Abstract
Background
No study has evaluated current scoring systems for their accuracy in predicting short- and long-term outcome of alcoholic hepatitis in a U.S. population.
Methods
We reviewed electronic records for patients with ALD admitted to Parkland Memorial Hospital between January 2002 and August 2005. Data and outcomes for 148 of 1761 admissions meeting pre-defined criteria were collected. The discriminant function (DF) was revised (INRdf) to account for changes in prothrombin time reagents that could potentially affect identification of risk using the prior DF threshold of > 32. Admission and theoretical peak scores using the Model for End-stage Liver Disease (MELD) were calculated. Analysis models compared 5 different scoring systems.
Results
INRdf was closely correlated with the old DF (r2 = 0.95). Multivariate analysis of data showed that survival at 28 days was significantly associated with admission values for white blood cell count (p = 0.006), a scoring system using a combination of age, bilirubin, coagulation status and creatinine (p < 0.001) as well as an elevated ammonia result within 2 days of admission (p = 0.006). When peak values for MELD were included, they were the most significant predictor of short-term mortality (p < 0.001) followed by INRdf (p = 0.006
Conclusion
On admission, 2 scoring systems that identify a subset of patients with severe alcoholic liver disease are able to predict > 50% mortality at 4 weeks as well as > 80% mortality at 6 months without specific treatment.
Keywords: discriminant function, Model of End-stage Liver Disease, prothrombin time, internal sensitivity index, alcoholic hepatitis scores
Introduction
Management of severe alcoholic hepatitis is not uniform in the U.S. despite decades of clinical research [1–3]. The value of corticosteroids for a sub-group of patients was often debated [4–6]. In 2008, the Cochrane Hepato-Biliary Group systematic review, with meta-analyses and trial sequential analyses, concluded that the current evidence base did not support the use of corticosteroids and that additional data were needed[7] while society guidelines advocated corticosteroids for more severe disease[8].
Factors that may influence short- (28 day) or intermediate-term (90 day or 6 month) survival in alcoholic hepatitis now include geographic location and availability of orthotopic liver transplantation. Limitations placed on transplantation arise from either lack of eligibility for liver transplantation, without 6 months of documented complete abstinence, or lack of financial resources or both. Identifying those likely to benefit from corticosteroids or alternative novel therapies in the short-term may be important for understanding not only intermediate- but also long-term (> 12 months) survival with or without rescue transplantation.
The criteria for defining steroid-responsive severe disease were originally established by Maddrey and colleagues[9]. The equation that discriminated those patients with the potential to benefit from corticosteroid therapy is often termed the Maddrey discriminant function (DF). The equation was a simple calculation based on the total bilirubin and the prothrombin time[9] which was then modified by Carithers et al so that prothrombin time (PT) measurements in different institutions could be compared and pooled in clinical trials[10].
Since the empiric generation of the Maddrey DF in the 1970s, measurements of total bilirubin have not changed. In contrast, measurement of the PT has undergone a series of improvements, designed to allow comparison of results between different laboratories [11–13]. New scoring systems to assess the severity of alcoholic hepatitis, with measures other than PT to assess coagulopathy, were developed in Scotland[14] and Spain[15] and compared with DF and the Model for End-stage Liver Disease (MELD) score in European[16–18] and Mexican[19] patients. We postulated that changes in PT reagents altered the DF threshold in a quantifiable manner. The current retrospective cross-sectional study was undertaken to examine this hypothesis and to identify factors that predicted survival in U.S. patients using 5 different scoring systems.
Materials and Methods
A UT Southwestern institutional review board approved, retrospective electronic chart review was performed of all patients with a diagnosis of alcoholic hepatitis between the dates of January 2002 and August 2005 at Parkland Memorial Hospital (PMH) an affiliated hospital of the University of Texas Southwestern Medical Center in Dallas, Texas. Subjects were identified by electronic health record query of all patients discharged with International Classification of Diseases, 9th revision codes 571.1 (acute alcoholic hepatitis), 571.2 (alcoholic cirrhosis) and 571.3 (alcoholic liver disease). A state institutional review board approved access to death certificate data from the Texas State Department of Health Statistics.
Patient selection criteria
Inclusion criteria were based on laboratory features consistent with jaundice from an acute decompensation in ALD (bilirubin > 5 mg/dL unaccounted for by another etiology or transfusion, AST increased and < 500 U/L with AST > ALT). Results from a clinical data repository were extracted; radiology, pathology and discharge summaries were reviewed for relevant information. Exclusion criteria were concomitant liver disease, persistent hyperbilirubinemia for > 2 months prior to admission, abstinence confirmed on multiple encounters, an alternative diagnosis or a previous index admission. Results of paracentesis in the first 2 days of the admission were used as a surrogate marker of clinical ascites and any elevated ammonia level in the first 2 days was used as a marker of deteriorated overall liver function. This time period allowed patients to improve or deteriorate when initially hospitalized, a strategy comparable to the observation period before using corticosteroids in management.
PT measurements
Conversion of PT measured in seconds, to International Normalized Ratio (INR), is dependent on the patient’s PT, the reference PT and the international sensitivity index (ISI) of the manufacturer’s reagents. The equation is INR = (patient PT / geometric mean of reference interval PT)ISI. The geometric mean reference interval changed from 10.69 (July to October 1997) to 11.13 (November 1997 to November 1998) and then to 11.71 (since December 1998). The ISI changed in December 1998 from 1.5 to 1.0. We calculated the effect of these changes in reagent sensitivity (ISI) and reference interval on the PT. The old and new values were related using the following equation, old PT = 4.087 + 0.5297(new PT). These effects were validated by analyzing > 1000 patient samples simultaneously using new versus old technology following implementation in 1998. We used this relationship to derive a new DF that used the INR (INRdf) with INRdf > 50 being the equivalent to DF > 32 prior to 1997 (see supplementary Figure A and supplementary Table A). The old DF was highly correlated to INRdf (r2 = 0.95), the correlation between admission MELD and either DF or INRdf was less strong (r2 = 0.71 and 0.79 respectively, supplementary Figure B).
Data collection and analysis
Demographics and laboratory test results were collected for all patients and entered into a computerized database. The Maddrey DF as modified for multi-site studies[10], an INR-based discriminant function (INRdf), MELD score with United Network for Organ Sharing modification[20], Glasgow alcoholic hepatitis score (GAHS)[14] and the age + serum bilirubin + INR + serum creatinine (ABIC) score[15] were calculated at admission. Peak bilirubin, PT, INR and creatinine levels were recorded and a theoretical peak MELD score was calculated from these data. Equations used for calculations of DF, INRdf, MELD and ABIC scores and GAHS are as shown (supplementary Table A).
Statistical analysis
Comparisons of demographics, laboratory values and mortality were performed among INRdf groups. Continuous variables were compared using Student’s t-test, Wilcoxon rank-sum (Mann-Whitney) tests or Spearman’s rank correlation; categorical variables were compared with chi-square tests or Fisher’s exact tests. Patient survival rates were estimated using the Kaplan-Meier method. Log-rank tests were conducted to examine if there were significant differences in survival among groups. Univariate Cox proportional hazards model was used to examine the association between risk factors and survival. Risk factors with p value less than 0.05 from univariate Cox regression were entered as candidate risk factors for stepwise Cox proportional hazards model, which was used to identify significant factors associated with survival. Variables were automatically excluded from multivariate analysis for collinearity. Statistical analysis was performed using Stata 12.1 (College Station, TX, USA) and SAS 9.3 (SAS Institute, Cary, NC, USA).
Results
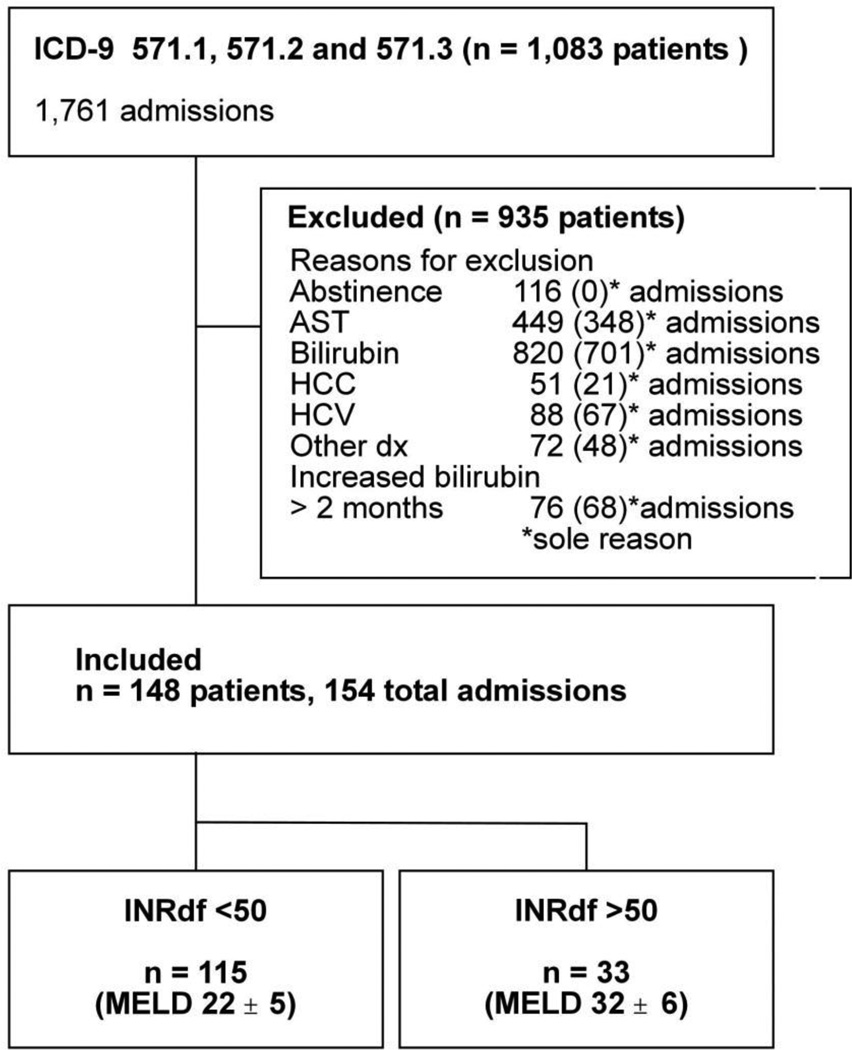
We reviewed the electronic charts of all patients with the diagnosis of acute alcoholic hepatitis, alcoholic cirrhosis or alcoholic liver disease not otherwise specified (ICD-9 571.1, 571.2 or 571.3) admitted to PMH between January 2002 and August 2005 (Figure 1). A total of 148 patients met the criteria for an index admission. Admissions for these 148 patients accounted for 9% (154/1,761) of the total hospitalizations with a diagnosis of alcoholic liver disease. Maximum bilirubin < 5 mg/dL was the commonest reason for exclusion followed by AST not matching entry criteria. AST was normal in 329 admissions (bilirubin also normal in 50, no recent alcohol in 21), ALT exceeded AST (n = 63) and AST > 500 IU/L (n = 57) were the findings. Based on the calculation of INRdf on admission, patients were classified into two mutually exclusive groups (Figure 1, Table I).
Figure 1.
Overall flow chart
Legend: Diagnoses resulting in exclusion, other than chronic hepatitis C and hepatocellular carcinoma, included acute and chronic hepatitis B, acute cholecystitis, acute kidney injury in an incarcerated patient, cholangiocarcinoma, chronic kidney disease with cirrhosis, cocaine ischemic liver injury, common bile duct transection, congestive heart failure, empyema of gall bladder, gas gangrene, hematoma, hemophagocytic histoplasmosis, incarcerated para-umbilical hernia, metastatic liver disease, motor vehicle accident, non-alcoholic fatty liver disease, pancreatitis, post-operative decompensation, primary biliary cirrhosis, renal cell carcinoma, secondary peritonitis, sepsis, Stevens-Johnson syndrome, subarachnoid hemorrhage, subdural hematoma and suspected drug-induced liver injury.
Table I.
Demographic and Clinical Characteristics on Admission
| Total | INRdf < 50 | INRdf > 50 | p-value* † | |
|---|---|---|---|---|
| n = 148 | n = 115 (78%) | n = 33 (22%) | ||
| Male | 118 (80%) | 91 (79%) | 27 (82%) | 0.74* |
| Age mean ± SD | 45 ± 9 | 46 ± 9 | 43 ± 7 | 0.054 |
| median, (range) | 45 (24 – 69) | 46 (24 – 69) | 44 (30 – 56) | |
| Race & ethnicity | ||||
| African-American | 14 (9%) | 12 (10%) | 2 (6%) | 0.41† |
| Caucasian | 67 (45%) | 55 (48%) | 12 (36%) | |
| Hispanic | 63 (43%) | 45 (39%) | 18 (55%) | |
| Native American | 4 (3%) | 3 (3%) | 1 (3%) | |
| DF mean ± SD | 44 ± 34 | 29 ± 14 | 94 ± 36 | < 0.001 |
| median, (range ) | 34 (0 – 193) | 28 (0 – 65) | 85 (55 – 193) | |
| DF > 32 | 80 (54%) | 47 (41%) | 33 (100%) | < 0.001* |
| MELD mean ± SD | 24 ± 6 | 22 ± 5 | 32 ± 6 | < 0.0001 |
| median, (range) | 22 (12 – 45) | 21 (12 – 35) | 30 (25 – 45) | |
| MELD < 21 | 64 (43%) | 64 (56%) | 0 | < 0.001† |
| MELD > 31 | 19 (13%) | 6 (5%) | 13 (39%) | <0.001† |
| GAHS mean ± SD | 8 ± 1 | 8 ± 1 | 9 ± 1 | < 0.0001 |
| median, (range) | 8 (5 – 11) | 8 (5 – 11) | 10 (7 – 11) | |
| GAHS > 9 | 27 (18%) | 10 (9%) | 17 (52%) | < 0.001* |
| ABIC mean ± SD | 7.5 ± 1.4 | 7.1 ± 1.2 | 8.9 ± 1.2 | < 0.0001 |
| median, (range) | 7.4 (4.3 – 12) | 7.0 (4.3 – 10.6) | 8.6 (7.3 – 12) | |
| ABIC > 9 | 24 (16%) | 8 (7%) | 16 (48%) | < 0.001* |
| ABIC < 6.71 | 43 (41%) | 43 (54%) | 0 | <0.001† |
| Paracentesis** | 54 (37%) | 43 (37%) | 11 (33%) | 0.67* |
| Hyperammonemia** | 90 (61%) | 64 (56%) | 26 (79%) | 0.016* |
Calculated from Chi-square test,
Fisher’s exact test, otherwise two-sample t-test.
70/148 (47%) subjects had paracentesis during the admission, 54/70 occurring within 2 days; 101/148 (68%) patients had a blood ammonia level within 2 days of admission.
Demographic and initial clinical characteristics
As shown in Table I, patients with INRdf < 50 (n = 115, 78%) had less severe alcoholic hepatitis with MELD scores of 22 ± 5 (mean ± SD) and DF < 32 in 59%. Patients with INRdf > 50 had more severe disease with initial MELD scores 32 ± 6 (n = 33, 22%); all of these patients had a DF > 32 and MELD > 21. The two groups were similar with respect to gender and ethnicity (Table I); INRdf > 50 patients were younger, this was not statistically significant (p = 0.054).
The calculated DF, ABIC and MELD scores and GAHS were significantly different between the groups, reflecting the contribution of bilirubin and INR to the calculations (Table I). There was a wide range of creatinine values on admission (INRdf < 50 = 0.4 – 3.7 mg/dL; INRdf > 50 = 0.6 – 6.0 mg/dL), explaining the lower correlation of INRdf with MELD than with the modified Maddrey DF (supplementary Figure B and Table B). An elevated ammonia within the first 2 days of admission was statistically more common in the patients with more severe disease (p = 0.016).
Hospitalization follow-up
The majority of patients developed worsening liver function during the index admission; bilirubin increased in 50%, PT in 51% and INR in 41% (supplementary Table B). The significant differences between the INRdf groups on admission persisted when peak values were compared. Renal function deteriorated in a subset of patients in both groups during the index hospitalization; overall creatinine increased in 56% of subjects, with higher frequency in those with more severe alcoholic hepatitis (supplementary Table B). Theoretical peak MELD scores (calculated from the highest values for each parameter) increased after admission in the majority of patients (58%). INRdf groups were statistically different when comparing peak MELD values and the distribution into MELD subgroups (< 21 and > 31).
Mortality
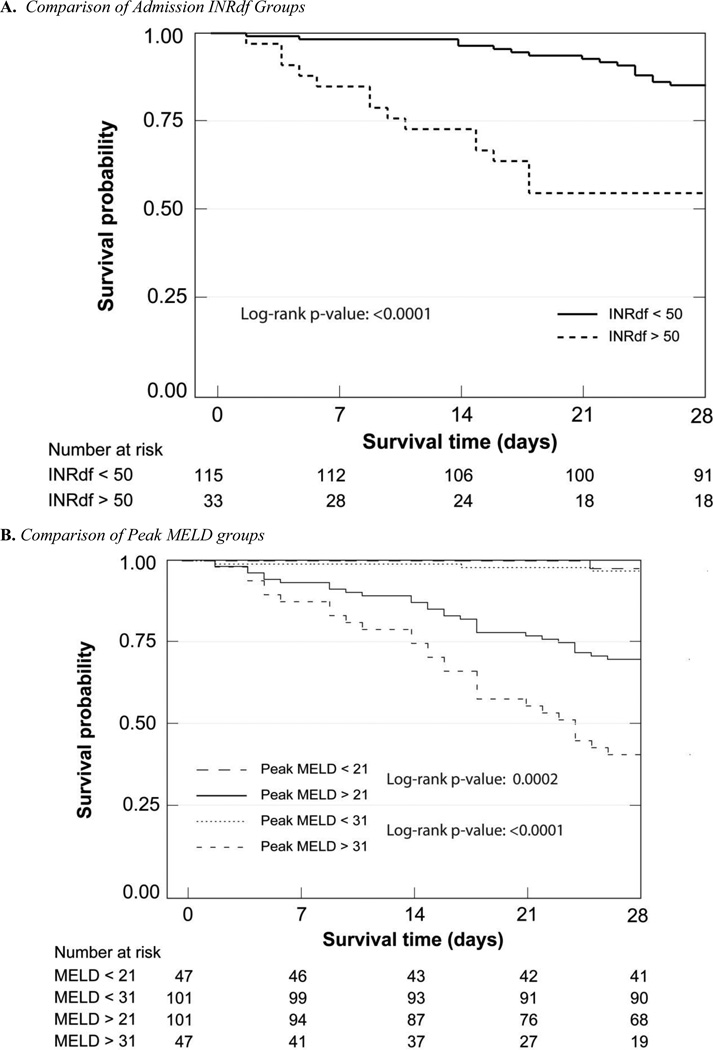
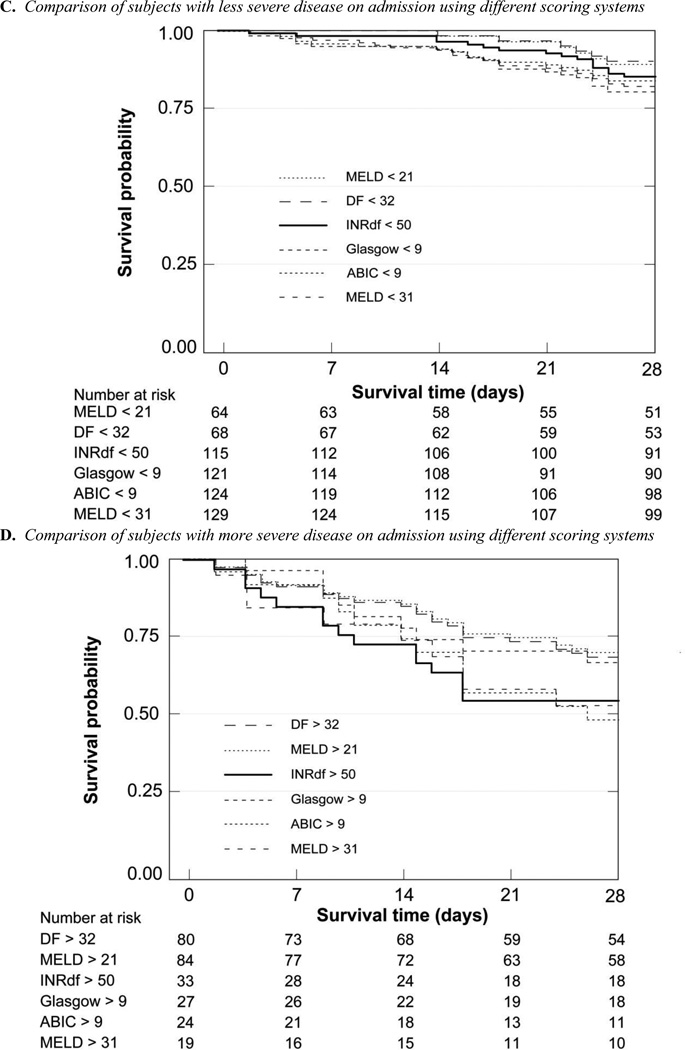
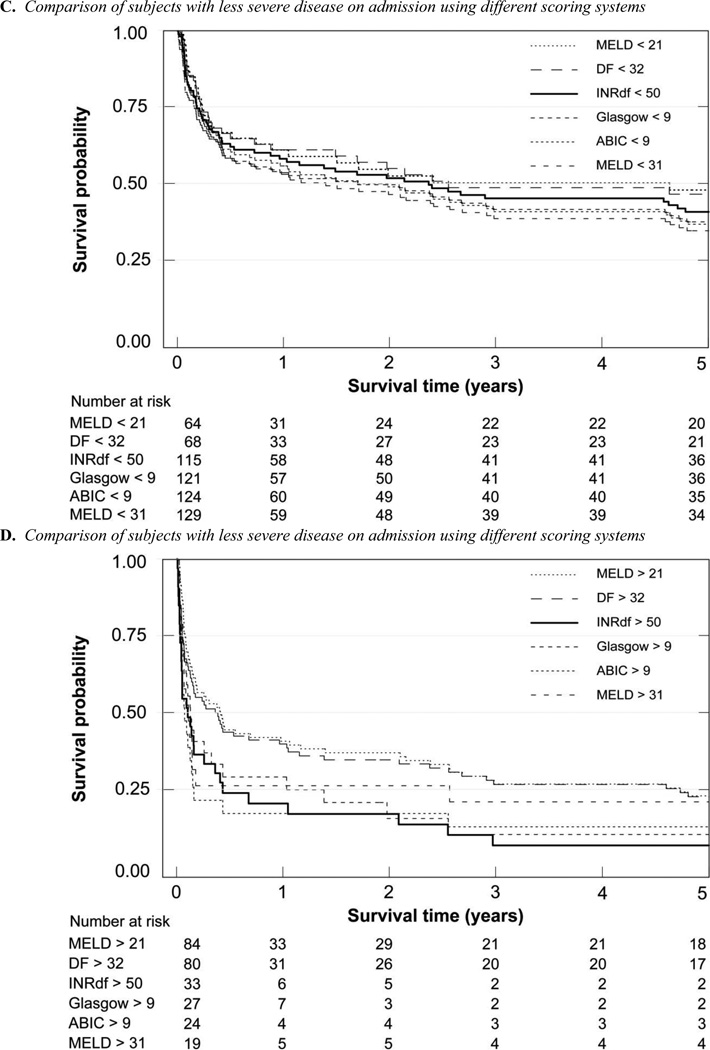
Death certificate data for all-cause mortality were obtained from the Texas State Department of Health Statistics. At each time point, (28 days, 90 days, 6 months, 5 years and overall) there were fewer survivors in the more severely affected groups (supplementary Table C, Figures 2 and 3); categories determined by INRdf, ABIC and MELD on admission gave similar results (40% – 44% alive at 28 days) with more subjects identified using INRdf (15/26 untreated with corticosteroids were deceased). Using DF > 32 for stratification was relatively less informative (66% alive at 28 days); the category included more subjects. The early end-points allow comparison with prior studies using 28 day or 90 day mortality to assess management strategies. When patients who received corticosteroids (n = 8) or corticosteroids and pentoxifylline (n = 1) were excluded, overall mortality was unchanged, reflecting the small number of subjects receiving specific treatments (supplementary Table C).
Figure 2.
Kaplan-Meier 28 Day Survival Analyses
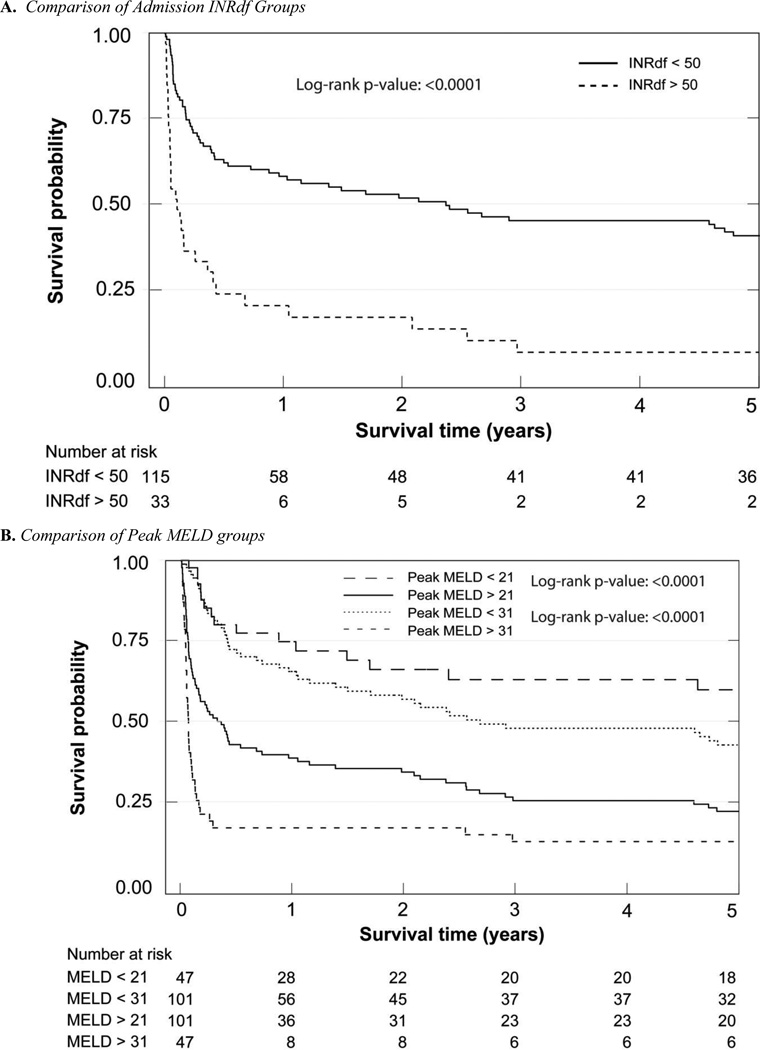
Figure 3.
Kaplan-Meier 5 year Survival Analyses
As expected, a peak MELD > 31 was highly predictive of mortality with 60% (28/47) succumbing by 28 days and 79% by 90 days. Regardless of its acuteness, those subjects with a creatinine that rose at least 0.3 mg/dL during the hospitalization (n = 48, with median peak creatinine 3.4 mg/dL, range 0.9 – 13.5 mg/dL), had a mortality that was high; 50% were known dead at 28 days and 69% at 90 days. In contrast, mortality was relatively low in those subjects with a creatinine that rose < 0.3 mg/dL during the hospitalization (n = 100, with median peak creatinine 0.9 mg/dL, range 0.45 – 4.35 mg/dL); 7% were known dead at 28 days and 19% at 90 days (5 and 7 unknown at 28 and 90 days respectively). None of the cohort was eligible for orthotopic liver transplantation, due to either financial or psychosocial barriers.
Survival analysis
The 28 day Kaplan-Meier survival curves of the different admission stratification groups were clearly separated; admission INRdf (p < 0.0001, chi = 36.20) and ABIC (p < 0.0001, chi = 23.47) were of higher statistical significance than admission MELD (log-rank p = 0.0012 for MELD < 21 and 0.0006 for MELD < 31). DF was the same as MELD > 31 (p = 0.0006) while the GAHS provided better differentiation (p = 0.0001, chi = 15.37). Theoretical peak MELD clearly demonstrated the high mortality in this group (MELD > 31, p < 0.0001, chi = 52.43) with less than half surviving 28 days, in sharp contrast to > 95% survival at 28 days when peak MELD was ≤ 31.
In the combined cohort of patients with DF > 32, only 9 patients received corticosteroids (2 with INRdf < 50, 7 with INRdf > 50). All but one was alive at 28 days, 5 had died by 90 days. The remaining 4 subjects (all with INRdf > 50) survived for at least a year after admission (for 13, 31, 70 and 71 months). Excluding the few subjects treated with steroids did not change the findings in the overall analysis.
Long term survival was different depending on severity of the alcoholic liver disease at the index admission as measured by INRdf, DF, ABIC, GAHS or MELD; peak MELD stratification was also informative (Figure 3). The dismal prognosis for all these patients is emphasized by the short median survival in those with a known 5 year outcome, even in the least affected groups (supplementary Table D). Only subjects with a peak MELD < 21 had a substantially longer median survival (65 months, see supplementary Table D). The co-existence of chronic hepatitis C and alcoholic liver disease further shortened survival (supplementary Table D). One abstinent subject with chronic hepatitis C underwent transplantation more than 12 months after admission for icteric liver disease.
Predictive factors
Cox proportional hazards model was used to identify factors predicting survival to 28 days from admission (Table II). In univariate analysis of continuous variables present on admission, higher bilirubin, creatinine, INR and PT levels, WBC counts and scoring systems other than GAHS were significantly associated with decreased survival. Categorical variables present on admission or within 2 days of admission (DF group, INRdf group, ABIC group, MELD group and elevated ammonia) were also significant predictors of decreased survival. When later data from the index admission were analyzed, peak MELD score and peak MELD group predicted decreased survival as did the difference in creatinine between admission and its peak value (Table II).
Table II.
Univariate Analysis of Predictors of 28 Day Survival*
| Variable | Parameter Estimate (± standard error) |
HR (95% CI) | p value | Area Under Curve† |
|---|---|---|---|---|
| Bilirubin | 0.03 (± 0.02) | 1.03 (1.00 – 1.07) | 0.044 | |
| Creatinine | 0.37 (± 0.15) | 1.45 (1.08 – 1.95) | 0.014 | |
| INR | 0.64 (± 0.15) | 1.90 (1.42 – 2.56) | < 0.001 | |
| PT | 0.070 (± 0.016) | 1.07 (1.04 – 1.11) | < 0.001 | |
| WBC | 0.063 (± 0.018) | 1.07 (1.03 – 1.10) | < 0.001 | |
| Hyperammonemia‡ | 1.65 (± 0.54) | 5.18 (1.81 – 14.82) | 0.002 | |
| DF score | 0.015 (± 0.00) | 1.02 (1.01 – 1.02) | < 0.001 | 0.74 |
| DF 32 category | 1.37 (± 0.45) | 3.92 (1.61 – 9.56) | 0.003 | |
| INRdf score | 0.026 (± 0.006) | 1.03 (1.01 – 1.04) | < 0.001 | 0.74 |
| INRdf 50 category | 1.44 (± 0.36) | 4.23 (2.09 – 8.66) | < 0.001 | |
| MELD score | 0.09 (± 0.02) | 1.10 (1.05 – 1.15) | < 0.001 | 0.74 |
| MELD 21 category | 1.24 (± 0.45) | 3.45 (1.42 – 8.42) | 0.006 | |
| MELD 31 category | 1.22 (± 0.40) | 3.40 (1.56 – 7.40) | 0.002 | |
| ABIC score | 0.34 (± 0.12) | 1.40 (1.11 – 1.77) | 0.005 | 0.68 |
| ABIC category | 0.95 (± 0.29) | 2.58 (1.46 – 4.56) | 0.001 | |
| Peak MELD score | 0.13 (± 0.019) | 1.13 (1.10 – 1.18) | < 0.001 | 0.91 |
| Peak MELD 21 category | 2.75 (± 1.02) | 15.66 (2.14 – 114.85) | 0.007 | |
| Peak MELD 31 category | 3.31 (± 0.61) | 27.27 (8.27 – 89.96) | < 0.001 | |
| Creatinine increase | 0.25 (± 0.044) | 1.29 (1.18 – 1.40) | < 0.001 |
Univariate analysis used Cox Proportional Hazards model. No significant effect (p > 0.05) of age, AST, ALT, alkaline phosphatase, GGT, albumin, total protein, BUN, sodium, hematocrit, platelets; GAHS (Area Under [Receiver Operating] Curve = 0.62) and GAHS category; paracentesis during first 2 days of admission.
Area under receiver operating curve (ROC);
Within 2 days of admission.
Stepwise Cox regression analysis identified independent predictors of 28 day survival (Table III). Of the “present on admission” variables, ABIC category and hyperammonemia within 2 days of admission were significant. INR was excluded for co-linearity. When peak MELD group was included in the analysis, it was the most significant independent predictor of outcome (p < 0.0001); however, 2 admission variables, total bilirubin and INRdf category, remained significant. Other variables including ABIC category and hyperammonemia were eliminated in the stepwise process when peak MELD was included. When 90 day survival was examined, hyperammonemia was no longer significant in multivariate analysis whereas DF on admission was significant. When MELD calculated with peak values during admission was included, the 3 significant factors were the same as at 28 days (supplementary table E).
Table IIIA.
Stepwise Cox Regression Analysis of Predictors of 28 Day Survival*
| Variable | Parameter Estimate (± standard error) |
HR (95% CI) | p value |
|---|---|---|---|
| Admission parameters | |||
| ABIC 9 category | 1.37 (± 0.37) | 3.94 (1.90 – 8.17) | < 0.001 |
| Hyperammonemia† | 1.42 (± 0.56) | 3.92 (1.60 – 13.41) | 0.012 |
| Admission & peak parameters | |||
| Peak MELD 31 category | 3.74 (± 0.67) | 42.03 (11.30 – 156.40) | < 0.001 |
| INRdf category | 1.13 (± 0.50) | 3.10 (1.57 – 8.33) | 0.024 |
| Admission bilirubin | −0.065(± 0.023) | 0.94 (0.90 – 0.98) | 0.005 |
Included all parameters with p < 0.05 on univariate analysis except INR (collinearity)
Within 2 days of admission
Discussion
The data reported here clearly identify a subgroup of patients with alcoholic hepatitis and a significantly worse short-, intermediate- and long-term prognosis. Thus, patients with INRdf > 50, ABIC > 9 or MELD > 31 on admission had the highest 28 day, 90 day and 6 months mortality. These newer scoring systems identified a small fraction with a high early mortality (56 – 60% at 28 days). In comparison, large randomized placebo-controlled trials of corticosteroids using DF > 32 as a marker of poor outcome at 28 days averaged 34% mortality without corticosteroids[21] and mortality was 46% in the control subjects of the randomized trial of pentoxifylline where in-hospital deaths occurred in 24/52 control patients after 33 ± 27 days (mean ± SD)[22]. Of note, these studies enrolled patients in the last decades of the 20th century before the changes in PT and INR measurements. INRdf is a simple calculation that increases the number of subjects identified as high risk (sensitivity) while retaining specificity (58% mortality at 28 days) while accounting for changes in analytical reagents.
The observation that an elevated ammonia level within 2 days of admission was independently predictive of death within 28 days suggests that it is a poor prognostic sign in patients admitted with icteric alcoholic liver disease. We used a value obtained from the electronic medical record, which may indicate that altered mentation resulted in ordering the ammonia level. Thus, a normal ammonia level can exclude hyperammonemic encephalopathy and an elevated one is consistent with a contribution of abnormal hepatic nitrogen metabolism to mental status. For ease of use, the INRdf may be preferable to ABIC since it relies only on addition of two numbers one of which is not manipulated before summing. In addition, INRdf was equivalent or superior to ABIC in most comparisons, identifying more patients with a poor prognosis at the time of admission.
Although the number of subjects with INRdf > 50 was relatively small (33 patients, 26 not receiving steroids), the findings demonstrate that these patients have a different prognosis than those with less severe disease. When we compared our primary data for patients not receiving corticosteroids with earlier randomized controlled trials [21], the ranges were similar for age, bilirubin, creatinine, albumin and AST. The PT was longer, DF higher and survival lower in the current subjects with INRdf > 50 or ABIC > 9 (supplementary Table F). In contrast, those identified by DF > 32 or GAHS > 9 had mortality rates that were equivalent to the past but more prolonged PT values than in the earlier studies.
While familiar with the limitations inherent in a retrospective, single institution study, we propose that the strengths of this report are substantial. They include the completeness of data collection, long-term follow-up of the vast majority of those with DF > 32 and the possibility of future external validation with electronic health record data. Clinical data at PHHS, apart from discharge summaries and pathology and radiology reports, were not electronic in this period and not extracted; consequently the cohort is potentially different from other studies of acute alcoholic hepatitis. In prospective studies, the acuteness of the presentation and the concurrence of symptoms and signs such as anorexia, nausea, right upper quadrant pain, tender hepatomegaly and jaundice are used to enroll patients. None is specific when assessed alone and diagnosis relies on interpretation of clinical data. We used a bilirubin of 5 mg/dL during the admission as a surrogate for jaundice; only 2 patients with DF > 32 were admitted with a bilirubin < 5 mg/dL (3.1 and 3.2 mg/dL) and neither had INRdf > 50. Of note, admission bilirubin was < 2.5 mg/dL in only 1 patient with DF < 32 (2.4 mg/dL) indicating that almost all the subjects were likely to be judged as jaundiced on admission.
Future efforts must focus on improving the immediate outcomes of this cohort of patients as well as the entire group. We have demonstrated that hospitalization with an acute decompensation of underlying chronic ALD that includes a bilirubin ≥ 5 mg/dL prior to discharge is a sentinel event. These are the patients for whom early liver transplantation is the one management that may prolong survival and only if that option is not eliminated by psychosocial or financial barriers. Prospective interventional trials of innovative management strategies are needed urgently, particularly for patients without a transplant option, since long-term survival is possible particularly with abstinence.
Supplementary Material
Acknowledgement
The authors are indebted to Dwain Thiele, M.D. for questioning the validity of the discriminant function in an era of changing prothrombin reagents, deriving the equation comparing results before and after and working with the coagulation laboratory members at Parkland Health and Hospital System to validate the relationship by comparing > 1000 results in tandem.
Grant Support: This study was supported in part by NIH U01-AA021893.
Abbreviations
- ABIC score
Age, Bilirubin, INR, Creatinine score
- DF
discriminant function
- GAHS
Glasgow alcoholic hepatitis score
- INRdf
modified DF using INR
- INR
international normalized ratio
- ISI
internal sensitivity index
- MELD
Model of End-Stage Liver Disease
- HCV
chronic hepatitis C
- PT
prothrombin time
- WBC
white blood cell
Footnotes
Disclosures:
The authors certify that we have no financial arrangements (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements, research support, major honoraria, etc.) with a company whose product figures in this manuscript or with a company making a competing product. This manuscript is not under review for consideration of publication elsewhere.
Author contributions:
Jennifer A. Cuthbert - study design; acquisition of data; review of clinical records; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content
Sami Arslanlar - acquisition of data; review of clinical records; review of the manuscript and important intellectual content
Jay Yepuri - acquisition of data; review of clinical records; review of the manuscript and important intellectual content
Marc Montrose - acquisition of data; review of death statistics; review of the manuscript and important intellectual content
Chul Ahn - analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Jessica P. Shah - study design; acquisition of data; review of clinical records; drafting and review of the manuscript for important intellectual content
References
- 1.Maddrey WC. Alcoholic hepatitis: pathogenesis and approaches to treatment. Scandinavian journal of gastroenterology Supplement. 1990;175:118–130. doi: 10.3109/00365529009093136. [DOI] [PubMed] [Google Scholar]
- 2.Mathurin P, Duchatelle V, Ramond MJ, Degott C, Bedossa P, Erlinger S, et al. Survival and prognostic factors in patients with severe alcoholic hepatitis treated with prednisolone. Gastroenterology. 1996;110:1847–1853. doi: 10.1053/gast.1996.v110.pm8964410. [DOI] [PubMed] [Google Scholar]
- 3.Mathurin P, Louvet A, Dharancy S. Treatment of severe forms of alcoholic hepatitis: where are we going? J Gastroenterol Hepatol. 2008;23(Suppl 1):S60–S62. doi: 10.1111/j.1440-1746.2007.05286.x. [DOI] [PubMed] [Google Scholar]
- 4.Christensen E, Gluud C. Glucocorticoids are ineffective in alcoholic hepatitis: a meta-analysis adjusting for confounding variables. Gut. 1995;37:113–118. doi: 10.1136/gut.37.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCullough AJ, O'Connor JF. Alcoholic liver disease: proposed recommendations for the American College of Gastroenterology. Am J Gastroenterol. 1998;93:2022–2036. doi: 10.1111/j.1572-0241.1998.00587.x. [DOI] [PubMed] [Google Scholar]
- 6.Mathurin P. Corticosteroids for alcoholic hepatitis--what's next? J Hepatol. 2005;43:526–533. doi: 10.1016/j.jhep.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis--a Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. Aliment Pharmacol Ther. 2008;27:1167–1178. doi: 10.1111/j.1365-2036.2008.03685.x. [DOI] [PubMed] [Google Scholar]
- 8.O'Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 9.Maddrey WC, Boitnott JK, Bedine MS, Weber FL, Jr, Mezey E, White RI., Jr Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193–199. [PubMed] [Google Scholar]
- 10.Carithers RL, Jr, Herlong HF, Diehl AM, Shaw EW, Combes B, Fallon HJ, et al. Methylprednisolone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial. Ann Intern Med. 1989;110:685–690. doi: 10.7326/0003-4819-110-9-685. [DOI] [PubMed] [Google Scholar]
- 11.Robert A, Chazouilleres O. Prothrombin time in liver failure: time, ratio, activity percentage, or international normalized ratio? Hepatology. 1996;24:1392–1394. doi: 10.1053/jhep.1996.v24.pm0008938167. [DOI] [PubMed] [Google Scholar]
- 12.Tripodi A, Poller L, van den Besselaar AM, Mannucci PM. A proposed scheme for calibration of international reference preparations of thromboplastin for the prothrombin time. On behalf of the Subcommittee on Control of Anticoagulation. Thrombosis and haemostasis. 1995;74:1368–1369. [PubMed] [Google Scholar]
- 13.Poller L, Keown M, Chauhan N, van den Besselaar AM, Tripodi A, Shiach C, et al. European Concerted Action on Anticoagulation. Evaluation of a method for International Sensitivity Index calibration of two point-of-care prothrombin time (PT) monitoring systems (CoaguChek Mini and TAS PT-NC) with fresh plasmas based on whole-blood equivalent PT. Clin Chem. 2002;48:1672–1680. [PubMed] [Google Scholar]
- 14.Forrest EH, Evans CD, Stewart S, Phillips M, Oo YH, McAvoy NC, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut. 2005;54:1174–1179. doi: 10.1136/gut.2004.050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dominguez M, Rincon D, Abraldes JG, Miquel R, Colmenero J, Bellot P, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol. 2008;103:2747–2756. doi: 10.1111/j.1572-0241.2008.02104.x. [DOI] [PubMed] [Google Scholar]
- 16.Sandahl TD, Jepsen P, Ott P, Vilstrup H. Validation of prognostic scores for clinical use in patients with alcoholic hepatitis. Scandinavian Journal of Gastroenterology. 2011;46:1127–1132. doi: 10.3109/00365521.2011.587200. [DOI] [PubMed] [Google Scholar]
- 17.Palaniyappan N, Subramanian V, Ramappa V, Ryder SD, Kaye P, Aithal GP. The utility of scoring systems in predicting early and late mortality in alcoholic hepatitis: whose score is it anyway? Int J Hepatol. 2012;2012:624675. doi: 10.1155/2012/624675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lafferty H, Stanley AJ, Forrest EH. The management of alcoholic hepatitis: a prospective comparison of scoring systems. Aliment Pharmacol Ther. 2013;38:603–610. doi: 10.1111/apt.12414. [DOI] [PubMed] [Google Scholar]
- 19.Altamirano J, Higuera-de laTijera F, Duarte-Rojo A, Martinez-Vazquez MA, Abraldes JG, Herrera-Jimenez LE, et al. The amount of alcohol consumption negatively impacts shortterm mortality in Mexican patients with alcoholic hepatitis. Am J Gastroenterol. 2011;106:1472–1480. doi: 10.1038/ajg.2011.141. [DOI] [PubMed] [Google Scholar]
- 20.UNOS. MELD/PELD Calculator Documentation. 2009 http://wwwunosorg/docs/MELD_PELD_Calculator_Documentationpdf.
- 21.Mathurin P, O'Grady J, Carithers RL, Phillips M, Louvet A, Mendenhall CL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: metaanalysis of individual patient data. Gut. 2011;60:255–260. doi: 10.1136/gut.2010.224097. [DOI] [PubMed] [Google Scholar]
- 22.Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637–1648. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.