Abstract
Background
Prior studies examining patterns of esophagogastroduodenoscopy (EGD) surveillance in patients with Barrett’s Esophagus (BE) demonstrate variable adherence to practice guidelines. In prior studies, memories of endoscopic experiences shaped patients’ overall perceptions and subsequent adherence behaviors, but the specific elements of that experience are unclear. We therefore sought to identify elements of the EGD experience that frame patients’ memories and overall perceptions of surveillance.
Methods
We conducted structured in-depth, qualitative interviews with BE patients in a single regional medical center. We recruited patients with a range of severity of BE (non-dysplastic, low-grade and high-grade dysplasia) who recently completed an EGD. Data collection continued until we reached thematic saturation (n=20). We applied principles of framework analysis to identify emerging themes regarding patients’ salient EGD experiences. We validated our coding scheme through multidisciplinary consensus meetings comprised of clinician (gastroenterologist and internist) and non-clinician investigators (sociologist and public health expert).
Results
Patient experiences can be conceptualized within a temporal model of surveillance EGD: prior to endoscopy, during the endoscopy procedure, and after endoscopy. Within this model, the most memorable aspects of the EGD experience include physician-patient communication prior to EGD, wait time at the endoscopy center, interpersonal interactions at the time of the EGD, level of pain or discomfort with the procedure, level of trust in the physician following EGD, and gaining a sense of control over BE.
Conclusions
We identified six salient memories before, during, and after the procedure that shape patients’ perceptions of the EGD experience. We offer recommendations for measuring the patient experience of EGD using a composite of validated survey items. Future studies should test the relation of patient experience measures and adherence to surveillance EGD.
Keywords: Qualitative research, perceptions, barriers, endoscopy, Barrett’s esophagus screening
Introduction
Barrett’s Esophagus (BE) is the premalignant lesion for esophageal adenocarcinoma (EA), a particularly fatal cancer with 5-year survival rate of 15-20% for stages IIb-IV.1 The risk of developing EA is 30 to 50 times higher among patients with BE.2-5 Evidence suggests that some patients with BE can reduce their risk of EA-related mortality if dysplasia or early stage cancer is detected and treated through surveillance esophagogastroduodenoscopy (EGD).6 Current guidelines from the American College of Gastroenterology and American Society for Gastrointestinal Endoscopy recommend surveillance EGD every 3 years in patients with BE without dysplasia, every year in patients with low grade dysplasia until they have had two endoscopies without dysplasia, and every 3 months in patients with high grade dysplasia or those who undergo treatment.7;8
The American Gastroenterological Association (AGA) in partnership with Choosing Wisely, an American Board of Internal Medicine (ABIM) Foundation initiative, has emphasized the importance of guideline-concordant use of surveillance EGD.9 Prior studies examining utilization patterns of surveillance EGD in patients with BE found overutilization, underutilization, and evidence-discordant adherence to clinical practice guidelines for BE surveillance. A recent study of three tertiary-care referral centers found high rates of adherence to surveillance EGD.10 However, an analysis of national Department of Veterans Affairs (VA) data found relatively low rates (23%) of guideline-concordant surveillance EGD in routine care.11 Prior work has explored the role of patients’ perceptions of cancer risk12 and health-related quality of life,13 and type of insurance on the utilization and psychosocial effects of surveillance EGD.10 Less attention has been given to how patients frame their experiences with EGD and how these ‘patient experiences’ shape intentions to pursue BE surveillance.
Patients’ adherence to surveillance EGD may be shaped by their memories of previous experiences with endoscopy.14 The psychological science literature describes how memories are imprecise and susceptible to perceptual bias especially when they relate to painful or emotional experiences.15 For example, memories formed during the moment of greatest discomfort (peak) and at the final moments (end) of an endoscopy shape perceptions of prior endoscopy more than memories of the start of the procedure, average discomfort level, or duration of endoscopy.16 Redelmeier et al. found that patients randomized to an intervention that purposefully reduced patients’ discomfort during the end experience of a prior colonoscopy were 40% more likely to adhere to follow-up colonoscopy recommendations compared to those randomized to standard procedure.17 Furthermore, a recent systematic review of studies describing patients’ perceptions of surveillance EGD found that global judgments about surveillance EGD were often shaped by patients’ perceptions of the prior endoscopy experience.12 These findings underscore the importance of how patients’ experiences shape their overall judgment of EGD—and intention to adhere to surveillance EGD. However, no previous study has described the salient elements that comprise the patient experience of EGD. The aim of this study is to define the patient experience of EGD from in-depth qualitative interviews with patients who recently underwent surveillance EGD.
Methods
Recruitment and Consent
This study was approved by the Michael E. DeBakey VA Medical Center and the Baylor College of Medicine Internal Review Board. Our sample of participants was recruited from a regional Department of Veterans Affairs Medical Center. Potential participants were identified using a clinical BE registry augmented by a detailed electronic medical record review to confirm BE diagnosis. Recruitment and data collection occurred between March 2011 and July 2012. Patients were considered for inclusion if they: 1) were between the ages of 18-80 years of age; 2) had non-dysplastic BE and received at least one surveillance EGD; or 3) had dysplastic BE (low-grade or high-grade dysplasia). Patients were excluded from the study if their records indicated that they: 1) had a severe medical or psychiatric co-morbidity; 2) were hospitalized at the time of recruitment; 3) had a gastroesophageal disorder requiring endoscopy for reasons other than BE surveillance (i.e. esophageal cancer, gastroduodenal cancer, gastroduodenal ulcers, radiation, caustic ingestion, infectious esophagitis, or HIV); or 4) had anemia, bleeding, cirrhosis, or metastatic cancer. During recruitment, potential participants were told that the investigators were interested in learning about patients’ experiences with EGD in order to improve patients’ health care experiences. Two potential participants called to opt-out of the study and four people declined to participate (one person did not want to participate in the interview but completed the survey, and three refused to sign consent forms). Participants were scheduled for in-person or telephone-based interviews, approximately one hour long, and consent was obtained prior to all interviews. Only the participant and researcher were present during interviews.
We applied a stratified purposeful sampling technique to recruit patients who met our inclusion and exclusion criteria. Recruitment was stopped at the point of thematic saturation, defined a priori as the point when 2 independent coders agreed that 3 consecutive transcripts within a given interview category (see below) rendered no new thematic concepts.18;19 Interviews were designed to elicit information about patients’ experiences with EGD, their expectations and perceptions of their physicians and the endoscopy experience, risk of developing esophageal cancer, necessity of EGD, and their intentions to adhere to future EGDs. The interview guide was pilot tested and revised prior to initiating data collection. Additional details on methodology and the full interview guide are published elsewhere.12 All interviews were conducted by either JA or MHL (both female) and were recorded, transcribed, and analyzed for content. We used Atlas.ti 6.2 to facilitate data analysis and management.
Analysis
Data were analyzed using framework analysis methodology which allows for the inclusion of existing concepts as well as emergent themes within a pre-established theoretical framework.20 Additionally, our analysis was guided by principles of ethnographic research, which emphasizes the value of reflecting participants’ detailed descriptions of phenomena, rather than the researcher’s interpretation of participants’ experiences.21 Two independent coders (JA holds a Ph.D. in Sociology and MHL holds a Ph.D. in Health Promotion and Behavioral Science) with expertise and experience in framework analysis independently created codes and indexed the data using Atlas.ti 6.2. Disagreements about coding decisions were resolved through group consensus,22 using a clinical investigator with experience in coding as a third coder (AN is a medical geriatrician) and tie-breaker. Our coding centered on four themes:
Perceptions of EGD- patients’ beliefs about the procedure itself including what it will involve and any risks associated with EGD. This includes patients’ descriptions of doctor-patient communication about the purpose and risks of EGD, and what they should expect.
Experience of EGD- experience of having an EGD, including waiting room, descriptions of problems during the procedure, and feelings related to the staff performing the procedure.
Outcome expectancy of EGD- what patients expect to happen from EGD. Respondents are probed about “physical” outcomes, e.g., feel throat soreness or damage to esophagus; “cognitive” outcomes, e.g., curiosity or worry about the results; or prognosis of BE. Expected outcomes may originate from personal experiences with EGD, hearsay from others’ experiences, or from communication with healthcare providers.
Motivation for follow-up- elaboration on the reasons for the participants’ belief they should go through the EGD schedule or why they have decided to opt out of surveillance. We probe factors beyond risk perceptions and outcome expectancies that frame participants’ intentions to adhere/not adhere to surveillance EGD.
After coding was completed, the full study team including HES and JH (gastroenterologists with significant endoscopy experience) and RS (communication scientist) reviewed qualitative coding and participated in the integration of codes into a model of the patient experience with EGD.
Results
We interviewed a total of 20 BE patients (see Table 1). The median age of participants was 62.9 years; all were male. Nine patients were diagnosed with BE with no dysplasia; 10 patients with BE with low-grade dysplasia; and one patient with BE with high-grade dysplasia. Approximately 35% of the patients completed five or more surveillance EGDs, while 25% completed only 1 prior EGD. The mean number of completed EGDs was 4.3 with a range of 1-20. None of these EGD procedures were performed using propofol or monitored anesthesia care; most procedures (90%) were performed under conscious sedation with midazolam (2-4mg IV) and/ or mepiridine (25-75mg IV), combined with topical anesthesia to the back of the throat. Only two patients received no systemic sedation during their most recent EGD because of patient preference or lack of a designated driver following the EGD.
Table 1.
Baseline Characteristics of the Study Population
| Characteristics | N (%) |
|---|---|
| Male gender, n (%) | 20 (100) |
| Age in years, mean (standard deviation); range | 62.9 (7.32); 50-80 |
| Race, n (%) | |
| White | 19 (95) |
| Black | 1 (5) |
| Clinical Factors | |
| Diagnosis, n (%) | |
| Barrett’s Esophagus with no dysplasia | 10 (50) |
| Barrett’s Esophagus with low-grade dysplasia | 9 (45) |
| Barrett’s Esophagus with high-grade dysplasia | 1 (5) |
| Number of prior endoscopies, n (%) | |
| 1 | 5 (25) |
| 2-5 | 8 (40) |
| 6-9 | 5 (25) |
| > 10 | 2 (10) |
We present salient aspects of patients’ experience with EGD within a temporal model (i.e., prior, during, and following the EGD procedure). Corresponding selected quotations are presented in Table 2.
Table 2.
Participants’ Quotations Regarding Salient Aspects of Endoscopy
| PRIOR | POSITIVE | NEGATIVE |
|---|---|---|
| Communication |
|
|
| Wait time | • I’ve waited there all day before….They have people wait and wait and wait without any idea when they’re going to go in and most of the time it’s 6 to 8 hours [until you get in]. |
|
| DURING | POSITIVE | NEGATIVE |
| Treatment by Physicians | •The doctor told me not to worry about anything and they would take care of it…they sedated me and I woke up and, I mean I didn’t even know anything had been done…. it worked out really well so I hope that the doctors that I see next time is gonna be the same way…she really did a very good job. |
|
| Pain or Lack of Pain |
|
|
| AFTER | POSITIVE | NEGATIVE |
| Trust |
|
• I don’t like the way they…said they were gonna sedate me and they didn’t sedate me…They would try to force it down my throat and I don’t like that, and I’m very leery. My trust just went out the window |
| Feeling in Control of BE |
|
Prior to EGD
Patients’ recollections of the pre-EGD experience involved two themes: doctor-patient communication and wait time at the endoscopy center on the day of the EGD procedure. Patients’ narratives of the EGD experience often involved perceptions of how their doctors prepared them for the endoscopy procedure. In describing their EGD experiences, some patients recalled their physician explaining details about EGD instrument, mechanics of the procedure, specific risks, and likelihood of encountering problems during the procedure (see Table 2).
On the other hand, several patients mentioned that they did not recall detailed conversations with a physician about endoscopy, and were left with many questions about what to expect; they discussed their uncertainty about the endoscopy instrument, the purpose of the procedure, and what to expect after the EGD (see Table 2). Some patients voiced concerns about the risks of EGD, including the fear that the endoscopy could cause “punctures of the tissue by the instrument” (P19); or more generally, one patient worried about “somebody screwing the procedure up” (P18). Regarding wait time at the endoscopy center, many participants cited prolonged wait time in the waiting room for the procedure as a salient and unpleasant aspect of the EGD experience. For some patients, the wait time in conjunction with the requirement to fast in preparation for the procedure was particularly burdensome (see Table 2).
During EGD
Patients’ narratives of the EGD experience often included mentions of interpersonal interactions with the physician performing the EGD and references to the physical sensation of EGD, particularly concerning the degree of discomfort they experienced (see Table 2). A few patients discussed how well they were treated by their physician, for instance, describing how a physician eased their anxiety upon arriving for the procedure and comforted them during the procedure. On the other hand, several participants’ salient memories of EGD involved feelings of not being treated well or feeling disrespected. Such mentions of “disrespect” often involved patients’ requests being “ignored,” and others reported they were incapable of movement during the procedure because they were “strapped down” (see Table 2).
Many BE patients discussed the physical sensation of the procedure as a salient aspect of EGD. All mentions of physical sensations involved degree of discomfort they experienced. While some participants indicated that the sedation was so effective that they slept through the procedure and felt little or no discomfort afterwards; most described EGD as a painful experience. Descriptions of pain range from mild to very severe. Patients typically attributed their painful experiences to a lack of anesthesia or analgesia, despite the fact that all but two participants received conscious sedation (see Table 2).
Following EGD
Narratives of salient aspects of the EGD experience often involved references to trust in physicians and gaining a sense of control over their BE. Patients who felt informed, respected, and experienced little or no discomfort during an EGD often discussed having a high degree of trust in their doctors and in the endoscopy center more generally. On the other hand, patients who felt under-informed, disrespected, or experienced pain during an EGD often discussed a loss of trust in their doctors. Based on our interviews, trust (or lack thereof) in one’s physician remains a strong sentiment long after the EGD is completed (see Table 2).
In terms of gaining a sense of control over BE, several patients’ narratives included references to cancer prevention, alleviating worry about progression of BE, and even symptom management. Many patients acknowledged that EGD allows them to monitor progression of BE to cancer, and increases the likelihood of identifying problems in their early stages. Other patients acknowledged that while they may tend to worry about BE, EGD gives them a sense of control over BE. Thus, for many patients, the most salient aspect of the EGD experience is the sense of control they receive from having the BE monitored (see Table 2).
Perception of EGD and Intention to Adhere
Two aspects in particular—interpersonal interactions with clinicians and the perception of discomfort caused by the EGD—shape patients’ stated intentions to return for future EGDs. Patients who reported positive interactions with clinicians at the time of the procedure and those who experienced little or no pain generally expressed high intention to adhere to future procedures. On the contrary, patients who felt disrespected at the time of the procedure and those who had particularly painful experiences often expressed lower intention to adhere to future EGDs; these patients described feeling anxiety about pending procedures, and cancelling appointments.
Discussion
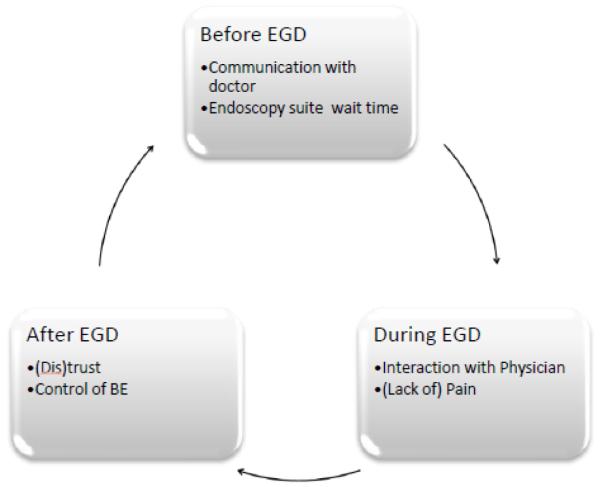
Qualitative analysis of in-depth interviews with BE patients about their salient memories of past EGDs revealed six aspects of surveillance EGD that were prominent in patients’ memories. Figure 1 illustrates a model of “the patient experience of EGD” temporally segmented into prior, during, and after the endoscopy procedure. Our findings suggest that patient experiences are framed by memories with strong emotions and particular impressions of the endoscopy experience occurring before, during, and after the procedure (see Figure 1).
Figure 1.
Model of the Patient Experience of Esophagogastroduodenoscopy (EGD)
Our analysis identified two particular memories associated with each of the temporal phases of the EGD experience. Memories of the pre-EGD experience involve communication that occurred prior to the procedure (including discussions about risks, sedation, the purpose of EGD, and what to expect on the day of the appointment) as well as experiences in the endoscopy suite’s waiting area just prior to the EGD. These accounts point to the importance of effective clinical communication, and are consistent with related research that showed greater satisfaction with colonoscopy was related to more effective provider-patient communication.23 Patient-clinician communication is an important mechanism for improving patients’ experiences and adherence behaviors.24-26
Patients’ salient memories of the EGD itself include feeling respected or disrespected and the level of discomfort experienced during the EGD. Consistent with previous research on important aspects of colonoscopy,27 the memory of pain is an important aspect of EGD that shapes patients’ overall perception of the procedure. These two aspects of EGD—feelings of respect/disrespect and pain—were most salient in shaping memories of the peak experience of EGD and therefore potentially impact future adherence behaviors.
Post-EGD memories involved references to keeping or losing trust in providers, particularly when they experienced a painful EGD. Patients’ trust in their physicians is an important predictor of their intentions to adhere to surveillance EGD.12 Some patients felt as though they had been deceived when they expected “full” sedation but still experienced discomfort or pain. Perceptions about sedation may be incongruous with the actual doses of sedation given as documented during the endoscopy procedure. Nevertheless, these perceptions may negatively impact trust in the endoscopist. Our findings point to the importance of clinical communication in shaping patients’ perceptions of the EGD experience.
Clinical Implications
Prior work suggests that patients’ experiences of colonoscopy, defined by their subjective (and often biased) recall of prior colonoscopy procedures, predicted adherence to surveillance colonoscopy.16;17 In the context of this prior work, our findings suggests that patient experiences with EGD may influence adherence to future endoscopy and subjective quality ratings for the endoscopist. Physicians who take a mindful approach to how they communicate about these specific elements may improve how patients evaluate the importance of EGD and their EGD experience.28 Results drawn from validated patient-reported measures describing the salient elements of the EGD experience can guide subsequent clinical communication. For example, we recommend developing a post-EGD survey using existing validated measures to elicit patients’ perceptions of the six elements of the EGD experience revealed in our analysis (see Table 3). When evaluating patient experiences, Manary and colleagues suggest using measures that describe a specific encounter, focusing on patient-clinician interactions, and doing so in a timely manner, instead of more global measures of patient satisfaction unrelated to a specific encounter.29 In prior studies of patient experiences of care, such measures were associated with adherence to prescribed treatment regimens and patient outcomes (i.e. readmissions, mortality).29
Table 3.
Components of a Potential Composite Measure of the Patient Experience of Esophagogastroduodenoscopy (EGD).
| Domain | Example of Validated Measure |
Scale Description | Number of Items |
|---|---|---|---|
| Patient- Physician Communication |
Consumer Assessment of Healthcare Providers and Systems (CAHPS): Measures for the Adult Visit Survey |
How Well Providers (or Doctors) Communicate with Patients: how well they explain things, listen, show respect, were knowledgeable, allowed adequate time during the visit, and provided easy instructions. |
6 items scored on a 3 point verbal scale. |
| Pain during procedure |
National Institutes of Health (NIH) Toolbox |
Pain Intensity Scale measures the patient’s immediate level of pain. |
1 item rated 0-10 using a numeric rating scale |
| Anxiety related to EGD |
Impact of Event Scale- Revised (IES-R) |
Measures the level of anxiety/stress the patient has experienced within 30 days of the traumatic event (EGD). |
22 items rated on a 5 point scale (0-4). Can use a subscale of this measure. |
| Trust in Endoscopist |
Trust in Physician Scale (TIPS) |
Measures two primary dimensions: trust in physician’s and trust in the Veteran’s Affairs hospital. |
9 items rated on 5 point Likert scale |
| Perceptions of Control |
Salience and Coherence of surveillance endoscopy |
Measures patient’s attitude and belief or their personal value placed on receiving an EGD. |
4 items rated on 5 point Likert scale |
Table 3 describes a potential post-EGD assessment of patient experiences using questions about their communication with health care providers and wait time experiences prior to their EGD.29 The CAHPS® (Consumer Assessment of Healthcare Providers and Systems) Survey includes a subscale to measure the patient’s experience while communicating with physicians.30 The remaining four elements of the patient experience could be assessed using questions taken from previously validated psychosocial scales. In a similar population, the Impact of Event Scale-Revised (IES-R)31 measured the level of anxiety patients experienced after the EGD. We recommend measuring trust with the Trust in Physician Scale (TIPS),32 which measures both trust in the physician and trust in one’s health care institution. Pain intensity experienced during the EGD could be measured using a single-item numerical rating scale.33;34 We recommend including a few questions to gauge the patients’ attitude about participating in surveillance EGDs; for example “I believe that surveillance endoscopies can help to protect my health” and “I think that the benefits of surveillance endoscopy outweigh any difficulty I might have in going through the test”.35 A composite patient experience survey using these measures could be easily implemented. It is not unusual for clinic staff to call patients after EGD. A short telephone survey would be both feasible and informative. Results of a patient experience survey may identify strengths and weaknesses in EGD care, which could influence organizational change, may improve adherence to surveillance EGD, and provide more clinically useful measures of patient satisfaction.29
Like most qualitative analyses, our study findings are limited in their generalizability beyond the study population. However, we sampled a representative group of typical BE patients including those with and without dysplasia. An additional limitation is that our sample population is mostly open access endoscopy; that is, some patients may not have spoken to a gastroenterologist before the day of procedure (therefore all discussion of EGD prior to the procedure may have been with a primary care practitioner), and they may not have an ongoing consistent clinical relationships with the specialist performing the procedure. While this is a common way of referral for endoscopy in many practice settings, the factors involved might impact patients’ experiences and their level of trust in providers. Despite these limitations, our findings provide novel perspectives on patients’ experiences of EGD and how their perceptions of those experiences relate to their stated intentions to undergo future procedures. Recent studies have highlighted the importance of measuring and understanding patients’ experiences as a potential factor impacting quality of care and health outcomes.25;29
In summary, our analysis of in-depth interviews uncovered patients’ most memorable experiences of surveillance EGD. These salient memories include wait time at the endoscopy center, provider-patient communication, discomfort during endoscopy, feelings of personal respect, trust in providers, and feelings of control over BE. By identifying these salient memories, we have gained a better understanding of factors that shape the patient experiences of EGD. These experiences can be measured using previously validated patient-reported scales. Future studies can explore the relationship of these validated measures of the patient experience and patients’ adherence to surveillance EGD as well as identify modifiable targets for interventions to improve the quality and patient-centeredness of endoscopy care.
Study Highlights.
- WHAT IS CURRENT KNOWLEDGE?
- Past research shows inconsistent findings regarding patients’ judgments of and adherence to surveillance EGD for Barrett’s Esophagus.
- Patients’ adherence to surveillance endoscopy is shaped by their memories of previous experiences with endoscopy.
- WHAT IS NEW HERE?
- While other studies have focused on patients’ risk perceptions and external factors, ours is the first to explore the importance of the patient experiences with EGD.
- Six distinct salient memories frame the patient experience with EGD: 1) patient-physician communication prior to EGD, 2) wait time at the endoscopy center, 3) interpersonal interaction with staff on day of EGD, 4) level of pain and discomfort with procedure, 5) level of trust with physician following EGD, and 6) feelings of control over BE after completing EGD.
- We have linked our findings to validated, self-report measures that can be used to create a composite measure of the patient experience with EGD.
Acknowledgments
GRANT SUPPORT: This work was supported by NIH grant RC4CA155844 awarded to Dr. El-Serag and the Texas Digestive Disease Center NIH DK58338. Additional resources and support was provided by the Houston VA Health Services Research & Development Center of Excellence (HFP90-020), American College of Gastroenterology Junior Faculty Development Award (J.K. Hou), and VA Office of Academic Affairs, Advanced Fellowship in Health Services Research (J. Arney). The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or National Institutes of Health
Footnotes
Guarantor of the article: Drs. Arney and Naik had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Specific author contributions: J. Arney participated in study design, interview protocol, acquisition, analysis, and interpretation of data and drafting of the manuscript; M. Hinojosa-Lindsey participated in study design, interview protocol, acquisition, analysis, and interpretation of data and drafting of results section; J. Hou contributed in study design, data interpretation, and editorial input in the manuscript. R. Street contributed to study design, interview protocol, data analysis, and revisions of the manuscript. H.B. El-Serag secured funding for the project, participated in study design, and provided critical revisions of the manuscript. A. Naik participated in study design, interview protocol, data analysis and interpretation, and critical revisions of the manuscript. All authors approved the final draft of this manuscript.
Potential competing interests: The authors report no conflicts of interest.
References
- 1.Sharma P, McQuaid K, Dent J, et al. A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology. 2004;127:310–30. doi: 10.1053/j.gastro.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Pohl H, Welch HG. The role of everdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–6. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.O’Connor JB, Falk GW, Richter JE. The incidence of adenocarcinoma and dysplasia in Barrett’s esophagus: report on the Cleveland Clinic Barrett’s Esophagus Registry. Am J Gastroenterol. 1999;94:2037–42. doi: 10.1111/j.1572-0241.1999.01275.x. [DOI] [PubMed] [Google Scholar]
- 5.Drewitz DJ, Sampliner RE, Garewal HS. The incidence of adenocarcinoma in Barrett’s esophagus: a prospective study of 170 patients followed 4.8 years. Am J Gastroenterol. 1997;92:212–5. [PubMed] [Google Scholar]
- 6.Cooper GS, Kou TD, Chak A. Receipt of previous diagnoses and endoscopy and outcome from esophageal adenocarcinoma: a population-based study with temporal trends. Am J Gastroenterol. 2009;104:1356–62. doi: 10.1038/ajg.2009.159. [DOI] [PubMed] [Google Scholar]
- 7.Hirota WK, Zuckerman MJ, Adler DG, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570–80. doi: 10.1016/j.gie.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Wang KK, Sampliner RE. Practice Parameters Committee of the American College of Gastroenterology. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788–97. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 9.American Board of Internal Medicine Foundation [Accessed May 13, 2013];Choosing Wisely: Five Things Physicians and Patients Should Question. Website 2013. http://www.choosingwisely.org/doctor-patient-lists/american-gastroenterological-association/
- 10.Crockett SD, Lipkus IM, Bright SD, et al. Overutilization of endoscopic surveillance in nondysplastic Barrett’s esophagus: A multicenter study. Gastrointest Endosc. 2012;75:23–31. doi: 10.1016/j.gie.2011.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Serag HB, Duan Z, Hinojosa-Lindsey M, et al. Practice patterns of surveillance endoscopy in a Veterans Affairs database of 29,504 patients with Barrett’s esophagus. Gastrointest Endosc. 2012;76(4):743–55. doi: 10.1016/j.gie.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinojosa-Lindsey M, Arney J, Heberlig S, et al. Patients’ intuitive judgments about surveillance endoscopy in Barrett’s Esophagus: A review and application to models of decision making [Published online: 5 FEB 2013] Dis Esophagus. doi: 10.1111/dote.12028. DOI: 10.1111/dote.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crockett SD, Lippmann QK, Dellon ES, et al. Health related quality of life in patients with Barrett’s Esophagus: A systematic review. Clin Gastroenterol Hepatol. 2009;7(6):613–23. doi: 10.1016/j.cgh.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erskine A, Morley S, Pearce S. Memory for pain: A review. Pain. 1990;41(3):255–65. doi: 10.1016/0304-3959(90)90002-U. [DOI] [PubMed] [Google Scholar]
- 15.Fredrickson BL, Kahneman D. Duration neglect in retrospective evaluations of affective episodes. J Pers Soc Psychol. 1993;65:44–55. doi: 10.1037//0022-3514.65.1.45. [DOI] [PubMed] [Google Scholar]
- 16.Redelmeier DA, Kahneman D. Patient’s memories of painful medical treatments: Real-time and retrospective evaluations of two minimally invasive procedures. Pain. 1996;66(1):3–8. doi: 10.1016/0304-3959(96)02994-6. [DOI] [PubMed] [Google Scholar]
- 17.Redelmeier DA, Katz J, Kahneman D. Memories of colonoscopy: A randomized trial. Pain. 2003;104:187–194. doi: 10.1016/s0304-3959(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 18.Morse J. The significance of saturation. Qualitative Health Research. 1995;5:147–9. doi:10.1177/104973239500500201. [Google Scholar]
- 19.Aita VA, Mcllvain HE, Crabtree BF, Miller WL, editors. Doing Qualitative Research. Sage; London: 1999. An armchair adventure in case study research. [Google Scholar]
- 20.Ritchie J, Spencer L. The Qualitative Researcher’s Companion. Sage Publications; London, UK: 2002. Qualitative data analysis for applied policy research; pp. 305–29. [Google Scholar]
- 21.Gubrium JF, Holstein JA. The New Language of Qualitative Method. Oxford University Press; New York: 1997. [Google Scholar]
- 22.Waitzken H. The Politics of Medical Encounters: How Patients and Doctors Deal with Social Problems. Yale University Press; New Haven, CT: 1991. [Google Scholar]
- 23.Larkins AS, Windsor AVC, Trebble TM. An evaluation of patient attitudes to the gastroenterology outpatient experience. Eur J Gastroenterol Hepatol. 2013;25:44–55. doi: 10.1097/MEG.0b013e3283589f80. 2013. [DOI] [PubMed] [Google Scholar]
- 24.Street RL, Makoul G, Arora NK, et al. How does communication heal? Pathways linking clinician-patient communication to health outcomes. Patient Educ Counsel. 2009;74(3):295–301. doi: 10.1016/j.pec.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Naik AD. On the Road to Patient-Centeredness. JAMA Internal Medicine. 2013;173(3):218–9. doi: 10.1001/jamainternmed.2013.1229. [DOI] [PubMed] [Google Scholar]
- 26.Naik AD, Kallen M, Walder A, et al. Improving Hypertension Control in Diabetes: The effect of collaborative and proactive health communication. Circulation. 2008;117(11):1361–8. doi: 10.1161/CIRCULATIONAHA.107.724005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McEntire J, Sahota J, Hynes T, et al. An evaluation of patient attitudes to colonoscopy and the importance of endoscopist interaction and the endoscopy environment to satisfaction and value. BMC Health Services Res. 2013;13:22. doi: 10.3109/00365521.2012.758768. [DOI] [PubMed] [Google Scholar]
- 28.Naik AD, Hinojosa-Lindsey M, Arney J, et al. Choosing Wisely and the perceived drivers of endoscopy use. Clin Gastroenterol Hepatol. 2013;11(7):753–5. doi: 10.1016/j.cgh.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manary MP, Boulding W, Staelin R, et al. The patient experience and health outcomes. N Engl J Med. 2013;368:201–3. doi: 10.1056/NEJMp1211775. [DOI] [PubMed] [Google Scholar]
- 30. [Accessed on June 24, 2013];Patient Experience Measures from the CAHPS Clinician and Group Surveys. 2012 www.cahps.ahrq.gov/clinician_group/cgsurvey/patientexperiencemeasurescgsurveys.pdf.
- 31.Essink-Bot ML, Kruijshaar ME, Bac DJ, et al. Different perceptions of the burden of upper GI endoscopy: An empirical study in three patient groups. Qual Life Res. 2007;16:1309–18. doi: 10.1007/s11136-007-9239-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon HS, Street RL, Jr., Sharf BF, et al. Racial differences in trust and lung cancer patients’ perceptions of physician communication. J Clin Oncol. 2006;24:904–9. doi: 10.1200/JCO.2005.03.1955. [DOI] [PubMed] [Google Scholar]
- 33. [Accessed on June 3, 2013];NIH Toolbox: For assessment of neurological and behavioral function. doi: 10.1212/WNL.0b013e3182872e5f. www.nihtoolbox.org. [DOI] [PMC free article] [PubMed]
- 34.Cook KF, Dunn W, Griffith JW, et al. Pain assessment using the NIH Toolbox. Neurology. 2013;80:S49. doi: 10.1212/WNL.0b013e3182872e80. Doi 10.1212/WNL.0b012e3182872e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vernon SW, Myers RE, Tilley BC. Development of an instrument to measure factors related to colorectal cancer screen adherence. Cancer Epidemiol Biomarkers Prev. 1997;6:825–32. [PubMed] [Google Scholar]