baseline) with Alzheimer’s Disease (AD), mild cognitive impairment (MCI), dementia due to multiple etiologies, frontotemporal dementia, Lewy body dementia, vascular dementia, vascular cognitive impairment, dementia due to a medical condition, dementia not otherwise specified, normal pressure hydrocephalus, and alcohol induced amnestic disorder. Patients excluded from this study were those who were diagnosed with a ‘normal’ diagnosis (no dementia), memory problems due to depression, B12 deficiency, Parkinson’s Disease, and Huntington’s Disease.
Patients are continually being introduced to the clinic and therefore are at various stages of clinical assessment. Of the original 119 patients who had been assessed at the RRMC at the onset of the current study, 25 subsequently discontinued their involvement prior to the one-year assessment for reasons including reported difficulty in traveling to appointments and the stress and inconvenience associated with follow-up care (25/119 = 21% drop out rate). An additional 19 patients had moved to long term care, 8 failed to return the questionnaire, 3 patients had moved outside of the geographic area, 3 were deceased, and 3 filled out an alternate form developed for patients who did not speak English as a first language. Another 26 patients had been with the clinic for less than one year and therefore had not completed the one-year follow-up assessment at the time of the current analyses. Consequently, at the time of this study there were 32 patients and their respective caregivers with complete information at one-year follow-up.
Measures
See Morgan et al. (2009) for a description of the full assessment procedures completed at clinic day and at follow-up assessments. Those instruments pertinent to this study are outlined below. Unless otherwise stated, if a patient’s scale was missing less than 25% of the items, then the case mean was substituted for the missing values. If 25% or more of a patient’s scale was missing, the entire scale for that patient was discarded (Fox-Wasylyshyn & El-Masri, 2005).
1) Quality of Life-Alzheimer’s Disease Scale (QOL-AD)
The QOL-AD is a 13-item measure of QOL in Alzheimer’s Disease (Logsdon et al., 1999). It is one of the shortest QOL instruments and most widely used internationally (Whitehouse et al., 2003). Each item is rated on a 4-point Likert scale, with 1 being poor and 4 being excellent. Total scores range from 13 to 52, with higher scores indicating greater QOL. This scale was designed to assess domains that have been identified as being important to QOL in people with AD and in other chronically ill populations. QOL-AD includes an appraisal of the patient’s physical condition, mood, interpersonal relationships, ability to participate in meaningful activities, financial situation, and an overall assessment of self and QOL. This measure obtains a rating of the patient’s QOL from both the patient and the caregiver. It is important to note that this study does not measure caregiver QOL, but rather the caregiver’s perception of the patient’s QOL. The reliability and validity of QOL-AD have been shown by Logsdon et al., (1999) and Thorgrimsen et al. (2003). Studies have found that the QOL-AD measure is appropriate for longitudinal studies and remains applicable throughout disease progression (Schölzel-Dorenbos et al., 2007; Thorgrimsen et al., 2003).
Although the patients and caregivers attending the RRMC were instructed that the patient should complete the QOL-AD form independently, some caregiver assistance was required for patients with more advanced cognitive deficits. For example, at the one-year follow-up assessment, 46% of patients completed the questionnaires with some assistance from a caregiver. The degree of assistance the patients received varied but the majority of cases simply required someone to read the questions to the patient. Thorgrimsen et al. (2003) and Hoe et al. (2007) noted similar requirements for their studies (i.e., scales were completed collaboratively by the patient and the interviewer).
2) The Modified Mini-Mental State Exam (3MS)
The 3MS (Teng & Chui, 1987) incorporates additional test items and other changes into the Mini-Mental State Examination (MMSE) (Folstein, Folstein, & McHugh, 1975). The MMSE includes 11 questions for a maximum score of 30 compared to a 3MS maximum score of 100. Lower 3MS scores indicate increased impairment. The 3MS has been described as a superior measure because it evaluates a broader variety of cognitive functions and is more sensitive in detecting dementia than the MMSE (Bland & Newman, 2001). The 3MS is administered by the team neuropsychologist and data were complete for all patients.
3) Neuropsychiatric Inventory Scale
The NPI is a proxy-rated scale that measures 12 psychiatric symptoms (Cummings et al., 1994). For this study only one component of the NPI scale (severity) was used in the analysis. The Neuropsychiatric Inventory-Severity scale (NPI-S) is a 12-item scale. Each item is rated on a 3-point Likert scale, with 1 being mild and 3 being severe. Total scores range from 12 to 36, with higher scores indicating more severe symptoms.
4) Centre for Epidemiologic Studies-Depression Scale (CES-D)
The CES-D is a patient self-reported 20-item scale developed to measure depressive symptoms such as sad mood, feelings of guilt and worthlessness, loss of appetite, and sleep disturbance (Radloff, 1977). Each item is rated on a 4-point scale with 0 being rarely or none of the time and 3 being most or all of the time. Total scores range from 0 to 60 with a higher score indicating more symptoms. The scale has demonstrated good validity and sensitivity in detecting depressive symptoms and change in symptoms over time (Weissman, Sholomskas, Pottenger, Prusoff, & Locke, 1977). If a patient’s CES-D scale was missing 1 item, the missing item was considered ‘0’. If ≥2 items were missing, the CES-D scale for that patient was discarded.
5) Instrumental Activities of Daily Living (IADL)
The IADL is a 9-item scale designed to measure the ability to perform daily tasks (Lawton & Brody, 1969). Each item is rated on a 3-point scale with 1 being completely unable to perform the task independently and 3 being able to complete the task independently. Total scores range from 9 to 27, with a higher score indicating a higher level of function.
6) Zarit Burden Scale (ZB)
A short (12-item) version of the Zarit Burden Interview was used to assess caregiver burden. The shortened version was created by Bédard et al. (2001) and validated by O’Rourke and Tuokko (2003). Each item is rated on a 5-point scale with 0 being never and 4 being nearly always. Total scores range from 0 to 48, with higher scores indicating greater burden.
7) Brief Symptom Inventory (BSI)
The BSI is a 53-item scale that requires the caregiver to rate the degree to which psychological symptoms have distressed them over the past 7 days (Derogatis & Melisaratos, 1983). Each item is rated on a 5-point scale with 0 being not distressed at all and 4 being extremely distressed. Scores are calculated by summing all responses and then dividing by the number of questions answered. This fraction is located in the BSI manual to determine the respondent’s Global Severity Index (GSI) score. GSI scores are standardized t scores with a mean of 50 and a standard deviation of 10. GSI scores on the BSI range from 35 to 80, with higher scores indicating greater distress. Data were excluded if an individual did not have complete data for the BSI.
8) Bristol Activities of Daily Living Scale (BADL)
The BADL is a caregiver-rated instrument containing 20 daily living abilities in four areas: mobility, instrumental activities of daily living, self-care, and orientation (Bucks, Ashworth, Wilcock, & Siegfried, 1996). Each item has four possible answers ranging from 0 (independence) to 3 (dependence.) Total scores from the BADL range from 0 to 60, with higher scores indicating greater dependence.
9) Functional Assessment Questionnaire (FAQ)
The FAQ is a 10-item screening tool for assessing independence in daily activities and universal skills among older adults (Pfeffer, Kurosaki, Harrah, Chance, & Filos, 1982). Each item is rated on a 4-point scale ranging from 0 (independence) to 3 (dependence.) Total scores from the FAQ range from 0 to 30, with higher scores indicating greater dependence.
10) Following the Statistics Canada protocol for calculating number of chronic conditions in the National Population Health Survey (Statistics Canada, 2010) we created an indicator of patient overall medical status at clinic day by summing the “yes” responses to a list of 21 chronic conditions in the patient questionnaire.
Statistical Analysis
Data were analyzed using SPSS 17.0. The relationship between patient and caregiver QOL-AD individual item and summary scores was assessed with Spearman’s rho and Pearson correlation respectively. Group comparisons between those with and without data at one year were examined using Chi-Square and independent samples t-test. Mean differences between QOL-AD total and item subscale change scores for both patients and caregivers was assessed with paired-samples t-test. Pearson correlations were used to analyze bivariate associations between the variables of interest and patient and caregiver QOL-AD. For regression analyses, conducted using the stepwise procedure, patient and caregiver-rated patient QOL-AD at clinic day and one year were the dependent variables. Both of the dependent variables were normally distributed. The independent variables included patient self-reported symptoms of depressed mood, instrumental activities of daily living, psychiatric symptom severity, patient age, years of formal education, level of cognitive impairment, gender, caregiver burden, caregiver psychological distress, and QOL at clinic day. These variables were identified based on previous literature (Albert et al., 2001; Hoe et al., 2007; Karlawish, Casarett, Klocinski, & Clark, 2001; Logsdon, Gibbons, McCurry, & Teri, 2002; Misotten et al., 2007; Ready, Ott, & Grace, 2004; Sands et al., 2004; Selwood et al., 2005; Winzelberg, Williams, Preisser, Zimmerman, & Sloane, 2005). A p value<0.05 was considered to be significant. If two or more variables were highly correlated (variance inflation factor [VIF] greater than approximately two), the most important of the variables, based on previous literature, was included in the regression analysis. Due to a small sample size at the one-year follow-up, variables included in the regression were chosen based on the results of the bivariate correlation analyses. Any variable significantly correlated with the dependent variable (caregiver or patient-rated QOL-AD at follow-up) and with a p value ≤ 0.2 was included in the regression analyses. The coefficient of multiple determination (r2) is reported for each model adjusting for the number of independent variables in the model.
Results
Comparison of patient and caregiver ratings of QOL
Means and standard deviations for all patient and caregiver measures are reported in Table 1, for the full sample (clinic day) and for the subsample with complete data (clinic day and one-year follow-up). The QOL-AD is a 13-item measure with total scores ranging from 13 to 52, with higher scores indicating greater QOL. The mean score and standard deviation (SD) for clinic day patient-rated and caregiver-rated QOL-AD total scores (n=119) are 34.9 (5.9) and 31.8 (6.3), respectively. The mean score and standard deviation for one-year patient and caregiver-rated QOL-AD total scores (n=32) are 35.5 (5.5) and 32.6 (6.2), respectively. At clinic day the means of the thirteen individual QOL-AD items (data not shown) were weakly (correlation <0.4) or moderately (correlation between 0.4 and 0.6) correlated (Salkind, 2004) between patient (n=102) and caregiver (n=114) ratings. There was a significant correlation (0.348, p<0.01) between patient QOL-AD total score and caregiver-rated patient QOL-AD total score at clinic day.
Table 1.
Means and standard deviations for patient and caregiver measures for the full sample at clinic day (baseline) and for the subsample with data at clinic day and one year.
| Clinic Day (n = 119) | Subsample with data at clinic day and one year (N=32)
|
|||||
|---|---|---|---|---|---|---|
| Clinic Day | One-year follow-up | |||||
|
| ||||||
| Patient | Caregiver | Patient | Caregiver | Patient | Caregiver | |
|
| ||||||
| Frequency (%) | ||||||
| Sex | ||||||
| Male | 44 (37%) | 40 (36%) | 17 (53.1%) | 13 (40.6) | 17 (53.1%) | 13 (40.6%) |
| Female | 75 (63%) | 71 (64%) | 15 (46.9%) | 19 (59.4) | 15 (46.9%) | 19 (59.4%) |
|
| ||||||
| Mean (SD) | ||||||
|
| ||||||
| Age | 74.4 (9.5) | 62.3 (14.7) | 71.5 (9.3) | 66.3 (12.8) | 72.5 (9.4) | 65.2 (12.3) |
|
| ||||||
| Education | 10.5 (3.0) | 10.9 (2.4) | 10.9 (2.4) | |||
|
| ||||||
| 3MS | 70.6 (18.7) | 78.0 (14.7) | 75.8 (18.7) | |||
|
| ||||||
| CES-D | 13.2 (9.2) | 12.9 (8.7) | 14.5 (7.6) | |||
|
| ||||||
| IADL | 21.1 (5.4) | 22.8 (4.1) | 22.3 (5.0) | |||
|
| ||||||
| FAQ1 | 14.2 (8.3) | 11.8 (6.9) | 14.0 (8.4) | |||
|
| ||||||
| NPI-S1 | 8.7 (6.1) | 7.3 (4.9) | 6.5 (4.0) | |||
|
| ||||||
| BSI | 51.6 (10.8) | 52.0 (12.6) | 53.5 (11.0) | |||
|
| ||||||
| Patient-rated QOL | 34.9 (5.9) | 34.5 (4.8) | 35.5 (5.5) | |||
|
| ||||||
| Caregiver-rated Patient QOL | 31.8 (6.3) | 34.3 (6.0) | 32.6 (6.2) | |||
|
| ||||||
| Caregiver Burden | 14.1 (9.2) | 13.2 (8.2) | 13.2 (9.1) | |||
Note. CES-D =Centre for Epidemiological Studies Depression Scale (range: 0–60); IADL = Instrumental Activities of Daily Living (range: 9–27); FAQ = Functional Assessment Questionnaire (range: 0–30); NPI-S = Neuropsychiatric Inventory Severity Scale (range: 12–36); BSI = Brief Symptom Inventory (GSI scores on the BSI range: 35–80); 3MS = Modified Mini-Mental State Exam (range: 0–100)
FAQ and NPI-S are completed by the caregiver based on their assessment of the patient
Similarly, at the one-year follow-up the individual QOL-AD items demonstrated a weak to moderate association between patient and caregiver ratings. For the subsample with complete data at clinic day and one year, the total score for patient QOL-AD (n=32) and caregiver-rated patient QOL-AD (n=30) exhibited a higher correlation at the one-year follow-up (0.665, p<0.01) than at clinic day (0.595, p=0.001).
Change in patient and caregiver ratings of QOL over time
The comparison of QOL-AD between clinic day and one-year assessment, for both the patient and caregiver, is shown in Table 2. Results from the paired-samples t-tests, performed to compare the two time points, are also displayed in Table 2. The only significant change in mean item score from the patients’ assessment was ‘memory’, which was rated as significantly higher at one year. Significant changes in caregiver QOL-AD items involved ‘memory’, ‘family’, ‘marriage, ‘friends’ and ‘ability to do chores around the house’. There was no significant change in total QOL score for patients or caregivers over the one-year time period.
Table 2.
Comparison of clinic day and one-year follow-up for patient and caregiver ratings of patient QOL.
| QOL-AD Item | Means (standard deviations)- clinic day vs. one year | Comparison between clinic day and one year | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Patient-Rated QOL-AD N=32 |
Caregiver-Rated Patient QOL-AD N=32+ |
Patient | Caregiver | |||
| Clinic day | 1 year | Clinic day | 1 year | |||
| Physical health | 2.72 (.52) | 2.53 (.76) | 2.57 (.73) | 2.50 (.63) | p=0.110 | p=0.573 |
| Energy | 2.22 (.75) | 2.16 (.68) | 2.10 (.71) | 2.07 (.58) | p=0.645 | p=0.769 |
| Mood | 2.56 (.68) | 2.41 (.76) | 2.47 (.63) | 2.30 (.70) | p=0.305 | p=0.169 |
| Living situation | 3.00 (.57) | 3.16 (.57) | 3.10 (.66) | 2.93 (.74) | p=0.134 | p=0.283 |
| Memory | 1.50 (.51) | 1.94 (.56) | 1.60 (.62) | 1.97 (.67) | p=0.001** | p=0.001** |
| Family | 3.28 (.58) | 3.28 (.46) | 3.30 (.65) | 2.90 (.71) | p=1.000 | p=0.008* |
| Marriage | 3.09 (.69) | 3.16 (.68) | 3.30 (.75) | 3.03 (.77) | p=0.572 | p=0.043* |
| Friends | 2.88 (.61) | 3.03 (.65) | 2.93 (.74) | 2.63 (.72) | p=0.134 | p=0.026* |
| Self as a whole | 2.69 (.64) | 2.78 (.55) | 2.67 (.84) | 2.47 (.73) | p=0.476 | p=0.297 |
| Ability to do chores around the house | 2.56 (.91) | 2.72 (.77) | 2.60 (.89) | 2.27 (.87) | p=0.305 | p=0.030* |
| Ability to do things for fun | 2.34 (.87) | 2.63 (.71) | 2.27 (.87) | 2.20 (.81) | p=0.095 | p=0.645 |
| Money | 2.72 (.73) | 2.78 (.75) | 2.63 (.89) | 2.60 (.93) | p=0.737 | p=0.856 |
| Life as a whole | 2.97 (.70) | 2.91 (.64) | 2.67 (.66) | 2.70 (.65) | p=0.601 | p=0.801 |
| Total score for QOL-AD | 34.53 (4.84) | 35.47 (5.47) | 34.20 (6.19) | 32.57 (6.22) | p=0.265 | p=0.128 |
Note. When comparing caregiver-rated patient QOL-AD at clinic day and one year, two caregivers did not provide enough information to be included in the mean differences analysis. Both caregivers failed to provide information at one year, therefore only 30 caregiver-rated patient QOL-AD ratings were used. QOL-AD: individual items are rated from 1–4 with total QOL-AD scores ranging from 13–52.
Comparison is significant at the 0.05 level.
Comparison is significant at the 0.01 level.
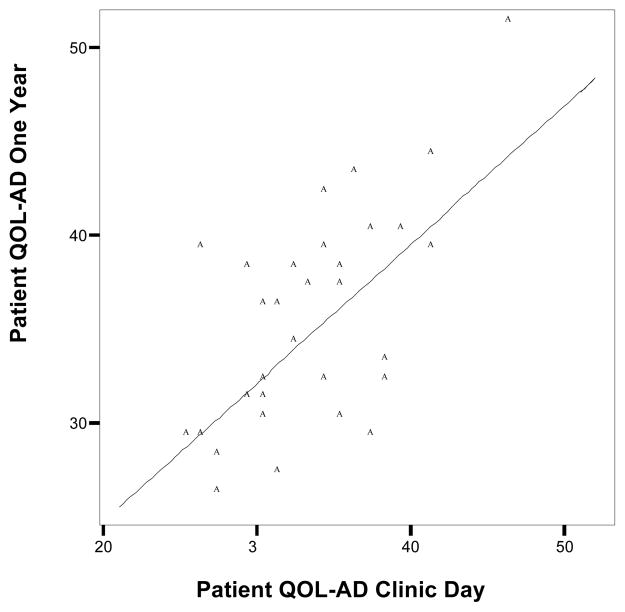
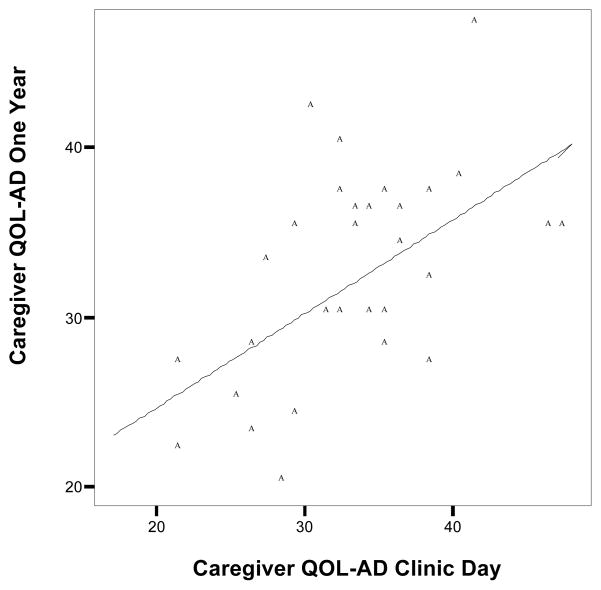
Figures 1 and 2 exhibit scatter plots of total QOL-AD scores at clinic day plotted against total QOL-AD scores at the one-year follow-up for patient and caregiver, respectively. At both time points there was a wide distribution of QOL-AD scores. The diagonal line is the no change line. The data points above this line demonstrate an improvement in total QOL over the one-year period. Points below this line are indicative of a decrease in total QOL-AD scores.
Figure 1.
Scatter plot of patient QOL-AD at clinic day against patient QOL-AD at one-year follow-up.
Figure 2.
Scatter plot of caregiver-rated patient QOL-AD at clinic day against caregiver-rated patient QOL-AD at one-year follow-up.
Comparisons between patients with and without data at one-year follow-up
The results of comparisons between patients who participated in the one-year follow-up and those without one-year data are reported in Table 3. The latter group includes those who discontinued the study, were institutionalized, died, moved outside the geographic area of the study, and those who continued in the study but did not return the form at one year or who completed an alternate form used with patients who did not speak English as a first language. The participants with one-year data were more likely to be younger, male, and have higher 3MS scores (less impaired). There was a trend toward higher education in the one-year follow-up group. Comparisons of overall medical status at clinic day show similar levels of comorbidities between those with and without one-year follow-up data.
Table 3.
Comparison of patients with and without data at one year.
| Clinic Day Variable | With data at one year (n=32) | Without data at one year 1 (n=61) | Comparison |
|---|---|---|---|
| Patient Age (mean, SD) | 71.5 (9.3) | 77.4 (8.5) | t(91)=3.08, p=0.003** |
| Patient Years of Formal | 10.9 (2.4) | 9.8 (3.1) | t(82)= −1.65, p=.103 |
| Education (mean, SD) | |||
| Patient Gender (n, % women) | 15 (46.9%) | 42 (68.9%) | Pearson Chi-square=4.27, p=0.039* |
| 3MS (mean, SD) | 78.0 (14.7) | 64.7 (20.0) | t(85)=−3.25, p=0.002** |
| Number of Chronic conditions (n, %) | |||
| One | 1 (3.1) | 2 (3.6) | Pearson Chi-square=1.21, p=0.877 |
| Two | 2 (6.3) | 5 (8.9) | |
| Three | 2 (6.3) | 7 (12.5) | |
| Four | 5 (15.6) | 8 (14.3) | |
| Five or more | 22 (68.8) | 34 (60.7) | |
Patients without data at one year includes those who discontinued the study (n=25), were institutionalized (n=19), died (n=3), moved outside the geographic area of the study (n=3), did not return the form at one year (n=8) or filled out the alternate form used when English is not the patient’s first language (n=3).
Comparison is significant at the 0.05 level.
Comparison is significant at the 0.01 level.
Predictors of patient and caregiver QOL ratings
Prior to regression analyses, predictors of QOL were included in bivariate correlation analyses. The results of these calculations are presented in Table 4. Patient-rated QOL at clinic day was significantly correlated with the CES-D, IADL, BADL and FAQ measures. Caregiver-rated QOL was significantly associated with the CES-D, IADL, NPI-S, BADL, FAQ, ZB, and GSI. It should be noted that the IADL, BADL and FAQ are similar measures that examine the patient’s functional abilities. The IADL is a patient-reported measure whereas the other two measures are completed by the caregiver.
Table 4.
Correlation coefficients between patient and caregiver QOL-AD, and demographic and clinical characteristics at clinic day (baseline).
| QOL Predictors (Clinic Day) | Correlation with patient-rated QOL-AD (p- value) | N | Correlation with caregiver-rated patient QOL-AD (p- value) | N |
|---|---|---|---|---|
| Patient Age at Clinic Day | 0.104 (p=0.297) | 102 | −0.119 (p=0.206) | 114 |
| Patient Years of Formal Education | 0.044 (p=0.666) | 98 | 0.140 (p=0.152) | 107 |
| 3MS at Clinic Day | −0.071 (p=0.489) | 97 | 0.104 (p=0.284) | 108 |
| CES-D | −0.517** (p<0.001) | 95 | −0.409** (p=0.001) | 97 |
| IADL | 0.382** (p<0.001) | 98 | 0.437** (p<0.001) | 107 |
| NPI-S | −0.093 (p=0.399) | 84 | −0.597** (p<0.001) | 96 |
| BADL | −0.280** (p=0.007) | 90 | −0.463** (p<0.001) | 102 |
| FAQ | −0.253* (p=0.010) | 102 | −0.494** (p<0.001) | 114 |
| ZB | −0.123 (p=0.221) | 101 | −0.614** (p<0.001) | 113 |
| GSI | −0.185 (p=0.066) | 100 | −0.332** (p=0.001) | 111 |
Note. CES-D = Centre for Epidemiologic Studies-Depression Scale; IADL = Instruments of Daily Living, Self-rated version; NPI-S = Neuropsychiatric Inventory-Severity; BADL = Bristol Activities of Daily Living Scale; FAQ = Functional Assessment Questionnaire; ZB = Zarit Burden Scale; GSI= Global Severity Index score of the Brief Symptom Inventory.
Correlation is significant at the 0.05 level.
Correlation is significant at the 0.01 level.
Table 5 exhibits the correlations between QOL predictors and patient and caregiver QOL ratings at the one-year follow-up. Patient QOL-AD was strongly correlated (>0.6) with the CES-D and moderately correlated (>0.4) with the IADL, FAQ, ZB and GSI. Caregiver-rated patient QOL ratings were strongly correlated with the ZB and moderately correlated with the CES-D, FAQ and GSI. Age, education and 3MS were not significantly related to patient or caregiver-rated QOL-AD.
Table 5.
Correlation coefficients between patient and caregiver-rated QOL-AD, and demographic and clinical characteristics at one year.
| QOL Predictors (One Year) | Correlation with patient-rated QOL-AD (p- value) | N | Correlation with caregiver-rated patient QOL-AD (p-value) | N |
|---|---|---|---|---|
| Patient Age at One Year | 0.026 (p=0.888) | 32 | 0.021 (p=0.912) | 30 |
| Patient Years of Formal Education | 0.104 (p=0.583) | 30 | 0.074 (p=0.706) | 28 |
| 3MS at One Year | 0.054 (p=0.787) | 28 | 0.080 (p=0.699) | 26 |
| CES-D | −0.655** (p<0.001) | 32 | −0.434* (p=0.017) | 30 |
| IADL | 0.468** (p=0.007) | 32 | 0.315 (p=0.090) | 30 |
| NPI-S | −0.281 (p=0.165) | 26 | −0.235 (p=0.269) | 24 |
| FAQ | −0.409* (p=0.022) | 31 | −0.463* (p=0.011) | 29 |
| ZB | −0.557** (p=0.001) | 31 | −0.725** (p<0001) | 29 |
| GSI | −0.461* (p=0.010) | 30 | −0.460* (p=0.014) | 28 |
| Clinic Day QOL-AD | 0.595** (p<0.001) | 32 | −0.578** (p=0.001) | 30 |
Note. CES-D = Centre for Epidemiologic Studies-Depression Scale; IADL = Instruments of Daily Living, Self-rated version; NPI-S = Neuropsychiatric Inventory-Severity; BADL = Bristol Activities of Daily Living Scale; FAQ = Functional Assessment Questionnaire; ZB = Zarit Burden Scale; GSI= Global Severity Index score of the Brief Symptom Inventory.
Correlation is significant at the 0.05 level.
Correlation is significant at the 0.01 level.
In order to determine the predictors of patient QOL at clinic day and one year, stepwise multivariate linear regression analysis was used. The independent variables included in the regression analyses were those found to be significant in the bivariate analyses (see Tables 4 and 5) as long as they had a p value ≤0.2. Predictor variables were excluded from the regression analyses if they were not significantly related to the outcome variable (caregiver- or patient-rated QOL-AD) or if they were highly correlated with other independent variables (multicollinearity). If there was any multicollinearity (variance inflation factor (VIF) greater than approximately 2) then one of the variables was removed from the analysis based on previous literature. Four different regression analyses were conducted (Table 6): (1) patient-rated QOL at clinic day, (2) caregiver-rated patient QOL at clinic day, (3) patient-rated QOL at one year, and (4) caregiver-rated patient QOL at one year. Any other variables that were highly correlated based on the value of the variance inflation factor (VIF greater than approximately 2) were removed to include only one from each correlated group of variables in the regression (ie., BADL, FAQ, and IADL). The BADL was completed by the caregiver at clinic day but not at the one-year follow-up and thus was not included in the regression analyses. Similarly, FAQ was also a caregiver reported measure and thus was excluded if highly correlated with another similar measure.
Table 6.
Multivariate stepwise linear regression of variables of interest.
| Variable | B (95%CI) | Beta | t | p |
|---|---|---|---|---|
|
Patient-rated QOL-AD at clinic day (n=89) Adjusted r2=0.33.
| ||||
| CES-D | −0.299 (−0.419 to −0.180) | −0.453 | −4.97 | <0.001 |
| IADL | 0.316 (0.099 to 0.533) | 0.264 | 2.90 | 0.005 |
|
| ||||
|
Caregiver-rated Patient QOL-AD at clinic day (n=71) Adjusted r2=0.49.
| ||||
| ZB | −0.265 (−0.426 to −0.103) | −0.360 | −3.26 | 0.002 |
| IADL | 0.298 (0.082 to 0.514) | 0.255 | 2.75 | 0.008 |
| NPIS | −0.306 (−0.549 to −0.063) | −0.278 | −2.52 | 0.014 |
|
| ||||
|
Patient-rated QOL-AD at one year (n=23) Adjusted r2=0.54.
| ||||
| CES-D | −0.380 (−0.656 to −0.104) | −0.478 | −2.87 | 0.009 |
| QOLPT-CD | 0.470 (0.058 to 0.882) | 0.396 | 2.38 | 0.027 |
|
| ||||
|
Caregiver-rated Patient QOL-AD at one year (n=27) Adjusted r2=0.52.
| ||||
| ZB | −0.486 (−0.672 to −0.300) | −0.732 | −5.38 | <0.001 |
Note. CES-D = Centre for Epidemiologic Studies-Depression Scale; IADL = Instruments of Daily Living, Self-rated version; ZB, = Zarit Burden Scale; QOLPT-CD = Quality of Life-Patient at clinic day.
In the first regression (patient QOL-AD at Clinic Day), GSI was excluded because it was not significantly related to patient QOL-AD. The BADL was excluded because it is highly correlated with IADL and FAQ. In the second regression (Caregiver-rated patient QOL-AD at Clinic Day), education and GSI were excluded because they were not significantly related to caregiver QOL-AD. The BADL and FAQ were excluded due to multicollinearity. In the third regression (patient-rated QoL at one year) GSI, NPIS, education and age were excluded since they were not significantly related to patient QOL-AD. The FAQ was excluded because of multicollinearity. In the fourth regression (caregiver-rated patient QOL-AD at one year), education, age and GSI were excluded because they were not significantly related to the outcome variable. The FAQ was left out because it was highly correlated with IADL and ZB.
Results of the first regression analysis indicate that patient depressive symptomatology (β=−0.453, p<0.001) and functional ability (β=0.264, p=0.005) were both significant predictors of patient-rated QOL-AD scores at clinic day. The model accounted for 33% of the variation in patient QOL-AD overall. Results of the second regression analysis reveal that caregiver burden (β=−0.360, p=0.002), patient functional ability (β=0.255, p=0.008), and neuropsychiatric symptom severity (β=−0.278, p=0.014) were significant predictors of caregiver-rated QOL-AD scores at clinic day. The model accounted for 49% of the variation in caregiver ratings of patient QOL. The third regression analysis demonstrates that patient depressive symptomatology (β=−0.478, p=0.009) and patient-rated QOL at clinic day (β=0.396, p=0.027) were significant predictors of patient-rated QOL at one year. The model accounted for 54% of the variation in patient QOL-AD at one year. The last regression analysis indicates that caregiver burden (β=−0.732, p<0.001) was the only significant predictor of caregiver-rated QOL-AD at one year. The model accounted for 52% of the variation in caregiver ratings of patient QOL at one year.
Discussion
This study has three main findings: (1) Individuals in the early stages of dementia and their caregivers rate patient QOL in a similar but not identical fashion; (2) Patient and caregiver ratings of QOL over a one-year period did not change and; (3) Patient self-rated QOL is mainly predicted by patient depressive symptomatology whereas caregiver ratings of patient QOL are most strongly associated with caregiver burden.
We found that patient and caregiver ratings of patient QOL are moderately associated at best. These results are consistent with previous research (Logsdon et al., 1999; Novella et al., 2001; Ready, Ott, & Grace, 2004; Sands et al., 2004; Schölzel-Dorenbos et al., 2007). Similar to the QOL-AD measure used in this study, the DQoL (Dementia Quality of Life) used by Sands was administered to both patients and their caregivers (Sands et al., 2004). Sands proposed that the disagreement between patient and caregiver QOL ratings might be due to the level of burden experienced by the caregiver. However, a study of patients with early stage dementia, who were assessed as having full insight (no anosognosia), found that patient and caregiver ratings of QOL were significantly correlated (Vogel, Mortensen, Hasselbalch, Andersen, & Waldemar, 2006). Our study involved patients in the relatively early stages of dementia and thus it is likely that most maintained insight into how their clinical symptoms such as energy, mood, and memory were affecting their quality of life. In our study it is possible that patient and caregiver ratings of patient QOL are correlated due to the level of insight of the patients but the associations are not strong due to the influence of burden on caregiver ratings of patient QOL.
The average QOL-AD scores reported in this study are similar to previous findings (Logsdon et al., 2002; Hoe et al., 2007; Selwood et al., 2005; Thorgrimsen et al., 2003).. The patient-rated QOL-AD ratings from the current study (34.53 and 35.47 at clinic day and one year respectively) are at the lower end of the range of patient-reported QOL-AD (34.4–39.8) of prior studies (Hoe et al., 2007; Logsdon et al., 2002; Selwood et al., 2005). The caregiver-rated patient QOL-AD ratings of the current study (34.20 and 32.57 at clinic day and one year respectively) are at the high end of the range of caregiver-reported QOL-AD (30.8–33.9) of prior studies (Hoe et al., 2007; Logsdon et al., 2002).
Consistent with previous findings (Brod et al., 1999; Sands et al., 2004), our results suggest that proxy ratings of patient QOL should not be used as the gold standard. Menne et al. (2009) suggest that individuals with dementia can reliably provide self-report information. Those with dementia are able to express values and preferences for everyday care and also about future treatment decisions (Whitlatch, Feinberg, & Tucke, 2005). Although patient and caregiver ratings are correlated it is not a high association and one rating should not be used as a substitute for the other. It is a more valid assessment of the patient’s subjective well-being if the patient is questioned directly. This finding implies that in a clinical setting the decisions about treatment options should involve the patient. Ready et al. (2004) have indicated that treatment decisions based on proxy reports may not achieve maximal benefit from a patient perspective because informants have different perceptions about patient QOL. Schölzel-Dorenbos et al. (2007) concluded that the QOL-AD assesses specific domains important for QOL including physical and mental health. If interventions are primarily dealing with health outcomes, then using patient rated QOL-AD is an appropriate method of incorporating patient perspectives into intervention decisions. Treatment interventions such as pharmacological agents are aimed at improving physical, behavioural, and psychological symptoms; one method to determine the efficacy of such treatments should be to include patient-rated QOL (Naglie, 2007).
When comparing differences between patients and caregivers on individual items of the QOL-AD, the trend was an increase in patient ratings and a decrease in caregiver ratings over one year. Future studies should look at similar comparisons over a longer follow-up period to see if the trends become significant. Patient QOL did not significantly change for either the patient or caregiver ratings over a period of one year. This finding corresponds with previous longitudinal studies (Missotten et al., 2007; Selwood et al., 2005) but differs from another study (Lyketsos et al., 2003). The study by Lyketsos (2003) involved patients with more severe dementia who resided in a long-term care facility and the patient’s QOL was rated by a caregiver proxy, which may limit its agreement with our study. The study by Missotten (2007) revealed an increase in QOL at the end of year one and a decrease in QOL by year two; overall, there was no significant change in QOL. The study also used family or professional caregiver proxy ratings of patient QOL and thus may not be a relevant comparison to the current study. Perhaps the most relevant comparison is the above study by Selwood (2005), which included patients with a broad range of dementia severity, and both self-rated and proxy measures of patient QOL, neither of which changed over one year.
On an individual basis approximately half of the patients in the current study reported an increase in QOL over one year, whereas the others reported a decrease in QOL. Caregiver ratings of patient QOL showed a similar pattern with approximately half of the caregivers rating patient QOL higher at one year than at clinic day. Lyketsos et al. (2003) reported that proxy ratings of patient QOL improved for 32%, remained the same for 17%, and declined for 51% of the patients. Similarly, Selwood et al. (2005) found that approximately half of people with dementia had either an increase or decrease in QOL. These findings indicate that although we can make generalizations about how QOL changes over time it still remains a very unique individual experience. This should give hope to patients and caregivers that a decline in QOL does not always occur. Perhaps with earlier detection and treatment of depressed mood the QOL of dementia patients can be maintained throughout disease progression. Our results suggest that patients who are capable of maintaining a positive mood will report a better QOL regardless of their level of cognitive impairment.
One limitation to this study is the relatively small sample sized used in the regression analyses. However, the results are in agreement with previous studies (Karlawish, Casarett, Klocinski, & Clark, 2001; Logsdon et al., 2002; Thorgrimsen et al., 2003) in that burden appears to play a significant role in caregiver ratings of patient QOL, with increased burden predicting lower ratings of caregiver-rated patient QOL. Caregiver burden was a significant predictor at both clinic day and one year indicating that the effect of the patient’s illness on the caregiver influences how the caregiver views the patient’s quality of life. The IADL measure was a predictor of caregiver-rated QOL at clinic day. It is perhaps a combination of caregiver burden and patient functional ability that determines how a caregiver views the patient’s QOL. This is to say that a patient’s inability to perform everyday tasks leads to increased demand on the caregiver. A more dependent family member may create a burdened environment and influence the caregiver’s perspective of patient QOL.
Patient-rated QOL was largely predicted by symptoms of depression and QOL at clinic day. Earlier studies have also shown that patients with more depressive symptoms rate their QOL lower than patients with fewer depressive symptoms (Logsdon et al., 2002; Sands et al., 2004; Selwood et al., 2005; Winzelberg] et al., 2005). Sevush and Leve (1993) concluded that patients who are willing and able to identify their dementia-related deficits will report higher levels of depression. Those who do not recognize their own deficits have lower levels of depression. Selwood et al., 2005 also found that the only major predictor of future QOL was baseline QOL. In our study one of the most important predictors of QOL at one year, as rated by the patient, was QOL at baseline. Our findings suggest that in order to maximize patient well-being in the early stages of dementia, steps should be taken to detect and treat symptoms of depression.
Demographic factors such as patient age, years of formal education, and gender were not significant predictors of patient QOL. Previous studies have reported similar results indicating that age and education at baseline do not predict the QOL experienced throughout the disease progression (Lyketsos et al., 2003; Missotten et al., 2007). In agreement with previous studies, level of cognitive impairment was not found to be a predictor of patient QOL (Lyketsos et al., 2003; Selwood et al., 2005; Thorgrimsen et al., 2003).
The findings from this study, compared to previous research, indicate that the relationship between rural patients with early stage dementia and the change in QOL appears to resemble that of an urban setting. However, treatment options to rural patients may be limited due to decreased availability, thus future studies should look at the services available to rural and remote patients and their caregivers.
In common with other studies (Lyketsos et al., 2003; Selwood et al., 2005), the number of patients with follow-up data at one year (32/119) was reduced due to study drop-out (21%), admission to long-term care (16%), as well as other factors (14%) including failure to complete questionnaires relocation, and death. Because of the longitudinal nature of the current study and the continuous enrollment of patients, many of the patients assessed at clinic day did not have one-year data available at the time of the analysis (22%). Given the clinic aim of assessment and diagnosis of early stage dementia, the findings of this study may not generalize to advanced stages of dementia.
The caregiver assistance required by many of the patients to complete the questionnaires is also a limitation to the study. This assistance may be a possible reason for the higher level of agreement between the person with dementia and the caregiver at the one-year follow-up QOL-AD assessment compared to clinic day. As the disease progresses, patients will require more assistance in completing the questionnaires but perhaps this could be provided by an independent individual other than the caregiver.
Conclusion
These data have shown that over a one-year period the QOL of patients with early stage dementia, as rated by the patient or their caregiver, did not change. Nearly half of the patients experienced an increase in QOL whereas the others experienced a decrease in QOL. This indicates that QOL is maintained in some individuals despite decreased cognition and functional ability. Predictors of patient self-rated QOL included symptoms of depression, functional ability, and QOL at clinic day. Treatment management should thus be aimed at early detection and treatment of depressed mood. The most important factors that influence caregiver ratings of patient QOL are caregiver burden and patient functional ability. This implies that support services for the caregiver should focus on managing caregiver burden.
Contributor Information
Marcie Heggie, Medical Student (2011), University of Saskatchewan, TEL: (306) 280-4697.
Debra Morgan, Professor, Canadian Centre for Health & Safety in Agriculture1, Chair, Rural Health Delivery, TEL: (306) 966-7905, FAX: (306) 966-8799.
Margaret Crossley, Professor, Department of Psychology, Director, Aging Research & Memory Clinic, TEL: (306) 966-5923, FAX: (306) 966-6630.
Andrew Kirk, Professor & Head, Division of Neurology, Department of Medicine, TEL: (306) 966-8372, FAX: (306) 966-8008.
Patrick Wong, Medical Student (2010), University of Saskatchewan.
Chandima Karunanayake, Professional Research Associate, Canadian Centre for Health & Safety in Agriculture, TEL: (306) 966-1647, FAX: (306) 966-8799.
Rob Beever, Research Officer/Data Analyst, Applied Research/Psychiatry, College of Medicine, TEL: (306) 966-8772, FAX (306) 966-8774.
References
- Albert SM, Jacobs DM, Sano M, Marder K, Bell K, Devanand D, et al. Longitudinal study of quality of life in people with advanced Alzheimer’s disease. Am J Geriatr Psychiatry. 2001;9:160–168. [PubMed] [Google Scholar]
- Alzheimer Society of Canada. [Accessed 26 March, 2010.];Rising Tide: The impact of dementia on Canadian society. Available at: http://www.alzheimer.ca/english/rising_tide/rising_tide_summary.htm.
- Bédard M, Molloy DW, Squire L, Dubois S, Lever JA, O’Donnell M. The Zarit Burden Interview: a new short version and screening version. Gerontologist. 2001;41:652–657. doi: 10.1093/geront/41.5.652. [DOI] [PubMed] [Google Scholar]
- Bland RC, Newman SC. Mild dementia or cognitive impairment: the Modified Mini-Mental State examination (3MS) as a screen for dementia. Canadian Journal of Psychiatry. 2001;46:506–510. doi: 10.1177/070674370104600604. [DOI] [PubMed] [Google Scholar]
- Bond J. Quality of life for people with dementia: Approaches to the challenge of measurement. Ageing and Society. 1999;19:561–579. [Google Scholar]
- Brod M, Stewart AL, Sands L, Walton P. Conceptualization and measurement of quality of life in dementia: the Dementia Quality of Life Instrument (DQoL) Gerontologist. 1999;39:25–35. doi: 10.1093/geront/39.1.25. [DOI] [PubMed] [Google Scholar]
- Bucks RS, Ashworth DL, Wilcock GK, Siegfried K. Assessment of activities of daily living in dementia: Development of the Bristol Activities of Daily Living scale. Age and Ageing. 1996;25:113–120. doi: 10.1093/ageing/25.2.113. [DOI] [PubMed] [Google Scholar]
- Cahill S, Begley E, Topo P, Saarikalle K, Macijauskiene J, Budraitiene A, et al. ‘I Know Where this is Going and I Know it won’t Go Back’: Hearing the Individual’s Voice in Dementia Quality of Life Assessments. Dementia. 2004;3:313–330. [Google Scholar]
- Canadian study of health and aging: study methods and prevalence of dementia. Canadian Medical Association Journal. 1994;150:899–913. [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychological Medicine. 1983;13:595–605. [PubMed] [Google Scholar]
- Edelman P, Fulton BR, Kuhn D, Chang CH. A comparison of three methods of measuring dementia-specific quality of life: perspectives of residents, staff, and observers. Gerontologist. 2005;45:27–36. doi: 10.1093/geront/45.suppl_1.27. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox-Wasylyshyn SM, El-Masri MM. Handling missing data in self-report measures. Research in Nursing & Health. 2005;28:488–495. doi: 10.1002/nur.20100. [DOI] [PubMed] [Google Scholar]
- Hendry F, McVittie C. Is Quality of Life a Healthy Concept? Measuring and Understanding Life Experiences of Older People. Qualitative Health Research. 2004;14:961–975. doi: 10.1177/1049732304266738. [DOI] [PubMed] [Google Scholar]
- Hendry K, Douglas D. Promoting Quality of Life for Clients Diagnosed with Dementia. Journal of the American Psychiatric Nurses Association. 2003;9:96–102. [Google Scholar]
- Hoe J, Katona C, Orrell M, Livingston G. Quality of life in dementia: care recipient and caregiver perceptions of quality of life in dementia: the LASER-AD study. International Journal of Geriatric Psychiatry. 2007;22:1031–1036. doi: 10.1002/gps.1786. [DOI] [PubMed] [Google Scholar]
- Karlawish JH, Casarett D, Klocinski J, Clark CM. The relationship between caregivers’ global ratings of Alzheimer’s disease patients’ quality of life, disease severity, and the caregiving experience. Journal of the American Geriatric Society. 2001;49:1066–1070. doi: 10.1046/j.1532-5415.2001.49210.x. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;3:179–186. [PubMed] [Google Scholar]
- Logsdon RG, Gibbons LE, McCurry SM, Teri L. Quality of life in Alzheimer’s disease: patient and caregiver reports. Journal of Mental Health & Aging. 1999;5:21–32. [Google Scholar]
- Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosomatic Medicine. 2002;64:510–519. doi: 10.1097/00006842-200205000-00016. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Gonzales-Salvador T, Chin JJ, Baker A, Black B, Rabins P. A follow-up study of change in quality of life among persons with dementia residing in a long-term care facility. International Journal of Geriatric Psychiatry. 2003;18:275–281. doi: 10.1002/gps.796. [DOI] [PubMed] [Google Scholar]
- McEachern W, Kirk A, Morgan D, Crossley M, Henry C. Utility of telehealth in following cognition in memory clinic patients from rural and remote areas. Canadian Journal of Neurological Sciences. 2008;35:643–646. doi: 10.1017/s0317167100009458. [DOI] [PubMed] [Google Scholar]
- Menne HL, Judge KS, Whitlatch CJ. Predictors of quality of life for individuals with dementia: implications for intervention. Dementia. 2009;8:543–560. [Google Scholar]
- Missotten P, Ylieff M, Di Notte D, Paquay L, De Lepeleire J, Buntinx F, et al. Quality of life in dementia: a 2-year follow-up study. International Journal of Geriatric Psychiatry. 2007;22:1201–1207. doi: 10.1002/gps.1814. [DOI] [PubMed] [Google Scholar]
- Morgan D, Crossley M, Kirk A, D’Arcy C, Stewart N, Biem J, et al. Improving access to dementia care: development and evaluation of a rural and remote memory clinic. Aging & Mental Health. 2009;13:17–30. doi: 10.1080/13607860802154432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Crossley M, Kirk A, McBain L, Stewart N, D’Arcy C, et al. Evaluation of telehealth for preclinic assessment and follow-up in an interprofessional rural and remote memory clinic. Journal of Applied Gerontology. doi: 10.1177/0733464810366564. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle W, Mcallister M, Venturato L, Adams T. Quality of life and dementia: The voice of the person with dementia. Dementia. 2007;6:175–191. [Google Scholar]
- Naglie G. Quality of life in dementia. Canadian Journal of Neurological Sciences. 2007;34:S57–61. doi: 10.1017/s0317167100005588. [DOI] [PubMed] [Google Scholar]
- Novella JL, Jochum C, Jolly D, Morrone I, Ankri J, Bureau F, et al. Agreement between patients’ and proxies’ reports of quality of life in Alzheimer’s disease. Quality of Life Research. 2001;10:443–452. doi: 10.1023/a:1012522013817. [DOI] [PubMed] [Google Scholar]
- O’Rourke N, Tuokko HA. Psychometric properties of an abridged version of the Zarit Burden Interview within a representative Canadian caregiver sample. Gerontologist. 2003;43:121–127. doi: 10.1093/geront/43.1.121. [DOI] [PubMed] [Google Scholar]
- Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. Journal of Gerontology. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- Rabins PV, Black BS. Measuring quality of life in dementia: purposes, goals, challenges and progress. International Psychogeriatrics. 2007;19:401–407. doi: 10.1017/S1041610207004863. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Ready RE, Ott BR. Integrating Patient and Informant Reports on the Cornell-Brown Quality-of-Life Scale. American Journal of Alzheimer’s Disease and Other Dementias. 2008;22:528–535. doi: 10.1177/1533317507307032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ready RE, Ott BR, Grace J. Patient versus informant perspectives of Quality of Life in Mild Cognitive Impairment and Alzheimer’s disease. International Journal of Geriatric Psychiatry. 2004;19:256–265. doi: 10.1002/gps.1075. [DOI] [PubMed] [Google Scholar]
- Ready RE, Ott BR, Grace J. Insight and Cognitive Impairment: Effects on Quality-of-Life Reports From Mild Cognitive Impairment and Alzheimer’s Disease Patients. American Journal of Alzheimer’s Disease and Other Dementias. 2006;21:242–249. doi: 10.1177/1533317506290589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkind NJ. Statistics for people who (think they) hate statistics. 2. Thousand Oaks, CA: Sage Publications; 2004. [Google Scholar]
- Sands LP, Ferreira P, Stewart AL, Brod M, Yaffe K. What explains differences between dementia patients’ and their caregivers’ ratings of patients’ quality of life? American Journal of Geriatric Psychiatry. 2004;12:272–280. [PubMed] [Google Scholar]
- Schölzel-Dorenbos CJ, Ettema TP, Bos J, Boelens-van der Knoop E, Gerritsen DL, Hoogeveen F, de Lange J, Meihuizen L, Dröes RM. Evaluating the outcome of interventions on quality of life in dementia: selection of the appropriate scale. International Journal of Geriatric Psychiatry. 2007;22:511–519. doi: 10.1002/gps.1719. [DOI] [PubMed] [Google Scholar]
- Selwood A, Thorgrimsen L, Orrell M. Quality of life in dementia-a one-year follow-up study. International Journal of Geriatric Psychiatry. 2005;20:232–237. doi: 10.1002/gps.1271. [DOI] [PubMed] [Google Scholar]
- Sevush S, Leve N. Denial of memory deficit in Alzheimer’s disease. American Journal of Psychiatry. 1993;150:748–751. doi: 10.1176/ajp.150.5.748. [DOI] [PubMed] [Google Scholar]
- Statistics Canada. [Accessed 31 March, 2010.];National Population Health Survey. Available at: http://www.statcan.gc.ca/bsolc/olc-cel/olc-cel?lang=eng&catno=82-567-X.
- Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. Journal of Clinical Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- Thorgrimsen L, Selwood A, Spector A, Royan L, de Madariaga Lopez M, Woods RT, Orrell M. Whose quality of life is it anyway? The validity and reliability of the Quality of Life-Alzheimer’s Disease (QoL-AD) scale. Alzheimer Disease & Associated Disorders. 2003;17:201–208. doi: 10.1097/00002093-200310000-00002. [DOI] [PubMed] [Google Scholar]
- Trigg R, Jones RW, Skevington SM. Can people with mild to moderate dementia provide reliable answers about their quality of life? Age & Ageing. 2007;36:663–669. doi: 10.1093/ageing/afm077. [DOI] [PubMed] [Google Scholar]
- Vogel A, Mortensen EL, Hasselbalch SG, Andersen BB, Waldemar G. Patient versus informant reported quality of life in the earliest phases of Alzheimer’s disease. International Journal of Geriatric Psychiatry. 2006;21:1132–1138. doi: 10.1002/gps.1619. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. American Journal of Epidemiology. 1977;106:203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- Whitlatch CJ, Feinberg LF, Tucke SS. Everyday care of persons with cognitive impairment and their family caregivers. The Gerontologist. 2005;45:370–380. doi: 10.1093/geront/45.3.370. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Patterson MB, Sami SA. Quality of life in dementia: ten years later. Alzheimer Disease & Associated Disorders. 2003;17:199–200. doi: 10.1097/00002093-200310000-00001. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Rabins PV. Quality of life and dementia. Alzheimer Disease & Associated Disorders. 1992;6:135–137. doi: 10.1097/00002093-199206030-00001. [DOI] [PubMed] [Google Scholar]
- Winzelberg GS, Williams CS, Preisser JS, Zimmerman S, Sloane PD. Factors associated with nursing assistant quality-of-life ratings for residents with dementia in long-term care facilities. Gerontologist. 2005;45:106–114. doi: 10.1093/geront/45.suppl_1.106. [DOI] [PubMed] [Google Scholar]
- World Health Organization. [Accessed 11 July, 2008.];ICD-10 Classification of mental and behavioural disorders. 2007 Available at: http://www.who.int/classifications/apps/icd/icd10online/