Abstract
Major advances in highly active antiretroviral therapies (HAART) have extended the lives of people living with HIV, but there still remains an increased risk of death by cardiovascular diseases (CVD). HIV proteins have been shown to contribute to cardiovascular dysfunction with effects on the different cell types that comprise the arterial wall. In particular, HIV-1 transactivating factor (Tat) has been shown to bind to endothelial cells inducing a range of responses that contribute to vascular dysfunction. It is well established that hemodynamics also play an important role in endothelial cell mediated atherosclerotic development. When exposed to low or oscillatory shear stress, such as that found at branches and bifurcations, endothelial cells contribute to proteolytic vascular remodeling by upregulating cathepsins, potent elastases and collagenases that contribute to altered biomechanics and plaque formation. Mechanisms to understand the influence of Tat on shear stress mediated vascular remodeling have not been fully elucidated. Using an in vivo HIV-Tg mouse model and an in vitro cone and plate shear stress bioreactor to actuate physiologically relevant pro-atherogenic or atheroprotective shear stress on human aortic endothelial cells, we have shown synergism between HIV proteins and pro-atherogenic shear stress to increase endothelial cell expression of the powerful protease cathepsin K, and may implicate this protease in accelerated cardiovascular disease in people living with HIV.
Keywords: cathepsin, HIV, Tat, arterial remodeling, shear stress
Introduction
Major advances in highly active antiretroviral therapies (HAART) have extended the lives of people living with HIV, but there still remains an increased risk of death by cardiovascular diseases (CVD). In 2010, the World Health Organization reported 34 million people living with Human Immunodeficiency Virus (HIV) infection globally, with 1.8 million cases resulting in death per year. HIV populations have demonstrated an increased risk of myocardial infarction, endothelial dysfunction, atherosclerotic lesions, carotid artery intima-medial thickness, and artery stiffness 1–9.
Studies have elucidated whether this increase in cardiovascular incidence is due to antiretroviral treatment or the virus itself. The SMART Study showed that HIV-positive patients’ risks for cardiovascular disease were increased when patients were taken off their antiretroviral therapies, even when CD4+ counts were kept below a certain threshold, demonstrating that some of these factors for cardiovascular disease are due to the virus itself10. We have recently shown that the antiretroviral AZT stiffens arteries in wildtype mice11, and have also shown that HIV proteins induce arterial stiffening in an HIV transgenic mouse model12, contributing to both sides of the debate. HIV proteins enter the bloodstream after being secreted by HIV-infected cells or shed from lysing cells without incorporation into new virions 13. Of particular interest is HIV-1 transactivating factor (Tat), a 14-kDa protein that binds to the secondary structure sequence TAR (Tat activation region), enabling the recruitment of cellular factors to activate transcription and elongation, performing regulatory functions and increasing infectivity of the virus. Tat has been shown to bind to endothelial cells and alter proliferation, apoptosis, matrix metalloprotease-2 production, migration, substrate adhesion, angiogenesis, leukocyte adhesion, and vascular permeability14–17.
It is well established that hemodynamics also play an important role in endothelial cell mediated atherosclerotic development. Atherosclerotic plaques preferentially form at branches and sharp turns in the arterial tree such as found on the outer wall of the carotid artery, lesser curvature of aortic arch, and abdominal aorta; sites where endothelial cells are exposed to low and oscillatory shear stress18. Among other effects, oscillatory shear stress has been shown to upregulate endothelial cell production of cathepsins, powerful elastases and collagenases that have been implicated in human cardiovascular disease, particularly cathepsins K and L19,20. These proteases remodel the extracellular matrix, allowing the plaque to grow, and change the mechanical properties of the arterial wall 21–25.
Here we tested the synergistic effects of oscillatory shear stress and HIV proteins on cathepsin production and activity by measuring cathepsin levels at distinct hemodynamic regions, characterized as pro-atherogenic or atheroprotective, in an HIV transgenic mouse model. This was then validated in human aortic endothelial cells using a shear stress bioreactor to actuate physiologically relevant pro-atherogenic or atheroprotective pulsatile shear stress profiles from the carotid artery, and co-stimulated with Tat, an HIV protein that activates inflammatory responses in endothelial cells. Altogether, this work tests the hypothesis that disturbed blood flow and low, oscillatory shear stress on the endothelium, with HIV proteins, specifically Tat, synergistically increase cathepsin activity that may participate in accelerated arterial wall remodeling.
Methods
HIV-Transgenic Mouse Model
Male hemizygous NL4-3Δ gag/pol transgenic and wild-type littermate (FVB/N) mice, 10–12 weeks old were euthanized with CO2 and the aorta removed and cleaned free of loose perivascular tissue. Unlike the homozygous HIV-Tg mice, which are smaller at birth, have decreased food intake compared to wild-type mice, and usually die within 40 days postnatal, the hemizygous mice appear normal at birth, but develop signs of disease, specifically renal failure known as HIV-associated nephropathy26. The aortic arch, thoracic aorta, and abdominal aorta were separated for immunohistochemistry and cathepsin zymography. All work was conducted under the regulation of Georgia Institute of Technology’s and Atlanta VA Medical Center’s Institutional Animal Care and Use Committee (IACUC).
Cathepsin Immunohistochemistry
Localization of cathepsin protein expression in the aortic wall was determined by immunohistochemistry of aortic arches. Aortic arches were embedded in OCT medium and the orientation of the greater and lesser curvature was carefully marked prior to snap freezing and storage at −80°C. Tissue sections (8 μm thick) were collected with a Leica CM3050 Cryostat and mounted on glass slides. Sections were fixed in acetone, rinsed with PBS, and then blocked in 3% BSA for an hour. Sections were immunolabeled with primary cathepsin K (Santa Cruz), cathepsin S, or mouse cathepsin L antibodies (R&D Systems) at 4°C overnight at a 1:50 or 1:100 dilution in 1% BSA and then probed with TRITC conjugated anti-rabbit or FITC conjugated-anti-goat fluorescent secondary antibodies (Life Technologies) at room temperature for an hour at a 1:100 dilution in the dark. Negative controls were incubated without primary antibody. Sections were then mounted using DAPI Pro-long Gold Antifade reagent (Life Technologies) and imaged using a Nikon Ti-E fluorescent microscope.
Cathepsin and MMP Zymography
Excised thoracic and abdominal aortas were separated below the diaphragm and stored on ice in PBS until placed in 50 μl of zymography lysis buffer (20 nM Tris–HCl at pH 7.5, 5 mM EGTA, 150 mM NaCl, 20 mM β-glycerol-phosphate, 10 mM NaF, 1 mM sodium orthovanadate, 1% Triton X-100, 0.1% Tween-20) with 0.1 mM leupeptin freshly added to stabilize enzymes during electrophoresis. Aortas were homogenized using disposable sample grinders (GE Healthcare), lysates were collected and cleared by centrifugation, and total protein concentration was determined by micro-bisinchoninic acid (BCA) assay (Pierce). Multiplex cathepsin zymography was performed as described previously 27,28 and MMP zymography according to Galis et al.29. Gel images were captured with an Imagequant 4010 (GE Healthcare), then images were inverted in Adobe Photoshop and densitometry performed using NIH ImageJ.
Endothelial Cell Culture
Human aortic endothelial cells (HAECs) (Lonza) were cultured in MCDB medium 131 (Mediatech) containing 10% fetal bovine serum (FBS), 1% L-glutamine, 1% penicillin/streptomycin, 1% endothelial cell growth serum (ECGS), and supplemental growth factors hydrocortisone (.001 mg/ml), fibroblast growth factor (.002 μg/ml), epidermal growth factor (.010 μg/ml), insulin-like growth factor (.002 μg/ml), VEGF (.001 μg/ml), and ascorbic acid (50μg/ml). Cells were maintained with 5% CO2 at 37°C in 10 cm dishes until confluent and used between passage 6 and 8. Upon confluence, medium was changed to Endothelial Growth Medium (EGM) (Lonza) for overnight, low serum culture prior to treatment with Tat (69.4 nM) (ImmunoDiagnostix) and exposure to shear stress profiles.
Shear Stress Bioreactor
The atheroprotective unidirectional waveforms and pro-atherogenic oscillatory waveforms that were used in this study were first characterized in humans by Dai et al30. The atheroprotective and pro-atherogenic waveforms are generated using a cone-and-plate shear system consisting of polytetrafluoroethylene (PTFE) cones with a 0.5 angle. The cones are connected to the drive shaft of a SM232AQ servomotor, which actuates the user-defined motion profile. A vacuum plate is used to secure the culture plates to a base platform while the cones are in motion. The shear stress bioreactor is contained in an incubator maintained at 37°C and 5% CO2.
Statistical Analysis
Each experimental condition was repeated with a minimum of three biological replicates, and each data point is presented as the mean value +/− standard error of the mean. Representative images are shown. Unpaired student t-tests were used to determine statistical significance between most experimental groups.
Results
Cathepsin K protein expression is higher in the lesser curvature of the aortic arch in HIV-Tg mice
Our previous findings showed increased cathepsin K activity in aortas of HIV-Tg mice as a mechanism for increased aorta stiffness 12, however we wanted to examine cathepsin expression in flow defined regions. We first investigated the aortic arch in HIV-Tg murine models since sites of atherosclerotic lesion development have been described hemodynamically, with the greater curvature being a site of atheroprotective high shear stress, and the lesser curvature exposed to pro-atherogenic, low and oscillatory shear stress 31. NL4-3Δ gag/pol heterozygote transgenic mice were sacrificed between 10–12 weeks and compared to littermate controls. Aortic arches were carefully embedded to denote the greater curvature and lesser curvature, cryopreserved, and immunohistochemical staining for cathepsins K, L, and S was performed. From the staining, it can be seen that there was greater overall red fluorescent intensity in HIV-Tg mice compared to littermate controls, indicating greater cathepsin K protein expression (n=3, representative images are shown for each stain) (Fig 1). These increases in expression were strongest in the intimal layer in wildtype littermate controls, but were throughout the medial and intimal layers within the HIV-Tg mice aortic arches. Asymmetry in cathepsin K staining was observed with the lesser curvature being higher compared to the greater curvature for both the HIV-Tg mice and the littermate controls. Taken together, these results suggest that both shear stress and HIV proteins could be contributing to upregulation of cathepsin K protein expression in artery wall.
Figure 1. HIV-Tg mice have greater cathepsin K expression in the lesser curvature of the aortic arch than the greater curvature.
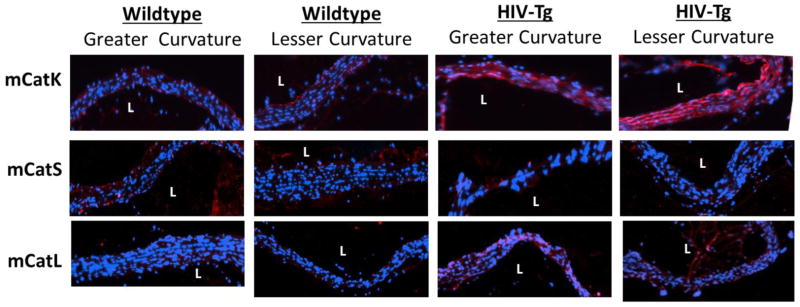
HIV-Tg mouse aortic arches were excised and oriented by their regions of lesser curvature (pro-atherogenic) and greater curvature (atheroprotected), where L denotes the lumen of the vessel. Immunohistochemical staining was carried out using antibodies for cathepsin K, mouse cathepsin L, and cathepsin S. Zoomed images show increased cathepsin K staining, indicated in red, in HIV-Tg mice compared to in littermate controls with increased staining in the lesser curvature compared to the greater curvature. There were not differences in staining for cathepsin L and cathepsin S between regions of greater and lesser curvature (n=3 for wildtype, n=4 for HIV Tg, representative images shown).
There were no observed asymmetrical differences for cathepsins S or mouse cathepsin L (n=3, representative images are shown for each stain).
Mature, active cathepsins K and S are increased at hemodynamically defined regions in aortas of HIV-Tg mice
Immunohistochemistry indicated that at regions of pro-atherogenic disturbed blood flow such as the lesser curvature, there was increased cathepsin K protein expression. Next it was important to confirm that the cathepsin K identified immunohistochemically was the mature, active form. Antibodies used for immunohistochemistry bind to and detect both the pro (inactive) and the mature (active) cathepsins, giving an overall, total cathepsin protein level, but does not accurately quantify active cathepsins that may be involved in proteolysis within the arterial wall. To investigate this, we used multiplex cathepsin zymography, an assay that can quantify amounts of active cathepsin K, L, and S in cells or tissue extracts. In situ labeling provided by immunohistochemistry showed asymmetry at the greater and lesser curvature of the aorta whereas zymography involves homogenization of the aortic tissue for gel electrophoresis.
The aortic arch region is too small to obtain the protein amounts necessary for detection of active cathepsins K, S, or L by zymography. Therefore we used thoracic and abdominal aorta segments, which have also been defined hemodynamically32,33 The thoracic aorta has been characterized dominantly by atheroprotective, high unidirectional shear stress, and the abdominal aorta with regions of pro-atherogenic, low and oscillatory shear stress18. Aortas were excised, cut into these two hemodynamically defined regions just below the diaphragm, cleaned, and homogenized. Total protein concentration was determined, and equal amounts of protein were loaded for cathepsin zymography to quantify differences in the amount of active, mouse cathepsins K, L, and S in the aorta wall in response to hemodynamics and HIV proteins (Fig 2).
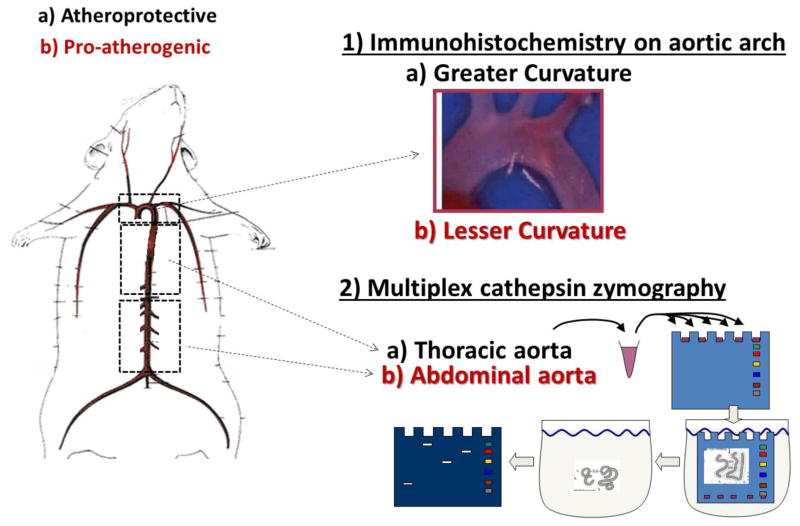
Figure 2. Schematic of aorta segmenting and experimental analysis.
The aortic arch has distinct regions that allow for investigation of pro-atherogenic shear stress region (lesser curvature) and atheroprotected shear stress region (greater curvature) within the same animal. To obtain more protein, the thoracic (atheroprotected) and abdominal (pro-atherogenic) aorta were excised, cleaned, segmented, and homogenized. The tissue lysate was prepared for multiplex zymography to quantify the amounts of active cathepsins K, L, and S by shear stress region. In the schematic, black text denotes atheroprotective shear stress regions (a-greater curvature of arch, thoracic aorta) and red text denotes pro-atherogenic shear stress regions (b – lesser curvature of arch, abdominal aorta).
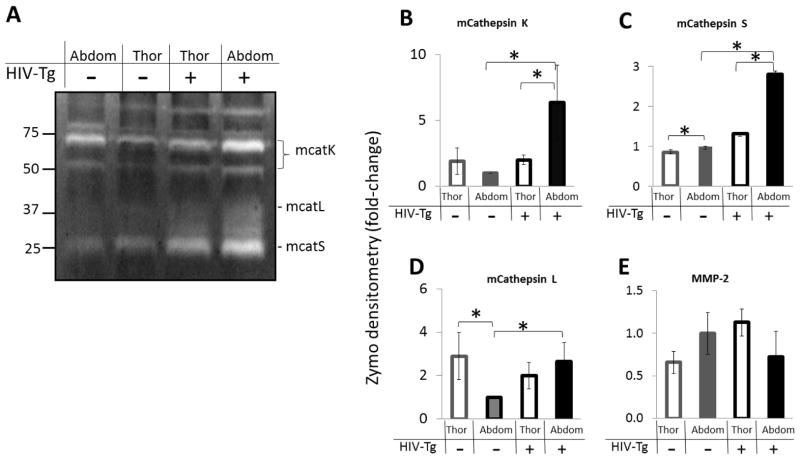
A representative zymogram of four mouse aortas tested is shown in Figure 3A with quantified densitometry in (Fig 3B–E). Abdominal aortas of HIV-Tg mice had higher levels of active cathepsin K compared to thoracic aortas of HIV-Tg mice and compared to the abdominal aortas of wildtype mice (7-fold increase) (n=4, p<.05). For wildtype mice, there was no significant difference in amounts of mature cathepsin K between the thoracic and abdominal aorta when averaged over the four mice (Fig 3B). Similarly, the highest amount of active cathepsin S was found in the abdominal aorta of HIV-Tg mice compared to thoracic HIV-Tg and wildtype mice aorta segments (n=4, p<.05) (Fig 3C).
Figure 3. Mouse cathepsins K, S and L activity are increased in abdominal aortas of HIV-Tg mouse aortas.
A) Multiplex cathepsin zymography was performed on HIV-Tg and littermate control mouse aortas excised and separated into the abdominal and thoracic aortas. Densitometry values are shown for B) mouse cathepsin K, C) mouse cathepsin S, D) mouse cathepsin L, and E) MMP-2 (* indicates p < 0.05, n=4, and data is mean ± SEM).
Mouse cathepsin L activity showed a 3-fold decrease in wildtype abdominal aortas compared to wildtype thoracic aortas, a discrepancy from what was observed for cathepsins K and S (Fig 3A,D). However, similarly to cathepsins K and S, there was by 2.4-fold increased cathepsin L activity in HIV-Tg abdominal aortas compared to wildtype abdominal aortas (n=4, p<.05) (Fig 3D). No significant differences were observed for MMP-2 due to aorta region or HIV-Tg status (Fig 3E).
To parse effects on cathepsin activity regulation due to shear stress from that of HIV proteins for each mouse, ratiometric comparisons were used. The influence of shear stress region was examined by dividing cathepsin activity in pro-atherogenic regions by that of atheroprotected regions within the same animal, as quantified from densitometric analysis of the zymography. If this ratio was higher than one, then cathepsin activity was higher in the abdominal aorta where pro-atherogenic flow dominates, if lower than one, then the activity was higher in the thoracic aorta which experiences more atheroprotective shear stresses, and when equal to one, there were no shear stress dependent differences. In HIV-Tg mice, the ratio for cathepsin K activity was 3.2 indicating a 3.2 fold increased cathepsin K in abdominal aortas compared to thoracic, but the ratio was close to one for the control mice (Fig 4A), indicating that the effect seen in the HIV-Tg mice was not seen in control mice (n=4, p<.05).
Figure 4. Ratiometric comparisons to parse regulation due to shear stress from that of HIV proteins.
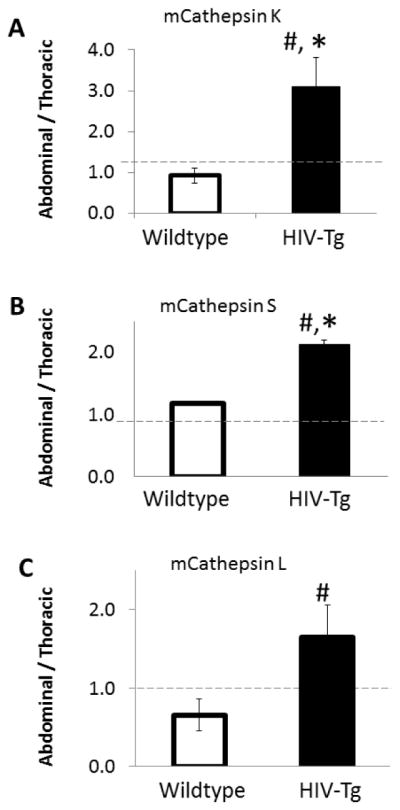
The influence of shear stress region was examined by dividing cathepsin activity in pro-atherogenic regions by that of atheroprotected regions within the same animal, as quantified from densitometric analysis of the zymography. If this ratio was higher than one, then cathepsin activity was higher in the abdominal aorta where pro-atherogenic flow dominates, if lower than one, then the activity was higher in the thoracic aorta, and when equal to one, there were no shear stress dependent differences. Analysis for A) mouse cathepsin K, B) mouse cathepsin S, and C) mouse cathepsin L are shown. (* indicates p < 0.05, n=4 and data is mean +/− SEM)(# indicates ratio is statistically increased p<.05, n=4).
Cathepsin S activity was 2-fold higher in pro-atherogenic regions (abdominal aorta) compared to atheroprotected regions (thoracic aorta) and also significantly higher than the wildtype ratio, which was close to one (Fig 4B, n=4, p<.05). For mouse cathepsin L we did not observe a significant increase in the ratio for HIV-Tg mice, however the HIV-Tg ratio was significantly higher than the ratio of the wildtype mice (Fig 4C, n=4, p<.05).
Co-stimulation with pro-atherogenic shear stress and Tat increase cathepsin K and V activity in human aortic endothelial cells
The HIV-1 protein Tat has been shown to stimulate human endothelial cell activation14,15,34–36, but neither Tat’s specific links to stimulating cathepsin production by endothelial cells nor any synergism with shear stress has been shown. There is a different cathepsin profile in human cells compared to mouse cells in that the nomenclature in mice is cathepsins K, S, and L, but in humans, they express cathepsins K, S, L, and L2, which is frequently termed cathepsin V (or cathepsin L2). Human cathepsin V (L2) and human cathepsin L share 80% homology37, but the mouse genome encodes for only one cathepsin L, which is 75% homologous and has similar biochemical properties with human cathepsin V; consequently, mouse cathepsin L and human cathepsin V are orthologs to compare across species, and human cathepsin L has no ortholog in mice38. To summarize, murine cathepsin L is compared to human cathepsin V, and there is no murine cathepsin V. We have also shown that cathepsins K, L, S, and V can all be detected simultaneously by the multiplex cathepsin zymography assay with human cathepsin V appearing at 37 kDa and human cathepsin L at 20 kDa28; cathepsins K and S migrate as in mice.
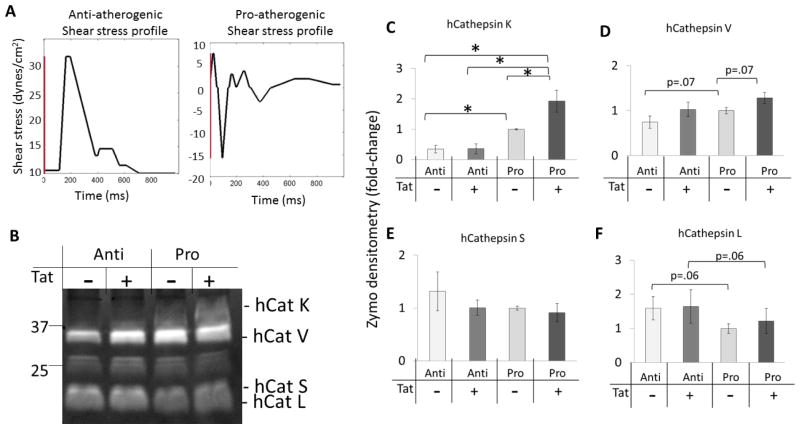
Tat circulates in the bloodstream at an estimated 2 ng/ml – 40 ng/ml range, and is secreted from infected T-cells and monocytes. Because macrophages may act as viral reservoirs within the arterial wall, it is believed that endothelial cells are exposed to even higher concentrations of Tat secreted locally by these HIV infected cells 39. Here we tested the hypothesis that Tat was sufficient to induce cathepsin activity in human endothelial cells cultured under fluid flow with atheroprotective or pro-atherogenic shear stress using a cone-and-plate bioreactor as described in the Methods section (Fig 5A), and lysed for multiplex cathepsin zymography (Fig 5B). Endothelial cells stimulated with HIV-1 Tat and cultured under pro-atherogenic shear stress, had higher levels of active human cathepsin K (Fig 5C) compared to vehicle control and atheroprotective conditions (n=5, p<.05). The amount of active cathepsin V was also increased compared to Tat-stimulated atheroprotective conditions (Fig 5D). Endothelial cells showed no increases in cathepsin S or L activity in response to Tat and shear stress stimulation. (Fig 5E,F; n=5, p<.05).
Figure 5. Human aortic endothelial cells stimulated with pro-atherogenic shear stress and HIV-1 Tat have increased cathepsin K activity.
A) Shear stress profiles that were programmed into Servomotors are shown. B) Endothelial cells were co-stimulated with HIV-1 Tat and atheroprotective (Anti) or pro-atherogenic (Pro) shear stress for 24 hours, then lysed for multiplex cathepsin zymography. Densitometry was quantified and shown for C) human cathepsin K, D) human cathepsin V, E) human cathepsin S, and F) human cathepsin L. Cathepsin K was significantly increased by pro-atherogenic shear stress and Tat co-stimulation compared to no Tat stimulation and atheroprotective shear stress. Though there was a trend, there were no significant differences for the other cathepsins investigated (* indicates p < 0.05 n=5 and data is mean +/− SEM).
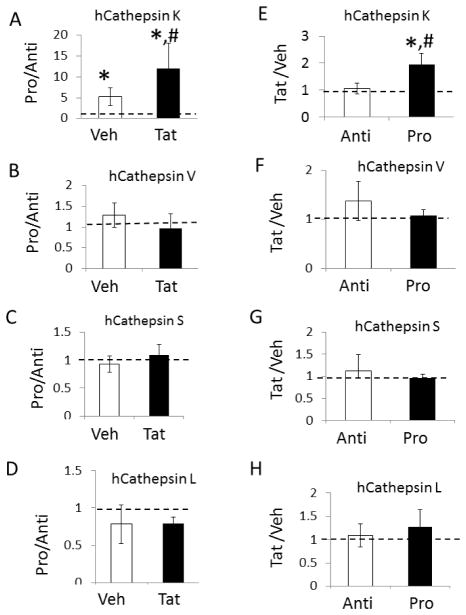
Again, we used a ratiometric analysis to parse the effects of Tat and shear stress on cathepsin activity in human aortic endothelial cells. In the presence of Tat, pro-atherogenic shear stress increases cathepsin K by 11-fold compared to atheroprotective shear stress, which is significantly greater than its upregulation in the absence of Tat (3-fold) (Fig 6A, n=5, p<.05). No significant differences were seen for cathepsins V, S, or L (Fig 6B–D). Under pro-atherogenic shear stress, Tat increased cathepsin K by 2-fold over cells under pro-atherogenic shear stress without Tat (vehicle); there was no significant difference for Tat stimulated endothelial cells exposed to atheroprotective shear stress (Fig 6E). Again, there were no differences for the other cathepsins V, S, or L (Fig 6F–H). This suggests that Tat and pro-atherogenic shear stress synergistically increase cathepsin K beyond pro-atherogenic shear stress or Tat alone.
Figure 6. Ratiometric comparisons to parse human endothelial cell regulation of cathepsins K, V, S and L due to shear stress from that of HIV proteins.
Influence of or region was examined with A ratio of cathepsin activity was used by dividing cathepsin activity from endothelial cells cultured under pro-atherogenic shear stress (Pro) by atheroprotective shear stress (Anti) and separated by co-stimulation with Tat or vehicle controls for cathepsins K, V, S, and L (A–D). Conversely, this ratio was established by dividing the Tat treatment for a shear stress profile by its vehicle control (E–H). Analysis shows 11-fold increases in cathepsin K activity when comparing Tat stimulated cells exposed to pro-atherogenic versus atheroprotective shear stress (* p<.05, n=5) (# indicates ratio is statistically increased p<.05, n=5) No statistically significant differences were observed for cathepsins S, L, or V.
Discussion
Our results show the upregulation of cathepsin K by pro-atherogenic shear stress and HIV proteins. This is significant because both of these stimuli are present in an individual living with HIV, but their synergism, to our knowledge, has never before been studied. The role of pro-atherogenic shear stress and HIV proteins inducing cathepsin activity could add important insight as to why people living with HIV are at increased risk of cardiovascular disease, as well as identify cathepsin inhibition as novel therapeutic targets.
Plaques preferentially form at sites of low and oscillatory shear stress, and cathepsin K has been shown to be upregulated in mouse endothelial cells exposed to pro-atherogenic, oscillatory shear stress compared to atheroprotective shear stress 25. Here, we showed that HIV proteins upregulate the amount of active cathepsin K in the aortas of HIV transgenic mice, specifically at sites of pro-atherogenic shear stress such as the lesser curvature of the aortic arch and the abdominal aorta. It is important to note that these effects were observed in a non-atherosclerotic mouse model, as these were FVB/N background, ApoE+/+, and fed regular chow, not Western or other high fat diets. This selectively isolated the influence of HIV proteins and hemodynamics from that of lipid accumulation and inflammation associated with plaque formation. These in vivo studies were corroborated by in vitro studies with human aortic endothelial cells cultured under either pro-atherogenic or atheroprotective fluid shear stress in the presence of the HIV protein Tat. In both the transgenic mouse model and cultured human endothelial cells, HIV proteins and pro-atherogenic shear stress worked synergistically to upregulate cathepsin K.
Increases in cathepsin K were visualized throughout the aorta of the mice with immunohistochemistry (Fig 2). It could be that the endothelial cells preferentially secrete cathepsin K basally, which was previously shown by us25, allowing its accumulation in the medial layer of the artery wall or that smooth muscle cells may also respond to HIV proteins. Our studies with human aortic smooth muscle cells in culture showed no increase in cathepsin activity when stimulated with Tat alone (data not shown). Perhaps, other HIV proteins expressed by the HIV-Tg mouse may stimulate smooth muscle cell cathepsin expression, but this work shows that for endothelial cells exposed to shear stress, Tat is sufficient.
In this study, cathepsin K was the most responsive of the cathepsins investigated, but cathepsins K, L, S, and V have each been shown to play important roles in pathological vascular remodeling in both animal and human studies. Double knockout mice deficient in both cathepsin K and ApoE had a (41.8%) reduction in plaque area and increased collagen in plaque formation 23. This suggests an important role for cathepsin K in atherosclerosis progression. Apo E−/− mice deficient in cathepsin L and S show similar trends; cathepsin L double knockout mice had significantly smaller atherosclerotic lesions compared with littermate controls, while cathepsin S double knockout mice had fewer acute plaque ruptures and smaller plaques than control 24. Cathepsins K, L, S, and V have also been shown to be increased in human atherosclerotic plaques 22,25,40.
It is interesting to note that cathepsin L was downregulated in wildtype abdominal aortas compared to wildtype thoracic aortas, which was different from cathepsins K and S (Fig 3); however, cathepsin L was significantly increased in the abdominal aorta of the HIV-Tg model compared to the wildtype abdominal aorta which was the same as cathepsins K and S. This indicates that there may be differential regulation and responses for different cysteine cathepsins in the absence of HIV proteins, but with HIV proteins present, cathepsins K, L, and S were all upregulated. Differential proteolytic regulation was also shown with no upregulation of MMP-2 in the aortas of our HIV-Tg mice (Fig 3).
Cathepsins S and L activities were upregulated in the HIV-Tg mouse (Fig 3, 4), but there were not statistically significant differences in the endothelial cells stimulated with Tat (Fig 5,6). This may be due to the other HIV proteins constitutively expressed in the HIV-Tg mouse model, whereas the endothelial cells were only stimulated with Tat in these experiments. In addition, aorta homogenates from the mice contain protein from endothelial cells, smooth muscle cells, and fibroblasts, which may be contributing to cathepsins S and L activities as mouse vascular smooth muscle cells have been shown to express cathepsins L and S during cardiovascular disease22,41 as have endothelial cells42. While we were able to parse the effect of Tat on endothelial cell cathepsin K activation, other HIV proteins may play a role in cathepsin S and L activity in the murine model, and this warrants further investigation.
This study is the first to investigate co-stimulation by shear stress and HIV-proteins in the context of HIV-mediated cardiovascular disease, and specifically using human aortic endothelial cells. These large artery human endothelial cells present a much more physiologically relevant cell type applicable to atherosclerosis, instead of the commonly used human umbilical vein endothelial cells (HUVECs) 15,34,35. We observed increased cathepsin K when these human aortic endothelial cells were exposed to pro-atherogenic shear stress and Tat stimulation, but other endothelial cells cultured from different parts of the vascular tree and smaller arteries may respond uniquely, as it has been established that endothelial cells derived from different regions have phenotypic diversity 43. This may also provide another rationale behind the differences in cathepsin activity observed between the thoracic and abdominal aorta regions in the HIV-Tg mouse model.
Many factors accelerating cardiovascular disease progression in HIV positive patients remain to be investigated. Here, we have parsed the effects of shear stress and HIV proteins, specifically Tat, on cathepsins K, S, V, and L activity of endothelial cells. These cells line the vascular wall, are directly exposed to blood fluid shear stress, and have first access to circulating HIV proteins shed from lysing, infected cells. Together, these factors may stimulate the endothelium to induce proteolytic remodeling by cathepsins and initiate earlier arterial wall remodeling indicative of HIV-mediated cardiovascular disease.
Acknowledgments
This work was completed partially with funding from a Creative and Novel Ideas in HIV Research (CNIHR) grant sponsored by the National Institutes of Health CFAR programme and the International AIDS Society (MOP, RLS, and RLG), NIH Award Number DP2OD007433 from the Office of the Director, National Institutes of Health (MOP), and National Science Foundation Graduate Research Fellowships (IKP and LMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Office of the Director, National Institutes of Health or the National Institutes of Health.
References
- 1.Meng Q, et al. Coronary artery calcification, atherogenic lipid changes, and increased erythrocyte volume in black injection drug users infected with human immunodeficiency virus-1 treated with protease inhibitors. American heart journal. 2002;144(4):642–8. doi: 10.1067/mhj.2002.125009. [DOI] [PubMed] [Google Scholar]
- 2.Spieker LE, et al. Rapid progression of atherosclerotic coronary artery disease in patients with human immunodeficiency virus infection. Heart and vessels. 2005;20(4):171–4. doi: 10.1007/s00380-004-0790-8. [DOI] [PubMed] [Google Scholar]
- 3.Chironi G, et al. Brief report: carotid intima-media thickness in heavily pretreated HIV-infected patients. Journal of acquired immune deficiency syndromes. 2003;32(5):490–3. doi: 10.1097/00126334-200304150-00004. [DOI] [PubMed] [Google Scholar]
- 4.Currier JS, et al. Carotid artery intima-media thickness and HIV infection: traditional risk factors overshadow impact of protease inhibitor exposure. AIDS. 2005;19(9):927–33. doi: 10.1097/01.aids.0000171406.53737.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsue PY, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS. 2009;23(9):1059–67. doi: 10.1097/QAD.0b013e32832b514b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McComsey GA, et al. Increased carotid intima media thickness and cardiac biomarkers in HIV infected children. AIDS. 2007;21(8):921–7. doi: 10.1097/QAD.0b013e328133f29c. [DOI] [PubMed] [Google Scholar]
- 7.van Vonderen MG, et al. Carotid intima-media thickness and arterial stiffness in HIV-infected patients: the role of HIV, antiretroviral therapy, and lipodystrophy. Journal of acquired immune deficiency syndromes. 2009;50(2):153–61. doi: 10.1097/QAI.0b013e31819367cd. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet D, et al. Arterial stiffness and endothelial dysfunction in HIV-infected children. AIDS. 2004;18(7):1037–41. doi: 10.1097/00002030-200404300-00012. [DOI] [PubMed] [Google Scholar]
- 9.Sevastianova K, et al. Arterial stiffness in HIV-infected patients receiving highly active antiretroviral therapy. Antiviral therapy. 2005;10(8):925–35. [PubMed] [Google Scholar]
- 10.El-Sadr WM, et al. CD4+ count-guided interruption of antiretroviral treatment. The New England journal of medicine. 2006;355(22):2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 11.Hansen L, et al. Azidothymidine (AZT) leads to arterial stiffening and intima-media thickening in mice. J Biomech. 2013;46(9):1540–7. doi: 10.1016/j.jbiomech.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen L, et al. Endothelial dysfunction, arterial stiffening, and intima-media thickening in large arteries from HIV-1 transgenic mice. Ann Biomed Eng. 2013;41(4):682–93. doi: 10.1007/s10439-012-0702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kline ER, Sutliff RL. The roles of HIV-1 proteins and antiretroviral drug therapy in HIV-1-associated endothelial dysfunction. J Investig Med. 2008;56(5):752–69. doi: 10.1097/JIM.0b013e3181788d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiodelli P, et al. Sialic acid associated with alphavbeta3 integrin mediates HIV-1 Tat protein interaction and endothelial cell proangiogenic activation. The Journal of biological chemistry. 2012;287(24):20456–66. doi: 10.1074/jbc.M111.337139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rusnati M, Presta M. HIV-1 Tat protein and endothelium: from protein/cell interaction to AIDS-associated pathologies. Angiogenesis. 2002;5(3):141–51. doi: 10.1023/a:1023892223074. [DOI] [PubMed] [Google Scholar]
- 16.Urbinati C, et al. Integrin alphavbeta3 as a target for blocking HIV-1 Tat-induced endothelial cell activation in vitro and angiogenesis in vivo. Arteriosclerosis, thrombosis, and vascular biology. 2005;25(11):2315–20. doi: 10.1161/01.ATV.0000186182.14908.7b. [DOI] [PubMed] [Google Scholar]
- 17.Urbinati C, et al. Substrate-immobilized HIV-1 Tat drives VEGFR2/alpha(v)beta(3)-integrin complex formation and polarization in endothelial cells. Arteriosclerosis, thrombosis, and vascular biology. 2012;32(5):e25–34. doi: 10.1161/ATVBAHA.111.242396. [DOI] [PubMed] [Google Scholar]
- 18.Ku DN. Blood flow in arteries. Annual Review of Fluid Mechanics. 1997;29:399–434. [Google Scholar]
- 19.Platt MO, Ankeny RF, Jo H. Laminar shear stress inhibits cathepsin L activity in endothelial cells. Arteriosclerosis, thrombosis, and vascular biology. 2006;26(8):1784–90. doi: 10.1161/01.ATV.0000227470.72109.2b. [DOI] [PubMed] [Google Scholar]
- 20.Platt MO, et al. Expression of cathepsin K is regulated by shear stress in cultured endothelial cells and is increased in endothelium in human atherosclerosis. Am J Physiol Heart Circ Physiol. 2007;292(3):H1479–86. doi: 10.1152/ajpheart.00954.2006. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, et al. Increased serum cathepsin S in patients with atherosclerosis and diabetes. Atherosclerosis. 2006;186(2):411–9. doi: 10.1016/j.atherosclerosis.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, et al. Cathepsin L expression and regulation in human abdominal aortic aneurysm, atherosclerosis, and vascular cells. Atherosclerosis. 2006;184(2):302–11. doi: 10.1016/j.atherosclerosis.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Lutgens E, et al. Disruption of the cathepsin K gene reduces atherosclerosis progression and induces plaque fibrosis but accelerates macrophage foam cell formation. Circulation. 2006;113(1):98–107. doi: 10.1161/CIRCULATIONAHA.105.561449. [DOI] [PubMed] [Google Scholar]
- 24.Sukhova GK, et al. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. The Journal of clinical investigation. 2003;111(6):897–906. doi: 10.1172/JCI14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Platt MO, et al. Expression of cathepsin K is regulated by shear stress in cultured endothelial cells and is increased in endothelium in human atherosclerosis. American journal of physiology Heart and circulatory physiology. 2007;292(3):H1479–86. doi: 10.1152/ajpheart.00954.2006. [DOI] [PubMed] [Google Scholar]
- 26.Kopp JB, et al. Progressive glomerulosclerosis and enhanced renal accumulation of basement-membrane components in mice transgenic for human-immunodeficiency-virus type-1 genes. Proc Natl Acad Sci U S A. 1992;89(5):1577–1581. doi: 10.1073/pnas.89.5.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li WA, et al. Detection of femtomole quantities of mature cathepsin K with zymography. Analytical Biochemistry. 2010;401(1):91–98. doi: 10.1016/j.ab.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 28.Wilder CL, et al. Manipulating substrate and pH in zymography protocols selectively distinguishes cathepsins K, L, S, and V activity in cells and tissues. Archives of biochemistry and biophysics. 2011;516(1):52–7. doi: 10.1016/j.abb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galis ZS, et al. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94(6):2493–503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai G, et al. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci U S A. 2004;101(41):14871–6. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suo J, et al. Hemodynamic shear stresses in mouse aortas: implications for atherogenesis. Arterioscler Thromb Vasc Biol. 2007;27(2):346–51. doi: 10.1161/01.ATV.0000253492.45717.46. [DOI] [PubMed] [Google Scholar]
- 32.Moore JE, Jr, et al. Hemodynamics in the abdominal aorta: a comparison of in vitro and in vivo measurements. J Appl Physiol (1985) 1994;76(4):1520–7. doi: 10.1152/jappl.1994.76.4.1520. [DOI] [PubMed] [Google Scholar]
- 33.Moore JE, Jr, et al. Pulsatile flow visualization in the abdominal aorta under differing physiologic conditions: implications for increased susceptibility to atherosclerosis. J Biomech Eng. 1992;114(3):391–7. doi: 10.1115/1.2891400. [DOI] [PubMed] [Google Scholar]
- 34.Cota-Gomez A, et al. The human immunodeficiency virus-1 Tat protein activates human umbilical vein endothelial cell E-selectin expression via an NF-kappa B-dependent mechanism. The Journal of biological chemistry. 2002;277(17):14390–9. doi: 10.1074/jbc.M108591200. [DOI] [PubMed] [Google Scholar]
- 35.Hofman FM, et al. Exogenous tat protein activates human endothelial cells. Blood. 1993;82 (9):2774–80. [PubMed] [Google Scholar]
- 36.Liu K, et al. HIV-1 Tat protein-induced VCAM-1 expression in human pulmonary artery endothelial cells and its signaling. American journal of physiology Lung cellular and molecular physiology. 2005;289(2):L252–60. doi: 10.1152/ajplung.00200.2004. [DOI] [PubMed] [Google Scholar]
- 37.Bromme D, et al. Human cathepsin V functional expression, tissue distribution, electrostatic surface potential, enzymatic characterization, and chromosomal localization. Biochemistry. 1999;38(8):2377–85. doi: 10.1021/bi982175f. [DOI] [PubMed] [Google Scholar]
- 38.Reiser J, Adair B, Reinheckel T. Specialized roles for cysteine cathepsins in health and disease. J Clin Invest. 2010;120(10):3421–31. doi: 10.1172/JCI42918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ensoli B, et al. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. Journal of virology. 1993;67(1):277–87. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yasuda Y, et al. Cathepsin V, a novel and potent elastolytic activity expressed in activated macrophages. J Biol Chem. 2004;279(35):36761–70. doi: 10.1074/jbc.M403986200. [DOI] [PubMed] [Google Scholar]
- 41.Sukhova GK, SG, Simon DI, Chapman HA, Libby P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. The Journal of Clinical Investigation. 1998;102:576–583. doi: 10.1172/JCI181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Platt MO, Ankeny RF, Jo H. Laminar shear stress inhibits cathepsin L activity in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26(8):1784–90. doi: 10.1161/01.ATV.0000227470.72109.2b. [DOI] [PubMed] [Google Scholar]
- 43.Antonov AS, et al. Prothrombotic phenotype diversity of human aortic endothelial cells in culture. Thromb Res. 1992;67(2):135–45. doi: 10.1016/0049-3848(92)90133-u. [DOI] [PubMed] [Google Scholar]