Abstract
The BCL-2 family is centrally involved in the mechanism of cell death after cerebral ischemia. It is well known that the proteins of the BCL-2 family are key regulators of apoptosis through controlling mitochondrial outer membrane permeabilization. Recent findings suggest that many BCL-2 family members are also directly involved in controlling transmission of Ca2+ from the endoplasmic reticulum (ER) to mitochondria through a specialization called the mitochondria-associated ER membrane (MAM). Increasing evidence supports the involvement of microRNAs (miRNA), some of them targeting BCL-2 family proteins, in the regulation of cerebral ischemia. In this mini-review, after highlighting current knowledge about the multiple functions of BCL-2 family proteins and summarizing their relationship to outcome from cerebral ischemia, we focus on the regulation of BCL-2 family proteins by miRNAs, especially miR-29 which targets multiple BCL-2 family proteins.
Keywords: BCL-2, cerebral ischemia, microRNA, miR-29, stroke
Introduction
Stroke is one of the leading causes of death worldwide and the most prominent cause of long-term disability. Although many clinical trials have been completed in stroke, none have demonstrated clinical protective efficacy except tissue plasminogen activator. Suggested reasons for the many failures include the complex interplay among signaling pathways and the potentially short therapeutic window for acute neuroprotection. It has been known for a long time that the BCL-2 family is involved in the choice between cell survival and cell death after cerebral ischemia (for review see Ouyang and Giffard (2004)). The proteins of the BCL-2 family are well known as key regulators of the mitochondrial pathway for induction of apoptosis. However recent studies suggest that several BCL-2 protein family members have additional novel functions (Hardwick et al., 2012; Hardwick and Soane, 2013) including controlling neuronal activity, autophagy, immune responses (Renault and Chipuk, 2013), mitochondrial dynamics (Martinou and Youle, 2011), calcium handling, and ER-mitochondria cross talk (Bonneau et al., 2013; Grimm, 2012; Rodriguez et al., 2011).
MicroRNAs (miRNAs) are a novel and abundant class of 19- to 22-nucleotide (nt) noncoding RNAs that control gene expression at the post-transcriptional level. miRNAs bind to messenger RNAs (mRNAs), known as their targets, based on sequence complementarity and direct the degradation or repression of translation of the targeted mRNAs. Recent evidence increasingly supports a role for miRNAs in response to cerebral ischemia, as we have reviewed recently (Ouyang et al., 2013a; Ouyang et al., 2013b). Numerous miRNAs target BCL-2 family proteins. This mini-review will focus on the novel role of miRNAs in the regulation of BCL-2 family expression and in ER-mitochondrial cross talk. We discuss recent experimental results showing that miR-29 targets multiple BCL-2 family members, including both pro-apoptotic and anti-apoptotic proteins, and influences the outcome of cerebral ischemia.
1. The BCL-2 family: versatile proteins that determine cell fate
The BCL-2 family proteins are characterized by the presence of BCL-2 homology (BH) domains. According to their function in apoptosis, BCL-2 family members are designated as anti- or pro-apoptotic, though as noted above, they are increasingly recognized as also having other physiological roles (Hardwick et al., 2012). All the anti-apoptotic proteins, including family prototype BCL-2, contain BH domains 1–4 and most contain a hydrophobic transmembrane (TM) domain for localization into biological membranes (Fig. 1A). Pro-apoptotic proteins are subdivided into two groups. The effector proteins such as BAX and BAK contain BH domains 1–3, and a TM domain whereas BH3-only proteins such as BAD and PUMA possess just the BH3 domain and no TM domain with some exceptions (Fig. 1A). It is well established that the proteins of the BCL-2 family are key regulators of the mitochondrial pathway of apoptosis. This section will summarize their roles in regulation of mitochondrial- and ER-dependent apoptosis focusing on ER-mitochondria crosstalk.
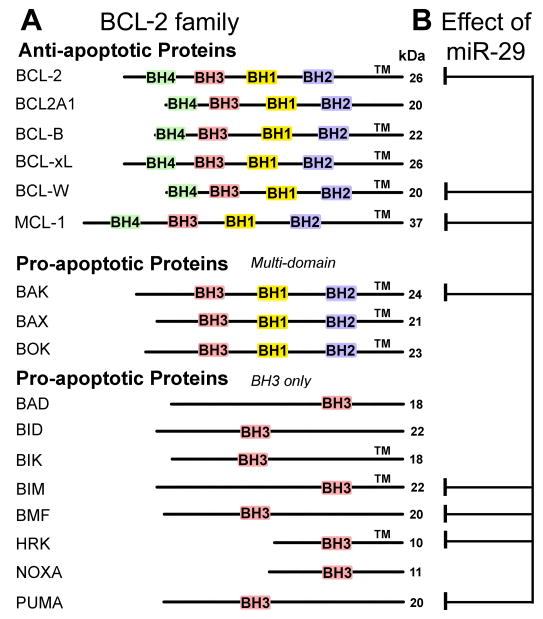
Fig. 1.
BCL-2 family members and those targeted by miR-29. A. The BCL-2 family is comprised of three subfamilies that contain one or more BCL-2 homology (BH) domains. The anti-apoptotic subfamily comprises proteins that contain all four BH domains. The multidomain pro-apoptotic subfamily lacks BH4 domains. Most members of these subfamilies also contain transmembrane domains (TM) and are therefore typically associated with membranes or pore formation in mitochondrial outer membranes. The BH3-only subfamily all contain the BH3 motif and most of them have no TM. The molecular weight of each protein is listed on the right (kDa). B. A single miRNA, miR-29, has many validated targets within the large BCL-2 family, including proteins in all three subfamilies.
1.1 BCL-2 family, mitochondria and apoptosis (Fig. 2A)
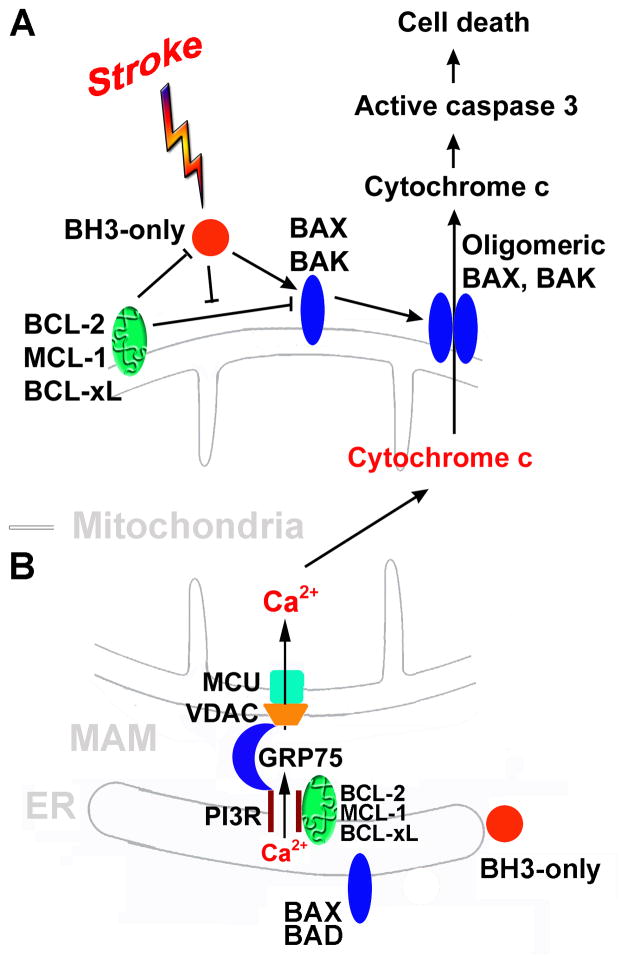
Fig. 2.
BCL-2 family-mediated cell death. A. The BCL-2 family regulates mitochondria-dependent apoptosis. BH3-only family members act as sentinels for many death stimuli including ischemia. They can mediate the activation of the multidomain pro-apoptotic BAX and BAK allowing oligomerization. Upon BAX and BAK oligomerization, the mitochondrial outer membrane is permeabilized releasing a apoptogenic proteins from the mitochondrial intermembrane space, such as cytochrome c, into the cytosol. Released cytochrome c triggers the activation of a downstream caspase cascade leading to cell death. BH3-only proteins can be sequestered by anti-apoptotic BCL-2 family members (e.g. BCL-2, MCL-1, BCL-xL,, etc.). B. The BCL-2 family regulates ER-mitochondria Ca2+ crosstalk at the MAM. The basic structure for release of Ca2+ at MAM is the IP3R on the ER, and VDAC and MCU on the mitochondrion. IP3R and VDAC are physically coupled by the chaperone GRP75/mortalin. Excessive increases in mitochondrial matrix Ca2+ triggers opening of the mitochondrial outer membrane permeability pore causing the release of cytochrome c and other pro-apoptotic factors into the cytoplasm. ER: endoplasmic reticulum; GRP75: glucose-regulated protein 75/mortalin; IP3R: inositol trisphosphate receptor; MAM: mitochondria-associated ER membrane; MCU: mitochondrial Ca2+uniporter; VDAC: voltage-dependent anion channel.
The BCL-2 family proteins play a crucial role in the control and the execution of the intrinsic, or mitochondrial, pathway of apoptosis, as well as influencing the extrinsic pathway and necrotic cell death. In response to diverse intracellular damage signals, the cell’s decision to undergo apoptosis is determined by interactions between these three factions of the BCL-2 protein family. The death stimuli activate BH3-only proteins which in turn lead to the activation of BAX and/or BAK either directly or by inhibiting anti-apoptotic members. Activated BAX/BAK then oligomerize at the mitochondria to induce outer mitochondrial membrane (OMM) permeabilization (Bender and Martinou, 2013) and release into the cytosol of apoptotic factors (e.g., cytochrome c and apoptosis-inducing factor (AIF)) which promote caspase activation and subsequent apoptosis execution. Anti-apoptotic members of the BCL-2 family are able to prevent OMM permeabilization via a direct inhibitory interaction with pro-apoptotic members (Chipuk et al., 2010).
Apoptosis regulation by the BCL-2 proteins is crucial for tissue homeostasis, for embryo development, for development of the immune and nervous systems, and for the maturation of blood cells (Strasser et al., 2011). Deregulation of BCL-2 proteins has a major role in tumor formation and in the cellular responses to anticancer therapy. Preclinical studies have shown that agents targeting anti-apoptotic BCL-2 family members have preclinical activity and some of them have already gone to Phase III of clinical trials (Table 1 in Kang and Reynolds (2009)).
1.2 The BCL-2 family and ER-mitochondrial crosstalk (Fig. 2B)
Although mitochondria are considered the primary site of action of BCL-2 family proteins, many of these proteins also localize to endoplasmic reticulum (ER) membranes. Mitochondria and ER exchange signals to regulate cell death, preeminent among them is calcium signaling. The physical association between the ER and mitochondria, known as the mitochondria-associated ER membrane (MAM), enables highly efficient and regulated transmission of Ca2+ from the ER to mitochondria under both physiological and pathological conditions (for a recent review see Ouyang and Giffard (2012)). Numerous proteins have been proposed to participate in the interaction and communication between the mitochondria and the ER. The central structure of MAM is the inositol 1,4,5-trisphosphate receptor (IP3R) on the ER and the voltage-dependent anion channel (VDAC) on the outer mitochondrial membrane. IP3R and VDAC are physically connected through glucose regulating protein 75/mortalin (GRP75) and facilitate the transfer of Ca2+ from the ER via IP3R to the mitochondria via VDAC.
Recent studies have shown that pro- and anti-apoptotic family members exert opposing effects on ER Ca2+ handling (Hardwick and Soane, 2013). The ability of BCL-2 and BAX to alter ER calcium levels does not depend on their putative pore-forming domains (Chami et al., 2004) but instead involves direct or indirect modulation of ER calcium channels.
Several groups have shown that BCL-2 and BCL-xL form a protein complex with the IP3R (Chen et al., 2004a; White et al., 2005), possibly modulating MAM opening. BCl-2 and IP3Rs were detected together in macromolecular complexes by coimmunoprecipitation (Chen et al., 2004b). White et al (White et al., 2005) found that BCL-xL interacts with the carboxyl terminus of the IP3R and sensitizes single IP3R channels in ER membranes to low IP3 concentrations, enhancing Ca2+ and IP3-dependent regulation of channel activity in vitro and in vivo, reducing ER Ca2+ content and stimulating mitochondrial energetics. The pro-apoptotic proteins BAX and tBID (a truncated and active form of BID) antagonize this effect by blocking the biochemical interaction of BCL-xL with the IP3R. This research group then found that the other structurally related proteins, BCL-2 and MCL-1, have a similar function. BCL-2, MCL-1, and BCL-xL bound with comparable affinity to the carboxyl termini of all three mammalian IP3R isoforms, lowered ER Ca2+ content and provided resistance to apoptosis (Eckenrode et al., 2010). The interaction of BCL-2 proteins with the IP3R likely involves multiple binding sites, as amino-terminal truncation of MCL-1 still allows efficient binding to the IP3R (Eckenrode et al., 2010). This group of anti-apoptotic proteins may interact with the IP3R with their BH4 domains (Rong et al., 2009). Targeting BCL-2 and IP3R interaction reversed BCL-2’s inhibition of apoptotic calcium signals (Rong et al., 2008).
Pro-apoptotic BCL-2 proteins also participate in ER calcium regulation. It has been reported that cells from BAX and BAK double knockout (DKO) mice have lower resting ER calcium levels and are protected from apoptotic stimuli that signal through calcium (Scorrano et al., 2003). Pro-apoptotic BAX and BAK were also reported to regulate the IP3R and calcium leak from the ER (Oakes et al., 2005). Although BAX and BAK have been shown to regulate type 1 IP3R (IP3R1) and Ca2+ leak, no direct interaction between BAX/BAK and the IP3R has been observed and these effects may be mediated through modulation of BCL-2/IP3R1 interaction and/or IP3R1 phosphorylation state (Oakes et al., 2005).
A role in regulating ER calcium release has also been reported for several BH3-only proteins. ER-localized BIK has been shown to be required for BAX/BAK-dependent ER Ca2+ release and cytochrome c release in response to genotoxic stress (Mathai et al., 2005). Similarly, PUMA has been shown to contribute to ER Ca2+ depletion-induced apoptosis by modulating BAX activity (Luo et al., 2005).
The release of calcium from the ER enters mitochondria through MAM and influences the apoptotic response to stimuli including cerebral ischemia (Fig. 2). However, ER calcium regulation is also involved in the non-apoptotic functions of these proteins such as regulation of mitochondrial energy metabolism and T-cell activation (Jones et al., 2007).
2. The important role of the BCL-2 family in cerebral ischemia
Animal models of ischemic stroke are used to study the basic pathophysiological processes and potential therapeutic interventions for this disease. Focal cerebral ischemia induced by middle cerebral artery occlusion (MCAO) in rats or mice is the rodent model most immediately relevant to human stroke. Global cerebral ischemia or transient forebrain ischemia, which induces a histopathological picture similar to that seen with cardiac arrest and resuscitation, is often induced by occluding both carotid arteries and inducing hypotension in rats for short durations of about 10 minutes. Glucose deprivation (GD) and combined oxygen-glucose deprivation (OGD) are common in vitro models of brain ischemia.
The BCL-2 family plays a prominent role in the mechanism of cell death after cerebral ischemia. Decreased BCL-2 was reported in cornu ammonis 1 (CA1) neurons after global ischemia (Martinez et al., 2007). We and others have reported that overexpressing anti-apoptotic BCL-2 family members protects against cerebral ischemia in vivo (Kitagawa et al., 1998; Zhao et al., 2003) and in vitro (Xu et al., 1999). The neuroprotection involves maintaining mitochondrial function and regulating ER-mitochondria calcium crosstalk (Ouyang and Giffard, 2012).
Pro-apoptotic BCL-2 family proteins also influence neuronal death after cerebral ischemia (Engel et al., 2011). PUMA (p53-upregulated modulator of apoptosis) is one of the most important BH3 only members of the BCL-2 family in cerebral ischemia. PUMA was discovered as a p53-induced BH3-only protein but can also be induced in a p53-independent manner (Jeffers et al., 2003; You et al., 2006). PUMA is potently pro-apoptotic, avidly binding all anti-apoptotic BCL-2 family proteins and it may also be capable of directly activating BAX/BAK (Jabbour et al., 2009). PUMA does not appear to be expressed in normal adult brain but is upregulated after global cerebral ischemia (Niizuma et al., 2009; Reimertz et al., 2003) and following focal cerebral ischemia (Kuroki et al., 2009; Luo et al., 2009). After global ischemia PUMA is upregulated in CA1 neurons, localizes to mitochondria, and binds BCL-xL and BAX (Niizuma et al., 2009). Selective CA1 injury induced by proteasomal inhibition was strongly reduced in PUMA knockout mice (Bonner et al., 2010; Tsuchiya et al., 2011). Other BH3-only BCL-2 family members are also involved in cerebral ischemia. BID cleavage into tBID is triggered by cerebral ischemia (Plesnila et al., 2001; Yin et al., 2002; Zhang et al., 2003) and caspase-8 has been suggested as a possible cause of BID cleavage after ischemia (Plesnila et al., 2001). Infarct volumes after MCAO were significantly reduced in bid−/− mice (Plesnila et al., 2001; Yin et al., 2002). BIM is upregulated quickly after focal cerebral ischemia, compatible with a contributory role in mitochondrial release of cytochrome c (Gao et al., 2005; Okuno et al., 2004; Shibata et al., 2002). It does not appear to be induced after global cerebral ischemia (Sanderson et al., 2009). Hippocampal damage was strongly reduced in bim−/− mice subjected to neonatal hypoxia/ischemia (Ness et al., 2006). Increased BAX and BH3-only proteins were reported in CA1 neurons after global ischemia (Martinez et al., 2007).
3. miRNAs regulate BCL-2 family proteins (Table 1)
Table 1.
BCL-2 family proteins are regulated by miRNAs
Bold with underline represents the miRNA that are regulated by cerebral ischemia
The discovery of posttranscriptional gene silencing by miRNAs has led to an explosion of new hypotheses in human disease. A short (5–7 nt long) sequence, referred to as the seed sequence, in the miRNA determines the specificity of binding to the mRNA, so miRNAs can bind multiple mRNAs and mRNAs can be bound by multiple miRNAs, creating a new and complex regulatory layer to post-transcriptional control of the proteome.
Recent research has shown that many miRNAs directly target BCL-2 family proteins (Table 1). BCL-2 is targeted by many miRNAs including miR-195, miR-24-2, and miR-365-2 (Zeng et al., 2012), miR-125b (Shi et al., 2012b), miR-885-3p (Huang et al., 2011), miR-181a-1*, miR-30e, and miR-34a (Khanna et al., 2011), miR-451 (Nan et al., 2010), and miR-181d (Wang et al., 2012). Chronic exposure of neurons to alcohol increases levels of miR-497, leading to apoptosis by targeting BCL-2 (Yadav et al., 2011). miR-15b, which is upregulated 72 hr following MCAO, targets BCL-2 as well (Shi et al., 2013). BCL-xL, another anti-apoptotic member of the BCL-2 family, is targeted by miR-491-5p (Guo et al., 2012). In addition, pro-apoptotic BAX is targeted by miR-128 (Adlakha and Saini, 2011) and BIM is decreased by miR-92a (Shi et al., 2013).
Given that a single miRNA can theoretically bind to and inhibit a large number of related targets, the potential for miRNA modulation in cerebral ischemia is promising (Ouyang et al., 2013b). The rest of this section will focus on single miRNAs that modulate multiple BCL-2 family proteins, including some of our data on the miR-29 family.
3.1 Single miRNAs target multiple BCL-2 family members
A few miRNAs target multiple members of the BCL-2 family. In addition to BCL-2 (Shi et al., 2012b), miR-125b decreases both pro-apoptotic BAK along with the anti-apoptotic gene MCL-1 (Zeng et al., 2012). miR-497 also targets BCL-W in addition to BCL-2 in Neuro-2A cells (Yin et al., 2010). Knockdown of miR-497, which targets both BCL-2 and BCL-W (Yin et al., 2010), is protective against MCAO-induced neuronal death. Our laboratory has focused extensively on miR-181a, which downregulates both BCL-2 and MCL-1, anti-apoptotic members of the BCL-2 family. It was demonstrated that miR-181a increased in vulnerable regions such as the ischemic core in focal ischemia (Ouyang et al., 2012b) or the hippocampal CA1 region after global ischemia (Moon et al., 2013), and decreased in the ischemia-resistant areas, the penumbra and hippocampal dentate gyrus (DG) area respectively. Antagomir to miR-181a reduced miR-181a levels in the brain and reduced infarct size in focal ischemia (Ouyang et al., 2012b) and CA1 neuronal loss in global cerebral ischemia (Moon et al., 2013). Furthermore, transfecting primary cultures with miR-181a inhibitor led to protection of astrocytes from ischemia-like stresses (Ouyang et al., 2012a). In addition to targeting anti-apoptotic members of the BCL-2 family, miR-181 also targets the endoplasmic reticulum protein GRP78/HSP78/BIP (Ouyang et al., 2012b). Therefore it is likely that the protective phenotype associated with miR-181a antagomir treatment results from enhanced protein levels of GRP78, BCL-2, and MCL-1.
3.2 miR-29 family
The miR-29 family consists of three members (a, b, and c) that map to two distinct genomic loci in clusters (Fig. 1A in Ouyang et al. (2013c)): miR-29 a/b-1 in chromosome 6 in mouse and 7 in human, and miR-29c/b-2 in chromosome 1 in both mouse and human. It has been demonstrated that miR-29a/b-1 is developmentally regulated in mouse brain with the highest expression observed in adults (Hebert et al., 2008; Kole et al., 2011).
The study of miR-29 began primarily in cancer research and focused on its role in regulation of apoptotic pathways. However a question that has stirred controversy for several years is whether miR-29 is pro-survival or pro-apoptotic (Pekarsky et al., 2006). While miR-29 expression is elevated in some cancers where it appears to function as an oncogene (Gebeshuber et al., 2009; Han et al., 2010), others have found miR-29 to have tumor suppressor functions (Pekarsky et al., 2006; Wang et al., 2008). This question is not only relevant in cancer research but is also important in ischemia research. While downregulation of miR-29 protected hearts against ischemia-reperfusion injury (Ye et al., 2011), upregulation of miR-29 protected neurons from apoptosis during neuronal maturation (Kole et al., 2011) and forebrain cerebral ischemia (Ouyang et al., 2013c).
Luciferase target assays conducted in our lab indicate that the miR-29 family targets both pro- and anti-apoptotic BCL-2 family members (Fig. 1B, Fig. 3). Using computational miRNA target prediction (targetscan.org), we found that miR-29 could potentially target mRNA 3′UTRs of at least five BCL-2 family members including pro-survival ones BCL-w (BCL2L2) and MCL-1, multi-domain pro-apoptotic proteins BAK (BAK1) and BH3-only pro-apoptotic proteins like PUMA (BBC3) and BMF in an evolutionarily conserved way (Fig. 3A).
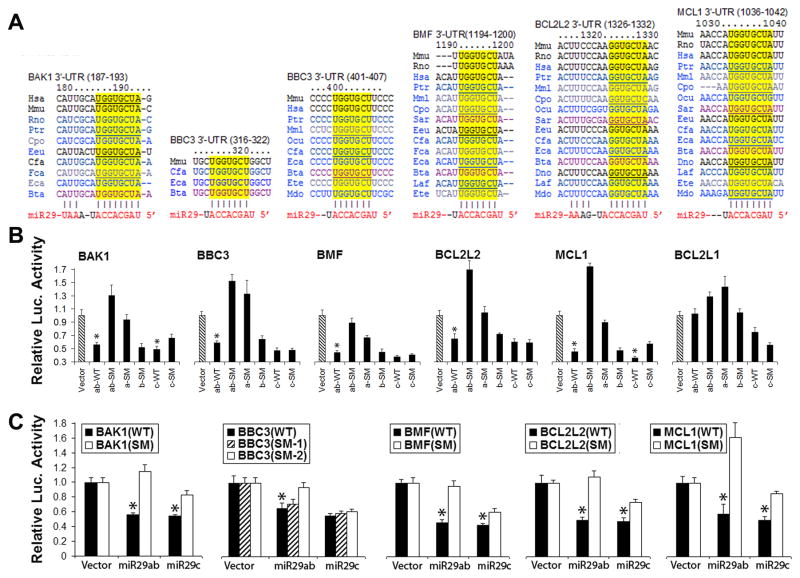
Fig. 3.
Luciferase assays validate multiple Bcl-2 family members as targets of the miR-29 family. A. Bioinformatics search suggests miR-29 could target 5 members of the BCL-2 family. The seed sequences of BAK1, BBC3, BMF, BCL2L2 and MCL1 3′UTRs targeted by miR-29 are highly conserved across species (from TargetScan). B. Dual luciferase activity assays using co-transfection with a plasmid containing luciferase followed by the 3′UTRs (WT) of BCL-2 family mRNAs and plasmids encoding either pri-miR-29 wild type or pri-miR-29 with seed mutations (SM) demonstrate that miR-29ab, but not miR-29c, recognizes the 3′UTRs of all of these five BCL-2 family members, but of Bcl2L1, which is not a predicted target. C. The same assay performed with the wild type 3′UTRs or their seed mutants (SM) of the five BCL-2 family members shows that miR-29ab reduces luciferase activity, validating that miR-29ab targets all of them. Assays were performed 3 times in triplicate. *P<0.01 compared to the miR-29ab-SM or 3′UTRs-SM group (the data shown for BBC3 data comes from Ouyang et al. (2013c); the additional data is in press in Ouyang et al. (2013d)).
To validate whether miR-29 directly recognizes the 3′UTRs of predicted BCL-2 family members, we cotransfected cells with luciferase control reporter, luciferase target reporter containing the wild type 3′UTR of BCL-2 family members and a miRNA (miR-29 or miR-29 mutant). For materials and methods on preparing pri-miRNA, 3′UTRs, and performing dual luciferase target validation assays see (Ouyang et al., 2013c). As shown in Fig. 3B, pri-miR-29ab (WT) represses expression from these 3′UTRs ~ 60% compared to vector controls. BCL2L1 (protein name BCL-xL) is not a predicated target of miR-29 and was used as a control.
Since pri-miR-29a and pri-miR-29b-1 are only about 100-nt apart, we made pri-miR-29a/b-1 constructs with different seed mutations to distinguish a and b functions. Seed mutation of miR-29ab (ab-SM) and miR-29a (a-SM) but not miR-29b (b-SM), de-repressed the targets indicating that within miR-29ab, miR-29a is the effective miRNA. Compared to vector controls, miR-29c (c-WT) is also able to repress these 3′UTRs significantly. However, seed mutation of miR-29c did not always de-repress the target indicating that the repression may not be specific. To further exclude off-target effects, we performed the complementary experiment of mutating the seed sequences of the 3′UTRs of BBC3 and BMF. This mutation abrogated repression of luciferase expression by miR-29ab and miR-29c. Thus we have demonstrated that all of the five BCL-2 family members are targets of miR-29ab and 4 out of 5 are targets of miR-29c. BBC3 (protein name PUMA) has two predicted target sites (Fig. 3A): the first is less broadly conservative than the second one. Fig. 3C shows that only the second site is the target for miR-29ab, and BBC3 is not a target for miR-29c. In summary, within miR-29ab, miR-29a is the effective component and only miR-29ab is the miRNA for all 5 members of the BCL-2 family.
The results strongly suggest that the reported both pro-apoptotic and anti-apoptotic effects of miR-29 likely result from different targets of miR-29 being inhibited in different cells or under different physiological or pathological settings. miR-29b is activated during neuronal maturation and targets several pro-apoptotic genes, BIM, BMF, HRK, PUMA, and BAK in the BCL-2 family (Kole et al., 2011). We found that miR-29a targets BH3-only protein PUMA and reduces neuronal vulnerability to forebrain ischemia (Ouyang et al., 2013c). However, the miR-29 family also has other demonstrated targets that contribute to protection, including DNA methyl transferase 3a (Pandi et al., 2013).
A recent report showed that loss of miR-29b at the infarct site is a key contributor to stroke lesion and the delivery of miR-29b mimic to the area decreased the stroke-induced brain lesion by half after MCAO (Khanna et al., 2013). In contrast, another study reported miR-29b increased with MCAO, but these authors measured miR-29b in whole brain after stroke, so it is unclear if the increase was primarily on the contralateral side (Shi et al., 2012a). A recent study demonstrated that miR-29c was significantly down-regulated after focal ischemia in the ischemic hemisphere in adult rats and after OGD in PC12 cells, and treatment with pre-miR-29c decreased the infarct volume as well as in vitro neuronal death (Pandi et al., 2013). Thus overall miR-29 family members have largely been found to be protective in the setting of cerebral ischemia.
4. Future directions
miRNAs may have greater therapeutic potential as candidates for the treatment of stroke than therapies targeting a single gene because of their faster post-transcriptional effect and their ability to simultaneously regulate many target genes, including transcription factors. Further work is needed to understand in which cells and under what physiological or pathological conditions the miR-29 family targets pro-apoptotic or anti-apoptotic proteins of the BCL-2 family. While pretreatment using miR-181a antagomir (Moon et al., 2013; Ouyang et al., 2012b) and miR-29a (Ouyang et al., 2013c) was effective in focal and global cerebral ischemia in rodents, testing treatment after the onset of ischemia is an essential step for the development of acute stroke treatment. Several miRNAs are already in clinical trials in liver diseases, suggesting that formulation and administration will be possible in a new disease setting or for a new miRNA target. However, in this regard, delivery into the CNS is often challenging, and remains part of the challenge in the clinical translation of miRNA therapy.
Highlights.
BCL-2 family regulates cerebral ischemic outcome
BCL-2 family regulates ER-mitochondrial crosstalk
MicroRNAs regulate the BCL-2 family
miR-29 targets multiple BCL-2 family members
Acknowledgments
Supported by NIH grants NS084396, NS053898, and NS080177 to RGG. The authors would like to thank William Magruder for help preparing the manuscript.
Footnotes
The authors have no conflicting financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adlakha Y, Saini N. MicroRNA-128 downregulates Bax and induces apoptosis in human embryonic kidney cells. Cellular and Molecular Life Sciences. 2011;68:1415–1428. doi: 10.1007/s00018-010-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender T, Martinou JC. Where killers meet--permeabilization of the outer mitochondrial membrane during apoptosis. Cold Spring Harb Perspect Biol. 2013;5:a011106. doi: 10.1101/cshperspect.a011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau B, Prudent J, Popgeorgiev N, Gillet G. Non-apoptotic roles of Bcl-2 family: the calcium connection. Biochim Biophys Acta. 2013;1833:1755–1765. doi: 10.1016/j.bbamcr.2013.01.021. [DOI] [PubMed] [Google Scholar]
- Bonner HP, Concannon CG, Bonner C, Woods I, Ward MW, Prehn JH. Differential expression patterns of Puma and Hsp70 following proteasomal stress in the hippocampus are key determinants of neuronal vulnerability. J Neurochem. 2010;114:606–616. doi: 10.1111/j.1471-4159.2010.06790.x. [DOI] [PubMed] [Google Scholar]
- Chami M, Prandini A, Campanella M, Pinton P, Szabadkai G, Reed JC, Rizzuto R. Bcl-2 and Bax exert opposing effects on Ca2+ signaling, which do not depend on their putative pore-forming region. J Biol Chem. 2004;279:54581–54589. doi: 10.1074/jbc.M409663200. [DOI] [PubMed] [Google Scholar]
- Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004a;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- Chen R, Valencia I, Zhong F, McColl KS, Roderick HL, Bootman MD, Berridge MJ, Conway SJ, Holmes AB, Mignery GA, Velez P, Distelhorst CW. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J Cell Biol. 2004b;166:193–203. doi: 10.1083/jcb.200309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chio CC, Lin JW, Cheng HA, Chiu WT, Wang YH, Wang JJ, Hsing CH, Chen RM. MicroRNA-210 targets antiapoptotic Bcl-2 expression and mediates hypoxia-induced apoptosis of neuroblastoma cells. Archives of Toxicology. 2013;87:459–468. doi: 10.1007/s00204-012-0965-5. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenrode EF, Yang J, Velmurugan GV, Foskett JK, White C. Apoptosis protection by Mcl-1 and Bcl-2 modulation of inositol 1,4,5-trisphosphate receptor-dependent Ca2+ signaling. J Biol Chem. 2010;285:13678–13684. doi: 10.1074/jbc.M109.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel T, Plesnila N, Prehn JH, Henshall DC. In vivo contributions of BH3-only proteins to neuronal death following seizures, ischemia, and traumatic brain injury. J Cereb Blood Flow Metab. 2011;31:1196–1210. doi: 10.1038/jcbfm.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Signore AP, Yin W, Cao G, Yin XM, Sun F, Luo Y, Graham SH, Chen J. Neuroprotection against focal ischemic brain injury by inhibition of c-Jun N-terminal kinase and attenuation of the mitochondrial apoptosis-signaling pathway. J Cereb Blood Flow Metab. 2005;25:694–712. doi: 10.1038/sj.jcbfm.9600062. [DOI] [PubMed] [Google Scholar]
- Gebeshuber CA, Zatloukal K, Martinez J. miR-29a suppresses tristetraprolin, which is a regulator of epithelial polarity and metastasis. EMBO Rep. 2009;10:400–405. doi: 10.1038/embor.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Zhang JP, Li B, Zeng C, You K, Chen MX, Yuan Y, Zhuang SM. MicroRNA-125b promotes apoptosis by regulating the expression of Mcl-1, Bcl-w and IL-6R. Oncogene. 2013;32:3071–3079. doi: 10.1038/onc.2012.318. [DOI] [PubMed] [Google Scholar]
- Grimm S. The ER-mitochondria interface: the social network of cell death. Biochim Biophys Acta. 2012;1823:327–334. doi: 10.1016/j.bbamcr.2011.11.018. [DOI] [PubMed] [Google Scholar]
- Guo R, Wang Y, Shi WY, Liu B, Hou SQ, Liu L. MicroRNA miR-491-5p Targeting both TP53 and Bcl-XL Induces Cell Apoptosis in SW1990 Pancreatic Cancer Cells through Mitochondria Mediated Pathway. Molecules. 2012;17:14733–14747. doi: 10.3390/molecules171214733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YC, Park CY, Bhagat G, Zhang J, Wang Y, Fan JB, Liu M, Zou Y, Weissman IL, Gu H. microRNA-29a induces aberrant self-renewal capacity in hematopoietic progenitors, biased myeloid development, and acute myeloid leukemia. J Exp Med. 2010;207:475–489. doi: 10.1084/jem.20090831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick JM, Chen YB, Jonas EA. Multipolar functions of BCL-2 proteins link energetics to apoptosis. Trends Cell Biol. 2012;22:318–328. doi: 10.1016/j.tcb.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick JM, Soane L. Multiple functions of BCL-2 family proteins. Cold Spring Harb Perspect Biol. 2013;5:a008722. doi: 10.1101/cshperspect.a008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert SS, Horre K, Nicolai L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, Kauppinen S, Delacourte A, De Strooper B. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression. Proc Natl Acad Sci U S A. 2008;105:6415–6420. doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Chuang AY, Ratovitski EA. Phospho-ΔNp63α/miR-885-3p axis in tumor cell life and cell death upon cisplatin exposure. Cell Cycle. 2011;10:3938–3947. doi: 10.4161/cc.10.22.18107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour AM, Heraud JE, Daunt CP, Kaufmann T, Sandow J, O’Reilly LA, Callus BA, Lopez A, Strasser A, Vaux DL, Ekert PG. Puma indirectly activates Bax to cause apoptosis in the absence of Bid or Bim. Cell Death Differ. 2009;16:555–563. doi: 10.1038/cdd.2008.179. [DOI] [PubMed] [Google Scholar]
- Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, MacLean KH, Han J, Chittenden T, Ihle JN, McKinnon PJ, Cleveland JL, Zambetti GP. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–328. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- Jones RG, Bui T, White C, Madesh M, Krawczyk CM, Lindsten T, Hawkins BJ, Kubek S, Frauwirth KA, Wang YL, Conway SJ, Roderick HL, Bootman MD, Shen H, Foskett JK, Thompson CB. The proapoptotic factors Bax and Bak regulate T Cell proliferation through control of endoplasmic reticulum Ca(2+) homeostasis. Immunity. 2007;27:268–280. doi: 10.1016/j.immuni.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15:1126–1132. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna A, Muthusamy S, Liang R, Sarojini H, Wang E. Gain of survival signaling by down-regulation of three key miRNAs in brain of calorie-restricted mice. Aging. 2011;3:223–236. doi: 10.18632/aging.100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna S, Rink C, Ghoorkhanian R, Gnyawali S, Heigel M, Wijesinghe DS, Chalfant CE, Chan YC, Banerjee J, Huang Y, Roy S, Sen CK. Loss of miR-29b following acute ischemic stroke contributes to neural cell death and infarct size. J Cereb Blood Flow Metab. 2013;33:1197–1206. doi: 10.1038/jcbfm.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Tsujimoto Y, Ohtsuki T, Kuwabara K, Matsushita K, Yang G, Tanabe H, Martinou JC, Hori M, Yanagihara T. Amelioration of hippocampal neuronal damage after global ischemia by neuronal overexpression of BCL-2 in transgenic mice. Stroke. 1998;29:2616–2621. doi: 10.1161/01.str.29.12.2616. [DOI] [PubMed] [Google Scholar]
- Kole AJ, Swahari V, Hammond SM, Deshmukh M. miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis. Genes Dev. 2011;25:125–130. doi: 10.1101/gad.1975411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki K, Virard I, Concannon CG, Engel T, Woods I, Taki W, Plesnila N, Henshall DC, Prehn JH. Effects of transient focal cerebral ischemia in mice deficient in puma. Neurosci Lett. 2009;451:237–240. doi: 10.1016/j.neulet.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Li H, Hui L, Xu W. miR-181a sensitizes a multidrug-resistant leukemia cell line K562/A02 to daunorubicin by targeting BCL-2. Acta Biochimica et Biophysica Sinica. 2012;44:269–277. doi: 10.1093/abbs/gmr128. [DOI] [PubMed] [Google Scholar]
- Luo X, He Q, Huang Y, Sheikh MS. Transcriptional upregulation of PUMA modulates endoplasmic reticulum calcium pool depletion-induced apoptosis via Bax activation. Cell Death Differ. 2005;12:1310–1318. doi: 10.1038/sj.cdd.4401659. [DOI] [PubMed] [Google Scholar]
- Luo Y, Kuo CC, Shen H, Chou J, Greig NH, Hoffer BJ, Wang Y. Delayed treatment with a p53 inhibitor enhances recovery in stroke brain. Ann Neurol. 2009;65:520–530. doi: 10.1002/ana.21592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez G, Musumeci G, Loreto C, Carnazza ML. Immunohistochemical changes in vulnerable rat brain regions after reversible global brain ischaemia. J Mol Histol. 2007;38:295–302. doi: 10.1007/s10735-007-9102-9. [DOI] [PubMed] [Google Scholar]
- Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathai JP, Germain M, Shore GC. BH3-only BIK regulates BAX, BAK-dependent release of Ca2+ from endoplasmic reticulum stores and mitochondrial apoptosis during stress-induced cell death. J Biol Chem. 2005;280:23829–23836. doi: 10.1074/jbc.M500800200. [DOI] [PubMed] [Google Scholar]
- Moon JM, Xu L, Giffard RG. Inhibition of microRNA-181 reduces forebrain ischemia induced neuronal loss. J Cereb Blood Flow Metab. 2013 doi: 10.1038/jcbfm.2013.157. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan Y, Han L, Zhang A, Wang G, Jia Z, Yang Y, Yue X, Pu P, Zhong Y, Kang C. MiRNA-451 plays a role as tumor suppressor in human glioma cells. Brain Research. 2010;1359:14–21. doi: 10.1016/j.brainres.2010.08.074. [DOI] [PubMed] [Google Scholar]
- Ness JM, Harvey CA, Strasser A, Bouillet P, Klocke BJ, Roth KA. Selective involvement of BH3-only Bcl-2 family members Bim and Bad in neonatal hypoxia-ischemia. Brain Res. 2006;1099:150–159. doi: 10.1016/j.brainres.2006.04.132. [DOI] [PubMed] [Google Scholar]
- Niizuma K, Endo H, Nito C, Myer DJ, Chan PH. Potential role of PUMA in delayed death of hippocampal CA1 neurons after transient global cerebral ischemia. Stroke. 2009;40:618–625. doi: 10.1161/STROKEAHA.108.524447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, Korsmeyer SJ. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2005;102:105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno S, Saito A, Hayashi T, Chan PH. The c-Jun N-terminal protein kinase signaling pathway mediates Bax activation and subsequent neuronal apoptosis through interaction with Bim after transient focal cerebral ischemia. J Neurosci. 2004;24:7879–7887. doi: 10.1523/JNEUROSCI.1745-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Giffard RG. Cellular neuroprotective mechanisms in cerebral ischemia: Bcl-2 family proteins and protection of mitochondrial function. Cell Calcium. 2004;36:303–311. doi: 10.1016/j.ceca.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Ouyang YB, Giffard RG. ER-Mitochondria Crosstalk during Cerebral Ischemia: Molecular Chaperones and ER-Mitochondrial Calcium Transfer. Int J Cell Biol. 2012;2012:493934. doi: 10.1155/2012/493934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Lu Y, Yue S, Giffard RG. miR-181 targets multiple Bcl-2 family members and influences apoptosis and mitochondrial function in astrocytes. Mitochondrion. 2012a;12:213–219. doi: 10.1016/j.mito.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Lu Y, Yue S, Xu LJ, Xiong XX, White RE, Sun X, Giffard RG. miR-181 regulates GRP78 and influences outcome from cerebral ischemia in vitro and in vivo. Neurobiol Dis. 2012b;45:555–563. doi: 10.1016/j.nbd.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Stary CM, White RE, Voloboueva LA, Giffard RG. The use of microRNAs to modulate redox and immune response to stroke. Antiox Redox Signaling. 2013a doi: 10.1089/ars.2013.5757. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Stary CM, Yang GY, Giffard R. microRNAs: innovative targets for cerebral ischemia and stroke. Curr Drug Targets. 2013b;14:90–101. doi: 10.2174/138945013804806424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Xu L, Lu Y, Sun X, Yue S, Xiong XX, Giffard RG. Astrocyte-enriched miR-29a targets PUMA and reduces neuronal vulnerability to forebrain ischemia. Glia. 2013c;61:1784–1794. doi: 10.1002/glia.22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Xu L, Yue S, Liu S, Giffard RG. Neuroprotection by astrocytes in brain ischemia: importance of microRNAs. Neuroscience Letters. 2013d doi: 10.1016/j.neulet.2013.11.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandi G, Nakka VP, Dharap A, Roopra A, Vemuganti R. MicroRNA miR-29c Down-Regulation Leading to De-Repression of Its Target DNA Methyltransferase 3a Promotes Ischemic Brain Damage. PLoS ONE. 2013;8:e58039. doi: 10.1371/journal.pone.0058039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarsky Y, Santanam U, Cimmino A, Palamarchuk A, Efanov A, Maximov V, Volinia S, Alder H, Liu CG, Rassenti L, Calin GA, Hagan JP, Kipps T, Croce CM. Tcl1 expression in chronic lymphocytic leukemia is regulated by miR-29 and miR-181. Cancer Res. 2006;66:11590–11593. doi: 10.1158/0008-5472.CAN-06-3613. [DOI] [PubMed] [Google Scholar]
- Plesnila N, Zinkel S, Le DA, Amin-Hanjani S, Wu Y, Qiu J, Chiarugi A, Thomas SS, Kohane DS, Korsmeyer SJ, Moskowitz MA. BID mediates neuronal cell death after oxygen/ glucose deprivation and focal cerebral ischemia. Proc Natl Acad Sci U S A. 2001;98:15318–15323. doi: 10.1073/pnas.261323298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimertz C, Kogel D, Rami A, Chittenden T, Prehn JH. Gene expression during ER stress-induced apoptosis in neurons: induction of the BH3-only protein Bbc3/PUMA and activation of the mitochondrial apoptosis pathway. J Cell Biol. 2003;162:587–597. doi: 10.1083/jcb.200305149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault TT, Chipuk JE. Getting away with murder: how does the BCL-2 family of proteins kill with immunity? Ann N Y Acad Sci. 2013;1285:59–79. doi: 10.1111/nyas.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez D, Rojas-Rivera D, Hetz C. Integrating stress signals at the endoplasmic reticulum: The BCL-2 protein family rheostat. Biochim Biophys Acta. 2011;1813:564–574. doi: 10.1016/j.bbamcr.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Rong YP, Aromolaran AS, Bultynck G, Zhong F, Li X, McColl K, Matsuyama S, Herlitze S, Roderick HL, Bootman MD, Mignery GA, Parys JB, De Smedt H, Distelhorst CW. Targeting Bcl-2-IP3 receptor interaction to reverse Bcl-2’s inhibition of apoptotic calcium signals. Mol Cell. 2008;31:255–265. doi: 10.1016/j.molcel.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong YP, Barr P, Yee VC, Distelhorst CW. Targeting Bcl-2 based on the interaction of its BH4 domain with the inositol 1,4,5-trisphosphate receptor. Biochim Biophys Acta. 2009;1793:971–978. doi: 10.1016/j.bbamcr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson TH, Kumar R, Murariu-Dobrin AC, Page AB, Krause GS, Sullivan JM. Insulin activates the PI3K-Akt survival pathway in vulnerable neurons following global brain ischemia. Neurol Res. 2009;31:947–958. doi: 10.1179/174313209X382449. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- Shi G, Liu Y, Liu T, Yan W, Liu X, Wang Y, Shi J, Jia L. Upregulated miR-29b promotes neuronal cell death by inhibiting Bcl2L2 after ischemic brain injury. Experimental Brain Research. 2012a;216:225–230. doi: 10.1007/s00221-011-2925-3. [DOI] [PubMed] [Google Scholar]
- Shi H, Sun B-l, Zhang J, Lu S, Zhang P, Wang H, Yu Q, Stetler RA, Vosler P, Chen J, Gao Y. miR-15b suppression of Bcl-2 contributes to cerebral ischemic injury and is reversed by sevoflurane preconditioning. CNS & neurological disorders drug targets. 2013;12:381–391. doi: 10.2174/1871527311312030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Zhang S, Feng K, Wu F, Wan Y, Wang Z, Zhang J, Wang Y, Yan W, Fu Z, You Y. MicroRNA-125b-2 confers human glioblastoma stem cells resistance to temozolomide through the mitochondrial pathway of apoptosis. International journal of oncology. 2012b;40:119–129. doi: 10.3892/ijo.2011.1179. [DOI] [PubMed] [Google Scholar]
- Shibata M, Hattori H, Sasaki T, Gotoh J, Hamada J, Fukuuchi Y. Temporal profiles of the subcellular localization of Bim, a BH3-only protein, during middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2002;22:810–820. doi: 10.1097/00004647-200207000-00006. [DOI] [PubMed] [Google Scholar]
- Strasser A, Cory S, Adams JM. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J. 2011;30:3667–3683. doi: 10.1038/emboj.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T, Bonner HP, Engel T, Woods I, Matsushima S, Ward MW, Taki W, Henshall DC, Concannon CG, Prehn JH. Bcl-2 homology domain 3-only proteins Puma and Bim mediate the vulnerability of CA1 hippocampal neurons to proteasome inhibition in vivo. Eur J Neurosci. 2011;33:401–408. doi: 10.1111/j.1460-9568.2010.07538.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Garzon R, Sun H, Ladner KJ, Singh R, Dahlman J, Cheng A, Hall BM, Qualman SJ, Chandler DS, Croce CM, Guttridge DC. NF-kappaB-YY1-miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer Cell. 2008;14:369–381. doi: 10.1016/j.ccr.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XF, Shi ZM, Wang XR, Cao L, Wang YY, Zhang JX, Yin Y, Luo H, Kang CS, Liu N, Jiang T, You YP. MiR-181d acts as a tumor suppressor in glioma by targeting K-ras and Bcl-2. Journal of Cancer Research and Clinical Oncology. 2012;138:573–584. doi: 10.1007/s00432-011-1114-x. [DOI] [PubMed] [Google Scholar]
- Wei C, Li L, Gupta S. NF-κB-mediated miR-30b regulation in cardiomyocytes cell death by targeting Bcl-2. Molecular and Cellular Biochemistry. 2013:1–7. doi: 10.1007/s11010-013-1878-1. [DOI] [PubMed] [Google Scholar]
- White C, Li C, Yang J, Petrenko NB, Madesh M, Thompson CB, Foskett JK. The endoplasmic reticulum gateway to apoptosis by Bcl-X(L) modulation of the InsP3R. Nat Cell Biol. 2005;7:1021–1028. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Lee JE, Giffard RG. Overexpression of bcl-2, bcl-XL or hsp70 in murine cortical astrocytes reduces injury of co-cultured neurons. Neurosci Lett. 1999;277:193–197. doi: 10.1016/s0304-3940(99)00882-4. [DOI] [PubMed] [Google Scholar]
- Yadav S, Pandey A, Shukla A, Talwelkar SS, Kumar A, Pant AB, Parmar D. miR-497 and miR-302b Regulate Ethanol-induced Neuronal Cell Death through BCL2 Protein and Cyclin D2. Journal of Biological Chemistry. 2011;286:37347–37357. doi: 10.1074/jbc.M111.235531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Li QJ, Gong ZB, Zhou L, You N, Wang S, An JZ, Wang DS, He Y, Dou KF. MicroRNA-34a Targets Bcl-2 and Sensitizes Human Hepatocellular Carcinoma Cells to Sorafenib Treatment. Technology in cancer research & treatment. 2013 doi: 10.7785/tcrt.2012.500364. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wu J, Guan H, Cai J, Fang L, Li J, Li M. MiR-136 promotes apoptosis of glioma cells by targeting AEG-1 and Bcl-2. FEBS Letters. 2012;586:3608–3612. doi: 10.1016/j.febslet.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Ye Y, Perez-Polo JR, Qian J, Birnbaum Y. The role of microRNA in modulating myocardial ischemia-reperfusion injury. Physiol Genomics. 2011;43:534–542. doi: 10.1152/physiolgenomics.00130.2010. [DOI] [PubMed] [Google Scholar]
- Yin KJ, Deng Z, Huang H, Hamblin M, Xie C, Zhang J, Chen YE. miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol Dis. 2010;38:17–26. doi: 10.1016/j.nbd.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin XM, Luo Y, Cao G, Bai L, Pei W, Kuharsky DK, Chen J. Bid-mediated mitochondrial pathway is critical to ischemic neuronal apoptosis and focal cerebral ischemia. J Biol Chem. 2002;277:42074–42081. doi: 10.1074/jbc.M204991200. [DOI] [PubMed] [Google Scholar]
- You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, Villunger A, Mak TW. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203:1657–1663. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng CW, Zhang XJ, Lin KY, Ye H, Feng SY, Zhang H, Chen YQ. Camptothecin Induces Apoptosis in Cancer Cells via MicroRNA-125b-Mediated Mitochondrial Pathways. Molecular Pharmacology. 2012;81:578–586. doi: 10.1124/mol.111.076794. [DOI] [PubMed] [Google Scholar]
- Zhang WH, Wang X, Narayanan M, Zhang Y, Huo C, Reed JC, Friedlander RM. Fundamental role of the Rip2/caspase-1 pathway in hypoxia and ischemia-induced neuronal cell death. Proceedings of the National Academy of Sciences. 2003;100:16012–16017. doi: 10.1073/pnas.2534856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem. 2003;85:1026–1036. doi: 10.1046/j.1471-4159.2003.01756.x. [DOI] [PubMed] [Google Scholar]