Abstract
Background
The cyanobacterial genus Nostoc includes several species forming centimetre-large gelatinous colonies in nutrient-poor freshwaters and harsh semi-terrestrial environments with extended drought or freezing. These Nostoc species have filaments with normal photosynthetic cells and N2-fixing heterocysts embedded in an extensive gelatinous matrix of polysaccharides and many other organic substances providing biological and environmental protection. Large colony size imposes constraints on the use of external resources and the gelatinous matrix represents extra costs and reduced growth rates.
Scope
The objective of this review is to evaluate the mechanisms behind the low rates of growth and mortality, protection against environmental hazards and the persistence and longevity of gelatinous Nostoc colonies, and their ability to economize with highly limiting resources.
Conclusions
Simple models predict the decline in uptake of dissolved inorganic carbon (DIC) and a decline in the growth rate of spherical freshwater colonies of N. pruniforme and N. zetterstedtii and sheet-like colonies of N. commune in response to a thicker diffusion boundary layer, lower external DIC concentration and higher organic carbon mass per surface area (CMA) of the colony. Measured growth rates of N. commune and N. pruniforme at high DIC availability comply with general empirical predictions of maximum growth rate (i.e. doubling time 10–14 d) as functions of CMA for marine macroalgae and as functions of tissue thickness for aquatic and terrestrial plants, while extremely low growth rates of N. zetterstedtii (i.e. doubling time 2–3 years) are 10-fold lower than model predictions, either because of very low ambient DIC and/or an extremely costly colony matrix. DIC uptake is limited by diffusion at low concentrations for all species, although they exhibit efficient HCO3– uptake, accumulation of respiratory DIC within the colonies and very low CO2 compensation points. Long light paths and light attenuation by structural substances in large Nostoc colonies cause lower quantum efficiency and assimilation number and higher light compensation points than in unicells and other aquatic macrophytes. Extremely low growth and mortality rates of N. zetterstedtii reflect stress-selected adaptation to nutrient- and DIC-poor temperate lakes, while N. pruniforme exhibits a mixed ruderal- and stress-selected strategy with slow growth and year-long survival prevailing in sub-Arctic lakes and faster growth and shorter longevity in temperate lakes. Nostoc commune and its close relative N. flagelliforme have a mixed stress–disturbance strategy not found among higher plants, with stress selection to limiting water and nutrients and disturbance selection in quiescent dry or frozen stages. Despite profound ecological differences between species, active growth of temperate specimens is mostly restricted to the same temperature range (0–35 °C; maximum at 25 °C). Future studies should aim to unravel the processes behind the extreme persistence and low metabolism of Nostoc species under ambient resource supply on sediment and soil surfaces.
Keywords: Gelatinous colonies, cyanobacteria, Nostoc commune, Nostoc flagelliforme, Nostoc pruniforme, Nostoc zetterstedtii, carbon use, carbon concentrating mechanisms, photosynthesis, light use, growth, long-lived, survival, desiccation tolerance, nutrient-poor
INTRODUCTION
The cyanobacterial genus Nostoc includes many species that are highly diverse with respect to morphology, functional properties, biotic relations and habitat distribution (Dodds et al., 1995). Nostoc species have filaments with normal photosynthetic cells and N2-fixing heterocysts and they periodically form resistant akinetes for survival and short motile filaments (hormogonia) for reproduction (Mollenhauer et al., 1994). Some species are free-living and many species engage in loose or obligate cooperation with land plants and fungi (e.g. lichens; Mollenhauer, 1988; Kaasalainen et al., 2012). A third, fascinating Nostoc type forms large gelatinous colonies of variable shape and structure in rice fields, freshwater lakes, ponds and streams and on alternating wet and dry soils or rock surfaces (Dodds et al., 1995; Gao et al., 2012). The large gelatinous species require special adaptations to obtain sufficient light, nutrients and dissolved inorganic carbon (DIC) (Sand-Jensen et al., 2009b) in water and to survive the extreme variations in temperature, water supply and irradiance on naked soils and rock surfaces (Tamaru et al., 2005; Li et al., 2011; Yu and Liu, 2013). While phototrophs in resource-rich environments have been treated in numerous original papers, reviews and books (Larcher, 2003; Lambers et al., 2008), studies on the adaptations of phototrophs to resource-poor and physically extreme environments are rare. The objective of this review is to evaluate the mechanisms behind the extremely low rates of growth and mortality and high persistence, longevity and protection against environmental hazards of gelatinous Nostoc colonies and their ability to economize with highly limiting resources.
The review focuses on four colonial Nostoc species: the sheet-like Nostoc commune, the closely related N. flagelliforme and two species that form spherical colonies, N. pruniforme and N. zetterstedtii. All four species resemble each other by forming centimetre-large gelatinous colonies, but they differ in distribution, habitat preference, growth rate, environmental tolerance and appearance (Fig. 1). Nostoc commune is a globally widespread terrestrial and semi-aquatic species forming inactive crusts when dry, but rapidly changing to 1–5-mm thick sheet-like gelatinous structures when wet (Scherer et al., 1984; Marsh et al., 2006; Novis et al., 2007). Nostoc commune has unprecedented tolerance of environmental extremes (Satoh et al., 2002; Tamaru et al., 2005; Sand-Jensen and Jespersen, 2012). It tolerates desiccation during dry seasons and can survive for >100 years on a museum shelf (Cameron, 1962). It tolerates freezing to –60 °C in Antarctic deserts (Davey, 1989; Novis et al., 2007) and to –269 °C in liquid helium in the laboratory (Sand-Jensen and Jespersen, 2012), but rapidly resumes metabolism when rewetted at physiologically suitable temperatures between 0 and 30 °C (Scherer et al., 1984; Taranto et al., 1993; Sand-Jensen and Jespersen, 2012; Møller et al., 2014). Nostoc flagelliforme is also widely distributed in arid and semi-arid areas throughout the world (Gao et al., 2012; Ye et al., 2012). It has a thread-like appearance but resembles N. commune in its genetics, growth habitat, colony composition and extreme tolerance to and rapid recovery from desiccation, freezing and salt stress (Gao and Zou, 2001; Qiu et al., 2004; Huang et al., 2005; Yong-Hong et al., 2005; Liu et al., 2010; Arima et al., 2011). We have observed Nostoc with the typical morphology of both N. commune and N. flagelliforme on the Great Alvar on Öland, Sweden (Sand-Jensen et al., 2010). Nostoc flagelliforme has been classified as a variety of N. commune in arid regions of Spain (Aboal et al., 2010) and recent DNA studies group those two ‘species’ together with N. sphaeroides and N. punctiforme in a common clade (Arima et al., 2011; Gao et al., 2011), which makes species identity dubious. Nonetheless, we maintain the distinction between N. commune and N. flagelliforme here in accordance with most available literature.
Fig. 1.

Images of colonies of rehydrated Nostoc commune (upper left), Nostoc zetterstedtii (upper right, usually more spherical) and N. pruniforme (lower middle).
Nostoc pruniforme forms a beautiful, dark green, spherical colony with a smooth surface like a plume. It is more common and widely distributed both geographically and ecologically in oligo- and mesotrophic freshwaters in temperate and sub-Arctic regions than its rare freshwater cousin, N. zetterstedtii (Dodds et al., 1995; Raun et al., 2009). Danish alkaline lakes housing N. pruniforme contain >0·7 mm DIC (Sand-Jensen et al., 2009b). In temperate lakes, N. pruniforme appears to grow in diameter from ∼0·2 cm in late spring to 2–3 cm in midsummer and it can rapidly die off, supposedly because of attacks by viruses or bacteria at high summer temperatures (K. Sand-Jensen, unpubl. data). In contrast, in a cold (4 °C), nutrient-poor spring in Oregon, USA, N. pruniforme reached a handball size of 15–17 cm in diameter and weighed 2·6 kg after sustained slow growth for 9–14 years (Dodds and Castenholz, 1987). Very large colonies are also found in cold transparent lakes in Greenland (K. Sand-Jensen, unpubl. data). In addition to their larger size and persistence, they differ from Danish temperate specimens by having a more solid surface and a denser gel. Two additional species, N. calcareous and N. sphaericus (Mollenhauer et al., 1994) resemble N. pruniforme but are not discussed further here.
The fourth species, N. zetterstedtii, is a rare, highly persistent organism whose colonies resemble firm blackberries. It lives in only 50–100 nutrient-poor soft-water lakes in Sweden and in a few other lakes in neighbouring countries (Bengtsson, 1986, 1995; Mollenhauer et al., 1999). Soft-water lakes usually have DIC concentrations of 0·02–0·3 mm and concentrations of total phosphorus <0·1–0·3 μm in open waters (Sand-Jensen et al., 2009b). Among the freshwater phototrophic species studied so far, Nostoc zetterstedtii has unprecedented low growth and mortality rates (Sand-Jensen and Møller, 2011). In oligotrophic Lake Värsjö, Sweden, it takes ∼3 years for N. zetterstedtii to double its colony mass and ∼30 years to reach the recorded maximum diameter of 7 cm (Sand-Jensen and Møller, 2011). There is no apparent mortality of grazers and pathogenic bacteria and viruses (Sand-Jensen and Møller, 2011). The persistence of N. zetterstedtii is confirmed by its ability to survive for 14 months in the dark at 5 °C with intact colony structure and unaltered fresh mass (K. Sand-Jensen, unpubl. data).
All mentioned Nostoc species must have common structural and functional properties linked to the large size and extensive matrix of the colonies. However, the species differ with respect to metabolism, growth, mortality, tolerance to environmental stress and habitat preference. In this review, I first examine some of the main structural properties of the three main species (N. commune, N. pruniforme and N. zetterstedtii) with supplementary data for N. flagelliforme related to the centimetre-large size and gelatinous matrix of the colonies. Second, I evaluate theoretically the diffusive flux of DIC to the colony surface for a set of environmental constraints on boundary layer thickness and external concentrations. Third, I examine the functional consequences of the large colony size for the use of DIC and light in photosynthesis and growth. Fourth, I compare the tolerance of Nostoc species to environmental stresses related to temperature, freezing and desiccation. Fifth, I evaluate the growth, mortality and longevity of the three Nostoc species and the ecological implications of these properties. The final section points out that costs and benefits of colony formation should be quantified in future studies. For this comparative analysis of the eco-physiology of gelatinous Nostoc colonies, I use published data and include supplementary unpublished data to ensure a comprehensive comparison.
SIZE, SHAPE AND COMPOSITION
Centimetre-thick colonies with photosynthetic filaments embedded in a gelatinous matrix exert large physical constrains on functional properties.
Size and shape
Large size of phototrophs imposes constraints on the use of light and uptake of inorganic carbon and nutrient ions from the surrounding water (Nielsen and Sand-Jensen, 1990; Agusti et al., 1994). For a body of a given shape, surface area (SA ≈ L2) scales to the second power of the linear dimension (L), volume (V ≈ L3) to the third power, and surface area:volume ratio (SA:V ≈ L–1) to the inverse of the linear dimension. These allometric relationships reflect that the supply rates of light and external resources are scaled to the outer surface while the requirements for production and maintenance of the cells and the gelatinous matrix are mainly scaled to the volume.
The spherical shape adopted by N. pruniforme and N. zetterstedtii is a simple shape of low SA:V ratio (3 r–1, where r is the radius of the sphere). Individual filaments of Nostoc embedded in the gelatinous matrix and free-living filaments of cyanobacteria and algae are cylindrical and maintain a high SA:V ratio inversely scaled to the transverse radius of the filament (2 r–1) but independent of filament length. The SA:V ratio of the flat sheet-like structure of Nostoc commune, multicellular algae and leaves of mosses and higher plants is inversely scaled to thickness (Lth) of the structure (SA:V = 2 Lth–1) but is independent of its surface area, volume and total mass as long as tissue thickness remains constant.
The gelatinous colonies of N. commune, N. pruniforme and N. zetterstedtii all have relatively thick tissues (0·1–17 cm) and very low SA:V ratios (0·4–20 cm–1) compared with the thin Nostoc trichomes within the gel (SA:V 4000–8000 cm–1) and the 0·01- to 0·3 cm-thick submerged leaves of mosses and angiosperms (SA:V 70–2000 cm–1) (Table 1). Nostoc commune sheets typically vary in thickness from 0·1 to 0·5 cm when wet and have SA:V ratios of 2–20 cm–1. The smooth spherical colonies of N. pruniforme vary from a diameter of ∼0·2 cm and an SA:V ratio of 30 cm–1 to an astonishing maximum diameter of 17 cm and an exceedingly low SA:V ratio of only 0·36 cm–1. Small colonies of N. zetterstedtii are smooth, have a diameter of ∼0·2 cm, just like N. pruniforme, while large colonies, reaching a recorded maximum diameter of 7 cm, have a granulated surface that can be regarded as consisting of small hemispheres (exposed area 2 πr2 and planform area πr2) distributed all over a large spherical surface. The granulated surface area has a ∼2-fold larger surface area relative to a smooth surface and the SA:V ratio is only 1·7 cm–1 for the largest N. zetterstedtii colonies. The granulated surface could, in theory, double the diffusion-limited uptake of external solutes for N. zetterstedtii by doubling the surface area compared with smooth colonies of N. pruniforme of the same diameter (see section ‘Size effects, diffusive supply and loss of solutes’). The granulated surface could also contribute to the formation of thinner diffusion boundary layers (DBL) around the surfaces of N. zetterstedtii when the granules increase micro-turbulence (Boudreau and Jørgensen, 2001). The micro-topography of the granulated surface can result in thinner DBLs at the protruding tips of the granules and thicker DBLs in the crevices between the granules (Carpenter and Williams, 1993; Jørgensen, 2001) so the diffusion geometry is difficult to interpret. Although diffusive fluxes remain experimentally unexplored, they are, nonetheless, highly relevant considering that N. zetterstedtii usually grows in lakes with lower concentrations of DIC, N and P than N. pruniforme (Sand-Jensen et al., 2009b).
Table 1.
Shape, range of linear dimensions and surface area:volume ratio (SA:V) of three species of Nostoc colonies, Nostoc trichomes, submerged leaves and single phototrophic unicells
| Species and phototrophic type | Shape | Diameter/thickness (cm) | SA:V (cm–1) |
|---|---|---|---|
| N. commune | Flat structure | 0·1 | 10 to 20 |
| 0·5 | 2 to 4 | ||
| N. pruniforme | Sphere | 0·2 | 30 |
| 17·0 | 0·36 | ||
| Nostoc zetterstedtii | Sphere, granulated surface | 0·2 | 30 |
| 7·0 | 0·85–1·7 | ||
| N. trichomes | Cylinders | 5 × 10–4 | 8000 |
| 10 × 10–4 | 4000 | ||
| Submerged leaves | Flat structure | 1 × 10–3 | 2000 |
| 30 × 10–3 | 70 | ||
| Single-celled algae or cyanobacteria | Sphere | 2 × 10–4 | 30 000 |
| 2 × 10–3 | 3000 |
Composition of colony matrix
A high proportion of Nostoc colonies is composed of the extracellular matrix, primarily of polysaccharides of high viscosity and molecular weight. The hydrolysed polysaccharides from N. flagelliforme were mainly composed of glucose (43 %), galactose (30 %), xylose (21 %), mannose (6 %) and small amounts of glucuronic and uronic acids (Jia et al., 2007; Pereira et al., 2009). Much the same main sugars were hydrolysed from hot water extracts of polysaccharides from field samples of N. commune, N. flagelliforme and N. sphaeroides, though small amounts of arabinose appeared in N. flagelliforme and rhamnose and fucose in N. sphaeroides (Huang et al., 1998). The composition of extracellular compared with intracellular polysaccharides was suggested to be quite different in N. commune and N. flagelliforme, perhaps due to a selective mechanism for excretion of polysaccharides (Mehta and Vaidya, 1978). Different studies of N. commune have shown the complex chemistry of the hydrolysed matrix, which includes six to nine monosaccharides, uronic acid, deoxy-sugars, pyruvate, acetate and peptides (Pereira et al., 2009). Scanning electron microscopy has revealed a net-like matrix of polysaccharides around Nostoc filaments (Cupac and Gantar, 1992), suggesting that their ultrastructure could influence colony shape. The chemical structure, the 3-D network and the presence of special peripheral groups, such as nosturonic acid, are probably essential for the outstanding moisture absorption and moisture retention capacity of Nostoc polysaccharides. These properties are highly interesting in biomedical applications involving living cells and tissue (Yu et al., 2010; Li et al., 2011).
When the gelatinous matrix of N. commune, N. flagelliforme and N. pruniforme is hydrated its water content is very high, leading to a soft structure that is easy to cut with a finger nail, as opposed to the very solid structure of N. zetterstedtii, which can only be cut, with great force, using a scalpel. Summer colonies of N. pruniforme from mesotrophic Lake Esrum with a fresh mass (FM) of 3·5 g have a mass specific density of ∼1·01 g FM cm–3 while colonies of N. zetterstedtii from oligotrophic Lake Värsjö have a specific density of ∼1·13 g FM cm–3 (Raun et al., 2009; Sand-Jensen et al., 2009b). Both species have gradually decreasing mass specific density for larger colonies, which tend to be hollow and water-filled in the centre of N. pruniforme and possess a massive, though less dense, structure without trichomes in N. zetterstedtii (Table 2). The carbon mass per surface area [CMA, equivalent to leaf mass per surface area for plants (LMA)] is closely scaled to structural and functional properties of macroalgal thalli (Markager and Sand-Jensen, 1994, 1996) and plant leaves (Nielsen and Sand-Jensen, 1989, 1991; Lambers and Poorter, 1992, 2004; Poorter et al., 2012). The CMA increased by a power of ∼0·25 relative to colony FM for N. zetterstedtii and only 0·05 for N. pruniforme, because the first species maintains massive colonies and the second species tends to develop hollow colonies with increasing size (Table 2). Thus, in two summer collections, CMA was ∼8-fold higher for N. zetterstedtii than N. pruniforme for colonies of 0·1 g FM, while the difference was ∼14-fold for colonies of 3·5 g FM.
Table 2.
Organic carbon content per surface area (CMA, μmol C cm–2) relative to colony fresh mass (FM,g) of three Nostoc species
| Species | Relationship | Source |
|---|---|---|
| N. commune | CMA = 184 ± 47 (mean ± s.e.) | K. Sand-Jensen, unpubl. data |
| N. pruniforme | Log CMA = 0·0474 log FM + 1·88 | C. Møller and K. Sand-Jensen, unpubl. data |
| CMA for 0·1 g FM colony = 69 | ||
| CMA for 4·0 g FM colony = 81 | ||
| N. zetterstedtii | Log CMA = 0·26 log FM + 3·13 | Sand-Jensen et al. (2009) Sand-Jensen and Møller (2011) |
| CMA for 0·1 g FM colony = 587 | ||
| CMA for 4·0 g FM colony = 1531 |
The typical differences in CMA values for 3·5 g FM colonies of N. zetterstedtii (∼1400 μmol organic C cm–2) and N. pruniforme (∼100 μmol organic C cm–2) have important implications. Assuming the same net photosynthesis relative to colony surface area of both species and the same proportion of net photosynthesis being incorporated into new biomass, the turnover rate of the colony mass will be 14-fold lower for N. zetterstedtii than for N. pruniforme. With the same assumption of constant photosynthesis per surface area independent of colony size, biomass turnover and relative growth rate should decline with colony weight in proportion to the increase in CMA; i.e. an 8- to 9-fold decline across the range of colony weights of N. zetterstedtii from 0·1 to 4 g FM. We have recorded a decline in the relative growth rate and the surplus of photosynthesis minus respiration per colony mass with increasing colony size in N. pruniforme, but did not find the same strong size dependence for N. zetterstedtii (Sand-Jensen and Møller, 2011). We propose that allocation to extracellular products and their diffusive loss to the environment may decline with colony size and that these processes are relatively more important for the metabolically less active N. zetterstedtii. Reduced loss of exudates with higher colony size could counteract the expected decline in growth rate in larger colonies (see section ‘Size effects, diffusive supply and loss of solutes’).
As emphasized, a very high proportion of the colony volume and fresh mass is made up of the water-rich colony matrix compared with the denser Nostoc filaments. Still very high proportions of the carbon content of the colony are bound in the matrix. In N. commune, polysaccharides in the matrix constitute >60 % of the colony dry mass (Hill et al., 1997). Among spherical Nostoc colonies, filaments are confined to an outer shell, which occupies a falling proportion of the volume with increasing colony size, such that the contribution of the matrix to the total organic content should also increase. If we assume that the organic carbon:chlorophyll a mass quotient in Nostoc filaments has a typical range of 30–50 at 15 °C and moderate irradiances (Geider, 1987; his Fig. 13), we estimate that the matrix constitutes 18–45 % of the organic carbon mass in the smallest colonies of N. pruniforme, while it is 37–62 % in large colonies of 3·5 g FM. The proportion of organic carbon in the matrix is much higher in N. zetterstedtii, being ∼50–70 % in small colonies weighing a few milligrams and ∼90 % in large colonies weighing 3·5 g FM. The smaller proportion of living Nostoc filaments in the colony mass in N. zetterstedtii than in N. pruniforme will, as already mentioned, result in less photosynthetic production of new organic material relative to colony mass and therefore much lower growth rates for N. zetterstedtii than for N. pruniforme.
Functional properties of colony matrix
The gelatinous matrix of Nostoc colonies is composed of a complex mixture of polysaccharides (Bertocchi et al., 1990; Yousimura et al., 2012), which is responsible for forming and maintaining the colony shape and protecting the cells against environmental hazards and pathogens (Tamaru et al., 2005; Knowles and Castenholz, 2008). It is fascinating, yet unexplored, how the different colony shapes come about. A variety of disaccharides, including sucrose and non-reducing trehalose, also participate in the protection of cells and matrix (Caiola et al., 1996; Hill et al., 1997; Potts, 1994, 1999, 2000). Poly- and disaccharides protect membranes and macromolecules against freezing, heating, desiccation and salt stress (Tamaru et al., 2005; Sakamoto et al., 2009; Yoshida and Sakamoto, 2009).
The formation and accumulation of trehalose in N. flagelliforme and N. punctiforme are under strict regulation in response to water limitation (Yong-Hong et al., 2005; Yoshida and Sakamoto, 2009), presumably because trehalose is an essential, though costly, product that diverts organic carbon away from growth processes. The functional importance of the gel is supported by the observation that strains of N. commune with no gel and strains where the gel has been physically removed from the filaments become susceptible to injury (Tamaru et al., 2005). Addition of exogenous polysaccharides to endolithic Nostoc sp. and Chlorella sp. increases their tolerance to desiccation (Knowles and Castenholz, 2008), probably by retaining a water layer around the cells and preventing membranes from cracking and macromolecules from undergoing destructive conformational changes. The main polysaccharides observed in the colony gel of N. commune have a strong capacity for in vitro moisture absorption and water retention, permitting a >10-fold increase in mass when desiccated colonies become rehydrated (Li et al., 2011; Sand-Jensen and Jespersen, 2012).
The gel has a strong adsorption capacity for heavy metal ions that are toxic to cyanobacteria in free ionic form (Bender et al., 1994; De Philippis et al., 2007; Pereira et al., 2009). Non-reducing sugars, UV-screening pigments and water stress proteins also assist in the environmental protection of Nostoc colonies (Hill et al., 1997; Potts, 1999). In response to increasing intensity and duration of UV exposure of N. flagelliforme, contents of protective scytonemin and mycosporine-like amino acids increase (Büdel et al., 1997; Yu and Liu, 2013). A large number (573) of the genes that were probed (6903) in N. punctiforme responded significantly to UVA exposure, 473 genes being up-regulated and only 100 being down-regulated (Soule et al., 2013). As expected, genes encoding antioxidant enzymes and the sunscreen scytonemin were up-regulated, while many genes involved in the synthesis of photosynthetic pigments were down-regulated. Also, Nostoc polysaccharides are capable of reducing oxidative damage by scavenging superoxide anions and hydroxyl radicals (Li et al., 2011).
Antibiotic production has been recorded in many Nostoc species (de Cano et al., 1986; Bloor and England, 1991; Gromov et al., 1991), including gelatinous colonies (Hameed et al., 2013). When N. zetterstedtii was homogenized in water and mixed 1:10 v/v with natural lake water containing bacteria, DNA synthesis stopped immediately (Sand-Jensen et al., 2009a). The polysaccharide nostoflan, isolated from N. flagelliforme, has in vitro and in vivo antiviral effects on a variety of enveloped viruses, including influenza A virus (Kanekiyo et al., 2005, 2007, 2008). Symbiotic Nostoc in a great variety of lichens either contains toxic microcystins or has the genes for producing them in 12 % of 803 lichen specimens collected worldwide (Kaasalainen et al., 2012). Microcystins occurred in 52 different variants in the 803 lichen specimens (Kaasalainen et al., 2012); they are small cyclic peptides and are known to be highly toxic to eukaryotes by inhibiting protein phosphatases (MacKintosh et al., 1990). Microcystins are active at multiple sites with gradually declining effects on nitrogenase activity, respiration, photosynthesis and growth of competing cyanobacteria. Three different classes of peptide compounds (anabaenopeptin, cryptophycin and nostocyclopeptides) could protect the toxin-producing cyanobacteria against bacteria, viruses, fungi, fish and ducks and could be deadly to mice and dogs around paddy fields (Nowruzi et al., 2012). Their most important ecological effects, however, involve the inhibition of pathogenic viruses and bacteria, potential grazers and competing phototrophs, not mice and dogs.
SIZE EFFECTS, DIFFUSIVE SUPPLY AND LOSS OF SOLUTES
As organisms increase in size, diffusion gradually becomes a less efficient means of supplying external dissolved substances.
Size effects
Because resource uptake in phototrophic organisms scales to surface area while resource demand scales to volume and mass, a first principle dictates that relative growth rate scales to SA:V and CMA (Nielsen and Sand-Jensen, 1990; Lambers and Poorter, 1992, 2004). Studies on N. pruniforme and N. zetterstedtii show that chlorophyll content and photosynthetic capacity relative to surface area are approximately independent of colony size because the Nostoc trichomes are mainly confined to the 1–2 mm-thick outer periphery of the spherical colony, while its dark central part is mostly devoid of trichomes and photosynthetic activity (Raun et al., 2009; Sand-Jensen et al., 2009b). If Nostoc colonies produce hollow spheres with an outer shell of uniform thickness and a water-filled central cavity, CMA would be independent of colony size, just as in the uniformly thick thalli of many macroalgae, plant leaves and the sheet-like structure of N. commune. This is also almost the situation for N. pruniforme in summer collections in Danish lakes, where CMA increases with colony mass to a power of only ∼0·05, corresponding to a 1·3-fold increase in CMA for a 100-fold increase in colony FM (Table 2). Spherical colonies could in this case maintain an almost unaltered growth rate with increasing size provided the extent of diffusive supply of limiting DIC and nutrients remained constant relative to surface area, or that this diffusive flux was not rate-limiting. Because colonies of N. zetterstedtii are massive, CMA is high and increases by a power of 0·75 with respect to colony size and thereby should contribute to a low and declining growth rate in larger colonies.
For growth performance, it is therefore crucial how resource fluxes and mass density respond to changes in environmental conditions and colony size and how photosynthates are allocated between new cells, colony matrix and protective organic compounds.
Resource supply by diffusion and turbulence
The diffusive flux (Fa) of a dissolved substance to a unit surface of a spherical colony is determined by colony size (e.g. radius r), thickness of the DBL surrounding the surface (δ), the concentration gradient across the boundary layer and the diffusion coefficient (D), according to eqn 1 (Table 3). The flux is highly dependent on size for small colonies, whereas for large colonies (r ≫ δ) the flux gradually approaches the two-dimensional situation for diffusion to a flat plate where the flux relative to surface area is independent of colony radius (eqn 2 in Table 3).
Table 3.
Equations 1–5 describe the diffusive flux relative to surface area (Fa), volume (Fv), shell volume (Fvs) and entire colony (Fcol) of smooth spherical colonies with a given radius (r) of a solute with diffusion coefficient D across a DBL (δ) with the concentration gradient Cm–Co, where Cm and Co are concentrations in the bulk medium and at the colony surface, respectively. Equation 6 is for a hollow sphere with an outer shell of thickness Ls. Equations 7 and 8 describe the concentration of a solute at the colony surface (Cr) produced at a rate of Pv per unit volume of a massive (eqn 7) or hollow colony (eqn 8) and lost by diffusion to stagnant external water of zero concentration
| No. | Equation |
|---|---|
| 1 | Fa = r–2 (r(r + δ) δ–1) D (Cm – Co) = r–1 (1 + r/δ) D (Cm – Co) |
| 2 | Fa ≈ δ–1 D (Cm – Co); (r ≫ δ) |
| 3 | Fa ≈ r–1 D Cm; (Co = 0) |
| 4 | Fcol = 4π r D Cm; (Co = 0) |
| 5 | Fv = 3 r–2 D Cm; (Co = 0) |
| 6 | Fvs ≈ r–1 Ls–1 D Cm; (Co = 0) |
| 7 | C (r) = 1/3 Pv D–1 r2; (Cm = 0) |
| 8 | C (r) ≈ Pv Ls r D–1; (Cm = 0) |
Equations are derived from Nobel (1983) and Denny (1993).
The flux of HCO3– was calculated to a unit surface of smooth spherical colonies like those of N. pruniforme (eqn 1) of variable size for realistic thicknesses (0·3, 1 and 3 mm) of the DBL in moving and quasi-stagnant water and differences in concentrations of 0·1, 0·5 and 2·0 mm (mm = mmol L–1 = μmol cm–3) between the external water and the colony surface representative of what would be expected in lakes of low, intermediate and high HCO3– concentrations, respectively (Fig. 2). Profiles of dissolved oxygen measured with microelectrodes above the top surface of a Nostoc parmelioides colony as a function of water velocity measured 1 mm above the colony suggested DBL thicknesses of 0·1 mm at 6–10 cm s–1, 0·2–0·3 mm at 1 cm s–1 and >0·4 mm in quasi-stagnant conditions (Fig. 3 in Dodds et al., 1995). The lower part of the colony resting on the surface has greater DBL thicknesses. Nostoc zetterstedtii could be expected to mostly experience concentration differences of 0·1–0·3 mm HCO3– and N. pruniforme 0·7–2 mm. When N. commune (and N. flagelliforme) is supplied with rainwater the DIC concentration is very low. However, when water with dissolved HCO3– is received from the carbonate-rich soils of the typical growth habitats, the expected DIC range is 0·4–2 mm (Sand-Jensen et al., 2010; Christensen et al., 2013). Because of the granulated surface of N. zetterstedtii, the flux is perhaps doubled relative to the smooth surface of N. pruniforme, while the flux to the sheet-like colonies of N. commune is much lower than the flux to small spherical colonies, but only slightly lower than the values for large colonies of N. pruniforme, because they physically approach the two-dimensional diffusion geometry. Only the diffusive flux of HCO3– has been calculated, because HCO3– constitutes >95 % of the DIC pool in alkaline water (>0·5 mm) and ∼85 % in soft water (0·1 mm) at air saturation with ∼0·015 mm CO2. All tested Nostoc colonies can use HCO3– actively (see later).
Fig. 2.
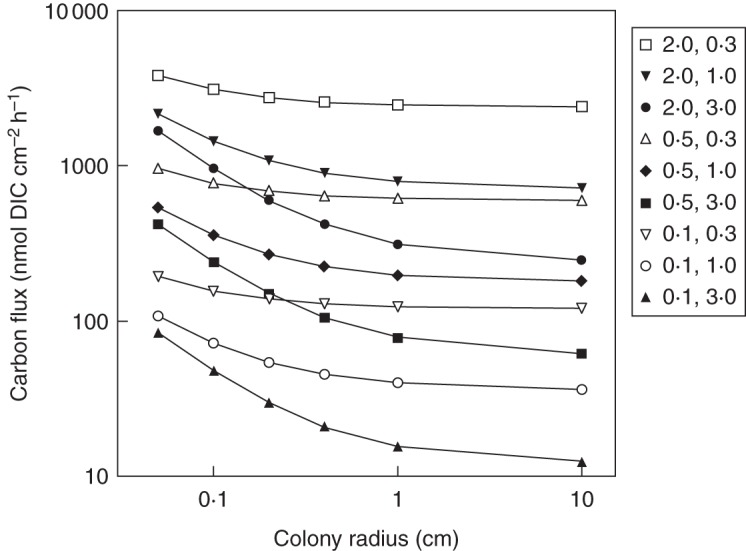
Calculated diffusive flux of HCO3– [J = r–2 [r′r (r′ – r) D (Cm – Co)]; Nobel, 1983] as a function of radius (r) for spherical colonies exposed to three ambient concentration levels (Cm: 0·1, 0·5 and 2·0 mm HCO3–) and three levels of boundary layer thickness (DBL: δ = r′ – r; 0·3, 1·0 and 3·0 mm). The combinations are indicated in the key in the figure, with Cm first and δ second. The molecular diffusion coefficient (D) is set at 1·0 × 10–9 m2 s–1 (Zeebe, 2011) and the HCO3– concentration at the colony surface is assumed to be zero.
Fig. 3.
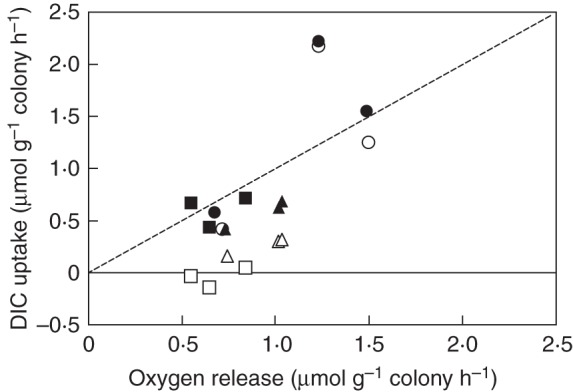
DIC exchange of N. zetterstedtii with water (open symbols) and with both water and the colony volume (filled symbols) as a function of oxygen exchange with water and colony volume during 18 h of photosynthesis in the light. The dotted line represents the 1:1 molar exchange of DIC and oxygen. Colonies were incubated at three DIC levels containing initially 0·99 mm (circles), 0·29 mm (triangles) and 0·10 mm (squares). After Sand-Jensen et al. (2009b); reproduced with permission of the Association for the Sciences of Limnology and Oceanography, Inc.
With an HCO3– gradient of 2 mm through a 1-mm-thick DBL, the potential flux is 1440 nmol C cm–2 h–1 for colonies with a radius of 1 mm (∼5 mg FM) and about half that value (790 nmol C cm–2 h–1) for colonies with a 10-fold higher radius of 10 mm (∼4·5 g FM) (Fig. 2). The potential flux for the two-dimensional diffusion geometry to a sheet-like structure like that of N. commune is 720 nmol C cm–2 h–1 and is independent of size. Fluxes decline 20-fold when the HCO3– gradient is 20-fold smaller (i.e. 0·1 mm across the DBL). In contrast, a thinner DBL of 0·3 mm increases the flux most for large colonies because they are more restricted by diffusion geometry than small colonies. Thus, for a gradient of 2 mm, the calculated flux increases to 3120 nmol C cm–2 h–1 for a small spherical colony (radius 1 mm), to 2470 nmol C cm–2 h–1 for a large spherical colony (radius 10 mm) and to 2400 nmol C cm–2 h–1 for a sheet-like structure.
What do these fluxes imply in terms of potential growth rate and biomass turnover? The answer is different for N. pruniforme, with carbon mass relative to surface area of ∼100 μmol C cm–2 for large colonies (radius 10 mm) compared with ∼1400 μmol C cm–2 for N. zetterstedtii of similar size. Assuming an inward flux during 12 daytime hours, a large HCO3– gradient of 2 mm across a DBL thickness of 1 mm, the potential turnover time of the biomass would be 11 d for N. pruniforme and 147 d for N. zetterstedtii, not accounting for night-time respiration, while a small gradient of 0·1 mm could only permit a potential turnover time of 210 d for N. pruniforme and 2940 d for N. zetterstedtii emphasizing the profound influence on carbon turnover of higher CMA for N. zetterstedtii compared with N. pruniforme. This difference should be enhanced by N. pruniforme's preference for hard-water lakes, ensuring a higher flux, and N. zetterstedtii's predominant existence in soft-water lakes. Thus, typical turnover times of N. pruniforme could be of the order of 11 d under suitable conditions in hard-water lakes containing 2 mm DIC for colonies measuring 10 mm in radius as opposed to 2940 d (8 years) for N. zetterstedtii of the same size growing in soft-water lakes with only 0·1 mm DIC available. If we account for the possibility of a 2-fold higher inward HCO3– flux for N. zetterstedtii because of the granulated colony surface, the turnover time would drop to 4 years, which is close to the observed values for N. zetterstedtii (3 years) in the soft-water Lake Värsjö (Sand-Jensen and Møller, 2011). Also, the turnover time of the biomass of N. pruniforme in laboratory experiments at 15–25 °C and in the shallow water of the hard-water Lake Esrum (2·5 mm DIC) during early summer was 10–14 d (Møller et al., 2014; C. Møller and K. Sand-Jensen, unpubl. data) and resembled the theoretical estimate of 11 d mentioned above. These calculations suggest that accounting for DIC concentrations, DBL thickness and CMA permits sound predictions of growth and biomass turnover of Nostoc colonies.
In addition to DIC, the metabolism and growth of Nostoc colonies could be constrained by the supply rate of dissolved inorganic phosphorus (DIP). Nitrogen can be fixed as elementary N2 and is less likely to be limiting, although growth stimulation by high external concentrations may occur, particularly for terrestrial species with unrestricted access to atmospheric CO2 (see later). Lakes and ponds with Nostoc species mostly have DIP concentrations in the water column ranging from undetectable by standard chemical methods to 0·3 mm (Sand-Jensen et al., 2009b), though higher concentrations can occur at the sediment surface. For a DIP gradient of 0·3 mm across a 1 mm thick DBL, the estimated influx was ∼0·22 nmol P cm–2 h–1 for small colonies (radius 1 mm) and 0·12 nmol P cm–2 h–1 for large colonies (radius 10 mm). These fluxes should permit a turnover time of the P pool within 6 d for small and 72 d for large colonies of N. zetterstedtii, supporting the hypothesis that growth is more likely to be constrained by the supply rate of DIC across gradients of 0·1 mm HCO3–. These calculations agreed with the observation that growth was unaffected by P richness in the sediments of soft-water Lake Värsjö (Sand-Jensen and Møller, 2011).
DIC supply is also important in dense epilithic communities growing on inert substrata and covered by relatively thick boundary layers (Sand-Jensen, 1983). Thus, short epilithic algal communities in marine waters have DBL thicknesses similar to those applied for Nostoc colonies and they appear to be constrained by the DIC flux to photosynthesis despite high HCO3– concentration (2 mm) in seawater. Larkum et al. (2003) showed that DBLs of epilithic algal communities covering dead coral surfaces were 0·18–0·59 mm thick under moderate flow (∼8 cm s–1) and >2 mm under quasi-stagnant conditions, and they generated high resistance to DIC influx, limiting primary production. Independent experiments with epilithic algal communities in other coral reef environments appeared to support the conclusion that DIC is a main limiting factor, as elevated nutrient levels had no effect on primary production and growth (Larkum and Koop, 1997; Miller et al., 1999), while large macroalgae in such nutrient-poor waters were stimulated by nutrient amendment (Lapointe et al., 1987).
Resource supply solely by diffusion
The flux to Nostoc colonies may be controlled entirely by diffusion in a stagnant water layer overlying the sediment. A stagnant surface layer may exist almost permanently in deep water with restricted turbulence and periodically in shallow water during calm weather. The colonies could be buried in a viscous boundary layer a few centimetres thick overlying the sediment (Boudreau and Jørgensen, 2001). When the surrounding water is virtually stagnant and the solute concentration is reduced to zero at the colony surface, the flux per unit surface area is inversely scaled to colony radius, emphasizing the immense advantage of being small (eqn 3 in Table 3). The calculated flux is 720 nmol C cm–2 h–1 for small colonies (radius 1 mm) in water containing 2 mm HCO3– or half the flux that occurs when DBL is 1 mm thick (eqn 1). For large colonies (radius 10 mm), however, the flux in stagnant water is only 72 nmol C cm–2 h–1, or 11-fold lower than in stirred water with a DBL of 1 mm. The potential biomass turnover time for large colonies as described above would then be 120 d for N. pruniforme under stagnant conditions in hard-water lakes and 32 350 d (89 years) for N. zetterstedtii in soft-water lakes. Both values are very high, stressing that the water overlying the sediment is either not entirely stagnant or is enriched with DIC (CO2 and HCO3–) from sediment decomposition. Maberly's (1985) measurements 10 cm above the sediments in a shallow bay covered by the moss Fontinalis antipyretica showed elevated CO2 concentrations relative to surface waters between 0·05 and 0·26 mm on 14 different dates. Concentrations are higher directly on the sediment surface and a near-bed CO2 concentration of, for example, 0·3 mm CO2 could permit a 6-fold higher influx of CO2 than a HCO3– gradient of 0·1 mm because the diffusion coefficient of CO2 is approximately twice that of HCO3– (Denny, 1993). The resulting turnover time could then be 6·3 years for large colonies of N. zetterstedtii if we assume that the granulated surface doubles the influx and 3·2 years if the inward flux continues in both light and darkness. Thus, we cannot exclude that large colonies of N. zetterstedtii could survive in an entirely diffusive environment provided available concentrations of CO2 and HCO3– were markedly higher than 0·1 mm. Small colonies a few millimetres in diameter can perform well by diffusive fluxes alone and are likely to be fully embedded in a diffusive boundary above the sediment surface.
Resource supply by diffusion relative to colony volume
The diffusive flux through stagnant water to a smooth, spherical colony follows the well-known formula often used for bacteria (eqn 4 in Table 3; Fenchel et al., 1998) because the colonies are usually so small relative to the thickness of DBL that the supply rate is entirely determined by diffusion. When metabolic activity and the demand for external resources are evenly distributed throughout the colony volume, the flux relative to unit volume (Fv) will be inversely scaled to the second power of radius, emphasizing the likelihood of extreme limitation as the organisms grow in size (eqn 5 in Table 3). The solutions to cope with this rapidly increasing risk of resource limitation with larger size would be either to reduce volume-specific requirements for metabolism (in the case of N. zetterstedtii) or develop a hollow sphere where all activity is restricted to an outer shell of thickness Ls and volume 4/3π (r3–(r – Ls)3) ≈ 4π (r – 0·5Ls)2 Ls. When r is large relative to Ls, volume is close to 4π r2 Ls and the flux relative to volume of the outer shell (Fvs) declines linearly with colony radius (eqn 6 in Table 3).
Thus, a 10-fold increase in colony radius will reduce the flux relative to the surface area and volume of a hollow spherical colony by the same order of magnitude, while the flux relative to the volume of a homogeneous, solid spherical colony declines 100-fold, emphasizing that if large colonies are indeed solid then their central parts must be composed of recalcitrant matrix material and either have no cells or cells of low metabolism. Because N. zetterstedtii colonies with a diameter of 10–20 mm absorb ∼96 % of incident irradiance from the surface to the centre (Sand-Jensen et al., 2009b), there is no scope for photosynthesis of Nostoc trichomes in the centre, but there is organic matter present here that could potentially be used by heterotrophic bacteria. The colony matrix is persistent and protected by bactericides (Sand-Jensen et al., 2009a), however, implying that there are no or very few active heterotrophic bacteria living within N. zetterstedtii colonies. The year-long persistence and low respiration rates of the colonies in complete darkness support this implication (see below).
Efflux of solutes
While the large size of gelatinous Nostoc colonies constrains the influx of external resources, it will also limit the efflux of valuable dissolved products derived from metabolism within the colonies. The possible implications of variations in this efflux have been overlooked so far. When the diffusive loss of respiratory CO2 and inorganic nutrients (e.g. phosphate) from the colony is constrained, they can better be retained and recycled within the colony (see section ‘Size effects, diffusive supply and loss of solutes’). Organic compounds that are produced and to a great extent released from the trichomes to form the extensive colony matrix can also be lost to the surrounding water and this loss can presumably be reduced in large colonies. Perhaps more importantly, antibiotics and toxins produced by Nostoc to reduce the risks of being attacked by harmful bacteria, viruses, mixotrophic algae and animals can be retained within the colony and here reach highly effective concentrations for a limited rate of production. Thus, with increasing colony size a constant production rate of the protective substance per colony volume will lead to increasing internal concentrations, or a constant internal concentration can be attained despite a declining production rate of the protective substance.
The formal analysis shows that a solute produced by a massive spherical organism at a uniform specific rate per unit volume (Pv) and lost by diffusion to stagnant surrounding water with a zero background concentration will have an effective concentration right at the surface of the colony that increases by the second power of the radius (eqn 7 in Table 3). If the concentration is kept constant, the production rate can decline by the second power of the radius. The mathematical formulas have been derived from Denny (1993), who treated an analogue biological example.
Photosynthetic production is constant relative to the surface area of N. pruniforme and N. zetterstedtii (Raun et al., 2009; Sand-Jensen et al., 2009b), in accordance with the fact that photosynthesis is confined to a surface shell of thickness Lsh and an approximate volume of 4π r2 Lsh. Because protective substances derive from photosynthetic products, they could have the same scaling properties. Thus, inserting shell volume instead of total colony volume in eqn 7 predicts that the concentration on the colony surface would increase in proportion to colony radius (eqn 8 in Table 3). Alternatively, the concentration would remain constant if the production rate of protective substances declined in proportion to increasing colony radius.
Therefore, the incorporation rate of inorganic carbon into new cells, colony matrix and extracellular products should all be determined as a function of colony size. This would permit direct determination of turnover rates of cells and colony matrix and evaluation of the relative cost of producing and releasing extracellular products. Despite extensive and elaborate work on the biochemistry of Nostoc colonies, this analysis has not been performed as yet.
FUNCTIONAL CONSEQUENCES OF THE USE OF LIGHT AND INORGANIC CARBON
Nostoc concentrates and circulates inorganic carbon efficiently within the large colonies, while the use of light suffers from extensive self-shading and absorption by non-photosynthetic elements.
Use of light
The large size, long internal light paths and high densities of trichomes within Nostoc colonies imply that light absorptance and internal self-shading are high. Mean light absorptance ranged from 55 % in N. commune to 96 % in N. zetterstedtii (Table 4). Moreover, because the cells experience high O2 and low CO2 concentrations within the colonies during photosynthesis, maximum photosynthesis relative to cell chlorophyll (i.e. the assimilation number), maximum quantum efficiency of photosynthesis and the ratio of photosynthesis to respiration should be much lower than among free-living unicells. To the extent that photons are absorbed by non-photosynthetic pigments and other coloured substances inside the colonies, maximum quantum efficiency will decline further.
Table 4.
Photosynthesis light variables of three Nostoc species at 15 °C
| Species |
Pmax |
Pmax/dark respiration | Icomp (μmol m–2 s–1) | Quantum efficiency (mmol O2/mol photons) | Absorption (%) | |
|---|---|---|---|---|---|---|
| (nmol O2 cm–2 h–1) | (mg O2 mg Chl–1 h–1) | |||||
| N. commune | 353–376 | 2·4 | 2·5 | 9·5 | 19 | 55 |
| N. pruniforme | 552–654 | 2·1 | 10 | 90 | ||
| N. zetterstedtii | 206–409 | 0·7 | 2·0–5·8 | 19·3 | 20 | 96 |
Data are means of measurements in several series.
Pmax, maximum net photosynthesis at light saturation; Icomp, light compensation point.
Sources: N. commune (K. Sand-Jensen, unpubl. data); N. pruniforme (Raun et al., 2009); N. zetterstedtii (Sand-Jensen et al., 2009b).
Quantum efficiency was indeed low for N. commune [average 19·1 mmol O2 (mol absorbed photon)–1] and N. zetterstedtii (11·2–39·9 in three series; Table 4). A similarly low efficiency [27 mmol O2 (mol photon)–1] was calculated for the thick-walled, balloon-like green macroalga Codium bursa (Geertz-Hansen et al., 1994) and microbial mats with extensive absorption (40–80 %) by structural components (Al-Najjar et al., 2012). In contrast, quantum efficiencies are much higher for free-living microalgae [70–120 mmol O2 (mol photon)–1], macroalgae and submerged plants [37–79 mmol O2 (mol photon)–1] (Frost-Christensen and Sand-Jensen, 1992).
Nostoc zetterstedtii had particularly low light-saturated photosynthesis relative to chlorophyll (average 0·5–0·7 mg O2 mg–1 chlorophyll a h–1) and values were also low for N. commune (average 2·4) and N. pruniforme (average 2·1). Likewise, photosynthesis relative to dark respiration (NP:R) was low for N. zetterstedtii (2·0–5·8) and N. commune (average 2·5), but not for N. pruniforme (average 10). Again free-living unicells have higher rates of photosynthesis relative to chlorophyll, typically between 2 and 20 (Harris, 1978) and NP:R ratios from 5 to 20 (Harris, 1978; Geider and Osborne, 1992). High self-shading within Nostoc colonies is an important constraint because photosynthesis at high irradiance increased 4-fold and NP:R increased 3-fold when self-shading was reduced by cutting colonies of N. zetterstedtii into 1–2-mm pieces (Sand-Jensen et al., 2009b).
Light attenuation by coloured substances, structural substances and dead or senescent cells within the colonies competes with photosynthetic pigments for photons, and thereby lowers quantum efficiency and increases the light compensation point (where photosynthesis outweighs respiration) compared with free-living unicells (Krause-Jensen and Sand-Jensen, 1998; Al-Najjar et al., 2012). Thus, light compensation points of N. zetterstedtii and N. commune (9·5–19·3 μmol photon m–2 s–1) exceeded those of most unicells (typically 0·8–9 μmol m–2 s–1; Langdon, 1988) and thin thalli and leaves of macroalgae and submerged plants (typically 2–12 μmol m–2 s–1; Sand-Jensen and Madsen, 1991). This comparison supports the hypothesis that inefficient light use in Nostoc colonies combined with higher respiratory costs of producing and maintaining the colony should lead to higher minimum light requirements for survival than for unicells, characeans and plants with thin photosynthetic tissues. Observations of lower light availability at the maximum depth limits of characeans, filamentous algae and mosses (3·1–7·8 % of surface light) than of N. pruniforme and N. zetterstedtii (9·4–12·5 % of surface light) in two Danish lakes support this hypothesis (Table 7 in Sand-Jensen et al., 2009b).
Table 7.
DIC consumed from the colony volume of three Nostoc species during extended light periods (∼20 h)
| Species | DIC consumed (μmol C g–1 fresh weight) |
Source | |
|---|---|---|---|
| Mean | 95 % CL | ||
| N. commune | 5·8 | 1·1 | K. Sand-Jensen (unpubl. data) |
| 6·5 | 0·5 | ||
| 9·0 | 1·7 | ||
| 10·2 | 2·3 | ||
| N. pruniforme | 8·67 | 1·33 | Raun (2006), Raun et al. (2009) |
| 8·57 | 0·84 | ||
| N. zetterstedtii | 16·3 | 2·9 | Sand-Jensen et al. (2009) |
| 24·5 | 5·6 | ||
Use of inorganic carbon
The diffusive supply of DIC from outside is constrained by the large size, low SA:V ratio and high density of filaments in the 1–3-mm-thick outer shells of gelatinous Nostoc colonies without direct contact with the surrounding medium. The effective diffusion path involves both the diffusive boundary layer surrounding the colony and the path through the gel to the sites of fixation in the Nostoc filaments, but the internal resistance has not been accounted for.
While increasing the resistance to influx of gas and solutes, the gel will also slow effluxes and contribute to the retention of night-time respiratory CO2 and facilitate accumulation of internal DIC pools. To ameliorate carbon limitation of photosynthesis and prevent excessive photorespiration, Nostoc colonies, like other cyanobacteria (Badger and Price, 1992, 2003), must attain reasonably high quotients of CO2 to O2 at the site of Rubisco activity within the cells. To achieve this, Nostoc colonies must be able to extract high proportions of the DIC pool in the water and have low compensation points of CO2 and HCO3– (Sültemeyer et al., 1998). The ability to accumulate DIC pools within the colony above immediate needs for photosynthesis and to retain respiratory CO2 from night-time respiration for later use during daytime photosynthesis could assist the carbon supply even further. Such efficient extraction and accumulation of carbon are known for free-living cyanobacteria (Kaplan et al., 1980; Sültemeyer et al., 1998) and phototrophs in lichen photosynthesis (Green et al., 1994), and the potential may also exist for Nostoc trichomes buried within the colony gel.
We found a high mean extraction capacity, of between 74 and 82 % of the initial DIC pool in the water (0·3–1·1 mm), during 20 h of photosynthesis in closed bottles with N. commune and N. zetterstedtii where DIC was gradually depleted and pH and O2 increased over time (Table 5). The mean extraction capacity was lower (58 %), but probably not fully expressed, in experiments with N. pruniforme, though it still reflected very active DIC uptake. Efficient DIC use is also known for N. flagelliforme and edible Nostoc Ge-Xian-Me (Gao and Zou, 2001; Qiu et al., 2004; Ye et al., 2012). Efficient active use of external DIC by N. commune and N. zetterstedtii was also reflected in the high final pH in the surrounding water (10·60–11·11) and the low final concentrations of HCO3– (6–75 μm) and CO2 (0·3–7 nm) as measures of HCO3– and CO2 compensation points (Table 6). Concentrations of HCO3– and CO2 at the sites of Rubisco within the cells must be markedly higher and molar quotients of O2 to HCO3– + CO2 lower than in the surrounding water (8–60) to ensure net carbon fixation and reduce photorespiration (Kaplan et al., 1980; Price et al., 2008).
Table 5.
Extraction capacity of the initial DIC pool (%) of three Nostoc species during photosynthesis for 20 h in closed bottles
| Species | Mean ± s.e. (%) | No. of measurements | Source |
|---|---|---|---|
| N. commune | 72 ± 4 | 16 | K. Sand-Jensen (unpubl. data) |
| 82 ± 3 | 12 | ||
| N. pruniforme* | 56 ± 3 | 5 | Raun (2006); Raun et al. (2009) |
| N. zetterstedtii | 74 ± 9 | 16 | Sand-Jensen et al. (2009) |
*Full extraction capacity probably not realized.
Table 6.
Final pH, final DIC, HCO3 and CO2 and final O2/HCO3– ratio of the four Nostoc species after 20 h of photosynthesis in closed bottles. Ranges are shown from several experimental series with many replicates
| Species | Initial DIC (μmol L–1) | Final pH | Final DIC (μmol L–1) | Final HCO3 (μmol L–1) | Final CO2 (nmol L–1) | O2/HCO3 | No. of series |
|---|---|---|---|---|---|---|---|
| N. commune | 106–310 | 10·68–11·11 | 12–90 | 6–34 | 1–7 | 9–60 | 5 |
| N. pruniforme* | 570 | 10·6 | ∼260 | ∼110 | <30 | ∼5 | 1 |
| N. zetterstedtii | 149–162 | 10·94–11·06 | 77–174 | 13–35 | 3–10 | 18–29 | 4 |
| N. flagelliforme | 3300 | 10·8 |
*Maximum final pH and minimum final DIC, HCO3 and CO2 probably not attained.
Sources: N. commune (K. Sand-Jensen unpubl. data); N. pruniforme (Raun, 2006; Raun et al., 2009); N. zetterstedtii (Sand-Jensen et al., 2009); N. flagelliforme (Gao and Zou, 2001).
The ability of all three Nostoc species to accumulate DIC to much higher concentrations within the colony than in the external water is supported by the relatively high rates of O2 release by photosynthesis for ∼20 h in water of low DIC (∼0·1 mm, 0·1 μmol cm–3) without any appreciable uptake of external DIC. Assuming that O2 is produced and inorganic C consumed from the colony itself during extended photosynthesis with a molar quotient of ∼1·0, we determined that a DIC pool of 16·3–24·5 μmol g–1 FM was available for photosynthesis in colonies of N. zetterstedtii and about half this pool size (5·8–10·2 μmol g–1 FM) was available in N. commune and N. pruniforme (Table 7). Direct measurements of the internal DIC pool in N. zetterstedtii yielded 19·0–24·7 μmol g–1 FM for colonies incubated in water containing 0·15 mm DIC and these measurements fully corresponded with estimates derived from the O2–DIC balance (Sand-Jensen et al., 2009b). Measured DIC concentrations were 150-fold higher within the colonies (average of cells and matrix) than in the external water and I expect that DIC concentrations would be even higher within the cells, resulting in a higher accumulation quotient than 150 between cells and water. Accumulation quotients of 500–1000 are known for free-living cyanobacteria (Kaplan et al., 1980; Badger and Price, 2003).
When I accounted for exchange of DIC during photosynthesis and respiration with both the colony matrix and the external water, I found mean molar exchange quotients of O2 relative to DIC in the light (1·19) and in the dark (0·96) close to 1·0 in experiments with N. zetterstedtii (Fig. 3). These direct measurements confirmed that a high proportion of DIC for photosynthesis in the light was consumed from a DIC pool in the colony, while in the dark a high proportion of respiratory DIC accumulated within the colony (Fig. 3). The measured internal DIC pools in N. zetterstedtii could support maximum photosynthesis for 11 and 23 h in 10- and 20-mm-diameter colonies, respectively, without uptake of external DIC. When N. commune was incubated for 20 h in relatively DIC-rich water containing initially 1·1 mm, it accumulated 16·7–18·7 μmol DIC g–1 FM of colony in excess of the immediate requirements for photosynthesis, while it consumed 9–10·2 μmol DIC g–1 FM from the colony to support photosynthesis when the initial DIC concentration in the water was only 0·2 mm (K. Sand-Jensen, unpubl. data). Photosynthetic ATP production provides ample energy for active transport of HCO3–, and at least N. commune can consume DIC for photosynthesis and at the same time accumulate DIC within the colony in the light when sufficient external DIC is available. Thus, the species does not solely rely on accumulation of respiratory DIC in the dark but can also take up external HCO3– for use in the following light period.
The ability of Nostoc colonies to build up substantial internal DIC pools had been overlooked in the past. This ability means that photosynthesis is less dependent on the immediate external DIC supply. Nonetheless, all three species were limited by the external DIC supply as photosynthetic rates at 0·1 mm DIC externally were ∼20 % of the maximum rate for N. commune, 11 % for small, 13 % for medium-sized and 25 % for large colonies of N. pruniforme and 60 % for N. zetterstedtii (Figs 4 and 5). As the internal DIC pool in N. zetterstedtii colonies is gradually exhausted during 18 h of light incubation in closed bottles, realized mean rates of O2 production are 1·8-fold lower in water initially containing 0·29 mm compared with water initially containing 0·99 mm DIC (Fig. 3). In N. pruniforme, which has higher rates of photosynthesis and DIC requirements per surface area than N. zetterstedtii and apparently 2-fold lower internal DIC pools, the dependency of photosynthesis on external DIC was stronger, showing half-saturation constants of 0·78 mm for small colonies (0·1 g FM) and 1·24 mm for larger colonies (2·5 g FM) in 2-h experiments under well-stirred conditions (Fig. 4). Large colonies have higher rates of photosynthesis (164 nmol O2 cm–2 h–1) at near-zero external DIC than small colonies (55 nmol O2 cm–2 h–1), probably because of a larger internal DIC pool (being scaled to colony volume) relative to photosynthetic activity (being scaled to colony surface area). The more favourable SA:V ratio in small colonies causes photosynthesis to increase more steeply with increasing external DIC (α-CO2, 319 nmol O2 cm–2 h–1 for a 1-mmol L–1 increase in external DIC) than in large colonies [α-CO2, 197 nmol O2 cm–2 h–1 (mmol DIC L–1)–1] (Fig. 4).
Fig. 4.
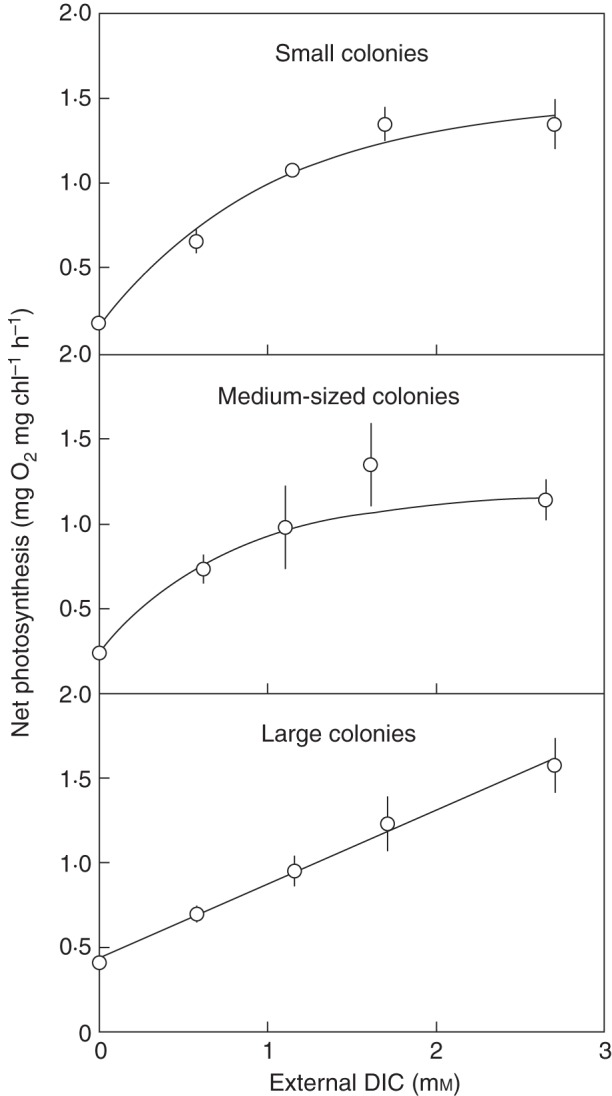
Mean net photosynthesis (± s.d. of four replicates) of small (0·18 g FM), medium (0·84 g FM) and large (2·71 g FM) spherical colonies of N. pruniforme as a function of external DIC concentration (>95 % HCO3–). The apparent half-saturation constant for small, medium and large colonies was 0·78, 0·78 and 1·24 mm HCO3–, the initial linear slope of net photosynthesis at low limiting DIC concentration was 319, 305 and 197 nmol O2 cm–2 h–1 (1 mmol DIC L–1)–1 and photosynthesis extrapolated to zero DIC was 55, 72 and 164 nmol O2 cm–2 h–1. From Raun (2006).
Fig. 5.
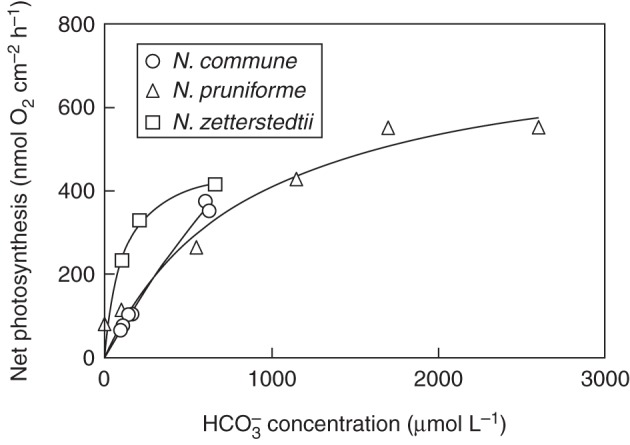
Mean DIC uptake during photosynthesis in high light as a function of mean DIC concentration in the water for N. commune, N. pruniforme and N. zetterstedtii. Sources: N. commune (K. Sand-Jensen, unpubl. data); N. pruniforme (Raun et al., 2009); N. zetterstedtii (Sand-Jensen et al., 2009b).
The terrestrial and semi-terrestrial N. commune and N. flagelliforme can use CO2 and HCO3– under water and CO2 alone in air. Under water, N. commune experiences limitation of photosynthesis at low DIC as rates rise to at least 1 mm. The diffusion coefficient of CO2 in air is ∼2 × 104 times higher than that of HCO3– in water, and for the same DBL thickness and with complete DIC depletion at the colony surface the CO2 gradient is ∼30–50 times lower in air (external CO2 ∼0·02 mm) than in water containing 0·6–1·0 mm HCO3–. Thus, the potential flux would be substantially higher (500- to 700-fold) in air than in water through a diffusive boundary layer of the same thickness overlying the colony surface, but this is followed by the same resistance through the colony to the Nostoc filaments in both terrestrial and aquatic habitats. At high irradiance and twice daily watering, growth of N. flagelliforme was doubled in CO2-enriched air (1500 ppm) compared with atmospheric air (350 ppm at the time; Gao and Yu, 2000). The realized photosynthetic rate of N. commune was the same in stirred air-saturated water of 1 mm DIC (0·98 mm HCO3– + 0·02 mm CO2) as in atmospheric air with 0·02 mm CO2 (Sand-Jensen and Jespersen, 2012). Thus, the optimal habitat of N. commune could be wet soils where water and inorganic carbon and nutrients are supplied to the lower colony surface, while CO2 and light are supplied to the upper surface. This is the habitat in 1-cm-deep water-filled depressions in the limestone pavements where we have observed massive growth of N. commune (Sand-Jensen et al., 2010).
TOLERANCE OF ENVIRONMENTAL EXTREMES
Freshwater Nostoc species do not experience extreme temperatures like those experienced by terrestrial species. Active growth of N. commune, N. pruniforme and N. zetterstedtii is, nonetheless, confined to the same temperature range (0–35 °C).
Temperature and desiccation tolerance
The terrestrial and semiterrestrial species N. commune and N. flagelliforme are exceptional in their tolerance of alternating freezing and thawing and of desiccation and rehydration at varying temperatures in their open habitats, located from the Arctic to tropical regions (Li, 1991; Sand-Jensen and Jespersen, 2012). In contrast, the freshwater species N. pruniforme and N. zetterstedtii are not exposed to freezing and drying or to extreme temperatures in their temperate and sub-Arctic environments. Nonetheless, it appears that active growth of all four species is confined to the same temperature interval between 0 and 35 °C and that the fastest growth takes place around 25 °C (Møller et al., 2014).
Both N. commune and N. flagelliforme are widely distributed species in arid and semiarid bare land throughout the world (Li, 1991; Dodds et al., 1995). Nostoc commune survives annual temperature ranges of ∼–60 to 25 °C under Arctic conditions and –30 to 50 °C under temperate conditions (Davey, 1989; Sand-Jensen and Jespersen, 2012). Surface temperatures in the open habitats of N. flagelliforme can reach 78 °C in summer and –40 °C in winter (Li, 1991). Both species can survive months or years of frost and drought as inactive desiccated crusts, and within minutes to hours can reactivate ion exchange, respiration, photosynthesis and N fixation when water and suitable temperatures again become available (Scherer et al., 1984; Satoh et al., 2002).
Experiments with the temperate species N. commune confirmed that photosynthesis and respiration were maintained after 36 h of desiccation of wet or already dry specimens at temperatures from –269 to 70 °C, while temperatures above 70 °C led to death (Sand-Jensen and Jespersen, 2012). During repeated daily cycles of drying for 18 h at –18, 20 and 40 °C and rewetting for 6 h at 20 °C, N. commune retained its photosynthetic capacity at –18 and 20 °C but died at 40 °C, presumably because of the greater costs of repair of macromolecules at this higher temperature, which would generate stronger thermal damage than lower temperatures (Sand-Jensen and Jespersen, 2012). In 24-day-long experiments under permanently submerged conditions, N. commune also died at 45 °C but survived at 35 °C; growth peaked at 25 °C and declined at the lower temperatures of 15 and 5 °C (Møller et al., 2014). Annual carbon fixation of rehydrated populations of N. commune from an Antarctic dry valley was also a strong positive function of increasing temperatures above zero and up to 20 °C, the highest temperature tested (Novis et al., 2007). Thus, there was no obvious sign of temperature adaptation or acclimation across geographical ranges.
Nostoc flagelliforme, like other terrestrial Nostoc species (Davey, 1989; Dodds et al., 1995; Sand-Jensen and Jespersen, 2012), showed great heat resistance when dry, while it was more susceptible when wet or immersed (Mei and Cheng, 1990). The regular protein pigment structure of photosystem I complexes was destroyed at 70 and 80 °C (Hu et al., 2005). Pretreatment of wet N. flagelliforme at 65 °C led to death and temperatures above 45 °C stopped photosynthesis (Mei and Cheng, 1990). The exact temperature effect on survival of N. commune and N. flagelliforme apparently depends on the duration and frequency of heat exposure and on the length of time available for repair of cellular damage between heat exposures (Sand-Jensen and Jespersen, 2012). Likewise, a longer time was required for maximal photosynthesis and respiration to recover after rehydration of specimens subjected to extended periods of desiccation (Scherer et al., 1984; Potts, 1994; 2000; Qiu and Gao, 1999). The recovery of rehydrated N. flagelliforme was light-dependent (Gao et al., 1998) and required potassium (Qiu and Gao, 1999), stressing that a suite of energy-demanding metabolic processes involving expensive protein and lipid synthesis and various cellular repair systems restores cellular structure and catalytic capacity (Angeloni and Potts, 1986; Taranto et al., 1993).
Desiccation is not an issue for freshwater species of Nostoc (except when they are washed ashore) and temperature variability is also much lower in freshwater than in terrestrial habitats. In Danish lakes, for example, the annual temperature range is typically 0–25 °C in shallow water and 2–15 °C in deep water (Møller et al., 2014). Nonetheless, the temperature dependence of long-term growth of N. pruniforme and N. zetterstedtii resembled that of hydrated N. commune in that all three species grew fastest at 25 °C and many times slower at 5 °C, while 45 °C led to die-off (Fig. 6 in Møller et al., 2014). So while terrestrial Nostoc species possess extraordinary desiccation tolerance there is no major difference in the relationship of active growth with temperature in terrestrial and aquatic specimens from north-temperate localities, though we cannot rule out that different relationships of growth with temperature have evolved in specimens living under Arctic or tropical climates. Because, under temperate conditions, temperature ranges between winter and summer are also profound it would not be a great surprise, however, if these relationships remained constant across geographical ranges. High temperature requirements (≥20 °C) of maximum photosynthesis of N. commune from an Antarctic valley with very short frost-free periods support the latter suggestion.
pH and salt tolerance
Being photosynthetic organisms capable of using both free CO2 and HCO3– at high efficiency, all four Nostoc species can push pH in the external water above 10·5 and in some cases even above 11 (Table 6). Exposure of rehydrated N. commune to a pH gradient for 36 h also showed that it tolerated pH ranging from 3 to 10, while it died at pH 2 (Sand-Jensen and Jespersen, 2012). Terrestrial Nostoc species should be able to survive exposure to rainwater of low pH (typically 3·8–4·8) and only very acidic habitats receiving sulphuric acid from the oxidation of metal sulphides are likely to be below pH 3. Therefore, when it comes to direct pH tolerance most terrestrial habitats should be open to colonization by Nostoc. Although N. commune can grow between garden tiles and on open sand soils fed by rainwater, it appears to prefer clay soils and calcareous rocks of high pH, perhaps because available HCO3– can support photosynthesis during prolonged illumination. The requirement of HCO3– for photosynthesis of aquatic Nostoc is accompanied by a preference for a pH above 7 in the water because of the buffering and alkalinization effect of HCO3–. Thus, at air saturation, freshwaters with pHs of 6·5, 7·5 and 8·5 have HCO3– concentrations of 0·05, 0·5 and 5 mm, respectively (Stumm and Morgan, 1981).
Because N. commune and N. flagelliforme often grow in arid habitats with salt accumulation they must be tolerant. Indeed, N. commune retained its photosynthetic capacity upon exposure to alkaline freshwater enriched with NaCl to 20 g kg–1, but not 30 g kg–1 as in oceanic water (Sand-Jensen and Jespersen, 2012). The closely related N. flagelliforme was also salt-resistant, but photosynthesis, respiration and photosystem II activity declined upon exposure to >12 g NaCl kg–1 (Ye and Gao, 2004; Yong-Hong et al., 2005). Salt resistance is probably based on the formation of sucrose and trehalose, both of which accumulate under desiccation and exposure to low salt concentrations (Sakamoto et al., 2009) and therefore play a dual role. Tolerance of freezing and desiccation is extraordinary, while salt tolerance is modest. Other cyanobacteria are more salt-tolerant, because of the synthesis of glucosylglycerol in species of moderate tolerance and glycine betaine and glutamate betaine in species showing high tolerance (Mackay et al., 1984).
ECOLOGICAL ADAPTATIONS AND STRATEGIES
Colonial Nostoc species live in resource-poor environments and succumb in competition with tall macroalgae and plants under richer conditions.
All four colonial Nostoc species discussed here live in resource-poor environments with a limited supply of water and inorganic nutrients on land and a limited supply of nutrients and DIC under water. Phosphorus could be a limiting nutrient for growth but experimental tests are lacking. All species can fix elemental N2, but this is a costly process that requires a rare co-factor (molybdenum). In suspension culture with N. flagelliforme, the variable availability of nitrogen and phosphorus influences the production of new cells and extracellular polysaccharides (Fei et al., 2012).
The terrestrial N. commune and N. flagelliforme grow on nutrient-poor, sparsely vegetated or bare soils and rock surfaces that alternate between being wet and dry. From April to September on the open limestone alvar on Öland, Sweden, measurements of humidity and temperature suggested that populations of N. commune were rehydrated and active at least 26 % of the time (Sand-Jensen and Jespersen, 2012). From October to March, populations were probably active during larger parts of the frost-free time.
Maximum recorded growth rates of N. commune floating on water and exposed to atmospheric air under laboratory conditions were 0·050–0·056 d–1 at 15–25 °C and markedly lower, at 0·020–0·025 d–1, at 5 and 35 °C (Møller et al., 2014). These growth rates correspond to doubling times of dry mass between 12 and 35 d. Growth rates are intermediate when compared with the extremely low rates (0·0008 d–1) of oligotrophic N. zetterstedtii and high rates (0·2–0·4 d–1) of nutrient-demanding thin macroalgae (e.g. Cladophora spp. and Enteromorpha spp.; Sand-Jensen and Borum, 1991; Nielsen and Sand-Jensen, 1991). According to Grime's (1979) classification of plant growth strategies in relation to resource richness and disturbance, the stress strategy (S) of N. zetterstedtii is linked to resource-poor, stabile habitats, the ruderal strategy (R) of thin green macroalgae is linked to resource richness and high disturbance, and the competitive strategy (C) of thick macroalgae is linked to resource richness and low disturbance. Resource-poor, highly disturbed habitats do not, in Grime's concept, support a viable strategy because the growth rate is very low when resources are limiting and disturbance leads to frequent loss of biomass, thus preventing long-term survival. However, N. commune (and N. flagelliforme) can survive disturbance by desiccation, freezing and extreme temperatures with restricted biomass loss in a quiescent stage and possesses a viable strategy in the resource-poor, disturbed terrestrial habitats that Grime considered unoccupied, at least by higher plants. A suite of drought-resistant lichens, mosses and a few flowering plants are capable of surviving in the same resource-poor, disturbed habitats as N. commune and N. flagelliforme (Sand-Jensen et al., 2010) and could be classified as belonging to a mixed S–D strategy, where S represents tolerance of limiting resources and D represents resistance to disturbance. Their biomass survives harsh physical conditions that are detrimental to most other phototrophs. Likewise, in the marine environment, crust-forming coralline red algae are adapted to an extremely low supply of light or nutrients, tolerate high disturbance by wave action and avoid intensive grazing, and thus have a viable S–D strategy with extremely slow growth and biomass turnover (Littler and Littler, 1980; Littler et al., 1986).
The freshwater N. zetterstedtii grows in soft-water, oligotrophic lakes and faces the strongest constraints on nutrients and DIC supply. Nostoc pruniforme grows in mesotrophic, alkaline lakes of higher DIC concentrations, where nutrients remain low from late spring to early autumn due to nutrient uptake by phytoplankton and other benthic phototrophs (Sand-Jensen et al., 2009b). Because both species are perennial, we cannot dismiss the untested possibility that they can take up nutrients in excess during the richer winter season for later use during summer when organic production by photosynthesis is high. This mechanism has been shown for perennial marine macroalgae (Chapman and Cragie, 1977; Lundberg et al., 1989; Lobban and Harrison, 1997). The persistence of Nostoc colonies may therefore offer an opportunity to withdraw external nutrients and produce photosynthates when conditions are favourable and store them within the colony until they are needed for growth and survival. The large size and low SA:V of the colonies can promote internal circulation of valuable substances and reduce their diffuse loss to the environment, as previously discussed (see section ‘Functional consequences of the use of light and inorganic carbon’).
Nostoc zetterstedtii is exceptional among aquatic phototrophic organisms due to its exceedingly low rates of growth, respiration and mortality as a consequence of the large spherical colony size and high CMA (Sand-Jensen and Møller, 2011). Because direct selection for low growth rate is not a viable evolutionary strategy (Lambers et al., 2008), low growth rate should, as emphasized, be regarded as an indirect consequence of high CMA and investment in a large arsenal of organic compounds as protection against environmental hazards, grazers and pathogens. The maximum growth rate of N. zetterstedtii in laboratory experiments was only 0·78 × 10–3 d–1, corresponding to a mean doubling time of colony biomass of 2·4 years (Møller et al., 2014). This value resembled the annual mean doubling time of 2·6–3·3 years in field experiments (Sand-Jensen and Møller, 2011). With such low growth rates, the largest colonies, measuring 7 cm in diameter, are 24–31 years old. We found no senescence and mortality in the examined population of colonies in the field during 13 608 incubation days. This finding implies that the mortality rate was <7·3 × 10–5 d–1 and longevity >24 years, in accordance with the high age of the largest colonies. Macerated N. zetterstedtii colonies diluted 10-fold in water and added to a sample of lake bacteria immediately stopped all cell division (Sand-Jensen et al., 2009a). Likewise, no grazing by small macroinvertebrates has been observed, and large potential grazers, such as crayfish, fish and swans, are probably unable to consume the large, firm colonies. Recent experiments showed that colonies survived for 14 months in complete darkness at 5 °C with integrity of photosynthesis and fresh mass of the colonies and extremely low daily respiration rates of 0·21 mmol C (mol organic carbon)–1 corresponding to a half-time of the colony carbon biomass of 9 years (K. Sand-Jensen, unpubl. data). I anticipate that Nostoc cells can utilize organic compounds from the colony matrix for basal respiration and long-term survival.
The ecological strategy of N. zetterstedtii is therefore an extreme example of a stress-selected species living in a very resource-poor environment, growing extremely slowly, being highly persistent thanks to efficient physical and chemical protection against pathogens and grazers, and having efficient recirculation of inorganic carbon (Fig. 3) (Sand-Jensen et al., 2009b). Measured growth rate in dry mass of N. zetterstedtii was ∼10-fold lower than rates predicted from general allometric relationships of growth rate with CMA of aquatic macroalgae (Markager and Sand-Jensen, 1996) and, likewise, of predicted growth rate with the thickness of photosynthetic tissue for a broad range of terrestrial and aquatic plants (Nielsen et al., 1996). This finding suggests that there are extra constraints on the growth of N. zetterstedtii linked to limited DIC supply and/or production of the colony matrix and the protective compounds. The proposed reduced loss of organic solutes from large colonies to the surrounding water has not been tested as yet, though it is plausible given the ability of N. zetterstedtii to maintain colony integrity and fresh mass during 14 months in darkness (K. Sand-Jensen, unpubl. data).
Nostoc pruniforme grows in oligo-mesotrophic lakes richer in DIC than the main habitats of N. zetterstedtii, and its growth, mortality and ecological strategy are also markedly different. Maximum growth rate of N. pruniforme in laboratory experiments was 75-fold higher than that of N. zetterstedtii, corresponding to a biomass doubling time of 12 d (Møller et al., 2014). Growth rates in a clear-water, DIC-rich lake during early summer resembled these laboratory rates (Møller and Sand-Jensen, unpubl. data 2013). A high DIC (2·0 mm) in the lake water was required to sustain a biomass turnover of 12 d, while incubation in low-DIC water (0·2 mm) reduced net photosynthesis 4-fold and prolonged biomass turnover (Møller et al., 2014). Field studies confirmed that N. pruniforme periodically undergoes mass mortality, presumably by attack of microbial pathogens. In terms of the ecological strategy, N. pruniforme has species traits belonging to a mixture of ruderal-, stress- and stress-disturbance-selected strategies. The expression of these traits is plastic depending on whether the species lives in cold, oligotrophic freshwaters and forms very large and old colonies or colonies that live for shorter periods in warmer, mesotrophic lakes and reach smaller sizes.
All four Nostoc species have one important trait in common. They live directly on the ground surface and are unable to survive in competition with tall, dense terrestrial or aquatic vegetation. This is a main reason why they are primarily confined to resource-poor habitats where tall, dense vegetation cannot develop. The Nostoc species therefore face the risk of disappearing when their habitats are subjected to nutrient enrichment. The freshwater Nostoc organisms in Scandinavian lakes have been further threatened by large-scale leaching of dissolved humic substances from low-buffered, acidic soils.
OUTLOOK
Most studies on the ecophysiology of phototrophs have focused on relatively fast-growing species from resource-rich habitats (Larcher, 2003; Lambers et al. 2008). With this review on large gelatinous Nostoc colonies I call attention to these competitively inferior species from nutrient-poor habitats. I introduce terrestrial and semiterrestrial Nostoc species possessing an unprecedented ability to enter quiescent, desiccated or frozen stages and rapidly resume metabolism when rehydrated. The molecular, biochemical and physiological sequences leading to quiescence and reactivation need to be fully explored and the metabolic costs of frequent desiccation, freezing and rehydration and substantial production of extracellular polysaccharides need quantification in the future. There is strong molecular, biomedical and ecological interest in these processes.
The rare freshwater species N. zetterstedtii has unprecedented low rates of metabolism, growth and mortality in its DIC- and nutrient-poor lake habitats. Its ability to take up external DIC and recirculate DIC within the colony is profound. It is challenging to determine whether the large colony size also creates advantages for effective circulation of nutrients within the colony and strong antibiotic effects for relatively low production costs. Future studies should also unravel fluid dynamics and nutrient and DIC conditions in the neglected near-bed habitats of N. pruniforme and N. zetterstedtii on lake sediments. This effort is needed in order to put the presented diffusion models for uptake and loss of solutes from spherical colonies of different size into proper physical and chemical perspectives in nature.
As free-living organisms and as symbionts in lichens, Nostoc species are both pioneers and permanent members of the vegetation of deserts, semideserts, dry grasslands and rock surfaces ranging in geographical distribution from polar to tropical regions. Their N input to these biomes can be of utmost importance (Dodds et al., 1995; Holst et al., 2009; Caputa et al., 2013). In a carefully mapped Low-Arctic tundra landscape, N2 fixation by cyanobacteria (0·68 kg ha–1) was twice the annual wet deposition of nitrogen (Stewart et al., 2011). It remains unexplored how the high water-absorbing capacity and N2 fixation of Nostoc can facilitate the colonization of bare or newly exposed mineral surfaces by mosses and higher plants, thereby forming more stable vegetation and more organic soils. With the exposure of new mineral surfaces behind retreating glaciers on a warming Earth, this ecosystem service of Nostoc deserves future attention.
ACKNOWLEDGEMENTS
This work was supported by grants from the Willum Kann Rasmussen Foundation to the Centre of Excellence for Lake Restoration Research (CLEAR) and from the Carlsberg Foundation. I thank Mikkel René Andersen, Michael Kühl and Claus Lindskov Møller for help and the referees for constructive suggestions.
LITERATURE CITED
- Aboal M, Cristobal CJ, Marin-Murcia JP. About the presence of N. commune var flagelliforme (Nostocaceae, Cyanophyceae) on clay soils from arid regions of south east Spain. Acta Botanica Malacitana. 2010;35:156–161. [Google Scholar]
- Agusti S, Enriquez S, Frost-Christensen H, Sand-Jensen K, Duarte CM. Light harvesting among photosynthetic organisms. Functional Ecology. 1994;8:273–279. [Google Scholar]
- Al-Najjar MAA, de Beer D, Kühl M, Polerecky L. Light utilization efficiency in photosynthetic microbial mats. Environmental Microbiology. 2012;14:982–992. doi: 10.1111/j.1462-2920.2011.02676.x. [DOI] [PubMed] [Google Scholar]
- Angeloni SV, Potts M. Polysome turnover in immobilized cells of Nostoc commune (Cyanobacteria) exposed to water stress. Journal of Bacteriology. 1986;168:1036–1039. doi: 10.1128/jb.168.2.1036-1039.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arima H, Horiguchi N, Tkaichi S, et al. Molecular, genetic and chemotaxonomic characterization of the terrestrial cyanobacterium Nostoc commune and its neighboring species. FEMS Microbiology Ecology. 2011;79:34–45. doi: 10.1111/j.1574-6941.2011.01195.x. [DOI] [PubMed] [Google Scholar]
- Badger MR, Price GD. The CO2 concentrating mechanism in cyanobacteria and microalgae. Physiologia Plantarum. 1992;84:606–615. [Google Scholar]
- Badger MR, Price GD. CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution. Journal of Experimental Botany. 2003;54:609–622. doi: 10.1093/jxb/erg076. [DOI] [PubMed] [Google Scholar]
- Bender J, Rodriguez-Eaton S, Ekanemesang UM, Phillips P. Characterization of metal-binding bio-flocculants produced by the cyanobacterial component of mixed microbial mats. Applied and Environmental Microbiology. 1994;60:2311–2315. doi: 10.1128/aem.60.7.2311-2315.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson R. The macroalga Nostoc zetterstedtii. Distribution and environmental requirements. Fauna och Flora. 1986;81:201–212. (in Swedish) [Google Scholar]
- Bengtsson R. Occurrence of Nostoc zetterstedtii (Sjöhjortron) in lakes in Småland and Blekinge, summer 1994. 1995. Report from Institute för vatten och luftvårdsforskning (IVL), Aneboda, Sweden (in Swedish)
- Bertocchi C, Navarini L, Cesaro A, Anastasio M. Polysaccharide from cyanobacteria. Carbohydrate Polymers. 1990;12:127–153. [Google Scholar]
- Bloor S, England RR. Antibiotic production by the cyanobacterium Nostoc muscorum. Journal of Applied Phycology. 1991;1:367–372. doi: 10.1016/0141-0229(91)90192-d. [DOI] [PubMed] [Google Scholar]
- Boudreau BP, Jørgensen BB, editors. The diffusive boundary layer. New York: Oxford University Press; 2001. [Google Scholar]
- Büdel B, Karsten U, Garcia-Pichel F. Ultraviolet-absorbing scytonemin and mycosporine-like amino acid derivates in exposed, rock-inhabiting cyanobacterial lichens. Oecologia. 1997;112:165–172. doi: 10.1007/s004420050296. [DOI] [PubMed] [Google Scholar]
- Caiola MG, Billi D, Friedmann EI. Effect of desiccation on envelopes of the cyanobacterium Chroococcidiopsis sp. (Chroococcales) European Journal of Phycology. 1996;31:97–105. [Google Scholar]
- Cameron RE. Species of Nostoc Vaucher occurring in the Sonaran desert in Arizona. Transactions of the American Microscopical Society. 1962;81:379–384. [Google Scholar]
- De Cano MS, De Mule MCZ, De Caire GZ, De Halperin DR. Growth control of Staphylococcus aureus and Candida albicans by Nostoc muscorum, Cyanophyta. Phyton. 1986;46:153–156. [Google Scholar]
- Caputa K, Cokson D, Sanborn P. Seasonal patterns of nitrogen fixation in biological soil crusts from British Columbia's Chilcotin grasslands. Botany. 2013;91:631–641. [Google Scholar]
- Carpenter RC, Williams SL. Effects of algal turf canopy height and microscale substratum topography on profiles of flow speed in a coral forereef environment. Limnology and Oceanography. 1993;38:687–694. [Google Scholar]
- Chapman ARO, Craigie JS. Seasonal growth in Laminaria longicruris: relations with dissolved inorganic nutrients and internal reserves of nitrogen. Marine Biology. 1977;40:197–205. [Google Scholar]
- Christensen JPA, Sand-Jensen K, Staehr PA. Fluctuating water levels control chemistry and metabolism of a charophyte-dominated pond. Freshwater Biology. 2013;58:1353–1365. [Google Scholar]
- Cupac S, Gantar M. Chemical composition and morphological structure of the mucilaginous sheath of the cyanobacterium Nostoc D. Biomedical Letters. 1992;47:133–138. [Google Scholar]
- Davey MC. The effect of freezing and desiccation on photosynthesis and survival of terrestrial Antarctic algae and cyanobacteria. Polar Biology. 1989;10:29–36. [Google Scholar]
- Denny MH. Air and water. The biology and physics of life's media. Princeton: Princeton University Press; 1993. [Google Scholar]
- Dodds WK, Castenholz RW. Effects of grazing and light on the growth of Nostoc pruniforme. British Journal of Phycology. 1987;33:219–227. [Google Scholar]
- Dodds WK, Gudder DA, Mollenhauer D. The ecology of Nostoc. Journal of Phycology. 1995;31:2–18. [Google Scholar]
- Fei Q, Xiao-yuan L, Bing-bing X, Yue B, Li-qui Z. Effect of nutrient level of phosphorus and nitrogen on the metabolism of the extracellular organic matter of Nostoc flagelliforme. Huanjing Kexue. 2012;33:1556–1563. [Google Scholar]
- Fenchel T, King GM, Blackburn TH. Bacterial biogeochemistry. The ecophysiology of mineral cycling. 2nd edn. New York: Academic Press; 1998. [Google Scholar]
- Frost-Christensen H, Sand-Jensen K. The quantum efficiency of photosynthesis in macroalgae and submerged angiosperms. Oecologia. 1992;91:377–384. doi: 10.1007/BF00317627. [DOI] [PubMed] [Google Scholar]
- Gao K. Chinese studies of the edible cyanobacterium, Nostoc flagelliforme: a review. Journal of Applied Phycology. 1998;10:37–49. [Google Scholar]
- Gao K, Ai H. Relationship of growth and photosynthesis with colony size in an edible cyanobacterium, Ge-Xian-Mi Nostoc (Cyanophyceae) Journal of Phycology. 2004;40:523–526. [Google Scholar]
- Gao K, Qiu B, Xia J, Yu A. Light dependency of the photosynthetic recovery of Nostoc flagelliforme. Journal of Applied Phycology. 1998;10:51–53. [Google Scholar]
- Gao K, Liu K, Qiu BS. An investigation of the genetic background of Nostoc flagelliforme by similarity analysis of its partial genomic DNA and phylogenetic comparison of deduced related species. Acta Physiologia Plantarum. 2011;33:1301–1318. [Google Scholar]
- Gao K, Ai YF, Giu BS. Drought adaptation of a terrestrial macroscopic cyanobacterium, Nostoc flagelliforme in arid areas: a review. African Journal of Microbiology Research. 2012;6:5728–5735. [Google Scholar]
- Gao X, Yu A. Influence of CO2, light, and watering on growth of Nostoc flagelliforme. Journal of Applied Phycology. 2000;10:37–49. [Google Scholar]
- Gao X, Zou D. Photosynthetic bicarbonate utilization by a terrestrial cyanobacterium, Nostoc flagelliforme (Cyanophyceae) Journal of Phycology. 2001;37:768–771. [Google Scholar]
- Geertz-Hansen O, Enriquez S, Duarte CM, et al. Functional implications of the form of Codium bursa, a balloon-like Mediterranean macroalga. Marine Ecology Progress Series. 1994;108:153–160. [Google Scholar]
- Geider R. Light and temperature dependence of the carbon to chlorophyll a ratio in microalgae and cyanobacteria: implications for physiology and growth of phytoplankton. New Phytologist. 1987;106:1–34. [Google Scholar]
- Geider R, Osborne BA. Algal photosynthesis. London: Chapman and Hall; 1992. [Google Scholar]
- Green TGA, Lange OL, Cowan IR. Ecophysiology of lichen photosynthesis: the role of water status and thallus diffusion resistance. Cryptogamic Botany. 1994;4:168–178. [Google Scholar]
- Grime JP. Plant strategies and vegetation processes. Chichester: Wiley and Sons; 1979. [Google Scholar]
- Gromov BV, Vepritskiy AA, Titova UN, Mamjayeva KA, Alexandra OV. Production of the antibiotic cyanobacterin LU-1 by Nostoc linckia Calu-892 (cyanobacterium) Journal of Applied Phycology. 1991;3:55–59. [Google Scholar]
- Hameed MSA, Hassan SH, Mohammed R, Gamal R. Isolation and characterization of antimicrobial active compounds from the cyanobacterium Nostoc commune Vauch. Journal of Pure and Applied Microbiology. 2013;7:109–116. [Google Scholar]
- Harris GP. Photosynthesis, productivity and growth: the physiological ecology of phytoplankton. Ergebnisse der Limnologie. 1978;10:1–171. [Google Scholar]
- Hill DL, Keenan TW, Helm RF, et al. Extracellular polysaccharide of Nostoc commune (Cyanobacteria) inhibits fusion of membrane vesicles during desiccation. Journal of Applied Phycology. 1997;9:237–248. [Google Scholar]
- Holst J, Butterbach-Bahl K, Liy CY, et al. Dinitrogen fixation by biological soil crusts in Inner Mongolian steppe. Biology and Fertility of Soils. 2009;45:679–690. [Google Scholar]
- Hu ZH, Xu YN, Gong YD, Kuong TY. Effects of heat treatment on the protein secondary structure and pigment environment in photosystem I complex. Photosynthetica. 2005;43:529–534. [Google Scholar]
- Huang H, Liu Y, Poulsen BS, Klaveness D. Studies on polysaccharides from three edible species of Nostoc (Cyanophyta) with different colony morphologies: comparison of monosaccharide compositions and viscosities of polysaccharides from field colonies and suspension cultures. Journal of Phycology. 1998;34:962–968. [Google Scholar]
- Huang H, Bai KZ, Zhong ZP, Li LB, Kuang TY. Energy transfer from phycobilisomes to photosystems of N. flagelliforme Born. et Filah during the rewetting course and its physiological significance. Journal of Integrative Plant Biology. 2005;47:703–708. [Google Scholar]
- Jia SR, Yu HF, Lin YX, Dai YJ. Characterization of extracellular polysaccharides from Nostoc flagelliforme cells in liquid suspension culture. Biotechnology and Bioprocess Engineering. 2007;12:271–275. [Google Scholar]
- Jørgensen BB. Life in the diffusive boundary layer. In: Boundreau BP, Jørgensen BB, editors. The diffusive boundary layer. New York: Oxford University Press; 2001. pp. 348–374. [Google Scholar]
- Kaasalainen U, Fewer DP, Jokela J, Wahlsten M, Sivonen K, Rikkinen J. Cyanobacteria produce a high variety of hepatotoxic peptides in lichen symbiosis. Proceedings of the National Academy of Sciences of the USA. 2012;109:5886–5891. doi: 10.1073/pnas.1200279109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo K, Le JB, Hayashi K, et al. Isolation of an antiviral polysaccharide, nostoflan, from a terrestrial cyanobacterium, Nostoc flagelliforme. Journal of Natural Products. 2005;68:1037–1041. doi: 10.1021/np050056c. [DOI] [PubMed] [Google Scholar]
- Kanekiyo K, Hayashi K, Takenaka H, Lee JB, Hayashi T. Antiherpes simplex virus target of an acidic polysaccharide, nostoflan, from the edible blue-green alga, Nostoc flagelliforme. Biological and Pharmaceutical Bulletin. 2007;30:1573–1575. doi: 10.1248/bpb.30.1573. [DOI] [PubMed] [Google Scholar]
- Kanekiyo K, Hayashi K, Lee JB, Takenaka H, Hayashi T. Structure and antiviral activity of an acidic polysaccharide from an edible blue-green alga, Nostoc flagelliforme. Yakugaku Zasshi. 2008;128:725–731. doi: 10.1248/yakushi.128.725. [DOI] [PubMed] [Google Scholar]
- Kaplan A, Badger MR, Berry JA. Photosynthesis and the intracellular inorganic carbon pool in the bluegreen alga Anabaena variabilis. Response to external CO2 concentration. Planta. 1980;149:219–226. doi: 10.1007/BF00384557. [DOI] [PubMed] [Google Scholar]
- Knowles EJ, Castenholz RW. Effects of exogenous extracellular polysaccharides on the desiccation and freezing tolerance of rock-inhabiting phototrophic microorganisms. FEMS Microbiology Ecology. 2008;66:261–270. doi: 10.1111/j.1574-6941.2008.00568.x. [DOI] [PubMed] [Google Scholar]
- Krause-Jensen D, Sand-Jensen K. Light attenuation and photosynthesis of aquatic plant communities. Limnology and Oceanography. 1998;43:396–407. [Google Scholar]
- Langdon C. On the causes of interspecific differences in the growth-irradiance relationship for phytoplankton. II. A general review. Journal of Plankton Research. 1988;10:1291–1312. [Google Scholar]
- Lambers H, Poorter H. Inherent variation in growth rate between higher plants: a search for physiological causes and ecological consequences. Advances in Ecological Research. 1992;23:187–261. [Google Scholar]
- Lambers H, Poorter H. Inherent variation in growth rate between higher plants: a search for physiological causes and ecological consequences. Advances in Ecological Research. 2004;34:283–362. [Google Scholar]
- Lambers H, Chapin FS, III, Pons TL. Plant physiological ecology. 2nd edn. Berlin: Springer; 2008. [Google Scholar]
- Lapointe BE, Littler MM, Littler DS. A comparison of nutrient-limited productivity in macroalgae from a Caribbean barrier reef and from a mangrove ecosystem. Aquatic Botany. 1987;28:243–255. [Google Scholar]
- Larcher W. Physiological plant ecology. 4th edn. Berlin: Springer; 2003. [Google Scholar]
- Larkum AWD, Koop K. ENCORE: algal productivity and a possible paradigm shift. In: Lessios HA, MacIntyre IG, editors. Proceedings of the 8th International Coral Reef Symposium, Vol. 1. Balboa, Panama: Smithsonian Tropical Research Institute; 1997. pp. 881–884. [Google Scholar]
- Larkum AWD, Koch E-MW, Kühl M. Diffusive boundary layers and photosynthesis of the epibenthic algal community of corals reefs. Marine Biology. 2003;142:1073–1082. [Google Scholar]
- Li H, Xu J, Liu Y, et al. Antioxidant and moisture-retention activities of the polysaccharide from Nostoc commune. Carbohydrate Polymers. 2011;83:1821–1827. [Google Scholar]
- Li SH. Ecology of the terrestrial alga Nostoc flagelliforme BERK. et CURT. in China. Journal of Phycology (Supplement) 1991;33:576–584. 45. [Google Scholar]
- Liu YH, Yu L, Ke WT, Gao X, Qui BS. Photosynthetic recovery of Nostoc flagelliforme (Cyanophyceae) upon rehydration after 2 years and 8 years dry storage. Phycologia. 2010;49:429–437. [Google Scholar]
- Littler MM, Littler DS. The evolution of thallus form and survival strategies in benthic marine macroalgae: field and laboratory tests of a functional form model. American Naturalist. 1980;116:25–44. [Google Scholar]
- Littler MM, Littler DS, Blair SM, Norris JN. Deep-water plant communities from an uncharted seamount off San Salvador Island, Bahamas: distribution, abundance, and primary productivity. Deep Sea Research. 1986;33:881–892. [Google Scholar]
- Lobban CS, Harrison PJ. Seaweed ecology and physiology. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Lundberg P, Weich RG, Jensen P, Vogel HJ. Phosphorus-31 and nitrogen-14 studies of the uptake of phosphorus and nitrogen compounds in the marine macroalga Ulva lactuca. Plant Physiology. 1989;89:1380–1387. doi: 10.1104/pp.89.4.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maberly SC. Photosynthesis by Fontinalis antipyretica. II. Assessment of environmental factors limiting photosynthesis and production. New Phytologist. 1985;100:141–155. [Google Scholar]
- Mackay MA, Norton RS, Borowitzka LJ. Organic osmoregulatory solutes in cyanobacteria. Journal of General Microbiology. 1984;130:2177–2191. [Google Scholar]
- MacKintosh C, Beattie KA, Klumpp S, Cohen P, Codd GA. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatase 1 and 2A from both mammals and higher plants. FEBS Letters. 1990;264:187–192. doi: 10.1016/0014-5793(90)80245-e. [DOI] [PubMed] [Google Scholar]
- Markager S, Sand-Jensen K. The physiology and ecology of light-growth relationship in macroalgae. In: Round FE, Chapman DJ, editors. Progress in Phycological Research. Bristol: Biopress; 1994. pp. 209–298. [Google Scholar]
- Markager S, Sand-Jensen K. Implications of thallus thickness for growth-irradiance relationships of marine macroalgae. European Journal of Phycology. 1996;31:79–87. [Google Scholar]
- Marsh J, Nouvet S, Sanborn P, Coxson D. Composition and function of biological crust communities along topographic gradients in grasslands of central interior British Columbia (Chilcotin) and southwestern Yukon (Kluane) Canadian Journal of Botany. 2006;84:713–731. [Google Scholar]
- Mehta VB, Vaidya BS. Cellular and extracellular polysaccharides of the blue-green alga Nostoc. Journal of Experimental Botany. 1978;29:1423–1430. [Google Scholar]
- Mei JX, Cheng ZJ. Effects of temperature on physiological activities of Nostoc flagelliforme Born et Flah (in Chinese with English summary) Journal of Western-north Normal University (National Science) 1990;1:75–85. [Google Scholar]
- Miller MW, Hay ME, Miller SL, et al. Effect of nutrients versus herbivores on reef algae: a new method for manipulating nutrients on coral reefs. Limnology and Oceanography. 1999;44:1847–1861. [Google Scholar]
- Mollenhauer D. Nostoc species in the field. Archiv für Hydrobiologie Supplementum. 1988;80:1–4. [Google Scholar]
- Mollenhauer D, Büdel B, Mollenhauer R. Algological studies. Archiv für Hydrobiologie Supplementum. 1994;105:189–209. [Google Scholar]
- Mollenhauer D, Bengtsson R, Lindström EA. Macroscopic cyanobacteria of the genus Nostoc: a neglected and endangered constituent of European inland aquatic biodiversity. European Journal of Phycology. 1999;34:349–360. [Google Scholar]
- Møller C, Vangsøe MT, Sand-Jensen K. Comparative growth and metabolism of gelatinous colonies of three cyanobacteria, Nostoc commune, N. pruniforme and N. zetterstedtii across temperatures. Freshwater Biology. 2014. in press.
- Nielsen SL, Sand-Jensen K. Regulation and photosynthetic rates of submerged rooted macrophytes. Oecologia. 1989;81:364–368. doi: 10.1007/BF00377085. [DOI] [PubMed] [Google Scholar]
- Nielsen SL, Sand-Jensen K. Allometric scaling of maximal photosynthetic growth rate to surface/volume ratio. Limnology and Oceanography. 1990;35:177–181. [Google Scholar]
- Nielsen SL, Sand-Jensen K. Variation in growth rates of submerged rooted macrophytes. Aquatic Botany. 1991;39:109–120. [Google Scholar]
- Nielsen SL, Enriquez S, Duarte CM, Sand-Jensen K. Maximum growth rates across photosynthetic organisms. Functional Ecology. 1996;10:167–175. [Google Scholar]
- Nobel PO. Biophysical plant physiology and ecology. New York: Freeman; 1983. [Google Scholar]
- Novis PM, Whitebread D, Gregorich, et al. Annual carbon fixation in terrestrial populations of Nostoc commune (Cyanobacteria) from an Antarctic dry valley is driven by temperature regime. Global Change Biology. 2007;13:1224–1237. [Google Scholar]
- Nowruzi B, Khavari-Nejad R-A, Sivonen K, et al. Identification and toxigenic potential of a Nostoc sp. Algae. 2012;27:303–313. [Google Scholar]
- Pereira S, Zille A, Micheletti E, Moradas-Ferreira P, De Philippis R, Temagnini P. Complexity of cyanobacterial exopolysaccharides: composition, structures, including factors of putative genes involved in their biosynthesis and assembly. FEMS Microbiology Reviews. 2009;33:917–941. doi: 10.1111/j.1574-6976.2009.00183.x. [DOI] [PubMed] [Google Scholar]
- De Philippis R, Paperi R, Sili C. Heavy metal sorption by released polysaccharides and whole cultures of two exopolysaccharide-producing cyanobacteria. Biodegradation. 2007;18:181–187. doi: 10.1007/s10532-006-9053-y. [DOI] [PubMed] [Google Scholar]
- Poorter H, Niklas KJ, Reich PB, Jacek O, Mommer L. Biomass allocation to leaves, stems and roots; meta-analyses of interspecific variation and environmental control. New Phytologist. 2012;193:30–50. doi: 10.1111/j.1469-8137.2011.03952.x. [DOI] [PubMed] [Google Scholar]
- Potts M. Desiccation tolerance of prokaryotes. Microbiological Reviews. 1994;58:755–805. doi: 10.1128/mr.58.4.755-805.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts M. Mechanisms of desiccation tolerance in cyanobacteria. European Journal of Phycology. 1999;34:319–328. [Google Scholar]
- Potts M. Nostoc. In: Whitton BA, Potts M, editors. The ecology of cyanobacteria. Dordrecht: Kluwer Academic Publishers; 2000. pp. 465–504. [Google Scholar]
- Price GD, Badger MR, Woodger FJ, Long BM. Advances in understanding the cyanobacterial CO2-concentrating-mechanisms (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. Journal of Experimental Botany. 2008;59:1441–1461. doi: 10.1093/jxb/erm112. [DOI] [PubMed] [Google Scholar]
- Qiu BS, Zhong AH, Zhou WB, et al. Effects of potassium on the photosynthetic recovery of the terrestrial cyanobacterium, Nostoc flagelliforme (Cyanophyceae) during rehydration. Journal of Phycology. 2004;40:323–332. [Google Scholar]
- Qiu B, Gao K. Dried populations of Nostoc flagelliforme (Cyanophyceae) require exogenous nutrients for their photosynthetic recovery. Journal of Applied Phycology. 1999;11:535–541. [Google Scholar]
- Raun AL. Copenhagen: University of Copenhagen (in Danish); 2006. CO2 concentrating mechanisms and bicarbonate use of the cyanobacterium, Nostoc pruniforme. Report from the Freshwater Biological Laboratory. [Google Scholar]
- Raun AL, Borum J, Sand-Jensen K. Active accumulation of internal DIC pools reduces transport limitation in large colonies of Nostoc pruniforme. Aquatic Biology. 2009;5:23–29. [Google Scholar]
- Sakamoto T, Yoshida T, Arima H, et al. Accumulation of trehalose in response to desiccation and salt stress in the terrestrial cyanobacterium Nostoc commune. Phycological Research. 2009;57:66–73. [Google Scholar]
- Sand-Jensen K. Physico-chemical parameters regulating growth of periphytic communities. In: Wetzel RG, editor. Periphyton of freshwater ecosystems. Dordrecht: Dr W. Junk; 1983. pp. 63–71. [Google Scholar]
- Sand-Jensen K, Borum J. Interactions among phytoplankton, periphyton and macrophytes in temperate freshwaters and estuaries. Aquatic Botany. 1991;41:137–175. [Google Scholar]
- Sand-Jensen K, Jespersen TS. Tolerance of the widespread cyanobacterium Nostoc commune to extreme temperature variations (-269 to 105°C), pH and salt stress. Oecologia. 2012;169:331–339. doi: 10.1007/s00442-011-2200-0. [DOI] [PubMed] [Google Scholar]
- Sand-Jensen K, Madsen TV. Minimum light requirements of submerged freshwater macrophytes in laboratory growth experiments. Journal of Ecology. 1991;79:749–764. [Google Scholar]
- Sand-Jensen K, Møller C. Unprecedented slow growth and mortality of the rare colonial cyanobacterium, Nostoc zetterstedtii, in oligotrophic lakes. Limnology and Oceanography. 2011;56:1976–1982. [Google Scholar]
- Sand-Jensen K, Revbech NP, Jørgensen BB. Microprofiles of oxygen in epiphyte communities in epiphyte communities on submerged macrophytes. Marine Biology. 1985;89:55–62. [Google Scholar]
- Sand-Jensen K, Borum J, Raun AL, Leth S, Hinke A. Co-operation in a spherical universe. Aktuel Naturvidenskab. 2009a;6:22–25. (in Danish) [Google Scholar]
- Sand-Jensen K, Raun AL, Borum J. Metabolism and resources of spherical colonies of Nostoc zetterstedtii. Limnology and Oceanography. 2009b;54:1282–1291. [Google Scholar]
- Sand-Jensen K, Baastrup-Spohr L, Winkel A, et al. Plant distribution patterns and adaptation in a limestone quarry on Öland. Svensk Botanisk Tidsskrift. 2010;104:23–31. [Google Scholar]
- Satoh K, Hirai M, Nishio J, et al. Recovery of photosynthetic systems during rewetting is quite rapid in a terrestrial cyanobacterium. Plant and Cell Physiology. 2002;43:170–176. doi: 10.1093/pcp/pcf020. [DOI] [PubMed] [Google Scholar]
- Scherer WS, Ernst A, Chen T-W, Böger P. Rewetting of drought-resistant blue-green algae: Time-course of water uptake and reappearance of respiration, photosynthesis and nitrogen fixation. Oecologia. 1984;62:418–423. doi: 10.1007/BF00384277. [DOI] [PubMed] [Google Scholar]
- Soule T, Gao QJ, Stout V, Garcia-Pichel F. The global response of Nostoc punctiforme ATCC 29133 to UVA stress, assessed in a temporal DNA microarray study. Photochemistry and Photobiology. 2013;89:415–423. doi: 10.1111/php.12014. [DOI] [PubMed] [Google Scholar]
- Stewart KJ, Coxson D, Grogan P. Nitrogen inputs by associative cyanobacteria across a low Arctic tundra landscape. Arctic, Antarctic and Alpine Research. 2011;43:267–278. [Google Scholar]
- Stumm W, Morgan JJ. Aquatic Chemistry. 2nd edn. New York: Wiley; 1981. [Google Scholar]
- Sültemeyer D, Klughammer B, Badger MR, Price GD. Fast induction of high-affinity HCO3– transport in cyanobacteria. Plant Physiology. 1998;116:183–192. [Google Scholar]
- Tamaru Y, Takani Y, Yoshida T, Sakamoto T. Crucial role of extracellular polysaccharides in desiccation and freezing tolerance in the terrestrial cyanobacterium Nostoc commune. Applied and Environmental Microbiology. 2005;71:7327–7333. doi: 10.1128/AEM.71.11.7327-7333.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taranto PA, Keenan TW, Potts M. Rehydration induces rapid onset of lipid biosynthesis in desiccated Nostoc commune (Cyanobacteria) Biochimica et Biophysica Acta. 1993;168:228–237. doi: 10.1016/0005-2760(93)90129-w. [DOI] [PubMed] [Google Scholar]
- Ye C, Gao K. Photosynthetic response to salt of aquatic-living colonies of the terrestrial cyanobacterium Nostoc flagelliforme. Journal of Applied Phycology. 2004;16:477–481. [Google Scholar]
- Ye CP, Zhang MC, Yang YF, Thirumaran G. Photosynthetic performance in aquatic and terrestrial colonies of Nostoc flagelliforme (Cyanophyceae) under aquatic and aerial conditions. Journal of Arid Environments. 2012;85:56–61. [Google Scholar]
- Yu HF, Liu R. Effect of UV-B radiation on the synthesis of UV-absorbing compounds in a terrestrial cyanobacterium, Nostoc flagelliforme. Journal of Applied Phycology. 2013;25:1441–1446. [Google Scholar]
- Yu HF, Jia SR, Dai YJ. Accumulation of exopolysaccharides in liquid suspension culture of Nostoc flagelliforme cells. Applied Biochemistry and Biotechnology. 2010;160:552–560. doi: 10.1007/s12010-008-8428-4. [DOI] [PubMed] [Google Scholar]
- Yong-Hong B, Zhong-Yang D, Zheng-Yu H, Min X. Response of Nostoc flagelliforme to salt stress. Acta Hydrobiologica Sinica. 2005;29:125–129. [Google Scholar]
- Yoshida T, Sakamoto T. Water-induced trehalose accumulation and control of trehalase in the cyanobacterium Nostoc punctiforme. Journal of General and Applied Microbiology. 2009;55:135–145. doi: 10.2323/jgam.55.135. [DOI] [PubMed] [Google Scholar]
- Yousimura H, Kotake T, Aohara T, Tsumuraya Y, Ikeuchi M, Ohmori M. The role of extracellular polysaccharides produced by the terrestrial cyanobacterium Nostoc sp. strain HK-01 in NaCl tolerance. Journal of Applied Phycology. 2012;24:237–243. [Google Scholar]
- Zeebe RE. On the molecular diffusion coefficients of dissolved CO2, HCO3–, and CO32– and their dependence on isotopic mass. Geochimica et Cosmochimica Acta. 2011;75:2483–2498. [Google Scholar]


