Abstract
The present study was designed to investigate the effect Hypericum Perforatum (HP), on behavioral changes, corticosterone, TNF-α levels and tryptophan metabolism and disposition in bilateral ovariectomized rats compared to 17α -ethinylestradiol. Behavioral analysis by measuring immobility time in forced swimming test and open field test, serum and hippocampal corticosterone and TNF-α along with hippocampal kynurenine/tryptophan ratio were determined in mature ovariectomized rats treated orally either by HP at three different doses 125, 250, and 500 mg/kg/day or by 17α-ethinylestradiol 30 µg/kg/day for 30 days. Ovariectomized rats showed significant increase in immobility time in the forced swimming test. Along with elevation in serum and hippocampal TNF-α and corticosterone levels associated with significant increase in hippocampal kynurenine/tryptophan ratio. Immobility time in the forced swimming test was decreased in rats treated by different doses of HP in a dose dependent manner and 17α-ethinylestradiol with no concomitant changes in the open field test. Only Rats treated with HP exhibited significant decrease in the elevated serum and hippocampal TNF-α and corticosterone, which couldn't explain the associated insignificant effect on hippocampaus kynurenine/tryptophan ratio in comparison to ovariectomized untreated rats. It is concluded that increased tryptophan metabolism toward kynurenine secondary to elevated corticosterone and TNF-α might be one of the pathohphysiological mechanisms that could explain depression like state observed in this rat model. Further, the observed attenuating effect of HP on TNF-α and corticosterone could contribute in its antidepressant effect in this animal model by other ways than their effects on tryptophan-kynurenine metabolism pathway.
Keywords: Corticosterone, Hypericum perforatum, Kyninurenine, TNF-α, Tryptophan
INTRODUCTION
The hormonal changes during perimenopausal and early postmenopausal period play a role in depression [1]. In spite of several studies reporting a tendency for depression in women with rapidly changing levels of reproductive hormones, a direct relationship between psychiatric symptoms and hormonal changes has not been clearly established. Stress, socioeconomic factors and partner status may influence both menopausal symptoms and the prevalence and clinical course of depressive disorders [2].
Depression in general, is associated with inflammatory processes and neural-immune interactions. Pro-inflammatory cytokines (tumor necrosis factor α [TNF-α], interleukins 1and 6) have been considered as neuromodulators of behavioral, neuroendocrine and neurochemical features of depressive disorders [3]. Among the different ways of bidirectional influence between the immune system and the central nervous system, TNF-α attracts a lot of scientific interest: although other cytokines also contribute to this interaction and, in part, amplify the effects of TNF-α [4]. Simen and his colleagues reported that deletion of either TNF receptor-1 or TNF receptor-2 leads to an antidepressant-like response in the forced swim test [5]. Postmenopausal women seem to have higher serum levels of TNFα compared to women of reproductive age [6].
One of the mechanisms by which chronic inflammation might trigger the age related disorders as menopause is transcriptional induction of indoleamine 2, 3-dioxygenase (IDO); rate-limiting enzyme of tryptophan (TRY)-kynurenine (KYN) pathway by pro-inflammatory cytokines. Activation of IDO shifts TRY metabolism from serotonin synthesis to formation of kynurenine. While diminished serotonin production is associated with depression, increased kynurenine might lead to neurodegenerative changes through induction of apoptosis, neurotoxic, and pro-oxidative effects. The other rate-limiting enzyme of the TRY-KYN pathway is TRY 2, 3-dioxygenase (TDO), which is activated by corticosterone [7].
Estrogen replacement therapy has been used as a valuable treatment for many years in treating depression in postmenopausal period [8]. Estrogen exerts potent effects on mood, mental state, and memory [9]. National Institutes of Health report cautioned that chronic hormone treatments could cause serious adverse effects [10]. Selective serotonin reuptake inhibitors are a first-line treatment for postmenopausal depression (PMD) [11] but an analysis of the Canadian Multicentre Osteoporosis Study cohort revealed an association between SSRI use and lower bone mineral density that was related to increased clinical fragility fracture risk [12]. Accordingly, the development of alternative therapies for the prevention and treatment of menopausal depression has been of interest.
Hypericum Perforatum (HP), popularly known as St. John's Wort, has been used for thousands of years in folk medicine and has been proved scientifically as an effective treatment for mild to moderate depression. Many clinical and experimental studies have shown its antidepressant properties [13,14].
The present study hypothesized that ovariectomy of mature rats might have behavioral, biochemical and neurochemical changes like that of depression. It was designed to investigate the effect of different doses of Hypericum Perforatum (HP) compared to 17α-ethinylestradiol, on the following; serum and hippocampus corticosterone and TNF-α levels, hippocampus tryptophan, kynurenine and kynurenine/tryptophan ratio.
METHODS
Drugs and Chemicals
Hypericum Perfratum (gift from Atos pharma group, Egypt) in the form of black powder which was dissolved in distilled water. Sodium pentobarbital (Sigma, St. Louis, MO) in the form of white powder which was dissolved in distilled water. 17α-ethinylestradiol powder (Sigma, St. Louis, MO) which was dissolved by drops of absolute ethanol and then diluted by distilled water (vehicle). The ethanol was left to evaporate overnight and the solution was kept in a dark bottle at 4℃.
Animals
Thirty six mature Wistar rats (220~230 g) were purchased from the Holding Company for Biological Products & Vaccines VACCERA, Helwan, Egypt. Rats were allowed at least 1 week to acclimatize to the lab conditions. Rats' chow was purchased from Meladco for Animal Food, El-Obour, Egypt. Pellets and tap water were provided ad libitum. Temperature was maintained at 25℃. A 12/12 h dark/light cycle was maintained. All procedures were done according to guidelines of ethical Committee, pharmacology department, faculty of Medicine, Ain Shams University.
Study design and drug treatment
Rats were divided into the following six groups (n=6/each); group 1 was sham operated, groups 2, 3, 4, 5, 6 were ovariectomized (OVX). Groups 2 and 3 were treated orally with the vehicle and 17α-ethinylestradiol (30 µg/kg) [15] respectively. Group 4, 5 and 6: were treated orally with three different doses of HP 125, 250 and 500 mg/kg respectively. Treatments were started immediately after ovariectomy and continue for 30 days [16,17].
Ovariectomy procedures
Following the method outlined by Kimura et al. [18], the ovaries were resected bilaterally in which each rat was anesthetized with sodium pentobarbital (50 mg/kg, i.p.) [19]. Sham-operated animals underwent the same procedure as the ovariectomized rat but without resection of the ovaries.
Measurements and samples preparation:
1. Body Weight determination
To study the effect of ovariectomy, body weights of animals were recorded at the beginning and at the end of the study.
2. Behavioral tests
One day before animal scarification behavioral testing was conducted between 09:00 and 12:00 hours and was recorded on videotape.
1) Open Field Test (OFT) [20]
Open field test was used to detect the general locomotor activity in rats. The rats were allowed to acclimatize to the test room 1 hr before conducting the experiment. Each rat was placed individually in the center of the quadrangular arena (60×60 cm with 45 cm height walls) the arena is divided into 16 equal squares illuminated by white light. The test duration was conducted for 5 minutes to each rat. The number of crossed squares (with at least three paws) was calculated using the modified Open Control software [21] with the aid of a USB camera used to record the whole sessions. The arena was cleaned by 10% alcohol after each rat.
2) Forced Swimming Test (FST)
In the FST, rats were forced to swim in a vertical glass cylinder (diameter 22 cm, height 50 cm) containing 35 cm of water maintained at 25℃. The test was carried on 2 days. On the first day, rats were trained to swim for 10~15 min. Water was changed after testing of each animal. One day later rats were re-exposed to the forced swimming for 5 minutes. Behavior was videotaped and immobility time was measured with a stopwatch. Immobility time is the time during which the animal floats on the surface with its front paws together and makes only those movements which were necessary to keep it afloat. According to an original version of the FST [22], the test was performed on each rat only once; one day before sacrificing the animal.
3. Hippocampal dissection and Blood samples collection
At the end of the experiment after blood collection rats were decapitated quickly by sharp scissors. The heads were rapidly cooled in ice. Brains were dissected out on cooled plate. Hippocampus was accessed from the medial side after dividing the brain at the mid sagittal plane into two hemispheres, and removal of the whole brain stem and cerebellum. Hippocampus was then, dissected out. Hippocampus was rapidly put into ice cold eppindorf tubes and stored at -80℃ until used. All the dissection procedures were carried out rapidly and under strict cooling conditions. Tissues were rinsed in ice-cold phosphate buffered saline (PBS; 0.02 mol/L; pH 7.0~7.2). The tissues were minced to small pieces then homogenized in 5~10 ml of PBS with a glass homogenizer on ice. After that, the homogenates were centrifuged for 5 minutes at 3000 rpm and the supernatant was removed.
Blood samples were collected from retrorbital plexus under pentobarbital anesthesia in test tubes. Samples were subsequently centrifuged for 15 min (3000 rpm), then serum was immediately frozen (-80℃) to be stored until used.
4. Serum and hippocampus TNF-α levels
Both were measured using rat TNF-α ELISA kit developed by Quantikine, USA and RayBiotech, Inc., USA respectively according to the manufacturer's instructions.
5. Serum and hippocampus corticosterone levels
Both were measured using rat corticosterone ELISA kits developed by Kamiya Biomedical Co, USA and Shanhai Crystal Day Biotech Co, LTD., China respectively according to the manufacturer's instructions.
6. Hippocampus tryptophan and kynurenine levels
They were measured using ELISA Kits for Tryptophan, Kynurenine from Rocky Mountain Diagnostics, USA and MyBioSource USA respectively.
Statistical Analysis
All values in the results were expressed as mean±SD. Statistical analysis was performed using "Graph Pad prism", USA, version 5.01 (2007). Statistical difference among groups was determined using one way ANOVA followed by Tukey's Multiple Comparisons test. Differences were considered statistically significant at p<0.05. Correlation-coefficient analysis was performed by the Pearson's r as needed.
RESULTS
Effects of 17α -ethinylestradiol and HP on body of OVX rats
Compared to the changes in the sham-operated group, ovariectomy induced significant body weight gain reaching 116%, 17α-ethinylestradiol and HP at three different doses 125, 250, 500 mg/kg produced significant (p<0.05) reduction in body weight in comparison to OVX group (Table 1).
Table 1.
Body weight changes after treatment with Hypericum Perforatum at three different doses (HP; 125, 250, 500 mg/kg) or 17α-ethinylestradiol (E; 30 µg/kg) in ovariectomized rats
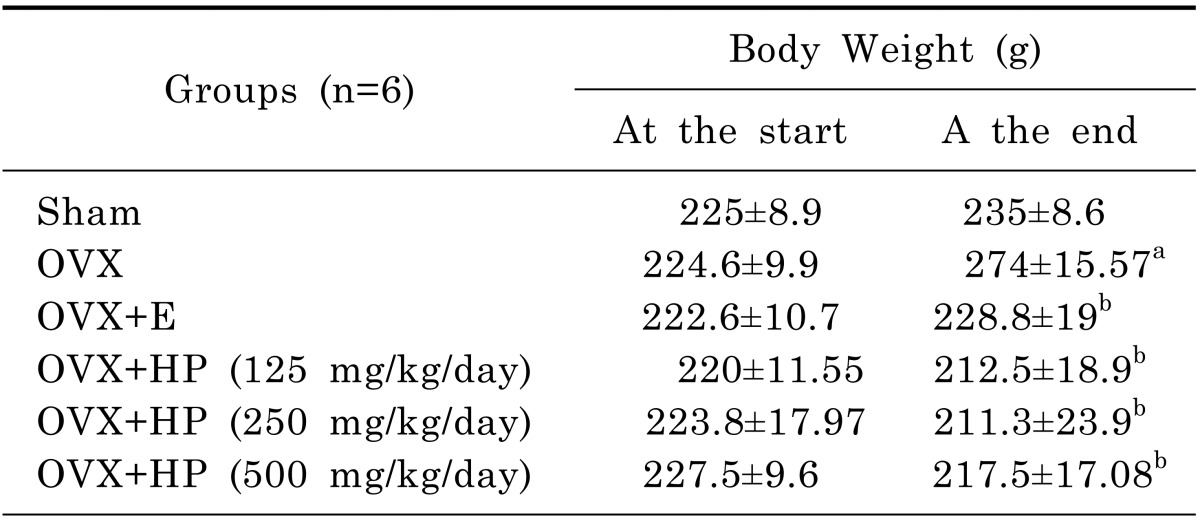
Data are mean±SD. n, number of animals; OVX, ovariectomized; HP, hypericum perforatum; E, 17α-ethinylestradiol. One way ANOVA followed by Tukey' Multiple Comparisons test: ap <0.05, compared to control sham operated group. bp<0.05 compared to OVX untreated group.
Effects of 17α-ethinylestradiol and HP on the behavior changes of OVX rats
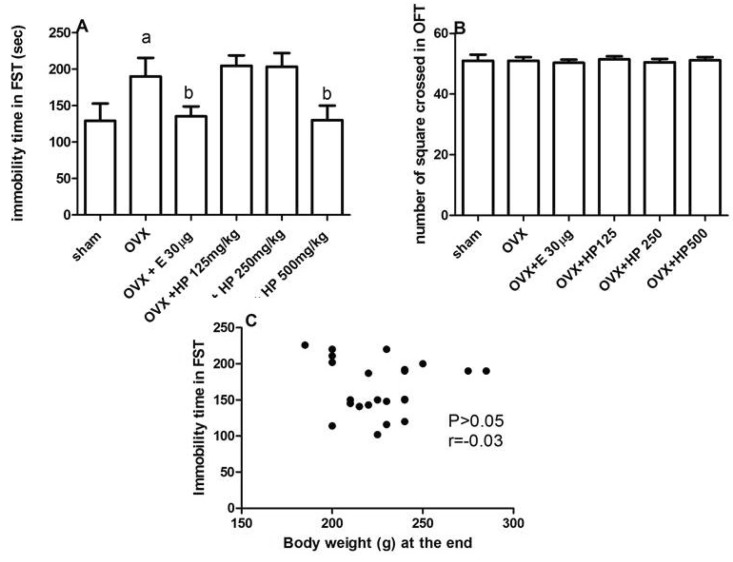
Fig. 1A revealed that, all OVX rats showed insignificant changes in Open field testing compared to sham rats. Fig. 1B showed that OVX rats exhibited depressive like behavior in the form of significant (p<0.05) prolongation in immobility time when exposed to FST in comparison to control sham operated group. Treatment with 17α-ethinylestradiol or HP significantly (p<0.05) ameliorated these depressive like behaviors in comparison to OVX untreated group. By Pearson's correlation coefficient there is insignificant (p>0.05) correlation between the immobility time recorded and the body weight of the rats, Fig. 1C.
Fig. 1.
Effect of treatment with 17α-ethinylestradiol (30 µg/kg) and Hpericum Perforatum (HP; 125, 250, 500 mg/kg) on the number of squares crossed in open field test (OFT) (A) and on immobility time of forced swimming test (FST) in seconds (B) of ovariectomized rats (OVX). Correlation between body weights at the end versus immobility time in FST in the different groups (C). Data are mean±SD, number of animals=6. One way ANOVA followed by Tukey's Multiple Comparisons test: ap<0.05, compared to control sham operated group. bp<0.05 compared to OVX untreated group and cp<0.05 compared to 17α-ethinylestradiol treated group. r, Pearson's correlation coefficient.
Effects of 17α -ethinylestradiol and HP on serum levels of TNFα and corticosterone of OVX rats
As shown in Table 2 OVX untreated rats showed significant (p<0.05) elevation in their serum levels of both TNF-α and corticosterone by 539.7% and 332.9% respectively in comparison to sham operated control group. In comparison to OVX untreated group, treatment with 17 α-ethinylestradiol showed significant (p<0.05) elevation of serum levels of TNF-α and corticosterone by 48.8% and 34.6% respectively. Meanwhile treatment by HP in the three different doses (125, 250, 500 mg/kg) showed significant (p<0.05) reduction in both parameters by 32.2%, 54.2% and 67.6% of TNF-α level and by 24.2%, 39.3% and 57% of corticosterone level in comparison to OVX untreated group respectively. It is worthy to mention that HP treated groups showed significant (p<0.05) dose dependent reduction of serum TNF-α level over 17α-ethinylestradiol group by 54.4%, 69.2% and 78.2% respectively, with significant (p<0.05) dose dependent reduction of serum corticosterone level by 43.7%, 54.9% and 68.1% respectively.
Table 2.
Serum TNFα and corticosterone after treatment with Hypericum Perforatum at three different doses (HP; 125, 250, 500 mg/kg) or 17α-ethinylestradiol (E; 30 µg/kg) in ovariectomized rats
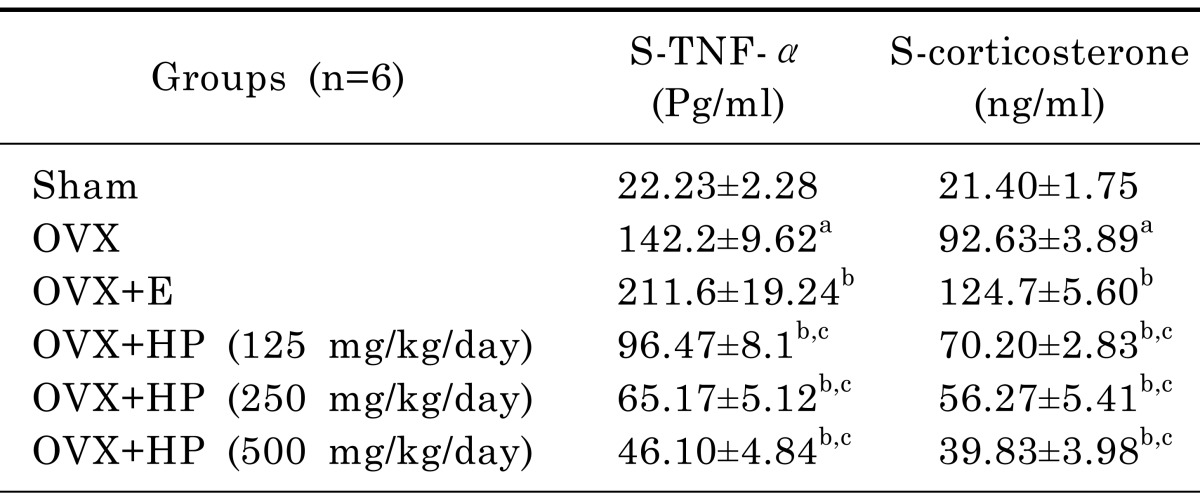
Data are mean±SD. n, number of animals; OVX, ovariectomized; HP, hypericum perforatum; E, 17α-ethinylestradiol. One way ANOVA followed by Tukey' Multiple Comparisons test: ap <0.05, compared to control sham operated group. bp<0.05 compared to OVX untreated group and cp<0.05 compared to 17α-ethinylestradiol treated group.
Effect of 17α-ethinylestradiol and HP on hippocampus levels of TNF-α and corticosterone of OVX rats
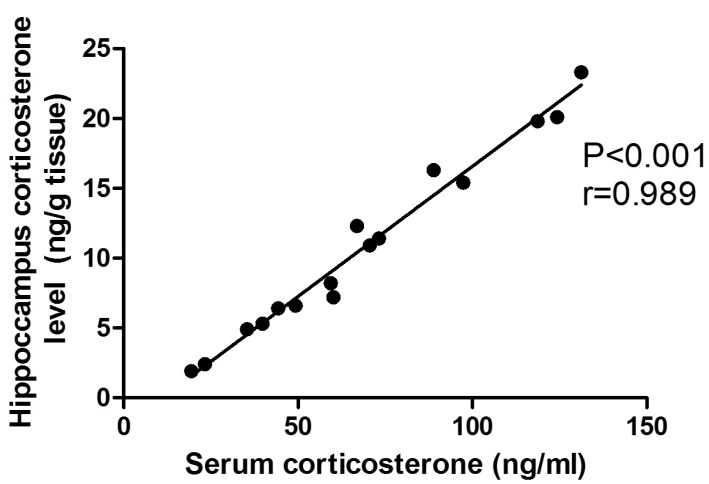
Table 3 showed that OVX rats had significant (p<0.05) elevation of hippocampal levels of TNF-α by 260.6%, and corticosterone by 622% in comparison to control sham operated group. Treatment with 17α-ethinylestradiol showed significant elevation of both TNF-α and corticosterone by 137.3% and 36.8% respectively in comparison to OVX untreated group. Treatment with HP in the three different doses (125, 250, 500 mg/kg) showed significant dose dependent reduction in both hippocampal TNFα and corticosterone by 24%, 46.1% & 57.2% and 25%, 52.4% & 64.1% respectively in comparison to OVX untreated group. Significant reduction of TNFα by 68%, 77.3% & 82% respectively and in corticosterone level by 45.3%, 65.2% & 73.8% respectively was observed in comparison to 17α-ethinylestradiol treated group. Fig. 2 illustrated positive correlation between hippocampal and plasma corticosterone level.
Table 3.
Effects of treatment with Hypericum Perforatum at three different doses (HP; 125, 250, 500 mg/kg) or 17α-ethinylestradiol (E; 30 µg/kg) on hippocampuslevels of TNFα and corticosterone in ovariectomized rats
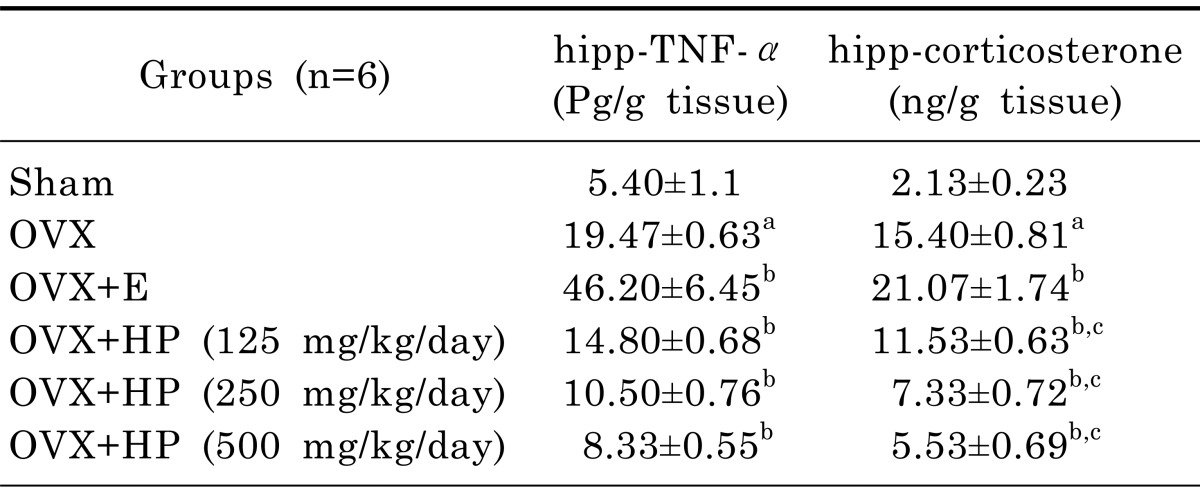
Data are mean±SD. n, number of animals; OVX, ovariectomized; HP, hypericum perforatum; E, 17α-ethinylestradiol. One way ANOVA followed by Tukey' Multiple Comparisons test: ap <0.05, compared to control sham operated group. bp<0.05 compared to OVX untreated group and cp<0.05 compared to 17α-ethinylestradiol treated group.
Fig. 2.
Correlation between serum versus hippocampus corticosterone level in the different group. r, Pearson's correlation coefficient, P significant level.
Effects of 17α -ethinylestradiol and HP on hippocampus tryptophan, kynurenine levels and kynurenine/tryptophan ratio of OVX rats
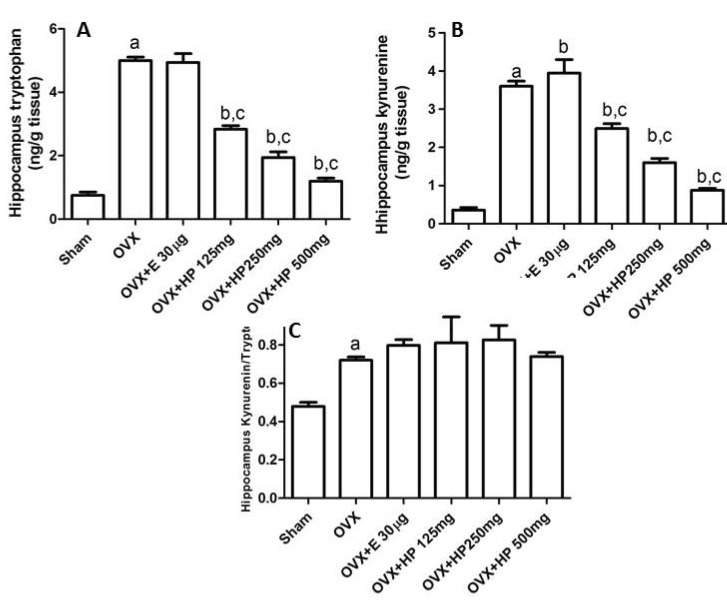
OVX untreated rats exhibited a significant (p<0.05) elevation of the hippocampal tryptophan, kynurenine and kynurenine/tryptophan ratio in comparison to control sham operated rats. Compared to ovariectomized untreated rats 17α-ethinylestradiol treated rats exhibited a significant (p <0.05) increase in the kynurenine level with insignificant (p>0.05) effect on the kynurenine/tryptophan ratio. Meanwhile HP in its three doses significantly (p<0.05) decreased both tryptophan and kynurenine levels with insignificant (p>0.05) effect on their ratio (Fig. 3).
Fig. 3.
Effects of treatment with 17α-ethinylestradiol (E; 30 µg/kg) and Hpericum Perforatum (HP; 125, 250, 500 mg/kg) on hippocampus. (A) Tryptophan, (B) kynurenine and (C) kynurenine/tryptophan ratio of OVX Wistar rats. Data are mean±SD, number of animals per group=6, OVX=ovariectomized. One way ANOVA followed by Tukey's Multiple Comparisons test: ap<0.05, compared to control sham operated group. bp<0.05 compared to OVX untreated group and cp<0.05 compared to 17α-ethinylestradiol treated group.
DISCUSSION
The present study hypothesized that the underlying mechanism of behavioral, biochemical, neurochemical changes of depression like state in OVX rats might be related to changes in tryptophan metabolism, corticosterone and TNF-α levels. Significant prolongations of immobility time in the forced swimming test 30 days after bilateral ovariectomy in the present study were observed. These observed results are supported by those of Okada and his colleagues [23]. Removal of the ovaries results in weight gain [24]. To exclude the possibility that body weight gain affect the immobility in FST, we evaluated the correlation between body weight and immobility in the OVX rats, using the Pearson correlation. There was no significant correlation between these two variables on the day of behavioral testing. Body weight at the end of the experiment in the OVX group was significantly greater than that in the sham-operated group. Conclusively, weight gain did not participate in the prolongation of immobility induced by Ovariectomy. Along with the insignificant affection of the general locomotor activities in rats which were reported with the open field test. Assumed that the duration of immobility in FST is closely related to helplessness, bilateral OVX induces a depressive-like state that may be analogous to some aspects of depression during menopause [25].
The present study demonstrates increased serum and hippocampal concentrations of TNF-α and corticosterone in OVX rats. Pro-inflammatory cytokines as TNF-α can cause depression by several mechanisms; activation of IDO leads to generation of neuroactive TRY metabolites, glucocorticoid receptor resistance, which amplifies the inflammatory response and leads to excessive production of corticotrophine releasing hormone [26] Hypothalamic pituitary axis dysregulation which is characterized by elevated circulating corticosterone concentrations is associated with mood disorders [27]. The present study revealed that hippocampal corticosterone concentration in rats is a linear function of its plasma concentration and that brain levels are usually less than plasma level. These support the hypothesis that corticosterone distributes passively into the brain [28].
Tryptophan does not have clear access to the brain from the plasma: 95% is protein-bound in the plasma, leaving 5% free to access the CNS [29]. It is transported across the blood-brain barrier via active transport in competition with the other large neutral amino acids [30]. Thus measurement of hippocampus level of TRY and KYN are more indicative of neurochemical changes.
As both the TNF-α and corticosterone are raised in OVX rats in the present study, it is not surprising that there was elevated KYN/TRY ratio in the hippocampal tissue of OVX rats as compared to sham operated. Elevated activity of the tryptophan-degrading enzyme IDO has emerged recently as one of the factors contributing to the pathogenesis of depression probably through the catabolism of TRY along the KYN pathway [31]. A measurement for in vivo IDO activity is the ratio of product/substrate (KYN/TRY). Thus, an increase in the ratio reflects greater enzyme activity, a decrease indicates lower activity [32]. Shift from serotonin synthesis to KYN pathway leads to decrease the former level which is one of the well-known neurochemical changes of depression [33]. Indeed TDO expression and activity can be increased by stress related glucocorticoid hormones [34].
In the current work 17α-ethinylestradiol treatment immediately after ovariectomy significantly shortened the duration of immobility which was similar to those previously reported by Galea et al. [35]. HP treatment significantly decreased the prolongation of immobility in a dose dependent manner. There have been many reports regarding the suppressive effects of HP by altering serotonin reuptake [36,37]. However, information is still limited regarding its effect on the immune system.
In the present study whereas treatment with 17α-ethinylestradiol exaggerated the effect of OVX on serum, hippocampal TNF-α, and corticosterone, HP significantly decreased their levels in a dose dependent manner. One sequel of prolonged corticosterone increase is a reduction in serotonin synthesis. Excessive corticosterone activates the enzyme TDO, shunting the dietary amino acid precursor tryptophan away from the serotonin pathway and into the kynurenine to niacin pathway resulting in inhibited serotonin production and reduced sensitivity of serotonin receptors [38]. Ethinylestradiol treatment significantly increased kynurenine with insignificant effect on the KYN/TRY ratio excluding its role in the antidepressant effect in OVX rats. Collectively, these led us to exclude the role of TNF-α, corticosterone and TRY metabolism in the antidepressant effect of 17α-ethinylestradiol.
HP in the present study treatment produced a significant decrease in plasma and hippocampal corticosterone levels, Franklin and his colleagues in 2005 reported that HP significantly reduced corticosterone in rat brain frontal cortex tissue by 30%. but these changes were not reflected in serum level [39].
In this study there were significant decreases in TRY and KYN levels with insignificant effect on KYN/TRY ratio in response to all doses of HP administration. Under normal circumstances the hepatic equivalent TDO metabolizes tryptophan into KYN. TDO is not induced by immune activation, but is constitutively active and is induced by glucocorticoids [40]. Under immune activation, IDO activity is increased, causing detectable increases in KYN and decreases in tryptophan. Two pathways for KYN metabolism one yield quinolinic acid, N-methyl-D-aspartate (NMDA) receptor agonist, which is neurotoxic due to increased oxidative stress and kynurenic acid an NMDA antagonist has been postulated to be neuroprotective [41]. Future work is needed to investigate HP effect on downstream of KYN metabolites.
Although HP insignificantly affect hippocampus KYN/TRY ratio it decreased serum, hippocampal TNF-α and corticosterone levels. Zhu and his colleagues reported that TNF-α can cause depression by mechanisms other than activation of IDO, it may increase glucocorticoid receptor resistance, or activate the serotonin transporter on neurons [42].
It is concluded from the present results that increased tryptophan metabolism toward kynurenine secondary to elevated corticosterone and TNF-α might be one of the pathophysiological mechanisms that could explain depression like state observed in this model of ovariectomized rats. Further, the observed attenuating effect of HP on TNF-α and corticosterone could contribute in its antidepressant effect in this animal model by other ways than their effects on tryptophan-kynurenine metabolism pathway, which need further exploration for example, estimation of serotonin.
ACKNOWLEDGEMENTS
This research was officially supported by the Medical Research Service of the Ain Shams.
ABBREVIATIONS
- OVX
ovariectomy
- IDO
indoleamine 2, 3-dioxygenase
- TRY
tryptophan
- KYN
kynurenine
- TNF-α
tumor necrosis factor α
- TDO
TRY 2, 3-dioxygenase
- PMD
postmenopausal depression
- HP
Hypericum Perforatum
- E
17 α ethinylestradiol
- OFT
open field test
References
- 1.Bromberger JT, Matthews KA, Schott LL, Brockwell S, Avis NE, Kravitz HM, Everson-Rose SA, Gold EB, Sowers M, Randolph JF., Jr Depressive symptoms during the menopausal transition: the Study of Women's Health Across the Nation (SWAN) J Affect Disord. 2007;103:267–272. doi: 10.1016/j.jad.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llaneza P, García-Portilla MP, Llaneza-Suárez D, Armott B, Pérez-López FR. Depressive disorders and the menopause transition. Maturitas. 2012;71:120–130. doi: 10.1016/j.maturitas.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Maes M, Smith R, Scharpe S. The monocyte-T-lymphocyte hypothesis of major depression. Psychoneuroendocrinology. 1995;20:111–116. doi: 10.1016/0306-4530(94)00066-j. [DOI] [PubMed] [Google Scholar]
- 4.Simen BB, Duman CH, Simen AA, Duman RS. TNFalpha signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biol Psychiatry. 2006;59:775–785. doi: 10.1016/j.biopsych.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Berthold-Losleben M, Himmerich H. The TNF-alpha system: functional aspects in depression, narcolepsy and psychopharmacology. Curr Neuropharmacol. 2008;6:193–202. doi: 10.2174/157015908785777238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cioffi M, Esposito K, Vietri MT, Gazzerro P, DAuria A, Ardovino I, Puca GA, Molinari AM. Cytokine pattern in postmenopause. Maturitas. 2002;41:187–192. doi: 10.1016/s0378-5122(01)00286-9. [DOI] [PubMed] [Google Scholar]
- 7.Oxenkrug GF. Metabolic syndrome, age-associated neuroendocrine disorders, and dysregulation of tryptophan-kynurenine metabolism. Ann N Y Acad Sci. 2010;1199:1–14. doi: 10.1111/j.1749-6632.2009.05356.x. [DOI] [PubMed] [Google Scholar]
- 8.Bhavnani BR, Strickler RC. Menopausal hormone therapy. J Obstet Gynaecol Can. 2005;27:137–162. doi: 10.1016/s1701-2163(16)30186-4. [DOI] [PubMed] [Google Scholar]
- 9.Fink G, Sumner B, Rosie R, Wilson H, McQueen J. Androgen actions on central serotonin neurotransmission: relevance for mood, mental state and memory. Behav Brain Res. 1999;105:53–68. doi: 10.1016/s0166-4328(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 10.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's HealthInitiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 11.Gyllstrom ME, Schreiner PJ, Harlow BL. Perimenopause and depression: strength of association, causal mechanisms and treatment recommendations. Best Pract Res Clin Obstet Gynaecol. 2007;21:275–292. doi: 10.1016/j.bpobgyn.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Richards JB, Papaioannou A, Adachi JD, Joseph L, Whitson HE, Prior JC, Goltzman D Canadian Multicentre Osteoporosis Study Research Group. Effect of selective serotonin reuptake inhibitors on the risk of fracture. Arch Intern Med. 2007;167:188–194. doi: 10.1001/archinte.167.2.188. [DOI] [PubMed] [Google Scholar]
- 13.Volz HP. Controlled clinical trials of hypericum extracts in depressed patients--an overview. Pharmacopsychiatry. 1997;30(Suppl 2):72–76. doi: 10.1055/s-2007-979522. [DOI] [PubMed] [Google Scholar]
- 14.Oztürk Y. Testing the antidepressant effects of Hypericum species on animal models. Pharmacopsychiatry. 1997;30(Suppl 2):125–128. doi: 10.1055/s-2007-979532. [DOI] [PubMed] [Google Scholar]
- 15.Picherit C, Coxam V, Bennetau-Pelissero C, Kati-Coulibaly S, Davicco MJ, Lebecque P, Barlet JP. Daidzein is more efficient than genistein in preventing ovariectomy-induced bone loss in rats. J Nutr. 2000;130:1675–1681. doi: 10.1093/jn/130.7.1675. [DOI] [PubMed] [Google Scholar]
- 16.Micioni Di, Vitale G, Massi M, Cifani C. Effect of hypericum perforatum extract in an experimental model of binge eating in female rats. J Obes. 2012;2012:956137. doi: 10.1155/2012/956137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perfumi M1, Panocka I, Ciccocioppo R, Vitali D, Froldi R, Massi M. Effects of a methanolic extract and a hyperforin-enriched CO2 extract of Hypericum perforatum on alcohol intake in rats. Alcohol Alcohol. 2001;36:199–206. doi: 10.1093/alcalc/36.3.199. [DOI] [PubMed] [Google Scholar]
- 18.Kimura F, Mitsugi N, Arita J, Akema T, Yoshida K. Effects of preoptic injections of gastrin, cholecystokinin, secretin, vasoactive intestinal peptide and PHI on the secretion of luteinizing hormone and prolactin in ovariectomized estrogenprimed rats. Brain Res. 1987;410:315–322. doi: 10.1016/0006-8993(87)90330-1. [DOI] [PubMed] [Google Scholar]
- 19.Park HJ, Han SM, Yoon WJ, Kim KS, Shim I. The effects of puerariae flos on stress-induced deficits of learning and memory in ovariectomized female rats. Korean J Physiol Pharmacol. 2009;13:85–89. doi: 10.4196/kjpp.2009.13.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roth KA, Katz RJ. Stress, behavioral arousal, and open field activity--a reexamination of emotionality in the rat. Neurosci Biobehav Rev. 1979;3:247–263. doi: 10.1016/0149-7634(79)90012-5. [DOI] [PubMed] [Google Scholar]
- 21.Aguiar P, Mendonça L, Galhardo V. OpenControl: a free opensource software for video tracking and automated control of behavioral mazes. J Neurosci Methods. 2007;166:66–72. doi: 10.1016/j.jneumeth.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 22.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 23.Okada M, Hayashi N, Kometani M, Nakao K, Inukai T. Influences of ovariectomy and continuous replacement of 17beta-estradiol on the tail skin temperature and behavior in the forced swimming test in rats. Jpn J Pharmacol. 1997;73:93–96. doi: 10.1254/jjp.73.93. [DOI] [PubMed] [Google Scholar]
- 24.Swithers SE, Sample CH, Katz DP. Influence of ovarian and non-ovarian estrogens on weight gain in response to disruption of sweet taste--calorie relations in female rats. Horm Behav. 2013;63:40–48. doi: 10.1016/j.yhbeh.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor M. Psychological consequences of surgical menopause. J Reprod Med. 2001;46(3 Suppl):317–324. [PubMed] [Google Scholar]
- 26.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bano S, Gitay M, Ara I, Badawy A. Acute effects of serotonergic antidepressants on tryptophan metabolism and corticosterone levels in rats. Pak J Pharm Sci. 2010;23:266–272. [PubMed] [Google Scholar]
- 28.Weber CC, Eckert GP, Müller WE. Effects of antidepressants on the brain/plasma distribution of corticosterone. Neuropsychopharmacology. 2006;31:2443–2448. doi: 10.1038/sj.npp.1301076. [DOI] [PubMed] [Google Scholar]
- 29.McMenamy RH. Binding of indole analogues to human serum albumin. Effects of fatty acids. J Biol Chem. 1965;240:4235–4243. [PubMed] [Google Scholar]
- 30.Oldendorf WH, Szabo J. Amino acid assignment to one of three blood-brain barrier amino acid carriers. Am J Physiol. 1976;230:94–98. doi: 10.1152/ajplegacy.1976.230.1.94. [DOI] [PubMed] [Google Scholar]
- 31.Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. The new '5-HT' hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:702–721. doi: 10.1016/j.pnpbp.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Schröcksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta. 2006;364:82–90. doi: 10.1016/j.cca.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Hindmarch I. Beyond the monoamine hypothesis: mechanisms,molecules and methods. Eur Psychiatry. 2002;17(Suppl 3):294–299. doi: 10.1016/s0924-9338(02)00653-3. [DOI] [PubMed] [Google Scholar]
- 34.Ara I, Bano S. Citalopram decreases tryptophan 2,3-dioxygenase activity and brain 5-HT turnover in swim stressed rats. Pharmacol Rep. 2012;64:558–566. doi: 10.1016/s1734-1140(12)70851-4. [DOI] [PubMed] [Google Scholar]
- 35.Galea LA, Wide JK, Barr AM. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behav Brain Res. 2001;122:1–9. doi: 10.1016/s0166-4328(01)00170-x. [DOI] [PubMed] [Google Scholar]
- 36.Hirano K, Kato Y, Uchida S, Sugimoto Y, Yamada J, Umegaki K, Yamada S. Effects of oral administration of extracts of Hypericum perforatum (St John's wort) on brain serotonin transporter, serotonin uptake and behaviour in mice. J Pharm Pharmacol. 2004;56:1589–1595. doi: 10.1211/0022357045039. [DOI] [PubMed] [Google Scholar]
- 37.Kientsch U, Bürgi S, Ruedeberg C, Probst S, Honegger UE. St. John's wort extract Ze 117 (Hypericum perforatum) inhibits norepinephrine and serotonin uptake into rat brain slices and reduces 3-adrenoceptor numbers on cultured rat brain cells. Pharmacopsychiatry. 2001;34(Suppl 1):S56–S60. doi: 10.1055/s-2001-15452. [DOI] [PubMed] [Google Scholar]
- 38.Linde K, Ramirez G, Mulrow CD, Pauls A, Weidenhammer W, Melchart D. St John's wort for depression--an overview and meta-analysis of randomised clinical trials. BMJ. 1996;313:253–258. doi: 10.1136/bmj.313.7052.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franklin M, Reed A, Murck H. Sub-chronic treatment with an extract of Hypericum perforatum (St John's wort) significantly reduces cortisol and corticosterone in the rat brain. Eur Neuropsychopharmacol. 2004;14:7–10. doi: 10.1016/s0924-977x(03)00038-5. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura T, Niimi S, Nawa K, Noda C, Ichihara A, Takagi Y, Anai M, Sakaki Y. Multihormonal regulation of transcription of the tryptophan 2,3-dioxygenase gene in primary cultures of adult rat hepatocytes with special reference to the presence of a transcriptional protein mediating the action of glucocorticoids. J Biol Chem. 1987;262:727–733. [PubMed] [Google Scholar]
- 41.Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- 42.Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31:2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]