Abstract
Background and Purpose
Neurophysiological models of rehabilitation and recovery suggest that a large volume of specific practice is required to induce the neuroplastic changes that underlie behavioral recovery. The primary objective of this meta-analysis was to explore the relationship between time scheduled for therapy and improvement in motor therapy for adults post-stroke by (1) comparing high-doses to low-doses and (2) using meta-regression to further quantify the dose-response relationship.
Methods
Databases were searched to find randomized controlled trials that were not dosage matched for total time scheduled for therapy. Regression models were used to predict improvement during therapy as a function of total time scheduled for therapy and years post-stroke.
Results
Overall, treatment groups receiving more therapy improved beyond control groups that received less, g = 0.35, 95% CI = [0.26, 0.45]. Furthermore, increased time scheduled for therapy was a significant predictor of increased improvement by itself and when controlling for linear and quadratic effects of time post-stroke.
Conclusions
There is a positive relationship between the time scheduled for therapy and therapy outcomes. These data suggest that large doses of therapy lead to clinically meaningful improvements, controlling for time post-stroke. Currently, trials report time scheduled for therapy as a measure of therapy dose. Preferable measures of dose would be active time in therapy or repetitions of an exercise.
Key Terms: Stroke, Rehabilitation, Dose, Therapy
Studies in experimental psychology, neuroscience, and rehabilitation science explore adaptations in neural tissue with respect to type, intensity, and frequency of a stimulus. Studies of experience-dependent synaptic-plasticity in nonhuman animals1,2 and humans3 demonstrate that large quantities of practice lead to cortical reorganization and improved behavioural function. Similar studies link neural changes with recovery of function and learning in adults post-stroke4,5. These data indicate that increased practice leads to greater skill, as long as practice is challenging, progressive and skill-based4,6. Meta-analyses7,8 also suggest a positive dose-response relationship.
Some define dose as the amount of time actively spent in practice9, or the number of repetitions of a movement10,11. For this paper, dose is defined as total time scheduled for therapy (e.g., 3 hrs/day * (10 days) = 30 hrs). Time scheduled for therapy may not accurately reflect actual practice time nor the number of movement repetitions (see Lang et al.12) so this measure is not ideal; however time scheduled for therapy is the only consistently reported metric in rehabilitation research studies.
Response may be defined as improved function or reduced impairment. For this paper response was defined as a standardized effect-size, Hedges' g, which shows improved function or reduced impairment on a standardized, validated behavioural test. Effect sizes reported here were based on the primary or secondary outcomes of randomized controlled trials (RCTs) found through the systematic review.
Our objective was to quantify the magnitude of functional improvement gained by increasing therapeutic time after stroke. Our meta-analysis builds on work addressing dose response in a binary manner: is more therapy better than less therapy7–9? To meet this objective, we purposely included papers with different types of therapy interventions because it is unclear at this time how the type of therapy provided affects responses13,14. By reviewing RCTs with different therapy times for treatment and control groups, we modeled the effect of increased time scheduled for therapy on standardized measures of recovery. We tested linear and quadratic effects of therapy time while controlling for linear and quadratic effects of years from the initial stroke to the beginning of the RCT. We chose this approach because it is unlikely that any effects are linear. We hypothesized that increased therapy time would positively affect outcomes7,8 whereas time post-stroke might negatively affect outcomes15.
Methods
The population of interest was adults post-stroke (PICO model: Population, Intervention, Comparison, Outcome16). Interventions were therapies without exogenous stimulation. Comparison groups included randomized controlled trials where the treatment and control groups received different amounts of time scheduled for therapy. In some (e.g.17–19), each group received the same therapy in different dosages. In others, groups received different types of therapy (e.g.20–22) in different dosages. Outcomes were restricted to validated behavioural measures of function or impairment. In two cases23,24 no appropriate parametric statistics for the primary outcome were presented, thus we used a secondary outcome.
Search Strategy
Manual and electronic searches identified relevant literature. Searches were conducted from the earliest available date in Medline, PSYCInfo, PubMed, and Google Scholar to April 9th, 2013. Search terms included "stroke" or "stroke rehab$" in combination with one of the terms "dose", "intens$", "constrain$", or "gait". Filters limited papers to randomized controlled trials (otherwise, "random" and "control" were search terms). Bibliographies of retrieved trials and review papers were searched.
Study Selection
An initial 832 titles were identified. After screening titles and abstracts, and removing duplicates, 138 papers were assessed (see Supplemental Appendix I). Details of the interventions and the time scheduled for therapy in the treatment and control groups was extracted. Exclusion criteria were: (a) lack of randomization with a control, (b) studied children (<18 years old), (c) >30% participants with neurological disorders other than stroke, (d) therapy in combination with a pharmaceutical treatment or electrical stimulation, (e) dose matched treatment and control conditions, and (f) unpublished or not translated into English. Thirty-seven trials remained (see Supplemental Table I) and were included in the assessment of study quality13,17–52. The Physiotherapy Evidence Database Scale was used to rate methodological quality (PEDro; www.pedro.org.au).
Quantitative Analysis
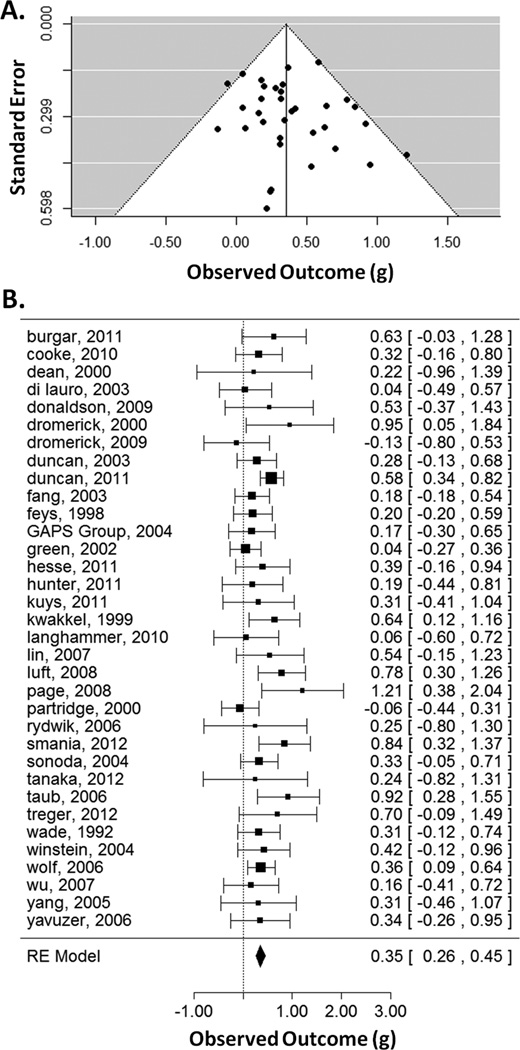
Means, standard deviations, and sample sizes for the treatment and control groups were entered into a spreadsheet. Standardized effect-sizes (Hedges' g) and variances (Vg) were calculated53. Effect sizes were computed from the terminal difference between treatment and control or the difference in improvement between treatment and control, divided by the standard deviation within groups. Subtraction was arranged so that effects favoring the treatment group were positive. Effect-size measures were analyzed using the "metafor" package54 in R (cran.r-project.org; Supplemental Table II). A funnel plot was constructed. There were three studies with large effect sizes and low levels of precision38,39,51. These studies were removed, leaving 34 studies for inclusion in the quantitative analysis (Supplemental Appendix II).
Custom scripts (Supplemental Appendix 3) tested a random-effects model for the overall effect of increased therapy dosage. The analysis was broken into two parts. Part one was congruent with previous analyses7,8, calculating a summary effect-size for groups who received more therapy compared to groups who received less. Part two elaborated on this analysis by using meta-regression models to quantify the dose-response relationship controlling for other factors. Four studies were omitted from regression models due to missing data19, 23, 30, 47 (see the "NAs" in Supplemental Table II); regression was based on 30 studies. Time post-stroke (Yrs.PS) was the average years from hospital admission to the onset of the intervention. Total time scheduled for therapy was calculated for the treatment and control groups based on descriptions in the text. Regression models then used the difference between groups in total time scheduled for therapy (ΔTime).
Constraint time in CIMT creates a problem for calculating ΔTime because it is not clear how time under constraint should be counted as time scheduled for therapy. To address this problem, we coded three different ΔTimes for CIMT studies. In the MIN Time calculation, 0% of constraint time counted as time scheduled for therapy. In the 50% Time calculation, 50% of constraint time counted as time scheduled. In the MAX Time calculation, 100% of constraint time counted as time scheduled. The results of the 50% Time calculation are presented here, as we assume that some, but not all, of constraint time was spent using the affected limb. (Details of all analyses are presented in Supplemental Appendix II.)
Results
Comparing high-dose to low-dose: There is an overall benefit of increased time in therapy
Across studies, there was a benefit for treatment groups receiving more therapy, g = 0.35, 95% CI = [0.26, 0.45] (Figure 1), which was significant, Zobs = 7.21, p < 0.001. The random-effects model had a τ2 = 0.01 (which is the maximum-likelihood estimate of between-study variance), I2 = 16.34 (which is the % of total variability due to heterogeneity), and H2 = 1.20 (the ratio of total variability to sampling variability). The test for heterogeneity was not significant, Q(33) = 37.34, p = 0.28. Thus, there was an overall benefit for more time scheduled for therapy compared to less.
Figure 1.
Funnel plot (A) showing effect-sizes (g) as a function of precision (standard error). Asymmetry was not significant. Forest plot (B) showing the effect-sizes and 95% confidence intervals for each study and the summary effect-size from the random-effects model. Positive values show a difference in favor of increased time scheduled for therapy. RE=random effects.
Descriptive statistics for the regression models
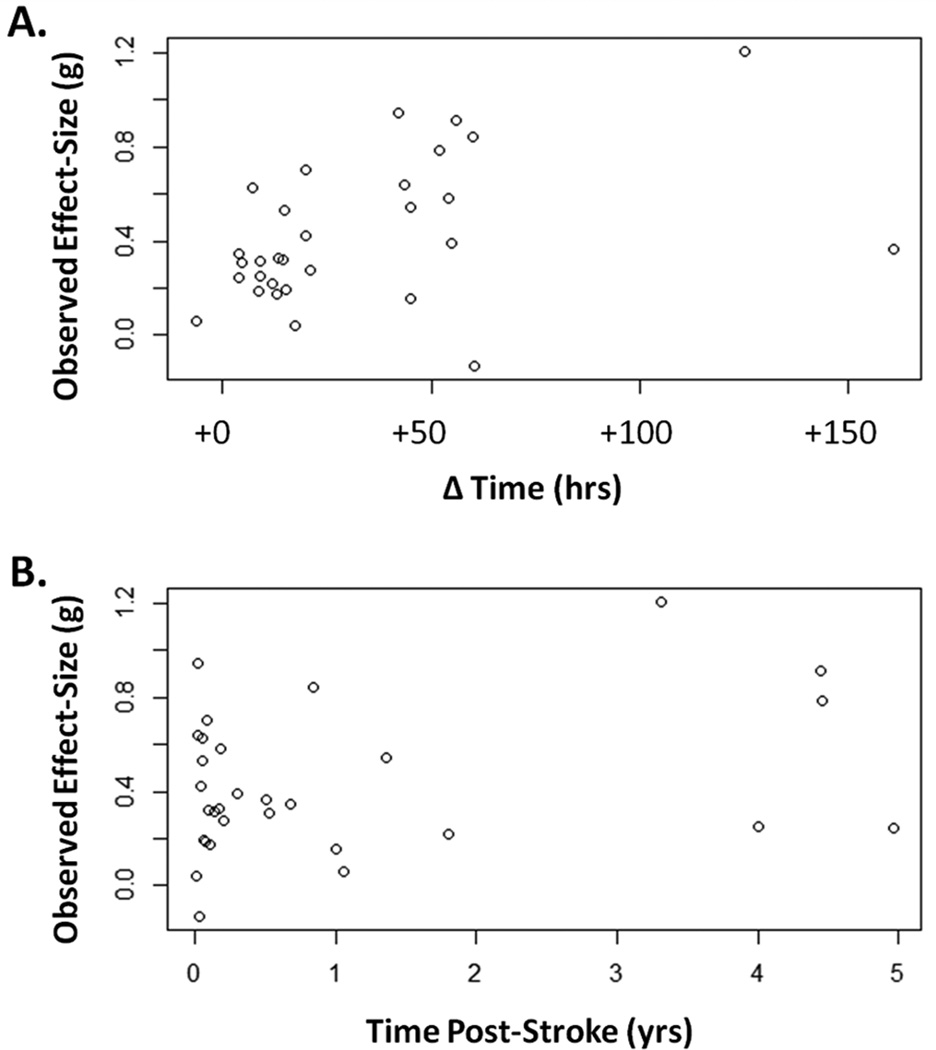
For the 30 studies included in the regression models, there were 1,750 total participants. The median number of participants in treatment groups was n = 21.5 and in control groups n = 19.5. In treatment groups time post-stroke was 1.01 ±1.49 yrs, [0.003, 5.14], shown as M ±SD [Min, Max]. In control groups time post-stroke was 1.02 ±1.63 yrs, [0.003, 5.38]. The duration of therapy in treatment groups was 49.56 ±68.12 days, [14, 365]. The duration of therapy in control groups was virtually identical, 49.60 ±68.10 days, [14, 365], as most studies were matched for treatment duration (see Supplemental Table I). Matching studies on treatment duration means that differences in total therapy time result from changes in the frequency and intensity of therapy for a given duration. Time scheduled for therapy in treatment groups was 57.41 ±44.88 hrs, [4.0, 160.8]. Time scheduled for therapy in control groups was 24.08 ±30.39 hrs, [0.0, 140.0]. The average ΔTime was 33.33 ±36.20 hrs, [−6.50, 160.80]. Observed effect-sizes as a function of ΔTime and Yrs.PS are shown in Figure 2.
Figure 2.
Observed effect-size (g) for each study as a function of additional time scheduled for therapy (A) and as a function of years post-stroke (B).
Quantifying dose: Increased scheduled therapy predicts greater recovery
In order to look at the linear effect of ΔTime, a series of models was tested. Model 1 tested the simple effect of ΔTime (in 10 hr units) as a predictor of effect size. This model was significant, Q(1) = 5.40, p = .02 and the parameter estimate of ΔTime was b = 0.037, 95% CI = [0.01, 0.07], p = .02. Model 2 tested the linear and quadratic effects of Yrs.PS. Model 2 was not significant, Q(2) = 1.44, p = 0.49, and the parameter estimates of Yrs.PS (b = 0.100, 95% CI = [−0.34, 0.54], p = 0.65) and Yrs.PS2 (b = −0.010, 95% CI = [−0.11, 0.08], p = 0.85), were not significant individually. Model 3, shown in Table 1, included the linear and quadratic effects of Yrs.PS with the linear effect of ΔTime. The omnibus test of moderators was non-significant, Q(3) = 6.73, p = .08, but the effect of ΔTime was significant. The test of residual homogeneity was not significant, Q(26) = 20.51, p = .77.
Table 1.
Details of Regression Model 3.
| Parameter Estimate |
95% Confidence Interval |
Z-value | P-value | |
|---|---|---|---|---|
| Intercept | 0.2735 | [0.09, 0.46] | 2.85 | .004 |
| Yrs.PS (yrs) | 0.0110 | [−0.45, 0.47] | 0.04 | .963 |
| Yrs.PS2 | 0.0078 | [−0.09, 0.11] | 0.15 | .879 |
| ΔTime (10 hrs) | 0.0344 | [0.00, 0.07] | 2.04 | .041 |
Note. Parameter estimates for Yrs.PS in years and the estimates for ΔTime in 10-hour units. We tested the interaction of Yrs.PS and ΔTime, which was marginally significant (p = 0.06; b = 0.027), suggesting the effect of increased time in therapy was larger for later post-stroke times. This interaction was marginal and did not improve the fit of the model, so the main effects model is presented.
Controlling for a nonlinear effect of ΔTime
Model 4 (Table 2) included linear and quadratic effects of both Yrs.PS and ΔTime. Overall, the test of moderators was non-significant, Q(4) = 8.21, p = .08. The test of residual homogeneity was not significant, Q(25) = 14.89, p = .94.
Table 2.
Details of Regression Model 4.
| Parameter Estimate |
95% Confidence Interval |
Z-value | P-value | |
|---|---|---|---|---|
| Intercept | 0.1680 | [−0.07, 0.41] | 1.36 | .172 |
| Yrs.PS (yrs) | 0.0338 | [−0.43, 0.49] | 0.14 | .885 |
| Yrs.PS2 | 0.0022 | [−0.10, 0.10] | 0.04 | .966 |
| ΔTime (10 hrs) | 0.0983 | [0.01, 0.19] | 2.07 | .038 |
| ΔTime2 | −0.0047 | [−0.01, 0.00] | −1.69 | .089 |
Note. Parameter estimates for Yrs.PS in years and estimates for ΔTime in 10-hour units. We also tested the interaction of Yrs.PS2 and ΔTime2, which was not significant (p = 0.12), so the main effects model is presented.
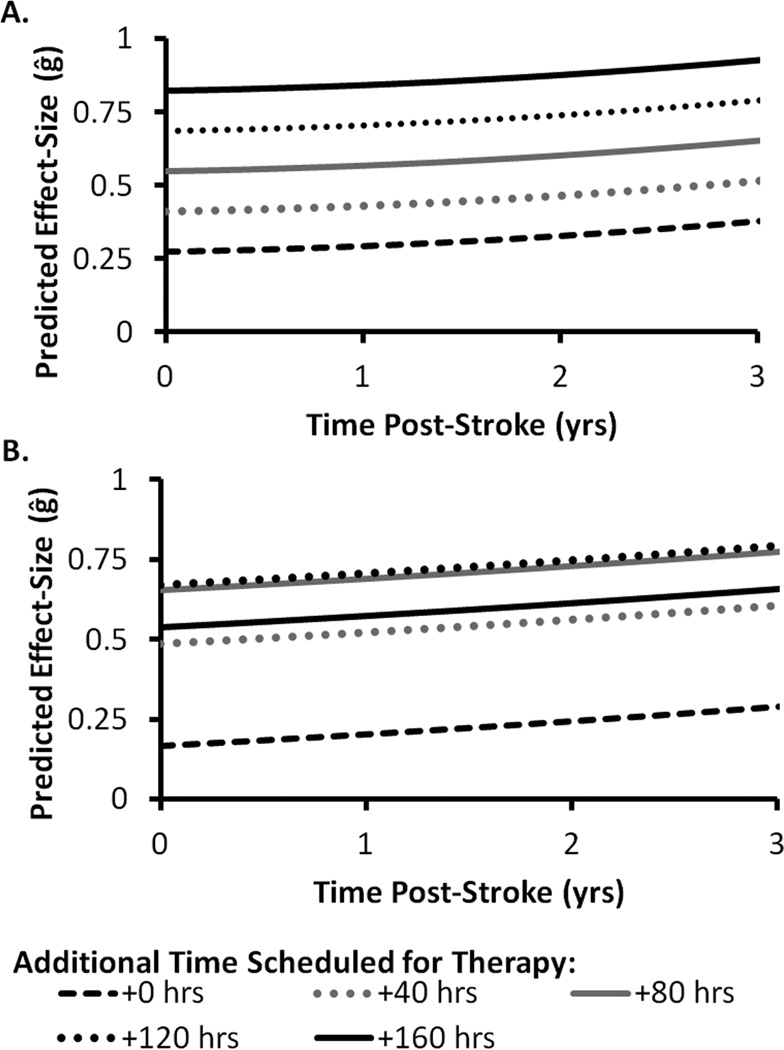
The linear effect of ΔTime was significant (p = .04) and ΔTime2 approached significance (p = .09). The predicted effect-sizes (ĝ) of Models 3 and 4 are shown in Figure 3. The non-significant effect of ΔTime2 suggests that the basic effect of ΔTime is positive and for every additional 10 hrs scheduled for therapy, the effect of ΔTime may become less positive. However, statistical power is an issue with this many moderators, so this effect should be interpreted with caution.
Figure 3.
Predicted effect-size (ĝ) as a function years post-stroke (x-axis) and select values of additional time scheduled for therapy (separate lines). Model 3 (A) includes the linear effect of time scheduled for therapy. Model 4 (B) includes the linear and quadratic effects of time scheduled for therapy. The dashed black line (+0 hrs) represents the predicted effect-size when no additional time is scheduled for therapy between treatment and control groups.
Discussion
This meta-analysis agrees with previous work7,8 suggesting a small overall benefit of augmented time in therapy (i.e., more is better). Kwakkel's review7 found smaller benefits of therapy dose (~0.20 for measures of ADL and walking speed) than our overall g = 0.35, which is likely due to differences in the methods for inclusion and analysis. It is difficult to compare our results directly to Langhorne's review8 because those authors measured odds-ratios and weighted mean differences, rather than standardized effect-sizes. However, those authors also found what they described as "modest effects" of increased therapy. Our analysis goes further to suggest reliable dose-response relationships between the time scheduled for therapy and improvement on clinical measures of function and impairment. In our analysis, neither the linear nor quadratic effects of time post-stroke were significant. However, there was a significant positive effect of time scheduled for therapy on outcomes (Model 1) even when controlling for time post-stroke (Model 3). Our evidence also suggests the potential for a nonlinear effect of time scheduled for therapy when controlling for the linear effect (Model 4).
We interpret these results as strong evidence of a positive relationship between dose and response. We were able to see a positive dose response relationship across studies rehabilitating different impairments and functions, employing different interventions, and measuring outcomes with different tools. All of these factors are potential sources of noise that could mask the dose-response relationship. Thus, we interpret these effects as evidence that time in therapy is a robust predictor of recovery across different types of therapy. Our data imply that providers of rehabilitation services should consider multiple ways to increase therapy time, both within and outside formal sessions. Furthermore, there was no interaction between time post-stroke and time scheduled for therapy. The lack of an interaction suggests that the benefit of large increases in therapy is similar across a range of post-stroke times regardless of whether a client is several months or several years post-stroke (post-stroke times ranged from 0.003 to 5.38 years).
Importantly, there are complications to this effect. For instance, if started too early, intensive therapy may hinder the rate of recovery20 or have no benefit over less intense therapies18. Also, too many hours of therapy may not be tolerable for participants, leading to drop-outs22. These nonlinearities are important considerations for clinicians that are not captured in the current analysis. As more data are added at different time points, these complexities in the dose-response relationship can be modeled more reliably.
Recovery following stroke is clearly a multidimensional problem, but it is reassuring to establish that time scheduled for therapy significantly predicted functional outcomes across studies. Our results also agree with experimental work in which dose was tightly controlled55–57. In those studies, the correlation between dose (measured in repetitions) and outcome was moderate (r = 0.5–0.6). In comparison, our meta-analysis is limited by using time scheduled for therapy as a predictor when ideally we could use active time in movement practice or movement repetitions. However, in the existing literature the only consistently reported metric was time scheduled for therapy. Within our own dataset, 23.5% of studies (8 out of 34 RCTs) provided a more certain/more detailed measure than time scheduled for therapy. These studies specified active time in therapy (such as time spent walking) or gave descriptive statistics about how much therapy time was fulfilled by participants (which may include active time plus rests, demonstrations, instructions, etc., but is still a more detailed measure than time scheduled). Thus, we recommend future RCTs report active time or repetitions of an exercise for a more accurate representation of the dose of therapy received.
With 30 studies in the meta-regression, we rapidly lost power to detect additional effects and interactions. Additional studies need to be included in the dataset to test additional predictors (e.g., stroke severity), higher order effects (e.g., cubic effects), or interactions. While the meta-data approach is powerful, dose-response relationships are likely more complex than what we present here. Future work can address this issue. We are currently conducting a systematic review that will result in a larger database of RCTs. These data will be analyzed with respect to terminal improvements and retention at long-term follow-ups (the current analysis is limited by only studying terminal effects) for treatment and control groups, separately. This approach allows the modeling of dosage effects for studies with different durations, intensities, and frequencies of treatment in more homogeneous treatment groups. Furthermore, the current meta-data and other experimental data55–57 warrant larger experimental studies to explore dose-response effects.
Supplementary Material
Acknowledgments
Funding sources: CEL received partial salary support from NIH R01 HD068290.
Footnotes
Disclosures: None.
References
- 1.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. Journal of Speech Language and Hearing Sciences. 2008;51:S225–S239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- 2.Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. Journal of Neurophysiology. 1996;75:2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- 3.Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proceedings of the National Academy of Science. 2002;99:1017–1022. doi: 10.1073/pnas.022615199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd LA, Vidoni ED, Wessel BD. Motor learning after stroke: Is skill acquisition a prerequisite for contralesional neuroplastic change. Neuroscience Letters. 2010;482:21–25. doi: 10.1016/j.neulet.2010.06.082. [DOI] [PubMed] [Google Scholar]
- 5.Schaechter JD, Fricker ZP, Perdue KL, Helmer KG, Vangel MG, Greve DN, et al. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Human Brain Mapping. 2009;30:3461–3474. doi: 10.1002/hbm.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taub E, Uswatte G, Mark VW, Morris DM, Barman J, Bowman MH, et al. Method for enhancing real-world use of a more affected arm in chronic stroke: Transfer package of constraint-induced movement therapy. Stroke. 2013;44:1383–1388. doi: 10.1161/STROKEAHA.111.000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwakkel G, van Peppen R, Wagenaar RC, Dauphinee SW, Richards C, Ashburn A, et al. Effects of augmented exercise therapy time after stroke: A meta-analysis. Stroke. 2004;35:2529–2539. doi: 10.1161/01.STR.0000143153.76460.7d. [DOI] [PubMed] [Google Scholar]
- 8.Langhorne P, Wagenaar RC, Partridge C. Physiotherapy after stroke: More is better? Physiotherapy Research International. 1996;1:75–88. doi: 10.1002/pri.6120010204. [DOI] [PubMed] [Google Scholar]
- 9.Kwakkel G, Wagenaar RC, Koelmann TW, Lankhorst GJ, Koetsier JC. Effects of intensity of rehabilitation after stroke: A research synthesis. Stroke. 1997;28:1550–1556. doi: 10.1161/01.str.28.8.1550. [DOI] [PubMed] [Google Scholar]
- 10.Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: Facts and theories. Restorative Neurology and Neuroscience. 2004;22:281–299. [PubMed] [Google Scholar]
- 11.Woldag H, Waldmann G, Hueschkel G, Hummelsheim H. Is the repetitive training of complex hand and arm movements beneficial for motor recovery in stroke patients. Clinical Rehabilitation. 2003;17:723–730. doi: 10.1191/0269215503cr669oa. [DOI] [PubMed] [Google Scholar]
- 12.Lang CE, MacDonald JR, Reisman DS, Boyd LA, Kimberley TJ, Schindler-Ivens SM, et al. Observation of amounts of movement practice provided during stroke rehabilitation. Archives of Physical Medicine and Rehabilitation. 2009;90:1692–1698. doi: 10.1016/j.apmr.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, et al. Body-weight supported treadmill rehabilitation after stroke. New England Journal of Medicine. 2011;364:2026–2036. doi: 10.1056/NEJMoa1010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG. Robot-assisted therapy for long-term upper-limb impairment after stroke. New England Journal of Medicine. 2010;362:1772–83. doi: 10.1056/NEJMoa0911341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ottenbacher KJ, Jannell S. The results of clinical trials in stroke rehabilitation research. Archives of Neurology. 1993;50:37–44. doi: 10.1001/archneur.1993.00540010033014. [DOI] [PubMed] [Google Scholar]
- 16.Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Medical Informatics and Decision Making. 2007;7:16. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgar CH, Lum PS, Scremin AME, Garber SL, Van der Loos HFM, Kenney D, et al. Robot-assisted upper-limb therapy in acute rehabilitation setting following stroke: Department of Veterans Affairs multisite clinical trial. Journal of Rehabilitation Research & Development. 2011;48:445–458. doi: 10.1682/jrrd.2010.04.0062. [DOI] [PubMed] [Google Scholar]
- 18.Di Lauro A, Pellegrino L, Savastano G, Ferraro C, Fusco M, Balzarano F, et al. A randomized trial on the efficacy of intensive rehabilitation in the acute phase of ischemic stroke. Journal of Neurology. 2003;250:1206–1208. doi: 10.1007/s00415-003-0185-2. [DOI] [PubMed] [Google Scholar]
- 19.Partridge C, MacKenzie M, Edwards S, Reid A, Jayawardena S, Guck N, et al. Is dosage of physiotherapy a critical factor in deciding patterns of recovery from stroke: A pragmatic randomized controlled trial. Physiotherapy Research International. 2000;5:230–240. doi: 10.1002/pri.203. [DOI] [PubMed] [Google Scholar]
- 20.Dromerick AW, Lang CE, Birkenmeier RL, Wagner JM, Miller JP, Videen TO, et al. Very early constraint-induced movement during stroke rehabilitation (VECTORS): A single-center RCT. Neurology. 2009;73:195–201. doi: 10.1212/WNL.0b013e3181ab2b27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuys SS, Brauer SG, Ada L. Higher-intensity treadmill walking during rehabilitation after stroke in feasible and not detrimental to walking pattern or quality: A pilot randomized trial. Clinical Rehabilitation. 2011;25:316–326. doi: 10.1177/0269215510382928. [DOI] [PubMed] [Google Scholar]
- 22.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, et al. Effect of constraint induced movement therapy on upper extremity function 3 to 9 months after stroke: The EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 23.Green J, Forster A, Bogle S, Young J. Physiotherapy for patients with mobility problems more than 1 year after stroke; A randomised controlled trial. Lancet. 2002;359:199–203. doi: 10.1016/S0140-6736(02)07443-3. [DOI] [PubMed] [Google Scholar]
- 24.Kwakkel G, Wagenaar RC, Twisk JWR, Lankhorst GJ, Koestier JC. Intensity of leg and arm training after primary middle-cerebral-artery stroke: A randomised trial. Lancet. 1999;354:191–196. doi: 10.1016/S0140-6736(98)09477-X. [DOI] [PubMed] [Google Scholar]
- 25.Cooke EV, Tallis RC, Clark A, Pomeroy VM. Efficacy of functional strength training on restoration of lower-limb motor function early after stroke: Phase I randomized control trial. Neurorehabilitation and Neural Repair. 2010;24:88–96. doi: 10.1177/1545968309343216. [DOI] [PubMed] [Google Scholar]
- 26.Dean CM, Richards CL, Malouin F. Task-related circuit training improves performance of locomotor tasks in chronic stroke: A randomized, controlled pilot trial. Archives of Physical Medicine and Rehabilitation. 2000;81:409–417. doi: 10.1053/mr.2000.3839. [DOI] [PubMed] [Google Scholar]
- 27.Donaldson C, Tallis R, Miller S, Sunderland A, Lemon R, Pomeroy V. Effects of conventional physical therapy and functional strength training on upper limb motor recovery after stroke: A randomized phase II study. Neurorehabilitation and Neural Repair. 2009;23:389–397. doi: 10.1177/1545968308326635. [DOI] [PubMed] [Google Scholar]
- 28.Dromerick AW, Edwards DF, Hahn M. Does the application of constraint-induced movement therapy during acute rehabilitation reduce arm impairment after ischemic stroke? Stroke. 2000;31:2984–2988. doi: 10.1161/01.str.31.12.2984. [DOI] [PubMed] [Google Scholar]
- 29.Duncan P, Studenski S, Richards L, Gollub S, Lai SM, Reker D, et al. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke. 2003;34:2173–2180. doi: 10.1161/01.STR.0000083699.95351.F2. [DOI] [PubMed] [Google Scholar]
- 30.Fang Y, Chen X, Li H, Lin J, Huang R, Zeng J. A study on additional early physiotherapy after stroke and factors affecting functional recovery. Clinical Rehabilitation. 2003;17:608–617. doi: 10.1191/0269215503cr655oa. [DOI] [PubMed] [Google Scholar]
- 31.Feys HM, De Weerdt WD, Selz BE, Cox Steck GA, Spichiger R, Vereeck LE, et al. Effect of a therapeutic intervention for the hemiplegic upper limb in the acute phase after stroke: A single-blind, randomized, controlled multicenter trial. Stroke. 1998;29:785–792. doi: 10.1161/01.str.29.4.785. [DOI] [PubMed] [Google Scholar]
- 32.The Glasgow Augmented Physiotherapy Study (GAPS) group. Can augmented physiotherapy input enhance recovery of mobility after stroke? A randomized clinical trial. Clinical Rehabilitation. 2004;18:529–537. doi: 10.1191/0269215504cr768oa. [DOI] [PubMed] [Google Scholar]
- 33.Hesse S, Welz A, Werner C, Quentin B, Wissel J. Comparison of an intermittent high-intensity vs continuous low intensity physiotherapy service over 12 months in community dwelling people with stroke: A randomized trial. Clinical Rehabilitation. 2011;25:146–156. doi: 10.1177/0269215510382148. [DOI] [PubMed] [Google Scholar]
- 34.Hunter SM, Hammett L, Ball S, Smith N, Anderson C, Clark A, et al. Dose-response study of mobilisation and tactile stimulation therapy for the upper extremity early after stroke: A phase I trial. Neurorehabilitation and Neural Repair. 2011;25:314–322. doi: 10.1177/1545968310390223. [DOI] [PubMed] [Google Scholar]
- 35.Langhammer B, Stanghelle JK. Exercise on a treadmill or walking outdoors? A randomized controlled trial comparing effectiveness of two walking exercise programmes later after stroke. Clinical Rehabilitation. 2010;24:46–54. doi: 10.1177/0269215509343328. [DOI] [PubMed] [Google Scholar]
- 36.Lin K-C, Wu C-Y, Wei T-H, Gung C, Lee C-Y, Liu J-S. Effects of modified constraint induced movement therapy on reach to grasp movements and functional performance after chronics stroke: A randomized controlled study. Clinical Rehabilitation. 2007;21:1075–1086. doi: 10.1177/0269215507079843. [DOI] [PubMed] [Google Scholar]
- 37.Luft AR, Macko RF, Forrester LW, Villagra F, Ivey F, Sorkin JD, et al. Treadmill exercise activates subcortical neural networks and improves walking after stroke: A randomized controlled trial. Stroke. 2008;39:3341–3350. doi: 10.1161/STROKEAHA.108.527531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Page SJ, Sisto S, Levine P, McGrath RE. Efficacy of a modified constraint-induced movement therapy in chronic stroke: A single-blinded randomized controlled trial. Archives of Physical Medicine and Rehabilitation. 2004;85:14–18. doi: 10.1016/s0003-9993(03)00481-7. [DOI] [PubMed] [Google Scholar]
- 39.Page SJ, Levine P, Leonard AC. Modified constraint-induced therapy in acute stroke: A randomized controlled pilot study. Neurorehabilitation and Neural Repair. 2005;19:27–32. doi: 10.1177/1545968304272701. [DOI] [PubMed] [Google Scholar]
- 40.Page SJ, Levine P, Leonard AC, Szaflarski JP, Kissela BM. Modified constraint-induced therapy in chronic stroke: Results of a single-blinded randomized controlled trial. Physical Therapy. 2008;88:333–340. doi: 10.2522/ptj.20060029. [DOI] [PubMed] [Google Scholar]
- 41.Rydwyk E, Eliasson S, Akner G. The effect of exercise of the affected foot in stroke patients - a randomized controlled pilot trial. Clinical Rehabilitation. 2006;20:645–655. doi: 10.1191/0269215506cre986oa. [DOI] [PubMed] [Google Scholar]
- 42.Smania N, Gandolfi M, Paolucci S, Iosa M, Ianes P, Recchia S, et al. Reduced-intensity modified constraint induced movement therapy versus conventional therapy for upper extremity rehabilitation after stroke: A multicenter trial. Neurorehabilitation & Neural Repair. 2012;26:1035–1045. doi: 10.1177/1545968312446003. [DOI] [PubMed] [Google Scholar]
- 43.Sonoda S, Saitoh E, Nagai S, Kawakita M, Kanada Y. Full-time integrated treatment program, a new system for stroke rehabilitation in Japan. American Journal of Physical Medicine & Rehabilitation. 2004;83:88–93. doi: 10.1097/01.PHM.0000107481.69424.E1. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka N, Saitou H, Takao T, Iizuka N, Okuno J, Yano H, et al. Effects of gait rehabilitation with a footpad type locomotion interface in patients with chronic post-stroke hemiparesis: A pilot study. Clinical Rehabilitation. 2012;26:686–695. doi: 10.1177/0269215511432356. [DOI] [PubMed] [Google Scholar]
- 45.Taub E, Uswatte G, Kay King D, Morris D, Crago JE, Chatterjee A. A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke. 2006;37:1045–1049. doi: 10.1161/01.STR.0000206463.66461.97. [DOI] [PubMed] [Google Scholar]
- 46.Treger I, Aidinof L, Lehrer H, Kalichman L. Modified constraint-induced movement therapy improved upper limb function in subacute poststroke patients: A small-scale clinical trial. Top Stroke Rehabil. 2012;19:287–293. doi: 10.1310/tsr1904-287. [DOI] [PubMed] [Google Scholar]
- 47.Wade DT, Collen FM, Robb GF, Warlow CP. Physiotherapy intervention late after stroke and mobility. BMJ. 1992;304:609–613. doi: 10.1136/bmj.304.6827.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winstein CJ, Rose DK, Tan SM, Lewthwaite R, Chui HC, Azen SP. A randomized controlled comparison of upper-extremity rehabilitation strategies in acute stroke: A pilot study of immediate and long-term outcomes. Archives of Physical Medicine and Rehabilitation. 2004;85:620–628. doi: 10.1016/j.apmr.2003.06.027. [DOI] [PubMed] [Google Scholar]
- 49.Wu C-Y, Chen C-L, Tang SF, Lin K-C, Huang Y-Y. Kinematic and clinical analyses of upper-extremity movements after constraint-induced movement therapy in patients with stroke: A randomized controlled trial. Archives of Physical Medicine and Rehabilitation. 2007;88:964–970. doi: 10.1016/j.apmr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 50.Yang Y-R, Yen J-G, Wang R-Y, Yen L-L, Lieu F-K. Gait outcomes after additional backward walking training in patients with stroke: A randomized controlled trial. Clinical Rehabilitation. 2005;19:264–273. doi: 10.1191/0269215505cr860oa. [DOI] [PubMed] [Google Scholar]
- 51.Yang Y-R, Wang R-Y, Chen Y-C, Kao M-J. Dual task exercise improves walking ability in chronic stroke: A randomized controlled trial. Archives of Physical Medicine and Rehabilitation. 2007;88:1236–1240. doi: 10.1016/j.apmr.2007.06.762. [DOI] [PubMed] [Google Scholar]
- 52.Yavuzer G, Eser F, Karakus D, Karaoglan B, Stam HJ. The effects of balance training on gait late after stroke: A randomized control trial. Clinical Rehabilitation. 2006;20:960–969. doi: 10.1177/0269215506070315. [DOI] [PubMed] [Google Scholar]
- 53.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Chichester, UK: John Wiley and Sons; 2009. [Google Scholar]
- 54.Viechtbauer W. Conducting meta-analysis in R with the metafor package. Journal of Statistical Software. 2010;26:3. [Google Scholar]
- 55.Birkenmeier RL, Prager EM, Lang CE. Translating animal doses of task-specific training to people with chronic stroke in 1-hour therapy sessions: A proof of concept study. Neurorehabilitation & Neural Repair. 2010;24:620–635. doi: 10.1177/1545968310361957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moore JL, Roth EJ, Killian C, Hornby TG. Locomotor training improves daily stepping activity and gait efficiency in individuals post-stroke who have reached a "plateau" in recovery. Stroke. 2010;41:129–135. doi: 10.1161/STROKEAHA.109.563247. [DOI] [PubMed] [Google Scholar]
- 57.Waddell JL, Birkenmeier RL, Moore JL, Hornby TG, Lang CE. The feasibility of high repetition, task-specific training for the paretic upper extremity. American Journal of Occupational Therapy. doi: 10.5014/ajot.2014.011619. [In press.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.