Abstract
Objective
HIV-associated neurocognitive disorders (HAND) remain prevalent in patients who receive highly active antiretroviral therapy (HAART) and may be associated with cumulative exposure to antiretroviral medications and other factors. We proposed that chronic toxic effects of antiretroviral drugs could contribute to cerebral small vessel disease (CSVD), which might be one of the key underpinnings of HAND.
Design
Clinicopathological cross-sectional study of HIV-infected adults in the California NeuroAIDS Tissue Network.
Methods
We employed multivariable logistic regression methods to determine associations between HAART exposure (protease inhibitor [PI]-based, non-PI-based, or no HAART) and CSVD occurrence (standard histopathology: moderate/severe, mild, or absent). We also associated HAND (relative to normal cognition) with CSVD, HIV-related neuropathologic changes, older age at death (≥50 years), sex, or hepatitis C virus infection.
Results
We found that both mild and moderate/severe CSVD were associated with PI-based HAART exposure after adjusting for diabetes mellitus [odds ratio (OR) 2.8 [95% confidence interval (CI) 1.03, 7.9] and 2.6 [95% CI 1.03, 6.7], respectively, n=134]. Moderate/severe CSVD was associated with diabetes after adjusting for HAART exposure (OR 7.4 [95% CI 1.6, 70.7], n=134). Notably, HAND was associated with mild CSVD (OR 4.8 [95% CI 1.1, 21.2], n=63), which remained statistically significant after adjusting for vessel mineralization, HIV encephalitis, microglial nodular lesions, white matter lesions, or older age.
Conclusion
PI-based HAART exposure may increase the risk of CSVD and thereby neurocognitive impairment in HIV-infected adults. Besides the possible direct toxicity to cerebral small vessels, PI-based HAART may contribute indirectly to CSVD by inducing metabolic abnormalities.
Keywords: aging, antiretroviral, cognition, HIV dementia, protease inhibitor, small vessel disease
Introduction
The benefit of highly active antiretroviral therapy (HAART) for preventing HIV-associated dementia (HAD), the severest form of HIV-associated neurocognitive disorders (HAND), is well documented [1]. Nonetheless, the milder forms of HAND, asymptomatic neurocognitive impairment (ANI) and mild neurocognitive disorder (MND), remain prevalent [2, 3]. HAD in the pre-HAART era occurred primarily in late stages of AIDS and was found to correlate with HIV encephalitis denoting robust HIV-1 replication [4, 5] and microglial activation [6] with aberrant cytokine expression [7] in the brain. In contrast, HAND in the current HAART era can affect even patients with low plasma viral loads and high CD4 cell counts [3, 8, 9], and more variability in the clinical course has been observed [3, 10]. Recent autopsy studies suggest that HAND is not necessarily associated with HIV encephalitis [11–13] and may be related to various comorbid factors such as cumulative exposure to antiretroviral (ARV) medications [14], aging-related cerebrovascular and neurodegenerative changes [15, 16], co-infection with hepatitis C virus (HCV), and substance use [17, 18].
Previous studies suggest that HIV-infected patients are at increased risk of ischemic cerebrovascular disease, potentially caused by infective vasculitis, brain opportunistic diseases, cardiac embolism, hypercoagulopathy, or HIV infection itself [19–26]. Among a variety of brain vessel pathologies found in this context [25], cerebral small vessel disease (CSVD) has been associated with ischemic stroke during life [21] and cerebral infarction at autopsy [23]. In patients receiving HAART, particular ARV drugs may directly cause injury to vessel walls or indirectly induce metabolic abnormalities (e.g., dyslipidemia and insulin resistance) that accelerate the development of atherosclerotic large vessel disease and thereby myocardial infarction [27, 28]. Nonetheless, the potential impact of HAART exposure on the development of ischemic cerebrovascular disease, including CSVD, remains controversial [20, 21, 24, 25, 29–31]. In a community-based study [32], the presence of punctate white matter (WM) lesions (hyperintensities) on magnetic resonance imaging (MRI) was found to be associated with older age and higher systolic blood pressure; moreover, there was a trend toward the direct association between WM lesions and HAART exposure. Whereas in the general population MRI WM lesions are thought to represent ischemic lesions caused by CSVD [33], the similar lesions in HIV-infected patients may also reflect foci of HIV-associated or inflammatory WM injury [34–37]. To our knowledge, in the literature there were no reports that specifically addressed CSVD occurrence in the postmortem brain in relation to HAART exposure.
We proposed that chronic toxic effects of ARV drugs on the cell components of vessel walls could contribute to CSVD, which might be one of the key underpinnings of HAND. More severe forms of CSVD may lead to neurocognitive impairment via cerebral blood flow restriction sufficient to produce microinfarcts and WM lesions. In mild CSVD, neurocognitive compromise may be associated with disturbance of cerebrovascular autoregulation (in response to fluctuations in systemic arterial pressure and in blood/tissue gas partial pressure and pH) and deficiency in functional hyperemia (adjustments of regional cerebral blood flow in response to local brain activity), which together could impair new protein synthesis in neurons required for synaptic plasticity and memory formation [38–42].
The present cross-sectional study was aimed at determining in HIV-infected adults associations between HAART exposure during life and CSVD at autopsy in the California NeuroAIDS Tissue Network (CNTN). We also examined the potential association between CSVD and HAND. We hypothesized that HAART exposure would be associated with higher likelihood of CSVD.
Methods
Study cohort
We assembled 144 HIV-infected autopsy cases that had detailed data on ARV medications and died during 1999–2011 in the CNTN. The University of California San Diego Human Research Protections Program approved the project and all study participants provided written informed consent to participate. The written consent to autopsy was also obtained. There were 120 men (83.3%). The age at death ranged from 26 to 70 years (median 45 years, interquartile range [IQR] 13.3 years); 47 (32.6%) cases aged ≥50 years (i.e., older age). Eighty-three cases (57.6%) were white, 31 (21.5%) Hispanic, 23 (16.0%) black, 5 (3.5%) Asian, and 2 (1.4%) others. The median HIV infection duration (i.e., in each case the estimated duration of HIV infection from the patient’s first awareness to death) was 11.9 years (IQR 8.9 years, n=116). Of 141 cases with available data, 16 (11.3%) had diabetes, 41 (29.1%) had systemic arterial hypertension, and 21 (14.9%) had dyslipidemia. Lifetime history of cigarette smoking was present in 45 (32.4%) of 139 cases and that of psychostimulant (primarily methamphetamine) dependence (determined according to either Psychiatric Research Interview for Substance and Mental Disorders [43] or Composite International Diagnostic Interview [44]) in 20 (28.6%) of 70 cases. HCV infection was found in 51 (41.5%) of 123 cases undergoing serological testing. Older age (≥50 years) was associated with higher proportions of diabetes, hypertension, and dyslipidemia (P=0.046, =0.01, and =0.045, respectively, Fisher’s exact test), but not with those of cigarette smoking, psychostimulant dependence, or HCV infection (P=0.6, =1.0, and =0.1, respectively, Fisher’s exact test).
The median postmortem interval was 13 hours (IQR 18.3 hours). There were 140 (97.2%) cases with AIDS-defining conditions, three pre-AIDS, and one undetermined. Additionally, the systemic autopsy findings frequently included pneumonia, malignant neoplasms (primarily carcinomas and non-Hodgkin lymphomas), hepatic cirrhosis, atherosclerotic cardiovascular disease, myocardial infarction, chronic pancreatitis, and sepsis. In the brain, opportunistic infections (i.e., cytomegalovirus [CMV] infection, cryptococcosis, progressive multifocal leukoencephalopathy [PML], and toxoplasmosis) were found in 32 (22.7%), primary non-Hodgkin lymphomas in 4 (2.8%), secondary non-Hodgkin lymphomas in 5 (3.5%), and metastatic carcinomas in 2 (1.4%) of 141 cases examined.
Antiretroviral treatment
In each autopsy case, individual drug components of the ARV regimen used at the last follow-up visit closest to death were recorded within a median of 10.2 weeks (IQR 27.5 weeks) prior to death. In our study, HAART was defined as regimens containing three or more ARV medications from at least two different drug classes.
Of 144 cases, 64 (44.4%) were on HAART regimens, 11 (7.6%) were on non-HAART regimens, 51 (35.4%) were no longer on ARV treatment, and 18 (12.5%) were ARV naive at their last visit. Of those 64 cases on HAART, 51 (79.7%) received protease inhibitor (PI)-based regimens. None of the non-HAART regimens contained PIs. Accordingly, our association analyses focused on PI exposure. The HAART status was categorized into PI-based, non-PI-based, and no HAART (i.e., non-HAART regimens, discontinuation of ARV treatment, and being ARV naive).
In the entire follow-up period, the median on-ARV duration (i.e., in each case the estimated total duration of exposure to any ARV regimens) was 1.5 years (IQR 5.2 years, n=132). The median off-ARV duration (i.e., in each case the HIV infection duration subtracted by the on-ARV duration) was 10.0 years (IQR 9.3, n=106).
Neurobehavioral assessment
Of 144 cases, 123 (85.4%) underwent comprehensive neuropsychological testing at a median of 25.3 weeks (IQR 35.4 weeks) prior to death. Seven domains of neurocognitive functioning were assessed: information processing speed, attention/working memory, learning, delayed recall, verbal fluency, abstraction/executive functioning, and motor/psychomotor speed, with statistical correction for demographic variables (i.e., age, sex, ethnicity, and education) [45]. Both the number and severity of deficits across the neuropsychological battery were considered. A clinical diagnosis of HAND was made according to standard criteria requiring at least mild impairment of at least two domains of neurocognitive functioning [46]. The neurocognitive diagnoses were distributed as follows: normal cognition (n=28, 22.8% of 123 cases), ANI (n=11, 8.9%), MND (n=16, 13.0%), and HAD (n=13, 10.6%). Also, there were 40 cases (32.5%) having neuropsychological impairment due to other causes (such severe neurological comorbidities that could not be ruled out as the primary cause of any neurocognitive impairment) and 15 cases (12.2%) whose diagnoses were inconclusive based on insufficient data; these cases were excluded from statistical analysis.
Histopathology
To systematically evaluate pathologic changes, the neuropathologist (V.S.), who was blinded to clinical and autopsy information, reviewed hematoxylin and eosin (H&E)-stained paraffin-embedded formalin-fixed tissue slides from the following brain regions: frontal (Brodmann’s area [BA] 8 and BA4), parietal (BA1–3 and BA7), temporal (BA21–22), hippocampus, basal ganglia (anterior and posterior), anterior cingulate and corpus callosum, occipital (BA17–18), hemispheric cerebellum, midbrain, pons, and medulla.
The present study focused on the following vessel pathologic changes. CSVD was defined as concentric intramural hyalinization of small arteries or arterioles in the forebrain WM, excluding those vessels in the ependymal vicinity. CSVD was graded as absent (normal), mild (partial-thickness involvement), or moderate/severe (full-thickness involvement with or without perivascular space dilatation) [42, 47]; in each case a grade of CSVD was assigned based on the severest lesion (Fig. 1). Vessel mineralization was defined as intramural deposition of basophilic amorphous material in small and medium-sized arteries [48, 49]. Perivascular hemosiderin denoted old perivascular leakage of blood [47, 50]. Atherosclerotic large vessel disease was not included because its severity was assessed on gross brain examination by the pathologist at each participating medical center in the CNTN, which was subject to inter-observer variation.
Fig. 1. Histopathology of cerebral small vessel disease (CSVD) in the forebrain white matter of HIV-infected adults.
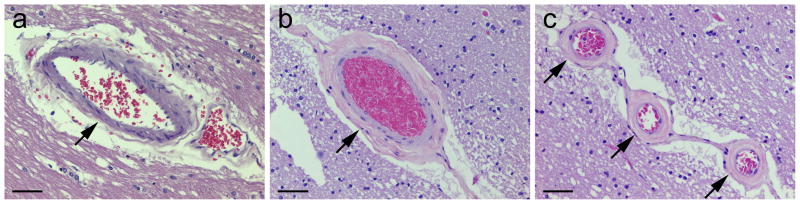
On hematoxylin and eosin staining, CSVD is defined as concentric intramural hyalinization of small arteries or arterioles and graded as absent [normal, with intactness of vascular smooth muscle cells] (a, arrow), mild [partial-thickness involvement] (b, arrow), or moderate/severe [full-thickness involvement] (c, arrows); scale bars 50 μm.
We examined the following parenchymal pathologic changes related to HIV infection [51]. HIV encephalitis was defined as multiple foci of microgliosis, multinucleated giant cells, and astrocytosis [52]. Microglial nodular lesions were defined as scattered microglial nodules in the absence of specific etiologies such as CMV infection, HIV encephalitis, and toxoplasmosis. WM lesions were defined as foci of myelin pallor with macrophage infiltration or astrocytosis in the forebrain in the absence of HIV encephalitis and PML. Acute pathologic changes, such as hypoxic-ischemic or hemorrhagic lesions and bacterial microabscesses, were not taken into account, as they were likely associated with conditions occurring in the period close to death.
Statistical analysis
We used nominal and ordinal logistic regression methods to test associations between categorical outcomes and predictors or covariates. For some categories with low frequencies, Firth’s penalized-likelihood approximation was employed to achieve reasonable estimates. Following univariable analyses, the multivariable analyses were performed as appropriate. The odds ratio (OR) with its 95% confidence interval (CI) was used to reflect the effect size. The two-tailed P value was considered significant at a threshold of P<0.05. The statistical analyses were performed using R (version 3.0.1, 2013, http://www.r-project.org).
Results
HAART and brain vessel pathology (Table 1)
Table 1.
HAART and covariates in relation to brain vessel pathology.a
| n (%) | Cerebral small vessel disease (CSVD) | Vessel mineralization | Perivascular hemosiderin | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Moderate/severe | Mild | Absent | Present | Absent | Present | Absent | |
| Overall | 65 (47.4) | 34 (24.8) | 38 (27.7) | 47 (34.6) | 89 (65.4) | 105 (75.0) | 35 (25.0) |
| HAART status | |||||||
| PI-based | OR 2.2d | OR 3.1*d | OR 0.8 | OR 1.2 | |||
| 24 (48.0) | 17 (34.0) | 9 (18.0) | 15 (30.6) | 34 (69.4) | 38 (76.0) | 12 (24.0) | |
| Non-PI-based | OR 3.2 | OR 1.0 | OR 1.9 | OR 2.1 | |||
| 9 (75.0) | 1 (8.3) | 2 (16.7) | 6 (50.0) | 6 (50.0) | 11 (84.6) | 2 (15.4) | |
| No HAARTb | 32 (42.7) | 16 (21.3) | 27 (36.0) | 26 (34.7) | 49 (65.3) | 56 (72.7) | 21 (27.3) |
| Older agec | OR 2.6* | OR 1.5 | OR 1.2 | OR 1.6 | |||
| Yes | 27 (60.0) | 10 (22.2) | 8 (17.8) | 17 (37.8) | 28 (62.2) | 37 (80.4) | 9 (19.6) |
| No | 38 (41.3) | 24 (26.1) | 30 (32.6) | 30 (33.0) | 61 (67.0) | 68 (72.3) | 26 (27.7) |
| Male | OR 1.0 | OR 1.3 | OR 1.7 | OR 0.8 | |||
| Yes | 53 (46.9) | 29 (25.7) | 31 (27.4) | 41 (36.6) | 71 (63.4) | 86 (74.1) | 30 (25.9) |
| No | 12 (50.0) | 5 (20.8) | 7 (29.2) | 6 (25.0) | 18 (75.0) | 19 (79.2) | 5 (20.8) |
| Diabetes mellitus | OR 6.6*d | OR 2.1 | OR 0.9 | OR 1.0 | |||
| Yes | 13 (81.3) | 2 (12.5) | 1 (6.3) | 5 (31.3) | 11 (68.8) | 12 (75.0) | 4 (25.0) |
| No | 51 (43.2) | 30 (25.4) | 37 (31.4) | 40 (34.2) | 77 (65.8) | 90 (74.4) | 31 (25.6) |
| Hypertension | OR 1.6 | OR 1.1 | OR 0.8 | OR 1.3 | |||
| Yes | 22 (56.4) | 8 (20.5) | 9 (23.1) | 12 (30.8) | 27 (69.2) | 31 (77.5) | 9 (22.5) |
| No | 42 (44.2) | 24 (25.3) | 29 (30.5) | 33 (35.1) | 61 (64.9) | 71 (73.2) | 26 (26.8) |
| Dyslipidemia | OR 0.9 | OR 1.2 | OR 0.4 | OR 1.1 | |||
| Yes | 9 (42.9) | 6 (28.6) | 6 (28.6) | 4 (20.0) | 16 (80.0) | 16 (76.2) | 5 (23.8) |
| No | 55 (48.7) | 26 (23.0) | 32 (28.3) | 41 (36.3) | 72 (63.7) | 86 (74.1) | 30 (25.9) |
| Cigarette smoking | OR 1.2 | OR 1.1 | OR 0.6 | OR 0.8 | |||
| Yes | 22 (48.9) | 11 (24.4) | 12 (26.7) | 11 (25.0) | 33 (75.0) | 32 (71.1) | 13 (28.9) |
| No | 40 (46.0) | 21 (24.1) | 26 (29.9) | 33 (37.9) | 54 (62.1) | 69 (76.7) | 21 (23.3) |
| Psychostimulant dependence | OR 0.4 | OR 0.7 | OR 0.7 | OR 1.8 | |||
| Yes | 6 (33.3) | 6 (33.3) | 6 (33.3) | 5 (27.8) | 13 (72.2) | 16 (84.2) | 3 (15.8) |
| No | 24 (51.1) | 14 (29.8) | 9 (19.1) | 17 (36.2) | 30 (63.8) | 36 (75.0) | 12 (25.0) |
| Hepatitis C virus infection | OR 1.9 | OR 0.8 | OR 0.9 | OR 1.2 | |||
| Yes | 27 (56.3) | 9 (18.8) | 12 (25.0) | 16 (32.7) | 33 (67.3) | 39 (78.0) | 11 (22.0) |
| No | 26 (38.2) | 20 (29.4) | 22 (32.4) | 23 (34.8) | 43 (65.2) | 52 (75.4) | 17 (24.6) |
HAART, highly active antiretroviral therapy; PI, protease inhibitor; OR, odds ratio.
Odds ratios were calculated by using univariable logistic regression methods in relation to ‘Absent’ outcomes (in columns) and ‘No’ predictors/covariates (in rows), *two-tailed P<0.05.
Included in the ‘No HAART’ category were patients who received non-HAART regimens or no longer received antiretroviral treatment at their last follow-up visit closest to death, and those who had never received antiretroviral treatment.
Age at death ≥50 years.
In the multivariable logistic regression analysis (Model: CSVD = HAART + Diabetes mellitus), both mild and moderate/severe CSVD were associated with PI-based HAART exposure after adjusting for diabetes (OR 2.8 and 2.6, respectively, P=0.04, both). Moderate/severe CSVD was associated with diabetes after adjusting for HAART exposure (OR 7.4, P=0.007).
Cerebral small vessel disease
Mild CSVD was present in 24.8% and moderate/severe CSVD in 47.4% of 137 cases examined. In univariable logistic regression analyses, PI-based HAART (relative to no HAART) was associated with higher likelihood of mild CSVD [relative to absent] (OR 3.1 [95% CI 1.2, 8.6], P=0.02), and the similar trend of moderate/severe CSVD approached statistical significance (OR 2.2 [95% CI 0.9, 5.6], P=0.08). Non-PI-based HAART was not significantly associated with either mild or moderate/severe CSVD (OR 1.0 [95% CI 0.1, 8.2] and 3.2 [95% CI 0.8, 17.9], P=1.0 and =0.1, respectively). Among potential covariates analyzed, older age and diabetes were associated with moderate/severe CSVD (OR 2.6 [95% CI 1.1, 6.6] and 6.6 [95% CI 1.5, 61.8], P=0.04 and =0.01, n=137 and =134, respectively). There were no significant associations between mild CSVD and any of the covariates (P>0.4). Race/ethnicity was not significantly associated with either mild or moderate/severe CSVD (overall P=0.1).
The multivariable analysis built using those variables found to be significant in univariable analyses (Model: CSVD = HAART + Older age + Diabetes + Hypertension, n=134) showed trends toward higher likelihood of mild and moderate/severe CSVD with PI-based HAART after adjusting for older age, diabetes, and hypertension (OR 2.7 [95% CI 1.0, 7.7] and 2.4 [95% CI 1.0, 6.2], P=0.05 and =0.06, respectively). Non-PI-based HAART was not significantly associated with either mild or moderate/severe CSVD after adjusting for older age, diabetes, and hypertension (P=0.9 and =0.2, respectively). Diabetes was associated with higher likelihood of moderate/severe CSVD after adjusting for HAART, older age, and hypertension (OR 5.7 [95% CI 1.2, 55.2], P=0.02), but not with mild CSVD (P=0.5). In contrast, older age was not significantly associated with either mild or moderate/severe CSVD after adjusting for HAART, diabetes, and hypertension (P=0.6 and =0.2, respectively), nor was hypertension after adjusting for HAART, older age, and diabetes (P=0.8, both). Therefore, older age and hypertension were excluded from the multivariable model.
In the multivariable analysis (Model: CSVD = HAART + Diabetes, n=134), PI-based HAART was associated with higher likelihood of mild and moderate/severe CSVD after adjusting for diabetes (OR 2.8 [95% CI 1.03, 7.9] and 2.6 [95% CI 1.03, 6.7], respectively, P=0.04, both). Non-PI-based HAART was not significantly associated with either mild or moderate/severe CSVD after adjusting for diabetes (P=1.0 and =0.1, respectively). Diabetes was associated with higher likelihood of moderate/severe CSVD after adjusting for HAART (OR 7.4 [95% CI 1.6, 70.7], P=0.007), but not with mild CSVD (P=0.4).
Vessel mineralization
Vessel mineralization in the globus pallidus was identified in 47 (34.6%) of 136 cases, in seven of which it was also present in other brain regions including the cerebellar WM, thalamus, hippocampus, dentate gyrus, and cerebral peduncle. The presence of vessel mineralization was not significantly associated with that of CSVD (χ2=0.17, df=2, P=0.9, chi-square test for independence). In univariable logistic regression analyses, no significant associations were found between vessel mineralization and PI-based or non-PI-based HAART (P=0.6 and =0.3, respectively) and the covariates (P>0.13).
Perivascular hemosiderin
Of 140 cases examined, perivascular hemosiderin was found in 105 (75%). In univariable logistic regression analyses, no significant associations were found between perivascular hemosiderin and PI-based or non-PI-based HAART (P=0.7 and =0.4, respectively) and the covariates (P>0.29).
HAART and brain parenchymal pathology (Table 2)
Table 2.
HAART and covariates in relation to brain parenchymal pathology.a
| n (%) | HIV encephalitis | Microglial nodular lesions | White matter lesions | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Present | Absent | Present | Absent | Present | Absent | |
| Overall | 22 (15.6) | 119 (84.4) | 12 (8.5) | 129 (91.5) | 17 (12.1) | 124 (87.9) |
| HAART status | ||||||
| PI-based | OR 1.0 | OR 1.6 | OR 1.8 | |||
| 8 (15.7) | 43 (84.3) | 6 (11.8) | 45 (88.2) | 9 (17.6) | 42 (82.4) | |
| Non-PI-based | OR 1.0 | OR 0.4 | OR 0.3 | |||
| 2 (15.4) | 11 (84.6) | 0 (0.0) | 13 (100.0) | 0 (0.0) | 13 (100.0) | |
| No HAARTb | 12 (15.6) | 65 (84.4) | 6 (7.8) | 71 (92.2) | 8 (10.4) | 69 (89.6) |
| Older agec | OR 0.3* | OR 0.2 | OR 2.0 | |||
| Yes | 3 (6.5) | 43 (93.5) | 1 (2.2) | 45 (97.8) | 8 (17.4) | 38 (82.6) |
| No | 19 (20.0) | 76 (80.0) | 11 (11.6) | 84 (88.4) | 9 (9.5) | 86 (90.5) |
| Male | OR 2.3 | OR 2.4 | OR 1.6 | |||
| Yes | 20 (17.1) | 97 (82.9) | 11 (9.4) | 106 (90.6) | 15 (12.8) | 102 (87.2) |
| No | 2 (8.3) | 22 (91.7) | 1 (4.2) | 23 (95.8) | 2 (8.3) | 22 (91.7) |
| Diabetes mellitus | OR 0.4 | OR 0.7 | OR 0.5 | |||
| Yes | 1 (6.3) | 15 (93.8) | 1 (6.3) | 15 (93.8) | 1 (6.3) | 15 (93.8) |
| No | 19 (15.6) | 103 (84.4) | 11 (9.0) | 111 (91.0) | 15 (12.3) | 107 (87.7) |
| Hypertension | OR 0.8 | OR 1.3 | OR 1.1 | |||
| Yes | 5 (12.5) | 35 (87.5) | 4 (10.0) | 36 (90.0) | 5 (12.5) | 35 (87.5) |
| No | 15 (15.3) | 83 (84.7) | 8 (8.2) | 90 (91.8) | 11 (11.2) | 87 (88.8) |
| Dyslipidemia | OR 1.5 | OR 1.1 | OR 0.8 | |||
| Yes | 4 (19.0) | 17 (81.0) | 2 (9.5) | 19 (90.5) | 2 (9.5) | 19 (90.5) |
| No | 16 (13.7) | 101 (86.3) | 10 (8.5) | 107 (91.5) | 14 (12.0) | 103 (88.0) |
| Cigarette smoking | OR 0.9 | OR 0.4 | OR 0.9 | |||
| Yes | 6 (13.3) | 39 (86.7) | 2 (4.4) | 43 (95.6) | 5 (11.1) | 40 (88.9) |
| No | 14 (15.4) | 77 (84.6) | 10 (11.0) | 81 (89.0) | 11 (12.1) | 80 (87.9) |
| Psychostimulant dependence | OR 0.8 | OR 1.3 | OR 1.1 | |||
| Yes | 2 (10.5) | 17 (89.5) | 1 (5.3) | 18 (94.7) | 3 (15.8) | 16 (84.2) |
| No | 6 (12.5) | 42 (87.5) | 2 (4.2) | 46 (95.8) | 7 (14.6) | 41 (85.4) |
| Hepatitis C virus infection | OR 0.8 | OR 0.3 | OR 0.4 | |||
| Yes | 8 (16.0) | 42 (84.0) | 2 (4.0) | 48 (96.0) | 3 (6.0) | 47 (94.0) |
| No | 13 (18.6) | 57 (81.4) | 9 (12.9) | 61 (87.1) | 10 (14.3) | 60 (85.7) |
HAART, highly active antiretroviral therapy; PI, protease inhibitor; OR, odds ratio.
Odds ratios were calculated by using univariable logistic regression methods in relation to ‘Absent’ outcomes (in columns) and ‘No’ predictors/covariates (in rows), *two-tailed P<0.05.
Included in the ‘No HAART’ category were patients who received non-HAART regimens or no longer received antiretroviral treatment at their last follow-up visit closest to death, and those who had never received antiretroviral treatment.
Age at death ≥50 years.
Of 141 cases examined, HIV encephalitis was present in 22 (15.6%), microglial nodular lesions in 12 (8.5%), and WM lesions in 17 (12.1%). In univariable logistic regression analyses, there were no significant associations between each of the parenchymal pathologic changes and PI-based or non-PI-based HAART (P>0.23 and >0.34, respectively). Among potential covariates analyzed, older age was associated with lower likelihood of HIV encephalitis (OR 0.3 [95% CI 0.1, 0.9], P=0.03, n=141), which remained significant after adjusting for HAART (P=0.049); there was no significant interaction effect between older age and HAART (P=0.8). No other significant associations were found between the parenchymal pathologic changes and any of the covariates (P>0.08).
Brain vessel or parenchymal pathology and HAND (Table 3)
Table 3.
Brain vessel or parenchymal pathology and covariates in relation to HAND.
| n (%) | HAND | Normal cognition | Odds ratio (OR)a |
|---|---|---|---|
|
|
|||
| Overallb | 40 (58.8) | 28 (41.2) | |
| Cerebral small vessel disease (CSVD) | |||
| Moderate/severe | 16 (57.1) | 12 (42.9) | 1.7 |
| Mild | 15 (78.9) | 4 (21.1) | 4.8* |
| Absent | 7 (43.8) | 9 (56.3) | |
| Vessel mineralization | |||
| Present | 14 (66.7) | 7 (33.3) | 1.4 |
| Absent | 24 (58.5) | 17 (41.5) | |
| Perivascular hemosiderin | |||
| Present | 30 (58.8) | 21 (41.2) | 0.8 |
| Absent | 9 (64.3) | 5 (35.7) | |
| HIV encephalitis | |||
| Present | 10 (66.7) | 5 (33.3) | 1.4 |
| Absent | 30 (58.8) | 21 (41.2) | |
| Microglial nodular lesions | |||
| Present | 3 (60.0) | 2 (40.0) | 1.0 |
| Absent | 37 (60.7) | 24 (39.3) | |
| White matter lesions | |||
| Present | 8 (66.7) | 4 (33.3) | 1.4 |
| Absent | 32 (59.3) | 22 (40.7) | |
| Older age at death (≥50 years) | |||
| Yes | 10 (58.8) | 7 (41.2) | 1.0 |
| No | 30 (58.8) | 21 (41.2) | |
| Male | |||
| Yes | 32 (59.3) | 22 (40.7) | 1.1 |
| No | 8 (57.1) | 6 (42.9) | |
| Hepatitis C virus infection | |||
| Yes | 14 (63.6) | 8 (36.4) | 1.5 |
| No | 21 (53.8) | 18 (46.2) | |
HAND, HIV-associated neurocognitive disorders.
Odds ratios were calculated by using univariable logistic regression methods in relation to ‘Normal cognition’ outcomes (in columns) and ‘Absent’ predictors or ‘No’ covariates (in rows), *two-tailed P<0.05.
In 123 HIV-infected patients who underwent comprehensive neuropsychological testing, the neurocognitive diagnoses were distributed as follows: normal cognition (n=28, 22.8%), asymptomatic neuropsychological impairment (n=11, 8.9%), mild neurocognitive disorder (n=16, 13.0%), and HIV-associated dementia (n=13, 10.6%). Also, there were 40 patients (32.5%) having neuropsychological impairment due to other causes (such severe neurological comorbidities that could not be ruled out as the primary cause of any neurocognitive impairment) and 15 patients (12.2%) whose diagnoses were inconclusive based on insufficient data; these cases were excluded from the statistical analysis of HAND.
In univariable logistic regression analyses, mild CSVD (relative to absent) was associated with higher likelihood of HAND [relative to normal cognition] (OR 4.8 [95% CI 1.1, 21.2], P=0.04, n=63). In multivariable analyses (Model: HAND = CSVD + single predictor/covariate), the direct association between mild CSVD and HAND remained significant after adjusting for vessel mineralization (OR 6.7 [95% CI 1.3, 34.0], P=0.02, n=61), HIV encephalitis (OR 4.6 [95% CI 1.03, 20.6], P=0.045, n=63), microglial nodular lesions (OR 5.1 [95% CI 1.1, 22.7], P=0.03, n=63), WM lesions (OR 4.8 [95% CI 1.1, 21.3], P=0.04, n=63), or older age (OR 4.8 [95% CI 1.1, 21.2], P=0.04, n=63). The interaction effects of CSVD and each of the pathologic changes (i.e., vessel mineralization, HIV encephalitis, and WM lesions) or older age on HAND were not statistically significant (P>0.58) and thereby were not included in the above multivariable models. For microglial nodular lesions, the data were insufficient for interaction analysis.
The apparent association between moderate/severe CSVD (relative to absent) and higher likelihood of HAND (relative to normal cognition) did not reach statistical significance in either univariable (P=0.4) or multivariable (P>0.36) analyses. There were no significant associations between HAND and the pathologic changes or covariates other than CSVD, in either univariable (P>0.4) or multivariable analyses after adjusting for CSVD (P>0.3).
Potential influence of HIV infection, on-ARV or off-ARV duration
In univariable logistic regression analyses, HIV encephalitis was associated with longer off-ARV duration (OR 1.1 [95% CI 1.02, 1.3] per one-year increase in duration, P=0.02, n=103) but not with either HIV infection or on-ARV duration (P=0.2, both). The HIV infection duration was not significantly associated with mild or moderate/severe CSVD, vessel mineralization, perivascular hemosiderin, microglial nodular lesions, or white matter lesions (P>0.2), nor was the on-ARV (P>0.26) or off-ARV (P>0.14) duration. The likelihood of HAND (relative to normal cognition) was lower in association with longer on-ARV duration (OR 0.8 [95% CI 0.7, 0.97] per one-year increase in duration, P=0.02, n=60) but not associated with either HIV infection (P=0.9) or off-ARV (P=0.2) duration.
Discussion
Generally, CSVD is associated with diabetes, hypertension, and aging [42]. In our cohort of HIV-infected adults, we found that moderate/severe CSVD was associated particularly with diabetes. In order to determine associations between CSVD and neurocognitive functioning, we considered only CSVD found within the forebrain. Additionally, to avoid a diagnostic challenge in differentiating CSVD from cerebral amyloid angiopathy, primarily affecting vessels in the leptomeninges and neocortex [53], on H&E-stained brain sections, only CSVD found in the WM was taken into account.
Of chief importance, we found direct associations between PI-based HAART exposure and higher likelihood of both mild and moderate/severe CSVD after statistically adjusting for diabetes. While the mechanisms remain unclear, it is possible that in the context of HIV infection, some drug components of PI-based HAART may be toxic to the cellular components of cerebral small vessels, such as vascular endothelial cells [54, 55] and smooth muscle cells, leading to vessel wall degeneration (i.e., CSVD). It is also possible that some drugs in PI-based HAART contribute indirectly to CSVD by inducing metabolic abnormalities such as dyslipidemia and insulin resistance, as they have been implicated in the premature development of atherosclerotic large vessel disease [27, 28]. Nonetheless, in our study there was no significant association between dyslipidemia and CSVD. Diabetes was found to be associated with moderate/severe CSVD even after statistically adjusting for HAART exposure, older age, and hypertension.
The clinical significance of CSVD in our study was substantiated by the finding that mild CSVD was associated with HAND even after statistically adjusting for vessel mineralization, HIV encephalitis, microglial nodular lesions, WM lesions, or older age. Nonetheless, with moderate/severe CSVD the apparent trend toward higher likelihood of HAND did not reach statistical significance. It is possible that a subset of neurocognitively impaired HIV-infected patients who concurrently had neurological or imaging evidence of cerebrovascular disease were not diagnosed with HAND by definitional criteria but were instead included, together with those having other neurological comorbidities (e.g., history of moderate or severe head injury, history of learning disability, brain opportunistic diseases, and severe substance use disorders), in a category of neuropsychological impairment due to other causes [46].
Vessel mineralization found primarily in the globus pallidus was consistently reported in pre-HAART AIDS autopsy cohorts [48, 49]. In contrast to CSVD, there was no significant association between vessel mineralization and HAART exposure in our cohort. Additionally, the presence of vessel mineralization was not significantly associated with that of CSVD. It is possible that vessel mineralization is pathophysiologically different from CSVD, in which the former may be mediated by the HIV-related effects on vessel walls in a brain region-specific manner, as HIV may directly infect vascular smooth muscle cells [56].
Our study did not find that HAART exposure was associated with lower likelihood of HIV encephalitis. It is possible that a subset of HIV-infected patients was failing ARV treatment (e.g., drug resistance and compliance issues), was consequently in advanced stages of immune suppression, developed HIV encephalitis, and died shortly afterward. Accordingly, HIV encephalitis was found at autopsy with no significant association with HAART status. In contrast, it may be that those patients who were able to longer maintain the benefit of HAART were less likely to develop HIV encephalitis, lived to older age, and eventually died with pathologic entities other than HIV encephalitis. In support of this scenario, we found lower likelihood of HIV encephalitis in patients who died at older age, even after statistically adjusting for HAART exposure.
In our study there was no significant association between HAND and HIV encephalitis, in agreement with other recent autopsy studies [11, 13]. Patients could develop HAND, particularly ANI and MND, even when their systemic HIV infection and immune function remain under control [3, 8, 9], whereas HIV encephalitis is characteristically associated with advanced immune suppression. In other words, a significant proportion of HAND is associated with pathophysiological sequences or pathologic changes in the brain other than HIV encephalitis. It is likely that CSVD associated with PI-based HAART exposure could occur before advanced immune suppression takes place. We found that mild CSVD was associated with HAND.
We did not find any significant association between non-PI-based HAART exposure and higher likelihood of CSVD. However, we could not entirely exclude the possible effect of non-PI-based HAART on the development of CSVD, given that the number of cases in this HAART category was relatively small.
Our study analyzed the effects of HAART regimens, but not individual drugs due to sample size issues. Nonetheless, we were aware that different ARV drugs even in the same class might carry differential degrees of toxicity on cerebral small vessels. This matter in question can be addressed in future studies using experimental animals [57] and in vitro cell systems [54]. Also, the toxic effects of ARV drugs might vary with the duration of drug use and drug metabolism. We found the estimated total duration of exposure to any ARV regimens in the entire follow-up period was not significantly associated with either mild or moderate/severe CSVD. Unfortunately, available data on the duration of each ARV regimen used were not sufficient for analysis.
Among HIV-infected patients studied, there might be significant variations in the duration of HIV infection and systemic viral burden, which might influence the development of CSVD as some investigators proposed that HIV might directly infect vascular smooth muscle cells [56]. In our study, the estimated duration of HIV infection was not significantly associated with either mild or moderate/severe CSVD. Data on plasma viral load were not sufficient for analysis. Additionally, whether HIV infection per se conferred a greater risk of CSVD was not addressed in our study because the number of HIV-seronegative autopsy cases was insufficient for analysis.
In conclusion, our clinicopathological study of HIV-infected adults in the CNTN revealed direct associations between PI-based HAART exposure and higher likelihood of both mild and moderate/severe CSVD after statistically adjusting for diabetes. Of clinical significance, mild CSVD was associated with HAND. These findings suggest that PI-based HAART exposure increases the risk of CSVD and thereby neurocognitive impairment. Some drug components of PI-based HAART may be toxic to vascular endothelium and smooth muscle cells, leading to vessel wall degeneration. Of particular interest are further studies on molecular mechanisms of ARV toxicity to the cell components of cerebral vessels and the identification of potential biomarkers for CSVD in HIV-infected patients. For instance, specific ARV drugs or drug combinations may affect the integrity and function of cerebral vessels by inducing premature senescence of vascular endothelium and smooth muscle cells [54, 58].
Acknowledgments
Source of Funding
This work was supported by the U.S. National Institutes of Health grants U24 MH100928 (California NeuroAIDS Tissue Network: I.G., C.L.A., R.J.E., D.J.M., A.U., B.G., W.T.), P50 DA026306 (I.G., C.L.A., R.J.E., A.U., V.S.), P30 MH062512 (I.G., C.L.A.), R01 MH096648 (D.J.M., C.L.A., V.S.), R01 MH097589 (E.M., C.L.A., V.S.), R01 MH094159 (C.L.A., B.S.), and R25 MH081482 (W.T.); the Don & Marilyn Short Fellowship in Parkinson’s Disease (V.S.); and the Medical Student Training in Research on Aging and Mental Disorders program (S.A.C, M.L.C.).
We are grateful to Dr. Scott L. Letendre for his advice on the analysis of antiretroviral treatment, as well as Craig Gibson, Wilmar Dumaop, and Andrea Bucci for their technical assistance. We thank attending neuropathologists at the participating medical centers in the CNTN: Drs William H. Yong (Cedars-Sinai), Marcia E. Cornford (Harbor-University of California Los Angeles, and Ronald C. Kim (University of California Irvine).
Footnotes
Conflicts of Interest
There were no conflicts of interest.
V.S. reviewed the literature, designed the study, performed histopathologic examination, interpreted the results, and wrote the first article draft. A.U. performed statistical analyses. S.A.C., M.L.C., B.S., and W.T. conducted tissue slide and data management. D.J.M. and E.M. provided diagnostic characterizations of HIV-infected cases. B.G. managed the patients’ database. I.G., R.J.E., and C.L.A. supervised the study design and result interpretation. All authors contributed to the article and approved the final article.
References
- 1.Price RW, Spudich S. Antiretroviral therapy and central nervous system HIV type 1 infection. J Infect Dis. 2008;197 (Suppl 3):S294–306. doi: 10.1086/533419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nath A, Schiess N, Venkatesan A, Rumbaugh J, Sacktor N, McArthur J. Evolution of HIV dementia with HIV infection. Int Rev Psychiatry. 2008;20:25–31. doi: 10.1080/09540260701861930. [DOI] [PubMed] [Google Scholar]
- 4.Wiley CA, Achim C. Human immunodeficiency virus encephalitis is the pathological correlate of dementia in acquired immunodeficiency syndrome. Ann Neurol. 1994;36:673–676. doi: 10.1002/ana.410360422. [DOI] [PubMed] [Google Scholar]
- 5.Cherner M, Masliah E, Ellis RJ, Marcotte TD, Moore DJ, Grant I, et al. Neurocognitive dysfunction predicts postmortem findings of HIV encephalitis. Neurology. 2002;59:1563–1567. doi: 10.1212/01.wnl.0000034175.11956.79. [DOI] [PubMed] [Google Scholar]
- 6.Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- 7.Anderson E, Zink W, Xiong H, Gendelman HE. HIV-1-associated dementia: a metabolic encephalopathy perpetrated by virus-infected and immune-competent mononuclear phagocytes. J Acquir Immune Defic Syndr. 2002;31 (Suppl 2):S43–54. doi: 10.1097/00126334-200210012-00004. [DOI] [PubMed] [Google Scholar]
- 8.Brew BJ. Evidence for a change in AIDS dementia complex in the era of highly active antiretroviral therapy and the possibility of new forms of AIDS dementia complex. AIDS. 2004;18 (Suppl 1):S75–78. [PubMed] [Google Scholar]
- 9.Cysique LA, Brew BJ. Prevalence of non-confounded HIV-associated neurocognitive impairment in the context of plasma HIV RNA suppression. J Neurovirol. 2011;17:176–183. doi: 10.1007/s13365-011-0021-x. [DOI] [PubMed] [Google Scholar]
- 10.Grant I. Neurocognitive disturbances in HIV. Int Rev Psychiatry. 2008;20:33–47. doi: 10.1080/09540260701877894. [DOI] [PubMed] [Google Scholar]
- 11.Everall I, Vaida F, Khanlou N, Lazzaretto D, Achim C, Letendre S, et al. Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J Neurovirol. 2009;15:360–370. doi: 10.3109/13550280903131915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelman BB, Chen T, Lisinicchia JG, Soukup VM, Carmical JR, Starkey JM, et al. The National NeuroAIDS Tissue Consortium brain gene array: two types of HIV-associated neurocognitive impairment. PLoS One. 2012;7:e46178. doi: 10.1371/journal.pone.0046178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelman BB, Lisinicchia JG, Morgello S, Masliah E, Commins D, Achim CL, et al. Neurovirological correlation with HIV-associated neurocognitive disorders and encephalitis in a HAART-era cohort. J Acquir Immune Defic Syndr. 2013;62:487–495. doi: 10.1097/QAI.0b013e31827f1bdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marra CM, Zhao Y, Clifford DB, Letendre S, Evans S, Henry K, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23:1359–1366. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soontornniyomkij V, Achim CL. Aging. In: Gendelman HE, editor. The Neurology of AIDS. New York: Oxford University Press; 2012. pp. 567–580. [Google Scholar]
- 16.Soontornniyomkij V, Moore DJ, Gouaux B, Soontornniyomkij B, Tatro ET, Umlauf A, et al. Cerebral β-amyloid deposition predicts HIV-associated neurocognitive disorders in APOE ε4 carriers. AIDS. 2012;26:2327–2335. doi: 10.1097/QAD.0b013e32835a117c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherner M, Letendre S, Heaton RK, Durelle J, Marquie-Beck J, Gragg B, et al. Hepatitis C augments cognitive deficits associated with HIV infection and methamphetamine. Neurology. 2005;64:1343–1347. doi: 10.1212/01.WNL.0000158328.26897.0D. [DOI] [PubMed] [Google Scholar]
- 18.Letendre SL, Cherner M, Ellis RJ, Marquie-Beck J, Gragg B, Marcotte T, et al. The effects of hepatitis C, HIV, and methamphetamine dependence on neuropsychological performance: biological correlates of disease. AIDS. 2005;19 (Suppl 3):S72–78. doi: 10.1097/01.aids.0000192073.18691.ff. [DOI] [PubMed] [Google Scholar]
- 19.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr. 2012;60:351–358. doi: 10.1097/QAI.0b013e31825c7f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruse B, Cysique LA, Markus R, Brew BJ. Cerebrovascular disease in HIV-infected individuals in the era of highly active antiretroviral therapy. J Neurovirol. 2012;18:264–276. doi: 10.1007/s13365-012-0092-3. [DOI] [PubMed] [Google Scholar]
- 21.Ortiz G, Koch S, Romano JG, Forteza AM, Rabinstein AA. Mechanisms of ischemic stroke in HIV-infected patients. Neurology. 2007;68:1257–1261. doi: 10.1212/01.wnl.0000259515.45579.1e. [DOI] [PubMed] [Google Scholar]
- 22.Ovbiagele B, Nath A. Increasing incidence of ischemic stroke in patients with HIV infection. Neurology. 2011;76:444–450. doi: 10.1212/WNL.0b013e31820a0cfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Connor MD, Lammie GA, Bell JE, Warlow CP, Simmonds P, Brettle RD. Cerebral infarction in adult AIDS patients: observations from the Edinburgh HIV autopsy cohort. Stroke. 2000;31:2117–2126. doi: 10.1161/01.str.31.9.2117. [DOI] [PubMed] [Google Scholar]
- 24.Rasmussen LD, Engsig FN, Christensen H, Gerstoft J, Kronborg G, Pedersen C, et al. Risk of cerebrovascular events in persons with and without HIV: a Danish nationwide population-based cohort study. AIDS. 2011;25:1637–1646. doi: 10.1097/QAD.0b013e3283493fb0. [DOI] [PubMed] [Google Scholar]
- 25.Benjamin LA, Bryer A, Emsley HC, Khoo S, Solomon T, Connor MD. HIV infection and stroke: current perspectives and future directions. Lancet Neurol. 2012;11:878–890. doi: 10.1016/S1474-4422(12)70205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singer EJ, Valdes-Sueiras M, Commins DL, Yong W, Carlson M. HIV stroke risk: evidence and implications. Ther Adv Chronic Dis. 2013;4:61–70. doi: 10.1177/2040622312471840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grunfeld C. Dyslipidemia and its treatment in HIV infection. Top HIV Med. 2010;18:112–118. [PMC free article] [PubMed] [Google Scholar]
- 28.Worm SW, Sabin C, Weber R, Reiss P, El-Sadr W, Dabis F, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010;201:318–330. doi: 10.1086/649897. [DOI] [PubMed] [Google Scholar]
- 29.d’Arminio A, Sabin CA, Phillips AN, Reiss P, Weber R, Kirk O, et al. Cardio- and cerebrovascular events in HIV-infected persons. AIDS. 2004;18:1811–1817. doi: 10.1097/00002030-200409030-00010. [DOI] [PubMed] [Google Scholar]
- 30.Corral I, Quereda C, Moreno A, Pérez-Elías MJ, Dronda F, Casado JL, et al. Cerebrovascular ischemic events in HIV-1-infected patients receiving highly active antiretroviral therapy: incidence and risk factors. Cerebrovasc Dis. 2009;27:559–563. doi: 10.1159/000214219. [DOI] [PubMed] [Google Scholar]
- 31.Worm SW, Kamara DA, Reiss P, Fontas E, De Wit S, El-Sadr W, et al. Evaluation of HIV protease inhibitor use and the risk of sudden death or nonhemorrhagic stroke. J Infect Dis. 2012;205:535–539. doi: 10.1093/infdis/jir788. [DOI] [PubMed] [Google Scholar]
- 32.McMurtray A, Nakamoto B, Shikuma C, Valcour V. Small-vessel vascular disease in human immunodeficiency virus infection: the Hawaii aging with HIV cohort study. Cerebrovasc Dis. 2007;24:236–241. doi: 10.1159/000104484. [DOI] [PubMed] [Google Scholar]
- 33.Ovbiagele B, Saver JL. Cerebral white matter hyperintensities on MRI: current concepts and therapeutic implications. Cerebrovasc Dis. 2006;22:83–90. doi: 10.1159/000093235. [DOI] [PubMed] [Google Scholar]
- 34.Archibald SL, Masliah E, Fennema-Notestine C, Marcotte TD, Ellis RJ, McCutchan JA, et al. Correlation of in vivo neuroimaging abnormalities with postmortem human immunodeficiency virus encephalitis and dendritic loss. Arch Neurol. 2004;61:369–376. doi: 10.1001/archneur.61.3.369. [DOI] [PubMed] [Google Scholar]
- 35.Miller RF, Lucas SB, Hall-Craggs MA, Brink NS, Scaravilli F, Chinn RJ, et al. Comparison of magnetic resonance imaging with neuropathological findings in the diagnosis of HIV and CMV associated CNS disease in AIDS. J Neurol Neurosurg Psychiatry. 1997;62:346–351. doi: 10.1136/jnnp.62.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heindel WC, Jernigan TL, Archibald SL, Achim CL, Masliah E, Wiley CA. The relationship of quantitative brain magnetic resonance imaging measures to neuropathologic indexes of human immunodeficiency virus infection. Arch Neurol. 1994;51:1129–1135. doi: 10.1001/archneur.1994.00540230067015. [DOI] [PubMed] [Google Scholar]
- 37.Filippi CG, Sze G, Farber SJ, Shahmanesh M, Selwyn PA. Regression of HIV encephalopathy and basal ganglia signal intensity abnormality at MR imaging in patients with AIDS after the initiation of protease inhibitor therapy. Radiology. 1998;206:491–498. doi: 10.1148/radiology.206.2.9457204. [DOI] [PubMed] [Google Scholar]
- 38.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 39.Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- 40.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120:287–296. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 43.Hasin DS, Trautman KD, Miele GM, Samet S, Smith M, Endicott J. Psychiatric Research Interview for Substance and Mental Disorders (PRISM): reliability for substance abusers. Am J Psychiatry. 1996;153:1195–1201. doi: 10.1176/ajp.153.9.1195. [DOI] [PubMed] [Google Scholar]
- 44.Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, et al. The Composite International Diagnostic Interview. An epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- 45.Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004;26:759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- 46.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deramecourt V, Slade JY, Oakley AE, Perry RH, Ince PG, Maurage CA, et al. Staging and natural history of cerebrovascular pathology in dementia. Neurology. 2012;78:1043–1050. doi: 10.1212/WNL.0b013e31824e8e7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anders KH, Guerra WF, Tomiyasu U, Verity MA, Vinters HV. The neuropathology of AIDS. UCLA experience and review. Am J Pathol. 1986;124:537–558. [PMC free article] [PubMed] [Google Scholar]
- 49.Gray F, Gherardi R, Keohane C, Favolini M, Sobel A, Poirier J. Pathology of the central nervous system in 40 cases of acquired immune deficiency syndrome (AIDS) Neuropathol Appl Neurobiol. 1988;14:365–380. doi: 10.1111/j.1365-2990.1988.tb01139.x. [DOI] [PubMed] [Google Scholar]
- 50.Gelman BB, Rodriguez-Wolf MG, Wen J, Kumar S, Campbell GR, Herzog N. Siderotic cerebral macrophages in the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1992;116:509–516. [PubMed] [Google Scholar]
- 51.Budka H. Neuropathology of human immunodeficiency virus infection. Brain Pathol. 1991;1:163–175. doi: 10.1111/j.1750-3639.1991.tb00656.x. [DOI] [PubMed] [Google Scholar]
- 52.Budka H, Wiley CA, Kleihues P, Artigas J, Asbury AK, Cho ES, et al. HIV-associated disease of the nervous system: review of nomenclature and proposal for neuropathology-based terminology. Brain Pathol. 1991;1:143–152. doi: 10.1111/j.1750-3639.1991.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 53.Attems J, Jellinger K, Thal DR, Van Nostrand W. Review: sporadic cerebral amyloid angiopathy. Neuropathol Appl Neurobiol. 2011;37:75–93. doi: 10.1111/j.1365-2990.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- 54.Lefèvre C, Auclair M, Boccara F, Bastard JP, Capeau J, Vigouroux C, et al. Premature senescence of vascular cells is induced by HIV protease inhibitors: implication of prelamin A and reversion by statin. Arterioscler Thromb Vasc Biol. 2010;30:2611–2620. doi: 10.1161/ATVBAHA.110.213603. [DOI] [PubMed] [Google Scholar]
- 55.Giwa MO, Williams J, Elderfield K, Jiwa NS, Bridges LR, Kalaria RN, et al. Neuropathologic evidence of endothelial changes in cerebral small vessel disease. Neurology. 2012;78:167–174. doi: 10.1212/WNL.0b013e3182407968. [DOI] [PubMed] [Google Scholar]
- 56.Eugenin EA, Morgello S, Klotman ME, Mosoian A, Lento PA, Berman JW, et al. Human immunodeficiency virus (HIV) infects human arterial smooth muscle cells in vivo and in vitro: implications for the pathogenesis of HIV-mediated vascular disease. Am J Pathol. 2008;172:1100–1111. doi: 10.2353/ajpath.2008.070457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reyskens KM, Fisher TL, Schisler JC, O’Connor WG, Rogers AB, Willis MS, et al. Cardio-metabolic effectsof HIV protease inhibitors (lopinavir/ritonavir) PLoS One. 2013;8:e73347. doi: 10.1371/journal.pone.0073347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ragnauth CD, Warren DT, Liu Y, McNair R, Tajsic T, Figg N, et al. Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation. 2010;121:2200–2210. doi: 10.1161/CIRCULATIONAHA.109.902056. [DOI] [PubMed] [Google Scholar]


