Abstract
Vaccines formulated with non-replicating pathogens require adjuvants to help bolster immunogenicity. The role of adjuvants in antibody production has been well studied, but how they influence memory CD8+ T cell differentiation remains poorly defined. Here we implemented dendritic cell (DC)-mediated immunization to study the effects of commonly used adjuvants, TLR ligands, on effector and memory CD8+ T cell differentiation in mice. Intriguingly, we found that the TLR4 ligand LPS was far more superior to other TLR ligands in generating memory CD8+ T cells upon immunization. LPS boosted clonal expansion similar to the other adjuvants, but fewer of the activated CD8+ T cells died during contraction, generating a larger pool of memory cells. Surprisingly, monophosphoryl lipid A (MPLA), another TLR4 ligand, enhanced clonal expansion of effector CD8+ T cells, but also promoted their terminal differentiation and contraction; thus, fewer memory CD8+ T cells formed and MPLA-primed animals were less protected against secondary infection compared to those primed with LPS. Furthermore, gene expression profiling revealed that LPS-primed effector cells displayed a stronger pro-memory gene expression signature, whereas the gene expression profile of MPLA-primed effector cells aligned closer with terminal effector CD8+ T cells. Lastly, we demonstrated that the LPS-TLR4-derived “pro-memory” signals were MyD88, but not Trif, dependent. This study reveals the influential power of adjuvants on the quantity and quality of CD8+ T cell memory, and that attention to adjuvant selection is crucial because boosting effector cell expansion may not always equate with more memory T cells or greater protection.
Keywords: LPS, MPLA, memory T cell, CD8+, dendritic cell vaccination
Introduction
Immunological memory is a cardinal property of the adaptive immune system (1, 2), and the ultimate goal of vaccination is to generate long-lived memory T, B cells and plasma cells (3, 4). Most successful vaccines that have used attenuated live pathogens offer several advantages in that they productively stimulate the innate and adaptive immune system without causing severe illness due to impaired replication abilities (5, 6). However, for many pathogens attenuated forms of vaccines are not available and therefore, a need for devising safe and effective vaccines for several types of infectious diseases exists. Nonliving forms of antigens are poorly immunogenic and require accessory adjuvants to activate the innate immune system, which enhance costimulation and the expression of cytokines that promote antibody production, activated T cell expansion and differentiation into effector and memory T cells (3, 4, 7). Although these approaches of combining antigens with adjuvants were successful in some cases, a number of trials failed to induce protective immunity (8-10). It has been recognized that the most commonly used adjuvants have been for the most part been developed empirically, without a clear understanding their cellular and molecular mechanisms of action. Despite the successful antibody response elicited by certain vaccines incorporating “alum’ as an adjuvant, these vaccine formulations, preferentially promotes Th2-biased response that may not provide best “help” to generate CD8+ T cell mediated protective immunity against most viral infections (3, 4, 7).
The explosion of knowledge in the field of innate immunity and the discovery of different types of pathogen recognition receptors (PRRs) has opened unprecedented opportunities for improved vaccine design (11-13). A growing body of literature shows in detail how different forms of adjuvants activate innate immune cells and enhance antigen presentation, cytokine and chemokine production and cell migration and interaction (3, 4, 7). But, choosing the proper adjuvants to elicit the optimal instructive signals that guide large, high quality and long-lasting CD8+ T cell memory remains challenging. Previous work has shown that inflammatory cytokines, elicited by pathogens or adjuvants, can directly influence the number and types of effector T cells formed during immune responses (14-17). IL-2, IL-12, IFNα/β and IFNγ act on CD8+ T cells and significantly enhance expansion and formation of cytotoxic CD8+ T cells (18-22). However, IL-12 and IFNγ have also been shown to negatively affect memory CD8+ T cell potential in activated CD8+ T cells (14-17, 23). In addition, recent work has also demonstrated that Wnt signaling and certain cytokines, such as IL-10 and IL-21, provide the positive signals for memory CD8+ T cell differentiation and maturation (24-27). Thus, it is crucial to further investigate not only how commonly used adjuvants enhance the expansion and activation of T cells during vaccination, but also how they augment the inflammatory milieu which can affect the quantity and quality of memory T cells formed.
In this study, we implemented a well-established DC-mediated immunization strategy (14, 15, 28) to compare the effects of different adjuvants on CD8+ T cell memory. Ultimately, we focused on two TLR4 ligands, LPS and MPLA, because we found that they both boosted expansion of antigen-specific CD8+ T cells, but their effects on memory CD8+ T cell numbers differed greatly. That is, a significantly larger fraction of LPS-primed CD8+ T cells populated the memory cell pool compared to those primed with MPLA. Compared to LPS-priming, MPLA-priming promoted the formation of a larger frequency of KLRG1hi IL-7Rlo terminal effector cells and also negatively affected IL-7Rhi memory precursor cell survival, leading to greater rates of effector T cell contraction. Furthermore, gene expression profiling revealed the intrinsic properties of IL-7Rhi memory precursor cells generated through LPS- and MPLA-priming were distinct and LPS induced a more memory-like, while MPLA induced a more effector-like pattern of gene expression. Moreover, the inflammatory milieus produced by LPS and MPLA were largely distinct and the “pro-memory” signals derived from LPS-TLR4 signaling were dependent on MyD88, but not Trif. These results highlight important ways in which the selection of adjuvants can influence the quantity and the quality of memory T cells that arise during vaccination.
Material and methods
Mice
Thy1.1+ P14 TCR tg mice have been described previously (29). To make “P14 chimeric mice”, ~5×104 Thy1.1+ P14 CD8+ T cells were transferred into naïve Thy1.2+ C57BL/6 (B6) mice that were purchased from National Cancer Institute (NCI, Charles River). After transfer the recipient mice typically contain ~5×103 donor naïve P14 CD8 T cells. IL-6−/−and IL-12Rβ2−/− mice were purchased from the Jackson Laboratory. MyD88−/−, Trif−/− and Interleukin-1β Converting Enzyme (ICE)−/− mice were obtained from Dr. Ruslan Medzhitov at Yale University. All animal experiments were performed under approved Institutional Animal Care and Use Committee protocols.
Generation of bone marrow-derived dendritic cells and DC immunization
Bone marrow-derived CD11c+ DCs were generated after 5 days of culture with GM-CSF and matured with LPS (50ng/ml) overnight as described previously (11, 30). Matured DCs were pulsed with GP33-41 peptide (200ng/ml) for 2h, washed with PBS and 1×106 DC-33 cells were injected intravenously (i.v.). Simultaneously, DC immunized mice were injected intraperitoneally (i.p.) with either PBS or one of the following TLR ligands: PAM3CSK4 (50μg), poly I:C (50μg), LPS (20μg), MPLA (30μg), Flagellin(20μg), LTA(20μg), R848 (20μg), CpG-A (50μg) or CpG-B (50μg). The dose for each adjuvant was primarily determined by either previous publications (14, 15, 31-34) and/or manufacture recommendations. Additional titrations were done in some cases to ensure comparable CD8 T cell responses at the effector phase as shown in Supplementary Figure 1 and no perceivable side effects. LPS was purchased from Sigma-Aldrich, St. Louis, MO; CpG-A and CpG-B were synthesized at Yale University Keck Facility; and the rest were all purchased from InvivoGen, San Diego, CA.
Antibodies and surface and intracellular staining
Lymphocyte isolation, GP33-41 peptide stimulations and surface/intracellular staining was performed as previously described (14). All antibodies were purchased from E-biosciences (San Diego, CA) except Anti-KLRG1 (2F1) hybridoma was a generous gift from Dr. Raulet (University of California, Berkley, CA) and was conjugated to alexa-647 (Invitrogen, Eugene, OR). All flow cytometry was analyzed on a FACSCalibur (BD) with FlowJo software (Treestar, San Carlos, CA).
Gene array analysis
Mice that contain small number of P14 CD8+ T cells were immunized with DC-33 either alone or in combination with LPS or MPLA. KLRG1loIL-7Rhi MPECs were purified by FACS sort, and mRNA isolated from MPECs was subjected to whole-genome expression profiling using Illumina MouseWG-6 v2.0 Expression BeadChip. Gene expressions of LCMV-MPEC and LCMV-SLEC CD8+ T cells were measured by Affymetrix GeneChip Mouse Genome 430 2.0 Array (GSE8678) (17). Microarray hybridization was carried out at the Yale Keck Microarray Facility. The data analyses were carried out using packages in R (35). Raw MPEC expression data from MPLA and LPS groups were normalized using the quantile method provided by the lumi package in R/Bioconductor (36). MPLA (LPS) signature genes were defined by two criteria: (1) a log2 (fold-change) ≥1 of MPLA (LPS) samples relative to LPS (MPLA) samples, and (2) a statistically-significant change in expression as determined by LIMMA (37) using a Benjamani-Hochberg false discovery rate cutoff of q < 0.05. Raw LCMV-MPEC and LCMV-SLEC microarray data in CEL file format were read in and normalized with the RMA method provided by the oligo package in R/Bioconductor (38). To test the association of MPLA and LPS signature genes with LCMV-MPEC and LCMV-SLEC expression profiles (GSE8678) (17), genes common to the two microarray platforms (according to gene symbols) were ranked based on their t-statistics from the comparison of LCMV-MPEC and LCMV-SLEC gene expression profiles using the limma package in R/Bioconductor. The enrichment of MPLA and LPS signature genes was then measured based on their relative positions in the ranked gene list using the geneSetTest in R/Bioconductor (39).
The microarray data of P14 CD8+ T cells immunized with DC-33 either alone or in combination with LPS or MPLA have been deposited in NCBI's Gene Expression Omnibus (40) and are accessible through GEO Series accession number GSE50764 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE50764).
Statistical Analyses
Where indicated, p values were determined by a two-tailed unpaired Student's t test. p values < 0.05 were considered significant. All graphs show averages of the mean ± SEM.
Results
Differential effects of TLR ligands on memory CD8+ T cell generation
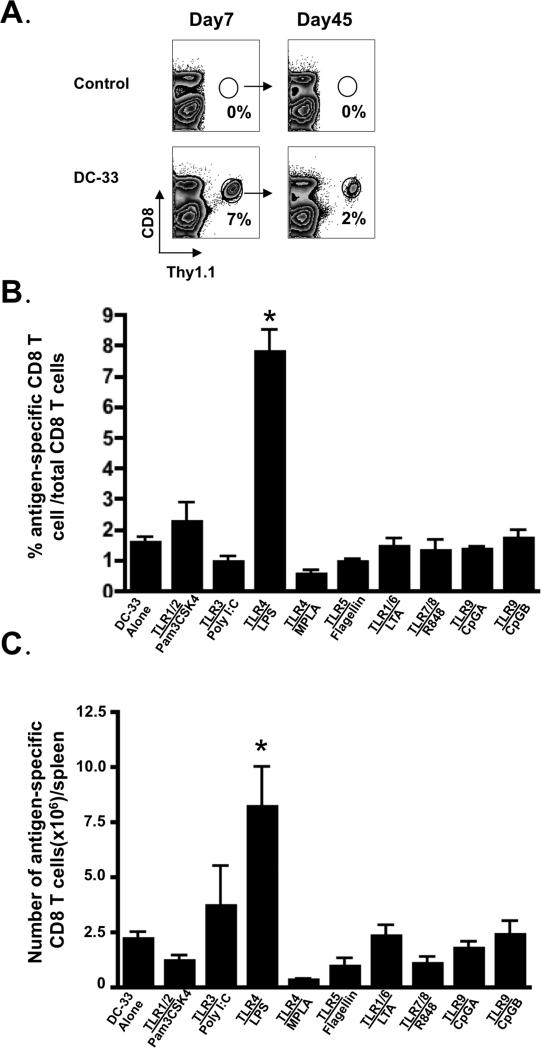
To investigate how commonly used adjuvants affect memory T cell formation, we first carried out a comprehensive and systematic analysis of various TLR ligands on memory CD8+ T cell development. In order to facilitate the detection of antigen-specific CD8+ T cell response in vivo, ‘P14 chimeric mice’ were generated in which a small number (~1×104) of naive Thy1.1+ P14 CD8+ T cells, which recognize the DbGP33-41 LCMV epitope, were transferred into wild type (WT) B6 mice. These mice were immunized with GP33-41 peptide-pulsed bone marrow derived DCs (referred to as DC-33) either alone or in combination with various TLR ligands. Both the DCs and adjuvants were administered systemically, with the DCs given i.v. and the TLR ligands given i.p. The bone marrow derived DCs were matured with LPS for 12 hrs using standard protocols (30) to induce high expression levels of CD11c, MHC Class I and II, and costimulatory molecules CD80/86. However, at the time of immunization, these DCs produced only low amounts of pro-inflammatory cytokines, such as IL-6, IL-12 and TNF, as their expression peaked at ~6hrs after LPS stimulation ((14, 28) and data not shown). Thus, this DC immunization system allowed us to vary the inflammatory milieu that the CD8+ T cells were exposed to systemically, through the injection of different adjuvants, while keeping antigenic stimulation relatively constant. Without DC immunization, the donor P14 CD8 T cells were undetectable in the recipient mice because of the small numbers of cell transferred, but with DC-33 immunization alone, (e.g., in the absence of TLR ligands) P14 effector and memory CD8 T cell responses that were readily detected at day 7 and 45 post immunization (Figure 1A). As expected, DC-33 immunization with the nine selected adjuvants— Pam3CSK4 (TLR1/2), Poly I:C (TLR3), LPS (TLR4), MPLA (TLR4), Flagellin (TLR5), LTA (TLR2/6), R848 (TLR7/8), CpG-B (TLR9), CpG-A (TLR9), boosted the overall expansion of the P14 effector CD8 T cells, with comparable efficiency, over that of DC immunization alone (Supplementary Figure 1). However, when analyzed 45 days later, P14 CD8+ T cells primed in the presence of LPS generated a significantly larger frequency and number of memory CD8+ T cells than the other adjuvants or DC-33 alone. Surprisingly, MPLA, which is a low-toxicity derivative of LPS and recently approved for use as an adjuvant in humans, generated the smallest number and percentage of splenic memory CD8+ T cells (Figure 1 B and C). These data suggested that priming with the TLR4 ligand LPS, but not MPLA, provided a unique set of signals that was beneficial for memory CD8+ T cell formation and survival.
Figure 1. TLR ligands differentially affected the number of memory CD8+ T cells that form following DC vaccination.
B6 mice containing a small number (~5×103/mouse) of naive P14 CD8+ T cells were immunized with either DC-33 i.v. alone or in combination with various TLR agonists i.p. as indicated (see methods for doses), an additional group of mice were served as untreated controls. (A) Spleens were harvested on day 7 and 45 days post immunization and the antigen-specific P14 CD8+ T cells plotted in the dot plots. (B) Frequency of CD8+ T cells and (C) numbers per spleen of antigen-specific P14 cells on day 45 post immunization were plotted in the bar graphs. Data shown are representative of at least three independent experiments (* denotes to p<0.05). Note, the baseline number of naïve P14 CD8 T cells without DC-33 immunization would be ~5×103 cells/ mouse.
Effects of TLR4 ligands LPS and MPLA on effector and memory CD8+ T cell differentiation
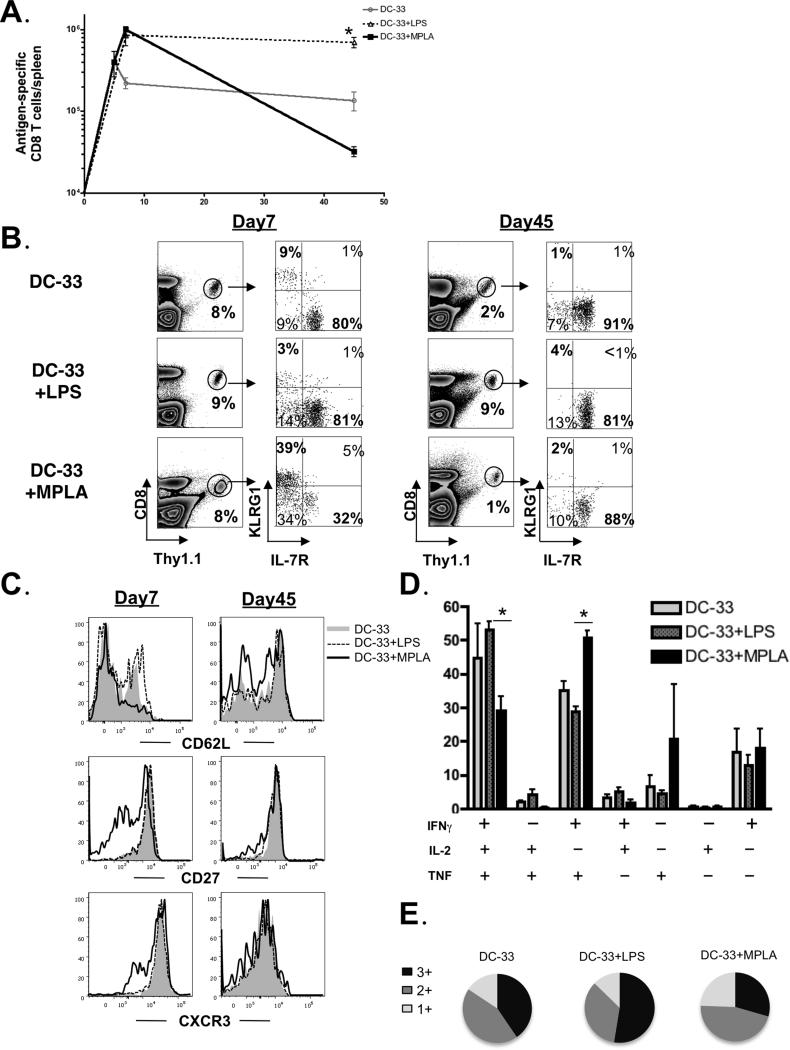
It was intriguing that the two TLR ligands LPS and MPLA, which signal through the same receptor, would have such opposing effects on memory CD8+ T cell generation. To further dissect how LPS or MPLA affect the development of memory CD8+ T cells in vivo, we immunized P14 chimeric mice with DC-33 alone or in conjunction with either LPS or MPLA. We first compared the effects of LPS and MPLA on the number and quality of effector and memory CD8+ T cells that formed during DC-33 immunization. The DC-33 vaccination generated ~1×106 P14 effector CD8+ T cells at the peak, ~90% of the effector CD8+ T cells induced by DC-33 contracted with ~10% of them developing into memory cells (Figure 2A). Remarkably, there was virtually no contraction of the effector CD8+ T cells generated by DC-33+LPS immunization, and this led to ~10 fold more memory cells than that formed during DC-33 alone (Figure 2A). The expansion induced by DC-33+MPLA was equivalent to that of LPS, but in contrast, the numbers of memory CD8+ T cells were substantially lower than any other condition (Figure 2A and Figure 1). Together, these data suggested that MPLA produced signals that could augment activated CD8+ T cell expansion, compared to DC-33 alone, but reduced their memory cell potential and long-term survival. In contrast, LPS could produce signals that potently enhanced formation of memory cells and their progenitors or alternatively, blocked the activated CD8+ T cells from receiving signals that diminish memory cell potential.
Figure 2. TLR4 ligands LPS and MPLA differentially regulated effector and memory CD8+ T cell differentiation.
B6 mice containing a small number of naive P14 CD8+ T cells were immunized with either DC-33 alone or in combination with LPS or MPLA. (A) Spleens were harvested at days 5, 7 and 45 post immunization, and antigen-specific P14 CD8+ T cells were enumerated and plotted in the line graphs. (B) The frequency of Thy1.1+ P14 cells of CD8+ T cells and expression of KLRG1 and IL-7R were analyzed at days 7 and 45 post immunization. (C) Day 7 effector and day 45 memory P14 CD8+ T cells generated after DC-33 alone (grey shaded) or in combination with LPS (black dashed line) or MPLA (black solid line) were compared for CD62L, CD27 and CXCR3 expression by flow cytometry. (D) Memory P14 CD8+ T cells were stimulated in vitro with GP-33 peptide and cytokine production (IFNγ, TNF and IL-2) was measured by flow cytometry. Bar graphs show the frequency of cells expressing each of the seven possible combinations of IFNγ, TNF and IL-2. (E) Pie graphs show the fraction of the total response comprising cells expressing three cytokines (3+), any two cytokines (2+), or any one cytokine (1+). Data shown are representative of three independent experiments (* denotes to p<0.05).
Next, we compared the phenotypes of CD8+ T cells at the effector phase among DC-33 alone, DC-33+LPS and DC-33+MPLA groups. Strikingly, DC-33+MPLA immunization induced the formation of effector CD8+ T cell subsets that could be distinguished, based on our past work, as KLRG1hiIL-7Rlo short-lived effector cells (SLECs) and KLRG1lo IL-7Rhi memory precursor effector cells (MPECs). Nearly all of the MPLA-induced KLRG1hi IL-7Rlo cells died between days 7 and 45 leaving behind a predominantly KLRG1lo IL-7Rhi memory CD8+ T cell population. This was due to direct apoptosis of KLRG1hi cells rather than conversion of the KLRG1hi → KLRG1lo cells (data not shown). In contrast, both DC-33 alone and DC-33+LPS induced a relatively homogenous KLRG1lo IL-7Rhi population and the resulting memory CD8+ T cells were of a similar phenotype (Figure 2B). Similar observations were obtained both in spleens and other tissues such as blood, lungs, livers and lymph nodes (data not shown). Consistent with increased expression of KLRG1, DC-33+MPLA induced effector CD8+ T cells also expressed less CD62L, CD27 and CXCR3 (Figure 2C). In addition, memory CD8+ T cells formed after DC-33+MPLA immunization were less “poly-functional” in comparison with that of either DC-33 or DC-33+LPS immunization (Figure 2D and E). Overall, immunization with LPS appeared to induce a greater fraction of IL-7Rhi effector cells that could survive and populate the memory pool compared to DC-33 or DC-33+MPLA immunization, suggesting that LPS-priming instilled the activated CD8+ T cells with memory cell developmental potential.
LPS, but not MPLA, enhanced memory CD8+ T cell mediated protective immunity
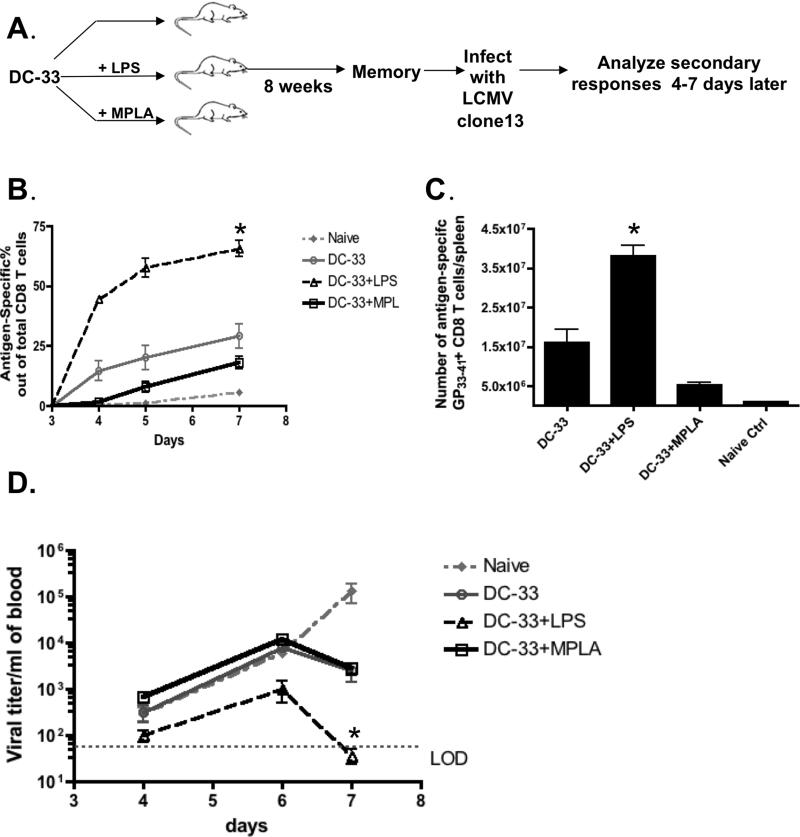
To better characterize how LPS and MPLA directly affected the development of memory CD8+ T cells in vivo, we immunized B6 mice with DC-33 alone or in combination with LPS or MPLA (Figure 3A). Note, in this system we could monitor the primary peptide-specific CD8 T cell responses by MHC-I DbGP33 tetramer staining in the peripheral blood (data not shown), however at most memory time points the antigen-specific CD8 T cells were often below the level of detection in the peripheral blood. To test the adjuvant effects on memory CD8+ T cell protection, the aforementioned three groups of mice were challenged with lymphocytic choriomeningitis Clone 13 strain (LCMV-Clone13) eight weeks after initial immunization and virus-specific CD8+ T cells were monitored in the peripheral blood between days 4 to 7 post infection (p.i.). Although all immunized groups mounted greater CD8+ T cell responses than the naive control group, the kinetics and magnitudes of the response were quite diverse (Figure 3B). Strikingly, mice immunized with DC-33+LPS generated more rapid and robust anti-viral CD8+ T cell response than any other groups, both in frequency (Figure 3B) and number (Figure 3C). Additionally, the LPS-primed memory CD8+ T cells demonstrated the greatest control over viral replication compared to the other groups because viral titers in the peripheral blood were ~5-10 fold lower at days 4-6 p.i. and were below the level of detection by day 7 p.i. (Figure 3D). Interestingly, priming with MPLA failed to improve CD8+ T cell mediated immune protection against LCMV infection in comparison to animals immunized with DC-33 alone (Figure 3B, C and D). To ensure the CD8+ T cell recall response was LCMV DbGP33-41 peptide-specific, we also examined another LCMV epitope DbNP396-404. This showed that the expansion of DbNP396-404 specific primary effector CD8+ T cells was similar among all groups of mice (Supplementary Figure 2). Collectively, these results suggest that the use of LPS as an adjuvant during immunization induced a larger number of memory CD8+ T cells and conferred greater immunological protection against secondary infection than that of MPLA.
Figure 3. LPS promoted memory CD8+ T cell mediated protective immunity.
(A) B6 mice were immunized with either DC-33 alone or in combination with LPS or MPLA. Eight weeks later, each group of mice was infected with LCMV-Clone13 (2×106 pfu/mouse) and virus-specific CD8+ T cells were monitored 4 and 7 days later. Groups of naïve, unimmunized mice were included as controls. (B) Line graphs show the frequency of DbGP33-41 tetramer+ CD8+ T cells in the blood on days 4 and 7 following Clone13 infection. (C) Bar graph shows the number of splenic DbGP33-41 tetramer+ CD8+ T cells at day 7 post infection (p.i.). (D) Viral titers were measured in the serum at days 4 and 7 p.i. and plotted as a line graph. Data shown are representatives of three independent experiments (* denotes to p<0.05).
LPS enhances activated CD8+ T cell survival, but not homeostatic turnover
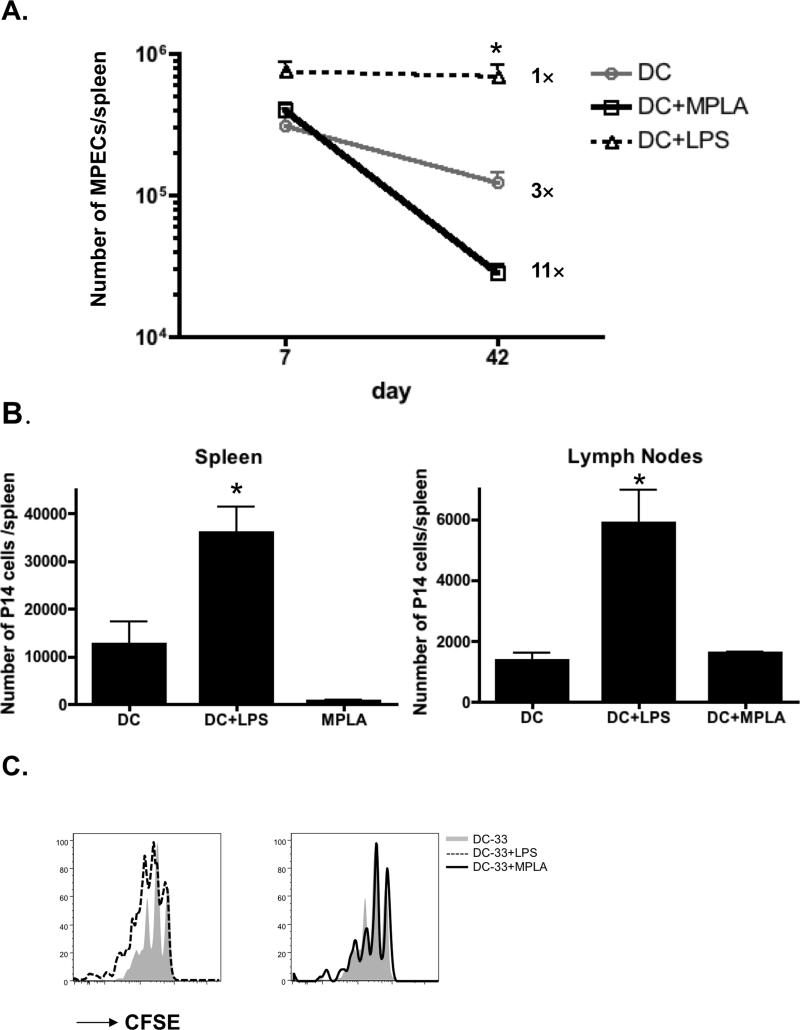
The different outcomes of the CD8+ T cell responses induced by either DC-33+LPS or DC-33+MPLA prompted us to further investigate whether the LPS mediated “pro-memory” effects on CD8+ T cells were due to improved survival of memory precursor cells or increased homeostatic proliferation of memory CD8+ T cells. To test these possibilities, we first measured the magnitude of contraction of KLRG1lo IL-7Rhi MPECs among DC-33 alone, DC-33+LPS and DC-33+MPLA groups. This demonstrated that ~33% of KLRG1lo IL-7Rhi MPECs formed at days 7 after DC-33 immunization survived and matured into functional memory CD8+ T cells in the spleens. Interestingly, almost 100% of KLRG1lo IL-7Rhi MPECs formed following DC-33+LPS immunization survived to memory phase without obvious contraction. Conversely, only 9% MPECs formed following DC-33+MPLA immunization survived contraction phase (Figure 4A), suggesting MPLA not only promoted SLEC formation but also impeded MPECs from surviving and transitioning to mature memory CD8+ T cells. To further confirm these distinct contraction rates were CD8+ T cell intrinsic, we next adoptively transferred the equal number of P14 effector CD8+ T cells that were generated from three groups of immunizations into naïve recipients and directly assessed their magnitude of contraction following transfer. In agreement with observations mentioned above, the number of LPS-primed effector cells recovered from the spleen four weeks later was ~4 and 50 fold more than the DC-33 alone and MPLA-primed CD8+ T cells, respectively (Figure 3B). A similar trend was observed in the lymph nodes although the numbers of donor cells recovered between the DC-33-alone and MPLA-primed CD8+ T cells were more comparable (Figure 4B). Furthermore, we found that the memory CD8+ T cells generated by all three immunizations underwent similar rates of homeostatic proliferation (Figure 4C). Lastly, we also compared the ability of these different memory CD8+ T cell populations to respond and protect against secondary infection by adoptively transferring equal numbers of P14 memory CD8+ T cells from mice immunized with DC-33 alone, DC-33+LPS or DC-33+MPLA one days prior into naïve mice that were subsequently infected with LCMV-Clone13. These experiments showed that all three groups of memory CD8+ T cells expanded and cleared the virus with very similar kinetics (Supplementary Figure 3). Collectively, these findings demonstrate that the LPS-mediated augmentation of the numbers of memory CD8+ T cells formed and the protection they confer, stems largely from the ability of LPS-priming to increase the lifespan of activated CD8+ T cells following immunization rather than increasing the rates of memory CD8+ T cell homeostatic turnover or their inherent ability to respond to secondary infection.
Figure 4. LPS enhanced memory CD8+ T cells survival, but not homeostatic turnover.
B6 mice containing a small number of naive P14 CD8+ T cells were immunized with either DC-33 alone (grey) or in combination with LPS (dashed) or MPLA (black). A) Line graph shows the numbers of KLRG1loIL-7Rhi P14 CD8+ T cells on day 7 and 42 post immunization. Note the increased rates of death within this subset of effector cells during the contraction phase in MPLA-primed mice. (B-D) At day 7 post immunization, equal numbers of effector P14 CD8+ T cells (1×106 cells/mouse) from each group were CFSE-labeled and adoptively transferred into naïve B6 recipients. (B) P14 CD8+ T cells recovered from spleens and lymph nodes on day 35 post-transfer were enumerated and plotted in the bar graphs. (C) CFSE dilution was analyzed in P14 CD8+ T cells 35 days later by flow cytometry and plotted in histograms (DC alone=grey shaded, DC+LPS=dashed line and MPLA=black line). Data shown are representative of at least two independent experiments (* denotes to p<0.05).
LPS promoted memory signature genes expression and memory T cell maturation
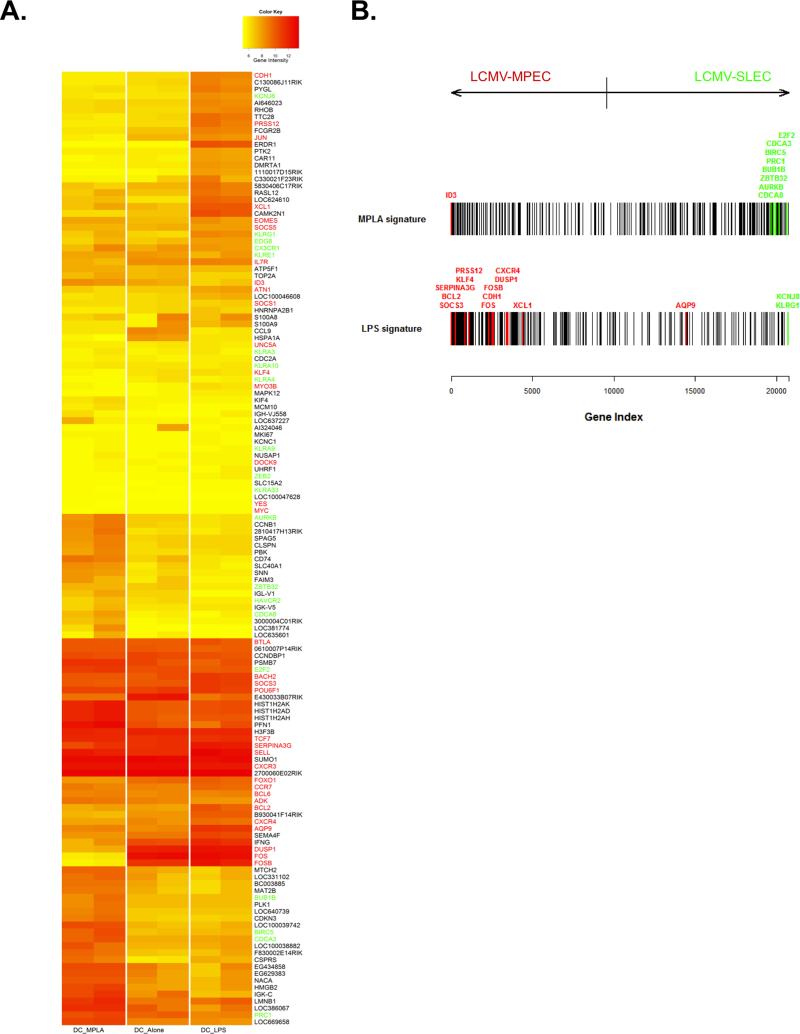
To gain further insights into how LPS- and MPLA-priming qualitatively impacted memory CD8+ T cell survival and differentiation, we compared the genome wide gene expression patterns of IL-7Rhi effector cells (i.e., MPECs) generated 7 days after DC-33 alone, DC-33+LPS and DC-33+MPLA immunization. We identified 96 genes with highest expression variance (Coefficient of Variation>0.8) among all three groups. We next generated a heat map that contained these 96 genes and 56 additional effector and memory signature genes derived from previously published studies (17, 41-43) (Figure 5A), in which 12 genes (i.e. aqp9, bcl2, cdh1, fosb, prss12, xcl1, aurkb, birc5, bub1b, cdca3, cdca8 and kcnj8) were overlapping between these two lists. Manual inspection of this data set suggested that gene expression pattern of the IL-7Rhi effector cells was closely clustered between DC-33 alone and DC-33+LPS group, whereas the DC-33+MPLA group appeared more distinct from the other two (Figure 5A). Among all three groups, several well-characterized memory signature genes were expressed at the similar levels, such as tcf7, sell, cxcr3, ccr7 and bcl6. Interestingly, some late-memory signature genes (41), such as, apq9, prss12 and cdh1, and pro-survival genes, such as bcl2 and serpina3g (encodes Spi-2a) were preferentially expressed in the MPECs of DC-33+LPS group (Figure 5A). This suggests that LPS may accelerate memory precursor cells maturation and/or promote their long-term survival even at this late effector phase. Conversely, the IL-7Rhi effector cells generated by MPLA-priming not only had reduced expression of the late-memory genes, but also preferentially up-regulated several terminal effector signature genes, such as birc5, cdca3, prc1 and zbtb32 (17, 41-43). To further assess the intrinsically distinct properties of MPECs induced by LPS- or MPLA-priming, we took most differentially expressed LPS- and MPLA- signature genes to examine their enrichment in the full ordered gene list ranked bi-directionally based on t-statistics from the comparison of LCMV-MPEC and LCMV-SLEC gene expression profiles (17, 41-43). This analysis clearly revealed a significant enrichment of the LCMVMPEC gene signature in the IL-7Rhi cells formed by LPS-priming whereas those primed by MPLA displayed significant enrichment of the LCMV-SLEC signature (Figure 5B). Together, these analyses demonstrate that the differential effects of LPS- and MPLA-priming on memory precursor cell differentiation involve transcriptional changes that correlate with, and likely direct, the long-term fate of the effector T cells. LPS positively induced several genes associated with the enhanced longevity observed in LCMV-specific IL-7Rhi memory precursor cells whereas MPLA induced greater expression of genes associated with terminal effector fates.
Figure 5. LPS promoted memory signature genes expression and memory T cell maturation.
B6 mice containing a small number of naive P14 CD8+ T cells were immunized with either DC-33 alone or in combination with LPS or MPLA. KLRG1loIL-7Rhi MPECs were purified by FACS sort and their mRNA was isolated and subjected to whole-genome expression profiling using Illumina MouseWG-6 v2.0 Expression BeadChips. (A) Heat map shows gene expression of 96 probe sets with highest variance (Coefficient of Variation > 0.8) and 54 known memory (in red) and effector (in green) signature genes across the CD8+ T cell populations primed via DC-33 alone, DC-33+LPS and DC-33+MPLA. Colors indicate log2 expression intensities. (B) Barcode plot shows the locations of signature genes of LPS- and MPLA- primed CD8+ T cells in the full ordered gene list ranked by the t-statistics to quantify the differential expression in LCMV-MPECs versus LCMV-SLECs. MPLA-signature genes (vertical bars in top barcode) are enriched among genes up-regulated in SLECs (towards the right) (P = 8.2e-06) whereas LPS-signature genes are enriched among genes up-regulated in the MPEC samples (towards the left) (P = 3.4e-13). Known memory signature genes are in red and effector signature genes are in green.
Differential cytokine milieus induced by LPS and MPLA modulate effector and memory CD8 T cell differentiation
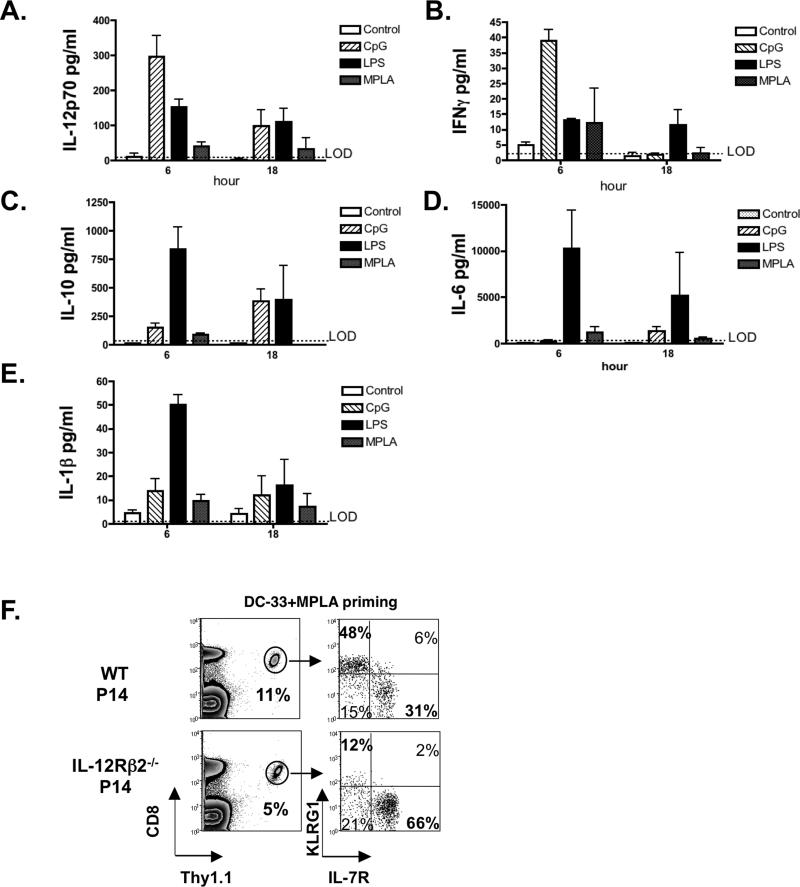
Given a large body of evidence has shown inflammatory cytokines directly influence effector and memory T cell fate decisions (2, 14-17, 26, 27, 44, 45), we attempted to discover the cytokines that may contribute to the different effects of LPS and MPLA on memory CD8 T cell development. We first performed multiplex cytokine arrays on serum samples from mice that vaccinated with DC-33, DC-33+LPS and DC-33+MPLA at 6 and 18 hours post immunization. Because previous work has clearly demonstrated that like MPLA, CpG-B can induce KLRG1hi terminal effector CD8 T cells (14, 17), we also analyzed mice immunized with DC-33+CpG-B as a “pro-effector” control. Among 22 cytokines and chemokines examined, we found that LPS and CpG-B elicited very different cytokine signatures. CpG-B preferentially induced IL-12 and IFNγ, (Figure 6 A, B) whereas LPS preferentially induced IL-6, IL-10 and IL-1β (Figure 6, CE). Consistent with previous studies (46, 47), MPLA was a poorer trigger of inflammatory cytokines, but modest inductions of IL-12p70, IFNγ and IL-1β were observed (Figure 6, A, B and E). Furthermore, LPS, MPLA and CpG-B induced small amounts of common γ chain cytokines IL-2, IL-7 and IL-15, and moderate, but similar, amounts of several other cytokines and chemokines, such as GM-CSF, IL-4 and IL-8 (data not shown). Collectively, this examination revealed differences in the cytokine milieus produced by different adjuvants that could contribute to their respective effects on the differentiation of different types of effector and memory CD8 T cells.
Figure 6. Distinct cytokine milieus produced by LPS, MPLA and CpG-B may differentially regulate effector and memory CD8 T cell differentiation.
(A-E) B6 mice containing a small number of naive P14 CD8+ T cells were immunized with DC-33 alone or in combination with LPS, MPLA or CpG-B. Serum samples were collected 6 and 18 hours post immunization. Cytokine and chemokine production was measured by Luminex multiplex assay. Bar graphs show the amounts of IL-12, IFNγ, IL-6, IL-10 and IL-1β produced post immunization. (F) B6 mice containing a small number of WT or IL-12Rβ2−/− P14 CD8+ T cells were immunized with DC-33 in combination with MPLA. At day 7, the frequency of splenic Thy1.1+ P14 CD8+ T cells and their expression of KLRG1 and IL-7R were analyzed. Data shown are representative of two independent experiments.
Although MPLA only triggered modest amounts of IL-12p70, we wondered whether the formation of KLRG1hi terminal effector CD8 T cells following MPLA priming was IL-12-dependent as found previously during CpG-B priming of CD8 T cells (14, 48). To this end, WT mice containing small numbers of either WT or IL-12Rβ −/−2 P14 CD8+ T cells were immunized with DC-33+MPLA and the formation of KLRG1hi effector CD8 T cells was analyzed 7 days later. This showed that IL-12 signaling was critical for the formation of KLRG1hi terminal effector cells during priming with MPLA (Figure 6F).
Although IL-6, IL-10 and IL-1β have been reported to promote memory T cell differentiation in certain infection or immunization models (26, 27, 49, 50), we did not observe significantly impaired memory CD8 T cell differentiation in either IL-6−/− or ICE−/− mice in comparison with wild type mice following DC-33+LPS immunization (Supplementary Figure 4). Because IL-10−/− mice succumbed to even low dose LPS treatment, we were unable to test if IL-10 was required for LPS induced “pro-memory” effects on CD8 T cells. These data collectively suggested that multiple cytokines elicited by LPS might act cooperatively to regulate memory CD8 T cell differentiation, and it is possible that the ratio(s) of IL-12 to IL-10 or IL-6 is ultimately what influences memory cell potential and development the most during LPS priming.
LPS promoted memory T cell differentiation in a MyD88-dependent, but Trif-independent manner
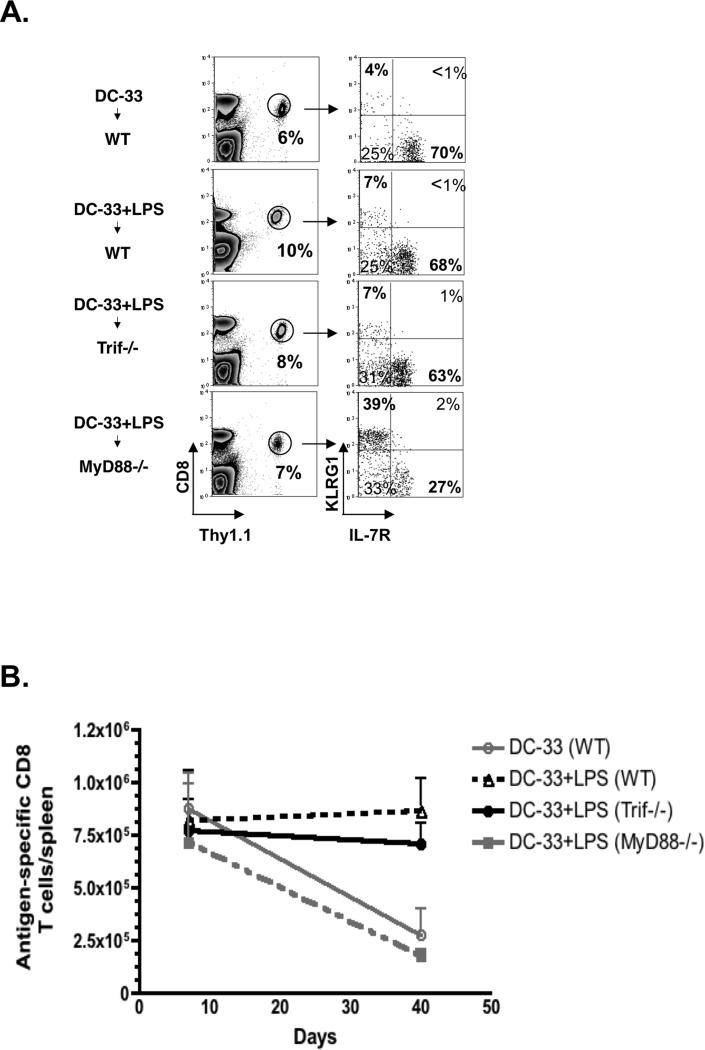
Given the above approach failed to identify a single cytokine that was responsible for the pro-memory effects of LPS priming, we considered the unique features TLR4 signaling downstream of LPS and MPLA. LPS activation of TLR4 activates both myeloid differentiation factor 88 (MyD88) and Toll-interleukin 1 receptor domain-containing adapter inducing interferon-beta (Trif) dependent signaling pathways (51, 52). MPLA, on the other hand, induces certain conformational changes in TLR4 that predominantly activates the Trif pathway with minimal activation of MyD88 pathway (47, 53). This accounts for why, MPLA provokes low levels of pro-inflammatory cytokines relative to LPS and has been considered a safe adjuvant for vaccines (7, 54). In order to dissect the roles of MyD88 and Trif in generating pro-memory signals downstream of LPS, we immunized MyD88−/− and Trif−/− mice that contained small numbers of WT P14 cells with DC-33+LPS. To our surprise, the development of KLRG1lo IL-7Rhi effector cells was impaired in MyD88−/− mice immunized with LPS, and rather a larger fraction of KLRG1hiIL-7Rlo terminal effector cells formed. In contrast, Trif deficiency had little effect on the formation of KLRG1lo IL-7Rhi MPECs following DC-33+LPS immunization (Figure 7A). In addition, our results further demonstrated that LPS mediated survival of effector CD8+ T cells during contraction phase was diminished in MyD88−/−, but not in Trif−/− mice (Figure 7B). It is important to note that given both the DCs and P14 cells used in the aforementioned experiments are derived from WT mice, these results demonstrate that the LPS-induced ‘pro-memory’ signals are derived from bystander host cells as opposed to the donor antigen-presenting DCs. Together these observations strongly indicate that activation of MyD88, but not Trif, is critical for production of signals that promote IL-7Rhi MPEC formation and long-term survival. These findings reveal previously unappreciated roles for these critical innate signaling pathways, and possibly unique qualities of the adjuvants LPS and MPLA, in generating memory CD8 T cells.
Figure 7. MyD88-dependent signals downstream of LPS-TLR4 promote memory CD8 T cell formation.
B6 (WT), Trif−/− and MyD88−/− mice that contained a small number of P14 CD8+ T cells were immunized with DC-33 alone or in combination with LPS. (A) At day 7, the frequency of Thy1.1+ P14 CD8+ T cells and their expression of KLRG1 and IL-7R were analyzed. (B) Line graph shows the numbers of splenic P14 CD8+ T cells at days 7 and 40 post immunization from the four different groups of animals. Data shown are representative of two independent experiments.
Discussion
Adjuvants have long been of great interest to vaccine developers. Better understanding of the molecular mechanisms how existing adjuvants work and how they might be improved will guide better vaccine design. Although a number of TLR agonists are currently under clinical trials for use as adjuvants in vaccine development, how they influence memory CD8+ T cell development has not been well studied. In this paper, we utilize a simple vaccination approach to study particularly how different TLR ligands affect the differentiation of effector and memory CD8+ T cells. Interestingly, we found that two TLR4 agonists LPS and MPLA had distinct effects on effector CD8+ T cell differentiation and memory CD8+ T cell development. LPS as an adjuvant promoted the formation of long-lived MPECs whereas MPLA was a more potent adjuvant for stimulating the development of terminal effector cells that died following immunization. The difference between the two adjuvants could be explained in part by their intrinsic effects on the longevity of the KLRG1lo IL-7Rhi effector cells that formed following immunization. Moreover, the ability of LPS to induce greater memory cell potential in the effector cells was directly associated with changes in the transcriptome of KLRG1lo IL-7Rhi effector cells. LPS accentuated the expression of pro-memory genetic signature in these cells whereas MPLA promoted the expression of genes associated with terminal effector cells. These differences likely stem from variations in the inflammatory milieus produced by the two adjuvants downstream of TLR4: MyD88-dependent signals promote formation and long-term survival of MPECs whereas Trif-dependent signals appear to promote the formation of SLECs.
The primary rationale for using adjuvants in vaccine formulation is to boost the magnitude of adaptive immunity. Recent studies have shown that larger number of memory CD8+ T cells provides better protection against infections, such as HIV and malaria (55, 56). One way to augment the number of memory CD8+ T cells is to increase CD8+ T cell expansion. This is based on multiple studies showing that the size of the memory CD8+ T cell pool is typically proportional to size of the clonal burst of effector T cells. Conceptually, larger numbers of memory CD8+ T cells may also be achieved through increased survival of effector CD8+ T cells during the contraction phase. The effect of LPS on memory CD8+ T cell formation appeared to both improve the expansion of effector CD8+ T cells and prolong their survival. Although LPS itself is not a candidate adjuvant for vaccines because of its ability to induce septic shock, it would be of interest to uncover the “pro-memory” signals derived from LPS as candidates for future vaccine design.
Given MPLA is a low-toxicity derivative of LPS with adequate immunostimulatory properties (47) it is an attractive adjuvant for human vaccines (7, 47, 54). MPLA and LPS both signal through TLR4, but MPLA binding induces conformational changes that bias activation of the Trif-dependent pathway whereas LPS signals through both MyD88- and Trif-dependent pathways (47). Our data show that optimal CD8 T cell memory is generated when both MyD88 and Trif are engaged simultaneously, but this concept should be extended to highlight that vaccines that incorporate multiple adjuvants to trigger a combination of innate-sensing pathways will likely yield more protective and longer-lasting immunity. For example, one study illustrated that vaccination with the adjuvants alum + MPLA provided greater CD8 T cell mediated protective immunity against influenza virus infection than vaccination with either adjuvant alone (57). Although Alum may not be able to directly activate MyD88 pathway, it can trigger the inflammasome to produce IL-1β, which could subsequently activate IL-1 receptor/MyD88 pathway (58-60). Our results showing reduced CD8 T cell survival after MPLA priming might also help explain why immunization with MPLA alone, without alum, provided poorer protection against influenza, despite that MPLA induced adequate primary response and CTL differentiation (57). In a separate study, vaccination with glucopyranosyl lipid adjuvant-stable emulsion (GLA-SE), a synthetic TLR4 agonist, required both MyD88 and Trif for generating protective TH1 responses against Mycobacterium tuberculosis (61). Lastly, a third study reported that nanoparticle based vaccines complexed with the adjuvants MPLA (TLR4) and R837 (TLR7) induced more potent and longer-lived antibody responses than those containing MPLA or R837 alone, and the synergistic effects of MPLA+R837 were dependent on simultaneous MyD88 and Trif signaling in the antigen-specific B cells (62). Together, these reports along with ours demonstrate that vaccines that stimulate multiple PAMP-sensing pathways in innate immune cells as well as B cells can lead to more protective long-term humoral and cellular immunity. Thus, the ground remains quite fertile for testing how various adjuvant combinations, and the inflammatory milieu they generate, affect both the number and quality of memory T and B cells.
Identifying “pro-memory” signals and understanding how they influence the types of memory T and B cells formed is a critical aspect of adjuvant research. Our results suggest that multiple cytokines downstream of TLR4-MyD88 pathway such as IL-6, IL-10 and IL-1β might work in a complimentary manner to promote memory CD8 T cell differentiation. Consistent with this idea, our recent work has shown that IL-10 and IL-21 cooperatively sustain memory cell potential and foster their functional maturation in a STAT3-dependent fashion during an acute viral infection (26). IL-10 can induce SOCS3 that can help insulate the CD8 T cells from signaling by “pro-effector” cytokines like IL-12 (26). Interestingly, IL-6 also signals through STAT3, and IL-6 and IL-10 are necessary for optimal memory CD8 T cell differentiation following a non-infectious immunization or Listeria infection, respectively (26, 27, 49). Therefore, it is likely that multiple signals during LPS priming confer its “pro-memory” effects and the ratio of “pro-memory vs. pro-effector” signals (such as IL10 vs. IL-12) may play a more important role than the absolute amounts of individual cytokines in memory CD8 T cell formation. This model helps to explain why even low amounts of IL-12 can induce KLRG1hi terminal effector T cell differentiation during MPLA priming when the MyD88-dependent IL-10 production is impaired.
Proper selection of adjuvants or viral vectors used to deliver the vaccine and are likely to be as important as the antigens one chooses to express because the inflammatory environment induced by these different factors can vary considerably and influence the types of memory CD8+ T cells that form. For instance, generating memory T cells that home preferentially to the lung, genital or gastrointestinal tracts or liver, are likely to provide the greatest protection against influenza, HIV or HCV, respectively, and therefore identifying the signals that govern the differentiation and homing of these types of memory T cells and how adjuvants or viral vectors may be exploited to enhance this process is a necessary aspect that needs to be included in modern vaccine research. In addition, although this study primarily examines the systemic effects of adjuvants on memory T cell formation, most vaccines are administrated at specific sites (e.g., oral, intramuscular or intranasal). By extension of this study, future work should examine how different types of adjuvants when administered through these routes influences the microenvironment and types of effector and memory T cells generated. Despite the success of currently approved adjuvants for generating immunity to viral and bacterial infections, a tremendous need and challenge remains for improved adjuvants that can enhance vaccines aimed-directly at generating potent T cell immunity for diseases such as HIV, HCV and malaria (7). By highlighting the disparate effects of adjuvants on the quality of memory CD8+ T cells and long-term immunity that forms during vaccination, this study emphasizes the importance of adjuvant selection and analysis of the types of memory T cells that form because boosting effector cell expansion may not always equate with better immunological memory.
Supplementary Material
Acknowledgments
This work was supported by the NIH RO1 AI066232-01 (S.M.K.) and BCRF (W.C.).
Abbreviations used in this paper
- TLR
Toll like receptor
- DC
dendritic cells
- LPS
Lipopolysaccharide
- MPLA
Monophosphoryl Lipid A
- LCMV
lymphocytic choriomeningitis virus
- KLRG1
Killer cell lectin-like receptor G1
Reference
- 1.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nature reviews. Immunology. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed R, Pulendran B. Learning vaccinology from viral infections. The Journal of experimental medicine. 2011;208:2347–2349. doi: 10.1084/jem.20112321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Letvin NL. Correlates of immune protection and the development of a human immunodeficiency virus vaccine. Immunity. 2007;27:366–369. doi: 10.1016/j.immuni.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. The New England journal of medicine. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 7.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. The New England journal of medicine. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 9.Graves P, Gelband H. Vaccines for preventing malaria (SPf66). The Cochrane database of systematic reviews. 2006:CD005966. doi: 10.1002/14651858.CD005966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ledford H. HIV vaccine may raise risk. Nature. 2007;450:325. doi: 10.1038/450325a. [DOI] [PubMed] [Google Scholar]
- 11.Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1–16. doi: 10.1007/3-540-32636-7_1. [DOI] [PubMed] [Google Scholar]
- 12.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 13.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nature immunology. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 14.Cui W, Joshi NS, Jiang A, Kaech SM. Effects of Signal 3 during CD8 T cell priming: Bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine. 2009;27:2177–2187. doi: 10.1016/j.vaccine.2009.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nature medicine. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 16.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nature immunology. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 17.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Whitmire JK, Tan JT, Whitton JL. Interferon-gamma acts directly on CD8+ T cells to increase their abundance during virus infection. The Journal of experimental medicine. 2005;201:1053–1059. doi: 10.1084/jem.20041463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- 22.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. The Journal of experimental medicine. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badovinac VP, Harty JT. Manipulating the rate of memory CD8+ T cell generation after acute infection. J Immunol. 2007;179:53–63. doi: 10.4049/jimmunol.179.1.53. [DOI] [PubMed] [Google Scholar]
- 24.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, Paulos CM, Muranski P, Restifo NP. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nature medicine. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novy P, Huang X, Leonard WJ, Yang Y. Intrinsic IL-21 signaling is critical for CD8 T cell survival and memory formation in response to vaccinia viral infection. J Immunol. 2011;186:2729–2738. doi: 10.4049/jimmunol.1003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity. 2011;35:792–805. doi: 10.1016/j.immuni.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foulds KE, Rotte MJ, Seder RA. IL-10 is required for optimal CD8 T cell memory following Listeria monocytogenes infection. J Immunol. 2006;177:2565–2574. doi: 10.4049/jimmunol.177.4.2565. [DOI] [PubMed] [Google Scholar]
- 28.Jiang A, Bloom O, Ono S, Cui W, Unternaehrer J, Jiang S, Whitney JA, Connolly J, Banchereau J, Mellman I. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27:610–624. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nature immunology. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 30.Chow A, Toomre D, Garrett W, Mellman I. Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature. 2002;418:988–994. doi: 10.1038/nature01006. [DOI] [PubMed] [Google Scholar]
- 31.Gupta S, Gould MP, DeVecchio J, Canaday DH, Auletta JJ, Heinzel FP. CpG-induced IFNgamma expands TLR4-specific IL-18 responses in vivo. Cellular immunology. 2006;243:75–82. doi: 10.1016/j.cellimm.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ngoi SM, Tovey MG, Vella AT. the TLR3-independent pathway boosts effector CD8 T cell differentiation through IFN-alpha/beta. Targeting poly. J Immunol. 2008;I181(C):7670–7680. doi: 10.4049/jimmunol.181.11.7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koay MA, Christman JW, Segal BH, Venkatakrishnan A, Blackwell TR, Holland SM, Blackwell TS. Impaired pulmonary NF-kappaB activation in response to lipopolysaccharide in NADPH oxidase-deficient mice. Infection and immunity. 2001;69:5991–5996. doi: 10.1128/IAI.69.10.5991-5996.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romero CD, Varma TK, Hobbs JB, Reyes A, Driver B, Sherwood ER. The Toll-like receptor 4 agonist monophosphoryl lipid a augments innate host resistance to systemic bacterial infection. Infection and immunity. 2011;79:3576–3587. doi: 10.1128/IAI.00022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Team RDC . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2010. [Google Scholar]
- 36.Du P, Kibbe WA, Lin SM. lumi: a pipeline for processing Illumina microarray. Bioinformatics. 2008;24:1547–1548. doi: 10.1093/bioinformatics/btn224. [DOI] [PubMed] [Google Scholar]
- 37.Smyth GK. Limma: linear models for microarray data. In: Gentleman R, Carey V, Dudoit S, Irizarry R, Huber W, editors. Bioinformatics and Computational Biology Solutions using R and Bioconductor. Springer; New York, NY: 2005. pp. 397–420. [Google Scholar]
- 38.Carvalho BS, Irizarry RA. A framework for oligonucleotide microarray preprocessing. Bioinformatics. 2010;26:2363–2367. doi: 10.1093/bioinformatics/btq431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nature genetics. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 40.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic acids research. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Best JA, Blair DA, Knell J, Yang E, Mayya V, Doedens A, Dustin ML, Goldrath AW. Transcriptional insights into the CD8(+) T cell response to infection and memory T cell formation. Nature immunology. 2013;14:404–412. doi: 10.1038/ni.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang CY, Best JA, Knell J, Yang E, Sheridan AD, Jesionek AK, Li HS, Rivera RR, Lind KC, D'Cruz LM, Watowich SS, Murre C, Goldrath AW. The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nature immunology. 2011;12:1221–1229. doi: 10.1038/ni.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Obar JJ, Jellison ER, Sheridan BS, Blair DA, Pham QM, Zickovich JM, Lefrancois L. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. J Immunol. 2011;187:4967–4978. doi: 10.4049/jimmunol.1102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joshi NS, Kaech SM. Effector CD8 T Cell Development: A Balancing Act between Memory Cell Potential and Terminal Differentiation. J Immunol. 2008;180:1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 46.Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, Larocque D, Van Mechelen M, Garcon N. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. 2009;183:6186–6197. doi: 10.4049/jimmunol.0901474. [DOI] [PubMed] [Google Scholar]
- 47.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 48.Pham NL, Badovinac VP, Harty JT. A default pathway of memory CD8 T cell differentiation after dendritic cell immunization is deflected by encounter with inflammatory cytokines during antigen-driven proliferation. J Immunol. 2009;183:2337–2348. doi: 10.4049/jimmunol.0901203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castellino F, Germain RN. Chemokine-guided CD4+ T cell help enhances generation of IL-6RalphahighIL-7Ralpha high prememory CD8+ T cells. J Immunol. 2007;178:778–787. doi: 10.4049/jimmunol.178.2.778. [DOI] [PubMed] [Google Scholar]
- 50.Ben-Sasson SZ, Wang K, Cohen J, Paul WE. IL-1beta Strikingly Enhances Antigen-Driven CD4 and CD8 T-Cell Responses. Cold Spring Harbor symposia on quantitative biology. 2013 doi: 10.1101/sqb.2013.78.021246. [DOI] [PubMed] [Google Scholar]
- 51.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nature reviews. Immunology. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 52.Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nature reviews. Immunology. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- 53.Ohto U, Fukase K, Miyake K, Satow Y. Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science. 2007;316:1632–1634. doi: 10.1126/science.1139111. [DOI] [PubMed] [Google Scholar]
- 54.Casella CR, Mitchell TC. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cellular and molecular life sciences : CMLS. 2008;65:3231–3240. doi: 10.1007/s00018-008-8228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, Abbink P, Coffey RT, Grandpre LE, Seaman MS, Landucci G, Forthal DN, Montefiori DC, Carville A, Mansfield KG, Havenga MJ, Pau MG, Goudsmit J, Barouch DH. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmidt NW, Butler NS, Harty JT. Plasmodium-host interactions directly influence the threshold of memory CD8 T cells required for protective immunity. J Immunol. 2011;186:5873–5884. doi: 10.4049/jimmunol.1100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.MacLeod MK, McKee AS, David A, Wang J, Mason R, Kappler JW, Marrack P. Vaccine adjuvants aluminum and monophosphoryl lipid A provide distinct signals to generate protective cytotoxic memory CD8 T cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7914–7919. doi: 10.1073/pnas.1104588108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kool M, Petrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN, Tschopp J. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 61.Orr MT, Duthie MS, Windish HP, Lucas EA, Guderian JA, Hudson TE, Shaverdian N, O'Donnell J, Desbien AL, Reed SG, Coler RN. MyD88 and TRIF synergistic interaction is required for TH1-cell polarization with a synthetic TLR4 agonist adjuvant. European journal of immunology. 2013;43:2398–2408. doi: 10.1002/eji.201243124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, Garcia-Sastre A, Compans R, Pulendran B. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.