Abstract
Background
Tumor-based biomarkers of outcome for patients with clear cell renal cell carcinoma (ccRCC) remain limited, especially for those with low-risk disease. Type IIa topoisomerase (TOPOIIa) is a well-known biomarker of DNA replication and a target for antineoplastic agents, but it has not been evaluated as a biomarker of ccRCC outcome.
Objective
To evaluate the association of TOPOIIa expression in ccRCC and risk of cancer-specific death following surgery.
Design, setting, and participants
Two independent cohort studies were studied in tertiary referral urology practices in the United States. We identified cohorts of 1378 (analytic) and 279 (validation) patients who underwent nephrectomy for clinically localized ccRCC and had paraffin tumor tissue available. TOPOIIa expression was assessed using immunohistochemistry and scored as the number of positive cells per square millimeter.
Outcome measurements and statistical analysis
Our primary end point was cancer-specific survival (CSS). We evaluated TOPOIIa expression as a continuous variable and dichotomized as low versus high. For associations with CSS, we used Kaplan-Meier curves and Cox regression models.
Results and limitations
In both cohorts, patients who had high TOPOIIa expression were approximately three times more likely to experience ccRCC death than those with low expression (hazard ratio [HR]: 2.75; 95% confidence interval [CI], 2.12–3.56; p = 1.79E-14 and HR: 3.45; 95% CI, 1.34–8.88; p = 0.0104, respectively). Multivariable adjustment for pathologic features of aggressiveness did not explain these associations, and stratified analysis suggests that the association is more pronounced among patients with low-risk disease as defined by the Mayo Clinic stage, size, grade, and necrosis score.
Conclusions
Higher TOPOIIa expression is independently associated with increased risk of cancer death among patients undergoing surgery for ccRCC, and the prognostic value is pronounced among patients with low-risk disease. Evaluation of TOPOIIa in ccRCC provides the opportunity to help guide postsurgical surveillance for ccRCC patients as well as inform the design of more targeted clinical trials and novel treatment strategies.
Keywords: Kidney neoplasms, Carcinoma, renal cell, Tumor biomarkers, Biologic, Survival
1. Introduction
Mortality rates for renal cell carcinoma (RCC) have been rising steadily for >3 decades [1]. During the same time period, there has been little change in 5-yr survival for patients diagnosed with RCC (approximately 65%) [2]. Moreover, the small observed increases in survival can be attributed in part to a lead-time bias associated with a rise in the incidental detection of small, clinically dubious tumors [3,4]. Taken together, these trends underscore the need to continue efforts to improve our understanding of the factors that predict RCC aggressiveness, particularly among the growing number of individuals diagnosed with low-risk RCC.
DNA topoisomerases are enzymes that manage the topologic state of DNA in the cell by introducing temporary single- or double-strand breaks in the DNA [5]. Through these strand breaks, the topoisomerase enzymes allow for a wide variety of essential DNA metabolic reactions including replication, transcription, recombination, and chromatin remodeling [6,7]. Several investigative teams have reported that higher intratumor expression levels of topoisomerase enzymes are an indicator of poor prognosis in a variety of human cancers [8–10]. Of interest, drugs targeting topoisomerase enzymes have been developed and represent some of the most successful drugs used to treat human malignancies [11]. Despite this well-known association with cancer aggressiveness, the potential role of topoisomerases in the pathogenesis and prognosis of RCC remains unknown.
Motivated by this gap in understanding, we used two large independent cohort studies to analyze and validate the hypothesis that higher tumor protein expression levels of the type IIa topoisomerase (TOPOIIa) are associated with increased risk of cancer-specific death following surgery for localized clear cell RCC (ccRCC). Moreover, we explore the specific hypothesis that this association is more pronounced among patients with low-risk ccRCC.
2. Patients and methods
2.1. Patient selection
After institutional review board approval, we identified 1663 patients treated with radical nephrectomy or nephron-sparing surgery (NSS) for unilateral, sporadic, noncystic, organ-confined (ie, N0 or Nx, M0) ccRCC between 1990 and 2006 from the Mayo Clinic Rochester Nephrectomy Registry. Of these, 1464 patients (88%) had paraffin-embedded tissue blocks available for immunohistochemical (IHC) staining and available outcome data, and this group represents our analytic cohort. For our validation cohort, we identified 415 patients from the Mayo Clinic Florida Nephrectomy Registry treated with radical nephrectomy or NSS for unilateral, sporadic, noncystic ccRCC between 2000 and 2011. Of these, 337 (81%) had tissue blocks and outcome data available, and this group represents our validation cohort. We discuss further loss of cases in both cohorts resulting from failure of IHC staining in the Results section. Of note, the underlying patient catchment areas for Mayo Rochester and Mayo Florida are separated by >1000 miles (1600 kilometers) and as such represent geographically and culturally unique populations within the United States.
2.2. Data collection
For both cohorts, we abstracted follow-up data from the registry efforts at each institution. Briefly, these data are routinely updated and maintained through a combination of active (mail-out questionnaires) and passive (medical record, linkage to national databases) surveillance by experienced clinical coordinators [12]. Loss to follow-up is <5% for both registry efforts. In addition, we abstracted data on relevant clinicopathologic covariates including age at surgery, gender, symptoms at presentation, Eastern Cooperative Oncology Group performance status, the 2010 American Joint Committee on Cancer (AJCC) primary tumor classification, regional lymph node involvement, distant metastases, the 2010 AJCC TNM stage groupings, tumor size, nuclear grade, and presence of coagulative tumor necrosis. To obtain the pathologic features in a standardized fashion, one urologic pathologist at each site (J.C.C. and K.J.W.) centrally reviewed the microscopic hematoxylin and eosin slides from all specimens without knowledge of patient outcome.
2.3. Type IIa topoisomerase expression
We identified a paraffin-embedded block with representative tumor tissue for each patient in both cohorts and obtained a 5-μm-thick slide for IHC. Technicians in our core facility performed IHC staining for TOPOIIa using a monoclonal antibody and the respective protocol from Leica Microsystems (Buffalo Grove, IL, USA). One of our study pathologists (J.C.C.) trained a certified cytotechnologist (T.H.) to review the stained slides to determine TOPOIIa expression in each tumor. The staining pattern was recorded as the average of the number of positive tumor cells in each of five representative high-powered fields using a Leica DMR microscope (Leica Microsystems, Wetzlar, Germany). With a 10/25 eyepiece and a ×40 objective, the Leica DMR has an object field diameter of 0.625 mm2, resulting in a high-powered field of 0.307 mm2. As such, TOPOIIa expression was quantified as the number of positive tumor cells per square millimeter. For the purposes of evaluating intrarater reliability, we selected a random sample of 50 cases from the analytic cohort for re-review by the same cytotechnologist. Similarly, to assess interrater agreement, we randomly sampled 100 cases from the analytic cohort for independent review by a urologic pathologist (K.J.W.).
2.4. Statistical methods
For our analysis in both cohorts, we explored the magnitude of the association of continuous TOPOIIa expression and RCC-specific death by using Cox proportional hazards regression models and summarized the results with hazard ratios (HRs) and 95% confidence intervals (CIs). Smoothing splines were used to explore the functional form of the continuous TOPOIIa, which was quantified as the number of positive cells per square millimeter, and it was determined that the square-root transformation of TOPOIIa had a linear relationship with cancer-specific survival (CSS). Thus, for both the analytic and validation cohorts, the continuous TOPOIIa variable was quantified as the number of positive cells per millimeter in the Cox regression models. In the Cox models, we first estimated the age-adjusted association of TOPOIIa expression with time to RCC-specific death. Then, to assess the association of TOPOIIa expression with RCC-specific death after controlling for other known predictors of ccRCC outcome, we constructed Cox models that adjusted for individual pathologic features of aggressiveness as well as a composite scoring system (Mayo Clinic stage, size, grade, and necrosis [SSIGN] score). We also evaluated TOPOIIa expression as a dichotomized variable (ie, high vs low). To estimate a cut point for dichotomizing TOPOIIa expression into high-versus-low expression, we used the analytic cohort and chose the cut point that maximized the concordance index. As a result, tumors with TOPOIIa expression <16.6 positive cells per square millimeter categorized as “low”; those ≥16.6 were categorized as “high.” In the validation cohort, we dichotomized TOPOIIa expression using the same cut point as for the analytic cohort. We analyzed concordance index values to compare the predictive ability of various models with and without the addition of the TOPOIIa expression variable. All concordance indices were internally validated using a bootstrap methodology proposed by Harrell et al [13] and therefore represent optimism-corrected estimates of prognostic accuracy. To further explore the potential prognostic value of TOPOIIa expression, we evaluated Kaplan-Meier curves and HR estimates from Cox models stratified by Mayo Clinic SSIGN score (ie, low = 0–3, intermediate = 4–7, and high = 8–11) [14–16].
Finally, we used Pearson correlation coefficients to evaluate intra- and interrater agreement for our method of quantifying TOPOIIa staining. Our statistical analyses were performed using the R programming language, v.2.15. All tests were two sided, and p values <0.05 were considered statistically significant.
3. Results
3.1. Association of type IIa topoisomerase with pathology and renal cell carcinoma–specific death (analytic cohort)
For our analytic cohort, 1378 of 1464 patients (94%) had successful staining of TOPOIIa, and the mean level of TOPOIIa expression was 13.7 positive tumor cells per square millimeter (median: 7.0; min = 0.0, max = 277.7). Of note, we observed no statistically significant differences in demographic or clinical features between the 1378 patients in our final cohort and the 285 who were excluded for lack of tissue, follow-up, or successful IHC staining. In our dichotomization of TOPOIIa, 332 patients (24.1%) had tumors classified as high (≥16.6 TOPOIIa-positive tumor cells per millimeter). In Table 1, we provide a comparison of standard clinicopathologic features by dichotomized TOPOIIa status (low vs high). Those tumors classified as TOPOIIa high had more aggressive pathologic features including larger size (p < 0.0001), higher grade (p < 0.0001), later stage (p < 0.0001), presence of necrosis (p < 0.0001), sarcomatoid features (p = 0.0006), and higher Mayo Clinic SSIGN score (p < 0.0001). Estimates of the age-adjusted and multivariable associations of TOPOIIa expression variables with cancer-specific death are summarized in Table 2a. When modeled as a continuous variable, we noted evidence of a linear increase in the risk of cancer-specific death with increasing TOPOIIa expression (HR: 1.266; 95% CI, 1.210–1.326; p < 2.0E-16) after adjusting for age; TOPOIIa was modeled as the number of positive tumor cells per millimeter. When we dichotomized TOPOIIa expression, patients with high TOPOIIa expression were nearly three times more likely to experience cancer-specific death than patients who had low TOPOIIa expression (HR: 2.750; 95% CI, 2.123–3.561; p = 1.79E-14) after adjusting for age. Multivariable adjustment for a variety of known predictors of ccRCC outcome resulted in slight attenuation of the association of TOPOIIa expression with risk of cancer-specific death (Table 2a). To quantify the prognostic ability of TOPOIIa, we provide estimates of the optimism-corrected concordance indices for models with and without adjustment for TOPOIIa in Table 2b. Of note, we observed similar HRs and 95% CIs when we modeled recurrence of disease as the end point of interest instead of death from RCC (data not shown).
Table 1.
Comparison of the clinicopathologic features of patients undergoing surgery for clear cell renal cell carcinoma in the analytic and validation cohorts
| Analytic cohort (n = 1378) | Validation cohort (n = 279) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| TOPOIIa low | TOPOIIa High | p value | TOPOIIa low | TOPOIIa High | p value | |
| Gender no. (%) | 0.5515 | 0.4427 | ||||
| Female | 358 (34.2) | 120 (36.1) | 81 (33.2) | 9 (25.7) | ||
| Male | 688 (65.8) | 212 (63.9) | 163 (66.8) | 26 (74.3) | ||
| Age at surgery, yr | 0.7822 | 0.2001 | ||||
| Mean | 63.0 | 63.4 | 62.8 | 65.5 | ||
| Median | 63.9 | 64.6 | 63.6 | 67.2 | ||
| Range | 19.8–90.2 | 27.8–88.2 | 27.2–92.1 | 44.8–85.6 | ||
| Tumor size, cm | <0.0001 | 0.4366 | ||||
| Mean | 5.8 | 6.8 | 4.9 | 5.1 | ||
| Median | 5.0 | 6.0 | 4.0 | 4.5 | ||
| Range | 0.5–29.0 | 0.8–24.0 | 0.3–14.5 | 2.0–12.0 | ||
| TNM stage, no. (%) | <0.0001 | 0.9031 | ||||
| Missing | 3 | 1 | 1 | 0 | ||
| I | 681 (65.3) | 163 (49.2) | 175 (72.0) | 26 (74.3) | ||
| II | 151 (14.5) | 37 (11.2) | 32 (13.2) | 0 (0.0) | ||
| III | 208 (19.9) | 125 (37.8) | 35 (14.4) | 9 (25.7) | ||
| IV | 3 (0.3) | 6 (1.8) | 1 (0.4) | 0 (0.0) | ||
| Nuclear grade, no. (%) | <0.0001 | 0.0013 | ||||
| Missing | 0 | 0 | 1 | 0 | ||
| 1 | 93 (8.9) | 10 (3.0) | 19 (7.8) | 0 (0.0) | ||
| 2 | 518 (49.5) | 105 (31.6) | 169 (69.5) | 19 (54.3) | ||
| 3 | 398 (38.0) | 175 (52.7) | 47 (19.3) | 13 (37.1) | ||
| 4 | 37 (3.5) | 42 (12.7) | 8 (3.3) | 3 (8.6) | ||
| Coagulative tumor necrosis, no. (%) | <0.0001 | 0.8161 | ||||
| Missing | 0 | 0 | 2 | 0 | ||
| No | 876 (83.7) | 201 (60.5) | 198 (81.8) | 28 (80.0) | ||
| Yes | 170 (16.3) | 131 (39.5) | 44 (18.2) | 7 (20.0) | ||
| Sarcomatoid differentiation, no. (%) | 0.0006 | 0.4911 | ||||
| No | 1038 (99.2) | 320 (96.4) | 240 (98.4) | 34 (97.1) | ||
| Yes | 8 (0.8) | 12 (3.6) | 4 (1.6) | 1 (2.9) | ||
| SSIGN category, no. (%) | <0.0001 | 0.0772 | ||||
| Missing | 161 | 40 | 2 | 0 | ||
| 0–3 | 669 (75.6) | 157 (53.8) | 191 (78.9) | 24 (68.6) | ||
| 4–7 | 185 (20.9) | 92 (31.5) | 46 (19.0) | 8 (22.9) | ||
| 8+ | 31 (3.5) | 43 (14.7) | 5 (2.1) | 3 (8.6) | ||
TOPOIIa = type IIa topoisomerase; SSIGN = stage, size, grade, and necrosis.
Table 2.
(a) Age-adjusted and multivariable associations of type IIa topoisomerase expression with clear cell renal cell carcinoma–specific death in the analytic cohort and validation cohorts; (b) optimism-corrected concordance indices in the analytic and validation cohorts*
| a.
| ||||
|---|---|---|---|---|
| Analytic cohort | Validation cohort | |||
|
| ||||
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Continuous TOPOIIa (square root) | ||||
| Adjusted for age | 1.266 (1.210–1.326) | <2.0E-16 | 1.239 (1.114–1.378) | 7.68E-05 |
| Adjusted for age + gender | 1.275 (1.217–1.336) | <2.0E-16 | 1.226 (1.101–1.366) | 0.000208 |
| Adjusted for age + sarcomatoid | 1.244 (1.188–1.303) | <2.0E-16 | 1.258 (1.133–1.397) | 1.84E-05 |
| Adjusted for age + TNM stage | 1.171 (1.117–1.229) | 8.88E-11 | 1.181 (1.071–1.302) | 0.000844 |
| Adjusted for age + tumor size | 1.171 (1.120–1.225) | 3.11E-12 | 1.257 (1.134–1.393) | 1.23E-05 |
| Adjusted for age + nuclear grade | 1.142 (1.089–1.199) | 5.93E-08 | 1.211 (1.088–1.348) | 0.000443 |
| Adjusted for age + necrosis | 1.112 (1.061–1.166) | 9.02E-06 | 1.161 (1.052–1.281) | 0.00310 |
| Adjusted for age + SSIGN score | 1.077 (1.018–1.139) | 0.00990 | 1.099 (0.995–1.215) | 0.0639 |
| Dichotomous TOPOIIa (high vs low) | ||||
| Adjusted for age | 2.750 (2.123–3.561) | 1.79E-14 | 3.445 (1.337–8.877) | 0.0104 |
| Adjusted for age + gender | 2.764 (2.134–3.580) | 1.33E-14 | 3.192 (1.233–8.264) | 0.0168 |
| Adjusted for age + sarcomatoid | 2.625 (2.022–3.407) | 4.08E-13 | 3.721 (1.431–9.677) | 0.00705 |
| Adjusted for age + TNM stage | 1.872 (1.438–2.438) | 3.19E-06 | 3.545 (1.335–9.415) | 0.0111 |
| Adjusted for age + tumor size | 2.397 (1.848–3.110) | 4.66E-11 | 4.006 (1.493–10.748) | 0.00585 |
| Adjusted for age + nuclear grade | 1.805 (1.385–2.353) | 1.24E-05 | 2.676 (1.014–7.060) | 0.0467 |
| Adjusted for age + necrosis | 1.726 (1.322–2.252) | 5.89E-05 | 3.703 (1.400–9.795) | 0.00835 |
| Adjusted for age + SSIGN score | 1.502 (1.107–2.037) | 0.00889 | 3.232 (1.114–9.376) | 0.0309 |
| b.
| ||||
|---|---|---|---|---|
| Analytic cohort | Validation cohort | |||
|
| ||||
| Without TOPOIIa | Including TOPOIIa | Without TOPOIIa | Including TOPOIIa | |
| Continuous TOPOIIa (square root): | ||||
| Age | 0.580 (0.00214) | 0.663 (0.000270) | 0.605 (0.0063) | 0.665 (0.0232) |
| Age + gender | 0.575 (0.00287) | 0.662 (0.00471) | 0.594 (0.0208) | 0.657 (0.0504) |
| Age + sarcomatoid | 0.606 (0.000114) | 0.676 (0.00455) | 0.649 (0.0214) | 0.685 (0.0266) |
| Age + TNM stage | 0.810 (0.00108) | 0.826 (0.000984) | 0.785 (0.0105) | 0.822 (0.0130) |
| Age + tumor size | 0.787 (1.34E-05) | 0.813 (0.000605) | 0.794 (0.00498) | 0.832 (0.0031) |
| Age + nuclear grade | 0.789 (0.000524) | 0.809 (0.00149) | 0.848 (0.00871) | 0.820 (0.00895) |
| Age + necrosis | 0.796 (0.000939) | 0.817 (0.00184) | 0.822 (0.00915) | 0.833 (0.0187) |
| Age + SSIGN score | 0.873 (0.00123) | 0.878 (0.000757) | 0.877 (0.00723) | 0.885 (0.00773) |
| Dichotomous TOPOIIa (high vs low): | ||||
| Age | 0.580 (0.00259) | 0.662 (3.27E-05) | 0.605 (0.0069) | 0.676 (0.0118) |
| Age + gender | 0.576 (0.00184) | 0.662 (0.00326) | 0.594 (0.0201) | 0.667 (0.0385) |
| Age + sarcomatoid | 0.606 (0.000739) | 0.676 (0.00110) | 0.651 (0.0192) | 0.687 (0.0215) |
| Age + TNM stage | 0.811 (0.000263) | 0.828 (0.000557) | 0.785 (0.0103) | 0.808 (0.0135) |
| Age + tumor size | 0.785 (0.00280) | 0.806 (0.00260) | 0.787 (0.0118) | 0.804 (0.0162) |
| Age + nuclear grade | 0.788 (0.000245) | 0.807 (0.00130) | 0.844 (0.0129) | 0.834 (0.0219) |
| Age + necrosis | 0.797 (1.55E-05) | 0.815 (0.000743) | 0.825 (0.00561) | 0.845 (0.0109) |
| Age + SSIGN score | 0.874 (0.000822) | 0.879 (0.000226) | 0.883 (0.00164) | 0.883 (0.00874) |
CI = confidence interval; HR = hazard ratio; SSIGN = stage, size, grade, and necrosis; TOPOIIa = type IIa topoisomerase.
The optimism, calculated from 150 bootstrap samples, is in parentheses and is an estimate of overfitting.
3.2. Association of type IIa topoisomerase with pathology and renal cell carcinoma–specific death (validation cohort)
For our validation cohort, 279 of 337 patients (83%) had successful staining of TOPOIIa, and the mean level of TOPOIIa expression was 9.9 positive tumor cells per square millimeter (median: 3.5; minimum = 0.0, maximum = 347.6). In our dichotomization of TOPOIIa, 35 patients (12.5%) had tumors classified as TOPOIIa high (≥16.6 TOPOIIa-positive tumor cells per square millimeter). Again, we provide a comparison of standard clinicopathologic features by dichotomized TOPOIIa status (low vs high) in Table 1. As with the analytic cohort, we noted evidence that TOPOIIa-high tumors have more aggressive pathologic features including higher grade (p = 0.0013). For comparison with our analytic cohort, we provide estimates of the age-adjusted and multivariable associations of TOPOIIa expression with cancer-specific death in Table 2a. Similar to the analytic cohort, we noted evidence of a linear increase in risk of cancer-specific death with increasing TOPOIIa expression (HR: 1.239; 95% CI, 1.114–1.378; p = 7.68E-5) after adjusting for age; TOPOIIa was modeled as the number of positive tumor cells per millimeter. Moreover, when using the same cut point we used in the analytic cohort to dichotomize TOPOIIa expression, we once again noted that patients with high TOPOIIa expression were more than three times more likely to experience cancer-specific death than patients with low TOPOIIa expression (HR: 3.445; 95% CI, 1.337–8.877; p = 0.0104) after adjusting for age. Interestingly, multivariable adjustment for known predictors of ccRCC outcome did not result in attenuation of the association of TOPOIIa expression with risk of cancer-specific death (Table 2a). We again observed similar HRs and 95% CIs when we modeled recurrence of disease as the end point of interest instead of death from RCC (data not shown).
3.3. Stratified analysis (analytic cohort only)
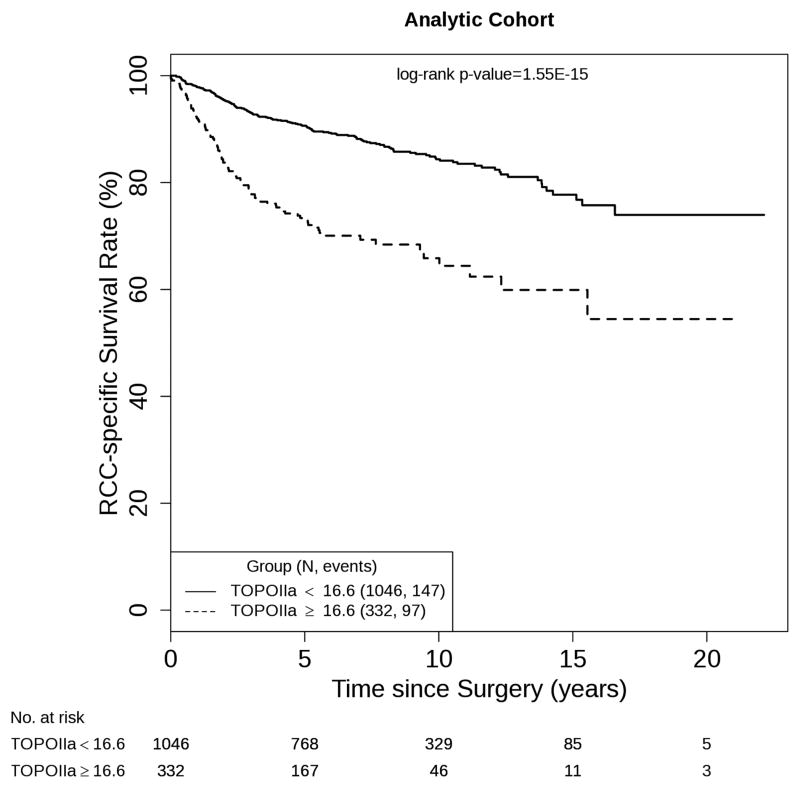
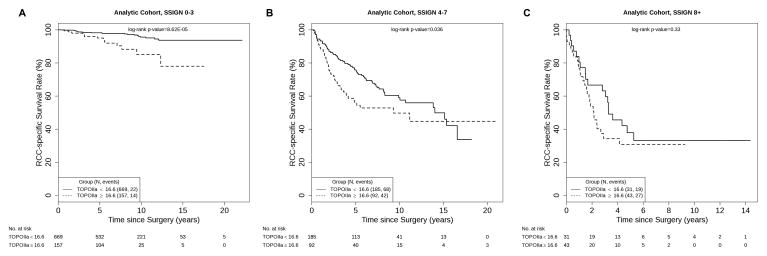
Figure 1 displays the overall disparity in CSS for patients with low- and high-TOPOIIa expression (log-rank p = 1.55E-15). To illustrate the potential prognostic value of TOPOIIa, we evaluated the ability of dichotomized TOPOIIa to further stratify patients following initial classification by Mayo Clinic SSIGN score. We noted that this was primarily evident among low-risk patients (Fig. 2a; log-rank p = 8.62E-5) and, to a slightly lesser extent, intermediate-risk patients (Fig. 2b; log-rank p = 0.0.036). In contrast, TOPOIIa expression had a limited ability to stratify patients already predicted to be at high risk of RCC-specific death based on SSIGN score (Fig. 2c; log-rank p = 0.33). In Table 3, we provide the age-adjusted HR and 95% CIs that correspond with our stratified Kaplan-Meier curves. Given that in our validation cohort only six patients who were classified as low risk using the SSIGN score experienced a ccRCC-specific death, we did not attempt stratified analyses in this cohort.
Fig. 1.
Estimated cancer-specific survival following surgery by dichotomized type IIa topoisomerase expression for 1378 patients with clear cell renal cell carcinoma (analytic cohort). RCC = renal cell carcinoma; TOPOIIa = type IIa topoisomerase.
Fig. 2.
Estimated cancer-specific survival following surgery by dichotomized type IIa topoisomerase expression among patients with (a) low, (b) intermediate, and (c) high Mayo Clinic stage, size, grade, and necrosis scores. RCC = renal cell carcinoma; SSIGN = stage, size, grade, and necrosis; TOPOIIa = type IIa topoisomerase.
Table 3.
Age-adjusted association (hazard ratio and 95% confidence interval) of high type IIa topoisomerase expression with cancer-specific death stratified by Mayo Clinic stage, size, grade, and necrosis score (analytic cohort only)
|
|
||||||
|---|---|---|---|---|---|---|
| Low risk (SSIGN = 0–3) | Intermediate risk (SSIGN = 4–7) | High risk (SSIGN = 8–11) | ||||
|
| ||||||
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
|
| ||||||
| TOPOIIa | ||||||
| Low | 1.0 (reference) | – | 1.0 (reference) | – | 1.0 (reference) | – |
| High | 3.55 (1.80–6.98) | 0.0002 | 1.52 (1.03–2.24) | 0.035 | 1.48 (0.81–2.71) | 0.20 |
SSIGN = stage, size, grade, and necrosis; HR = hazard ratio; CI = confidence interval; TOPOIIa = type IIa topoisomerase
3.4. Inter- and intrarate reliability for type IIa topoisomerase staining level
We observed a high level of intrarater reliability for the quantitation of TOPOIIa staining. The correlation coefficient among the 50 cases that were read twice in a blinded fashion by our cytotechnologist (T.H.) was 0.77 (p < 0.001). Similarly, we noted a high level of interrater reliability for the quantitation of TOPOIIa staining. The correlation coefficient among the 100 cases read by our cytotechnologist (T.H.) and an experienced urologic pathologist (K.J.W.) was 0.70 (p < 0.001).
4. Discussion
Key advancements in the management of ccRCC patients continue to center on the need to more accurately pinpoint postoperative risk for cancer-related death to better inform patient surveillance and streamline the design of the next generation of clinical trials [17]. Related to this, there is a parallel need to identify molecular features within ccRCC tumor tissues that represent markers of disease aggressiveness, predictors of treatment response, and rational targets for the development of novel therapeutics [18]. We present the first data supporting the finding that higher expression of TOPOIIa is associated with an increased risk of cancer-related death following surgery for clinically localized ccRCC. Aspects of our report that increase the value of our findings include (1) these associations remained after adjustment for known predictors of ccRCC aggressiveness, (2) we noted a specific association among the patients with low-risk disease (the largest growing subset of ccRCC patients), and (3) we report high inter- and intrarater agreement with regard to quantifying TOPOIIa in ccRCC tissues.
Implications of our current results warrant further discussion. Primary among these is the potential for TOPOIIa (or any single biomarker) to emerge as a meaningful clinical tool in the postsurgical management of ccRCC patients. In fact, although we have shown that TOPOIIa remains statistically associated with cancer-specific death after adjustment for SSIGN score and age (p = 0.0099), the clinically relevant improvement in concordance index deserves further discussion. The incorporation of TOPOIIa into a prognostic model with age and SSIGN score will improve prediction for roughly 5 in 1000 patients. However, we have demonstrated in Table 3 and Figure 2 that this improvement in prediction will largely take place for patients who would otherwise be classified as low risk using standard clinicopathologic indices. We have previously advocated for the sequential or stepwise use of tumor-based biomarkers in determining postsurgical ccRCC prognosis [19]. In other words, rather than seeking to immutably integrate a particular biomarker into an existing algorithm, we support the use of biomarkers on an as-needed basis. The most cost-effective value for any tumor-based biomarker rests on its ability to first determine prognosis for a patient using readily available routine pathology-based indices and algorithms. This effort can then be followed by further prognostic refinement by biomarker testing, where physicians and their patients deem it necessary. Along these lines, our data suggest that any value of TOPOIIa as a prognostic marker would most likely be in the specific subset of patients with low-risk disease. Patients are often uncomfortable with the notion they are at low risk for developing metastatic disease after surgery. For many, this fear is exacerbated by the absence of any guidelines as to how they can lower their risk further; the lack of a screening marker for early detection of recurrent disease; and the reality that if metastatic disease develops, no therapies offer durable success. In contrast, patients who have intermediate- or high-risk ccRCC are often placed on more rapid surveillance protocols (ie, imaging at 6 mo rather than 1 yr) or even encouraged to enroll in adjuvant trials. Our data support that staining and analysis of TOPOIIa could be offered to patients who have low-risk disease to provide additional information regarding the probability that they are among the 5–10% who will progress to metastatic disease and die from their cancer. Ultimately, the clinical value of TOPOIIa (or any biomarker) will most likely be realized when adjuvant therapies for ccRCC are approved and these biomarkers can be examined for their ability to predict response to therapy.
Limitations of our current study warrant further discussion. Chief among these is that our sample size for the validation cohort was smaller than the analytic cohort. That said, it is worth noting that for our primary analysis (estimating association with TOPOIIa), our validation cohort was adequately powered to report the same associations we observed in the analytic cohort as statistically significant. Additional limitations include patient populations from tertiary referral centers with limited racial or ethnic diversity (>95% white); the focus on only one enzyme in the topoisomerase family; quantitation of TOPOIIa staining that did not incorporate a measure of staining intensity; exclusion of individuals because of lack of data on SSIGN score; tumor tissue or failed IHC staining; and follow-up that, although standardized, was observational and not performed as part of a clinical trial. Nevertheless, the strengths of this investigation include the two-stage cohort design, use of the same cut point in both cohorts, centralized pathology review, adjustment for well-known predictors of ccRCC outcome, additional stratified analyses, use of a commercially available monoclonal antibody, and demonstration of high inter- and intrarater agreement for quantification of TOPOIIa expression.
5. Conclusions
We provide the first evidence that higher expression of TOPOIIa in ccRCC tissues is associated with an increased risk of cancer-specific death independent of other known pathologic predictors of RCC outcome. Moreover, this association is more pronounced among patients with low-risk disease.
Take-home message.
The ability to identify which patients who undergo surgery for clear cell renal cell carcinoma (ccRCC) will progress and die from their disease remains challenging. Evaluation of type IIa topoisomerase expression can be used to augment standard pathologic indices and help identify ccRCC patients who have aggressive disease.
Footnotes
Author contributions: Alexander S. Parker had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Parker, Eckel-Passow, Parasramka, Joseph, Wu, Cheville, Leibovich.
Acquisition of data: Parker, Hilton, Parasramka, Joseph, Wu, Cheville, Leibovich.
Analysis and interpretation of data: Parker, Eckel-Passow, Serie, Hilton, Leibovich.
Drafting of the manuscript: Parker, Eckel-Passow, Parasramka, Joseph, Wu, Cheville, Leibovich, Hilton, Serie.
Critical revision of the manuscript for important intellectual content: Parker, Parasramka, Joseph.
Statistical analysis: Parker, Eckel-Passow, Serie.
Obtaining funding: Parker, Leibovich.
Administrative, technical, or material support: None.
Supervision: Parker, Leibovich.
Other (specify): None.
Financial disclosures: Alexander S. Parker certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Alexander S. Parker and John C. Cheville have patents pertaining to cancer prognostic markers including B7-H1, survivin, and Ki-67. Funding/Support and role of the sponsor: This research was funded by a grant from the National Institutes of Health (NIH) and National Cancer Institute (NCI) [R01CA134466-4]. NIH and NCI participated in the collection, management, analysis, and interpretation of the data in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sun M, Thuret R, Abdollah F, et al. Age-adjusted incidence, mortality, and survival rates of stage-specific renal cell carcinoma in North America: a trend analysis. Eur Urol. 2011;59:1–4. doi: 10.1016/j.eururo.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Cho E, Adami HO, Lindblad P. Epidemiology of renal cell cancer. Hematol Oncol Clin North Am. 2011;25:651–65. doi: 10.1016/j.hoc.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–31. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 4.Leslie JA, Prihoda T, Thompson IM. Serendipitous renal cell carcinoma in the post-CT era: continued evidence in improved outcomes. Urol Oncol. 2003;21:39–44. doi: 10.1016/s1078-1439(02)00205-3. [DOI] [PubMed] [Google Scholar]
- 5.Deweese JE, Osheroff N. The DNA cleavage reaction of topoisomerase II: wolf in sheep’s clothing. Nucleic Acids Res. 2009;37:738–48. doi: 10.1093/nar/gkn937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nitiss JL. DNA topoisomerase II and its growing repertoire of biological functions. Nat Rev Cancer. 2009;9:327–37. doi: 10.1038/nrc2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakshi RP, Galande S, Muniyappa K. Functional and regulatory characteristics of eukaryotic type II DNA topoisomerase. Crit Rev Biochem Mol Biol. 2001;36:1–37. doi: 10.1080/20014091074165. [DOI] [PubMed] [Google Scholar]
- 8.Depowski PL, Rosenthal SI, Brien TP, Stylos S, Johnson RL, Ross JS. Topoisomerase IIalpha expression in breast cancer: correlation with outcome variables. Mod Pathol. 2000;13:542–7. doi: 10.1038/modpathol.3880094. [DOI] [PubMed] [Google Scholar]
- 9.Erguden HÇ, Koksal D, Demirag F, et al. The association of topoisomerase 2α expression with prognosis in surgically resected non-small cell lung cancer (NSCLC) patients. J Thorac Dis. 2012;4:352–7. doi: 10.3978/j.issn.2072-1439.2012.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Resende MF, Vieira S, Chinen LT, et al. Prognostication of prostate cancer based on TOP2A protein and gene assessment: TOP2A in prostate cancer. J Transl Med. 2013;11:36. doi: 10.1186/1479-5876-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fortune JM, Osheroff N. Topoisomerase II as a target for anticancer drugs: when enzymes stop being nice. Prog Nucleic Acid Res Mol Biol. 2000;64:221–53. doi: 10.1016/s0079-6603(00)64006-0. [DOI] [PubMed] [Google Scholar]
- 12.Parker AS, Cheville JC, Lohse CM, Igel T, Leibovich BC, Blute ML. Loss of expression of von Hippel-Lindau tumor suppressor protein associated with improved survival in patients with early-stage clear cell renal cell carcinoma. Urology. 2005;65:1090–5. doi: 10.1016/j.urology.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 13.Harrell FE, Lee KL, Mark DB. Multivariate prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168:2395–400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 15.Fujii Y, Saito K, Iimura Y, et al. External validation of the Mayo Clinic cancer specific survival score in a Japanese series of clear cell renal cell carcinoma. J Urol. 2008;180:1290–5. doi: 10.1016/j.juro.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Zigeuner R, Hutterer G, Chromecki, et al. External validation of the Mayo Clinic stage, size, grade, and necrosis (SSIGN) score for clear-cell renal cell carcinoma in a single European centre applying routine pathology. Eur Urol. 2010;57:102–9. doi: 10.1016/j.eururo.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 17.Atkins MB, Avigan DE, Bukowski RM, et al. Innovations and challenges in renal cancer: consensus statement from the first international conference. Clin Can Res. 2004;10:6277S–81S. doi: 10.1158/1078-0432.CCR-040720. [DOI] [PubMed] [Google Scholar]
- 18.Lam JS, Shvarts O, Leppert JT, Figlin RA, Belldegrun AS. Renal cell carcinoma 2005: new frontiers in staging, prognostication and targeted molecular therapy. J Urol. 2005;173:1853–62. doi: 10.1097/01.ju.0000165693.68449.c3. [DOI] [PubMed] [Google Scholar]
- 19.Parker AS, Leibovich BC, Lohse CM, et al. Development and evaluation of BioScore: a biomarker panel to enhance prognostic algorithms for clear cell renal cell carcinoma. Cancer. 2009;115:2092–103. doi: 10.1002/cncr.24263. [DOI] [PMC free article] [PubMed] [Google Scholar]