Abstract
There is a need to seek new treatment(s) for Alzheimer's disease (AD). A recent study showed that AD patients may have decreased levels of functional GABA receptors. Propofol, a commonly used anesthetic, is a GABA receptor agonist. We therefore set out to perform a proof of concept study to determine whether chronic treatment with propofol (50 mg/kg/week) can improve cognitive function in both aged wild-type (WT) and AD transgenic (Tg) mice. Propofol was administrated to the WT and AD Tg mice once a week for 8 or 12 weeks, respectively. Morris water maze was used to assess the cognitive function of the mice following the propofol treatment. Activation of caspase-3, caspase-9, and caspase-8 was investigated using western blot analysis at the end of the propofol treatment. In the mechanistic studies, effects of propofol, amyloid-β protein (Aβ), and GABA receptor antagonist flumazenil on caspase-3 activation and opening of the mitochondrial permeability transition pore were assessed in H4 human neuroglioma and mouse neuroblastoma cells by western blot analysis and flow cytometry. Here we showed that the propofol treatment improved cognitive function and attenuated brain caspase-3 and caspase-9 activation in both aged WT and AD Tg mice. Propofol attenuated Aβ-induced caspase-3 activation and opening of the mitochondrial permeability transition pore in the cells, and flumazenil inhibited the propofol's effects. These results suggested that propofol might improve cognitive function via attenuating the Aβ-induced mitochondria dysfunction and caspase activation, which explored the potential that anesthetic propofol could improve cognitive function in elderly and AD patients.
Keywords: Aging, Alzheimer's disease, amyloid-β protein, anesthesia, apoptosis, mitochondria, neurodegeneration, propofol
INTRODUCTION
Alzheimer's disease (AD), an aging associated disorder, is the most common form of dementia, and is characterized by global cognitive decline and the robust accumulation of amyloid deposits and neurofibrillary tangles in the brain (reviewed in [1]). The current treatments for AD are acetylcholinesterase inhibitor donepezil (Aricept), rivastigmine tartrate (Exelon), galantamine hydrobromide (Razadyne), and noncompetitive antagonist of the N-methyl-D-aspartate (NMDA) receptor memantine hydrochloride (Namenda), which were approved by the Food and Drug Administration in 1996, 1998, 2001, and 2003, respectively (reviewed in [2]). Numerous additional medications and substances have also been investigated, aiming to treat or prevent AD. Recently, bapineuzumab, a humanized anti-amyloid-β (Aβ) monoclonal antibody [3], and solanezumab, another humanized monoclonal antibody [4] failed to improve cognitive function in AD patients. Therefore, seeking innovative AD therapeutics is important.
Accumulation and deposition of Aβ and caspase activation have been reported as parts of AD neuropathogenesis (reviewed in [1]). Specifically, increasing evidence suggests a role for caspase activation and apoptosis in AD neuropathogenesis ([5–14], reviewed in [15, 16]), even though the contribution of apoptosis to neuronal loss in AD remains debatable (reviewed in [17, 18]). This is most likely due to the long duration of AD and very rapid clearance of apoptotic cells from organs. A recent study suggests that caspase activation even without apoptosis can contribute to AD neuropathogenesis [19]. Mitochondrial dysfunction may also contribute to AD neuropatho-genesis ([20], reviewed in [1, 21]). Specifically, it has been reported that mitochondrial dysfunction causes caspase activation [22–24], which then leads to AD neuropathogenesis [19, 25–27]. Furthermore, a recent study has shown that AD patients may have an age-dependent decrease of gamma-aminobutyric acid (GABA) currents in the AD brain, and this reduction was associated with decreased mRNA and protein levels of GABA receptor subunits [28]. These findings suggest that enhancing GABA neurotransmission could be a strategic potential of AD treatment.
Propofol (2, 6-disopropylphenol), an intravenous anesthetic, is a GABA receptor agonist [29]. Propofol can attenuate the caspase-3 activation and Aβ oligomerization induced by the anesthetic isoflurane [30]. Moreover, preliminary data have shown that propofol may improve cognitive function in humans [31]. However, the effects of propofol on learning and memory function in animals still remain controversial [32–36]. Moreover, these studies assessed the acute effects of propofol on the learning and memory in rodents. The effects of chronic treatment with propofol on the learning and memory function in rodents have not been assessed.
We therefore set out to test a hypothesis that weekly treatment with propofol can attenuate the aging and AD gene mutation-associated caspase-3 activation and enhance cognitive function in aged wild-type (WT) mice and AD transgenic (Tg) mice. The rationale to establish a weekly treatment of propofol in mice is to promote further studies, which may ultimately lead to utilization of a chronic treatment of propofol to improve cognitive function in humans. The AD Tg mice [B6.Cg-Tg (APPswe, PSEN1dE9) 85Dbo/J] have the same genetic background as the WT mice (C57BL/6J) but with two mutant genes linked to familial AD: APP and PSEN1 [37, 38]. The senile plaques and increases in levels of soluble and insoluble Aβ40 and Aβ42 were detected in these AD Tg mice as early as 4 months of age [37]. The radial arm water maze test showed that the AD Tg mice developed cognitive impairments starting at 6 months of age as compared to the WT controls, and the cognitive impairments were exacerbated at 12 months [38].
Finally, in H4 human neuroglioma cells and mouse neuroblastoma cells, we investigated the potential mitochondria and GABA receptor-associated underlying mechanisms by which propofol might attenuate the caspase-3 activation.
MATERIALS AND METHODS
Mice and cells
The animal protocol was approved by the Standing Committee on Animals at Massachusetts General Hospital, Boston, Massachusetts. WT mice (C57BL/6J), The Jackson Lab, Bar Harbor, ME) and AD Tg mice [B6.Cg-Tg (APPswe, PSEN1dE9) 85Dbo/J (The Jackson Laboratory) were used in the study. There were 10 WT or 10 AD Tg mice in the propofol treatment group or control group, respectively. We employed H4 human neuroglioma cells (H4 naïve cells) and mouse neuroblastoma cells (N2A cells) for the mechanistic studies.
Treatment for mice
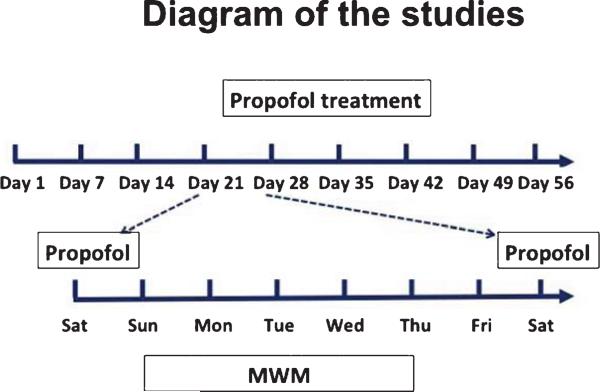
As demonstrated in the diagram (Fig. 1), the WT mice, at the age of 18 months, were randomized by weight and gender into experimental groups, which received propofol (APP Pharmaceuticals, Inc., Schaumburg, IL) treatment [50 mg/kg, intraperitoneal (IP) injection], and control groups, which received the same volume of saline, once a week every Saturday (day 1, 7, 14 and 21 in the diagram) for four weeks. The treatment with 50 mg/kg propofol induced sedation in the mice, as evidenced by decreased function of the righting reflex. After the fourth (the last) dosing (day 21 in the diagram), the mice were tested in the Morris water maze (MWM) from Sunday to Thursday. The mice received another round of propofol or saline treatment on Saturday (day 28 in the diagram) for another four weeks (day 28, 35, 42, and 49), followed by MWM test from Sunday to Thursday (day 50 to 54 in the diagram). The AD Tg mice, at 19 months, were randomized by weight and gender into experimental groups, which received 50 mg/kg propofol (IP) weekly for four weeks, and control groups, which received saline weekly for four weeks, and assessed the cognitive function in the mice. We continued the treatment with 50 mg/kg propofol and the assessment of learning and memory function for an additional 8 weeks (total of 16 weeks).
Fig. 1.
The diagram of the studies. The treatment of propofol occurred on Saturdays (day 1, 7, 14, 21, 28, 35, 42, and 49). The MWM was performed from Sunday to Thursday.
Treatment for H4 naïve cells and N2A cells
In the in vitro caspase activation studies, H4 naïve cells were treated with DMSO or 5 μM Aβ42 for 1 h, followed by 100 μM propofol or saline for 6 h as described in our previous studies with modification [30]. N2A cells were treated with DMSO or 5 μM Aβ42 (Yale University, New Haven, CT) for 1 h, followed by the treatment with 100 μM propofol or saline for 3 h in the mPTP studies as described in our previous studies [39]. In the flumazenil (Sigma, St. Louis, MO) experiments, 20 μM flumazenil [40] was added immediately after Aβ administration in the H4 naïve cells and in the N2A cells.
Tissue preparation
One day after the last propofol or saline treatment (e.g., day 56 in the diagram), mice were decapitated, and the brain cortex and hippocampus were harvested. The harvested cortex or hippocampus was homogenized on ice with an immunoprecipitation buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM ethylenediaminetetraacetic acid, 0.5% Nonidet P-40) plus protease inhibitors (1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin A). The lysates were centrifuged at 14,000 rpm for 15 min, and quantified for total protein concentration by a bicinchoninic acid protein assay kit (Pierce, Iselin, NJ). The harvested H4 naïve cells and brain tissues were subjected to western blot analyses as described in our previous studies [30, 39].
Western blot analysis
A caspase-3 antibody (1:1000 dilution; Cell Signaling Technology, Danvers, MA) was used to recognize full-length caspase-3 (35–40 kDa) and caspase-3 fragment (17–20 kDa) resulting from cleavage at aspartate position 175. Caspase-8 antibody (Cell Signaling) and caspase-9 antibody (Cell Signaling) were used to detect caspase-8 (18 kDa) and caspase-9 (17 kDa), respectively. Antibody anti-β-Actin (1:10,000, Sigma) was used to detect β-Actin (42 kDa). Each band in the western blot represented an independent experiment. The results were averaged from 6 to 12 independent experiments. We quantified the western blots in two steps. First, we used β-Actin levels to normalize protein levels (e.g., determining the ratio of caspase-3 fragment to β-Actin amount) and to control for loading differences in the total protein amount. Second, we presented protein level changes in brain tissues of mice or cells treated with propofol as a percentage of those in the control group. 100% of protein level changes refer to control levels for the purpose of comparison to experimental conditions.
Flow cytometric analysis of mitochondrial permeability transition pore (mPTP) opening
Opening of mPTP (MitoProbe Transition Pore Assay Kit, Invitrogen, Carlsbad, CA) was detected by flow cytometry as described in our previous studies [41]. Briefly, in normal conditions, the non-fluorescent acetoxymethyl ester (AM) of calcein dye (calcein AM) and cobalt can enter the cells. The acetoxymethyl ester groups are cleaved from calcein via non-specific esterase, and calcein can then show fluorescence signal in both the cytosol and mitochondria. Cobalt can quench the cytosolic calcein signal. However, cobalt cannot enter healthy mitochondria freely, and therefore cannot quench the mitochondrial calcein signal. When the opening of mPTP occurs, cobalt enters through the pore and subsequently quenches the mitochondrial calcein signal. Flow cytometry was used to detect the amount of cells that exhibit quenched calcein signal inside the mitochondria. The location of the curves indicates the amount of such cells, which suggests the opening of mPTP. Inomycin was used as a positive control for the opening of mPTP in the experiments. Dead cells and debris were excluded from analysis by gates set on a forward and side angle light scatter.
Morris water maze (MWM)
MWM was carried out to assess spatial learning and memory function as previously described with modification [42–44]. Briefly, all mice were trained to swim to a hidden platform in four trials per day for 5 days (day 1–5) starting one day after the fourth treatment with propofol. The mice were given 90 s to find the platform and allowed to stay for 10–15 s before being removed from the pool. If a mouse could not find the platform within 90 s, it was gently guided to the platform and allowed to remain there for 10–15 s. The platform was placed in the target quadrant for all trials within one MWM test, but the starting points were random for each mouse. The WT mice had two MWM tests and the AD Tg mice had four MWM tests. The platform was randomly placed in different target quadrants in each of these MWM tests. We trained the mice by using a cue on the wall (cued training). We measured the time it took for each mouse to reach the platform (escape latency) and used this as the learning score. On the fifth day, we also tested the memory of each mouse by removing the platform and measuring the number of times the mouse crossed the platform area with the same cue to obtain the memory score. We compared the learning and memory score and swimming speed between the mice in the propofol groups and saline groups.
Statistics
Given presence of background caspase activation in cells or brain tissues of mice, we did not use absolute values to describe these changes. Instead, the caspase activation was presented as a percentage of that of the control group. Data were expressed as mean ± standard deviation (SD). The number of samples in vitro varied from 6 to 10, and the samples were normally distributed (tested by normality test). For MWM test, escape latency (expressed as mean ± SD) were recorded to assess ability of learning. Escape latency was analyzed by two-way ANOVA for interaction between treatment and time. Post-hoc test (Bonferroni) was used to compare the difference in escape latency between the control and anesthesia group in each day of the MWM. Platform crossing times, which assessed the ability of memory, was analyzed by Mann-Whitney test. Student-t test was used to analyze the difference between Aβ, propofol, and/or flumazenil on caspase-3 activation. p-values less than 0.05 and 0.01 were considered statistically significant. SAS software (Cary, NC) and Prism 6 software (La Jolla, CA) were used to analyze the data.
RESULTS
Propofol improved cognitive function in aged WT mice
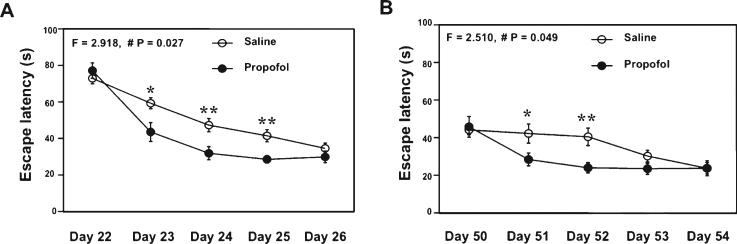
The effects of propofol on learning and memory have been investigated but remain controversial [32–36]. We therefore set out to determine whether weekly treatment with propofol can enhance cognitive function and attenuate the aging and AD gene mutation-associated caspase-3 activation in the brain tissues of aged WT mice and AD Tg mice. In the MWM test (Fig. 2), the propofol treatment decreased the escape latency of MWM as compared to saline treatment in the aged WT mice at four weeks (Fig. 2A) and 8 weeks (Fig. 2B) after the treatment. Two-way ANOVA with repeated measurement illustrated a significant interaction of time and group in the escape latency during the reference training between the propofol and saline treatment (Fig. 2A, F = 2.918, p = 0.027; Fig. 2B, F = 2.510, p = 0.049). The post hoc test showed that the mice that received the propofol treatment had shorter escape latency than the mice that had saline treatment at day 23, day 24, day 25 (Fig. 2A), and day 51 and day 52 (Fig. 2B). The propofol treatment did not significantly alter the platform crossing times in the MWM test (data not shown). There was no significant difference in swimming speed between the mice receiving propofol treatment and the control mice (data not shown). These results suggested that the weekly treatment with propofol might specifically improve the spatial learning function in the aged mice.
Fig. 2.
Propofol improves learning and memory function in aged WT mice. A) Propofol (black circle) decreases escape latency as compared to saline (white circle) after four week treatment in 18 month-old WT mice. B) Propofol (black circle) decreases escape latency as compared to saline (white circle) after another four week treatment in 19 month-old WT mice. WT, wild-type. n = 10.
Propofol attenuated caspase-3 activation through the mitochondrial pathway in aged WT mice
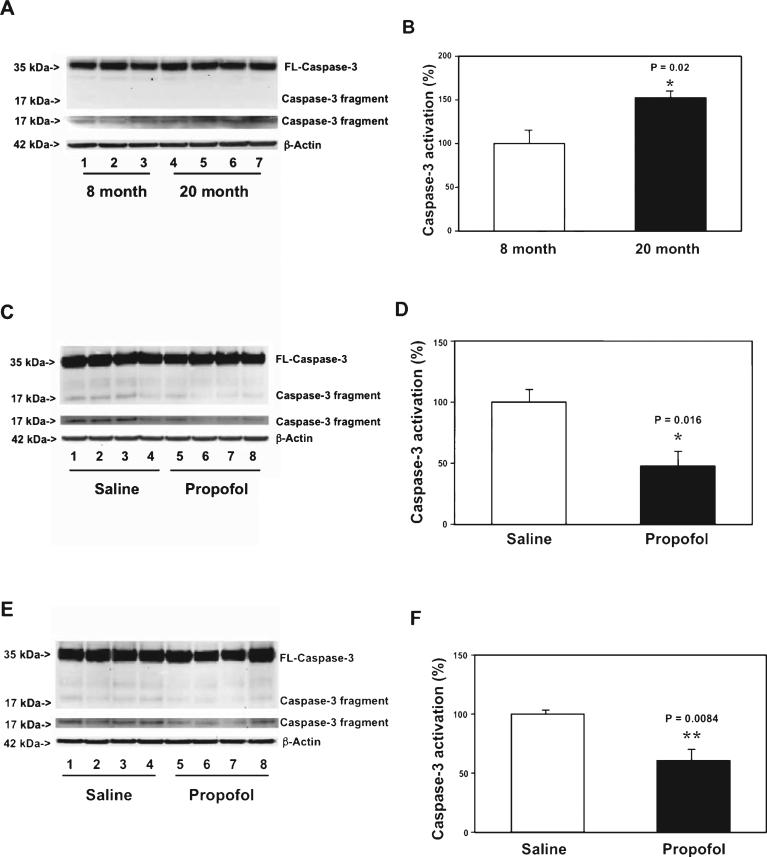
Caspase activation in the brain, even without apoptosis, has been reported to be part of AD neuropathogenesis and to contribute to cognitive dysfunction [19]. It is also known that aging is a risk factor of AD [1]. Given that the chronic treatment of propofol could improve the learning function in the aged mice, next, we determined the potential underlying mechanisms by assessing the effects of the chronic treatment of propofol on caspase-3 activation in brain tissues. First, we assessed and compared the caspase-3 activation in the cortex of 8 month-old WT mice and 20 month-old mice. Immunoblotting of caspase-3 showed that there was a visible increase in the levels of caspase-3 fragment in the cortex of 20 month-old mice as compared to that of 8 month-old mice (Fig. 3A). The quantification of the western blot, based on the ratio of caspase-3 fragment to full-length caspase-3, illustrated that there was a greater baseline caspase-3 activation in the cortex of 20 month-old mice than that in the cortex of 8 month-old mice (Fig. 3B): 152% versus 100%, p = 0.020. These data suggested that greater caspase-3 activation in brain tissues could be associated with aging. Then, we assessed the effects of the chronic treatment with propofol on the aging-associated caspase-3 activation. The brain tissues were harvested one day after the final propofol treatment. Immunoblotting of caspase-3 found that the mice following the chronic propofoltreatmenthadlowervisiblelevelsofcaspase-3 fragment in the cortex as compared to the mice following saline treatment (Fig. 3C). The quantification of the caspase-3 activation, based on the ratio of caspase-3 fragment to the full-length-caspase-3, showed that propofol attenuated the caspase-3 activation (Fig. 3D): 48% versus 100%, p = 0.016. Moreover, propofol also attenuated the caspase-3 activation in the hippocampus ofthemiceascomparedtothesalinetreatment(Fig.3E, F): 61% versus 100%, p = 0.0084. Taken together, these findingssuggestedthatanestheticpropofolmightattenuate the aging-associated caspase-3 activation in both the cortex and hippocampus of mice.
Fig. 3.
Propofol attenuates the aging-associated caspase-3 activation in cortex and hippocampus of WT mice. A) There is a greater caspase-3 activation in the cortex of 20 month-old WT mice (lanes 4-7) as compared to 8 month-old WT mice (lanes 1-3). There is no significant difference in amounts of β-Actin between the two groups. B) Quantification of the western blot shows that there is a greater caspase-3 activation in the cortex of 20 month-old WT mice (black bar) as compared to that of 8 month-old mice (white bar). C) Propofol (lanes 5-8) decreases caspase-3 activation as compared to saline (lanes 1-4) in the cortex of 20 month-old WT mice. There is no significant difference in amounts of β-Actin in saline- or propofol-treated mice. D) Quantification of the western blot shows that propofol (black bar) decreases the caspase-3 activation as compared to saline (white bar) in the cortex of the 20 month-old WT mice. E) Propofol (lanes 5-8) decreases caspase-3 activation as compared to saline (lanes 1-4) in the hippocampus of 20 month-old WT mice. There is no significant difference in amounts of β-Actin in saline- or propofol-treated mice. F. Quantification of the western blot shows that propofol (black bar) decreases the caspase-3 activation as compared to saline (white bar) in the hippocampus of 20 month-old WT mice. FL, full length; WT, wild-type. n = 6.
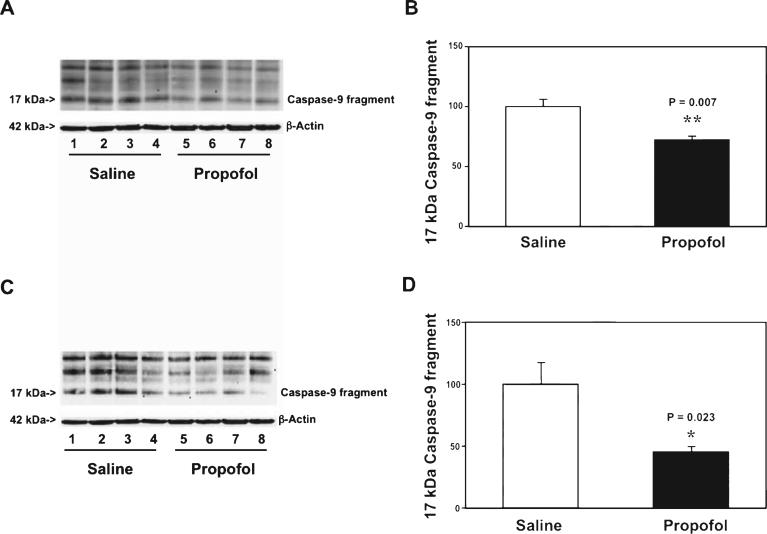
Next, we asked whether propofol could specifically attenuate the mitochondria- or death receptor-associated caspase-3 activation. We therefore assessed the effects of propofol on caspase-9 (the marker of mitochondria-associated caspase activation) [45] and caspase-8 (the marker of death receptor-associated caspaseactivation)[45]inthebraintissuesofthemice.The immunoblotting of caspase-9 showed that there was a visible reduction in the caspase-9 fragment level in the cortex of mice following propofol treatment as compared to saline treatment (Fig. 4A). The quantification of the western blot demonstrated that there was a reduction of caspase-9 fragment level in the cortex of mice following propofol treatment as compared to the saline treatment (Fig. 4B): 72% versus 100%, p = 0.007. We also found that there was a reduction of caspase-9 fragment level in the hippocampus of the mice following the propofol treatment as compared to the saline treatment (Fig. 4C, D): 45% versus 100%, p = 0.023. There was no significant difference in the caspase-8 fragment level in the cortex and hippocampus of the mice following either the propofol treatment or the saline treatment (data not shown). Collectively, these findings suggested that propofol might specifically affect the mitochondria-associated caspase activation, leading to attenuation of the caspase-3 activation in brain tissues (cortex and hippocampus) of the aged mice.
Fig. 4.
Propofol attenuates the aging-associated caspase-9 activation in the cortex and hippocampus of WT mice. A) Propofol (lanes 5-8) decreases caspase-9 activation as compared to saline (lanes 1-4) in the cortex of 20 month-old WT mice. There is no significant difference in amounts of β-Actin in saline- or propofol-treated mice. B) Quantification of the western blot shows that propofol (black bar) attenuates the caspase-9 activation in the cortex of 20 month-old mice. C) Propofol (lanes 5-8) decreases caspase-9 activation as compared to saline (lanes 1-4) in the hippocampus of 20 month-old WT mice. There is no significant difference in amounts of β-Actin in saline- or propofol-treated mice. D) Quantification of the western blot shows that propofol (black bar) decreases the caspase-9 activation as compared to saline (white bar) in the hippocampus of 20 month-old WT mice. WT, wild-type. n = 6.
Propofol improved cognitive function in AD Tg mice
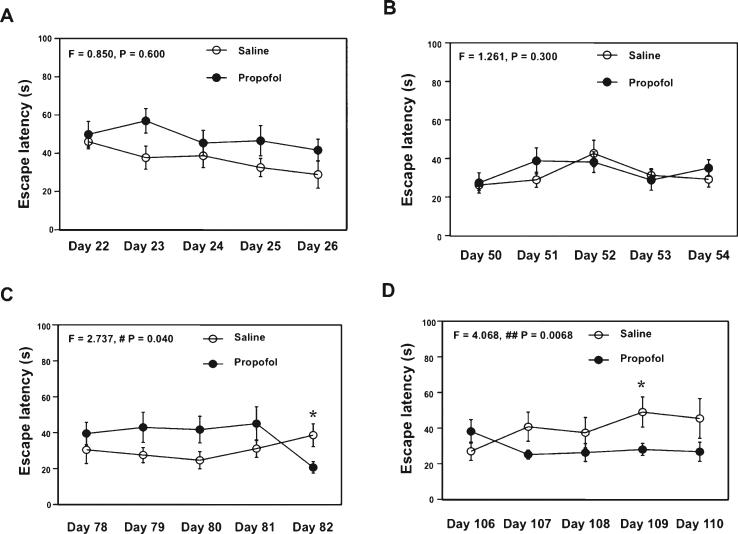
Given the fact that the chronic propofol treatment could improve cognitive function and attenuate the brain caspase-3 activation in WT aged mice, we assessed the effects of chronic treatment with propofol on cognitive function in the AD Tg mice. We treated the mice with 50 mg/kg propofol weekly. After four weeks (the 1st four week) of treatment, the propofol treatment did not significantly alter the escape latency of MWM (Fig. 5A, F = 0.850, p = 0.600, two-way ANOVA with repeated measurement). The propofol treatment did not significantly alter the escape latency of MWM after another four weeks (the 2nd four week) of treatment (Fig. 5B, F = 1.261, p = 0.300, twoway ANOVA with repeated measurement). However, after another four weeks (the 3rd four week) of treatment with propofol, the propofol treatment started to decrease the escape latency of MWM (Fig. 5C, F = 2.737, p = 0.040, two-way ANOVA with repeated measurement). Specifically, the propofol treatment decreased the escape latency at day 82 after the propofol treatment (post hoc test). Finally, two-way ANOVA with repeated measurement illustrated a significant interaction of time and group between the propofol treatment and control at 16 weeks (the 4th four week) of treatment, and the propofol treatment reduced the escape latency in the MWM test (Fig. 5D, F = 4.068, p = 0.0068). The propofol treatment decreased the escape latency at day 109 after the propofol treatment (post hoc test). There was no significant difference in swimming speed between the mice that received propofol treatment and the control mice (data not shown). Collectively, these results suggested that treatment with propofol at the dose of 50 mg/kg/week might improve the cognitive function in the AD Tg mice.
Fig. 5.
Propofol improves learning and memory function in aged AD Tg mice. A) Propofol (black circle) does not decrease escape latency as compared to saline (white circle) after the four week treatment (1st four week, 50 mg/kg propofol) in 18 month-old AD Tg mice. B) Propofol (black circle) does not decrease escape latency as compared to saline (white circle) after another four week treatment (2nd four week, 50 mg/kg) in 19 month-old AD Tg mice. C) Propofol (black circle) decreases escape latency as compared to saline (white circle) after another four week treatment (3rd four week, 50 mg/kg propofol) in 20 month-old AD Tg mice. D) Propofol (black circle) decreases escape latency as compared to saline (white circle) after another four week treatment (final four week, 50 mg/kg) in 21 month-old AD Tg mice. AD, Alzheimer's disease, Tg, transgenic. n = 10.
Propofol attenuated caspase-3 activation through the mitochondrial pathway in AD Tg mice
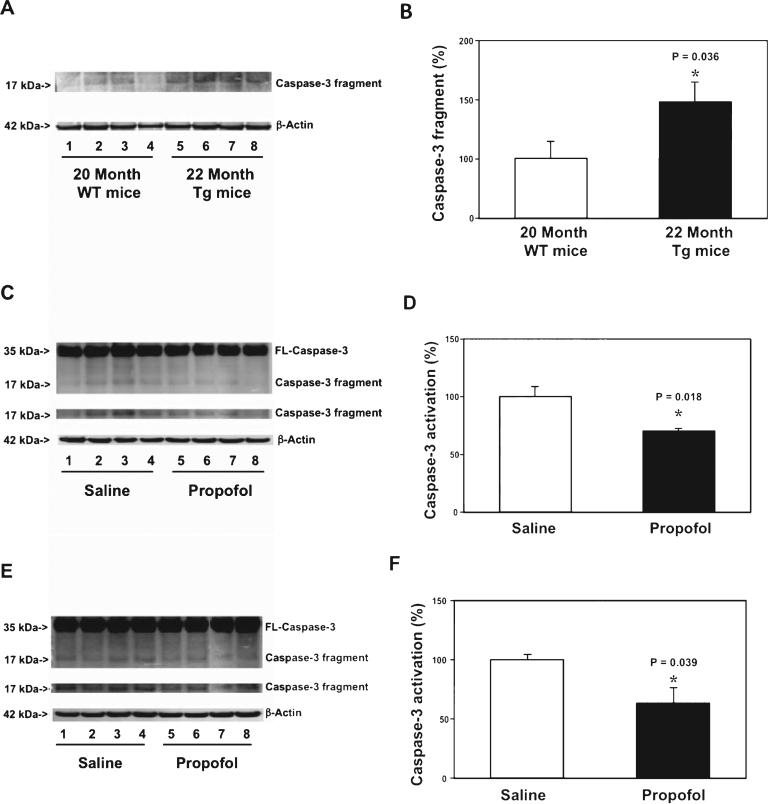
Next, we assessed the effects of the chronic propofol treatment on caspase-3 activation in the brain tissues of AD Tg mice. The brain tissues were harvested one day after the last propofol treatment. Immunoblotting of caspase-3 showed that there was a visible increase in the caspase-3 fragment levels in the cortex of 22 month-old AD Tg mice as compared to that of 20 month-old WT mice (Fig. 6A). The quantification of the western blot illustrated that there was a greater baseline caspase-3 activation in the cortex of 22 month-old AD Tg mice than that of 20 month-old WT mice (Fig. 6B): 144% versus 100%, p = 0.036. These data suggested that greater caspase-3 activation in brain tissues could be associated with AD gene mutation (e.g., APP and PSEN1).
Fig. 6.
Propofol attenuates caspase-3 activation in the cortex and hippocampus of aged AD Tg mice. A) There is a greater caspase-3 activation in the cortex of aged AD Tg mice (lanes 5-8) as compared to that in the cortex of aged WT mice (lanes 1-4). There is no significant difference in amounts of β-Actin between the two groups. B) Quantification of the western blot shows that there is a greater caspase-3 activation in the cortex of the aged AD Tg mice (black bar) as compared to that in the cortex of aged WT mice (white bar). C) Propofol (lanes 5-8) decreases caspase-3 activation as compared to saline (lanes 1-4) in the cortex of aged AD Tg mice. There is no significant difference in amounts of β-Actin in saline-or propofol-treated AD Tg mice. D) Quantification of the western blot shows that propofol (black bar) decreases the caspase-3 activation as compared to saline (white bar) in the cortex of aged AD Tg mice. E) Propofol (lanes 5-8) decreases caspase-3 activation as compared to saline (lanes 1-4) in the hippocampus of aged AD Tg mice. There is no significant difference in the amounts of β-Actin in saline- or propofol-treated AD Tg mice. F) Quantification of the western blot shows that propofol (black bar) decreases caspase-3 activation as compared to saline (white bar) in the hippocampus of aged AD Tg mice. FL, full length; AD, Alzheimer's disease, WT, wild-type; Tg, transgenic. n = 6.
Then, we assessed the effects of the chronic propofol treatment on the AD gene mutation-associated caspase-3 activation in the 22 month-old AD Tg mice. Immunoblotting of caspase-3 found that the mice following the propofol treatment had a lower visible level of caspase-3 fragment in the cortex as compared to the mice following saline treatment (Fig. 6C). The quantification of the caspase-3 activation showed that the propofol treatment attenuated the caspase-3 activation in the cortex of the AD Tg mice (Fig. 6D): 70% versus 100%, p = 0.018. Moreover, propofol also attenuated the caspase-3 activation in the hippocampus of the AD Tg mice as compared to the saline treatment (Fig. 6E, F): 63% versus 100%, p = 0.039. Taken together, these findings suggested that the chronic treatment with propofol might attenuate the AD gene mutation-associated caspase-3 activation in both the cortex and hippocampus.
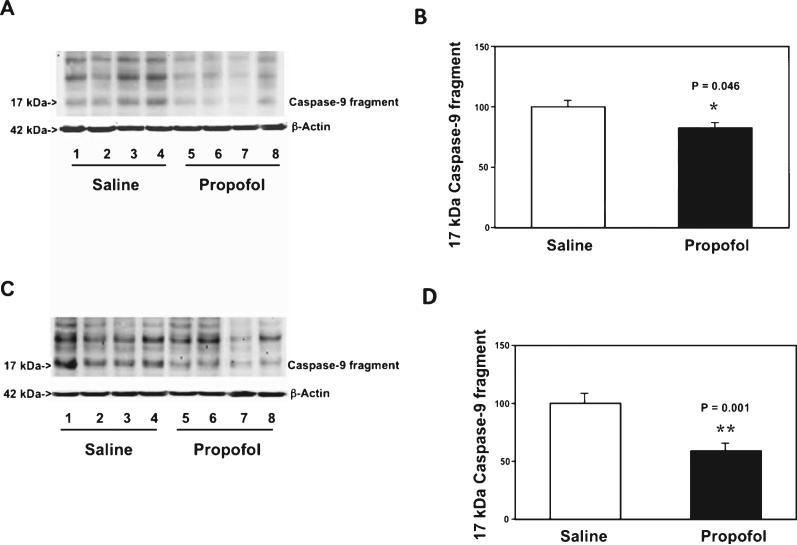
Next, we found that the chronic propofol treatment specifically attenuated the caspase-9 (the marker of mitochondria-associated caspase activation) in the cortex (Fig. 7A, B): 82% versus 100%, p = 0.046, and hippocampus (Fig. 7C, D) of the AD Tg mice: 59% versus 100%, p = 0.001. The propofol treatment did not attenuate the caspase-8 activation (the maker of death receptor-associated caspase activation) in the cortex and hippocampus of the AD Tg mice (data not shown). Taken together, these findings suggested that the chronic propofol treatment might specifically affect the mitochondria-associated caspase activation, leading to attenuation of the caspase-3 activation in the brain tissues (cortex and hippocampus) of AD Tg mice.
Fig. 7.
Propofol attenuates the caspase-9 activation in the cortex and hippocampus of aged AD Tg mice. A) Propofol (lanes 5-8) decreases caspase-9 activation as compared to saline (lanes 1-4) in cortex of aged AD Tg mice. There is no significant difference in amounts of β-Actin in saline- or propofol-treated mice. B) Quantification of the western blot shows that propofol (black bar) attenuates the caspase-9 activation (white bar) in the cortex of aged AD Tg mice. C) Propofol (lanes 5-8) decreases caspase-9 activation as compared to saline (lanes 1-4) in hippocampus of aged AD Tg mice. There is no significant difference in amounts of β-Actin in saline- or propofol-treated mice. D) Quantification of the western blot shows that propofol (black bar) decreases caspase-9 activation as compared to saline (white bar) in the hippocampus of aged AD Tg mice. AD, Alzheimer's disease, Tg, transgenic. n = 6.
Propofol attenuated Aβ-induced caspase-3 activation and mPTP opening through GABA receptors
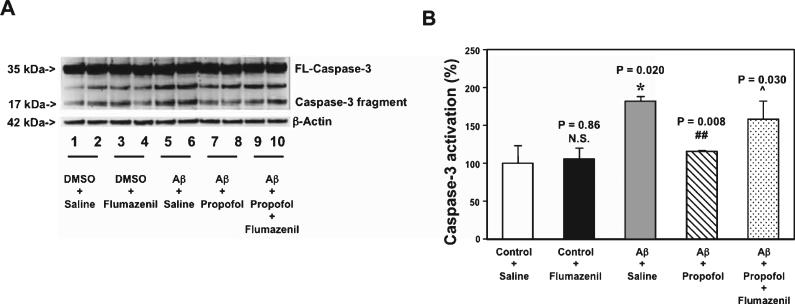
Aβ accumulation is observed in brain tissues of aged mice [46] and AD Tg mice [37, 38], and Aβ has been shown to induce caspase-3 activation in H4 human neuroglioma cells (H4 naïve cells) [47]. We therefore set out an in vitro study to assess the effects of propofol on the Aβ-induced caspase-3 activation in the H4 naïve cells. We found that the treatment with 5 μM Aβ for six hours induced caspase-3 activation in the H4 naïve cells (lane 5 and 6 in Fig. 8A, and grey bar in Fig. 8B, p = 0.020). The treatment with 100 μM of propofol (lanes 7 and 8 in Fig. 8A, and net bar in Fig. 8B, p = 0.008) attenuated the Aβ-induced caspase-3 activation in the cells. Propofol is a GABA receptor agonist [29] and flumazenil is the GABA receptor antagonist [48]. Treatment with flumazenil alone did not significantly alter the caspase-3 activation (lanes 3 and 4 in Fig. 8A, the black bar in Fig. 8B), however, the treatment of flumazenil mitigated the effects of propofol on attenuating the Aβ-induced caspase-3 activation (lanes 9 and 10 in Fig. 8A, and dot bar in Fig. 8B, p = 0.030). These data suggested that propofol might attenuate the Aβ-induced caspase-3 activation, possibly via its action on GABA receptors.
Fig. 8.
Propofol attenuates Aβ-induced caspease-3 activation through GABA receptors in H4 naïve cells. A) Five μM Aβ (lanes 5-6) induces caspase-3 activation as compared to saline (lanes 1-2). 100 μM propofol (lanes 7-8) attenuates the caspase-3 activation induced by 5 μM Aβ (lanes 5-6) in H4 naïve cells. 20 μM flumazenil (lanes 9-10) inhibits the attenuation effect of 100 μM propofol (lanes 7-8) on the Aβ-induced caspase-3 activation (lanes 5-6). 20 μM flumazenil alone (lanes 3-4) has no significant effects on caspase-3 activation as compared to saline (lanes 1-2) in H4 naïve cells. There is no significant difference in amounts of β-Actin among all the groups. B) Quantification of the western blot shows that 5 μM Aβ (grey bar) induces caspase-3 activation as compared to saline (white bar), 100 μM propofol (net bar) attenuates the Aβ-induced caspase-3 activation (grey bar) in H4 naïve cells. Twenty μM flumazenil (dot bar) inhibits the attenuation effect of 100 μM propofol (net bar) on the Aβ-induced caspase-3 activation (grey bar). There is no difference on caspase-3 activation between the treatment of saline (white bar) and 20 μM flumazenil alone (black bar) in H4 naïve cells. FL, full length, Aβ, amyloid-β protein. n = 6.
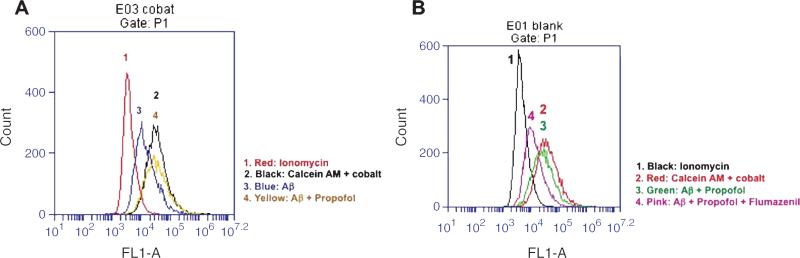
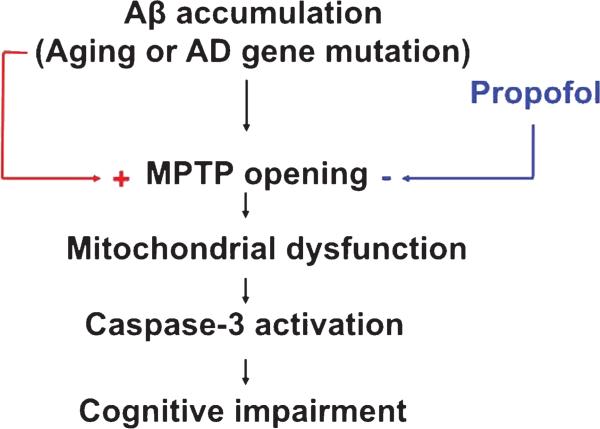
Mitochondrial dysfunction is associated with caspase activation and apoptosis [49]. Specifically, the opening of mitochondrial permeability transition pore (mPTP) has been shown to be the upstream mechanism of anesthetic-induced caspase activation and neurotoxicity in the mouse neuroblastoma cells [41]. We therefore assessed the effects of propofol and Aβ on the opening of mPTP in the mouse neuroblastoma cells. Flow cytometric analysis of immunocytochemistry staining of calcein AM and cobalt showed that the treatment with 100 μM of propofol attenuated the opening of the mPTP induced by the treatment with 5 μM of Aβ, as evidenced by an increase in the intensity of fluorescence in the cells treated by propofol (Fig. 9A, peak 4) as compared to that detected in Aβ-treated cells (Fig. 9A, peak 3). Moreover, we found that flumazenil reversed the protective effect of propofol on the Aβ-induced mPTP opening (Fig. 9B, peak 4 versus peak 3) in the mouse neuroblastoma cells. Taken together, these findings suggested that aging or AD gene mutation-associated Aβ accumulation may induce the opening of the mPTP, which caused mitochondrial dysfunction, leading to caspase-3 activation and cognitive impairment. Anesthetic propofol might attenuate caspase activation and improve cognitive function by blocking the Aβ-induced mPTP opening and mitochondrial dysfunction (Fig. 10).
Fig. 9.
Propofol attenuates Aβ-induced mPTP opening through GABA receptor in N2A cells. A) Flow cytometric analysis shows changes in calcein levels in mitochondria of N2A cells stained with calcein AM or calcein AM plus cobalt, which indicate the opening of the mPTP. Peak 1: positive control cells (treatment of calcein AM plus cobalt and ionomycin); peak 2: negative control (treatment of calcein AM plus cobalt); peak 3: cells treated with calcein AM plus cobalt and 5 μM Aβ; peak 4: cells treated with calcein AM plus cobalt and 5 μM Aβ and 100 μM propofol. The changes in intensity of fluorescence between Aβ treated (peak 3), Aβ plus propofol (peak 4), positive control (peak 1), and negative control (peak 2) suggest that Aβ induces the opening of the mPTP, propofol attenuates the Aβ-induced opening of the mPTP, as demonstrated by that the position of peak of Aβ treatment is shifted to right following the propofol treatment. B) Peak 1: positive control (treatment of calcein AM plus cobalt and ionomycin); peak 2: negative control (treatment of calcein AM plus cobalt); peak 3: cells treated with calcein AM plus cobalt and 5 μM Aβ and 100 μM propofol; peak 4: cells treated with calcein AM plus cobalt and 5 μM Aβ plus 100 μM propofol and 20 μM flumazenil. The changes in intensity of fluorescence between Aβ plus propofol (peak 3), Aβ plus propofol and flumazenil (peak 4), positive control (peak 1), and negative control (peak 2) suggest that flumazenil inhibits the attenuation effect of propofol on the Aβ-induced opening of the mPTP in N2A cells. mPTP, mitochondrial permeability transition pore, Aβ, amyloid-β protein. n = 6.
Fig. 10.
Hypothesized pathway of the effects of propofol in improving learning and memory. Aging- or AD gene mutation-associated Aβ accumulation may open the mPTP, which causes mitochondrial dysfunction and caspase-3 activation, leading to learning and memory impairment. Propofol attenuates the Aβ-induced mPTP opening and thus mitigates the apoptosis, which eventually improves learning and memory function in the mice. mPTP, mitochondrial permeability transition pore, Aβ, amyloid-β protein.
DISCUSSION
In the current study, we assessed whether weekly treatment of anesthetic propofol could improve cognitive function and attenuate brain caspapse-3 activation in both aged WT mice and AD Tg mice. We found that the weekly treatment of propofol in both aged WT mice and AD Tg mice was able to improve the cognitive function (Figs. 2 and 5) and attenuate brain caspase-3 activation (Figs. 1 and 4) as compared to the weekly treatment of saline control. Note that the current studies only served as pilot investigation and established a system to further assess the effects of chronic treatment with propofol on neurochemistry and neurobehavioral changes. The long-term goal is to use the established pre-clinical system to further assess the effects of propofol on brain function and the underlying mechanisms.
In the mechanistic investigations, we first found that there was a greater brain caspase-3 activation in aged WT mice as compared to young WT mice and in aged AD Tg mice as compared to aged WT mice (Figs. 3 and 6). Then, we found that a treatment with 50 mg/kg/weekly for 8 and 16 weeks decreased the caspase-3 activation in both cortex and hippocampus of the WT and AD Tg mice, respectively (Figs. 3 and 6), potentially through the mitochondrial pathway of caspase activation (Figs. 4 and 7).
Aging [46] and the mutations of the AD gene [37] are associated with accumulation of Aβ. The in vitro studies showed that propofol was able to attenuate the Aβ-induced caspase-3 activation and opening of the mPTP, and these effects were inhibited by GABA receptor antagonist flumazenil (Figs. 8 and 9). Taken together, we hypothesized that propofol might decrease the Aβ-induced caspase-3 activation in aged and AD Tg mice brain through its action on GABA receptors, leading to the improvement of cognitive function in the aged and AD Tg mice (Fig. 10). Future studies to test this hypothesis are warranted, which could lead to novel treatment(s) for AD and cognitive impairment.
Previous studies reported that there were reduced mRNA levels of GABA receptor in both the prefrontal cortex [50] and hippocampus [51, 52] of AD brains. A recent study found that there was a reduction of GABA current in AD patients’ brains, which was associated with reduction of both mRNA and protein levels of principal GABA receptor subunits in the brains [28]. All of these studies suggested that manipulating GABAergic signaling could be a potential treatment of AD. Consistently, we found that propofol, an anesthetic which is a GABA agonist, improved cognitive function in the aged and AD Tg mice. The future studies may include the experiments to assess whether other GABA agonists have similar effects.
Opening of the mPTP in mitochondria has been reported to lead to caspase activation [22–24, 53], which may contribute to cognitive dysfunction [19, 25–27]. In the current studies, anesthetic propofol mitigated the Aβ-induced mPTP opening and flumazenil inhibited the effects of propofol. Taken together, these findings suggested that regulation of GABA neuro-transmission and mPTP opening by anesthetics may lead to improvement of cognitive function. Future studies to test this hypothesis may provide targeted interventions to improve the cognitive dysfunction. The outcomes from these pre-clinical studies might promote further studies, which could lead to the development of employing a weekly treatment of propofol to improve cognitive function in AD and senior patients. Moreover, the findings that propofol could improve cognitive function suggest that propofol would be a better choice of anesthetic when providing anesthesia care for AD and senior patients.
The studies have several limitations. First, we did not determine the dose-dependent effects of propofol on caspase-3 activation and cognitive function in the aged and AD Tg mice, because these AD Tg and aged mice are expensive and not easy to obtain. Different doses of propofol may cause neurotoxic [54–56] and neuroprotective [57–59] effects. It is possible that different doses of propofol may neither attenuate the caspase-3 activation nor improve the cognitive function in the aged and AD Tg mice. Nevertheless, the outcomes from the current studies demonstrated a potential new concept, which may lead to a better understanding of the neuropathogenesis of aging and AD-associated cognitive dysfunction and novel treatment of the cognitive dysfunction. These findings will hopefully promote more studies to further determine whether: 1) chronic treatment of propofol can be used to treat cognitive dysfunction in humans; and 2) propofol is a better anesthetic for the anesthesia care of senior and AD patients, who are vulnerable to develop cognitive dysfunction. Second, we did not assess whether there is a cause-effect relationship of propofol on GABA action, caspase-3 activation, and cognitive function in the mice by determining whether flumazenil can rescue the in vivo effects of propofol. This is mainly because the C57BL/6 background mice exhibit a high incidence of seizures [60]. Flumazenil itself may have side effect of seizures in rodents [61] and we cannot find a better GABA antagonist to perform the in vivo studies at the current time. Third, the difference in cognitive function determined in MWM between propofol and saline treatment was not dramatic. The future studies should include other methods, e.g., Fear Conditioning Test and Radial Arm Water Maze, to further assess whether chronic treatment with propofol can improve cognitive function in aged and AD Tg mice. Finally, we only observed the behavioral changes up to 20 months in WT mice and 22 months in AD Tg mice, because four WT mice died after 20 months and three AD Tg mice died after 22 months, and consequently we did not have enough mice to generate meaningful data in their older age.
In conclusion, we found that the chronic treatment with the anesthetic propofol was able to attenuate the caspase-3 activation in both the cortex and hippocampus of aged WT mice and AD Tg mice and to improve the cognitive function in these mice. Propofol might act on GABA receptors to attenuate the Aβ-induced mPTP opening, leading to the reduction in the caspase-3 activation. The studies, serving to generate concept and hypothesis, will promote more research to determine the effect of chronic treatment of propofol on brain function, which may ultimately lead to new therapeutic strategies for aging- and AD-associated cognitive dysfunction and better anesthesia care for senior and AD patients.
ACKNOWLEDGMENTS
This research was supported by R21AG038994, R01 GM088801 and R01 AG041274 from National Institutes of Health, Bethesda, Maryland, Investigator-initiated Research grant from Alzheimer's Association, Chicago, Illinois, and Cure Alzheimer's Fund, Wellesley, Massachusetts to Zhongcong Xie. The flow cytometric analysis was performed in the Cell Biology Core of Harvard Clinic Nutrition Research Center (P30 DK040561).
Authors’ disclosures available online (http://www.jalz.com/disclosures/view.php?id=2138).
REFERENCES
- 1.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 2.Aisen PS, Cummings J, Schneider LS. Symptomatic and nonamyloid/tau based pharmacologic treatment for Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006395. doi: 10.1101/cshperspect.a006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, Reichert M, Ketter N, Nejadnik B, Guenzler V, Miloslavsky M, Wang D, Lu Y, Lull J, Tudor IC, Liu E, Grundman M, Yuen E, Black R, Brashear HR. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, Raman R, Sun X, Aisen PS, Siemers E, Liu-Seifert H, Mohs R. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 5.Holtzman DM, Deshmukh M. Caspases: A treatment target for neurodegenerative disease? Nat Med. 1997;3:954–955. doi: 10.1038/nm0997-954. [DOI] [PubMed] [Google Scholar]
- 6.Lunkes A, Trottier Y, Mandel JL. Pathological mechanisms in Huntington's disease and other polyglutamine expansion diseases. Essays Biochem. 1998;33:149–163. doi: 10.1042/bse0330149. [DOI] [PubMed] [Google Scholar]
- 7.Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, Yuan J, Moskowitz MA. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18:3659–3668. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim TW, Pettingell WH, Jung YK, Kovacs DM, Tanzi RE. Alternative cleavage of Alzheimer-associated presenilins during apoptosis by a caspase-3 family protease. Science. 1997;277:373–376. doi: 10.1126/science.277.5324.373. [DOI] [PubMed] [Google Scholar]
- 9.Loetscher H, Deuschle U, Brockhaus M, Reinhardt D, Nelboeck P, Mous J, Grunberg J, Haass C, Jacobsen H. Presenilins are processed by caspase-type proteases. J Biol Chem. 1997;272:20655–20659. doi: 10.1074/jbc.272.33.20655. [DOI] [PubMed] [Google Scholar]
- 10.Barnes NY, Li L, Yoshikawa K, Schwartz LM, Oppenheim RW, Milligan CE. Increased production of amyloid precursor protein provides a substrate for caspase-3 in dying motoneurons. J Neurosci. 1998;18:5869–5880. doi: 10.1523/JNEUROSCI.18-15-05869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovacs DM, Mancini R, Henderson J, Na SJ, Schmidt SD, Kim TW, Tanzi RE. Staurosporine-induced activation of caspase-3 is potentiated by presenilin 1 familial Alzheimer's disease mutations in human neuroglioma cells. J Neurochem. 1999;73:2278–2285. doi: 10.1046/j.1471-4159.1999.0732278.x. [DOI] [PubMed] [Google Scholar]
- 12.Su JH, Anderson AJ, Cummings BJ, Cotman CW. Immunohistochemical evidence for apoptosis in Alzheimer's disease. Neuroreport. 1994;5:2529–2533. doi: 10.1097/00001756-199412000-00031. [DOI] [PubMed] [Google Scholar]
- 13.Su JH, Deng G, Cotman CW. Bax protein expression is increased in Alzheimer's brain: Correlations with DNA damage, Bcl-2 expression, and brain pathology. J Neuropathol Exp Neurol. 1997;56:86–93. doi: 10.1097/00005072-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Tesco G, Koh YH, Kang EL, Cameron AN, Das S, Sena-Esteves M, Hiltunen M, Yang SH, Zhong Z, Shen Y, Simpkins JW, Tanzi RE. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron. 2007;54:721–737. doi: 10.1016/j.neuron.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattson MP. Contributions of mitochondrial alterations, resulting from bad genes and a hostile environment, to the pathogenesis of Alzheimer's disease. Int Rev Neurobiol. 2002;53:387–409. doi: 10.1016/s0074-7742(02)53014-2. [DOI] [PubMed] [Google Scholar]
- 16.Raina AK, Hochman A, Ickes H, Zhu X, Ogawa O, Cash AD, Shimohama S, Perry G, Smith MA. Apoptotic promoters and inhibitors in Alzheimer's disease: Who wins out? Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:251–254. doi: 10.1016/S0278-5846(03)00020-4. [DOI] [PubMed] [Google Scholar]
- 17.LeBlanc AC. The role of apoptotic pathways in Alzheimer's disease neurodegeneration and cell death. Curr Alzheimer Res. 2005;2:389–402. doi: 10.2174/156720505774330573. [DOI] [PubMed] [Google Scholar]
- 18.Cribbs DH, Poon WW, Rissman RA, Blurton-Jones M. Caspase-mediated degeneration in Alzheimer's disease. Am J Pathol. 2004;165:353–355. doi: 10.1016/S0002-9440(10)63302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burguillos MA, Deierborg T, Kavanagh E, Persson A, Hajji N, Garcia-Quintanilla A, Cano J, Brundin P, Englund E, Venero JL, Joseph B. Caspase signalling controls microglia activation and neurotoxicity. Nature. 2011;472:319–324. doi: 10.1038/nature09788. [DOI] [PubMed] [Google Scholar]
- 20.Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu X, Perry G, Smith MA, Wang X. Abnormal mitochondrial dynamics in the pathogenesis of Alzheimer's disease. J Alzheimers Dis. 2013;33(Suppl 1):S253–S262. doi: 10.3233/JAD-2012-129005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 23.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan PG, Rabchevsky AG, Waldmeier PC, Springer JE. Mitochondrial permeability transition in CNS trauma: Cause or effect of neuronal cell death? J Neurosci Res. 2005;79:231–239. doi: 10.1002/jnr.20292. [DOI] [PubMed] [Google Scholar]
- 25.Gervais FG, Xu D, Robertson GS, Vaillancourt JP, Zhu Y, Huang J, LeBlanc A, Smith D, Rigby M, Shearman MS, Clarke EE, Zheng H, Van Der Ploeg LH, Ruffolo SC, Thornberry NA, Xanthoudakis S, Zamboni RJ, Roy S, Nicholson DW. Involvement of caspases in proteolytic cleavage of Alzheimer's amyloid-beta precursor protein and amyloidogenic A beta peptide formation. Cell. 1999;97:395–406. doi: 10.1016/s0092-8674(00)80748-5. [DOI] [PubMed] [Google Scholar]
- 26.Stadelmann C, Deckwerth TL, Srinivasan A, Bancher C, Bruck W, Jellinger K, Lassmann H. Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer's disease. Evidence for apoptotic cell death. Am J Pathol. 1999;155:1459–1466. doi: 10.1016/S0002-9440(10)65460-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louneva N, Cohen JW, Han LY, Talbot K, Wilson RS, Bennett DA, Trojanowski JQ, Arnold SE. Caspase-3 is enriched in postsynaptic densities and increased in Alzheimer's disease. Am J Pathol. 2008;173:1488–1495. doi: 10.2353/ajpath.2008.080434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Limon A, Reyes-Ruiz JM, Miledi R. Loss of functional GABA(A) receptors in the Alzheimer diseased brain. Proc Natl Acad Sci U S A. 2012;109:10071–10076. doi: 10.1073/pnas.1204606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hales TG, Lambert JJ. The actions of propofol on inhibitory amino acid receptors of bovine adrenomedullary chromaffin cells and rodent central neurones. Br J Pharmacol. 1991;104:619–628. doi: 10.1111/j.1476-5381.1991.tb12479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Zhen Y, Dong Y, Xu Z, Yue Y, Golde TE, Tanzi RE, Moir RD, Xie Z. Anesthetic propofol attenuates the isoflurane-induced caspase-3 activation and abeta oligomerization. PLoS One. 2011;6:e27019. doi: 10.1371/journal.pone.0027019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pryor K, Blackstock-Bernstein AS, Feiler D, Vortsman E, Root JC. Administration of propofol after learning improves memory performance in human subjects via loss of competitive consolidation: Evidence that propofol amnesia occurs at the induction of consolidation. American Association of Anesthesiologists, ANESTHESIOLOGY 2012. 2012 Abstract No. BOC09. [Google Scholar]
- 32.O'Gorman DA, O'Connell AW, Murphy KJ, Moriarty DC, Shiotani T, Regan CM. Nefiracetam prevents propofol-induced anterograde and retrograde amnesia in the rodent without compromising quality of anesthesia. Anesthesiology. 1998;89:699–706. doi: 10.1097/00000542-199809000-00021. [DOI] [PubMed] [Google Scholar]
- 33.Pang R, Quartermain D, Rosman E, Turndorf H. Effect of propofol on memory in mice. Pharmacol Biochem Behav. 1993;44:145–151. doi: 10.1016/0091-3057(93)90292-2. [DOI] [PubMed] [Google Scholar]
- 34.Ren Y, Zhang FJ, Xue QS, Zhao X, Yu BW. Bilateral inhibition of gamma-aminobutyric acid type A receptor function within the basolateral amygdala blocked propofol-induced amnesia and activity-regulated cytoskeletal protein expression inhibition in the hippocampus. Anesthesiology. 2008;109:775–781. doi: 10.1097/ALN.0b013e31818a37c4. [DOI] [PubMed] [Google Scholar]
- 35.Veselis RA, Pryor KO, Reinsel RA, Li Y, Mehta M, Johnson R., Jr Propofol and midazolam inhibit conscious memory processes very soon after encoding: An event-related potential study of familiarity and recollection in volunteers. Anesthesiology. 2009;110:295–312. doi: 10.1097/ALN.0b013e3181942ef0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hauer D, Ratano P, Morena M, Scaccianoce S, Briegel I, Palmery M, Cuomo V, Roozendaal B, Schelling G, Campolongo P. Propofol enhances memory formation via an interaction with the endocannabinoid system. Anesthesiology. 2011;114:1380–1388. doi: 10.1097/ALN.0b013e31821c120e. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, Purcell SM, Betensky RA, Raju S, Prada C, Greenberg SM, Bacskai BJ, Frosch MP. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis. 2006;24:516–524. doi: 10.1016/j.nbd.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 38.Xiong H, Callaghan D, Wodzinska J, Xu J, Premyslova M, Liu QY, Connelly J, Zhang W. Biochemical and behavioral characterization of the double transgenic mouse model (APPswe/PS1dE9) of Alzheimer's disease. Neurosci Bull. 2011;27:221–232. doi: 10.1007/s12264-011-1015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Dong Y, Wu X, Lu Y, Xu Z, Knapp A, Yue Y, Xu T, Xie Z. The mitochondrial pathway of anesthetic isoflurane-induced apoptosis. J Biol Chem. 2010;285:4025–4037. doi: 10.1074/jbc.M109.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jazvinscak Jembrek M, Svob Strac D, Vlainic J, Pericic D. The role of transcriptional and translational mechanisms in flumazenil-induced up-regulation of recombinant GABA(A) receptors. Neurosci Res. 2008;61:234–241. doi: 10.1016/j.neures.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Xu Z, Wang H, Dong Y, Shi HN, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol. 2012;71:687–698. doi: 10.1002/ana.23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bianchi SL, Tran T, Liu C, Lin S, Li Y, Keller JM, Eckenhoff RG, Eckenhoff MF. Brain and behavior changes in 12-month-old Tg2576 and nontransgenic mice exposed to anesthetics. Neurobiol Aging. 2008;29:1002–1010. doi: 10.1016/j.neurobiolaging.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng H, Dong Y, Xu Z, Crosby G, Culley DJ, Zhang Y, Xie Z. Sevoflurane anesthesia in pregnant mice induces neurotoxicity in fetal and offspring mice. Anesthesiology. 2013;118:516–526. doi: 10.1097/ALN.0b013e3182834d5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen X, Dong Y, Xu Z, Wang H, Miao C, Soriano SG, Sun D, Baxter MG, Zhang Y, Xie Z. Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology. 2013;118:502–515. doi: 10.1097/ALN.0b013e3182834d77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: The calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 46.Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. Beta-secretase protein and activity are increased in the neo-cortex in Alzheimer disease. Arch Neurol. 2002;59:1381–1389. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- 47.Xie Z, Dong Y, Maeda U, Moir RD, Xia W, Culley DJ, Crosby G, Tanzi RE. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J Neurosci. 2007;27:1247–1254. doi: 10.1523/JNEUROSCI.5320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ondo WG, Hunter C. Flumazenil, a GABA antagonist, may improve features of Parkinson's disease. Mov Disord. 2003;18:683–685. doi: 10.1002/mds.10426. [DOI] [PubMed] [Google Scholar]
- 49.Thornberry NA, Lazebnik Y. Caspases: Enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 50.Luchetti S, Huitinga I, Swaab DF. Neurosteroid and GABA-A receptor alterations in Alzheimer's disease, Parkinson's disease and multiple sclerosis. Neuroscience. 2011;191:6–21. doi: 10.1016/j.neuroscience.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 51.Rissman RA, Mobley WC. Implications for treatment: GABAA receptors in aging, Down syndrome and Alzheimer's disease. J Neurochem. 2011;117:613–622. doi: 10.1111/j.1471-4159.2011.07237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mizukami K, Grayson DR, Ikonomovic MD, Sheffield R, Armstrong DM. GABAA receptor beta 2 and beta 3 subunits mRNA in the hippocampal formation of aged human brain with Alzheimer-related neuropathology. Brain Res Mol Brain Res. 1998;56:268–272. doi: 10.1016/s0169-328x(97)00347-1. [DOI] [PubMed] [Google Scholar]
- 53.Criollo A, Galluzzi L, Maiuri MC, Tasdemir E, Lavandero S, Kroemer G. Mitochondrial control of cell death induced by hyperosmotic stress. Apoptosis. 2007;12:3–18. doi: 10.1007/s10495-006-0328-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pearn ML, Hu Y, Niesman IR, Patel HH, Drummond JC, Roth DM, Akassoglou K, Patel PM, Head BP. Propofol neurotoxicity is mediated by p75 neurotrophin receptor activation. Anesthesiology. 2012;116:352–361. doi: 10.1097/ALN.0b013e318242a48c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whittington RA, Virag L, Marcouiller F, Papon MA, El Khoury NB, Julien C, Morin F, Emala CW, Planel E. Propofol directly increases tau phosphorylation. PLoS One. 2011;6:e16648. doi: 10.1371/journal.pone.0016648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krzisch M, Sultan S, Sandell J, Demeter K, Vutskits L, Toni N. Propofol anesthesia impairs the maturation and survival of adult-born hippocampal neurons. Anesthesiology. 2013;118:602–610. doi: 10.1097/ALN.0b013e3182815948. [DOI] [PubMed] [Google Scholar]
- 57.Bayona NA, Gelb AW, Jiang Z, Wilson JX, Urquhart BL, Cechetto DF. Propofol neuroprotection in cerebral ischemia and its effects on low-molecular-weight antioxidants and skilled motor tasks. Anesthesiology. 2004;100:1151–1159. doi: 10.1097/00000542-200405000-00017. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, Dong Y, Xu Z, Xie Z. Propofol and magnesium attenuate isoflurane induced caspase-3 activation via inhibiting mitochondrial permeability transition pore. Med Gas Res. 2012;2:20. doi: 10.1186/2045-9912-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rossaint J, Rossaint R, Weis J, Fries M, Rex S, Coburn M. Propofol: Neuroprotection in an in vitro model of traumatic brain injury. Crit Care. 2009;13:R61. doi: 10.1186/cc7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Minkeviciene R, Rheims S, Dobszay MB, Zilberter M, Hartikainen J, Fulop L, Penke B, Zilberter Y, Harkany T, Pitkanen A, Tanila H. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci. 2009;29:3453–3462. doi: 10.1523/JNEUROSCI.5215-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schulze-Bonhage A, Elger CE. Induction of partial epileptic seizures by flumazenil. Epilepsia. 2000;41:186–192. doi: 10.1111/j.1528-1157.2000.tb00138.x. [DOI] [PubMed] [Google Scholar]