Abstract
Gonadal steroid hormones play important roles during critical periods of development to organize brain structures that control sexually dimorphic neuroendocrine responses and behaviors. Specific receptors for androgens and estrogens must be expressed at appropriate times during development in order to mediate these processes. The present study was performed to test for sex differences in the relative expression of estrogen receptor-α (ERα) and androgen receptor (AR) mRNA during the window of time in gestation that is critical for behavioral masculinization and differentiation of the ovine sexually dimorphic nucleus (oSDN) in the sheep. In addition, we examined whether ERα and AR mRNA expression is localized within the nascent oSDN and could be involved in its development. Using quantitative RT-PCR, we found that females expressed more ERα mRNA than males in medial preoptic area and medial basal hypothalamus during the mid-gestational critical period for brain sexual differentiation. No sex differences were found for AR mRNA in any tissue examined or for ERα in amygdala and frontal cortex. Using radioactive in situ hybridization, we found that the distributions of ERα and AR mRNA overlapped with aromatase mRNA, which delineates the boundaries of the developing oSDN and identifies this nucleus as a target for both androgens and estrogens These data demonstrate that the transcriptional machinery for synthesizing gonadal steroid receptors is functional in the fetal lamb brain during the critical period for sexual differentiation and suggest possible mechanisms for establishing dimorphisms controlled by gonadal steroids may exist at the level of steroid hormone receptor expression.
Keywords: estrogen receptor-alpha, androgen receptor, medial preoptic area, medial basal hypothalamus, amygdala, sheep, fetus
Introduction
Sexual differentiation of sheep is dependent on exposure to the gonadal hormone testosterone (T) before birth (1). The broad critical period for differentiation occurs between gestational day (GD) 30 and 90 of the 147-d term pregnancy (1,2). Female lambs born to ewes treated with T during the full 60-day critical period are born with completely virilized genitals (2). Behaviorally, these T-treated females exhibit more male-typical copulatory behaviors and less female-typical proceptive and receptive behaviors than controls, indicating that behavior is both masculinized and defeminized (3,4). Prenatal exposure of females to T throughout this period also reduces hypothalamic sensitivity to the major feedback systems involved in control of cyclic GnRH and gonadotropin secretion, including estrogen negative and positive feedback and progesterone (P) negative feedback (5-7). In addition, exposure to T throughout the entire critical period was shown to masculinize sexually dimorphic cell groups in the arcuate nucleus of the hypothalamus (8,9) and the central component of the medial preoptic nucleus, also known as the ovine sexually dimorphic nucleus (oSDN) (10). These anatomical structures are presumed to be involved in the regulation of GnRH secretion and the expression of male-typical sexual behavior, respectively.
Treatments with T for shorter periods demonstrate that timing and duration of steroid exposure are important and reveal that multiple overlapping critical periods exist. Early administration of T from GD30 to GD60 results in development of a penis and scrotum devoid of testes in genetically female offspring. By contrast, late treatment between GD60 and GD90 causes slight enlargement of the clitoris but does not alter the basic structure of the female genitalia (11). Both early and late treatments alter the timing of puberty and the LH positive feedback response to estradiol (E2) in females, but neither treatment alone completely defeminizes hormone feedback or receptive behavior (11,12). In contrast, late treatment is sufficient to masculinize courtship and mounting activity (4,13) and enlarge the volume of the oSDN (14). Thus, the prenatal critical period for masculinization of the sheep brain and sexual behavior appears to occur later than and separate from the period for development of the male genitals and is more time-limited than the critical period for gonadotropin control.
Sexual differentiation is initiated by the secretion of T by the fetal testis. It is well documented that T can be metabolized to E2 and dihydrotestosterone (DHT) (15,16); metabolites that act on separate estrogen (ER) and androgen receptor (AR) pathways, respectively. Studies in sheep suggest that both androgens and estrogens organize neuroendocrine mechanisms and behavioral activity, but their relative contributions are not yet fully understood. For example, estrogen is believed to defeminize GnRH control because prenatal treatment of females with T (androgenic and estrogenic actions) disrupts or abolishes the LH surge response to late follicular phase concentrations of E2, but prenatal DHT treatment (androgenic actions only) do not (17). In contrast, the development of male-typical play behavior and pubertal timing are dependent primarily on androgens, not estrogens (18-20). There is some evidence that prenatal estrogen is involved in masculinization of copulatory behavior in rams, but not required for the development of male-typical sexual partner preferences (21). Though steroid hormones have been established as causal agents in sex specific brain development, the specific molecular mechanisms mediating these phenomena have not been fully elucidated in sheep. If estrogens and androgens act through their receptors to organize a male typical brain, then either the dimorphic brain regions or areas functionally connected to these regions should express ER and AR during the masculinization process. Additionally, dimorphisms at the level of receptor expression may play a role in determining the degree of brain masculinization. Previous studies that established steroid receptors are expressed in the developing fetal lamb brain used near term fetuses (22) or gross dissections of the diencephalon that may have obscured possible sex differences (23-26). The present study used younger fetuses and a more discrete dissection of the medial preoptic area (MPOA) in order to test for sex differences in the expression levels of ERα and AR messenger RNA (mRNA) during the window of time that is critical for behavioral masculinization and oSDN differentiation in the sheep. In addition, we employed in situ hybridization to examine whether ERα and AR mRNA expression overlaps with aromatase mRNA, which defines the boundaries of the developing oSDN.
Material and methods
Animals
Adult Polypay ewes, purchased from the sheep facility at Oregon State University (OSU), were mated during the breeding season after synchronization of estrus with intravaginal progestagen pessaries (Eazi-breed CIDR Sheep Insert; Zoetis, New York, NY) for 7 days. Ewes received an i.m. injection of 125 μg prostaglandin F2α on day 6 (Estrumate: Schering-Plough Animal Health, Elkhorn, NE) and were bred on day 9. The day of mating was confirmed by the presence of the stud ram's raddle marks on the ewe's rump and recorded as Day 1 of gestation. To collect fetuses of various gestational ages, dams were sedated with ketamine and diazepam i.v. and then maintained under general anesthesia with inhaled isoflorane and oxygen. The uterus was exposed through a mid-ventral laparotomy and incised to expose the fetus and obtain blood from the umbilical artery. Fetuses were then removed, weighed and exsanguinated. A total of 16 male and 16 female lamb fetuses were used in this experiment. All animal procedures were approved by the Institutional Animal Care and Use Committees of the Oregon Health and Science University and OSU in accordance with Public Health Services Policy on Humane Care and Use of Laboratory Animals.
Tissue Collection
Tissues used in this study were obtained from male and female fetal lambs of the following days of gestation ± 2 days: 65, 85, 100 and 135 and intended to span the critical period for organization of the oSDN i.e., GD 60 to 90. A full term pregnancy is approximately 147 days long. Fetal brains were rapidly isolated, sectioned mid-sagitally and dissected using surface landmarks. The left half of the brain was used to dissect out frontal cortex (FCTX), medial preoptic area (MPOA), medial basal hypothalamus (MBH) and amygdala (AMYG). The FCTX was dissected from the anterior pole of the cerebral cortex. The MPOA consisted of a block of tissue extending rostrocaudally from the lamina terminalis anteriorly to the caudal aspect of the optic chiasm. The block of tissue is situated between the optic chiasm and the anterior commissure in the dorsal-ventral plane and extended laterally ∼1 mm. The MBH consisted of a block of tissue immediately caudal to MPOA extending to the rostral aspect of the mammillary bodies. This dissection extended dorsoventrally from the ventral border of the thalamus to the floor of the third ventricle and laterally to the hypothalamic sulcus. The AMYG was a wedge of tissue dissected from the ventromedial left temporal lobe. The FCTX was a piece of tissue 2 mm deep dissected from the anterior pole of the left frontal lobe. Fresh tissues were rapidly frozen and stored at -80°C until used to extract RNA. The right half of the brain was dissected into a block of tissue which extended from the lamina terminalis to the posterior border of the mammillary bodies. This tissue block was then immersion fixed overnight at 4°C in 4% paraformaldehyde, cryoprotected in 20% sucrose, frozen and stored at -80°C until cryostat sectioned.
Real time polymerase chain reaction (RT-PCR) quantification
Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. RNasin® RNAse inhibitor (Promega) was added after extraction and samples were stored at -80°C until used. Total RNA (500ng) in each sample was converted to cDNA by reverse transcription using the First Strand Superscript III Kit (Invitrogen) according to the manufacturer's instructions. The resulting cDNA samples were stored at -20°C. Real time PCR reactions were run in triplicate for each sample using PowerSYBR Green Master Mix (Invitrogen). Primers (Table 1) for ovine ERα, AR and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were specifically designed to cross exon junctions using Clone Manager software ver. 8 (Sci-Ed Software, Cary, NC). All reactions were run in an ABI Fast 7500 Thermal Cycler (Applied Biosystems, Life Technologies). Reactions were amplified using the following conditions: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The primer efficiencies for ERα and AR were 91% and 89%, respectively. Melting curves showed a single peak. Control reactions (no template and RT minus) were run at the same time to verify the primers were specific for amplified product and that the RNA was not contaminated with genomic DNA. Quantification of gene expression was performed by the relative standard curve method (27), normalized against the reference gene GAPDH and reported as the fold difference relative to the mean expression levels of male fetuses at each gestational age (28). Note that no significant variations in GAPDH expression were observed between sexes.
Table 1. Ovine ERα, AR and GAPDH primer sequences used in RT-PCR.
| Sense | Antisense | Accession # | |
|---|---|---|---|
| ERα | GGACCACATCCACCGCGTCC | TGCCTTTGTTGCTCATGTGCCT | AY033393.1 |
| AR | CATGGGCTGGCGGTCCTTC | CCGGGACTTGTGCATGCGGT | AF105713.1 |
| GAPDH | TCATCCATGACCACTTTG | AGTAGAAGCAGGGATGATGT | NM_001190390.1 |
In situ hybridization
Fixed brains from fetal lambs (n = 2 males and 1female per gestational age) were sectioned coronally at 20 or 40 μm thick, then mounted onto Superfrost® plus microscope slides (Fischer Scientific; Pittsburgh, PA), desiccated and stored frozen at – 80°C. Four parallel series of sections were taken throughout the MPOA-anterior hypothalamus and used for in situ hybridization and for thionin staining. Partial cDNA clones for ovine ERα (accession number U30299; nt 53-335), AR (accession number AF105713; nt 1-338) and aromatase (accession number AF193858; nt 61-285) (29-31) were used to synthesize P33-labeled antisense and sense RNA probes with the appropriate DNA-dependent RNA polymerase according the manufacturer's instructions (Promega Corp.; Madison, WI). In situ hybridization was conducted according to our previously published protocol (32). Following hybridization, the sections were exposed for 8-14 days to Hypermax B films and then developed in Kodak® D19 developer. Control hybridizations performed using P33-labeled sense probes showed no specific signal above background (data not shown).
Steroid radioimmunoassay
Fetal serum at all gestational time points was analyzed for concentrations of E2 and T using radioimmunoassay (RIA) procedures that have been validated and published previously (33). Briefly, serum samples (500 μl) were extracted with 5 ml diethyl ether, dried under forced air and re-dissolved in 200 μl of column solvent (Hexane:benzene:methanol = 62:20: 13). The sample was then added to a 1 × 6 cm all-glass column containing Sephadex LH-20 for separation of different steroids. Each collected column fraction contained a specific isolated steroid and was dried under an air stream before being subjected to RIA. Hormone values were corrected for extraction-chromatography losses determined by radioactive trace recovery at the same time with sample extraction; recovery usually was between 60 and 80%. The sensitivity/tube of the E2 and T RIA was 1 and 5 pg, respectively. The overall interassay variation was less than 15% and the intraassay variations did not exceed 10%.
Statistical analysis
Sex comparisons of steroid receptor expression data were analyzed with a t-test corrected for differences among several means (34). Comparisons between ages were not performed because the rapid brain growth during gestation leads to tissue dilution which may confound interpretation of age-related expression data. Hormone concentrations were analyzed by two-way ANOVA, with post-hoc comparisons made using Fisher's least squares difference (LSD) test. Data were log10 transformed when necessary to equalize variances among groups. All data are expressed as mean + SEM; statistical significance for all analyses was defined as P<0.05.
Results
RT- PCR
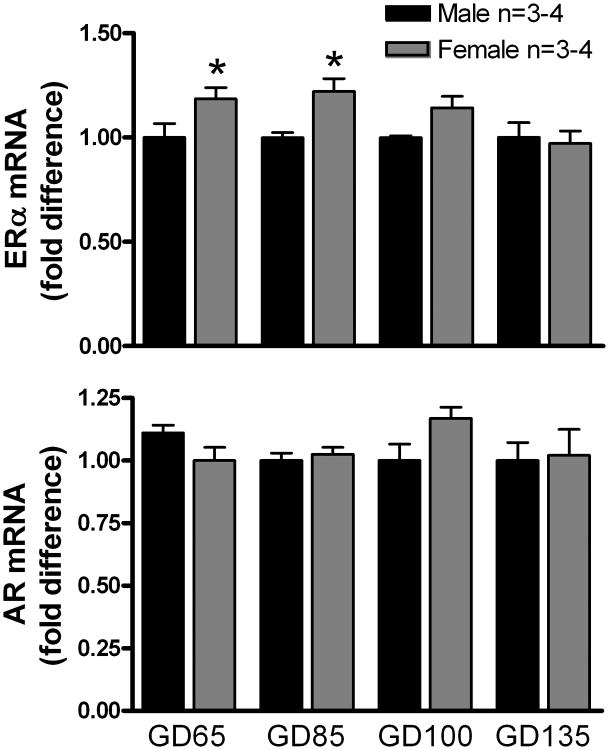
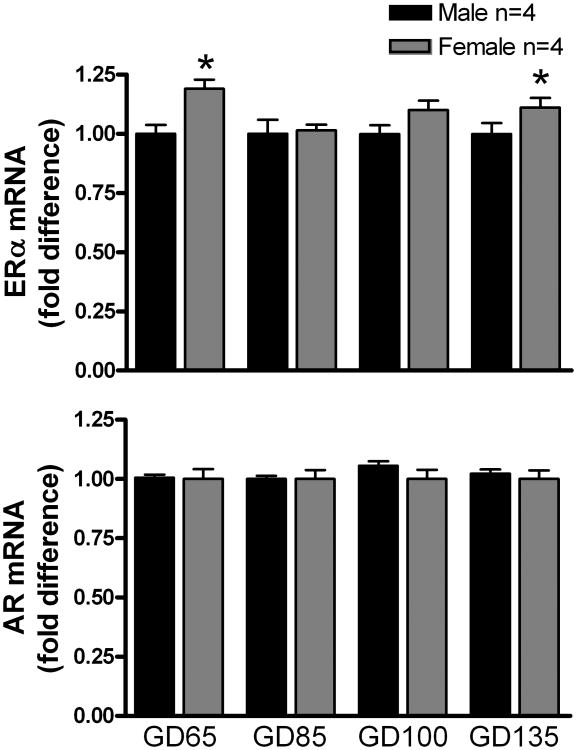
ERα and AR mRNA was detected by RT-PCR in all brain tissues that were examined. In the MPOA (Fig. 1), ERα mRNA expression was significantly (P < 0.05) higher in females than in males at GD 65 and GD 85. No sex differences in AR expression were observed in MPOA. In the MBH (Fig. 2), ERα mRNA expression was significantly (P < 0.05) higher in females than in males at GD 65 and GD 135, but no sex differences were observed at the other ages. No sex differences in AR expression were observed in MPOA. No sex differences were apparent in either ERα or AR mRNA expression in either the AMYG (Supplemental Fig. 1) or the FCTX (Supplemental Fig. 2).
Figure 1.
Expression of ERα and AR mRNA in the MPOA of male and female fetal lambs. Expression of mRNA is normalized against the reference gene GAPDH and reported as the fold difference relative to the mean expression levels of male fetuses at each gestational age. Data are the mean ± SEM fold differences relative to mean expression of 65-day-old male MPOA. Data were analyzed by a t-test for multiple means. *P < 0.05, female versus male.
Figure 2.
Expression of ERα and AR mRNA in the MBH of male and female fetal lambs. See legend to Figure 1 for other details. *P < 0.05, female versus male.
In situ hybridization
ERα and AR mRNA was expressed in both male and female fetal lambs MPOA as early as GD 65 (Fig.3). The strongest signals were evident in the central component of the medial preoptic nucleus (MPNc), which represents the nascent oSDN and occupies the central portion of the MPOA as defined by aromatase mRNA expression (Fig. 3). Hybridization signal was also found in the bed n. of the stria terminalis. The expression patterns for both receptors were similar at all ages throughout gestation and overlapped with aromatase mRNA expression within the boundaries of the oSDN.
Figure 3.
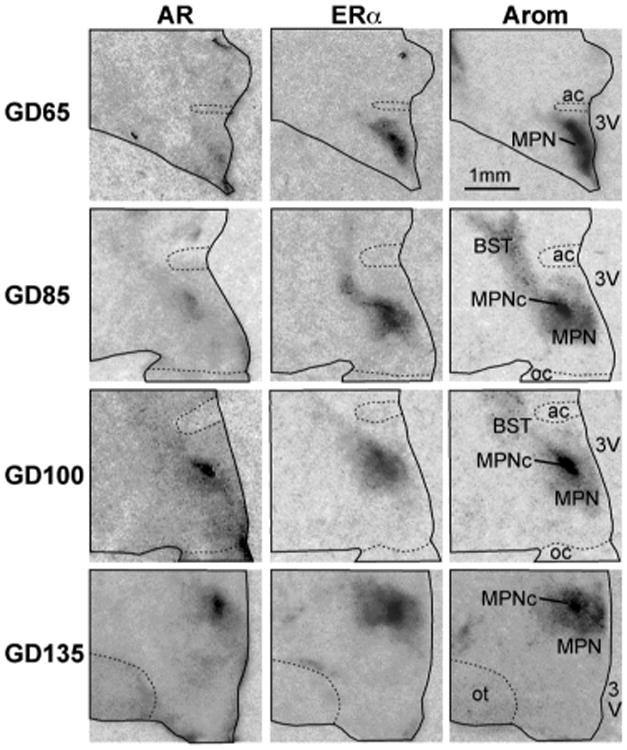
Digitized autoradiographic film images of coronal sections through the right half of the fetal lamb preoptic region showing the pattern of expression for ERα and AR mRNAs at gestational days (GD): 65, 85, 100 and 135. Note that the dark signal for aromatase mRNA in the central area of the medial preoptic n. (MPNc) represents the nascent oSDN. All sections are from males, no significant differences were found in the patterns of the signal in females. Sections were cut at 20 μm thickness except for GD135 which was cut at 40 μm. All images were enlarged 380× (see bar for scale). Abbreviations: ac, anterior commissure; BST, bed n. of the stria terminalis; MPN, medial preoptic n.; oc, optic chiasm; ot, optic tract; 3V, third ventricle. Brightness and contrast were adjusted to optimize images.
Serum steroid concentrations
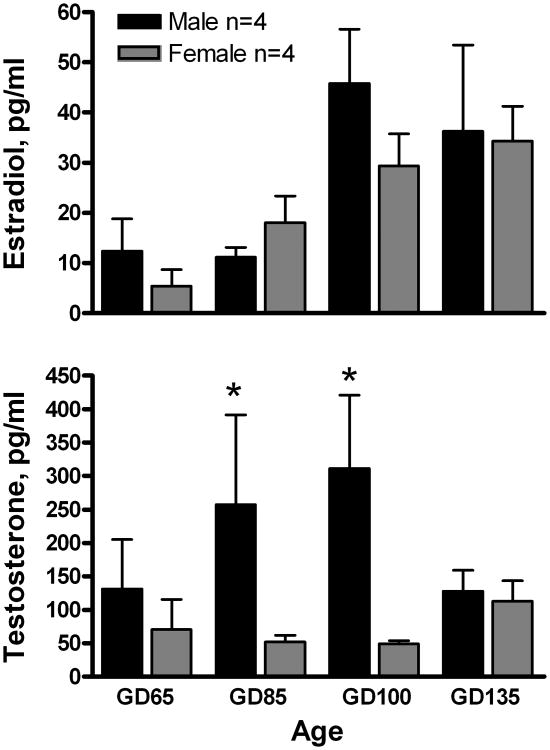
The average serum estradiol levels increased with developmental age (F[3,24] = 8.5; P <0.001), but did not exhibit an effect of sex or an age × sex interaction, Fig. 4. Serum testosterone exhibited a significant effect of sex (F[1,24] = 12.5; P <0.01), but no age effect or age x sex interaction. The mean concentrations of testosterone at GD 85 and GD 100 were significantly (P < 0.05) higher in male than in female lamb fetuses, Fig. 4.
Figure 4.
Developmental changes in mean (± SEM) concentrations of E2 and T in umbilical artery serum from male and female fetal lambs. Data were log10 transformed and analyzed by 2-way ANOVA followed by Fisher's LSD test. *P < 0.05, male versus female.
Discussion
We found that ERα and AR mRNAs are expressed in fetal lamb brain regions that regulate reproductive function and behavior. ERα mRNA levels were significantly higher in the MPOA and MBH of females than of males during the critical period for sexual differentiation. No sex differences were found for AR mRNA expression in any tissue examined or for ERα in amygdala and frontal cortex. In addition, our anatomical study demonstrated that both ERα and AR are expressed within the central component of the fetal medial preoptic n., which represents the nascent oSDN as defined by aromatase mRNA expression. These results complement our previous observation that aromatase mRNA expression has a similar pattern of distribution in the fetal MPOA but not quantitatively different between sexes (28). The simultaneous expression of aromatase, ERα and AR suggest that both androgens and estrogens, either synthesized in the brain or derived from the circulation, are available to mediate hormone dependent developmental processes that lead to functional and structural changes in brain areas important for male-typical differentiation. However unlike ERα, AR expression levels are similar in males and females during the critical period. The absence of a sex difference in AR expression suggests that testosterone dependent dimorphisms in these brains regions are probably mediated by sex differences in hormone levels, rather than dimorphisms in receptor expression levels.
Sex differences in ERα levels have been demonstrated previously in several species (35-38). In adult rats, ER binding in periventricular preoptic area, MPOA and ventromedial nucleus of the hypothalamus (VMN) is higher in females than in males (39). A bias toward higher levels in females has also been reported in ERα mRNA levels (37). In adult sheep, ERα mRNA expression in the VMN and arcuate n. is higher in females than in males, but no sex differences are observed in the MPOA nuclei (35). In fetal rats, sex differences in ERα emerge within 24 hr. after birth in the MPOA and appear by postnatal day 2 in the VMN and arcuate n. (ARC) (36,38,40,41). These differences persist for at least 2 weeks and encompass the postnatal critical period for sexual differentiation of the brain in rats. Similarly, our observations show that in sheep the sex difference in ERα expression appears to coincide with the critical period, which occurs prenatally (i.e. between GD 30 and 90) in this species. The existence of higher ERα mRNA levels in the brain of females suggests that the male might be less sensitive to estrogens during this period. However, ERα mRNA has been shown to be reduced by estrogens in the brains of adult and fetal animals (37,42,43) indicating that lower levels of mRNA could represent receptor activation and autologous down-regulation of gene expression. Further studies to examine the effect of exogenous estrogen treatment on ERα expression will be required to distinguish between these possibilities.
We found that sex differences in ERα expression vary during development, appearing only transiently as they seem to do in MBH at GD 65 and then again at GD 135. Previous studies have proposed that there could be multiple critical periods during which gonadal steroids organize different sexually dimorphic neuroendocrine and behavioral functions in sheep (44). The present observations are consistent with the view that this may be due, in part, to temporal variations in ERα expression.
Our results differ from previous reports that found ERα mRNA expression in fetal lambs does not exhibit sex differences (22,23,25,26). In general, these earlier studies used older fetuses (i.e. > 120 days of gestation) and/or gross dissections of diencephalon that included both preoptic and hypothalamic tissue. We used younger fetuses to encompass the critical period for sexual differentiation of the oSDN and separate dissections of the MPOA and MBH to provide better anatomical resolution. These differences may explain why we detected subtle but significant region-specific sex differences.
No sex differences in AR mRNA expression were found in the fetal sheep brain. These results suggest that the developing brain of both sexes could be influenced by androgens under normal or pathological conditions. Similar to our results, earlier studies in rhesus monkeys, a long gestation species like sheep, failed to find evidence of sex differences in brain AR content between male and female fetuses (45,46). A study conducted with guinea pigs found that AR content at midgestation was transiently higher in males than in females (47). In rats, AR mRNA has been detected in the preoptic area and hypothalamus one day before birth and then increases over the first 10 days of life (48). On postnatal day 10, a sex difference emerges in the MPOA and bed n. of the stria terminalis, but not in the MBH, with males having greater levels of receptor mRNA than females. Rats are less mature at birth than sheep and developmental stages that occur in rats postnatally occur prenatally in sheep. In contrast to fetuses, MPOA but not hypothalamus contains greater levels of AR expression in adult rams than ewes (49) consistent with the role of this region in the regulation of male sexual behaviors. The MPOA tissue block used in the present study contained several regions/nuclei in which AR mRNA expression may be regulated differentially. For instance, while sex differences in adults were found for the AR mRNA content of MPOA and bed n. of the stria terminalis, no difference was found in the anterior hypothalamus (49). Since all of these nuclei were included in our dissection a sex difference could have been negated and should be confirmed using quantitative anatomical studies.
The results of the present in situ hybridization study demonstrate that ERα, AR and aromatase mRNAs, are present in the developing oSDN. The adult oSDN is larger and contains more neurons in males than in females, and is enlarged in females exposed prenatally to testosterone (10,50). Prenatal exposure of male lamb fetuses to an aromatase inhibitor does not interfere with oSDN masculinization (51), however, exposure to the anti-androgen flutamide does (our unpublished observations). These results indicate that the control of cell number and volume in the oSDN most likely does not rely on aromatase and estrogen metabolites of testosterone in sheep but rather requires androgenic actions. Studies in rats have shown previously that androgens directly stimulate functional differentiation of cultured hypothalamic neurons by increasing the number of aromatase-positive neurons and the expression level per neuron (52,53). Similar androgenic effects could be responsible for masculine differentiation of the oSDN. Both androgens and estrogens contribute to circuit formation by stimulating morphological differentiation of central neurons (54-58). In the developing male oSDN in vivo, there may be higher tissue levels of androgens as well as estrogens, the latter being due to higher local aromatization of the increased circulating testosterone characteristic of males during the critical period. Thus, in addition to a larger oSDN, we would predict that axon outgrowth and neuronal connectivity would be more pronounced in males than in females.
Our results provide information about the relative steady-state amounts of ERα and AR transcript. While mRNA is required for synthesis of receptor protein, it is not yet possible to relate steroid receptor mRNA levels to receptor protein and binding. Mismatches have been reported between the developmental profiles of mRNA and protein abundance for steroid receptors in the sheep brain (24,26) suggesting that additional regulation occurs at the translational and posttranslational levels. In addition to ERα and AR, a second form of the estrogen receptor ERβ is expressed in the sheep brain (26,35,59) but was not measured in the present study. Though not shown to play a role in SDN development, ERβ has been implicated in the estrogen-dependent organization of gonadotropin signaling pathways in rats (36,60). Whether ERβ plays any role in the sexual differentiation of the sheep brain remains to be determined in future experiments.
In summary, we compared the expression levels of ERα and AR within the preoptic area, hypothalamus, amygdala and cerebral cortex of male and female lamb fetuses. We found females expressed more ERα than males in MPOA and MBH during the critical period for brain sexual differentiation. Our anatomic evidence suggests that the developing oSDN in the MPOA is a target site for both androgens and estrogens. We speculate that testicular testosterone acting through both receptors produces the sexually dimorphic brain morphology and circuitry that underlie sex differences in the neuroendocrine responses and sexual behaviors of adult ewes and rams.
Supplementary Material
Supplemental Figure 1. Expression of ERα and AR mRNA in the AMYG of male and female fetal lambs. See legend to Figure 1 for other details.
Supplemental Figure 2. Expression of ERα and AR mRNA in the CTX of male and female fetal lambs. See legend to Figure 1 for other details.
Acknowledgments
This work was supported by NIH R01OD011047, formerly RR014270 (CER).
Footnotes
There are no conflicts of interest for any of the authors.
References
- 1.Ford JJ, D'Occhio MJ. Differentiation of sexual behavior in cattle, sheep, and swine. J Anim Sci. 1989;67:1816–1823. doi: 10.2527/jas1989.6771816x. [DOI] [PubMed] [Google Scholar]
- 2.Wood RI, Foster DL. Sexual differentiation of reproductive neuroendocrine function in sheep. Rev Reprod. 1998;3:130–140. doi: 10.1530/ror.0.0030130. [DOI] [PubMed] [Google Scholar]
- 3.Fabre-Nys C, Venier G. Sexual differentiation of sexual behaviour and preovulatory LH surge in ewes. Psychoneuroendocrinology. 1991;16:383–396. doi: 10.1016/0306-4530(91)90003-c. [DOI] [PubMed] [Google Scholar]
- 4.Clarke IJ. The sexual behaviour of prenatally androgenized ewes observed in the field. J Reprod Fertil. 1977;49:311–315. doi: 10.1530/jrf.0.0490311. [DOI] [PubMed] [Google Scholar]
- 5.Foster DL, Padmanabhan V, Wood RI, Robinson JE. Sexual differentiation of the neuroendocrine control of gonadotrophin secretion: concepts derived from sheep models. Reprod Suppl. 2002;59:83–99. [PubMed] [Google Scholar]
- 6.Padmanabhan V, Sarma HN, Savabieasfahani M, Steckler TL, Veiga-Lopez A. Developmental reprogramming of reproductive and metabolic dysfunction in sheep: native steroids vs. environmental steroid receptor modulators. Int J Androl. 2010;33:394–404. doi: 10.1111/j.1365-2605.2009.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson JE, Forsdike RA, Taylor JA. In utero exposure of female lambs to testosterone reduces the sensitivity of the gonadotropin-releasing hormone neuronal network to inhibition by progesterone. Endocrinology. 1999;140:5797–5805. doi: 10.1210/endo.140.12.7205. [DOI] [PubMed] [Google Scholar]
- 8.Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE. Identification of neurokinin B-expressing neurons as an highly estrogen- receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology. 2000;141:4218–4225. doi: 10.1210/endo.141.11.7743. [DOI] [PubMed] [Google Scholar]
- 9.Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The Kisspeptin/Neurokinin B/Dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;151:301–311. doi: 10.1210/en.2009-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roselli CE, Stadelman H, Reeve R, Bishop CV, Stormshak F. The ovine sexually dimorphic nucleus of the medial preoptic area is organized prenatally by testosterone. Endocrinology. 2007;148:4450–4457. doi: 10.1210/en.2007-0454. [DOI] [PubMed] [Google Scholar]
- 11.Wood RI, Mehta V, Herbosa CG, Foster DL. Prenatal testosterone differentially masculinizes tonic and surge modes of luteinizing hormone secretion in the developing sheep. Neuroendocrinology. 1995;62:238–247. doi: 10.1159/000127010. [DOI] [PubMed] [Google Scholar]
- 12.Roberts EK, Padmanabhan V, Lee TM. Differential effects of prenatal testosterone timing and duration on phenotypic and behavioral masculinization and defeminization of female sheep. Biol Reprod. 2008;79:43–50. doi: 10.1095/biolreprod.107.067074. [DOI] [PubMed] [Google Scholar]
- 13.Clarke IJ, Scaramuzzi RJ. Sexual behaviour and LH secretion in spayed androgenized ewes after a single injection of testosterone or oestradiol-17b. J Reprod Fertil. 1978;52:313–320. doi: 10.1530/jrf.0.0520313. [DOI] [PubMed] [Google Scholar]
- 14.Roselli CE, Estill C, Stadelman HL, Meaker M, Stormshak F. Separate critical periods exist for testosterone-induced differentiation of the brain and genitals in sheep. Endocrinology. 2011;152:2409–2415. doi: 10.1210/en.2010-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roselli CE, Resko JA, Stormshak F. Estrogen synthesis in fetal sheep brain: effect of maternal treatment with an aromatase inhibitor. Biol Reprod. 2003;68:370–374. doi: 10.1095/biolreprod.102.007633. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen PN, Billiards SS, Walker DW, Hirst JJ. Changes in 5alpha-pregnane steroids and neurosteroidogenic enzyme expression in the perinatal sheep. Pediatr Res. 2003;53:956–964. doi: 10.1203/01.PDR.0000064905.64688.10. [DOI] [PubMed] [Google Scholar]
- 17.Masek KS, Wood RI, Foster DL. Prenatal dihydrotestosterone differentially masculinizes tonic and surge modes of luteinizing hormone secretion in sheep. Endocrinology. 1999;140:3459–3466. doi: 10.1210/endo.140.8.6913. [DOI] [PubMed] [Google Scholar]
- 18.Jackson LM, Timmer KM, Foster DL. Sexual differentiation of the external genitalia and the timing of puberty in the presence of an antiandrogen in sheep. Endocrinology. 2008;149:4200–4208. doi: 10.1210/en.2007-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sachs BD, Harris VS. Sex differences and developmental changes in selected juvenile activities (play) of domestic lambs. Anim Behav. 1978;26:684. [Google Scholar]
- 20.Orgeur P. Sexual play behavior in lambs androgenized in utero. Physiol Behav. 1995;57:185–187. doi: 10.1016/0031-9384(94)00227-v. [DOI] [PubMed] [Google Scholar]
- 21.Roselli CE, Schrunk JM, Stadelman HL, Resko JA, Stormshak F. The effect of aromatase inhibition on the sexual differentiation of the sheep brain. Endocrine. 2006;29:501–512. doi: 10.1385/ENDO:29:3:501. [DOI] [PubMed] [Google Scholar]
- 22.Gorton LM, Mahoney MM, Magorien JE, Lee TM, Wood RI. Estrogen receptor immunoreactivity in late-gestation fetal lambs. Biol Reprod. 2009;80:1152–1159. doi: 10.1095/biolreprod.108.073189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roselli CE, Resko JA, Stormshak F. Expression of steroid hormone receptors in the fetal sheep brain during the critical period for sexual differentiation. Brain Res. 2006;1110:76–80. doi: 10.1016/j.brainres.2006.06.070. [DOI] [PubMed] [Google Scholar]
- 24.Wood CE, Keller-Wood M. Ontogeny of androgen receptor expression in the ovine fetal central nervous system and pituitary. Neurosci Lett. 2008;439:153–156. doi: 10.1016/j.neulet.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexander BM, Singh P, Austin KJ, Cockrum RR, Cammack KM, Hess BW, Moss GE, Nathanielsz PW, Ford SP. Effect of maternal fatness on fetal steroids and semi-quantitative real-time PCR expression of receptor genes in sheep. Anim Reprod Sci. 2009;116:58–64. doi: 10.1016/j.anireprosci.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaub CE, Gersting JA, Keller-Wood M, Wood CE. Development of ER-alpha and ER-beta expression in the developing ovine brain and pituitary. Gene Expr Patterns. 2008;8:457–463. doi: 10.1016/j.gep.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Larionov A, Krause A, Miller W. A standard curve based method for relative real time PCR data processing. BMC Bioinformatics. 2005;6:62. doi: 10.1186/1471-2105-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roselli CE, Stormshak F. Ontogeny of cytochrome P450 aromatase mRNA expression in the developing sheep brain. J Neuroendocrinol. 2011;24:443–452. doi: 10.1111/j.1365-2826.2011.02260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ing NH, Spencer TE, Bazer FW. Estrogen enhances endometrial estrogen receptor gene expression by a posttranscriptional mechanism in the ovariectomized ewe. Biol Reprod. 1996;54:591–599. doi: 10.1095/biolreprod54.3.591. [DOI] [PubMed] [Google Scholar]
- 30.Roselli CE, Stormshak F, Resko JA. Distribution of aromatase mRNA in the ram hypothalamus: An In Situ hybridization study. J Neuroendocrinol. 2000;12:656–664. doi: 10.1046/j.1365-2826.2000.00496.x. [DOI] [PubMed] [Google Scholar]
- 31.Mateescu RG, Thonney ML. Gene expression in sexually dimorphic muscles in sheep. J Anim Sci. 2002;80:1879–1887. doi: 10.2527/2002.8071879x. [DOI] [PubMed] [Google Scholar]
- 32.Roselli CE, Abdelgadir SE, Ronnekleiv OK, Klosterman SA. Anatomic distribution and regulation of aromatase gene expression in the rat brain. Biol Reprod. 1998;58:79–87. doi: 10.1095/biolreprod58.1.79. [DOI] [PubMed] [Google Scholar]
- 33.Resko JA, Ellinwood WE, Pasztor LM, Buhl AE. Sex steroids in the umbilical circulation of fetal rhesus monkeys from the time of gonadal differentiation. J Clin Endocrinol Metab. 1980;50:900–905. doi: 10.1210/jcem-50-5-900. [DOI] [PubMed] [Google Scholar]
- 34.Bruning JL, Kintz BL. Computational Handbook of Statistics. Glenview, Illinois: HarperCollins Publishers; 1987. [Google Scholar]
- 35.Scott CJ, Tilbrook AJ, Simmons DM, Rawson JA, Chu S, Fuller PJ, Ing NH, Clarke IJ. The distribution of cells containing estrogen receptor-alpha (ERalpha) and ERbeta messenger ribonucleic acid in the preoptic area and hypothalamus of the sheep: comparison of males and females. Endocrinology. 2000;141:2951–2962. doi: 10.1210/endo.141.8.7622. [DOI] [PubMed] [Google Scholar]
- 36.Cao J, Patisaul HB. Sexually dimorphic expression of hypothalamic estrogen receptors a and b and Kiss1 in neonatal male and female rats. J Comp Neurol. 2011;519:2954–2977. doi: 10.1002/cne.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shughrue PJ, Bushnell CD, Dorsa DM. Estrogen receptor messenger ribonucleic acid in female rat brain during the estrous cycle: A comparison with ovariectomized females and intact males. Endocrinology. 1992;131:381–388. doi: 10.1210/endo.131.1.1612018. [DOI] [PubMed] [Google Scholar]
- 38.Yokosuka M, Okamura H, Hayashi S. Postnatal development and sex difference in neurons containing estrogen receptor-alpha immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. J Comp Neurol. 1997;389:81–93. doi: 10.1002/(sici)1096-9861(19971208)389:1<81::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 39.Brown TJ, Hochberg RB, Zielinski JE, MacLusky NJ. Regional sex differences in cell nuclear estrogen-binding capacity in the rat hypothalamus and preoptic area. Endocrinology. 1988;123:1761–1770. doi: 10.1210/endo-123-4-1761. [DOI] [PubMed] [Google Scholar]
- 40.DonCarlos LL, Handa RJ. Developmental profile of estrogen receptor mRNA in the preoptic area of male and female neonatal rats. Dev Brain Res. 1994;79:283–289. doi: 10.1016/0165-3806(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 41.Kuhnemann S, Brown TJ, Hochberg RB, MacLusky NJ. Sex differences in the development of estrogen receptors in the rat brain. Horm Behav. 1994;28:483–491. doi: 10.1006/hbeh.1994.1046. [DOI] [PubMed] [Google Scholar]
- 42.Kuhnemann S, Brown TJ, Hochberg RB, MacLusky NJ. Sexual differentiation of estrogen receptor concentrations in the rat brain: effects of neonatal testosterone exposure. Brain Res. 1995;691:229–234. doi: 10.1016/0006-8993(95)00640-c. [DOI] [PubMed] [Google Scholar]
- 43.DonCarlos LL, McAbee M, Ramer-Quinn DS, Stancik DM. Estrogen receptor mRNA levels in the preoptic area of neonatal rats are responsive to hormone manipulation. Dev Brain Res. 1995;84:253–260. doi: 10.1016/0165-3806(94)00179-4. [DOI] [PubMed] [Google Scholar]
- 44.Foster DL, Jackson LM, Padmanabhan V. Reproduction in Domestic Ruminants. Nottingham: Nottingham University Press; 2007. Novel concepts about normal sexual differentiation of reproductive neuroendocrine function and the developmental origins of female reproductive dysfunction: the sheep model; pp. 83–107. [DOI] [PubMed] [Google Scholar]
- 45.Handa RJ, Connolly PB, Resko JA. Ontogeny of cytosolic androgen receptors in the brain of the fetal rhesus monkey. Endocrinology. 1988;122(5):1890–1896. doi: 10.1210/endo-122-5-1890. [DOI] [PubMed] [Google Scholar]
- 46.Pomerantz SM, Sholl SA. Analysis of sex and regional differences in androgen receptors in fetal rhesus monkey brain. Dev Brain Res. 1987;36:151–154. doi: 10.1016/0165-3806(87)90074-5. [DOI] [PubMed] [Google Scholar]
- 47.Toyooka KT, Connolly PB, Handa RJ, Resko JA. Ontogeny of androgen receptors in fetal guinea pig brain. Biol Reprod. 1989;41:204–212. doi: 10.1095/biolreprod41.2.204. [DOI] [PubMed] [Google Scholar]
- 48.McAbee MD, DonCarlos LL. Ontogeny of region-specific sex differences in androgen receptor messenger ribonucleic acid expression in the rat forebrain. Endocrinology. 1998;139:1738–1745. doi: 10.1210/endo.139.4.5940. [DOI] [PubMed] [Google Scholar]
- 49.Scott CJ, Clarke IJ, Rao A, Tilbrook AJ. Sex differences in the distribution and abundance of androgen receptor mRNA-containing cells in the preoptic area and hypothalamus of the ram and ewe. J Neuroendocrinol. 2004;16:956–963. doi: 10.1111/j.1365-2826.2005.01261.x. [DOI] [PubMed] [Google Scholar]
- 50.Roselli CE, Larkin K, Resko JA, Stellflug JN, Stormshak F. The volume of a sexually dimorphic nucleus in the ovine medial preoptic area/anterior hypothalamus varies with sexual partner preference. Endocrinology. 2004;145:478–483. doi: 10.1210/en.2003-1098. [DOI] [PubMed] [Google Scholar]
- 51.Roselli CE, Stormshak F. The neurobiology of sexual partner preferences in rams. Horm Behav. 2009;55:611–620. doi: 10.1016/j.yhbeh.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beyer C, Green SJ, Hutchison JB. Androgens influence sexual differentiation of embryonic mouse hypothalamic aromatase neurons in vitro. Endocrinology. 1994;135:1220–1226. doi: 10.1210/endo.135.3.8070366. [DOI] [PubMed] [Google Scholar]
- 53.Hutchison JB, Beyer C, Hutchison R, Wozniak A. Sexual dimorphism in the developmental regulation of brain aromatase. J Steroid Biochem Molec Biol. 1995;53:307–313. doi: 10.1016/0960-0760(95)00068-b. [DOI] [PubMed] [Google Scholar]
- 54.Beyer C, Hutchison JB. Androgens stimulate the morphological maturation of embryonic hypothalamic aromatase-immunoreactive neurons in the mouse. Dev Brain Res. 1997;98:74–81. doi: 10.1016/s0165-3806(96)00170-8. [DOI] [PubMed] [Google Scholar]
- 55.Lustig RH. Sex hormone modulation of neural development in vitro. Horm Behav. 1994;28:383–395. doi: 10.1006/hbeh.1994.1035. [DOI] [PubMed] [Google Scholar]
- 56.Diaz H, Lorenzo A, Carrer HF, Cáceres A. Time lapse study of neurite growth in hypothalamic dissociated neurons in culture: sex differences and estrogen effects. J Neurosc Res. 1992;33:266–281. doi: 10.1002/jnr.490330210. [DOI] [PubMed] [Google Scholar]
- 57.Toran-Allerand CD. On the genesis of sexual differetiation of the central nervous system: Morphogenetic consequences of steroidal exposure and possible role of alfa-fetoprotein. Prog Brain Res. 1984;61:63–98. doi: 10.1016/s0079-6123(08)64429-5. [DOI] [PubMed] [Google Scholar]
- 58.Estrada M, Uhlen P, Ehrlich BE. Ca2+ oscillations induced by testosterone enhance neurite outgrowth. J Cell Sci. 2006;119:733–743. doi: 10.1242/jcs.02775. [DOI] [PubMed] [Google Scholar]
- 59.Deitch HR, Mershon JL, Clark KE. Estrogen receptor beta is the predominant isoform expressed in the brain of adult and fetal sheep. Am J Obstet Gynecol. 2001;184:1077–1079. doi: 10.1067/mob.2001.115223. [DOI] [PubMed] [Google Scholar]
- 60.Orikasa C, Kondo Y, Hayashi S, McEwen BS, Sakuma Y. Sexually dimorphic expression of estrogen receptor beta in the anteroventral periventricular nucleus of the rat preoptic area: implication in luteinizing hormone surge. Proc Natl Acad Sci U S A. 2002;99:3306–3311. doi: 10.1073/pnas.052707299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Expression of ERα and AR mRNA in the AMYG of male and female fetal lambs. See legend to Figure 1 for other details.
Supplemental Figure 2. Expression of ERα and AR mRNA in the CTX of male and female fetal lambs. See legend to Figure 1 for other details.