Abstract
Background and Aim
Aluminium (Al3+) inhibits root growth of sensitive plant species and is a key factor that limits durum wheat (Triticum turgidum) production on acid soils. The aim of this study was to enhance the Al3+ tolerance of an elite durum cultivar by introgression of a chromosomal fragment from hexaploid wheat (Triticum aestivum) that possesses an Al3+ tolerance gene.
Methods
A 4D(4B) substitution line of durum wheat ‘Langdon’ was backcrossed to ‘Jandaroi’, a current semi-dwarf Australian durum. In the second backcross, using ‘Jandaroi’ as the recurrent parent, a seedling was identified where TaALMT1 on chromosome 4D was recombined with the Rht-B1b locus on chromosome 4B to yield an Al3+-tolerant seedling with a semi-dwarf habit. This seedling was used in a third backcross to generate homozygous sister lines with contrasting Al3+ tolerances. The backcrossed lines were characterized and compared with selected cultivars of hexaploid wheat for their Al3+ and Na+ tolerances in hydroponic culture as well as in short-term experiments to assess their growth on acid soil.
Key Results
Analysis of sister lines derived from the third backcross showed that the 4D chromosomal fragment substantially enhanced Al3+ tolerance. The ability to exclude Na+ from leaves was also enhanced, indicating that the chromosomal fragment possessed the Kna1 salt tolerance locus. Although Al3+ tolerance of seminal roots was enhanced in acid soil, the development of fine roots was not as robust as found in Al3+-tolerant lines of hexaploid wheat. Analysis of plant characteristics in the absence of Al3+ toxicity showed that the introgressed fragment did not affect total grain yield but reduced the weight of individual grains.
Conclusions
The results show that it is possible to increase substantially the Al3+ tolerance of an elite durum wheat cultivar by introgression of a 4D chromosomal fragment. Further improvements are possible, such as introducing additional genes to enhance the Al3+ tolerance of fine roots and by eliminating the locus on the chromosomal fragment responsible for smaller grain weights.
Keywords: Triticum turgidum, Triticum aestivum, durum wheat, 4D chromosomal fragment, aluminium tolerance, acid soil, root growth, salt tolerance, TaALMT1, Kna1, malate, rhizosheath
INTRODUCTION
Compared with bread wheat (Triticum aestivum; hexaploid, chromosomal make up of AABBDD), durum wheat (Triticum turgidum ssp. durum; tetraploid, chromosomal make up of AABB) is a minor crop that occupies about 8 % of the total area devoted to wheat production worldwide (Joppa, 1993). This is despite the relatively high price for durum grain on the international market that can yield a better return to farmers than bread wheat and other crops. However, durum grain yields are likely to be constrained in many regions because of its poor tolerance of abiotic stresses such as aluminium (Al3+) (Bona et al., 1993) and Na+ (Zubaidi et al., 1999) and by its susceptibility to biotic stresses such as crown rot (Balmas et al., 1995).
Acid soils are prevalent around the world where plant growth is restricted as a consequence of the low pH dissolving Al minerals into the toxic Al3+ cation (von Uexküll and Mutert 1995). In susceptible species, Al3+ inhibits root growth by damaging cells at the root apex, which affects the plant's ability to take up water and nutrients, resulting in reduced crop yields. Soil acidity and its accompanying Al3+ toxicity can be ameliorated by the application of lime (CaCO3) but, because lime moves slowly down the soil profile, it can take many years to neutralize sub-surface acidity. A common strategy for improving cereal yields on acid soils is to combine liming practices with Al3+-tolerant germplasm (Scott et al., 1997).
Early work by Mesdag and Slootmaker (1969) and Slootmaker (1974) showed that hexaploid wheat was considerably more Al3+ tolerant than durum wheat when grown on acid soils and that durum's tolerance was similar to that of Al3+-sensitive cultivars of barley (Hordeum vulgare L). Bona et al. (1993) screened cereal germplasm in acid soil over a short time (4 d) and ranked the Al3+ tolerance of the species as follows: rye (Secale cereale L.) > oats (Avena sativa L.) > millet (Panicum miliaceum) > bread wheat > barley > durum wheat. This susceptibility of durum to Al3+ was confirmed in long-term pot trials where grain yield was reduced in two durum cultivars when grown on an acid soil whereas grain yield was unaffected in two bread wheat cultivars grown on the same soil (Bona et al., 1995). In field trials, durum wheat yielded similarly to triticale (× Triticosecale) and hexaploid wheat on limed soil but performed much worse than these species on acid soil (Carmago et al., 1992). A range of studies using hydroponic and soil culture found that different genotypes of bread wheat varied considerably in their level of Al3+ tolerance, whereas all durum genotypes were sensitive (Foy and da Silva, 1991; Camargo et al., 1992; Moustakas et al., 1992; Cosic et al., 1994). One of the more comprehensive studies screened >600 durum genotypes of diverse origins and found that none possessed significant levels of Al3+ tolerance (Ryan et al., 2010). In contrast, Foy (1996) reported that several durum lines had Al3+ tolerance levels similar to those of tolerant hexaploid wheat, and Dai et al. (2010) identified T. turgidum subspecies that varied in Al3+ tolerance. This germplasm is a potential source of genes for enhancing the acid soil tolerance of durum wheat, but it needs to be characterized genetically and confirmed to be T. turgidum and not T. aestivum.
An Al3+ tolerance mechanism common in many plant species relies on the efflux of organic anions such as malate, citrate and oxalate from roots (Delhaize et al., 2012b). These organic anions are thought to chelate Al3+ around the root apex, forming harmless complexes that allow the root to continue growing unimpeded. Within the cereals, this mechanism has been described in bread wheat, rye, rice, triticale and barley, where variation in ability to secrete organic anions from root apices underlies genotypic differences in Al3+ tolerance (Delhaize et al. 2012b). In tolerant wheat, Al3+ activates the efflux of malate, and the gene (TaALMT1) controlling the mechanism has been isolated (Sasaki et al., 2004). TaALMT1 is located on chromosome 4DL (Ma et al., 2005; Raman et al., 2005) and encodes an Al3+-activated anion channel on the plasma membrane that facilitates malate efflux from root apices (Sasaki et al., 2004; Yamaguchi et al., 2005). Tolerant alleles of TaALMT1 are widely distributed within bread wheat germplasm, and the gene is located in a region where a major Al3+ tolerance locus has been mapped on chromosome 4D (Riede et al., 1996; Ma et al., 2005; Raman et al., 2005). When used to modify a range of plant species genetically, TaALMT1 increases malate efflux and confers Al3+ tolerance (Sasaki et al., 2004; Delhaize et al., 2004; Pereira et al., 2010).
The Al3+ sensitivity of durum wheat in comparison with bread wheat can be largely attributed to durum lacking chromosome 4D where TaALMT1 is located. Al3+ tolerance loci located on chromosomes other than the D group have been described in bread wheat (Aniol and Gustafson, 1984; Aniol et al., 1990; Papernik et al., 2001; Navakode et al., 2009; Ryan et al., 2009). However, these other loci confer lower levels of Al3+ tolerance than TaALMT1 and are either absent from the durum germplasm or do not contribute sufficient tolerance to durum lines to be detected in the screens applied. Durum wheat is an attractive target for genetic modification with TaALMT1 to enhance its level of Al3+ tolerance but, as an alternative that avoids public concerns regarding genetic modification, a strategy using introgression of a fragment of chromosome 4D into durum was investigated. Joppa and William (1988) described the development of durum lines where specific chromosomes were substituted with the corresponding homeologous D chromosomes derived from ‘Chinese Spring’. A line where chromosome 4B was substituted with chromosome 4D [4D(4B) substitution line] was used to introgress the salt tolerance locus Kna1, also located on chromosome 4D, into a durum genetic background (Dvorak and Gorham, 1992; Luo et al., 1996). The pairing homeologous (ph1c) mutation was used to generate recombinations between chromosomes 4B and 4D with the aim of introgressing a relatively small fragment of chromosome 4D that included the Kna1 locus. Some of the initial recombinant lines were also screened for Al3+ tolerance and this confirmed the presence of a major Al3+ tolerance locus on the long arm of chromosome 4D proximal to the Kna1 locus (Luo and Dvorak, 1996). These lines were in genetic backgrounds that are poorly adapted for agriculture and were not investigated in detail for their Al3+ tolerance and growth on acid soils. In the current study, we used the 4D(4B) substitution line to develop backcrossed germplasm that incorporates a fragment of the 4D chromosome into a modern elite cultivar of durum. The backcrossed lines were characterized and compared with selected cultivars of hexaploid wheat for their Al3+ and Na+ tolerances in hydroponic culture as well as in short-term experiments to assess their growth on acid soil.
MATERIALS AND METHODS
Germplasm
A durum wheat (Triticum turgidum ssp. durum) line with the ‘Langdon’ genetic background where the 4B chromosome is substituted with the 4D chromosome of hexaploid wheat (Joppa and William, 1988) was crossed with the Australian durum ‘Jandaroi’ and backcrossed three times using ‘Jandaroi’ as the recurrent, female parent. ‘Jandaroi’ is an elite, high-yielding cultivar that matures rapidly, has a semi-dwarf habit due to the Rht-B1b mutation, and high straw strength. In contrast, the 4D(4B) substitution line has poor agronomic traits and possesses the wild-type Rht-D1a allele that confers a tall habit, but also possesses the TaALMT1 gene from ‘Chinese Spring’ (Sasaki et al., 2004). The TaALMT1 allele in ‘Chinese Spring’ has a large duplication in its promoter region that is associated with an intermediate level of Al3+ tolerance (Sasaki et al., 2004). Initial experiments established that the 4D(4B) substitution line was considerably more Al3+ tolerant than ‘Jandaroi’ (data not shown) and that a screen based on root growth in hydroponic culture could be used to select seedlings in the backcrosses as described below. A cleaved amplified polymorphic sequence (CAPS) marker based on the TaALMT1 gene (Sasaki et al., 2004) was used to confirm that the Al3+-tolerant selections possessed a fragment of the 4D chromosome containing the TaALMT1 gene. In the BC2F1 generation, an Al3+-tolerant plant with a semi-dwarf habit was identified and used to generate subsequent lines. Analysis of the BC3F3 generation enabled Al3+-tolerant lines homozygous for the TaALMT1 locus and Al3+-sensitive sister lines that were null for the TaALMT1 locus to be identified based on segregation ratios for Al3+ tolerance. Selected lines were grown on to generate the BC3F4 generation of grain which was used in the hydroponics and soil experiments. Other germplasm used in this study included the hexaploid wheat lines ‘Chinese Spring’, ET8, ES8 and ‘Fronteria’ obtained from CSIRO Plant Industry (Canberra, Australia) and putative Al3+-tolerant durum lines obtained from the Australian Winter Cereals Collection (Tamworth, Australia). ‘Chinese Spring’ is the original source of the 4D chromosome in the 4D(4B) substitution line (Joppa and William, 1988), ET8 and ES8 are near isogenic lines that differ in Al3+ tolerance at a single genetic locus (Sasaki et al., 2004), and ‘Fronteira’ is a cultivar that possesses Al3+-tolerant root hairs (Delhaize et al., 21012a).
Screening germplasm for Al3+ tolerance by hydroponics
Seeds were surface-sterilized in approx. 1 % (w/v) sodium hypochlorite for 15 min, rinsed thoroughly in water, and incubated for 1–2 d in the dark on moist filter paper at 4 °C. Seed was then incubated at 25 °C and, once germinated, were grown in hydroponics at pH 4·3 using a nutrient solution described previously (Delhaize et al., 2004). Seedlings were grown in the hydroponic culture in a glasshouse with natural light and temperatures set at 25 °C for the day and 18 °C for the night. For the derivation of backcrossed germplasm, seedlings were screened with 5 μm AlCl3 added to the nutrient solutions and after 7 d seedlings able to grow roots were transferred to soil. To quantify Al3+ tolerance, seedlings were grown for 7 d at various AlCl3 concentrations, and the net growth of the three longest roots was measured where initial root length at planting was subtracted from final root length. Lateral roots generally had not emerged after 7 d growth in hydroponics, and some experiments assessed growth over 14 d during which lateral roots were well developed in all lines grown in the control (no Al3+) treatment. For fine roots and total root lengths, the root systems were scanned and measured using WinRhizo software as described below.
Short-term soil experiments
Plants were grown in an acidic red ferrosol obtained from the Robertson region of New South Wales, Australia (34°35′S, 150°36′E). One batch of soil (Batch A) was either unamended or amended with 0·5, 1 or 4 g of lime (CaCO3) per kg of soil. The pH and soluble Al concentrations in the soil were determined in 0·01 m CaCl2 extracts (one part dry soil to five parts solution) and values for the various treatments were as follows: unamended soil, pH 4·3 and 17·5 mg Al kg–1; 0·5 g lime treatment, pH 4·4 and 9·3 mg Al kg–1; 1 g lime treatment, pH 4·5 and 6·5 mg Al kg–1; 4 g lime treatment, pH 4·9 and 0·8 mg Al kg–1. A second batch of less toxic soil (Batch B) was prepared by amending the soil with 0·6, 2·2 or 3·2 g of lime per kg soil. The values for the various treatments of Batch B were as follows: 0·6 g lime treatment, pH 4·6 and 2·9 mg Al kg–1; 2·2 g lime treatment, pH 4·7 and 0·8 mg Al kg–1; 3·2 g lime treatment, pH 4·8 and 0·6 mg Al kg–1. Seedlings were surface sterilized and germinated as described above, and a single pre-germinated seed of each of two genotypes was planted into a pot that contained 1·3 kg of soil. Seedlings were grown in a glasshouse with natural light and temperatures set at 25 °C for the day (16 h) and 18 °C for the night (8 h). Pots were watered by weight to 80 % field capacity every day and, after 7 d of growth, seedlings were harvested and root length was measured. The combined length of the three longest seminal roots was measured with a ruler, and fine root (diameters <0·36 mm; comprising mainly lateral roots) length and total root length were measured by WinRhizo Pro V (2002) software after scanning the roots.
Rhizosheath screen
The rhizosheath is defined as the soil that remains adhered to roots when removed from the surrounding soil (Watt et al., 1994). For this experiment, we used the acidic ferrosol soil and limed treatments as described above. Rhizosheaths were assayed as described previously (Delhaize et al., 2012a). The seedlings were grown in a cabinet set at 25 °C with a 16 h light/8 h dark regime and were arranged in randomized block designs. To reduce drying of the soil surface, trays of water were placed within the cabinet to maintain the humidity at about 70 %. Pre-germinated seed with 3–6 mm roots were planted into the moist soil (80 % field capacity) with five replicates for each line. Fifteen pots were placed in trays (two trays per replicate) and covered with transparent plastic lids to prevent excessive moisture loss during the experiment. The surface of the pots was moistened on the second day (approx. 10 mL) and, after 3 d growth, plants were harvested. Soil was tipped out of the pots and seedlings were gently removed from the soil. The three seminal roots were cut off directly into a small tray, weighed with the adhering soil still intact and then their lengths were measured. Rhizosheaths are expressed as gram per metre of root, and included the weight of both the fresh root and the moist soil.
Assay of malate efflux and TaALMT1 expression
Seedlings were grown in hydroponic culture as described above in nutrient solution without Al3+. After 4 d growth, root apices (approx. 3 mm long) were excised and assayed for malate efflux as described by Ryan et al. (1995). Total RNA was extracted from ten root apices collected from each genotype using an RNeasy minikit (Qiagen). cDNA was synthesized with the SuperScript III First-Strand Synthesis System (Invitrogen) following methods provided with the kit. Gene expression was determined by quantitative real-time PCR (qRT-PCR) using the SYBR Green Supermix (Bio-Rad) kit on a Bio-Rad CFX96 Real Time System. Conditions and primers used for amplification of the TaALMT1 transcript along with GAPDH and PT1 as reference genes are described by Delhaize et al. (2004) and Tovkach et al. (2013).
Dgas marker
To assess whether durum lines previously identified as being Al3+ tolerant were actually T. turgidum and not T. aestivum, we used primers CTTCTGACGGGTCAGGGGCAC and CTGAATGCCCCTGCGGCTTAAG in a PCR that amplified the Dgas44 sequence (Bryan et al. 1998) in genomic DNA extracted from leaves. Dgas44 is a repetitive sequence specific to the D-genome and can be used to distinguish hexaploid wheat from tetraploid wheats that lack the D-genome (McNeil et al., 1994). Triticum aestivum lines generate a fragment of approx. 290 bp in a PCR whereas species lacking the D-genome do not generate a fragment.
Growth characteristics of backcrossed lines in the absence of Al3+ toxicity
Following the third backcross, three pairs of sister lines that differed in Al3+ tolerance were planted into potting mix of pH 6·2 that lacked Al3+ toxicity. Seedlings were grown in a glasshouse with natural light and temperatures set at 25 °C for the day (16 h) and 18 °C for the night (8 h). For each genotype, 20 seedlings were planted and harvested at maturity. The various pairs of lines were planted at different times, and pots of a particular pair of lines were placed together on a bench in a randomized arrangement. At harvest, the plant height, the number of spikes and the weight of seed were measured.
Analysis of Na+ exclusion
Plants were screened for their ability to exclude Na+ from leaves as an indirect measure of salt tolerance using a hydroponic culture method described by Munns et al. (2000). Briefly, after the second leaf had fully emerged, NaCl (5 m) was added to the nutrient solution over 3 d (twice a day) so that a final concentration of 150 mm was reached. At the same time, CaCl2 was added to achieve an Na+ to Ca2+ ratio of 15:1. After approx. 10 d, the third fully emerged leaf was harvested, dried and extracted with 0·5 m nitric acid. The Na+ and K+ extracted from the leaf were measured using inductively coupled plasma spectrometry.
RESULTS
Germplasm development
A report by Foy et al. (1996) described durum lines with Al3+ tolerance comparable with that of Al3+-tolerant cultivars of hexaploid wheat. The use of Al3+-tolerant germplasm of the same species would greatly facilitate the introgression of this trait into elite germplasm. We confirmed that some of these lines were Al3+ tolerant using hydroponic culture; however, analysis with a Dgas44 marker specific to the D-genome indicated that the lines were likely to be hexaploid wheat and were most probably misclassified as being durum (data not shown). In the absence of well-characterized natural variation for Al3+ tolerance in durum germplasm, we used the 4D(4B) substitution line as a starting point to cross into an elite Australian cultivar.
The 4D(4B) substitution line that possesses a full 4D chromosome was crossed to ‘Jandaroi’ and then progeny were backcrossed three more times to ‘Jandaroi’. The various backcross generations were assessed for segregation of tolerant seedlings from sensitive seedlings (Table 1). It was apparent that the 4D chromosome was effectively transmitted to the ‘Jandaroi’ genetic background since the backcrossed F1 seedlings at each generation showed at least 1:1 segregation (tolerant:sensitive). The segregation ratio of the F2 generations from each backcross followed Mendelian genetics consistent with tolerance being a single dominant locus. In the BC2F1 generation we identified an Al3+-tolerant plant with a semi-dwarf habit and inferred that the 4D chromosome had recombined with the 4B chromosome as explained below. This plant was used for a subsequent backcross to develop sister lines in the BC3 generation that were all semi-dwarf.
Table 1.
Segregation ratios for Al3+ tolerance (5 μm AlCl3) of backcrossed progeny derived from crosses between the 4D(4B) substitution line and ‘Jandaroi’
| Tolerant | Sensitive | Expected ratio | P-value (χ2) | |
|---|---|---|---|---|
| F1 | 5 | 1* | All tolerant | n.d. |
| BC1F1 | 4 | 2 | 1:1 | n.d. |
| BC2F1 | 10 | 4 | 1:1 | n.d. |
| BC3F1 | 5 | 5 | 1:1 | n.d. |
| BC1F2 | 40 | 20 | 3:1 | 0·14 |
| BC2F2 | 38 | 21 | 3:1 | 0·06 |
| BC3F2 | 57 | 18 | 3:1 | 0·84 |
| Combined F2 populations | 135 | 59 | 3:1 | 0·26 |
*The appearance of a sensitive seedling is likely to be the result of an unsuccessful cross.
n.d., not determined, i.e. P-values were not calculated due to the small sample size.
Al3+ tolerance in hydroponics
After the third backcross of ‘Jandaroi’ to the 4D(4B) substitution line, we developed six Al3+-tolerant lines (T lines) that were homozygous for the TaALMT1 gene and six Al3+-sensitive sister lines (S lines) that lacked the TaALMT1 gene. When screened in hydroponics for Al3+ tolerance, root growth of all six T lines was significantly greater than that of the six S lines (Fig. 1A). At this concentration, the backcrossed T lines had a similar level of tolerance to both the 4D(4B) substitution line and hexaploid wheat ‘Chinese Spring’, the original source of the 4D chromosome.
Fig. 1.
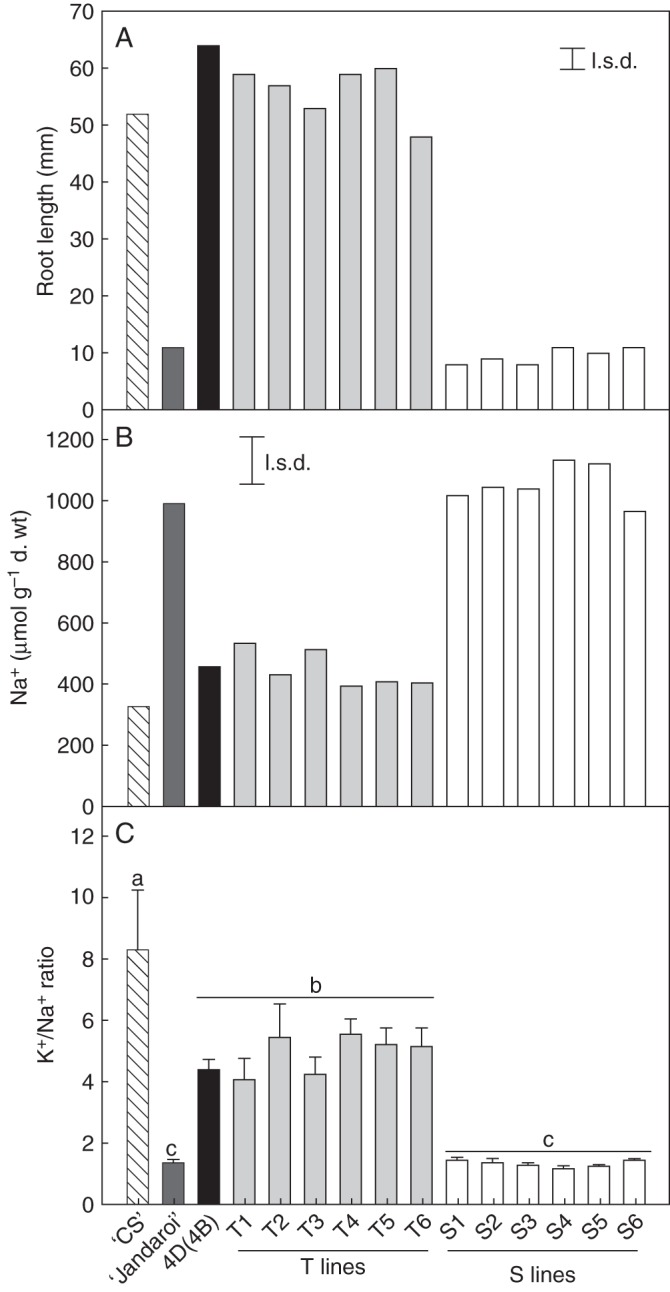
A 4D chromosomal fragment containing the TaALMT1 gene confers enhanced Al3+ tolerance and Na+ exclusion to durum. (A) Root length of seedlings grown for 7 d in nutrient solution that contained 10 μm AlCl3. (B) Na+ concentration and (C) K+/Na+ ratio in the third leaf of seedlings grown for 10 d in nutrient solution that contained 150 mm NaCl. The T lines are BC3 lines that possess a 4D chromosomal fragment, while the S lines are sister lines that lack the 4D chromosomal fragment. Control lines include hexaploid wheat ‘Chinese Spring’ (‘CS’), durum wheat ‘Jandaroi’ (recurrent parent used for backcrosses) and the 4D(4B) substitution line of durum in the ‘Langdon’ background. The least significant difference (l.s.d.) at P = 0·05 is shown for (A) and (B), whereas for (C) data failed an equal variance test and were log10 transformed prior to ANOVA. Significant differences between lines (P<0·05) are indicated by the different letters. Error bars for (C) show ± s.e.m. (n = 5).
The Kna1 locus that confers Na+ tolerance in hexaploid wheat is also located on chromosome 4D. Therefore, we tested whether the chromosome segment introgressed into the T lines also maintained this gene. The T lines were all semi-dwarf in growth habit and, because the 4D(4B) substitution line has the wild-type allele of the Ph1 locus that ensures pairing between homologous chromosomes, it was likely that a spontaneous recombination had occurred between the 4D and 4B chromosomes during the backcrosses such that the chromosomal fragment containing the wild-type allele of Rht-D1a was replaced with a chromosomal fragment containing the mutant Rht-B1b allele. Since the Kna1 locus is located distal to TaALMT1 (Luo and Dvorak, 1996) and the Rht-D1 locus proximal to TaALMT1 (Raman et al., 2005), it was likely that a hybrid 4D/4B chromosome was created that possessed both the Kna1 and TaALMT1 loci along with the Rht-B1b locus that confers a semi-dwarf growth habit. We tested the six T lines and six S lines for their ability to exclude Na+ from leaves, a trait associated with salt tolerance conferred by Kna1 (Gorham et al., 1990). All T lines had a low Na+ concentration in the third leaf as found for the 4D(4B) substitution line and ‘Chinese Spring’, whereas all S lines had higher Na+ concentrations that were similar to the parental ‘Jandaroi’ (Fig. 1B). High K+ to Na+ ratios are also associated with salt tolerance, and all T lines and parental lines with the 4D chromosomal fragment yielded a much higher K+/Na+ ratio than ‘Jandaroi’ and the S lines (Fig. 1C).
Hydroponics was used to assess Al3+ tolerance using two ranges of treatments and compared with parental lines as well as hexaploid lines that varied in their Al3+ tolerance. In the lower range of Al3+ treatments (Fig. 2A; 0–20 μm AlCl3), seminal root growth of all T lines was stimulated at 5 μm AlCl3 and all maintained root growth even at 20 μm AlCl3. In contrast, root growth of ‘Jandaroi’, the S line and ES8 (Al3+-sensitive hexaploid isogenic line of Al3+-tolerant ET8) was severely inhibited even at 5 μm, although ES8 was more tolerant than the sensitive durum lines at this concentration. The differences between the genotypes were also apparent at the higher range of Al3+ treatments (0–90 μm AlCl3), with all tolerant lines behaving similarly up to the 60 μm treatment (Fig. 2B). At the highest treatment, root growth of all lines was severely inhibited and ET8 had slightly better growth than the other lines.
Fig. 2.
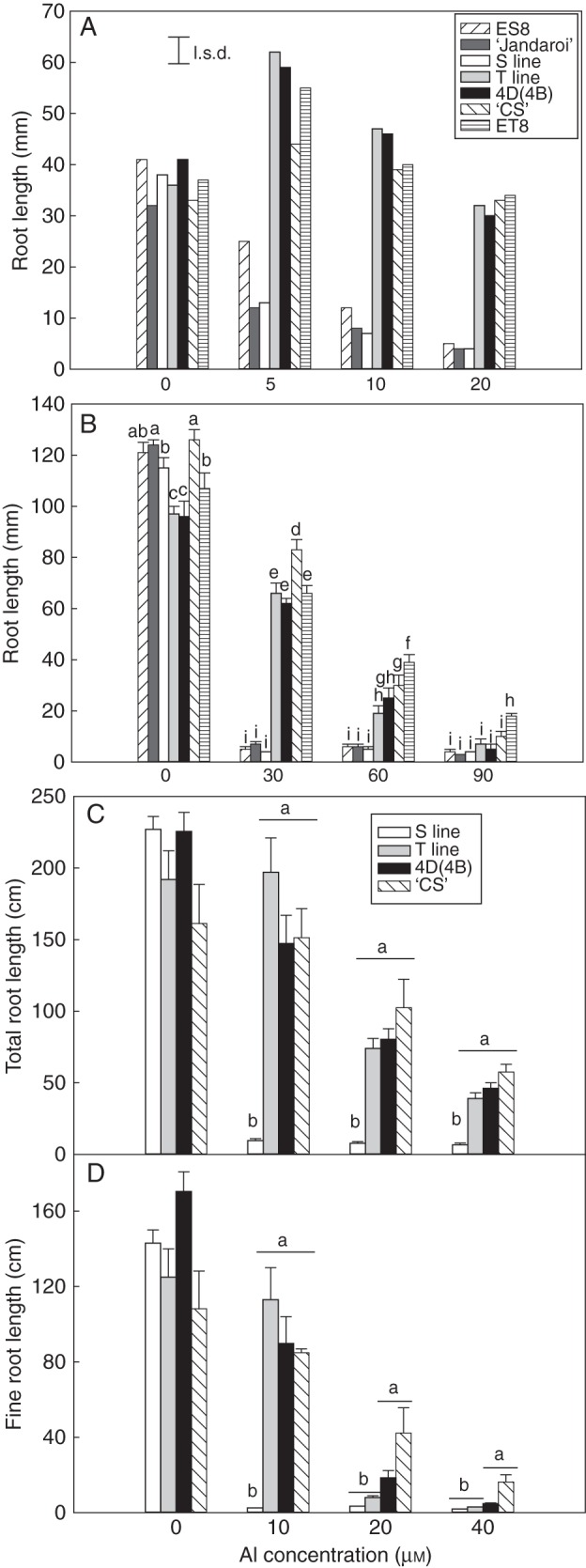
The effect of Al3+ on root growth in hydroponic culture. Plants were grown for 7 d in nutrient solution that contained AlCl3 ranging from (A) 0 to 20 μm and (B) 0 to 90 μm. To assess the effect of Al3+ on growth of fine roots, seedlings were grown in nutrient solution that contained AlCl3 ranging from 0 to 40 μm, and lengths of (C) total roots and (D) fine roots were measured after 14 d growth. The germplasm included hexaploid lines (‘Chinese Spring’, ES8 and ET8) and durum lines [substitution line 4D(4B), ‘Jandaroi’, lines T1 and S1 for (A) and (B) and composite T and S lines for (C) and (D) where three lines of each class of T and S were combined]. For (A) and (B), the combined length of the three longest roots was measured, while for (C) and (D) roots were scanned and measured using WinRhizo software. The error bars show the s.e.m. [n = 6 for (A) and (B); n = 4–12 for (C) and (D)] and the least significant difference (l.s.d.) at P = 0·05. For (B), (C) and (D), data were log10 transformed prior to two-way ANOVA (B) or one-way ANOVA within each treatment group (C and D). The different letters indicate significant differences at P < 0·05.
To assess the effect that Al3+ had on fine root development, hydroponics experiments were run over 14 d, since, in the above experiments run for 7 d, lateral roots had only just started to develop. Seminal root length (three longest roots; Supplementary Data Fig. S1A) and total root length of the T lines, substitution line 4D(4B) and ‘Chinese Spring’ (Fig. 2C) were all similar and were considerably longer than those of the S lines at all Al3+ treatments. Similarly, in the 10 μm AlCl3 treatment, fine roots of the S line were severely inhibited whereas all lines with the TaALMT1 gene maintained growth of fine roots (Fig. 2D). Fine roots were more sensitive than the seminal roots in the Al3+-tolerant lines (Fig. 2D; Supplementary Data Fig. S1A) and differences between Al3+-tolerant lines were only apparent at the two highest Al3+ treatments where the T and S lines had shorter fine roots than ‘Chinese Spring’ and substitution line 4D(4B). The average diameter of roots increased as the Al3+ concentration increased, and this was particularly apparent for the durum lines (Supplementary Data Fig. S1B).
Malate efflux and TaALMT1 gene expression
When assayed for malate efflux, the lines ET8, ‘Chinese Spring’, 4D(4B) and T all released malate from root apices when exposed to Al3+, whereas malate efflux was absent from ‘Jandaroi’ and the S line (Fig. 3A). Malate efflux from root apices of the T line was marginally less than in the other Al3+-tolerant lines [ET8, ‘Chinese Spring’ and 4D (4B)], but this was not always apparent in different experiments (data not shown). TaALMT1 expression in root apices was analysed by qRT-PCR from plants grown in the absence of Al3+ since the gene is constitutively expressed (Sasaki et al., 2004). Similar to malate efflux, TaALMT1 expression was only apparent in root apices of the Al3+-tolerant lines, with the T line showing a similar level of expression to the 4D(4B) substitution line (Fig. 3B). These data confirmed that the TaALMT1 gene introgressed into the ‘Jandaroi’ genetic background was expressed and capable of conferring Al3+-activated malate efflux.
Fig. 3.
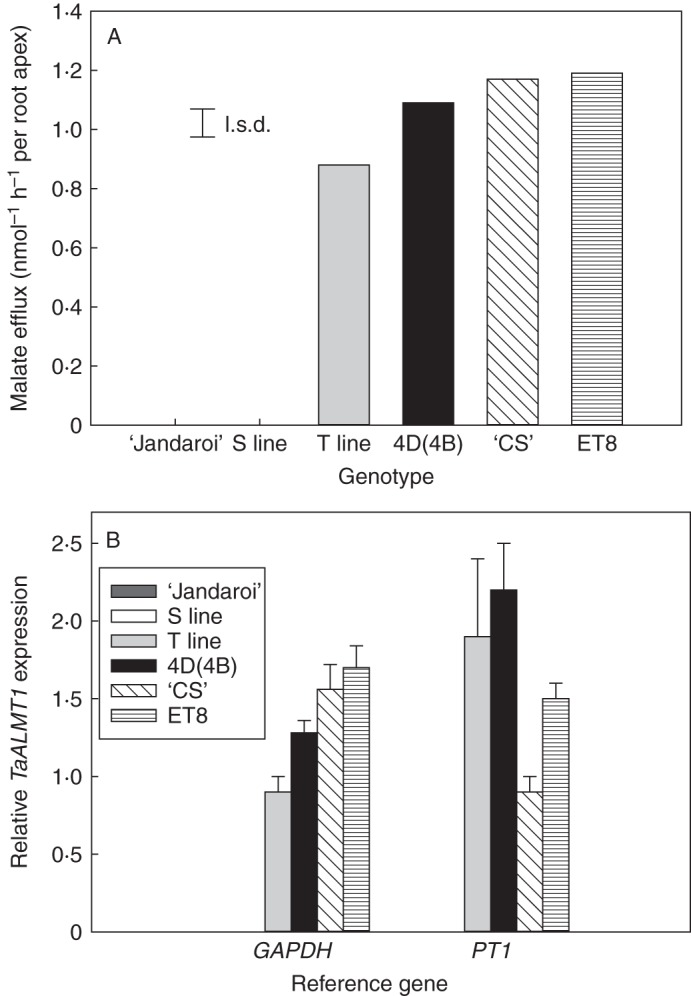
A 4D chromosomal fragment confers Al3+-activated malate efflux from durum root apices that is associated with TaALMT1 expression. (A) Al3+-activated malate efflux from root apices of lines S6 and T6 and control lines consisting of ‘Jandaroi’, the 4D(4B) substitution line, ‘Chinese Spring’ (‘CS’) and ET8. Apices were incubated in 100 μm AlCl3 added to basal solution (0·2 mm CaCl2, pH 4·3). Excised root apices incubated in basal solution alone exuded little or no malate for all genotypes. The least significant difference (l.s.d.) at P = 0·05 is shown (n = 4) for lines that had detectable malate efflux. (B) TaALMT1 expression in root apices of the same lines. RNA from excised root apices was extracted, transcribed to cDNA, and analysed for TaALMT1 expression by qRT-PCR. Expression is expressed relative to GAPDH or PT1 reference genes, and error bars show the s.e.m. (n = 4). Malate efflux and TaALMT1 expression were not detected above background in either line S6 or ‘Jandaroi’.
Soil experiments
Root growth of the backcrossed lines was assessed in an acid soil that had been amended with different amounts of lime. Liming increased the soil pH and this was associated with reduced concentrations of soluble Al (Materials and Methods). Three pairs of T and S lines were initially compared by measuring the combined length of the three longest seminal roots. In the unlimed soil, all the T lines along with the 4D(4B) substitution line had longer roots than the S lines and ‘Jandaroi’ (Supplementary Data Fig. S2). With the addition of lime, the difference between genotypes was reduced so that at the higher lime treatments (1 and 4 g lime kg–1 soil) all lines had similar root growth. A pair of T and S lines was then assessed in more detail and compared with control lines comprising Al3+-tolerant hexaploid and parental lines. The combined lengths of the three longest roots (seminals) of lines that possessed TaALMT1 [T, 4D(4B) substitution line, ET8 and ‘Chinese Spring’] were considerably greater in the acid soil than in lines that lacked TaALMT1 (S and ‘ Jandaroi’) and this difference disappeared at the highest liming treatment (Fig. 4A). When total root length was measured, both ET8 and ‘Chinese Spring’ had the greatest root length on the acid soil and differences between genotypes disappeared at the highest liming treatment (Fig. 4C). Notably, differences between the T and S lines were attenuated for total root length as compared with the three longest roots. Indeed for all lime treatments, the T and S lines did not differ from one another for total root length, whereas the 4D(4B) substitution line had greater total root length than S and ‘Jandaroi’ at all treatments. Fine root growth (primarily lateral roots) was inhibited more than that of the seminal roots for the durum and substitution lines. Fine root length in the T line did not differ from that of the S line at any treatment, whereas both hexaploid lines maintained fine root growth even in the unamended soil (Fig. 4B). On a less toxic batch of acid soil where seminal root growth of the S line was only marginally reduced, fine roots were 75 % less in the S line compared with the T line (Supplementary Data Fig. S3: lime treatment 0·6 g kg–1). The average root diameter was also increased in the S line at the lowest lime treatment, consistent with Al3+ toxicity and the inhibition of fine root development (Supplementary Data Fig. S3).
Fig. 4.
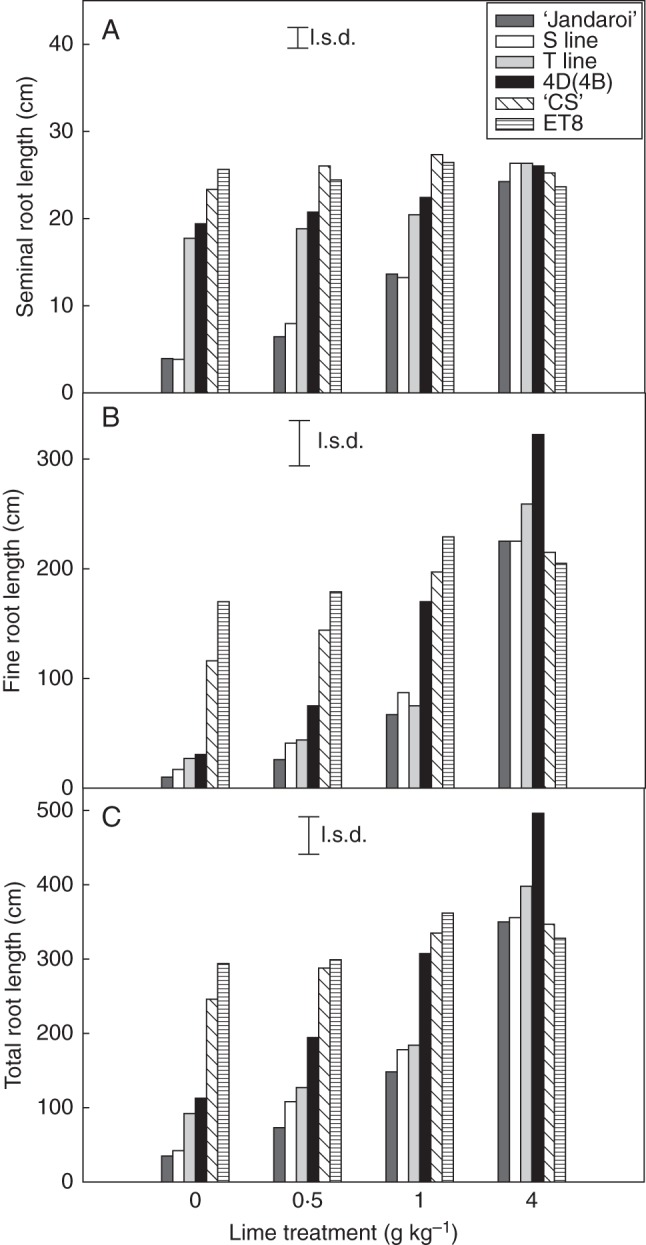
A 4D chromosomal fragment enhances growth of seminal roots but not fine roots of durum wheat grown on an acid soil. Growth of (A) the three longest seminal roots, (B) fine roots and (C) total roots was assessed for lines T1, S1, 4D(4B), ‘Jandaroi’, ‘Chinese Spring’ and ET8 grown on an unamended acid soil (Batch A: Materials and Methods) and the same soil that had been limed at various rates. Plants were harvested 7 d after sowing and the data show treatment means (n = 5) and the least significant difference (l.s.d.; P = 0·05).
We observed that roots grown in the soil at the highest lime treatment had copious rhizosheaths that were largely absent from the other treatments. The size of the rhizosheath of wheat germplasm grown in acid soil is correlated with root hair length, and rhizosheaths can be quantified using a simple assay (Delhaize et al., 2012a). As a positive control, ‘Fronteira’, a hexaploid wheat that maintains a rhizosheath on acid soil, was included in the experiment. ‘Fronteira’ had the largest rhizosheath in all treatments (Fig. 5A), whereas all other lines were similar to one another and only developed a sizable rhizosheath at the highest lime treatment. Photographs show that root hairs were largely absent from durum roots grown in acid soil (Fig. 5B), whereas roots growing in the limed soil (4 g kg–1) developed abundant root hairs (Fig. 5C).
Fig. 5.
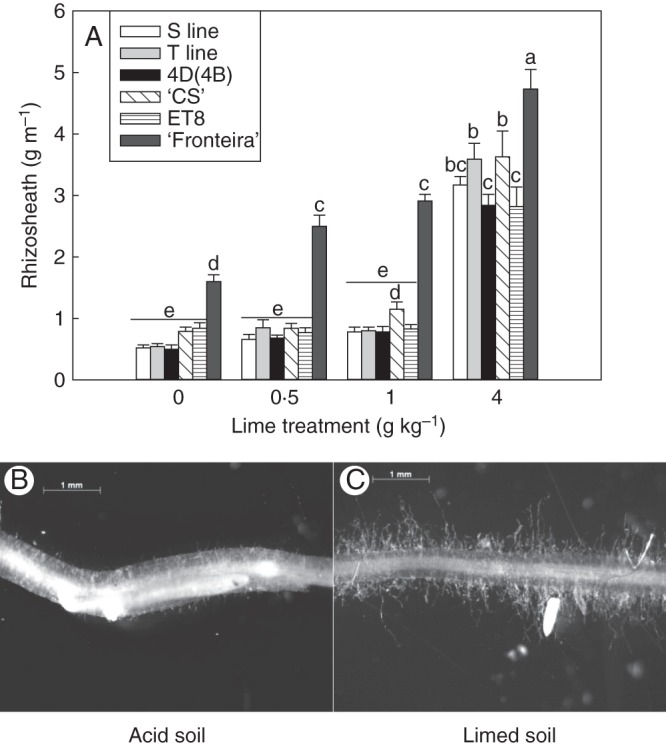
Comparison of rhizosheaths formed on roots of hexaploid and durum lines grown on an acid soil. (A) The durum lines [T1, S1 and 4D(4B) substitution line] were compared with hexaploid lines (‘Fronteria’, ‘Chinese Spring’ and ET8) for their ability to form rhizosheaths on unamended acid soil and the same soil that had been limed at the rates indicated. Data failed an equal variance test and were log10 transformed prior to ANOVA, and significant differences between lines (P < 0·05) are indicated by the different letters (n = 5). Photographs of representative root segments taken from the T1 line typical of all durum lines grown in (B) unamended acid soil and (C) the same soil that had been limed to 4 g kg–1.
Growth characteristics of BC3 lines in the absence of Al3+ toxicity
Three pairs of T and S lines were planted into a non-acid soil (pH 6·2) in a glasshouse and assessed at maturity for plant height, spike number and grain weight. Since the pairs of lines were planted at different times, they could only be compared within a pair and not between pairs. The T and S lines for a given pair did not differ from one another for spike number and grain weight, but for two pairs of lines the T line was marginally taller than the S line, indicating little or no effect of the introgressed 4D chromosomal fragment on these parameters (Fig. 6). Although total grain yield per plant was similar for both the T and S lines, individual grain weights measured from two pairs of lines indicated that the T lines had smaller grains than the S lines for two pairs of lines (Fig. 6D).
Fig. 6.
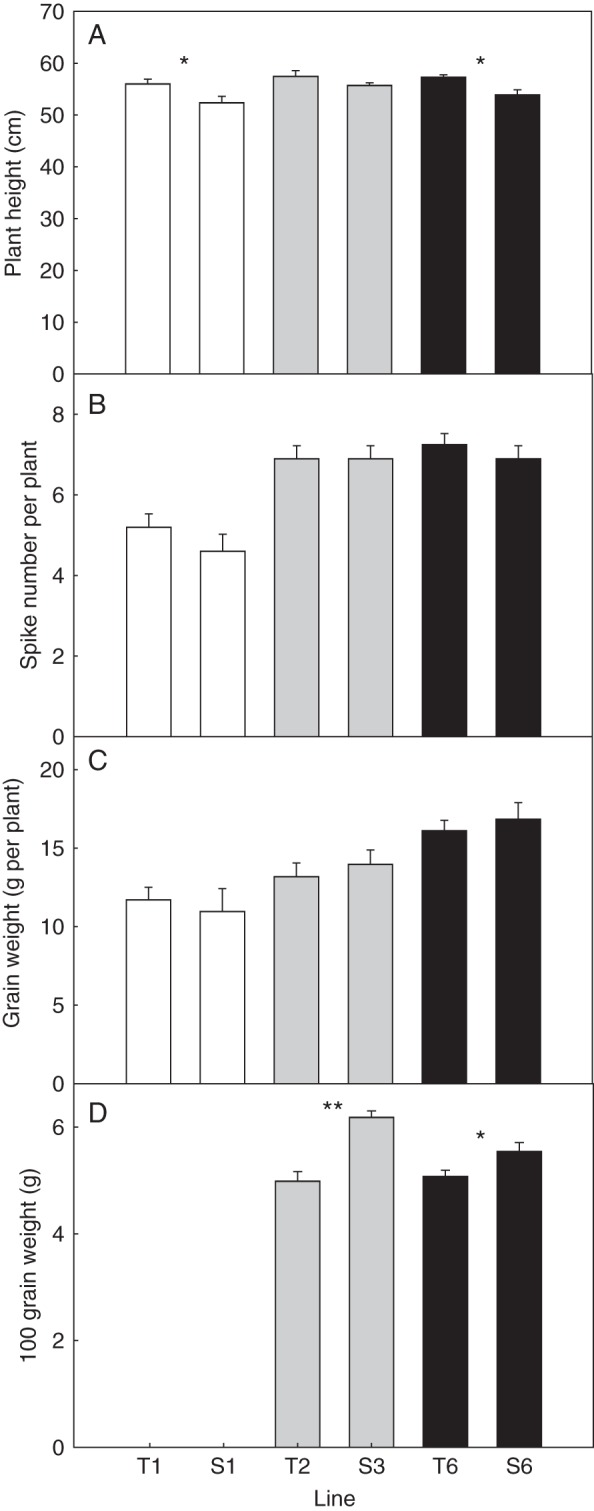
Effect of a 4D chromosomal fragment on plant height, spike number and grain weight in durum grown in the absence of Al3+ toxicity. Three pairs of T and S sibling lines were planted into soil (pH 6·2) and assessed for (A) plant height, (B) spike number, (C) grain yield at maturity and (D) the weight of 100 grains. Since the pairs of lines were sown at different times, comparisons could only be made between individual lines of each pair. For (D), data were only collected from two pairs of lines. Differences between T and S lines as identified using a two-tailed Student's t-test are denoted by * (P < 0·05) and ** (P < 0·001). Error bars denote the s.e.m. (n = 20 for A, B and C; n = 5–7 for D).
DISCUSSION
This study shows that it was possible to enhance markedly the Al3+ tolerance of an elite durum cultivar by introgression of a fragment of the 4D chromosome harbouring the TaALMT1 gene. Furthermore, since the fragment conferred Na+ exclusion, a trait associated with salt tolerance, we inferred that it also possessed the Kna1 locus. The Kna1 locus was previously found to confer a low shoot K+/Na+ ratio and mapped to the distal region of chromosome 4DL (Dubcovsky et al., 1996). Although the ability to exclude Na+ from leaves is associated with salt tolerance, future work will need to verify that the Kna1 locus is able to improve growth of the elite durum cultivar under saline conditions. The original source of the 4D chromosome is ‘Chinese Spring’ and this is a tall cultivar that possesses a wild-type Rht-D1a allele. The 4D and 4B chromosomes do not normally recombine in the absence of the ph1c mutation; however, we identified a spontaneous recombination event between chromosomes 4D and 4B that yielded an Al3+-tolerant plant with a semi-dwarf habit. Most modern wheat cultivars are semi-dwarf, and this is an important agronomic trait that reduces lodging and improves the harvest index. The pairing of homeologous chromosomes resulting in recombination in the absence of ph1c can occur where typically a whole chromosome arm is translocated to generate a hybrid chromosome (Friebe et al., 1996). Further analysis of the T lines with molecular markers and by genome in situ hybridizations is underway to establish the nature of the putative translocation in these lines.
Previous studies found that the full 4D chromosome is poorly transmitted through pollen, resulting in non-Mendelian segregation in F1 progeny arising from crosses when a male parent heterozygous for the 4D chromosome is used as the donor of the 4D chromosome (Dvorak and Gorham, 1992). This is potentially a problem for breeding and may require a large number of crosses at each backcross to ensure that at least some F1 plants possess the 4D chromosome. However, in the current study, transmission of the 4D chromosome into the ‘Jandaroi’ genetic background was not compromised and appeared to follow a 1:1 segregation at each backcross, although the numbers were too small for statistical analysis (Table 1). Analysis of the F2 generation at the various backcrosses showed that inheritance of the 4D chromosome followed Mendelian genetics, which provided further evidence that transmission of the 4D chromosome through pollen was not compromised in these lines.
Although the 4D chromosomal fragment improved seminal root growth of durum plants grown in acid soil, the growth of fine roots was inhibited (Fig. 4). In contrast, growth of fine roots in Al3+-tolerant hexaploid lines in these same soils was only marginally restricted (Fig. 4). This can be attributed in part to the greater sensitivity of the fine roots towards Al3+ for the durum lines, as found in hydroponic culture when the T line is compared with ‘Chinese Spring’ at the two highest Al3+ treatments (Fig. 2D). Greater sensitivity of the durum lines was also apparent from root diameters, where the T lines had thicker roots than ‘Chinese Spring’ in the Al3+ treatments. Thickening of roots is a typical symptom of Al3+ toxicity along with reduced root elongation. ‘Chinese Spring’ possesses an Al3+ tolerance locus on chromosome 3B in addition to TaALMT1 on chromosome 4D (Navakode et al., 2009) and this can explain its greater tolerance than the durum lines. Fine root growth of durum lines in soil was more severely affected than in hydroponics, and the T line was inhibited to a similar extent as the S line even in a limed treatment where seminal root growth of the S line was inhibited by about 50 % (Fig. 4, lime treatment of 1 g kg–1). A difference in growth of fine roots between T and S lines was apparent on a soil that was only marginally toxic to seminal root growth (<20 % inhibition of the S line; Supplementary Data Fig. S3), indicating that although the TaALMT1 locus protected these roots it appeared not to be as effective as the protection afforded to seminal roots. It is possible that some factor other than Al3+ toxicity also inhibited fine root development of the durum lines grown in acid soil. Acid soils can also possess high concentrations of soluble Mn2+ that can be toxic to plants. Although Mn2+ toxicity is usually apparent in shoots, in a previous study root growth of two durum lines was also sensitive to Mn2+ when compared with hexaploid wheat (Khabaz-Saberi et al., 2010).
Another phenotype associated with Al3+ toxicity in wheat is the inhibition of root hair development that can occur despite the root system maintaining its ability to elongate in the presence of Al3+. Variation in Al3+ tolerance of root hairs was recently described for hexaploid wheat, and this trait is controlled by genes distinct from TaALMT1 (Delhaize et al., 2012a). Rhizosheath size was found to be a reliable surrogate for root hair length in hexaploid germplasm grown on acid soil, and in the current study we found that all durum lines had small rhizosheaths on acid soil regardless of whether they possessed the 4D chromosomal fragment or not. This finding is consistent with TaALMT1 not being the major determinant for the Al3+ tolerance of root hairs, as found previously.
Analysis of the T and S lines for plant height and grain yield in pot trials indicated that the pairs of lines were similar for these attributes. However, despite having similar grain yields, the T lines had smaller grains. This suggests that a locus or loci on the introgressed 4D chromosomal fragment was conferring this attribute. Grain size can influence the early vigour of seedlings, and small grains in this context are undesirable. Since spike numbers were similar for both T and S lines, we calculated that the T line generated more grains per spike than the S line, resulting in similar total grain yield.
Although we have shown that a 4D chromosomal fragment derived from hexaploid wheat has the potential to enhance the tolerance of durum wheat to two major abiotic stresses, further improvements are possible. Hexaploid germplasm possesses other genes that confer Al3+ tolerance to root growth and, although the effects of these are minor compared with TaALMT1, it may be possible to combine them with TaALMT1 to enhance root growth further on acid soils. For instance, TaMATE1B was found to confer Al3+ tolerance by citrate efflux and the gene is located on chromosome 4B (Tovkach et al., 2013). Introgression of this gene into durum should be straightforward using a molecular marker, but would require that it be recombined with the 4D/4B chimeric chromosome in the current germplasm. Further work is being undertaken using molecular markers to define the size of the 4D chromosomal fragment introgressed into the T lines which will indicate whether recombining the TaMATE1B and TaALMT1 genes on the same chromosome is a viable strategy. In addition, root hair development was severely inhibited by Al3+ in the durum lines, and the hexaploid wheat ‘Fronteira’ is a potential source of tolerance. Root hairs are important for the uptake of mineral nutrients, particularly phosphate, and maintaining long root hairs in acid soil will be important for ensuring efficient use of fertilizers. There is no direct evidence to indicate that the smaller grain size of the T lines was due to TaALMT1, and it is more likely to be caused by another locus or loci on the 4D chromosomal fragment. To circumvent this potential problem, we have initiated crosses using a ph1c mutant with the aim of introgressing a smaller fragment of the 4D chromosome that may avoid the locus or loci responsible for small grain size. Finally, it will be important to assess the germplasm in the field on acid and saline sites as well as establishing the effect that the chromosomal fragment has on grain quality.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Dr Richard James for providing advice on the Na+ exclusion assays. C.H. thanks the China Scholarship Council for financial support to undertake this work.
LITERATURE CITED
- Aniol A. Genetics of tolerance to aluminium in wheat (Triticum aestivum L. Thell). Plant and Soil. 1990;123:223–227. [Google Scholar]
- Aniol A, Gustafson JP. Chromosome location of genes controlling aluminum tolerance in wheat, rye, and triticale. Canadian Journal of Genetics and Cytology. 1984;26:701–705. [Google Scholar]
- Balmas V, Burgess LW, Summerell BA. Reaction of durum wheat cv. Yallaroi to crown and root rot caused by Fusarium graminearum Group 1 and Fusarium crookwellense. Australasian Plant Pathology. 1995;24:233–237. [Google Scholar]
- Bona L, Wright RJ, Baligar VC, Matuz J. Screening wheat and other small grains for acid soil tolerance. Landscape and Urban Planning. 1993;27:175–178. [Google Scholar]
- Bona L, Baligar VC, Wright RJ. Soil acidity effects on agribotanical traits of durum and common wheat. In: Date RA, Grundon NJ, Rayment GE, Probert ME, editors. Plant–soil interactions at low pH: principals and management. Dordrecht: Kluwer; 1995. pp. 425–428. [Google Scholar]
- Bryan GJ, Dixon A, Gale MD, Wiseman G. A PCR-based method for the detection of hexaploid bread wheat adulteration of durum wheat and pasta. Journal of Cereal Science. 1998;28:135–145. [Google Scholar]
- Camargo CEDeO, DosSantos RR, Pettinelli A. Durum wheat: tolerance to aluminum toxicity in nutrient solution and in the soil. Bragantia. 1992;51:69–76. [Google Scholar]
- Cosic T, Poljak M, Custic M, Rengel Z. Aluminium tolerance of durum wheat germplasm. Euphytica. 1994;78:239–243. [Google Scholar]
- Dai SF, Yan ZH, Liu DC, Wei YM, Zheng YL. Comparative analysis of six Triticum turgidum L. subspecies for acid and aluminum tolerance.Agricultural Science in China. 2010;9:642–650. [Google Scholar]
- Delhaize E, Ryan PR, Hebb DM, Yamamoto Y, Sasaki T, Matsumoto H. Engineering high-level aluminum tolerance in barley with the ALMT1 gene. Proceedings of the National Academy of Sciences; USA. 2004. pp. 15249–15254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, James RA, Ryan PR. Aluminium tolerance of root hairs underlies genotypic differences in rhizosheath size of wheat (Triticum aestivum) grown on acid soil. New Phytologist. 2012a;195:609–619. doi: 10.1111/j.1469-8137.2012.04183.x. [DOI] [PubMed] [Google Scholar]
- Delhaize E, Ma JF, Ryan PR. Transcriptional regulation of aluminium tolerance genes. Trends in Plant Science. 2012b;17:341–348. doi: 10.1016/j.tplants.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Dubcovsky J, Santa MG, Epstein E, Luo MC, Dvorak J. Mapping of the K+/Na+ discrimination locus Kna1 in wheat. Theoretical and Applied Genetics. 1996;2:448–454. doi: 10.1007/BF00223692. [DOI] [PubMed] [Google Scholar]
- Dvorak J, Gorham J. Methodology of gene transfer by homoeologous recombination into Triticum turgidum: transfer of K+/Na+ discrimination from T. aestivum. Genome. 1992;35:639–646. [Google Scholar]
- Foy CD. Tolerance of durum wheat lines to an acid, aluminium-toxic subsoil. Journal of Plant Nutrition. 1996;19:1381–1394. [Google Scholar]
- Foy CD, da Silva AR. Tolerances of wheat germplasm to acid subsoil. Journal of Plant Nutrition. 1991;14:1277–1295. [Google Scholar]
- Friebe B, Jiang J, Raupp WJ, McIntosh RA, Gill BS. Characterizations of wheat-alien translocations conferring resistance to diseases and pests: current status. Euphytica. 1996;91:59–87. [Google Scholar]
- Gorham J, Wyn Jones RG, Bristol A. Partial characterization of the trait for enhanced K+–Na+ discrimination in the D genome of wheat. Planta. 1990;180:590–597. doi: 10.1007/BF02411458. [DOI] [PubMed] [Google Scholar]
- Joppa LR. Chromosome engineering in tetraploid wheat. Crop Science. 1993;33:908–913. [Google Scholar]
- Joppa LR, Williams ND. Langdon durum disomic substitution lines and aneuploid analysis in tetraploid wheat. Genome. 1988;30:222–228. [Google Scholar]
- Khabaz-Saberi H, Rengel Z, Wilson R, Setter TL. Variation of tolerance to manganese toxicity in Australian hexaploid wheat. Journal of Plant Nutrition and Soil Science. 2010;173:103–112. [Google Scholar]
- Luo MC, Dvorak J. Molecular mapping of an aluminum tolerance locus on chromosome 4D of Chinese Spring wheat. Euphytica. 1996;91:31–35. [Google Scholar]
- Luo MC, Dubcovsky J, Goyal S, Dvorak J. Engineering of interstitial foreign chromosome segments containing the K+/Na+ selectivity gene Kna1 by sequential homoeologous recombination in durum wheat. Theoretical and Applied Genetics. 1996;93:1180–1184. doi: 10.1007/BF00230144. [DOI] [PubMed] [Google Scholar]
- Ma H-X, Bai G-H, Carver BF, Zhou l-L. Molecular mapping of a quantitative trait locus for aluminum tolerance in wheat cultivar Atlas 66. Theoretical and Applied Genetics. 2005;112:51–57. doi: 10.1007/s00122-005-0101-5. [DOI] [PubMed] [Google Scholar]
- McNeil D, Lagudah ES, Hohmann U, Appels R. Amplification of DNA sequences in wheat and its relatives: the Dgas44 and R350 families of repetitive sequences. Genome. 1994;37:320–327. doi: 10.1139/g94-044. [DOI] [PubMed] [Google Scholar]
- Mesdag J, Slootmaker AJ. Classifying wheat varieties for tolerance to high soil acidity. Euphytica. 1969;18:36–42. [Google Scholar]
- Moustakas M, Yupsanis T, Symeonidis L, Karataglis S. Aluminum toxicity effects on durum wheat cultivars. Journal of Plant Nutrition. 1992;15:627–638. [Google Scholar]
- Munns R, Hare RA, James RA, Rebetzke GJ. Genetic variation for improving the salt tolerance of durum wheat. Australian Journal of Agricultural Research. 2000;51:69–74. [Google Scholar]
- Navakode S, Weidner A, Lohwasser U, Röder MS, Börner A. Molecular mapping of quantitative trait loci (QTLs) controlling aluminium tolerance in bread wheat. Euphytica. 2009;166:283–290. [Google Scholar]
- Papernik LA, Bethea AS, Singleton TE, Magalhaes JV, Garvin DF, Kochian LV. Physiological basis of reduced Al tolerance in ditelosomic lines of Chinese Spring wheat. Planta. 2001;212:829–834. doi: 10.1007/s004250000444. [DOI] [PubMed] [Google Scholar]
- Pereira JF, Zhou GF, Delhaize E, Richardson T, Zhou MX, Ryan PR. Engineering greater aluminium resistance in wheat by over-expressing TaALMT1. Annals of Botany. 2010;106:205–214. doi: 10.1093/aob/mcq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman H, Zhang K, Cakir M, et al. Molecular mapping and characterization of ALMT1, the aluminium-tolerance gene of bread wheat (Triticum aestivum L.) Genome. 2005;48:781–791. doi: 10.1139/g05-054. [DOI] [PubMed] [Google Scholar]
- Riede CR, Anderson JA. Linkage of RFLP markers to an aluminum tolerance gene in wheat. Crop Science. 1996;36:905–909. [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ. Characterisation of Al-stimulated efflux of malate from the apices of Al-tolerant wheat roots. Planta. 1995;196:103–110. [Google Scholar]
- Ryan PR, Raman H, Gupta S, Horst WJ, Delhaize E. A second mechanism for aluminum resistance in wheat relies on the constitutive efflux of citrate from roots. Plant Physiology. 2009;149:340–351. doi: 10.1104/pp.108.129155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Raman H, Gupta S, Sasaki T, Yamamoto Y, Delhaize E. The multiple origins of aluminium resistance in hexaploid wheat include Aegilops tauschii and more recent cis mutations to TaALMT1. The Plant Journal. 2010;64:446–455. doi: 10.1111/j.1365-313X.2010.04338.x. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Yamamoto Y, Ezaki B, et al. A wheat gene encoding an aluminum-activated malate transporter. The Plant Journal. 2004;37:645–653. doi: 10.1111/j.1365-313x.2003.01991.x. [DOI] [PubMed] [Google Scholar]
- Scott BJ, Conyers MK, Poile GJ, Cullis BR. Subsurface acidity and liming affect yield of cereals. Australian Journal of Agricultural Research. 1997;48:843–854. [Google Scholar]
- Slootmaker LAJ. Tolerance to high soil acidity in wheat related species, rye and triticale. Euphytica. 1974;23:505–513. [Google Scholar]
- Tovkach A, Ryan PR, Richardson AE, et al. Transposon-mediated alteration of TaMATE1B expression in wheat confers constitutive citrate efflux from root apices. Plant Physiology. 2013;161:880–892. doi: 10.1104/pp.112.207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Uexküll HR, Mutert E. Global extent, development and economic impact of acid soils. Plant and Soil. 1995;171:1–15. [Google Scholar]
- Watt M, McCully ME, Canny MJ. Formation and stabilization of rhizosheaths of Zea mays L. Effect of soil-water content. Plant Physiology. 1994;106:179–186. doi: 10.1104/pp.106.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Sasaki T, Sivaguru M, et al. Evidence for the plasma membrane localization of Al-activated malate transporter (ALMT1) Plant and Cell Physiology. 2005;46:812–816. doi: 10.1093/pcp/pci083. [DOI] [PubMed] [Google Scholar]
- Zubaidi A, McDonald GK, Hollamay GJ. Shoot growth, root growth and grain yield of bread and durum wheat in South Australia. Australian Journal of Experimental Agriculture. 1999;39:709–720. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


