Abstract
There are two developmentally regulated alternatively spliced forms of Disabled-1 (Dab1) in the chick retina: an early form (Dab1-E) expressed in retinal precursor cells and a late form (Dab1-L) expressed in neuronal cells. The main difference between these two isoforms is the absence of two Src family kinase (SFK) recognition sites in Dab1-E. Both forms retain two Abl/Crk/Nck recognition sites implicated in the recruitment of SH2 domain-containing signaling proteins. One of the Dab1-L-specific SFK recognition sites, at tyrosine(Y)-198, has been shown to be phosphorylated in Reelin-stimulated neurons. Here, we use Reelin-expressing primary retinal cultures to investigate the role of the four Dab1 tyrosine phosphorylation sites on overall tyrosine phosphorylation, Dab1 phosphorylation, SFK activation and neurite formation. We show that Y198 is essential but not sufficient for maximal Dab1 phosphorylation, SFK activation and neurite formation, with Y232 and Y220 playing particularly important roles in SFK activation and neuritogenesis, and Y185 having modifying effects secondary to Y232 and Y220. Our data support a role for all four Dab1 tyrosine phosphorylation sites in mediating the spectrum of activities associated with Reelin-Dab1 signaling in neurons.
Keywords: retina, alternative splicing, tyrosine phosphorylation, site-directed mutagenesis, Disabled-1
Introduction
The Reelin–Dab1 signaling pathway plays a key role in neuronal cell migration and in the positioning of neurons within laminated structures. Reelin is a secreted glycoprotein that binds to the very low density lipoprotein receptor (VLDLR) and apolipoprotein E receptor 2 (ApoER2), resulting in receptor clustering and membrane recruitment of Dab1.1–3 Dab1 is a cytosolic adaptor protein that undergoes Reelin–induced tyrosine phosphorylation thereby activating Src family kinases (SFKs).2,4–6 Dab1 tyrosine phosphorylation is critical for transduction of the Reelin signal, since mice that express a mutant form of Dab1 lacking all tyrosine phosphorylation sites show identical neuronal positioning defects to those seen in Reelin−/− (reeler), Dab1−/− (scrambler/yotari/mdab1-1) and VLDLR−/−/ApoER2−/− mutant mice.3,7–10
There are five tyrosine residues in Dab1 (Y185, Y198, Y200, Y220 and Y232) that correspond to four conserved tyrosine phosphorylation sites. Y185 and Y198/Y200 are within two SFK consensus phosphorylation sites (YQXI), whereas Y220 and Y232 are part of Abl family kinase/Crk/Nck consensus phosphorylation/recognition sites (YXVP).11 Y198 has been identified as the primary (and main) residue to undergo Reelin-mediated phosphorylation, with Y220 serving as a secondary and less phosphorylated residue.6 More recently, phosphorylation at both Y220 and Y232 was shown to be important for binding to members of the Crk family.12 Furthermore, phosphorylation of both these tyrosine residues is required for the detachment of migrating neurons from radial glial fibers.13 Other reports indicate that Lis1 binding to Dab1 is dependent on phosphorylation at either Y198 or Y22014 and that Nckβ binding requires either Y220 or Y232 phosphorylation.15 Combined, these data tyrosine residues may be important in the regulation of downstream effectors of Reelin–Dab1 signaling.
Most studies addressing Reelin–Dab1 function have been carried out in the brain. However, Reelin and Dab1 are also expressed in the retina.16–18 Early in development, the retina consists of neuroectodermal progenitor cells, which differentiate into the six classes of neuronal cells (ganglion, amacrine, bipolar, horizontal, rod, cone) and one class of glial cells (Müller) that make up the mature tissue. First to differentiate in the retina are the ganglion cells, followed by amacrine, horizontal, photoreceptor, bipolar and Müller glial cells.19 Reelin−/− and Dab1−/− mutant mice have a number of retinal defects, including abnormal patterning of type AII amacrine cell projections in the inner nuclear layer, a reduction in the number of type AII amacrine dendrites, a decrease in rod bipolar cell numbers and abnormal synaptic layering of rod bipolar cells.20
We have found two main forms of Dab1 in the developing chick retina: an early form (Dab1-E) expressed in progenitor cells and a late form (Dab1-L, also known as Dab1555 or Dab1) expressed in ganglion and amacrine cells.16 Dab1-L contains two SFK and two Abl consensus recognition sites, whereas Dab1-E only has two Abl consensus recognition sites. One of the two Abl recognition sites (Y185) in Dab1-E represents a conversion from a SFK to an Abl recognition site as a consequence of developmentally regulated alternative splicing. Expression of Dab1-L in primary retinal cultures results in increased tyrosine phosphorylation, Dab1 phosphorylation, SFK activation, induction of GAP43 and the formation of neurite-like processes.16 These biochemical and morphological alterations are dependent on Dab1 phosphorylation at Y198, as cells that express either a Dab1-LY198F mutant protein or Dab1-E, which lacks both the Y185 and Y198 SFK phosphorylation sites, fail to form processes and do not show induction of phosphotyrosine or SFK activation. In contrast, cells which express Dab1-L singly mutated at Y185, Y200 or Y220 show induction of phosphotyrosine and form neurite-like processes.
The presence of multiple tyrosine phosphorylation sites in Dab1 suggests the possibility of hierarchical phosphorylation of Dab1 upon Reelin stimulation. Here, we examine our primary retinal cultures for Reelin expression, Reelin response, VLDLR expression and Dab1 alternative splicing. We use a series of GFP-Dab1-L constructs harboring combinations of single, double and triple Y→phenylalanine(F) substitutions to determine the contribution of each Dab1 tyrosine phosphorylation site on cellular morphology, phosphotyrosine levels, Dab1 phosphorylation and SFK activation.
Results
Analysis of Dab1, Reelin and VLDLR in retinal cultures
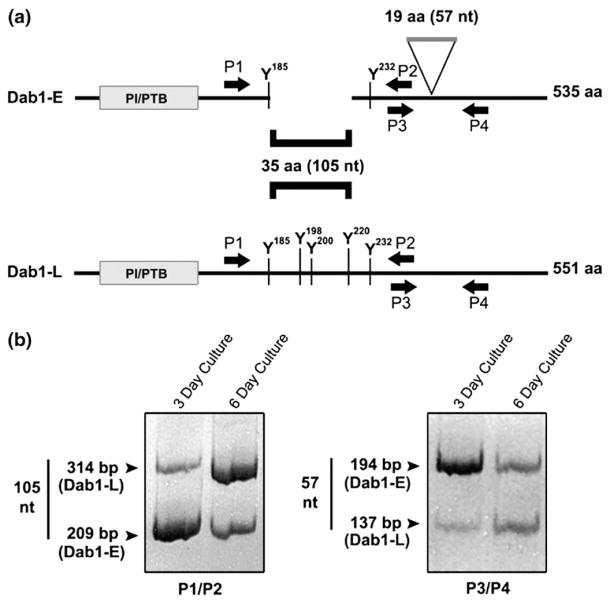
Enzymatically dissociated retinal cells, such as those used in this study, have been shown to reassociate into multicellular aggregates which maintain many of the characteristics of the retinal tissue from which they originate, including differentiation of retinal progenitor cells into neuronal cells.21 Our cultures are set up at ED5 when 80% of the cells consist of proliferating neuroectodermal progenitor cells.22 To examine whether these cultures have the ability to naturally undergo Dab1 alternative splicing, RT-PCR analysis was carried out using primers flanking the 105 nt region specific to Dab1-L (P1, P2) and primers flanking the 57 nt region specific to Dab1-E (P3, P4). As shown in Figure 1, the predominant form of Dab1 mRNA expressed in a three day retinal culture is Dab1-E. Three days later, comparatively stronger Dab1-L-specific bands are observed, indicating that transition from Dab1-E to Dab1-L expression can effectively take place in culture.
Figure 1.
RT-PCR analysis of Dab1-E and Dab1-L-specific products in primary retinal cultures. (a) Schematic representation of Dab1-E and Dab1-L deletion and insertion regions. The locations of the primer pairs, P1/P2 and P3/P4, used for deletion/insertion analysis are indicated. (b) cDNAs synthesized from total RNA prepared from retinal cells cultured for three days and six days were amplified using P1 and P2 primers for the 35 amino acid (105 nt) region and P3 and P4 primers for the 19 amino acid (57 nt) region.
To verify that primary retinal cultures express Reelin, we carried out Western blot analysis of extracts prepared from transfected cultures. Two major bands were observed upon immunostaining with anti-Reelin antibody: (i) a 180 kDa band (arrowhead), consistent with the size of the proteolytically activated N-terminal Reelin fragment; and (ii) a larger band of approximately 400 kDa (arrow) representing the full-length unprocessed form of Reelin (Figure 2(a)).23 VLDLR was also easily detectable in extracts prepared from retinal cultures (Figure 2(b)). Together with the observed Dab1-E to Dab1-L transition as a function of time in culture, expression of Reelin and VLDLR in our primary retinal cultures suggests that these cells represent a biologically relevant neuronal model system for studying Reelin–Dab1 signaling events.
Figure 2.
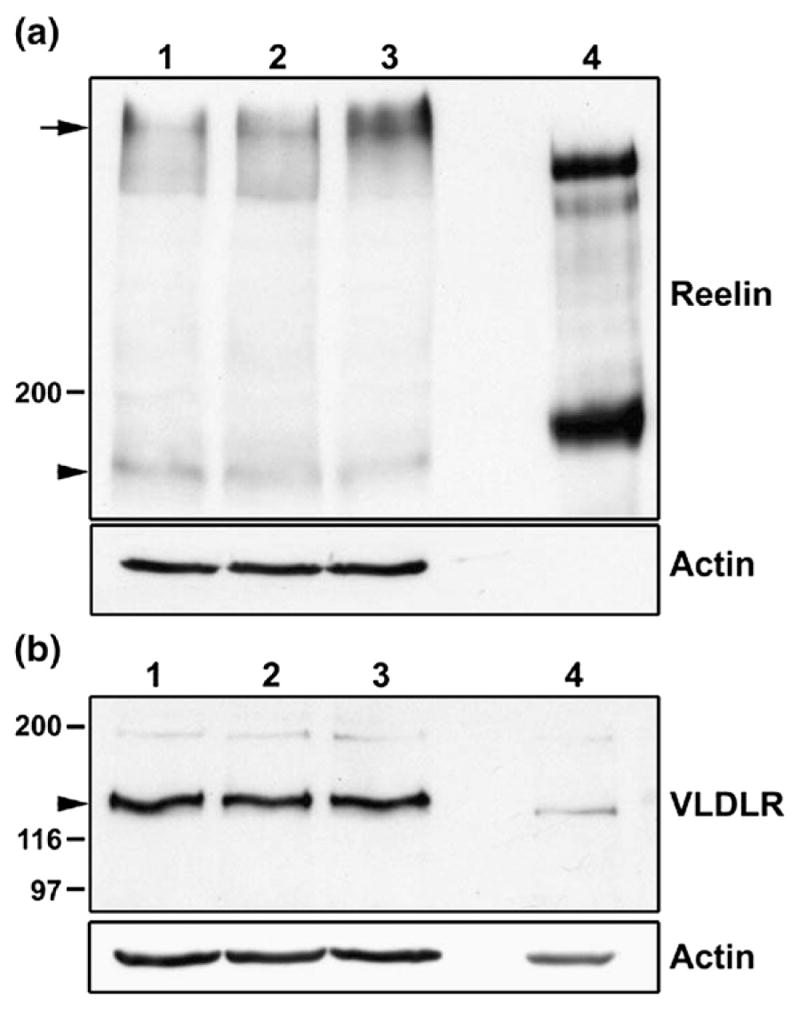
Analysis of Reelin and VLDLR expression in retina. (a) Western blot analysis of cell lysates prepared from primary retinal cultures transfected with GFP (lane 1), GFP–Dab1-E (lane 2) or GFP–Dab1-L (lane 3) expression constructs (50 μg protein/lane). Supernatant (4 μl) from HEK293T cells transfected with the pCrl Reelin expression construct served as the positive control (lane 4). Proteins were electrophoresed through an SDS–8% polyacrylamide (low Bis-acrylamide) gel for 2 h and transferred to nitro-cellulose. Membranes were immunostained with anti-Reelin antibody and the signal detected by chemiluminescence using ECL reagent. Signals for unprocessed (~400 kDa) and activated Reelin (~180 kDa) are as indicated by the arrow and arrowhead, respectively. The diffuse pattern observed for the top band in retinal tissue likely reflects a glycosylation state.25 (b) Western blot analysis of cell lysates from GFP (lane 1), GFP–Dab1-E (lane 2) and GFP–Dab1-L- (lane 3) transfected cells, with ED5 chick retinal tissue (lane 4) serving as control. Proteins were electrophoresed through an SDS–8% polyacrylamide gel and VLDLR was detected with mouse anti-VLDLR antibody. Both filters were immunostained with anti-actin antibody in order to ensure that a similar amount of protein was loaded in each lane (with the exception of lane 4 in (a)).
Alteration in SFK induction and neurite formation in the presence of Reelin antibody CR-50
Transfection experiments were carried out by calcium phosphate-mediated DNA precipitation three days after plating ED5 retinal cells. This transfection method works most efficiently for proliferating cells as the nuclear envelope, a barrier for nuclear uptake of plasmids, breaks down during mitosis.24 An estimated 5–10% of cells in our retinal cultures are transfected using this method. Based on Western blotting, levels of GFP-Dab1 in transfected retinal cultures are about four times higher than endogenous Dab1 (Figure 3). However, there is considerable cell-to-cell variation in the level of green fluorescent protein (GFP) expressed by transfected cells based on immunofluorescence microscopy. Cells expressing very high levels of GFP–Dab1 appeared under stress, often demonstrating blebbing. These cells were excluded from our immunofluorescence analysis.
Figure 3.
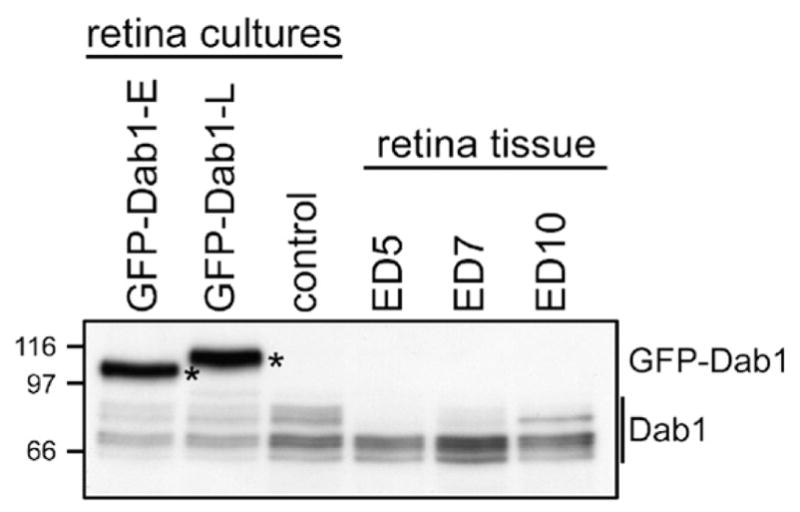
Analysis of GFP–Dab1 and endogenous Dab1 levels in transfected retinal cells and retinal tissue. Western blot analysis of whole cell lysates prepared from primary retinal cultures transfected with GFP–Dab1-E (lane 1), -L (lane 2), control untransfected retinal cultures (lane 3), and retinal tissue at ED5 (lane 4), ED7 (lane 5) and ED10 (lane 6). Proteins were electrophoresed through an SDS–8% polyacrylamide gel and transferred to nitrocellulose. The membrane was immunostained with anti-Dab1 antibody, which recognizes both GFP–Dab1 (indicated by asterisks) and endogenous forms of Dab1 (indicated by a line). To calculate relative GFP–Dab1 and combined endogenous Dab1 band intensities, the autoradiogram was scanned with the Ultroscan XL-laser densitometer and analyzed using the Gel Scan XL V2.1 program.
We have shown that cells transfected with a GFP–Dab1-L expression construct gain a differentiated morphology characterized by elongated processes and SFK activation, whereas cells transfected with a GFP–Dab1-E expression construct retain an undifferentiated appearance.16 To determine whether the phenotypic and biochemical alterations observed in GFP–Dab1-L-transfected retinal cells are mediated through Reelin, we treated these cultures with the Reelin inhibiting antibody CR-50.25,26 CR-50 has been shown to interfere with Reelin homopolymerization, thereby preventing Reelin-mediated signaling.27 The phospho-SFK (pSFK) signal was significantly reduced with few processes observed in GFP–Dab1-L-expressing cells treated with CR-50 (Figure 4; CR-50-treated). In contrast, GFP–Dab1-L-transfected cultures treated with monoclonal antibody to the transcription factor AP-2α displayed robust induction of SFK and formed numerous processes (Figure 4; mock). The reduction in SFK activation and the suppression of Dab1-L-induced morphology by CR-50 suggest that Reelin signaling is required for Dab1-L function in the retina.
Figure 4.
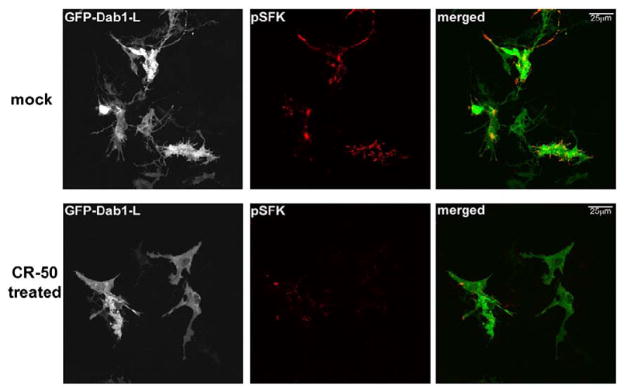
Analysis of CR-50-treated GFP–Dab1-L-expressing retinal cells. GFP–Dab1-L-trans-fected retinal cells were treated with an unrelated antibody, mouse monoclonal anti-activator protein 2 (AP-2 transcription factor) (Developmental Studies Hybridoma Bank, University of Iowa) (mock) or with mouse monoclonal anti-Reelin CR-50 antibody. Cells were immunostained with anti-phospho-SFK(Y416) (pSFK) antibody followed by Alexa 555-conjugated goat anti-mouse secondary antibody. The GFP signal in transfected cells was detected by epifluorescence.
Exogenous Reelin does not affect either tyrosine phosphorylation or SFK activation in Dab1-transfected cells
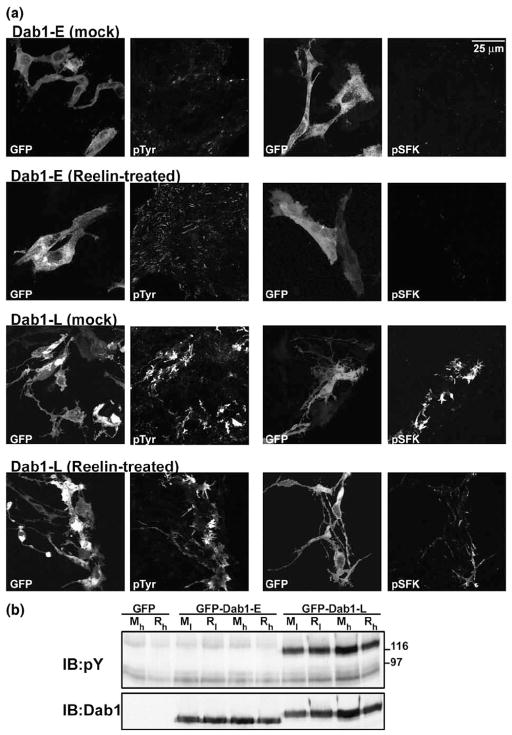
As Reelin may be limiting in transfected retinal cultures, we treated GFP–Dab1-E and –L transfectants with recombinant Reelin prepared from pCrl-transfected HEK293T cells (Figure 2(a), lane 4). After 25 min exposure to exogenous Reelin, transfected cells were processed for immunofluorescence analysis. As shown in Figure 5(a), there were no apparent differences in morphology, phosphotyrosine levels and SFK activation in Reelin-treated GFP–Dab1-E and GFP–Dab1-L transfectants compared to mock-treated transfectants. Western blot analysis of mock- versus Reelin-treated GFP, GFP–Dab1-E and GFP–Dab1-L-transfected retinal cultures revealed undetectable GFP–Dab1-E phosphorylation and no further induction of GFP–Dab1-L phosphorylation upon Reelin treatment (Figure 5(b)). These results indicate that Dab1-E tyrosine phosphorylation is not induced even in the presence of elevated levels of Reelin. Furthermore, Reelin does not appear to be present in limiting amounts in our cultures.
Figure 5.
Treatment of retinal cultures with Reelin. (a) GFP–Dab1-E and GFP–Dab1-L transfected primary retinal cultures were treated with Reelin-enriched medium (1/15 dilution of 30X-concentrated supernatants obtained from pCrl-transfected HEK193T cells) or mock-transfected medium for 25 min. Cultures were fixed and stained with mouse anti-phosphotyrosine antibody (left panels) or mouse anti-phospho-SFK(Y416) antibody (right panels), followed by goat anti-mouse Alexa 555-conjugated secondary antibody. The GFP signal in transfected cells was detected by epifluorescence. (b) Western blot analysis of GFP, GFP–Dab1-E and GFP–Dab1-L-transfected retinal cultures treated with Reelin-enriched medium (R, high (h) or low (l) dose) or mock-transfected medium (M) for 25 min. The filter was sequentially immunostained with anti-phosphotyrosine antibody and anti-Dab1 antibody. For high doses, supernatants from pCrl-transfected HEK293Tcells were concentrated 30× and used at a 1:15 dilution. For low doses, 30×-concentrated supernatants were used at a 1:30 dilution.
Reelin–Dab1-mediated neurite formation and SFK induction require multiple Dab1 tyrosine phosphorylation sites
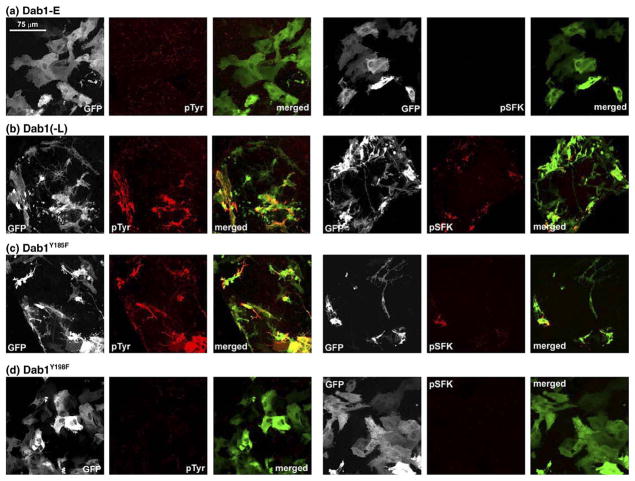
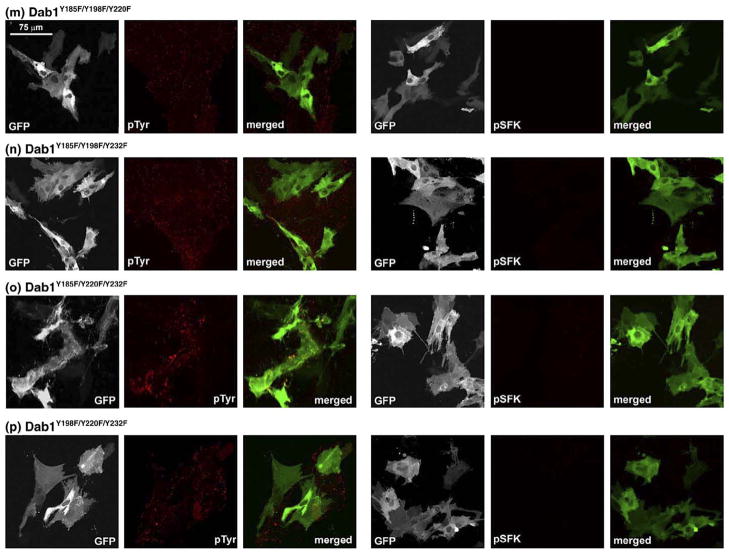
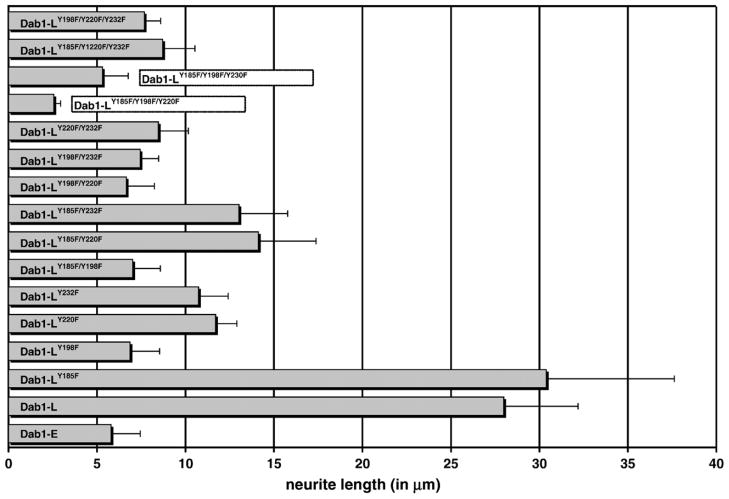
To determine the relative importance of the four Dab1 tyrosine phosphorylation sites in phosphotyrosine induction, SFK activation and neurite formation, we transfected retinal cells with GFP–Dab1-L constructs singly mutated at Y185F, Y198F, Y220F or Y232F. Transfected cultures were immunostained with anti-phosphotyrosine and pSFK antibodies and analyzed by confocal microscopy. Cells expressing GFP–Dab1(-L)Y198F had an undifferentiated epithelial-like morphology, showed little phosphotyrosine immunoreactivity and no induction of SFK activity (Figures 6(d) and 7), similar to that observed with GFP–Dab1-E transfectants (Figures 6(a) and 7). In contrast, cells expressing the GFP-Dab1Y185F (Figure 6(c)) mutant construct had similar properties to that of cells expressing wild-type GFP–Dab1-L (Figures 6(b) and 7), including strong phosphotyrosine immunoreactivity and the formation of numerous thin elongated processes. The average lengths of processes in GFP–Dab1-E, –Dab1-L, –Dab1Y198F and –Dab1Y185F transfectants are indicated in Figure 8. Interestingly, cells expressing either GFP–Dab1Y220F or GFP–Dab1Y232F displayed a morphology that was neither Dab1-E-like nor Dab1-L-like, but rather resembled an intermediate phenotype with numerous short processes (Figures 6(e) (f), 7 and 8). Similar to Dab1-L, cells expressing GFP-Dab1Y220F or GFP-Dab1Y232F showed increased levels of phosphotyrosine as well as SFK activation. These data suggest that while Y198 plays a major role in Reelin-mediated Dab1 tyrosine phosphorylation, induction of SFKs and associated changes in morphology, Y220 and Y232 are required for the extensive neurite formation observed with Dab1-L expression.
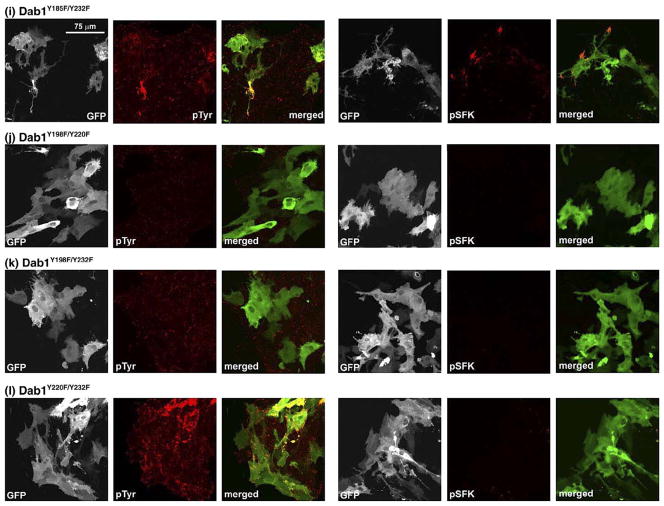
Figure 6.
Analysis of primary chick retinal cultures transfected with chicken GFP–Dab1-E (a), GFP–Dab1-L (b) and single ((c)–(f)), double ((g)–(l)) and triple ((m)–(p)) GFP–Dab1-LY→F mutants. GFP–Dab1-expressing cells (shown in green) were fixed and stained with mouse anti-phosphotyrosine or mouse anti-phospho-SFK(Y416) antibodies, followed by goat anti-mouse Alexa 555-conjugated secondary antibody (shown in red). The GFP signal was detected by epifluorescence. The absence of phosphotyrosine and pSFK background signal in most panels reflects the fact that image stacks were collected under non-saturating conditions, and that, for consistency, parameters for image stack collection were set using GFP–Dab1-L-transfected cells. To better visualize cellular morphology, representative sections of (a), (b), (d), (f), (h), (l) and (o) are magnified in Figure 7.
Figure 7.
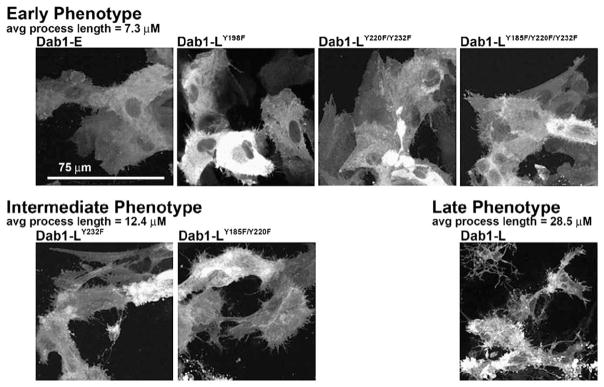
Morphology of retinal cells transfected with GFP–Dab1 constructs. The early (Dab1-E-like) phenotype characterized by an undifferentiated epithelial-like appearance was observed in retinal cells transfected with GFP–Dab1-E, GFP–Dab1-LY198F, GFP–Dab1-LY220F/Y232F and GFP–Dab1-LY185F/Y220F/Y232F expression constructs. The intermediate phenotype characterized by the formation of numerous short processes was observed in cells transfected with GFP–Dab1-LY232F and GFP–Dab1-LY185F/Y220F constructs. The late phenotype defined by numerous elongated processes was observed in retinal cells transfected with the Dab1-L construct. The grey scale used for this Figure enhances the visualization of fine details such as thin processes. Values listed for average process length were obtained from Figure 8.
Figure 8.
Lengths of neurites in cells transfected with GFP–Dab1 constructs. The lengths of a minimum of 20 neurites from GFP-positive cells from wild-type and mutant GFP–Dab1 transfected cultures were measured as described in Materials and Methods. Standard deviations are indicated by the error bars.
To verify that the induction of tyrosine phosphorylation was primarily mediated through Y198 and to further examine the role of tyrosine residues in SFK activation and cellular morphology, Dab1 Y→F double and triple mutants were analyzed. As expected, cells expressing mutants that included the Y198F substitution (Dab1Y185F/Y198F, Dab1Y198F/Y220F, Dab1Y198F/Y232F, Dab1Y185F/Y198F/Y220F, Dab1Y185F/Y198F/Y232F, Dab1Y198F/Y220F/Y232F) (Figure 6(g), (j), (k), (m), (n) and (p)) displayed identical morphology and properties to those expressing the GFP–Dab1Y198F single substitution. GFP–Dab1Y185F/Y220F and GFP–Dab1Y185F/Y232F-expressing cells had a similar appearance to that of GFP–Dab1Y220F and GFP–Dab1Y232F-expressing cells, along with similar levels of phosphotyrosine and activated SFK (Figures 6(h), (i), 7 and 8). Interestingly, a number of similarities were noted when cells transfected with the GFP–Dab1Y198F construct (e.g. see Figures 6(d) and 7) were compared to cells transfected with the GFP-Dab1Y185F/Y220F/Y232F triple mutant construct (which has an intact Y198) (Figures 6(o), 7 and 8), with the former showing greatly reduced phosphotyrosine levels, no induction of pSFK and a Dab1-E-like morphology, while the latter had reduced levels of phosphotyrosine, and showed little SFK activation or neurite formation. Cells expressing GFP–Dab1Y220F/Y232F (Figures 6(l) and 7) appeared to have higher levels of phosphotyrosine compared to cells expressing GFP–Dab1Y185F/Y220F/Y232F. These data demonstrate a role for multiple tyrosine residues in Dab1 signaling. Immunofluorescence data are summarized in Table 1.
Table 1.
Summary of immunofluorescence data
| Clone | pTyr | pSFK(Y416) | Phenotype |
|---|---|---|---|
| Dab 1-E | − | − | E |
| Dab 1-L | + | + | L |
| Dab 1-L Y185F | + | + | L |
| Dab 1-L Y198F | − | − | E |
| Dab 1-L Y220F | + | + | I |
| Dab 1-L Y232F | + | + | I |
| Dab 1-L Y185F/Y198F | − | − | E |
| Dab 1-L Y185F/Y220F | + | + | I |
| Dab 1-L Y185F/Y232F | + | + | I |
| Dab 1-L Y198F/Y220F | − | − | E |
| Dab 1-L Y198F/Y232F | − | − | E |
| Dab 1-L Y220F/Y232F | + | Low | E |
| Dab 1-L Y185F/Y198F/Y220F | − | − | E |
| Dab 1-L Y185F/Y198F/Y232F | − | − | E |
| Dab 1-L Y185F/Y220F/Y232F | Low | Low | E |
| Dab 1-L Y198F/Y220F/Y232F | − | − | E |
E, early phenotype. L, late phenotype. I, intermediate phenotype.
The most critical residue for Dab1 tyrosine phosphorylation is Y198
The increase in phosphotyrosine levels observed in Dab1-L-expressing cells can be attributed at least in part to phosphorylation of the Dab1 protein itself.16 To investigate whether the correlation between phosphotyrosine levels and Dab1 phosphorylation can be extended to Dab1 mutants, Western blots were prepared using whole cell extracts from retinal cells transfected with wild-type and mutant Dab1 constructs. Phosphorylated proteins were detected using the anti-phosphotyrosine antibody 4G10, as well as by the relative migration rates of the GFP–Dab1 proteins (Figure 9(a)). We also immunoprecipitated the GFP fusion proteins from cells transfected with wild-type and mutant constructs and immunostained the blot with the 4G10 anti-phosphotyrosine antibody (Figure 9(b)). There was a good correlation between the Western blot/immunoprecipitation data (measuring Dab1 phosphorylation) (Figure 9) and the immunofluorescence data (measuring tyrosine phosphorylation) (Table 1). GFP–Dab1-L proteins produced from all Dab-LY198F mutant constructs showed considerably reduced tyrosine phosphorylation, whereas tyrosine phosphorylation of (GFP)–Dab1-LY185F, Dab1-LY220F, Dab1-LY232F, Dab1-LY85F/Y220F and Dab1-LY185F/Y232F was similar to that of Dab1-L. A positive signal was also detected in cells transfected with the GFP–Dab1-LY220F/Y232F construct although the intensity of the signal was weaker than in the GFP–Dab1-L lane. The GFP–Dab1-LY185F/Y220F/Y232F-expressing cells appeared negative for Dab1 phosphorylation by Western blot analysis even though weak phosphotyrosine staining was detected by immunofluorescence (Figure 6(o)). Immunoprecipitation of Dab1-LY185F/Y220F/Y232F protein followed by immunostaining with anti-phosphotyrosine antibody confirmed weak Dab1 phosphorylation in Dab1-LY185F/Y220F/Y232F-transfected cells (Figure 9(b)).
Figure 9.
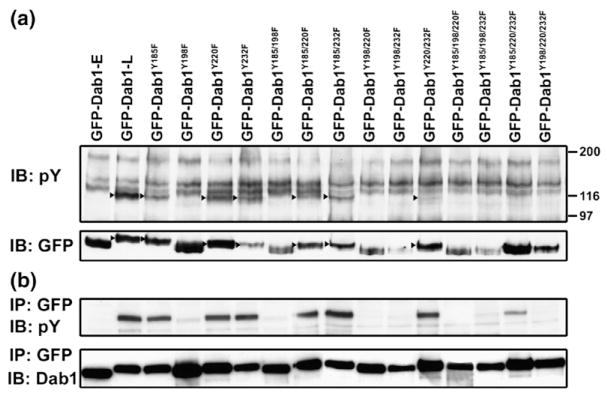
Dab1 phosphorylation in transfected retinal cultures. (a) Western blot analysis of GFP–Dab1-transfected retinal cultures. Transfected cells were lysed and immunoblotted (IB) with anti-phosphotyrosine antibody 4G10 and anti-GFP antibody. Tyrosine-phosphorylated GFP–Dab1 proteins (indicated by the arrowheads) migrated at a slower rate compared to non-phosphorylated proteins. This Western blot analysis also confirms that single, double and triple Y→F GFP–Dab1-L proteins are full-length. (b) GFP–Dab1 proteins were immunoprecipitated (IP) from lysed transfected cells using anti-GFP antibody. Immunoprecipitated proteins were run on an SDS–8% polyacrylamide and detected using anti-phosphotyrosine antibody 4G10 and anti-Dab1 antibody.
Quantitative analysis of pSFK in GFP–Dab1 transfected cells
Immunofluorescence analysis allows us to study induction of tyrosine phosphorylation, SFK activation and neurite formation in individual GFP-transfected cells. However, this technique is not particularly quantitative. In an attempt to quantify relative levels of activated SFK in retinal cells transfected with the different GFP–Dab1-L mutant constructs, Western blotting was carried out using whole cell lysates prepared from transfected cultures. Because primary retinal cultures express endogenous pSFK (Figure 10(a), lane 1), we were only able to observe consistent lane-to-lane variation in pSFK signal intensity when the transfection efficiency was in the upper range (~7–10%). Comparison of GFP–Dab1-E- versus GFP–Dab1-L-transfected cultures in Figure 10(a) reveals 20–25% higher levels of pSFK in the GFP–Dab1-L transfectants in two out of three sets of experiments.
Figure 10.
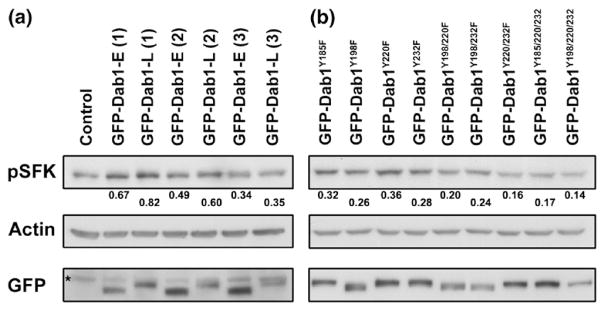
SFK activation in transfected retinal cultures. (a) Western blot analysis of three independent sets of GFP–Dab1-E and -L-transfected retinal cultures. The control is from untransfected retinal cultures harvested at the same stage as the transfected cultures. (b) Western blot analysis of primary retinal cultures transfected with GFP–Dab1 mutant constructs. Transfected and control cells were lysed in RIPA buffer and proteins electrophoresed in duplicate SDS–8% polyacrylamide gel and transferred to PVDF membranes. The first membrane was sequentially immunostained with rabbit anti-pSFK and goat anti-actin antibodies. The second membrane was immunostained with anti-GFP antibody to ensure that the transfection efficiency was similar for each construct. Band intensities were measured with a densitometer as described for Figure 3. Relative areas under the peaks for pSFK bands are shown below each lane of the pSFK blot. The asterisk in (a) (GFP blot) indicates the position of a non-specific band.
Next, we analyzed pSFK levels in nine GFP–Dab1-L mutant constructs. A reduction in signal intensity was observed in the GFP–Dab1Y198F-transfected cells compared to cells transfected with the GFP–Dab1Y185F, GFP–Dab1Y220F and GFP–Dab1Y232F constructs (based on Figure 10(b) and two other experiments that are not shown). The pSFK signal in the GFP–Dab1Y198F/Y220F and GFP–Dab1Y198F/Y232F double transfectants was similar or slightly lower than that of GFP–Dab1Y198F transfected cells. There was no evidence of pSFK induction in either the GFP–Dab1Y220F/Y232F or GFP–Dab1Y185F/Y220F/Y232F-transfected cells, suggesting that the weak pSFK signal detected in these transfectants by immunofluorescence is near background level. In fact, the signal obtained for GFP–Dab1Y220F/Y232F, GFP–Dab1Y185F/Y220F/Y232F and GFP–Dab1Y198F/Y220F/Y232F-transfected cells was significantly lower than that of GFP–Dab1Y198F transfectants, even though only background levels of pSFK were detected by confocal microscopy upon mutation of the single Y198 residue. These results could be explained by the fact that different anti-pSFK antibodies were used for immunofluorescence analysis (monoclonal 9A6) and Western blotting (ABR rabbit pTyr418) as we were not able to obtain a signal with clone 9A6 on Western blots. These two antibodies may differ in their ability to recognize different pSFKs. Thus, activation of one or more member of the SFK family may be particularly affected by mutation of the Y220/Y232 residues. This inconsistency notwithstanding, both the immunofluorescence and Western blot data underline the importance of Y220 and Y232, in addition to Y198, in SFK activation.
Discussion
We have systematically evaluated the role of the four Dab1-L tyrosine phosphorylation sites in Reelin-responsive/VLDLR-positive retinal cultures. An advantage of the retinal culture system is that Dab1-L expression in these cells is accompanied by clear-cut biochemical and morphological events, including induction of tyrosine phosphorylation, SFK activation and neurite formation. Thus, this culture system allows analysis of tyrosine residues in relation to both Dab1 phosphorylation and downstream events in a neuronal context. Our study demonstrates hierarchical and specific roles for each of the four Dab1 tyrosine phosphorylation sites. We show that: (i) Y198 is a primary and essential residue required for Dab1 signaling, including induction of phosphotyrosine, SFK activation and formation of neurites; (ii) either Y232 or Y220 is necessary for Dab1-L-mediated activation of SFKs; (iii) Y232 and Y220 are both required for the formation of the elongated processes characteristic of Dab1-L-transfected cells; and (iv) Y185 has modifying effects that are secondary to Y220 and Y232.
While Y198, Y220 and/or Y232 have previously been implicated in Reelin–Dab1 signaling (e.g. by protein binding assays, Dab1 mutation analysis in non-neuronal cells, analysis of mice expressing Dab1 mutated at all five tyrosine residues), this is the first report demonstrating a role for all four tyrosine phosphorylation sites in the context of a naturally differentiating Reelin-responsive neuronal culture system. In support of a role for Y185 in Dab1 signaling, immunoprecipitation data indicate that Dab1 is weakly phosphorylated in Dab1Y185F/Y220F/Y232F transfectants, whereas Dab1 phosphorylation is easily detected in Dab1Y220F/Y232F transfectants. These results are supported by immunofluorescence data, which demonstrate reduced levels of phosphotyrosine in Dab1Y185F/Y220F/Y232F transfectants compared to Dab1Y220F/Y232F transfectants. It is noteworthy that presence or absence of Y185 in the context of the double Y220F/Y232F mutation does not appear to have a significant effect on SFK activation or neurite formation as both the double and triple mutants show similar low levels of activated SFK and a Dab1-E-like morphology. However, this may simply reflect the quantitative limits of our detection systems.
Others have shown that Y198 is an important residue in Reelin-mediated tyrosine phosphorylation. Using antibodies specific to phospho-Y185, -Y198, -Y220 and -Y232, Keshvara et al.6 demonstrated that Y198 and Y220 are the major sites of Reelin-induced Dab1 phosphorylation in embryonic brain. In agreement with these data, Magdaleno et al.28 have reported that Dab1Y198 is phosphorylated upon Reelin stimulation of cultured reeler cortical ventricular zone neurons. Similar to our study, Ballif et al.12 have analyzed single, double, triple and quadruple GFP-fused Dab1 Y→F mutants. As the aim of their study was to specifically identify Dab1 phosphorylation sites involved in Crk interaction, an activated Fyn kinase expression construct was co-transfected along with the Dab1 constructs into the embryonic kidney HEK293T cell line, thus precluding the identification of tyrosine residues required for endogenous SFK activation. In contrast to our results, mutant Dab1Y198F in HEK293T transfected cells did not significantly reduce Dab1 tyrosine phosphorylation. Furthermore, the Dab1Y220F/Y232F double mutant was not tyrosine phosphorylated in HEK293T cells. These conflicting data demonstrate the importance of biological context when studying signal transduction pathways.
The EGFP–Dab1 constructs used in this study are under the control of the strong CMV promoter. A wide range of GFP signal intensities was observed in our GFP–Dab1 transfected cells, from complete saturation to barely detectable. Cells with intense GFP signals showed morphological alterations associated with apoptosis (i.e. membrane blebbing) and were not included in our immunofluorescence analyses. While it is possible that GFP–Dab1 can multimerize, and become tyrosine phosphorylated, when over-expressed in retinal cultures, Pramatarova et al.15 reported that Dab1GFP, in contrast to Dab1RFP (red fluorescent protein), is not tyrosine-phosphorylated (and therefore is unlikely to exist of a multimer) in transfected Rat-2 fibroblasts. In support of a physiologically relevant response for GFP–Dab1-transfected retinal cells, mutation of a single amino acid in Dab1-L (Y198F) results in abrogation of morphological (neurite formation) and biochemical (SFK activation) properties associated with Dab1-L. Furthermore, the reduction in SFK activation observed in GFP–Dab1-L-transfected cells upon CR-50 treatment suggests a requirement for Reelin in the activation of downstream effectors of Dab1.
Tyrosine phosphorylated Dab1 has been shown to recruit a number of SH2-containing proteins implicated in actin and microtubule-mediated cytoskeletal reorganization and neuronal migration. Specific Dab1 tyrosine phosphorylated sites bind specific SH2-containing proteins; e.g. proteins associated with Dab1 phosphotyrosine-198 include SFKs (Fyn, Src and Yes), phosphoinositol 3′-kinase (PI3K) and phospholipase C-gamma1 (PLCγ1), while those associated with Dab1 phosphotyrosine-220/232 include the Crk and Nck families of adaptor proteins (CrkII, CrkL and Nckβ).12,15,29–32 Recent data indicate that phosphorylation of Y220 and Y232 is required for the release of neurons from radial glial fibers and may be required for the regulation of α3 integrin levels which are critical for the detachment of migrating neurons from the radial glia.13 It is noteworthy that the dynein/dynactin-associated protein, Lis1, requires phosphorylation at both Y198 and Y220 in order to interact with Dab1,14 indicating that some downstream effectors require phosphorylation at both SFK and Abl/Crk/Nck tyrosine phosphorylation/recognition sites. Lis1 is involved in microtubule-dependent transport, neuronal migration and axonal outgrowth.33 These combined results suggest that phosphorylation of multiple tyrosine residues is required to mediate the full spectrum of downstream cytoskeletal-modulating and cell migratory signals that accompany Reelin–Dab1 signaling. Future studies will involve using our primary retinal cultures transfected with Dab1 mutant constructs to address the activity of additional putative Reelin–Dab1 effectors that are downstream of Dab1 phosphorylation and SFK activation.
Previous studies have demonstrated a role for Dab1 and Reelin in dendrite formation. For example, a reduction in the density of amacrine dendrites has been observed in Reelin−/− and Dab1−/− mice.20 Reelin and Dab1 phosphorylation have also been shown to be important for dendritic outgrowth of hippocampal pyramidal cells and dentate granule cells.34 A recent report indicates that knock-down of Dab1 in migrating neurons of the developing mouse cortex results in reduced dendritogenesis, with an accompanying reduction in the number of neuronal cells located in the last 40 μm of the embryonic day 20/21 cortex.35 Here, we report a strong correlation between SFK activation and neurite formation in GFP–Dab1-L-transfected primary retinal cultures, suggesting a direct link between these biochemical and morphological events. SFK activation has been consistently associated with Dab1 signaling in previous studies using both biochemical and biological assays. For example, Dab1 was originally cloned based on its interaction with Src.32 SFK inhibitors abolish Reelin-induced Dab1 phosphorylation in cultured neurons.4,5 Furthermore, combined absence of Src and Fyn in mice mimics many aspects of Reelin deficiency.36 Our data extend these findings, supporting a role for Dab1 in neurite/dendrite formation through SFK activation.
In summary, our analysis sheds light on how individual Dab1 tyrosine phosphorylation sites contribute to the regulation of SFK activation and neurite formation. We have shown that Y198 is important for induction of tyrosine phosphorylation and/or Dab1 phosphorylation, SFK activation and neurite formation. However, Y198 is not sufficient for maximal tyrosine phosphorylation, SFK activation and neurite formation, as Y220 and Y232 are also required for these processes, with Y185 playing a modifying role in Dab1 phosphorylation. This study establishes retinal cultures as an effective and relevant neuronal model system for the analysis of Reelin-mediated Dab1 biochemical functions. A particular advantage of our model system is that it allows the linking of Dab1 downstream signaling events (such as tyrosine phosphorylation and SFK activation) to neuronal cell morphology, thereby providing a multi-faceted reporter system to examine alteration or disruption of Reelin–Dab1 signaling.
Materials and Methods
RT-PCR analysis of Dab1 in retinal cultures
Cultures were prepared using the retinas of chick embryos at day five of incubation (embryonic day five, ED5). Total RNA was extracted from cells cultured for either three days or six days using the hot phenol method. Five μg RNA were reverse-transcribed with Superscript reverse transcriptase (Invitrogen) and oligo(dT) primer. PCR was carried out using 1/20 of the cDNA generated from this reaction. P1 and P2 primers were used for the analysis of the 105 nt region deleted in Dab1-E, whereas P3 and P4 primers were used for the analysis of the 57 nt region specific to Dab1-E.
Generation of Dab1-L phosphorylation mutants
Generation of site-directed single, double and triple Dab1 Y→F mutants was carried out by sequential PCR.37 Partially complementary primers containing a point mutation corresponding to a Y→F substitution (TA(T/C)→TT(T/C)) were used in conjunction with pEGFP-C1 vector primers located upstream of the EcoRI site and downstream of the BamHI site to generate DNA fragments corresponding to full-length chDab1-L, each mutated at a specific tyrosine residue. DNA fragments were annealed, extended and amplified using pEGFP-C1 vector primers. The DNA was digested with EcoRI and BamHI and cloned into pEGFP-C1. Double mutants were generated from corresponding single mutants while triple mutants were generated from corresponding double mutants. Constructs were sequenced to ensure incorporation of the appropriate mutation. Expression of full-length GFP-chDab1-L mutant proteins was confirmed by transfecting each expression construct into HeLa cells followed by Western blot analysis using anti-GFP antibody (data not shown).
Chicken Dab1Y185F, Dab1Y198F, Dab1Y220F and Dab1Y185F/Y198F mutants have been described.16 In total, ten new mutants were generated: Dab1Y232F, Dab1Y185F/Y220F, Dab1Y198F/Y220F, Dab1Y185F/Y232F, Dab1Y198F/Y232F, Dab1Y220F/Y232F, Dab1Y185F/Y198F/Y220F, Dab1Y185F/Y198F/Y232F, Dab1Y185F/Y220F/Y232F and Dab1Y198F/Y220F/Y232F. Primers used to generate these mutants have been described,16 with the exception of those specific for Dab1Y232F (sense strand, 5′-AGGTGTTTTT-GATGTGCCA-3′; antisense strand, 5′-ACATCAAAAA-CACCTTCCTT-3′) (mutated residue corresponding to Y→F substitution is italicized).
Transfection and immunofluorescence analysis of chick retinal cells
Primary retinal cultures were prepared from ED5 chick retinas trypsinized prior to plating onto glass coverslips (1/12 of a retina per 12 mm coverslip). Cells were grown in Dulbecco’s Modified Eagle Medium containing 10%(v/v) fetal calf serum and incubated in a 5%(v/v) CO2 humidified chamber. Cells were transfected with GFP expression constructs three days after plating with 1 μg DNA/ml of culture medium. The DNA was introduced into cells by calcium phosphate-mediated DNA precipitation, followed by DNA removal after 16 h. 30 h later, cells were fixed with 4%(w/v) paraformaldehyde in phosphate-buffered saline (PBS) for 10 min and permeabilized for 5 min in 0.5%(v/v) Triton X-100/PBS. Cells were immunostained overnight with mouse anti-phosphotyrosine (PT-66) (1:250) (Sigma) or mouse anti-phospho-SFK(Y416) (9A6) (1:50) (Upstate) antibodies followed by Alexa 555-conjugated goat anti-mouse secondary antibody (Molecular Probes) (1:200) for 1 h. The coverslips were mounted on slides using glycerol containing 1 mg/ml of p-phenylene-diamine and 1 μg/ml of 4′, 6′-diamidino-2-phenylindole (DAPI). Micrographs were collected using a Zeiss LSM 510 confocal laser scanning microscope with a plan apochromat 40× oil immersion lens. Images from individual channels were collected sequentially in order to avoid signal bleed-through. Image stacks (z-series) were collected at 0.3 μm intervals and were overlaid to create a single image showing the three-dimensional nature of GFP– (detected by epifluorescence), phosphotyrosine–and/or phospho–SFK(Y416)-positive cells. Neurite lengths were measured from randomly selected fluorescent micrographs using Adobe Photoshop CS. The distance between the neurite base at the edge of the cell to the tip of the neurite was measured using the Photoshop ‘Measure’ tool. This distance was then converted into neurite length by interpolation using the appropriate scale bar.
For Reelin inhibition experiments, Dab1-L-transfected retinal cells were treated with CR-50 antibody (a gift from Dr T. Curran, St. Jude Children’s Research Hospital, TN). After the removal of DNA from transfected cells, 2 μl of CR-50 antibody (200 μg/ml) were added to each well twice a day. Cells were fixed and stained with anti-phospho-SFK(416) antibody. For Reelin treatment of retinal cultures, HEK293T cells were transfected with the pCrl Reelin expression construct (pCrl was obtained from Dr T. Curran, St. Jude Children’s Research Hospital, TN) or pcDNA3 (mock). The medium was replaced with OPTI-MEM I (Invitrogen) 24 h after transfection. Supernatants were collected 36 h later and concentrated 30× using Amicon Ultra filters (100,000 molecular mass cut-off) (Millipore). Retinal cultures were treated with diluted concentrates (1/15 or 1/30) for 25 min.
Western blot analysis
For Dab1, Reelin and VLDLR expression analysis, retinal cultures were lysed in RIPA buffer (50 mM Tris–HCl (pH 7.5), 500 mM NaCl, 10 mM MgCl2, 0.5%(w/v) sodium deoxycholate, 0.1%(w/v) SDS, 1%(v/v) Triton X-100) containing 1 mM sodium orthovanadate, 1 mM sodium fluoride and 1× Complete (Roche) protease inhibitor cocktail. For detection of VLDLR and GFP–Dab1, 50 μg of protein extracts were electrophoresed through an SDS–8%(w/v) polyacrylamide gel. For Reelin detection, extracts were electrophoresed through an SDS–8% polyacrylamide gel containing low Bis-acrylamide (112:1). Proteins were transferred to nitrocellulose or PVDF membranes by electroblotting followed by immunostaining with mouse anti-Reelin antibody (1:700) (clone 142; Calbiochem), mouse anti-VLDLR antibody (1:200) (clone 6A6; Santa Cruz Biotechnology), mouse anti-phosphotyrosine antibody (1:5000) (clone 4G10; Upstate), rabbit anti-pSFK (1:1000) (Affinity BioReagents), mouse anti-GFP antibody (1:1000) (clone GFP-20; Sigma), rabbit anti-Dab1 antibody (1:5000) (Rockland Immunochemicals) or goat anti-actin antibody (1:500) (I-19; Santa Cruz Biotechnology). The signal was visualized using the ECL chemiluminescence detection system (GE Healthcare).
For GFP immunoprecipitation experiments, 4 μl anti-GFP antibody was incubated with 200 μg of whole cell extracts prepared from GFP–Dab1-E, GFP–Dab1-L and single, double or triple-mutant GFP–Dab1-L-transfected cells at 4 °C for 2 h. Immune complexes were bound to protein A-Sepharose beads (GE Healthcare) and eluted in SDS-sample buffer. Immunoprecipitates were resolved by SDS–PAGE, blotted to nitrocellulose membranes and immunostained with anti-phosphotyrosine antibody (clone 4G10).
Acknowledgments
We are grateful to Ken Roy and Dr Lei Li for expert technical assistance and to Christina Fok for help in the preparation of the manuscript. We thank Dr Tom Curran (St. Jude Children’s Research Hospital, Memphis, TN) for the pCrl Reelin expression construct and the CR-50 antibody. We also thank Dr Xuejun Sun and the Cell Imaging Facility at the Cross Cancer Institute for assistance with cell imaging. This work was supported by the Canadian Institutes of Health Research. S.K. was supported by a Dr Cyril Kay Graduate Studentship from the Alberta Cancer Foundation. Z.G. was supported by a Department of Oncology endowed graduate studentship and an Alberta Cancer Foundation Graduate Studentship.
Abbreviations used
- SFK
Src family kinase
- pSFK
phospho-SFK
- VLDLR
very low density lipoprotein receptor
- Dab1
Disabled-1
- GFP
green fluorescent protein
- RT-PCR
reverse-transcription PCR
References
- 1.Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, Herz J. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- 2.Strasser V, Fasching D, Hauser C, Mayer H, Bock HH, Hiesberger T, Herz J, Weeber EJ, Sweatt JD, Pramatarova A, Howell B, Schneider WJ, Nimpf J. Receptor clustering is involved in Reelin signaling. Mol Cell Biol. 2004;24:1378–1386. doi: 10.1128/MCB.24.3.1378-1386.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- 4.Arnaud L, Ballif BA, Forster E, Cooper JA. Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development. Curr Biol. 2003;13:9–17. doi: 10.1016/s0960-9822(02)01397-0. [DOI] [PubMed] [Google Scholar]
- 5.Bock HH, Herz J. Reelin activates SRC family tyrosine kinases in neurons. Curr Biol. 2003;13:18–26. doi: 10.1016/s0960-9822(02)01403-3. [DOI] [PubMed] [Google Scholar]
- 6.Keshvara L, Benhayon D, Magdaleno S, Curran T. Identification of reelin-induced sites of tyrosyl phosphorylation on disabled 1. J Biol Chem. 2001;276:16008–16014. doi: 10.1074/jbc.M101422200. [DOI] [PubMed] [Google Scholar]
- 7.D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- 8.Howell BW, Herrick TM, Hildebrand JD, Zhang Y, Cooper JA. Dab1 tyrosine phosphorylation sites relay positional signals during mouse brain development. Curr Biol. 2000;10:877–885. doi: 10.1016/s0960-9822(00)00608-4. [DOI] [PubMed] [Google Scholar]
- 9.Sheldon M, Rice DS, D’Arcangelo G, Yoneshima H, Nakajima K, Mikoshiba K, et al. Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice. Nature. 1997;389:730–733. doi: 10.1038/39601. [DOI] [PubMed] [Google Scholar]
- 10.Ware ML, Fox JW, Gonzalez JL, Davis NM, Lambert de RC, Russo CJ, et al. Aberrant splicing of a mouse disabled homolog, mdab1, in the scrambler mouse. Neuron. 1997;19:239–249. doi: 10.1016/s0896-6273(00)80936-8. [DOI] [PubMed] [Google Scholar]
- 11.Songyang Z, Shoelson SE, Chaudhuri M, Gish G, Pawson T, Haser WG, et al. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 12.Ballif BA, Arnaud L, Arthur WT, Guris D, Imamoto A, Cooper JA. Activation of a Dab1/CrkL/C3G/Rap1 pathway in Reelin-stimulated neurons. Curr Biol. 2004;14:606–610. doi: 10.1016/j.cub.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 13.Sanada K, Gupta A, Tsai LH. Disabled-1-regulated adhesion of migrating neurons to radial glial fiber contributes to neuronal positioning during early corticogenesis. Neuron. 2004;42:197–211. doi: 10.1016/s0896-6273(04)00222-3. [DOI] [PubMed] [Google Scholar]
- 14.Assadi AH, Zhang G, Beffert U, McNeil RS, Renfro AL, Niu S, et al. Interaction of reelin signaling and Lis1 in brain development. Nature Genet. 2003;35:270–276. doi: 10.1038/ng1257. [DOI] [PubMed] [Google Scholar]
- 15.Pramatarova A, Ochalski PG, Chen K, Gropman A, Myers S, Min KT, Howell BW. Nck beta interacts with tyrosine-phosphorylated disabled 1 and redistributes in Reelin-stimulated neurons. Mol Cell Biol. 2003;23:7210–7221. doi: 10.1128/MCB.23.20.7210-7221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katyal S, Godbout R. Alternative splicing modulates Disabled-1 (Dab1) function in the developing chick retina. EMBO J. 2004;23:1878–1888. doi: 10.1038/sj.emboj.7600185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee EJ, Kim HJ, Lim EJ, Kim IB, Kang WS, Oh SJ, et al. AII amacrine cells in the mammalian retina show disabled-1 immunoreactivity. J Comp Neurol. 2004;470:372–381. doi: 10.1002/cne.20010. [DOI] [PubMed] [Google Scholar]
- 18.Rice DS, Curran T. Disabled-1 is expressed in type AII amacrine cells in the mouse retina. J Comp Neurol. 2000;424:327–338. doi: 10.1002/1096-9861(20000821)424:2<327::aid-cne10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci USA. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rice DS, Nusinowitz S, Azimi AM, Martinez A, Soriano E, Curran T. The reelin pathway modulates the structure and function of retinal synaptic circuitry. Neuron. 2001;31:929–941. doi: 10.1016/s0896-6273(01)00436-6. [DOI] [PubMed] [Google Scholar]
- 21.Seigel GM. The golden age of retinal cell culture. Mol Vis. 1999;5:4. [PubMed] [Google Scholar]
- 22.Dutting D, Gierer A, Hansmann G. Self-renewal of stem cells and differentiation of nerve cells in the developing chick retina. Brain Res. 1983;312:21–32. doi: 10.1016/0165-3806(83)90117-7. [DOI] [PubMed] [Google Scholar]
- 23.Tissir F, Goffinet AM. Reelin and brain development. Nature Rev Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- 24.Dean DA, Byrd JN, Jr, Dean BS. Nuclear targeting of plasmid DNA in human corneal cells. Curr Eye Res. 1999;19:66–75. doi: 10.1076/ceyr.19.1.66.5344. [DOI] [PubMed] [Google Scholar]
- 25.D’Arcangelo G, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Curran T. Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J Neurosci. 1997;17:23–31. doi: 10.1523/JNEUROSCI.17-01-00023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, et al. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- 27.Utsunomiya-Tate N, Kubo K, Tate S, Kainosho M, Katayama E, Nakajima K, Mikoshiba K. Reelin molecules assemble together to form a large protein complex, which is inhibited by the function-blocking CR-50 antibody. Proc Natl Acad Sci USA. 2000;97:9729–9734. doi: 10.1073/pnas.160272497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magdaleno S, Keshvara L, Curran T. Rescue of ataxia and preplate splitting by ectopic expression of Reelin in reeler mice. Neuron. 2002;33:573–586. doi: 10.1016/s0896-6273(02)00582-2. [DOI] [PubMed] [Google Scholar]
- 29.Ballif BA, Arnaud L, Cooper JA. Tyrosine phosphorylation of Disabled-1 is essential for Reelin-stimulated activation of Akt and Src family kinases. Brain Res Mol Brain Res. 2003;117:152–159. doi: 10.1016/s0169-328x(03)00295-x. [DOI] [PubMed] [Google Scholar]
- 30.Beffert U, Morfini G, Bock HH, Reyna H, Brady ST, Herz J. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3beta. J Biol Chem. 2002;277:49958–49964. doi: 10.1074/jbc.M209205200. [DOI] [PubMed] [Google Scholar]
- 31.Chen K, Ochalski PG, Tran TS, Sahir N, Schubert M, Pramatarova A, Howell BW. Interaction between Dab1 and CrkII is promoted by Reelin signaling. J Cell Sci. 2004;117:4527–4536. doi: 10.1242/jcs.01320. [DOI] [PubMed] [Google Scholar]
- 32.Howell BW, Gertler FB, Cooper JA. Mouse disabled (mDab1): a Src binding protein implicated in neuronal development. EMBO J. 1997;16:121–132. doi: 10.1093/emboj/16.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith DS, Niethammer M, Ayala R, Zhou Y, Gambello MJ, Wynshaw-Boris A, Tsai LH. Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian Lis1. Nature Cell Biol. 2000;2:767–775. doi: 10.1038/35041000. [DOI] [PubMed] [Google Scholar]
- 34.Niu S, Renfro A, Quattrocchi CC, Sheldon M, D’Arcangelo G. Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway. Neuron. 2004;41:71–84. doi: 10.1016/s0896-6273(03)00819-5. [DOI] [PubMed] [Google Scholar]
- 35.Olson EC, Kim S, Walsh CA. Impaired neuronal positioning and dendritogenesis in the neocortex after cell-autonomous Dab1 suppression. J Neurosci. 2006;26:1767–1775. doi: 10.1523/JNEUROSCI.3000-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuo G, Arnaud L, Kronstad-O’Brien P, Cooper JA. Absence of Fyn and Src causes a reeler-like phenotype. J Neurosci. 2005;25:8578–8586. doi: 10.1523/JNEUROSCI.1656-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cormack B, Castano I. Introduction of point mutations into cloned genes. Methods Enzymol. 2002;350:199–218. doi: 10.1016/s0076-6879(02)50964-2. [DOI] [PubMed] [Google Scholar]