Abstract
Ischemic neuroprotection afforded by sevoflurane preconditioning has been previously demonstrated, yet the underlying mechanism is poorly understood and likely affects a wide range of cellular activities. Several individual microRNAs have been implicated in both the pathogenesis of cerebral ischemia and cellular survival, and are capable of affecting a range of target mRNA. Conceivably, sevoflurane preconditioning may lead to alterations in ischemia-induced microRNA expression that may subsequently exert neuroprotective effects. We first examined the microRNA expression profile following transient cerebral ischemia in rats and the impact of sevoflurane preconditioning. Microarray analysis revealed that 3 microRNAs were up-regulated (>2.0 fold) and 9 were down-regulated (< 0.5 fold) following middle cerebral artery occlusion (MCAO) compared to sham controls. In particular, miR-15b was expressed at significantly high levels after MCAO. Preconditioning with sevoflurane significantly attenuated the upregulation of miR-15b at 72h after reperfusion. Bcl-2, an anti-apoptotic gene involved in the pathogenesis of cerebral ischemia, has been identified as a direct target of miR-15b. Consistent with the observed downregulation of miR-15b in sevoflurane-preconditioned brain, post-ischemic Bcl-2 expression was significantly increased by sevoflurane preconditioning. We identified the 3’-UTR of Bcl-2 as the target for miR-15b. Molecular inhibition of miR-15b was capable of mimicking the neuroprotective effect of sevoflurane preconditioning, suggesting that the suppression of miR-15b due to sevoflurane contributes to its ischemic neuroprotection. Thus, sevoflurane preconditioning may exert its anti-apoptotic effects by reducing the elevated expression of miR-15b following ischemic injury, allowing its target proteins, including Bcl-2, to be translated and expressed at the protein level.
Keywords: Sevoflurane, Preconditioning, Cerebral ischemia, microRNA, Bcl-2
INTRODUCTION
The molecular basis of neural cell death and survival following ischemic injury has been an intense focus of research over the past several decades. Targeting of single molecules has yielded limited success in conferring longterm survival of neural tissue, leading to the search for more pluripotent therapeutic strategies. Recently, microRNAs have been identified as a class of single-stranded, noncoding RNA molecules comprised of 18–23 nucleotides that function as gene expression regulators at the posttranscriptional level [1, 2]. These endogenous small RNA molecules induce cleavage or translation blockage of target mRNAs by complete or incomplete complementary binding to the 3′ untranslated region (UTR) of mRNAs. Since one microRNA has the potential to regulate hundreds of mRNAs, it is conceivable that microRNAs are involved in almost every cellular process such as development, growth and metabolism. Furthermore, changes in microRNA expression may result in different pathologies including tumor, inflammation and apoptosis. Accumulating evidence has begun to suggest that microRNAs contribute to the pathogenesis of cerebral ischemia [3–13]. However, the particular microRNAs that could emerge as potential therapeutic targets are not well defined and are likely to be highly context-specific under different neuroprotective strategies.
Cerebral ischemia is a prevalent brain injury in the general population, but also contributes to serious complications during the perioperative period. Interestingly, volatile anesthetics such as sevoflurane have been effectively used to induce a neuroprotective ischemic tolerant state [14]. Thus, the clinical use of volatile anesthetics to reduce the incidence of perioperative brain ischemia has become an emerging priority of volatile anesthetic treatment. Sevoflurane preconditioning has shown direct neuroprotective effects on brain against experimental ischemic injury both in vitro [15–21] and in vivo [14, 22–27] settings. Previous work in our and other laboratories have reported that sevoflurane preconditioning protects brain ischemia/reperfusion injury by affecting a variety of cellular events, including attenuation of inflammation [24], release of reactive oxygen species (ROS) [18, 25, 28], opening mitochondrial ATP-sensitive potassium channels [18, 22, 26] and improving blood-brain-barrier (BBB) integrity [27]. However, the precise molecular mechanisms underlying sevoflurane preconditioning remain unclear. Given the multifaceted nature of the cellular effects of sevoflurane preconditioning, we hypothesized that microRNAs may be differentially expressed in the ischemic tolerant state. In this work, we describe the microRNA expression profile following transient cerebral ischemia in rats and the impact of sevoflurane preconditioning. We focus on assessing the functional activity of the microRNA miR-15b in targeting degradation of the pro-survival Bcl-2 mRNA as a mechanism involved in the ischemic injured state, and further explore the effects of sevoflurane preconditioning on suppressing the expression of miR-15b.
MATERIALS AND METHODS
Animals and model of transient focal cerebral ischemia
Adult male Sprague-Dawley rats (SD, 260– 280g, 8 weeks old) were provided by Shanghai SLAC Laboratory Animal Co. Ltd., Shanghai, China. The rats were housed in the same temperature- and humidity-controlled animal facility with a 12 h light/dark cycle. All animal experiments were performed in accordance with institutional guidelines and all efforts were made to minimize the number of animals.
Transient focal cerebral ischemia was induced by filament occlusion of the middle cerebral artery (MCAO) as previously described [24]. Rats were anesthetized with 1–2% isoflurane (Abbott, U.S.A.) in air and mechanically ventilated with an endotracheal tube. After a midline cervical incision, the left common carotid artery was exposed and a 4-0 nylon monofilament coated with a silicone tip was introduced into the external carotid artery, and advanced 1.9–2.0 cm along the internal carotid artery until occluding the origin of the middle cerebral artery. The animals underwent left MCAO for 120 minutes and then reperfusion for the indicated duration. In sham-operated groups, rats were anesthetized, and only branches of external cervical artery were dissected, and then the wound was sutured (ischemia was not induced). All rats were randomly distributed into the corresponding groups.
Breath rate, end tidal CO2 (EtCO2), artery blood gas and rectal temperature were monitored throughout the experiment. Breath rate, EtCO2 and concentration of isoflurane were monitored with a Datex-Ohmeda AS/3 monitoring device. Rectal temperature was maintained at 37.0±0.5°C during and shortly after surgery with a temperature-regulated heat lamp. The left femoral artery and vein were cannulated for artery blood gas. To confirm the success of MCAO, changes in regional cerebral blood flow (rCBF) were evaluated in rats by laser Doppler flowmetry.
Assessment of neurological deficits
Animals subjected to MCAO underwent neurological evaluation at 24 hr and 48 hr after ischemia. Each rat was assigned a score according to a five-point behavioral rating scale [22]: 0, no deficit; 1, forelimb weakness, and torso turning to the ipsilateral side when held by tail; 2, circling to the affected side; 3, unable to bear weight on the affected side; and 4, no spontaneous locomotor activity or barrel rolling. Any animal without a deficit was excluded from the study. A single observer blinded to group assignment performed neurological testing.
Sevoflurane preconditioning in vivo
In the sevoflurane-preconditioned groups, the rats received 1.0 minimum alveolar concentration (MAC) sevoflurane (2.4% sevoflurane in air, Baxter, U.S.A.) in an anesthetic chamber for 30 min per day on 4 consecutive days, monitored by a Datex-Ohmeda AS/3 monitoring device [24]. Animals in sham-operated and vehicle groups were exposed to ambient air. At 24h following the last exposure, rats were anesthetized and exposed to transient focal cerebral ischemia.
Microarray analysis
Rats were randomly divided between sham-operated (n=2) and MCAO (n=3/time point) groups for the determination of differential microRNA expression, and rats were euthanized at 24h or 72h after MCAO. RNA samples were prepared from the ischemic cortex for microarray analysis to determine global microRNA expression changes following transient cerebral ischemia in rats. Total RNA was extracted with TRIzol reagent according to the manufacturer's instructions and subjected to Affymetrix Gene Chip microRNA microarray analysis to determine relative microRNA expression.
Quantitative RT-PCR validation of microRNA expression
In order to determine the effects of sevoflurane preconditioning following MCAO, eighteen rats were randomized into three experimental groups: sham-operated, vehicle and sevoflurane preconditioning (n=3/group /time point) groups. Rats were euthanized at 24h or 72h after MCAO and tissues were prepared from the ischemic cortex for real time PCR and western blots.
Total RNA was extracted and the integrity and purity was verified. miR-15b was subsequently chosen as the candidate microRNA for validation based on the results from the array analysis. U6 RNA was chosen as internal control. The 20 µl reverse transcriptase reactions contained 1 µg of RNA samples using miScript Reverse Transcription Kit (QIAGEN, U.S.A.) following the manufacturer's instructions. The RT-PCR reactions contained miScript SYBR Green PCR Kit (QIAGEN, U.S.A.), and miScript Primer Assay specific for miR-15b (QIAGEN, U.S.A.) and U6 (QIAGEN, U.S.A.).
Reverse transcriptase reactions were incubated for 60 min at 37°C, 95 °C for 5 min and then placed on ice. Real-time PCR was performed using a realplex analysis system (Eppendorf, Germany). The following real-time PCR protocol was used: PCR initial activation step (95 °C for 15 min), amplification and quantification program repeated for 40 cycles (94 °C for 15 s and 55 °C for 30 s and 70 °C for 30s with a single fluorescence measurement), and melting curve program. The microRNA expression data were normalized with respect to U6 RNA.
Western blot
The tissue used in this part was harvested as described above in section 3.5, and all procedures were performed as previously described [24]. The primary antibody used in this study was rabbit anti Bcl-2 (Cell Signaling Technology, U.S.A.). The blots were semi-quantified using gel densitometry with Quantity One software (Bio-Rad, U.S.A.), and the amount was normalized to beta-actin values in the same lane.
Immunofluorescence staining
At 72h after reperfusion, rats (n=3/group) were sacrificed in deep anesthesia by intracardiac perfusion with 0.9% saline and subsequent 4% paraformaldehyde. Rat brains were removed and cryoprotected in 20% sucrose and then in 30% sucrose in phosphate-buffered saline. Brain tissues were coronally sectioned with a cryostat and double immunostaining was performed on 30 µm free-floating sections. Rabbit anti-Bcl-2 antibody (Novus, U.S.A.) and mouse anti-NeuN antibody (Millipore, U.S.A.) were used as the primary antibodies in this study. After blocking with 10% goat serum albumin in phosphate-buffered saline for 1 hour, we incubated the sections with primary antibodies for 1 hour at 37°C and then at 4°C overnight. After washing, DyLightTM 488-conjugated goat anti- mouse IgG (Jackson ImmunoResearch Laboratories, U.S.A.) or Cy3-conjugated Donkey anti-rabbit IgG secondary antibody (Jackson ImmunoResearch Laboratories, U.S.A.) was incubated for 1h at 37°C. Sections were then stained with DAPI (Thermo Scientific, U.S.A.) for 2 minutes at room temperature for counterstaining.
Intracerebroventricular injection
The rats were anesthetized and mounted in a stereotactic frame, and intracerebroventricular injections using a 10-microL Hamilton syringe were made at the following coordinates: 0.8 mm posterior to the bregma, 1.5 mm lateral, and 3.7 mm deep. The selective Bcl-2 inhibitor TW-37 0, 2.5, 5°g (SelleckBio.com, Houston, TX, U.S.A) in 5 °l DMSO was infused for 5 min and the needle was kept in this position for an additional 15 min after injection and then withdrawn slowly from the brain. Either TW-37 or vehicle injection was done at 1 h prior to MCAO or sham operation.
Oxygen-glucose deprivation and sevoflurane preconditioning in neuron cultures
Cortical neurons were dissociated, suspended in Neurobasal medium supplemented with B27, and plated in 6 well dishes at 2 × 105 cells/cm2 for activity assays, 1.6 × 105 cells/cm2 for cell death assays, and 8 × 104 cells/cm2 for immunocytochemistry. Experiments were performed on day in vitro (DIV) 11–12 to ensure development of a mature neuronal phenotype with expression of glutamate receptors [29] and where cultures consisted of >97% neurons determined by neuron- and glial-specific immunocytochemistry [30]. Oxygen–glucose deprivation (OGD) was used as an in vitro model of ischemia. Briefly, maintenance medium was replaced with medium lacking glucose and other components known to be substrates for glycolysis; culture dishes were placed in an airtight chamber (Billups-Rothenberg) and flushed with 100% argon gas for 3 min; and neurons were incubated at 37 °C for 1 h. Following OGD, medium was replaced and incubated (reperfused) for the times indicated. Control cultures had their medium changed with maintenance medium the same number of times as OGD-treated cultures and were incubated at 37 °C in humidified 95% air and 5% CO2 for equivalent periods.
To induce sevoflurane preconditioning, neurons were exposed to 1 MAC of sevoflurane for 30 min, repeating for 3 times, with 15-min washout after each exposure. At 24 h after sevoflurane preconditioning, neurons were subjected to OGD for 1 h and collected for protein analysis at 2 or 6 h after OGD or for cell viability assays at 24 h after OGD.
Cell death/viability assessment in cultured neurons
Fluorescence of Alamar blue, an indicator that changes from blue to red and fluoresces when reduced by cellular metabolic activity, was used to measure the viability of cultured neurons at 24h after OGD [31, 32]. One-half of the culture medium was replaced with MEM-Pak containing 10% (vol/vol) Alamar blue, and cultures were incubated for 1.5 h at 37 °C in humidified 95% air and 5% CO2. Fluorescence was determined in a Millipore CytoFluor 2300 automated plate-reading fluorimeter, with excitation at 530nm and emission at 590 nm.
OGD-induced cell death was confirmed at 24h after OGD by measuring lactate dehydrogenase (LDH) release from damaged cells into the culture medium. In brief, 10-µl aliquots of medium taken from the cell culture wells were added to 200 µl of LDH reagent (Sigma). Using a spectrophotometer plate reader (Molecular Devices), the emission was measured at 340 nm, which is proportional to the amount of LDH in the medium. The percentage of cell death was calculated as described previously [31].
Luciferase Assay
The 3’-UTR activity of Bcl-2 was assessed in neuronal cultures transfected with a luciferase reporter plasmid containing the putative Bcl-2 3’-UTR target site for miR-15b. The Bcl-2 3’-UTR fragment was PCR-amplified with the primers (sense 5’-CCT GTG GAT GAC TGA GTA CC-3’, antisense 5’-GAG ACA GCC AGG AGA AAT CA-3’) and subcloned into the cloning cassette of pGL3-Basic (Promega). The integrity of the constructs and the incorporated mutations were confirmed by sequencing. For Bcl-2-LUC reporter activity assay, neurons cultured in 24-well plates were transfected with 2 µg of reporter DNA together with 100 ng of pRL-TK (Promega) using Lipofectamine 2000 (Invitrogen). Twenty-four hours later, cultures were subjected to OGD for 60 min. Luciferase assays were performed at 2 h after OGD using the Dual-Luciferase reporter assay system (Promega) and a Turner TD-20e luminometer according to the manufacturers’ instructions. Firefly outputs were normalized to Renilla outputs to control for transfection efficiency.
Lentiviral vector transfection
The lentiviral vectors for overexpression of mouse miR-15b were purchased from Applied Biological Materials (abm, Cat # mm10673, Richmond, BC, Canada). The vectors contain both the pre-miRNA and GFP inserts under the same CMV promoter. The transcription termination site is after the pre-miRNA, thus both GFP and the pre-miRNA are transcribed together, making GFP an actual transcription reporter for the 15b miRNA. The pre-miRNA region of the 15b mRNA folds over on itself and forms a stem loop structure which is processed in the cell by the Drosha/Pasha enzymes and cleaved from the GFP portion of the mRNA (www.abmgood.com). For lentivirus vector transduction in primary neurons, the neuronal cultures were transduced for 2 days with the lentiviral vectors at the range of 10 to 100 TU per cell.
Statistical analysis
Data were expressed as mean ± standard error (SE) of the mean. Differences in miR-15b and Bcl-2 expression under various experimental conditions or infarct volume after MCAO were determined with analysis of variance (ANOVA) followed by post hoc Fisher’s probable least-squares difference (PLSD) tests. It was considered statistically significant when the P value was less than 0.05.
RESULTS
miR-15b is induced following ischemia and directly inhibits bcl-2
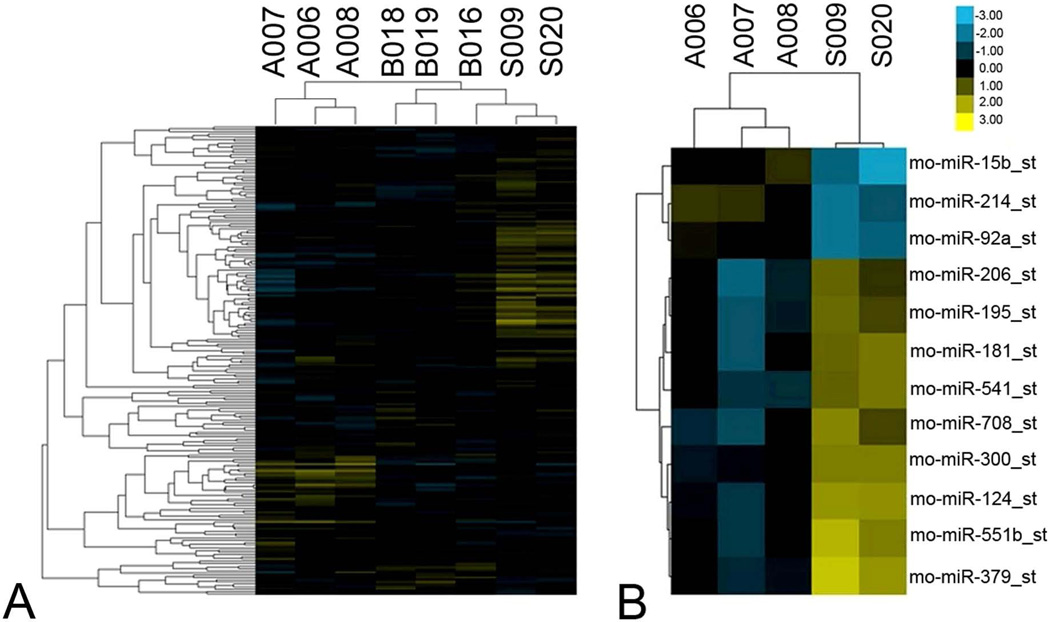
Recently, microRNAs have presented themselves as potentially potent effectors of cell death and survival [33]. As more pharmacological inhibitors are becoming available, we wished to determine which, if any, microRNAs might contribute to neural damage following cerebral ischemia. Using comprehensive microRNA profiling, we performed a clustering analysis of data to compare sham-operated controls to MCAO rats at 24 and 72 h post-reperfusion (Figure 1A). Differential expression of specific microRNAs was observed at both 24 and 72 h following MCAO (Figure 1B, Table 1), including 3 microRNAs upregulated by more than 2 fold, and 9 microRNAs downregulated to less than half the level of sham operated controls. microRNA15-b, in particular, exhibited robust upregulation in ischemic rats.
Figure 1. microRNA profiles differentiate ischemic rats from sham-operated rats.
(A) Comparative microRNA expression of sham-operated and MCAO rats at 24h and 72h following transient focal cerebral ischemia (n=3/group). (B) Differentially expressed microRNAs between ischemic and sham-operated rats at 72 h after MCAO (n=3/group). Each column of the heatmap figure represents a particular microarray sample (24h after reperfusion: B016, B018, B019; 72h after reperfusion: A006, A007, A008; sham: S009, S020). And each row is a microRNA (miR-15b, -214, -92a, -206, -195, -181, -541, -708, -300, -124, -551b, -379). Heatmap coloring is based on microRNA expression levels. Blue is down-regulated and yellow is up-regulated.
Table 1.
Differentially expressed miRNAs in brain after MCAO.
| miRNA | 24h (fold over sham) | 72h (fold over sham) |
|---|---|---|
| Upregulated microRNAs | ||
| miR-15b | 1.086 | 2.918 |
| miR-214 | 1.219 | 2.560 |
| miR-92a | 1.055 | 2.371 |
| Downregulated microRNAs | ||
| miR-551b | 0.439 | 0.330 |
| miR-206 | 0.620 | 0.490 |
| miR-195 | 0.522 | 0.478 |
| miR-300-3p | 0.676 | 0.458 |
| miR-181d | 0.604 | 0.457 |
| miR-541 | 0.653 | 0.441 |
| miR-708 | 0.510 | 0.437 |
| miR-124-star | 0.630 | 0.386 |
| miR-379 | 0.538 | 0.330 |
The finding the miR-15b was robustly upregulated following MCAO supports the recent studies indicating that miR-15b contributes to the progression of many pathological conditions [34, 35]. We thus hypothesized that miR-15b upregulation would be found in regions undergoing pathological events following MCAO. In order to verify the microarray profiling, we performed quantitative RT-PCR analysis of miR-15b expression in sham-operated and MCAO rats at 72h following ischemic injury, using tissue encompassing the infarct region (Figure 2A). Consistent with the microarray data, quantitative RT-PCR demonstrated a significant upregulation of miR-15b (2.61±0.42) following MCAO compared to sham-operated controls (Figure 2B).
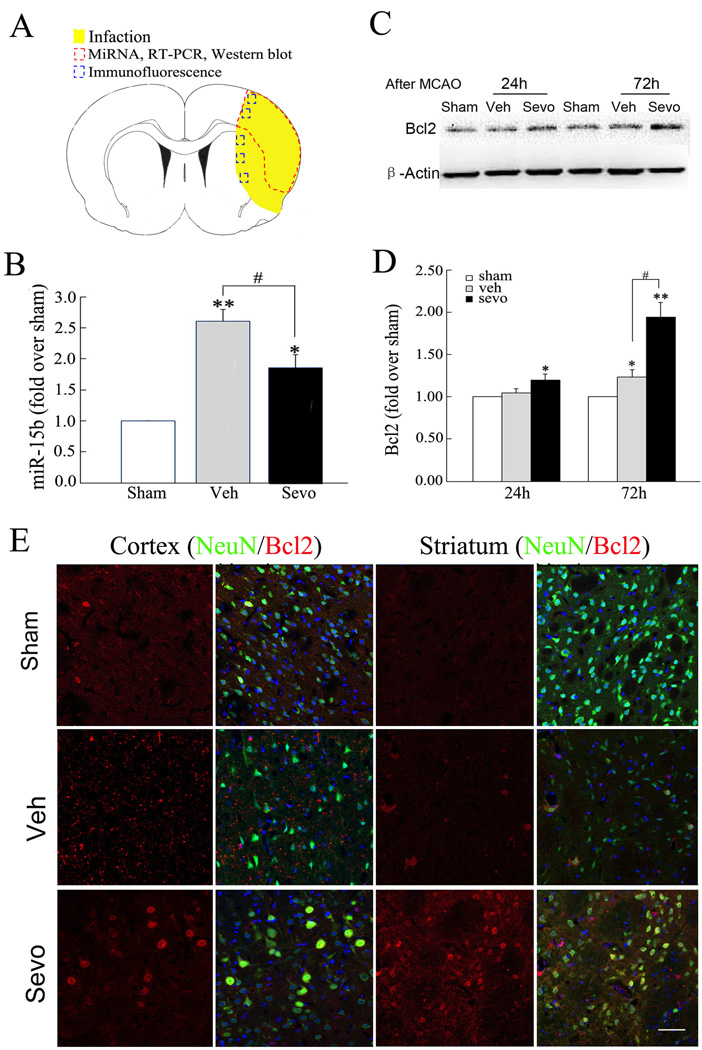
Figure 2. Sevoflurane preconditioning suppresses the ischemic induction miR-15b and allows Bcl-2 protein expression.
(A) The dashed red circle indicates the region of infarct in the cortex from which tissue was examined for the microarray data and RT-PCR, whereas the blue boxes indicate the regions in the cortex and striatum used for Bcl2 expression experiments. (B) Validation of microarray data and effects of sevoflurane preconditioning using quantitative RT-PCR. The level of miR-15b was up-regulated in vehicle relative to sham-operated rats at 72h after MCAO. Real-time PCR analysis of miR-15b in vehicle and sevoflurane preconditioning groups was carried out (n=6/group). Triplicate assays were performed for each RNA sample and the relative amount of each microRNA was normalized to U6 RNA. (C) Representative Western blot of Bcl-2 in ipsilateral cortex at 24 or 72 hours after reperfusion. (D) Semi-quantitative analysis of Bcl-2. All data are mean± SE. n=6/group. *P<0.05, **P<0.01 compared to sham-operated rats; #P<0.05 compared to vehicle rats. (E) Expression of Bcl-2 protein in neurons preconditioned with sevoflurane increases at 72 h after ischemia. Representative images of NeuN (green) and Bcl2 (red) immuno-fluorescence double-labeling in a peri-infarct region in ipsilateral (Ipsi) cerebral cortex (Ctx) and striatum (Str) in sham-operated, vehicle- and sevoflurane preconditioning rats at 72 hours after reperfusion. Scale bar =100 µm.
To establish that the upregulation of miR-15b exerts a functional effect on target protein expression, we wished to assess the levels of a target of miR-15b in the border region between the infarction zone and the penumbra. Recent studies in non-neuronal model systems have indicated that Bcl-2 mRNA is a direct target for miR-15b activity and subsequent inhibition of translation [34–36]. We therefore hypothesized that Bcl-2 protein levels would be suppressed under ischemic conditions that upregulate miR-15b. Consistent with this hypothesis, we observed that only limited Bcl-2 protein levels could be detected in neurons following MCAO (Figure 2C–E). These findings established a correlative relationship between miR-15b upregulation and ischemic infarction, and an inverse correlation between miR-15b upregulation and Bcl-2 protein expression.
miR-15b expression is attenuated by the neuroprotective use of sevoflurane preconditioning
To further implicate miR-15b as a pathological effector in ischemic injury, we sought to determine if known neuroprotective strategies used prior to the ischemic event could suppress the upregulation of miR-15b. Preconditioning with the volatile anesthetic sevoflurane has been reported by our group and others to exert neuroprotection against subsequent ischemic events by inhibition of an active cell death process [14, 17, 24, 25, 27]}. Using 30 min of 1.0 MAC sevoflurane administered daily for 4 consecutive days followed by MCAO induced 24 h after the last sevoflurane treatment, we observed a significant attenuation in the induction of miR-15b (Figure 2B). Furthermore, sevoflurane preconditioning increased the level of Bcl-2 protein in neurons following MCAO (Figure 2C–E), concurrent to the observation of attenuated miR-15B expression. Bcl-2 protein was rarely observed in sham-operated rats, but was expressed to limited extent in neurons following MCAO, primarily in the ipsilateral cortex, or border region of the infarct. However, preconditioning with sevoflurane robustly increased the expression of Bcl-2 in neurons not only in the ischemic cortex, but in the ipsilateral striatum as well (Figure 2E). These results indicate that suppression of miR-15b may play a role in the neuroprotection function of sevoflurane preconditioning, as suppression of miR-15b correlates with neuroprotection afforded by sevoflurane preconditioning, and miR-15b expression is inversely related to Bcl-2 protein expression following cerebral ischemia.
miR-15b targets the 3’-UTR of Bcl-2 to suppress protein expression
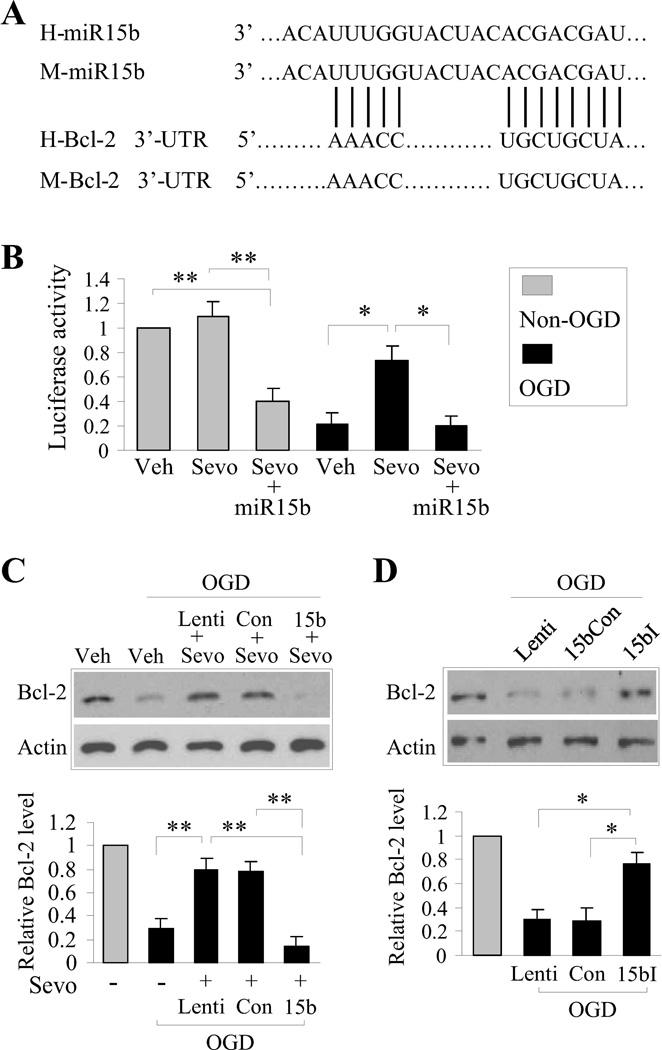
Given that the above results indicate that 1) miR-15b correlates with ischemic injury and suppression of bcl-2, and 2) sevoflurane suppresses miR-15b and promotes Bcl-2 protein expression, we wished to more definitively address the activity of miR-15b following ischemic injury, using a reporter assay as well as Bcl-2 protein expression as functional readouts. The mechanism by which miR-15b suppresses target protein translation relies primarily on its 5’-end “seed” region, which is highly conserved across species. This region of miR-15b is complementary to the 3’-UTR of Bcl-2 (Figure 3A, [36]). In order to test whether miR-15b targets this region of Bcl-2 under ischemic conditions and suppresses protein translation, we constructed a luciferase reporter vector containing the putative Bcl-2 3’-UTR target site for miR-15b positioned downstream of the luciferase gene. This allows the transcription of the luciferase mRNA with the 3’-UTR of Bcl-2. Using an in vitro model of neuronal ischemia, we transduced the luciferase reporter vector into cortical cultures and exposed them to 1 h of OGD followed by 2 h of reperfusion. Under normal conditions, luciferase activity was readily detected, indicating that the addition of the 3’-UTR of Bcl-2 did not affect exogenously expressed luciferase protein function. Following OGD, significant suppression of luciferase activity was observed compared to non-OGD cultures (Figure 3B), which reflects a loss of protein function, or of the protein itself. Interestingly, preconditioning the cultures with 1 MAC of sevofluane (30 min each time, 3 times, 24 h prior to OGD) significantly attenuated the loss of luciferase activity after OGD (Figure 3B). To determine if suppression of miR-15b expression was responsible for the retention of luciferase activity in sevoflurane preconditioned cultures, we overexpressed exogenous miR-15b in the presence of the luciferase-3’-UTR reporter in cultures and subjected them to sevoflurane preconditioning followed by OGD. With forced overexpression of miR-15b, sevoflurane preconditioning no longer sustained luciferase activity following OGD, indicating that sevoflurane preconditioning actively suppresses miR-15b activity and that miR-15b targets the 3’-UTR derived from Bcl-2 mRNA. We confirmed these results by examining the endogenous target of miR-15b, Bcl-2 mRNA. Similar to the results obtained using the luciferase reporter, OGD effectively suppressed Bcl-2 expression (Figure 3C). Sevoflurane preconditioning led to increased levels of Bcl-2 protein following OGD when compared to OGD alone, even in the presence of the luciferase reporter or a scrambled plasmid control. However, forced overexpression of miR-15b led to robust suppression of Bcl-2, even in the context of sevoflurane preconditioning (Figure 3C). Taken together, these results suggest that 1) ischemia-induced miR-15b actively suppresses protein translation by targeting the 3’-UTR of Bcl-2, and 2) functional suppression of miR-15b is required for Bcl-2 expression following sevoflurane preconditioning.
Figure 3. miR-15b targets the 3’-UTR of Bcl-2 under neuronal ischemic conditions and is suppressed by sevoflurane preconditioning.
(A) Bcl-2 is a direct target of posttranscriptional repression by miR-15b. The unique site of complementary microRNA::mRNA is conserved in human and mouse. The sites of target mutagenesis are indicated. (B) Luciferase assay was performed in OGD/non-OGD neurons with sevoflurane- or vehicle- preconditioning, transfected with luciferase construct alone or cotransfected with miR-15b precursor or the scramble precursor control. Firefly luciferase activity was normalized to Renilla luciferase activity for each sample. (C) Bcl-2 protein level was assessed in OGD/non-OGD neurons with sevoflurane- or vehicle- preconditioning, transfected with luciferase construct alone or contransfected with miR-15b or the scramble precursor. (D) Western blots analysis of Bcl-2 was performed in OGD/non-OGD neurons transfected with luciferase construct alone or contransfected with miR-15b inhibitor or the scramble inhibitor control. All data are mean ± SEM, from at least three independent experiments, *P<0.05, **P<0.01.
Inhibition of miR-15b mimics sevoflurane preconditioning
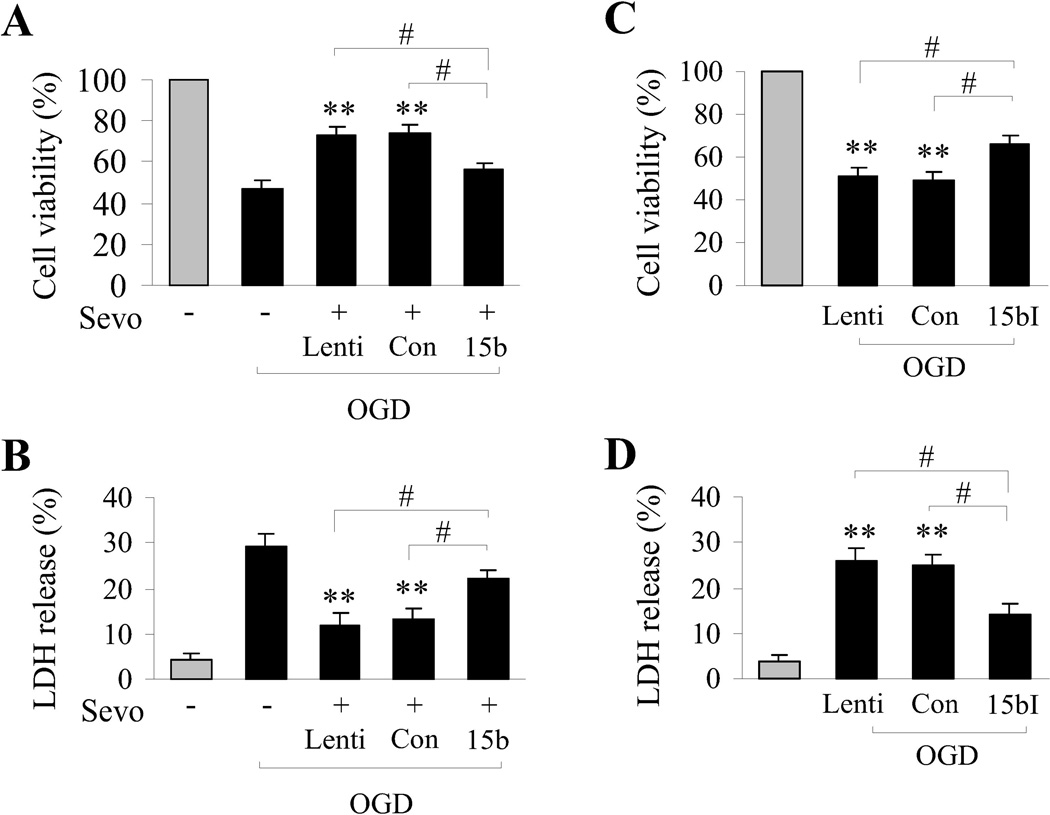
The use of sevoflurane as a preconditioning/neuroprotective stimulus is attractive in the context of ischemic injury during surgical procedures. However, for the general population, volatile anesthetic preconditioning presents multiple limitations, including prophylactic administration, unknown off-target effects and dosage control. Thus, identifying molecular mechanisms of neuroprotective therapies such as sevoflurane preconditioning may result in better therapeutic interventions for wider patient populations. Given the above results demonstrating that miR-15b is robustly downregulated as a result of sevoflurane preconditioning against ischemia, we hypothesized that inhibition of miR-15b may mimic the neuroprotective effects of sevoflurane preconditioning. Our results described above indicate that sevoflurane preconditioning suppressed miR-15b expression and allowed Bcl-2 protein expression, and that the sevoflurane-induced expression of Bcl-2 could be inhibited by forced overexpression of exogenous miR-15b. Using the in vitro neuronal cultures, we then sought to mimic the preconditioning effects of sevoflurane by using a lentiviral vector (miR-15b-I) encoding a short siRNA targeting, and thus inhibiting, miR-15b. When cultures were transfected with miR-15b-I, Bcl-2 protein expression was significantly increased following OGD compared to ischemic cultures tranfected with control vectors (Figure 3D). This result indicates that direct inhibition of miR-15b in ischemic cultures mimics the increased Bcl-2 protein expression observed in sevoflurane-preconditioned ischemic cultures. In addition, we examined the cell viability afforded by sevoflurane preconditioning compared to direct molecular inhibition of miR-15b with miR-15b-I. Using Alamar blue reduction as a viability assay and lactate dehydrogenase (LDH) release as a measure of cell toxicity, we found that sevoflurane preconditioning (Figure 4A,B) or molecular inhibition of miR-15b (Figure 4C,D) significantly protected against OGD cell toxicity. Consistent with the data demonstrating that sevoflurane-induced Bcl-2 expression could be mitigated by high levels of miR-15b, forced overexpression of miR-15b also significantly decreased the neuroprotective effects against OGD afforded by sevoflurane preconditioning (Figure 4A,B). These results indicate that targeted molecular inhibition of miR-15b mimics the increased Bcl-2 expression and neuroprotection against ischemia attained by sevoflurane preconditioning.
Figure 4. Molecular inhibition of miR-15b is neuroprotective against neuronal ischemia in vitro.
(A,B) Neuroprotection afforded by sevoflurane preconditioning is significantly decreased by transduction of miR-15b (15b) in terms of cell viability (A) and cell death (B). (C,D) Transduction with the miR-15b inhibitor (15bI) effectively promotes cell viability (C) and decreases cell death (D) following OGD. Viability and death were measured by Alamar blue uptake and LDH release. Asterisks indicate comparisons between OGD-only cultures to cultures treated with OGD and transduced with the indicated lentiviral vectors. All data are mean ± SEM, from at least three indepebdent experiments, **P<0.01; and #P<0.05 compared with transduction of either miR-15b (15b) or the inhibitor miR-15B-I (15bI).
Bcl-2 protein is required for ischemic neuroprotection afforded by sevoflurane preconditioning in vivo
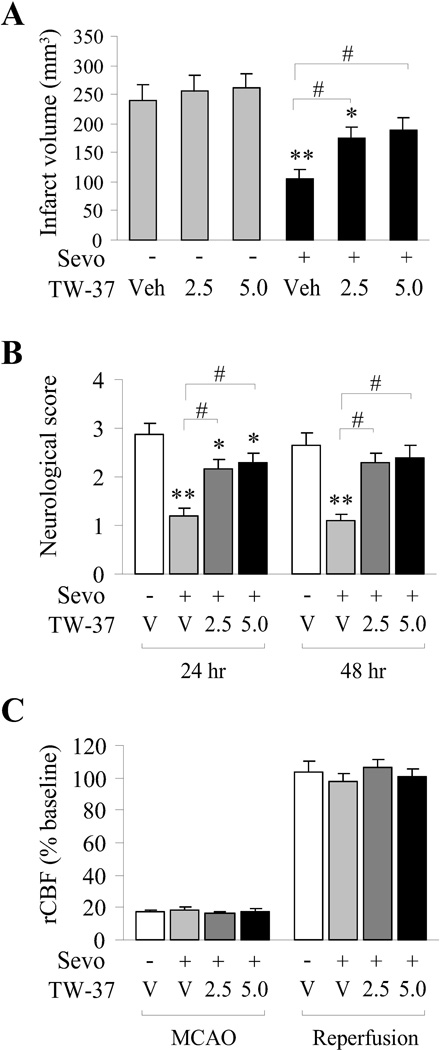
Currently, no pharmacological inhibitors directly targeting miR-15b are available for use in brain, and molecular inhibition of microRNAs in vivo is compromised by experimental limitations. In order to address the implications of sevoflurane-mediated upregulation of Bcl-2 protein, we took advantage of a novel Bcl-2 inhibitor, TW-37, which functions as a Bcl-2 inhibitor at the protein level (by binding to its BH3 domain). Sevoflurane preconditioning significantly decreased ischemic infarct volume and improved neurological scores following MCAO (Figure 5A,B). However, intracerebroventricular injection of TW-37 following sevoflurane preconditioning and 1 h before MCAO significantly increased the infarct volume at both 24 and 48 hr following reperfusion (Figure 5A,B). Interestingly, injection of TW-37 in the absence of sevoflurane preconditioning had no effect on ischemic infarct even at the higher dose (Figure 5A). These effects were observed in the absence of any difference among groups in regional cerebral blood flow (rCBF) or other physiological parameters (data not shown). Thus, the function of Bcl-2 at the protein level is a necessary component for full neuroprotective effects of sevoflurane preconditioning against cerebral ischemia.
Figure 5. Bcl2 inhibitor significantly inhibits neuroprotection afforded by sevoflurane preconditioning.
A) Effects of sevoflurane preconditioning and Bcl-2 inhibitor (TW-37) on infarct volume. (B) Effects of sevoflurane preconditioning and Bcl-2 inhibitor (TW-37) on neurological scores. Neurological function was tested in rats at 24 and 48 hours after ischemia. (C) Changes in rCBF were not different between groups during ischemia and during reperfusion. Compared with the vehicle group without TW-37 or sevoflurane preconditioning, All data are mean ± SEM, n=10/group, **p<0.01, *p<0.05; compared with the sevoflurane preconditioned group, #p<0.05.
DISCUSSION
In the present study, we have identified the upregulation of miR-15b as a major contributor to neural ischemic injury. Taken together with the novel findings that 1) the neuroprotective paradigm of sevoflurane preconditioning robustly suppresses miR-15b expression following ischemia, 2) targeted inhibition of miR-15b can mimic the protective effects of sevoflurane preconditioning, and 3) Bcl-2 mRNA is effectively targeted by miR-15b via its 3’-UTR under neural ischemic conditions, we propose the targeting of miR-15b expression as a novel neuroprotective strategy against ischemic injury.
Our data indicate that a broad range of microRNAs is altered following cerebral ischemia. We focused on miR-15b primarily due to its robust upregulation under ischemic conditions (Figure 1A) as well as its previously identified role in apoptosis and the specific targeting the mRNA of the pro-survival gene Bcl-2 in cancer cells [34, 35]. Previous work by our group indicated that antisense knockdown of endogenous Bcl-2 mRNA exacerbated cerebral ischemic injury in the rat and blocked the neuroprotection afforded by ischemic preconditioning [37, 38]. Indeed, we have demonstrated that by direct molecular inhibition of miR-15b we could mimic protection afforded by sevoflurane preconditioning and allow increased expression of Bcl-2 protein, as well as potentially other relevant molecules. This step forward presents an innovative aspect to therapeutic intervention compared to previous antisense therapies targeting single molecules. The processes of cell death and survival are now generally accepted to be multifaceted in nature, particularly under complex pathophysiological states such as ischemia and reperfusion. Stemming from these past few decades’ worth of research, the concept of the “silver bullet” approach to therapeutics (i.e., specific targeting of one gene product) has been acknowledged to be severely limited in scope for the treatment of pathological states such as ischemia. Rather, a multimodal approach (“silver shotgun”) appears necessary, where multiple gene products would be manipulated differently at different times via a coordinated process. Manipulation of microRNAs, which target diverse sets of mRNAs, may present an interesting opportunity to encompass a wider range of therapeutic effects. Consistent with this, it is likely that further studies targeting the other differentially expressed microRNAs in ischemic brain (miR-15b, -214, -92a, -551b, -206, -195, -300-3p, -181d, -541, -708, -124-star, and -379) will reveal alternative ways to affect cerebral ischemic outcomes, particularly if the time frame of these alterations is better defined.
Identification of miR-15b as a target for therapeutic intervention against stroke is interesting. However, the implications of microRNAs on cell function have only recently started to undergo investigation. Given the highly complex signaling pathways now evident in virtually every aspect of cellular function, it is likely that a microRNA with several targets could impinge on a multitude of cell function – possibly affecting posttranslational modifications, degradation, synthesis and biochemical processes via the effective knockdown of key components required in different signaling pathways. In context of the above study, it is highly likely that sevoflurane preconditioning exerts neuroprotective effects via integration of multiple pathways that may be equally necessary for the full impact of neuroprotection. Given the wide range of cellular events observed to contribute to sevoflurane protection against ischemic injury in both the heart and brain, the suppression of miR-15b may account for only a portion of the overall cellular protection afforded by preconditioning. Our current study demonstrates that suppression of miR-15b can mimic sevoflurane preconditioning, but it remains highly likely that combinatorial use of miR-15b suppression with other cellular targets would increase therapeutic effectivity.
Bioinformatic analysis indicates that the 5′-end “seed” region portion of miR-15b is complementary to 3′UTR of Bcl-2 in various species, including human, mouse and rat [36]. Cimmino and colleagues observed that transfection of miR-15b greatly reduced Bcl-2 protein, but not Bcl-2 mRNA, and the luciferase activity of Bcl-2 3’-UTR-based reporter construct in a leukemia cell line [39]. Likewise, forced over-expression of miR-15b in SGC7901/VCR cells reduced Bcl-2 protein and the luciferase activity of a Bcl-2 3′ untranslated region-based reporter construct [35]. We have further extrapolated these finding to the neural ischemic context. Taken together, these findings suggest that Bcl-2 is a direct target of miR-15b, and that miR-15b negatively regulates the antiapoptotic gene Bcl-2 at the posttranscriptional level. Further analysis on other mRNAs containing similar UTRs or differential protein profiles in the presence of the miR-15b inhibitor could help identify more downstream targets of miR-15b, and thus may lead to novel insights into the molecular mechanisms of ischemic injury.
Cerebral ischemia in rats induces a broad change in the microRNA profile, including significant upregulation of miR-15b. Sevoflurane preconditioning has been well documented to exert neuroprotection against ischemic injury, both in vivo [15–21] and in vitro [14, 22–27]. Interestingly, we have made the novel observations that preconditioning with sevoflurane significantly attenuated the expression and functional activity of miR-15b after reperfusion, and that forced overexpression of miR-15b abrogated the neuroprotective effects of sevoflurane. The reduction of miR-15b expression correlated with increased protein expression of Bcl-2, which may account for at least a significant portion of the neuroprotective effects afforded by sevoflurane preconditioning against ischemic injury in rats. Thus, the suppression of miR-15b (and subsequent upregulation of Bcl-2, among other unknown targets) may contribute to the neuroprotective effects of sevoflurane preconditioning. While the use of anesthetic preconditioning can be clinically translated to reduce ischemic risk or severity in surgical populations [40, 41], defining pluripotent molecular targets such as miR-15b underlying the therapeutic effects of volatile anesthestics may allow for a more precise, and more generally applicable, method in stroke treatment. Further studies investigating miR-15b and the other differentially expressed microRNAs as a mechanism underlying sevoflurane actions will help to expand the understanding of the effects of volatile anesthetics on injurious states as well as provide insight into the molecular mechanisms leading to ischemic neural survival. These findings not only highlight the broad effect of cerebral ischemia on microRNA expression profiles, but also offer insights into mechanisms of sevoflurane preconditioning and facilitate our access to novel therapeutic strategy against ischemic injury.
CONCLUSION
Preconditioning with 1.0 MAC sevoflurane exerts neuroprotective effects against focal cerebral injury in rats by downregulating the ischemia-induced elevation of miR-15b and promoting the sustained expression of its target, Bcl-2.
ACKNOWLEGEMENTS
This work was supported by the National Natural Science Foundation of China grants #30870794 and 81020108021 (to Y.G.), #81271275 and 81070947 (to B.S.), and #81150110494 (to P.V), NIH/NINDS grants NS56118 and NS45048 (to J.C.), the Ph. D. Programs Foundation of Ministry of Education #2010007111 (to W.L.), and the Open Research Fund Program of the Institutes of Brain Science of Fudan University (to Y.G. and H.S.). B.S. was also supported by a grant from the Natural Science Foundation of Shandong (#ZR2012HZ006).
LIST OF ABBREVIATIONS
- BBB
improving blood-brain-barrier
- EtCO2
end tidal CO2
- MCAO
middle cerebral artery occlusion
- rCBF
regional cerebral blood flow
- ROS
reactive oxygen species
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest with other individuals or organizations.
REFERENCES
- 1.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10(10):704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang Y, Shen XJ, Zou Q, Wang SP, Tang SM, Zhang GZ. Biological functions of microRNAs: a review. J Physiol Biochem. 2011;67(1):129–139. doi: 10.1007/s13105-010-0050-6. [DOI] [PubMed] [Google Scholar]
- 3.Benoit ME, Tenner AJ. Complement protein C1q-mediated neuroprotection is correlated with regulation of neuronal gene and microRNA expression. J Neurosci. 2011;31(9):3459–3469. doi: 10.1523/JNEUROSCI.3932-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buller B, Liu X, Wang X, Zhang RL, Zhang L, Hozeska-Solgot A, Chopp M, Zhang ZG. MicroRNA-21 protects neurons from ischemic death. FEBS J. 2010;277(20):4299–4307. doi: 10.1111/j.1742-4658.2010.07818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee ST, Chu K, Jung KH, Yoon HJ, Jeon D, Kang KM, Park KH, Bae EK, Kim M, Lee SK, Roh JK. MicroRNAs induced during ischemic preconditioning. Stroke. 2010;41(8):1646–1651. doi: 10.1161/STROKEAHA.110.579649. [DOI] [PubMed] [Google Scholar]
- 6.Lusardi TA, Farr CD, Faulkner CL, Pignataro G, Yang T, Lan J, Simon RP, Saugstad JA. Ischemic preconditioning regulates expression of microRNAs and a predicted target, MeCP2, in mouse cortex. J Cereb Blood Flow Metab. 2010;30(4):744–756. doi: 10.1038/jcbfm.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sepramaniam S, Armugam A, Lim KY, Karolina DS, Swaminathan P, Tan JR, Jeyaseelan K. MicroRNA 320a functions as a novel endogenous modulator of aquaporins 1 and 4 as well as a potential therapeutic target in cerebral ischemia. J Biol Chem. 2010;285(38):29223–29230. doi: 10.1074/jbc.M110.144576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi H, Li P, Liang W, Chen J, Gao Y. Mechanisms of microRNA-mediated regulation of angiogenesis. Front Biosci (Elite Ed) 2010;2:1304–1319. doi: 10.2741/e191. [DOI] [PubMed] [Google Scholar]
- 9.Siegel C, Li J, Liu F, Benashski SE, McCullough LD. miR-23a regulation of X-linked inhibitor of apoptosis (XIAP) contributes to sex differences in the response to cerebral ischemia. Proc Natl Acad Sci U S A. 2011;108(28):11662–11667. doi: 10.1073/pnas.1102635108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weng H, Shen C, Hirokawa G, Ji X, Takahashi R, Shimada K, Kishimoto C, Iwai N. Plasma miR-124 as a biomarker for cerebral infarction. Biomed Res. 2011;32(2):135–141. doi: 10.2220/biomedres.32.135. [DOI] [PubMed] [Google Scholar]
- 11.Yin KJ, Deng Z, Huang H, Hamblin M, Xie C, Zhang J, Chen YE. miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol Dis. 2010;38(1):17–26. doi: 10.1016/j.nbd.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng L, Liu J, Wang Y, Wang L, Weng S, Tang Y, Zheng C, Cheng Q, Chen S, Yang GY. MicroRNA-210 as a novel blood biomarker in acute cerebral ischemia. Front Biosci (Elite Ed) 2011;3:1265–1272. doi: 10.2741/e330. [DOI] [PubMed] [Google Scholar]
- 13.Ziu M, Fletcher L, Rana S, Jimenez DF, Digicaylioglu M. Temporal differences in microRNA expression patterns in astrocytes and neurons after ischemic injury. PLoS One. 2011;6(2):e14724. doi: 10.1371/journal.pone.0014724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Payne RS, Akca O, Roewer N, Schurr A, Kehl F. Sevoflurane-induced preconditioning protects against cerebral ischemic neuronal damage in rats. Brain Res. 2005;1034(1–2):147–152. doi: 10.1016/j.brainres.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Kehl F, Payne RS, Roewer N, Schurr A. Sevoflurane-induced preconditioning of rat brain in vitro and the role of KATP channels. Brain Res. 2004;1021(1):76–81. doi: 10.1016/j.brainres.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 16.Luo Y, Ma D, Ieong E, Sanders RD, Yu B, Hossain M, Maze M. Xenon and sevoflurane protect against brain injury in a neonatal asphyxia model. Anesthesiology. 2008;109(5):782–789. doi: 10.1097/ALN.0b013e3181895f88. [DOI] [PubMed] [Google Scholar]
- 17.Sigaut S, Jannier V, Rouelle D, Gressens P, Mantz J, Dahmani S. The preconditioning effect of sevoflurane on the oxygen glucose-deprived hippocampal slice: the role of tyrosine kinases and duration of ischemia. Anesth Analg. 2009;108(2):601–608. doi: 10.1213/ane.0b013e31818e2018. [DOI] [PubMed] [Google Scholar]
- 18.Velly LJ, Canas PT, Guillet BA, Labrande CN, Masmejean FM, Nieoullon AL, Gouin FM, Bruder NJ, Pisano PS. Early anesthetic preconditioning in mixed cortical neuronal-glial cell cultures subjected to oxygen-glucose deprivation: the role of adenosine triphosphate dependent potassium channels and reactive oxygen species in sevoflurane-induced neuroprotection. Anesth Analg. 2009;108(3):955–963. doi: 10.1213/ane.0b013e318193fee7. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Lei B, Popp S, Meng F, Cottrell JE, Kass IS. Sevoflurane immediate preconditioning alters hypoxic membrane potential changes in rat hippocampal slices and improves recovery of CA1 pyramidal cells after hypoxia and global cerebral ischemia. Neuroscience. 2007;145(3):1097–1107. doi: 10.1016/j.neuroscience.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Dai ZG, Dong XW, Guo SX, Liu Y, Wang ZP, Zeng YM. Duplicate preconditioning with sevoflurane in vitro improves neuroprotection in rat brain via activating the extracellular signal-regulated protein kinase. Neurosci Bull. 2010;26(6):437–444. doi: 10.1007/s12264-010-6024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zitta K, Meybohm P, Bein B, Ohnesorge H, Steinfath M, Scholz J, Albrecht M. Cytoprotective effects of the volatile anesthetic sevoflurane are highly dependent on timing and duration of sevoflurane conditioning: findings from a human, in-vitro hypoxia model. Eur J Pharmacol. 2010;645(1–3):39–46. doi: 10.1016/j.ejphar.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Adamczyk S, Robin E, Simerabet M, Kipnis E, Tavernier B, Vallet B, Bordet R, Lebuffe G. Sevoflurane pre- and post-conditioning protect the brain via the mitochondrial K ATP channel. Br J Anaesth. 2010;104(2):191–200. doi: 10.1093/bja/aep365. [DOI] [PubMed] [Google Scholar]
- 23.Codaccioni JL, Velly LJ, Moubarik C, Bruder NJ, Pisano PS, Guillet BA. Sevoflurane preconditioning against focal cerebral ischemia: inhibition of apoptosis in the face of transient improvement of neurological outcome. Anesthesiology. 2009;110(6):1271–1278. doi: 10.1097/ALN.0b013e3181a1fe68. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Lu S, Yu Q, Liang W, Gao H, Li P, Gan Y, Chen J, Gao Y. Sevoflurane preconditioning confers neuroprotection via anti-inflammatory effects. Front Biosci (Elite Ed) 2011;3:604–615. doi: 10.2741/e273. [DOI] [PubMed] [Google Scholar]
- 25.Yang Q, Dong H, Deng J, Wang Q, Ye R, Li X, Hu S, Xiong L. Sevoflurane preconditioning induces neuroprotection through reactive oxygen species-mediated up-regulation of antioxidant enzymes in rats. Anesth Analg. 2011;112(4):931–937. doi: 10.1213/ANE.0b013e31820bcfa4. [DOI] [PubMed] [Google Scholar]
- 26.Ye Z, Guo Q, Wang N, Xia P, Yuan Y, Wang E. Delayed neuroprotection induced by sevoflurane via opening mitochondrial ATP-sensitive potassium channels and p38 MAPK phosphorylation. Neurol Sci. 2012;33(2):239–249. doi: 10.1007/s10072-011-0665-6. [DOI] [PubMed] [Google Scholar]
- 27.Yu Q, Chu M, Wang H, Lu S, Gao H, Li P, Gan Y, Shi H, Liang W, Chen J, Gao Y. Sevoflurane preconditioning protects blood-brain-barrier against brain ischemia. Front Biosci (Elite Ed) 2011;3:978–988. doi: 10.2741/e303. [DOI] [PubMed] [Google Scholar]
- 28.Hanouz JL, Zhu L, Lemoine S, Durand C, Lepage O, Massetti M, Khayat A, Plaud B, Gerard JL. Reactive oxygen species mediate sevoflurane- and desflurane-induced preconditioning in isolated human right atria in vitro. Anesth Analg. 2007;105(6):1534–1539. doi: 10.1213/01.ane.0000286170.22307.1a. table of contents. [DOI] [PubMed] [Google Scholar]
- 29.Mattson MP, Kumar KN, Wang H, Cheng B, Michaelis EK. Basic FGF regulates the expression of a functional 71 kDa NMDA receptor protein that mediates calcium influx and neurotoxicity in hippocampal neurons. J Neurosci. 1993;13(11):4575–4588. doi: 10.1523/JNEUROSCI.13-11-04575.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao G, Pei W, Lan J, Stetler RA, Luo Y, Nagayama T, Graham SH, Yin XM, Simon RP, Chen J. Caspase-activated DNase/DNA fragmentation factor 40 mediates apoptotic DNA fragmentation in transient cerebral ischemia and in neuronal cultures. J Neurosci. 2001;21(13):4678–4690. doi: 10.1523/JNEUROSCI.21-13-04678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Jin K, Chen M, Pei W, Kawaguchi K, Greenberg DA, Simon RP. Early detection of DNA strand breaks in the brain after transient focal ischemia: implications for the role of DNA damage in apoptosis and neuronal cell death. J Neurochem. 1997;69(1):232–245. doi: 10.1046/j.1471-4159.1997.69010232.x. [DOI] [PubMed] [Google Scholar]
- 32.Halliwell B, Aruoma OI. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett. 1991;281(1–2):9–19. doi: 10.1016/0014-5793(91)80347-6. [DOI] [PubMed] [Google Scholar]
- 33.Yang BF, Lu YJ, Wang ZG. MicroRNAs and apoptosis: implications in the molecular therapy of human disease. Clin Exp Pharmacol Physiol. 2009;36(10):951–960. doi: 10.1111/j.1440-1681.2009.05245.x. [DOI] [PubMed] [Google Scholar]
- 34.Guo CJ, Pan Q, Li DG, Sun H, Liu BW. miR-15b and miR-16 are implicated in activation of the rat hepatic stellate cell: An essential role for apoptosis. J Hepatol. 2009;50(4):766–778. doi: 10.1016/j.jhep.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 35.Xia L, Zhang D, Du R, Pan Y, Zhao L, Sun S, Hong L, Liu J, Fan D. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int J Cancer. 2008;123(2):372–379. doi: 10.1002/ijc.23501. [DOI] [PubMed] [Google Scholar]
- 36.Willimott S, Wagner SD. Post-transcriptional and post-translational regulation of Bcl2. Biochem Soc Trans. 2010;38(6):1571–1575. doi: 10.1042/BST0381571. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Simon RP, Nagayama T, Zhu R, Loeffert JE, Watkins SC, Graham SH. Suppression of endogenous bcl-2 expression by antisense treatment exacerbates ischemic neuronal death. J Cereb Blood Flow Metab. 2000;20(7):1033–1039. doi: 10.1097/00004647-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu S, Nagayama T, Jin KL, Zhu L, Loeffert JE, Watkins SC, Graham SH, Simon RP. bcl-2 Antisense treatment prevents induction of tolerance to focal ischemia in the rat brain. J Cereb Blood Flow Metab. 2001;21(3):233–243. doi: 10.1097/00004647-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005;102(39):13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koerner IP, Brambrink AM. Brain protection by anesthetic agents. Curr Opin Anaesthesiol. 2006;19(5):481–486. doi: 10.1097/01.aco.0000245271.84539.4c. [DOI] [PubMed] [Google Scholar]
- 41.Bienengraeber MW, Weihrauch D, Kersten JR, Pagel PS, Warltier DC. Cardioprotection by volatile anesthetics. Vascul Pharmacol. 2005;42(5–6):243–252. doi: 10.1016/j.vph.2005.02.005. [DOI] [PubMed] [Google Scholar]