Abstract
Background
Thyroid cancer incidence is increasing worldwide. Incorporating 22 years of incidence data through 2009, we extend examination of these trends among a wide array of subgroups defined by patient (age, sex, race/ethnicity, and nativity), tumor (tumor size and stage), and neighborhood (socioeconomic status and residence in ethnic enclaves) characteristics, to identify possible reasons for this increase.
Methods
Thyroid cancer incidence data on 10,940 men and 35,147 women were obtained from the California Cancer Registry for 1988–2009. Population data were obtained from the 1990 and 2000 US Census. Incidence rates and 95% confidence intervals (CI) were calculated and incidence trends evaluated using Joinpoint regression to evaluate the timing and magnitude of change (annual percent change (APC) and rate ratios).
Results
The incidence of papillary thyroid cancer continues to increase in both men (APC=5.4, 95% CI: 4.5–6.3 for 1998–2009) and women (APC=3.8, 95% CI: 3.4–4.2 for 1998–2001 and APC=6.3, 95% CI: 5.7–6.9 for 2001–2009). Increasing incidence was observed in all subgroups examined.
Conclusions
While some variation in the magnitude or temporality of the increase in thyroid cancer incidence exists across subgroups, the patterns (1) suggest that changes in diagnostic technology alone do not account for the observed trends and (2) point to the importance of modifiable behavioral, lifestyle, or environmental factors in understanding this epidemic.
Impact
Given the dramatic and continued increase in thyroid cancer incidence rates, studies addressing the causes of these trends are critical.
Keywords: thyroid cancer, incidence rates, temporal trends, surveillance, California
Introduction
Substantial increases in the incidence of thyroid cancer over the last 30 years have been observed in many countries worldwide (1–5). In the US, significant increases in thyroid cancer incidence rates began in 1980 and the rate of increase has accelerated since 1997 (6). While thyroid cancer is three times more common in women than men, the rate and temporality of this increase is similar in men and women (6). In addition, the increase appears to be largely limited to papillary thyroid cancer (4, 5, 7–10) and most prominent among small tumors <1 cm (7, 8, 11). While some authors have attributed this increase solely to improvements in diagnostic technology and imaging (7, 12–14) leading to the over-diagnosis of occult tumors (14, 15), most have concluded that other factors are also involved (2, 3, 8, 10, 11, 14, 16–22).
Here we present a comprehensive evaluation of temporal trends in thyroid cancer incidence by patient, tumor, and selected neighborhood/contextual factors in the diverse California population. This work extends previous analyses which have examined trends in other populations through 2005, by several calendar years, to 2009, thus, evaluating current trends. In addition, it expands the scope of previous work by evaluating variation in thyroid cancer incidence by previously unexamined individual-level (i.e., nativity) and contextual-level (i.e., residence in an ethnic enclave among Hispanics and Asian/Pacific Islanders (APIs)) factors which reflect degree of acculturation (23). The contextual-level acculturation variable has, to our knowledge, only been compiled for census-regions within California, as has denominator data specific to the joint distribution of age, ethnicity, and nativity which is necessary for computing nativity-specific rates and evaluating trends by our individual-level acculturation measure. Since acculturation has been found to modify the predictive nature of socioeconomic status (SES) in other cancer studies (24), the evaluation of California data in this regard may provide additional insight into what is known about thyroid cancer trends. Finally, we address the impact that improved completeness of tumor size data has on the observed trends.
Materials and Methods
Cancer incidence data were obtained from the California Cancer Registry (CCR), which is part of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program. Reporting of cancer cases to the CCR is mandated by law, and data quality, completeness, and timeliness of reporting meet SEER standards (25). Cases were included in the present analysis if they were residents of California when diagnosed with a first primary invasive thyroid cancer (International Classification of Diseases for Oncology version 3 [ICD-O-3] site code C73.9) between January 1, 1988 and December 31, 2009. Primary analyses focused on papillary thyroid carcinoma including its follicular variant (ICD-O-3 histology codes 8050, 8260, 8340–8344 and 8350).
For each cancer case, we obtained the following information from the CCR: age at diagnosis, date of diagnosis, sex, race/ethnicity, birthplace (when available), census tract of residence at diagnosis, tumor size, and stage at diagnosis. These data are routinely abstracted from the medical record. The North American Association of Central Cancer Registries (NAACCR) Hispanic and Asian Pacific Islander Identification Algorithms (NHIA and NAPIIA, respectively), which are based on surname, maiden name, and birthplace, were used to improve classification of Hispanic and specific API ethnicities (26, 27). Birthplace/nativity data were augmented as described below. Census tract of residence was used to determine neighborhood SES. For Hispanics and APIs, residence in an ethnic enclave, was determined as described below. Disease stage was classified according to the SEER classification system as localized, regional, distant, or unknown. The SEER staging system for papillary thyroid cancer (and its follicular variant) is independent of tumor size. Local refers to a tumor confined to the thyroid gland. Regional indicates spread to the lymph nodes or extranodal tissue in the neck. Distant refers to metastatic spread elsewhere in the body.
General population data was obtained from the 1990 and 2000 Census Summary File 3 (SF-3). Population counts by sex, race/ethnicity, and five-year age group for California were used. For nativity-specific population estimates, data from the 1990 and 2000 Census’s 5% Integrated Public-Use Microdata Sample was used to estimate age- and nativity-specific population counts for each ethnic group by smoothing with a spline-based function (28, 29). For intercensal years, the foreign-born population was estimated using cohort component interpolation and extrapolation methods, adjusting the estimates to the age and year populations provided by the California Department of Finance for years 1988–1989 and by the National Center for Health Statistics for years 1990–2004. At the time of analysis, 2010 Census data by nativity were not yet available and extrapolation of nativity-stratified population estimates beyond 2004 would have resulted in unstable estimates..
Analyses by Nativity
Information on birthplace is routinely collected by the CCR from hospital medical records and augmented with data from death certificates. Our prior research shows that these data, when available, are highly accurate at the level of US- and foreign-born (30, 31). However, they are missing on approximately 30% to 35% of Hispanic and API cases. As described previously, we have developed an optimized system for imputing birthplace with minimal bias using the first five digits of the Social Security number, which correspond to the state and year of issuance (24, 32, 33).
Analyses by Neighborhood Characteristics
A neighborhood SES quintile was assigned to each person based on their census tract of residence at the time of diagnosis and a previously developed index that incorporates Census data on education, income, occupation, unemployment, and housing costs (34). Census data from 1990 was used for determining the SES index for the period 1988–1992 and 2000 Census data was used for the period between 1998 and 2002. Quintiles of SES were based on ranking all California census tracts according to this index within each time period.
Residence in an ethnic enclave, defined as a geographic unit with a higher concentration of a foreign-born race/ethnic-specific population and language(s) than other geographic units, was determined for each Hispanic or API person’s census tract based on a previously developed index that incorporates 2000 census data on race/ethnicity, nativity, immigration history, English and native language fluency, and linguistic isolation (24, 35). The census-tract level index is based on averaging the data at the block group level for each tract. Each Hispanic and API person was assigned to an enclave quintile based on their census block-group of residence at diagnosis.
Analyses of neighborhood SES and residence in an ethnic enclave were limited to persons diagnosed in the five years around the 1990 and 2000 decennial censuses (i.e., 1988–1992 and 1998–2002).
Statistical Analyses
SEER*Stat software (36) was used to compute age-adjusted and age-specific incidence rates per 100,000 persons, standardized to the 2000 US standard million population, and corresponding 95% confidence intervals (CI). Incidence rate ratios (IRR) and corresponding CIs were calculated to estimate the magnitude of the difference between incidence rates for various time periods, with the earlier time period being the reference group. Time trends in incidence rates between 1988 and 2009 were also examined using Joinpoint Regression (37, 38). This software calculates the annual percent change (APC) in incidence rates by fitting a series of least squares regression lines to the natural logarithm of the age-adjusted incidence rates (the dependent variable), using calendar year as the independent regression variable. This method allows for the identification of all changes in the slope of the regression (trend) line that represents statistically significant contributions to the explanatory model based on the Permutation Test and the Bayesian Information Criterion; the points at which the trend lines change are termed the “joinpoints.” Results from the Joinpoint analysis are presented in Table 2 as a given number of “trends” (i.e., joinpoints) of a given magnitude (i.e., the APC) for a group of years for each subgroup examined.
Table 2.
Temporal trends in the annual percent change (APC) in age-adjusted papillary thyroid cancer incidence rates between 1988 and 2009 in California, by individual and tumor characteristics.
| Trend 1
|
Trend 2
|
Trend 3
|
Trend 4
|
Trend 5
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| years | APC | years | APC | years | APC | years | APC | years | APC | |
| Men | ||||||||||
| All | 1988–1998 | 1.1 (−0.3–2.5) | 1998–2009 | 5.4 (4.5–6.3) | ||||||
| Age | ||||||||||
| <20 | 1988–2009 | 1.3 (−1.4–4.1) | ||||||||
| 20–34 | 1988–2009 | 2.6 (1.8–3.4) | ||||||||
| 35–49 | 1988–1996 | −2.1 (−5.8-1.8) | 1996–2009 | 4.9 (3.4–6.5) | ||||||
| 50–64 | 1988–2009 | 4.3 (3.6–5.0) | ||||||||
| ≥65 | 1988–2009 | 4.6 (3.6–5.6) | ||||||||
| Race/ethnicity/nativity | ||||||||||
| non-Hispanic white | 1988–1995 | −0.4 (−3.2-2.4) | 1995–2009 | 5.4 (4.6–6.3) | ||||||
| non-Hispanic black | 1988–2009 | 4.1 (2.3–6.1) | ||||||||
| Hispanic | 1988–2009 | 3.9 (2.5–5.2) | ||||||||
| US-born | 1988–2004a | 3.3 (0.4–6.2) | ||||||||
| foreign-born | 1988–2004a | 2.2 (−1.7–6.3) | ||||||||
| Asian/Pacific Islander | 1988–2006 | 1.1 (−0.3–2.5) | 2006–2009 | 16.8 (2.3–33.5) | ||||||
| US-bornb | 1988–2004a | 2.9 (−2.4–8.5) | ||||||||
| foreign-bornb | 1988–2004a | −0.2 (−2.3-1.9) | ||||||||
| Native Americanc | -- | |||||||||
| Tumor size | ||||||||||
| <1 cm | 1988–2009 | 7.8 (6.9–8.8) | ||||||||
| 1.0–1.4 cm | 1988–2009 | 5.7 (4.5–7.0) | ||||||||
| 1.5–1.9 cm | 1988–1993 | −7.1 (−17.2-4.3) | 1993–2009 | 8.1 (6.5–9.8) | ||||||
| 2.0–2.9 cm | 1988–2009 | 4.0 (3.1–4.9) | ||||||||
| 3.0–3.9 cm | 1988–2009 | 3.5 (2.2–4.9) | ||||||||
| 4.0–4.9 cm | 1988–2009 | 3.6 (2.4–4.8) | ||||||||
| ≥5.0 cm | 1988–2009 | 4.4 (3.3–5.6) | ||||||||
| unknown | 1988–1997 | −11.5 (−14.0- -9.0) | 1997–2009 | −2.2 (−4.3-0.0) | ||||||
| SEER stage | ||||||||||
| local | 1988–1995 | −0.6 (−4.3-3.2) | 1995–2009 | 5.5 (4.5–6.5) | ||||||
| regional | 1988–2003 | 1.5 (0.1–2.8) | 2003–2009 | 10.2 (5.9–14.6) | ||||||
| distant | 1988–2009 | 1.5 (−0.1–3.1) | ||||||||
| unknown | 1988–2009 | −1.9 (−4.5-0.8) | ||||||||
| Tumor size / stage | ||||||||||
| <2.0 cm / local | 1988–2009 | 7.8 (7.0–8.6) | ||||||||
| <2.0 cm / advancedd | 1988–2009 | 5.8 (5.0–6.6) | ||||||||
| ≥2.0 cm / local | 1988–2009 | 3.2 (2.2–4.2) | ||||||||
| ≥2.0 cm / advanced | 1988–2009 | 4.8 (3.9–5.6) | ||||||||
| unknown/local | 1988–2009 | −9.9 (−12.3- -7.5) | ||||||||
| unknown/advanced | 1988–2000 | −10.5 (−13.8- -7.2) | 2000–2009 | 3.1 (−3.4– 10.0) | ||||||
| Women | ||||||||||
| All | 1988–2001 | 3.8 (3.4–4.2) | 2001–2009 | 6.3 (5.7–6.9) | ||||||
| Age | ||||||||||
| <20 | 1988–2009 | 2.1 (0.6–3.7) | ||||||||
| 20–34 | 1988–2009 | 3.0 (2.6–3.5) | ||||||||
| 35–49 | 1988–2001 | 3.9 (3.2–4.6) | 2001–2009 | 6.1 (5.1–7.2) | ||||||
| 50–64 | 1988–1992 | 7.3 (2.7–12.1) | 1992–1995 | −0.6 (−12.3–12.6) | 1995–1999 | 9.3 (3.5–15.5) | 1999–2003 | 4.6 (0.2–9.3) | 2003–2009 | 8.4 (7.2–9.6) |
| ≥65 | 1988–2009 | 6.0 (5.3–6.7) | ||||||||
| Race/ethnicity | ||||||||||
| non-Hispanic white | 1988–1993 | 1.6 (−0.7–3.8) | 1993–2009 | 5.4 (5.1–5.8) | ||||||
| non-Hispanic black | 1988–2009 | 5.3 (3.8–6.8) | ||||||||
| Hispanic | 1988–2009 | 4.8 (4.3–5.2) | ||||||||
| US-born | 1988–2004a | 4.7 (3.2–6.2) | ||||||||
| foreign-born | 1988–2004a | 4.0 (2.6–5.5) | ||||||||
| Asian/Pacific Islander | 1988–2003 | 2.2 (1.3–3.1) | 2003–2009 | 7.1 (4.8–9.5) | ||||||
| US-bornb | 1988–2004a | 4.1 (2.3–6.0) | ||||||||
| foreign-bornb | 1988–2004a | 1.1 (0.1–2.1) | ||||||||
| Native Americanc | 1988–2009 | 4.4 (0.9–8.0) | ||||||||
| Tumor size | ||||||||||
| <1 cm | 1988–2009 | 9.0 (8.5–9.5) | ||||||||
| 1.0–1.4 cm | 1988–2004 | 6.3 (5.7–6.8) | 2004–2009 | 12.1 (10.0–14.3) | ||||||
| 1.5–1.9 cm | 1988–2009 | 5.2 (4.5–5.9) | ||||||||
| 2.0–2.9 cm | 1988–2009 | 3.8 (3.4–4.2) | ||||||||
| 3.0–3.9 cm | 1988–1994 | 8.5 (4.1–13.2) | 1994–2009 | 3.6 (2.8–4.4) | ||||||
| 4.0–4.9 cm | 1988–1995 | 10.5 (5.1–16.2) | 1995–2009 | 4.0 (2.8–5.1) | ||||||
| ≥5.0 cm | 1988–2009 | 5.0 (4.0–6.0) | ||||||||
| unknown | 1988–1995 | −9.7 (−12.4- -6.8) | 1995–2009 | −5.1 (−6.5- -3.7) | ||||||
| SEER stage | ||||||||||
| local | 1988–1998 | 3.4 (2.5–4.3) | 1998–2009 | 6.0 (5.4–6.6) | ||||||
| regional | 1988–2000 | 2.9 (1.6–4.1) | 2000–2009 | 8.2 (6.8–9.6) | ||||||
| distant | 1988–1998 | 9.3 (4.9–13.9) | 1998–2009 | −2.9 (−5.6- -0.1) | ||||||
| unknown | 1988–1995 | 11.3 (−0.3–24.2) | 1995–2009 | −7.3 (−11.3- -3.2) | ||||||
| Tumor size / stage | ||||||||||
| <2.0 cm / local | 1988–2009 | 7.8 (7.5–8.1) | ||||||||
| <2.0 cm / advancedd | 1988–2005 | 6.2 (5.2–7.2) | 2005–2009 | 14.9 (9.2–20.8) | ||||||
| ≥2.0 cm / local | 1988–2005 | 3.8 (3.3–4.4) | 2005–2009 | −0.1 (−4.0–4.1) | ||||||
| ≥2.0 cm / advanced | 1988–2009 | 5.6 (5.0–6.2) | ||||||||
| unknown/local | 1988–1991 | −5.8 (−14.8-4.3) | 1991–1994 | −22.0 (−38.1- -1.7) | 1994–2009 | −4.5 (−5.7- -3.1) | ||||
| unknown/advanced | 1988–2009 | −6.6 (−8.1- -5.0) | ||||||||
nativity data can only be calculated for the period 1988–2004
includes Chinese, Japanese, Filipino, Vietnamese, Korean, and South Asian persons
American Indian/Alaskan Native
combines regional and distant stages
The potential impact of the set of {kij} missing or unknown tumor sizes, for years i=1988, …, 2009 and age groups j=1, …, 18, on temporal trends in rates by tumor size was assessed by determining how best to apportion the kij cases to the seven levels of tumor size, from <0.1 to 5+ cm, in such a way as to minimize differences in the magnitude of temporal trends. Specifically, a set of seven non-negative proportions (p1, p2, …, p7) that sum to 1, corresponding to the seven levels of tumor size, was sought such that, when pkkij cases were added to the existing case count for year I and age group j, the differences in AAPC for the resulting rates over time among the seven tumor sizes was minimized. Constrained nonlinear optimization was used to determine the proportions, where the criterion to be minimized was deviance between two nested Poisson Generalized Linear Regression models with a log link. The more general model allowed a separate slope for each tumor size, while the nested model required a common slope among the seven tumor sizes. (Each model allowed different intercepts for each tumor size.)
Results
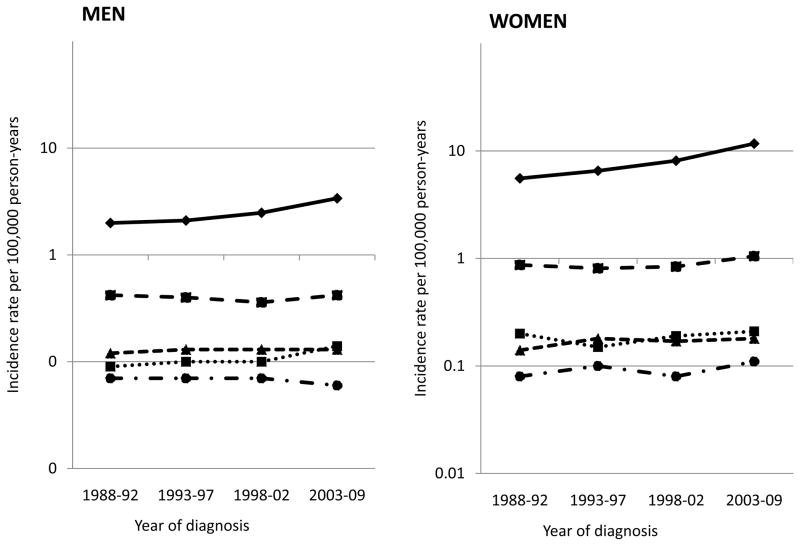
Between 1988 and 2009, 10,940 men and 35,147 women were diagnosed with thyroid cancer in California. Among men and women, 80% and 86%, respectively, of all thyroid cancers were of papillary histology, incidence of which increased significantly during this time period (Figure 1). Among men, no increase was observed for follicular, medullary or anaplastic thyroid cancers; however, tumors for which histology was not specified increased significantly (from 0.09 per 100,000 men in 1988–92 to 0.14 in 2003–2009; IRR=1.66, 95% CI: 1.17–2.41). Among women, a significant increase was observed for follicular tumors (IRR=1.21, 95% CI: 1.10–1.34), but not for medullary, anaplastic, or tumors of unspecified histology. The incidence of papillary thyroid cancer increased from 1.99 to 3.31 per 100,000 men (IRR=1.66, 95% CI: 1.57–1.77) and from 5.56 to 11.37 per 100,000 women (IRR=2.04, 95% CI: 1.97–2.12) between 1988–92 and 2003–09 (Table 1). Among men, this increase began in 1998, while among women, significant annual increases were observed during the entire study period but accelerated as of 2001 (APC1998–2001=3.8, 95% CI: 3.4–4.2 and APC2001–2009=6.3, 95% CI: 5.7–6.9; Table 2).
Figure 1. Average annual age-adjusted thyroid cancer incidence rates (per 100,000) in California, by sex and histologic type.
Papillary: solid line with diamonds; follicular: dashed line with squares; medullary: small dashed line with triangles; anaplastic: dotted & dashed line with circles; “not otherwise specified”: dotted line with squares. Annual percent change, 1988–2009 (unless otherwise specified), for men and women, respectively: papillary: 1988–98 1.1 and 1998–09 5.4*, and 1988–01 3.8* and 2001–09 6.3*; follicular: 0.0 and 1.2*; medullary 0.4 and 1.0; anaplastic −0.9 and 1.2; and “not otherwise specified” histology: 3.0* and 0.3. * denotes p<0.05.
Table 1.
Average annual age-adjusted papillary thyroid cancer incidence rates (per 100,000) for 1988–1992 and 2003–2009 and incidence rate ratios (IRR) in California, by individual and tumor characteristics.
| Men
|
Women
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Na | Incidence rate
|
IRR | Na | Incidence rate
|
IRR | |||
| 1988–1992 | 2003–2009 | 1988–1992 | 2003–2009 | |||||
| All | 8,739 | 1.99 (1.88–2.11) | 3.31 (3.20–3.41) | 1.66 (1.57–1.77) | 30,295 | 5.56 (5.39–5.74) | 11.37 (11.19–11.56) | 2.04 (1.97–2.12) |
| Age | ||||||||
| <20 | 144 | 0.15 (0.10–0.21) | 0.15 (0.12–0.20) | 1.04 (0.66–1.66) | 821 | 0.76 (0.64–0.89) | 1.00 (0.90–1.11) | 1.32 (1.08–1.60) |
| 20–34 | 1,501 | 1.42 (1.27–1.59) | 2.03 (1.87–2.2) | 1.43 (1.24–1.64) | 7,453 | 7.12 (6.75–7.50) | 11.21 (10.81–11.62) | 1.57 (1.48–1.68) |
| 35–49 | 2,877 | 2.98 (2.71–3.27) | 4.34 (4.1–4.59) | 1.45 (1.30–1.62) | 11,289 | 9.21 (8.74–9.71) | 18.93 (18.42–19.45) | 2.05 (1.94–2.18) |
| 50–64 | 2,636 | 3.68 (3.28–4.12) | 6.81 (6.45–7.19) | 1.85 (1.63–2.10) | 7,035 | 7.64 (7.08–8.24) | 19.06 (18.47–19.67) | 2.49 (2.30–2.71) |
| ≥65 | 1,582 | 3.24 (2.82–3.71) | 3.24 (2.82–3.71) | 1.99 (1.70–2.32) | 3,697 | 4.73 (4.30–5.20) | 12.02 (11.47–12.59) | 2.54 (2.29–2.82) |
| Race/ethnicity/nativity | ||||||||
| non-Hispanic white | 5,619 | 2.20 (2.06–2.35) | 3.87 (3.71–4.03) | 1.75(1.62–1.89) | 16,546 | 5.80 (5.58–6.03) | 12.03(11.75–12.32) | 2.07 (1.98–2.17) |
| non-Hispanic black | 264 | 0.83 (0.56–1.18) | 1.67 (1.37–2.01) | 2.01 (1.33–3.09) | 1,003 | 2.78 (2.31–3.31) | 6.01 (5.49–6.57) | 2.16 (1.78–2.66) |
| Hispanic | 1,628 | 1.59 (1.32–1.89) | 2.68 (2.48–2.89) | 1.69 (1.40–2.06) | 7,683 | 5.24 (4.83–5.66) | 11.03 (10.68–11.40) | 2.11 (1.94–2.30) |
| US-bornc | 465 | 2.02 (1.40–2.85) | 2.49 (1.98–3.09) | 1.23 (0.81–1.89) | 2215 | 5.48 (4.58–6.52) | 9.13 (8.22–10.12) | 1.67 (1.36–2.05) |
| foreign-bornc | 511 | 1.75 (1.01–3.44) | 1.90 (1.54–2.32) | 1.09 (0.74–1.64) | 2475 | 5.19 (4.49–5.99) | 8.43 (7.81–9.09) | 1.62 (1.38–1.92) |
| Asian/Pacific Islander | 1,134 | 2.52 (2.10–3.00) | 3.54 (3.24–3.85) | 1.40 (1.15–1.72) | 4,682 | 7.77 (7.10–8.49) | 13.03 (12.50–13.57) | 1.68 (1.52–1.85) |
| US-bornc,d | 142 | 2.23 (1.37–3.53) | 3.43 (2.68–4.33) | 1.54 (0.90–2.66) | 512 | 7.99 (6.33–10.01) | 12.40 (10.91–14.03) | 1.55 (1.20–2.02) |
| foreign-bornc,d | 491 | 2.70 (2.18–3.33) | 2.66 (2.33–3.04) | 0.99 (0.77–1.27) | 2057 | 7.85 (7.07–8.74)c | 9.17 (8.62–9.76) | 1.17 (1.03–1.32) |
| Native Americane | 23 | 0.82 (0.25–1.97)b | 1.34 (0.67–2.47) | 1.00 (0.27–5.77) | 113 | 3.00 (1.62–5.17) | 6.36 (4.74–8.37) | 2.12 (1.13–4.21) |
| Tumor size | ||||||||
| <1 cm | 1,814 | 0.23 (0.19–0.27) | 0.83 (0.78–0.88) | 3.66 (3.06–4.40) | 7,871 | 0.84 (0.78–0.92) | 3.53 (3.43–3.63) | 4.18 (3.83–4.57) |
| 1.0–1.4 cm | 932 | 0.15 (0.12–0.18) | 0.39 (0.35–0.42) | 2.56 (2.05–3.22) | 4,280 | 0.60 (0.55–0.66) | 1.82 (1.75–1.90) | 3.03 (2.73–3.37) |
| 1.5–1.9 cm | 895 | 0.17 (0.14–0.20) | 0.38 (0.34–0.41) | 2.22 (1.18–2.76) | 3,903 | 0.68 (0.62–0.74) | 1.48 (1.41–1.55) | 2.17 (1.96–2.41) |
| 2.0–2.9 cm | 1,486 | 0.30 (0.26–0.35) | 0.54 (0.50–0.59) | 1.80 (1.53–2.12) | 5,740 | 1.08 (1.00–1.15) | 2.01 (1.94–2.09) | 1.87 (1.73–2.03) |
| 3.0–3.9 cm | 992 | 0.20 (0.16–0.23) | 0.36(0.33–0.40) | 1.85 (1.51–2.27) | 2,900 | 0.50 (0.45–0.55) | 1.02 (0.96–1.07) | 2.05(1.82–2.32) |
| 4.0–4.9 cm | 618 | 0.13 (0.10–0.16) | 0.24 (0.21–0.27) | 1.84 (1.43–2.39) | 1,367 | 0.21 (0.17–0.24) | 0.51 (0.47–0.55) | 2.46 (2.05–2.97) |
| ≥5.0 cm | 887 | 0.16 (0.13–0.19) | 0.35 (0.31–0.38) | 2.20 (1.76–2.78) | 1,381 | 0.23 (0.19–0.27) | 0.52 (0.48–0.56) | 2.27 (1.91–2.71) |
| unknown | 1,115 | 0.66 (0.60–0.73) | 0.23 (0.20–0.26) | 0.34 (0.29–0.40) | 2,853 | 1.43 (1.35–1.52) | 0.49 (0.45–0.53) | 0.34 (0.31–0.38) |
| SEER stage | ||||||||
| local | 4,560 | 1.02 (0.94–1.10) | 1.78 (1.70–1.86) | 1.75 (1.60–1.92) | 19,769 | 3.60 (3.46–3.74) | 7.52 (7.37–7.68) | 2.09 (2.00–2.18) |
| regional | 3,250 | 0.75 (0.68–0.82) | 1.22 (1.16–1.28) | 1.63 (1.47–1.81) | 8,662 | 1.60 (1.51–1.69) | 3.36 (3.26–3.47) | 2.11 (1.97–2.25) |
| distant | 709 | 0.17 (0.14–0.21) | 0.25 (0.22–0.28) | 1.45 (1.14–1.84) | 1,264 | 0.22 (0.19–0.26) | 0.36 (0.33–0.40) | 1.63 (1.36–1.97 ) |
| unknown | 220 | 0.06 (0.04–0.08) | 0.06 (0.05–0.08) | 1.06 (0.69–1.65) | 600 | 0.15 (0.12–0.18) | 0.12 (0.10–0.14) | 0.78 (0.66–1.10) |
| Tumor size / stage | ||||||||
| <2.0 cm / local | 2309 | 0.33 (0.29–0.38) | 1.06 (1.00–1.12) | 3.20(2.76–3.73) | 12058 | 1.57 (1.48–1.67) | 5.16 (5.04–5.29) | 3.28 (3.07–3.50) |
| <2.0 cm / advancedf | 1313 | 0.21 (0.18–0.25) | 0.53(0.49–0.57) | 2.49 (2.07–3.02) | 3934 | 0.53 (0.48–0.59) | 1.66 (1.59–1.73) | 3.11 (2.78–3.48) |
| ≥2.0 cm / local | 1863 | 0.40 (0.35–0.45) | 0.66 (0.62–0.71) | 1.67 (1.45–1.93) | 6306 | 1.21 (1.12–1.29) | 2.14 (2.06–2.22) | 1.77 (1.64–1.92) |
| ≥2.0 cm / advanced | 2088 | 0.38 (0.34–0.44) | 0.82 (0.76–0.87) | 2.13 (1.84–2.47) | 4981 | 0.77 (0.71–0.84) | 1.89 (1.82–1.97) | 2.45 (2.23–2.69) |
| unknown/local | 388 | 0.29 (0.25–0.34) | 0.06 (0.05–0.08) | 0.21 (0.15–0.28) | 1405 | 0.82 (0.75–0.89) | 0.22 (0.20–0.25) | 0.27 (0.24–0.32) |
| unknown/advanced | 558 | 0.32(0.28–0.37) | 0.12 (0.10–0.15) | 0.38(0.30–0.48) | 1011 | 0.51 (0.46–0.57) | 0.18 (0.15–0.20) | 0.35 (0.29–0.41) |
1988–2009
1988–1997; number of cases was too small to calculate stable rates for the 1988–1992 time period alone
for periods 1988–1992 and 1998–2004
includes Chinese, Japanese, Filipino, Vietnamese, Korean, and South Asian persons
American Indian/Alaskan Native
combines regional and distant stages
The remainder of the analyses were limited to papillary thyroid cancer. The patterns observed for men and women for all subgroups were generally similar. Table 1 presents the incidence rates for the earliest (1988–1992) and latest (2003–2009) time periods examined and the IRRs comparing rates for these two time periods stratified by several individual and tumor characteristics. Table 2 presents temporal changes in the APC over the entire time period studied. For women, significant increases in papillary thyroid cancer are seen across all subgroups examined (including age, race/ethnicity, birthplace, tumor size, and stage) with the exception of tumors of unknown size or stage, for which significant decreases are observed. Overall, for both men and women, the magnitude of the temporal increase in rates was greater with increasing age and slightly lower among APIs than other racial/ethnic groups (Table 1), the latter due to the lower IRRs for foreign-born API men and women compared to their US-born counterparts. However, during the latter part of the study period, the rate of increase accelerated for men aged 35–49 and women aged 35–64 and white and API men and women (Table 2). For Hispanic men and women, increases were similar regardless of nativity, while for API men and women, the overall rate of increase was substantially greater among those who were US-born. While we attempted to examine the effects of nativity within the disaggregated API ethnic groups, the number of cases in many of the cells became small and the differences were not statistically significant (data not shown).
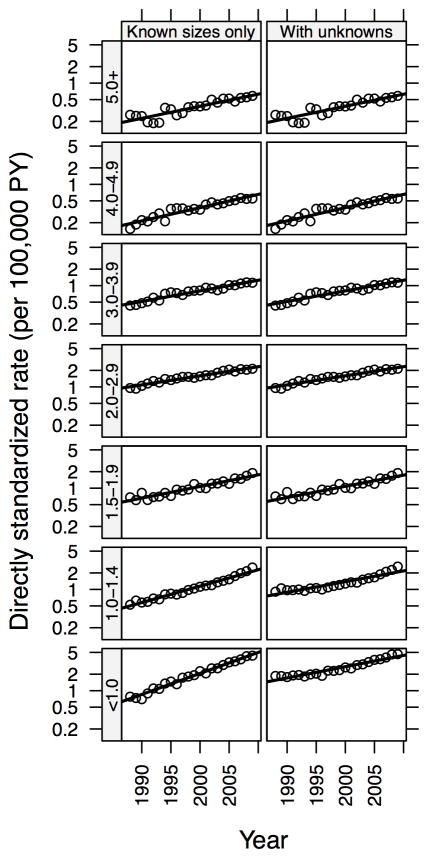
Tumors 1.5 cm or smaller increased at a greater rate than larger tumors, although significant temporal increases were seen for all size tumors, including those ≥5.0 cm. Indeed, the greatest increase in incidence occurred among the earliest stage tumors (<2 cm / local stage; IRR=3.28, 95% CI: 3.07–3.50 for women and IRR=3.20, 95% CI: 2.76–3.73 for men) but with a large and statistically significant increase also seen among the latest stage tumors (≥2 cm / advanced stage; IRR=2.45, 95% CI: 2.23–2.69 for women and IRR=2.13, 95% CI: 1.84–2.47 for men). There was also a significant acceleration in the rate of increase of small but advanced tumors (<2 cm / advanced (regional or distant) stage) among women since 2005. While tumors of unknown stage account for only 2% of all papillary thyroid cancers, tumors of unknown size account for 10% overall, declining from 27% in 1988–92 to 5% in 2003–09. Thus, we examined whether the increase in tumors of any size might be due to the decrease in tumors of unknown size. We found that with an optimal choice of proportions of unknowns allocated to each of the size categories, the differences in category-specific rate increases over time, as measured by the reduction in deviance in a model with separate slopes compared to a model restricted to a common slope, can be reduced by approximately 90% in women, and disappear altogether in men (see Figure 2 for women). Hence, while there does appear to be differences in the rates of increase over time across all of the size categories, the magnitude of the rate change for specific tumor sizes may not be as dramatic as what is depicted by analysis of the cases restricted to data with known size categories.
Figure 2.
Reduction in differences in average annual percent change by tumor size in females due to optimal allocation of unknown tumor sizes.
Table 3 presents the incidence rates by neighborhood characteristics for the two five-year periods around the decennial censuses (1988–1992 and 1998–2002) and the IRRs comparing rates for these two time periods. The incidence of papillary thyroid cancer increased similarly across all levels of SES. In addition, the tumor size-specific patterns of increase were similar for persons of low and high SES. For Hispanics, rates increased similarly regardless of SES or residence in an ethnic enclave. For APIs, the increase in rates was slightly, but not statistically significantly, greater among those of higher SES but did not vary according to residence in an ethic enclave.
Table 3.
Average annual age-adjusted papillary thyroid cancer incidence rates (per 100,000) for 1988–1992 and 1998–2002 and incidence rate ratios (IRR) in California, by neighborhood characteristics.
| Men
|
Women
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Na | Incidence rate
|
IRR | Na | Incidence rate
|
IRR | |||
| 1988–1992 | 1998–2002 | 1988–1992 | 1998–2002 | |||||
| All race/ethnicities | ||||||||
| Neighborhood socioeconomic status (SES) | ||||||||
| Q1 (lowest) | 400 | 1.37 (1.15–1.63) | 2.04 (1.78–2.32) | 1.48 (1.19–1.85) | 1,565 | 4.59 (4.20–5.00) | 7.21 (6.76–7.69) | 1.57 (1.41–1.75) |
| Q2 | 526 | 1.71 (1.49–1.95) | 1.97 (1.74–2.21) | 1.15 (0.96–1.38) | 1,922 | 5.13 (4.76–5.52) | 7.08 (6.67–7.50) | 1.38 (1.26–1.52) |
| Q3 | 589 | 1.78 (1.56–2.03) | 2.15 (1.93–2.40) | 1.21 (1.02–1.43) | 2,280 | 5.76 (5.37–6.17) | 8.44 (8.00–8.89) | 1.46 (1.34–1.60) |
| Q4 | 808 | 2.31 (2.05–2.58) | 3.01 (2.74–3.29) | 1.30 (1.13–1.51) | 2,506 | 6.00 (5.62–6.41) | 9.14 (8.69–9.60) | 1.52 (1.40–1.65) |
| Q5 (highest) | 958 | 2.75 (2.47–3.05) | 3.54 (3.25–3.84) | 1.29 (1.13–1.48) | 2,640 | 6.55 (6.13–6.98) | 10.04 (9.56–10.53) | 1.53 (1.41–1.66) |
| Tumor size / SES | ||||||||
| <2.0 cm / low SES | 485 | 0.37 (0.31–0.44) | 0.79 (0.71–0.89) | 2.14 (1.74–2.64) | 2,511 | 1.74 (1.61–1.87) | 3.83 (3.65–4.01) | 2.20 (2.02–2.41) |
| <2.0 cm / high SES | 697 | 0.79 (0.69–0.90) | 1.44 (1.32–1.58) | 1.83 (1.55–2.16) | 2,635 | 2.71 (2.53–2.91) | 5.30 (5.06 –5.55) | 1.95 (1.79–2.12) |
| ≥2.0 / low SES | 702 | 0.67 (0.59–0.76) | 1.01 (0.92–1.12) | 1.52 (1.29–1.79) | 2,317 | 1.97 (1.83–2.11) | 3.09 (2.93–3.25) | 1.57 (1.44–1.71) |
| ≥2.0 / high SES | 776 | 0.97 (0.85–1.10) | 1.55 (1.42–1.69) | 1.60 (1.37–1.87) | 1,881 | 2.14 (1.98–2.32) | 3.65 (3.45–3.87) | 1.70 (1.55–1.88) |
| unknown / low SES | 328 | 0.59 (0.52–0.68) | 0.23 (0.19–0.29) | 0.39 (0.30–0.50) | 939 | 1.48 (1.36–1.60) | 0.65 (0.58–0.73) | 0.44 (0.38–0.51) |
| unknown / high SES | 293 | 0.77 (0.66–0.88) | 0.29 (0.23–0.35) | 0.37 (0.29–0.48) | 630 | 1.41 (1.28–1.55) | 0.62 (0.54–0.71) | 0.44 (0.37–0.52) |
| Hispanics | ||||||||
| Residence in an ethnic enclave | ||||||||
| Q1 (least dense) | 47 | 0.08 (0.04–0.14) | 0.23 (0.16–0.32) | 2.93 (1.43–6.44) | 206 | 0.46 (0.35–0.59) | 0.97 (0.82–1.15) | 2.11 (1.56–2.89) |
| Q2 | 79 | 0.19 (0.13–0.28) | 0.30 (0.22–0.40) | 1.55 (0.95–2.55) | 306 | 0.57 (0.46–0.70) | 1.25 (1.09–1.43) | 2.19 (1.71–2.83) |
| Q3 | 91 | 0.18 (0.12–0.27) | 0.39 (0.30–0.50) | 2.15 (1.32–3.56) | 409 | 0.82 (0.68–0.98) | 1.62 (1.43–1.82) | 1.98 (1.59–2.46) |
| Q4 | 127 | 0.36 (0.26–0.48) | 0.50 (0.40–0.63) | 1.41 (0.96–2.10) | 626 | 1.22 (1.05–1.42) | 2.59 (2.35–2.85) | 2.12 (1.77–2.54) |
| Q5 (most dense) | 208 | 0.65 (0.50–0.83) | 0.94 (0.78–1.13) | 1.45 (1.06–2.01) | 1,017 | 2.34 (2.08–2.62) | 4.68 (4.33–5.06) | 2.00 (1.74–2.31) |
| SES / enclavea | ||||||||
| low / most dense | 205 | 0.64 (0.49–0.82) | 0.95 (0.78–1.14) | 1.47 (1.07–2.04) | 1011 | 2.36 (2.09–2.64) | 4.69 (4.33–5.07) | 1.99 (1.73–2.29) |
| low / less dense | 199 | 0.22 (0.17–0.29) | 0.43 (0.36–0.51) | 1.92 (1.40–2.65) | 924 | 0.94 (0.83–1.06) | 1.95 (1.80–2.11) | 2.07 (1.79–2.39) |
| high / most dense | -- | -- | -- | -- | -- | -- | -- | -- |
| high / less dense | 145 | 0.18 (0.13–0.23) | 0.29 (0.23–0.35) | 1.62 (1.13–2.33) | 623 | 0.61 (0.52–0.70) | 1.30 (1.18–1.43) | 2.15 (1.80–2.57) |
| Asians/Pacific Islanders | ||||||||
| Residence in an ethnic enclavea | ||||||||
| Q1 (least dense) | 10 | 0.05 (0.02–0.11) | -- | -- | 57 | 0.16 (0.10–0.24) | 0.24 (0.17–0.34) | 1.54 (0.87–2.77) |
| Q2 | 35 | 0.13 (0.08–0.21) | 0.11 (0.06–0.18) | 0.86 (0.41–1.79) | 149 | 0.32 (0.24–0.43) | 0.61 (0.50–0.75) | 1.89 (1.32–2.75) |
| Q3 | 56 | 0.08 (0.04–0.14) | 0.29 (0.21–0.38) | 3.78 (1.85–8.41) | 214 | 0.50 (0.39–0.63) | 0.83 (0.70–0.98) | 1.67 (1.24–2.25) |
| Q4 | 86 | 0.22 (0.14–0.32) | 0.28 (0.29–0.50) | 1.79 (1.09–2.98) | 355 | 0.80 (0.66–0.97) | 1.48 (1.30–1.68) | 1.85 (1.47–2.35) |
| Q5 (most dense) | 217 | 0.62 (0.48–0.78) | 0.91 (0.76–1.08) | 1.47 (1.09–1.99) | 799 | 1.91 (1.68–2.16) | 3.17 (2.91–3.46) | 1.66 (1.43–1.94) |
| SES / enclaveb | ||||||||
| low / most dense | 101 | 0.61 (0.43–0.83) | 0.83 (0.63–1.08) | 1.37 (0.89–2.12) | 419 | 2.05 (1.74–2.40) | 3.13 (2.75–3.54) | 1.53 (1.24–1.88) |
| low / less dense | 82 | 0.10 (0.07–0.14) | 0.15 (0.11–0.19) | 1.47 (0.91–2.44) | 375 | 0.40 (0.33–0.48) | 0.64 (0.56–0.73) | 1.61 (1.29–2.01) |
| high / most dense | 116 | 0.67 (0.46–0.94) | 1.02 (0.81–1.27) | 1.53 (1.00–2.39) | 380 | 1.74 (1.42–2.11) | 3.26 (2.88–3.67) | 1.88 (1.49–2.38) |
| high / less dense | 105 | 0.14 (0.10–0.20) | 0.29 (0.22–0.36) | 2.02 (1.30–3.15) | 400 | 0.53 (0.44–0.64) | 1.07 (0.95–1.21) | 2.02 (1.62–2.52) |
Least dense = living in a census tract with a lower density of people of the same race/ethnicity, suggesting greater acculturation at the individual-level; most dense = living in a densely ethnic census tract, suggesting less acculturation.
SES: low (Q1–Q3), high (Q4–Q5); enclave: less dense (Q1–Q4), most dense (Q5); low/most dense implies a low SES, less acculturated living context; high/less dense implies a high SES, more acculturated living context..
Discussion
During the last 20 years in California, there has been a significant, rapid, and continued increase in thyroid cancer incidence rates in all subgroups defined by patient (age, sex, race/ethnicity, and nativity), tumor (tumor size and stage), or contextual (SES and, among Hispanics and APIs, residence in ethnic enclaves) characteristics. While some variation in the magnitude or temporality of these increases exists across subgroups, these patterns suggest that changes in diagnostic technology or surveillance patterns alone cannot account for this epidemic. Other modifiable behavioral, lifestyle, or environmental factors are most likely to be involved.
Prior concerns that the trends in thyroid cancer incidence were largely related to changes in diagnostic technology were well founded. The 1980s saw substantial changes in the technologies used to diagnose thyroid cancer: ultrasound imaging in the early part of the decade and ultrasound-guided fine needle aspiration (FNA) in the latter part (8). These technologies achieved widespread use by the mid-1980s and early 1990s, respectively (7). The greater diagnostic ability of these screening technologies combined with the relatively slow-growing nature of many thyroid cancers and the presence of “occult” microcarcinomas, is undoubtedly responsible for a portion of the increased incidence of thyroid cancer over time, particularly in the 1990s. Indeed, the increase in tumors <1 cm and 1.0–1.4 cm was greater than that of larger tumors. However, as observed here and elsewhere (8, 11, 19), there has also been a statistically significant temporal increase in larger tumors, including those >5 cm, and tumors classified as regional or distant SEER stage. But interpretation of the trends by tumor size is complicated by the consistent improvement in the completeness of tumor size information in cancer registry data over the course of the last twenty years. In our sensitivity analysis, we found that optimal allocation of the tumors of unknown size into the specific size categories had the largest impact on the smallest tumor size categories, reducing the magnitude of the increasing trend, particularly for tumors <1 cm. Substantially less impact was seen for the trends in larger tumors. Thus, changing patterns in the completeness of tumor size data over time accounts for a portion of the observed tumor size-specific trends, suggesting that analyses that do not account for this change in reporting, such as the unadjusted tumor size data presented here as well as in previously published data based on SEER, must be viewed with caution and that the impact of diagnostic technology on the temporal trends in thyroid cancer is probably less than previously suggested.
In addition, if screening or diagnostic technology were the sole cause of the temporal increases, one would expect the increases to occur first and to the greatest extent among subgroups of the population who have greater medical care utilization and access (e.g., women, persons of white race/ethnicity, and persons of higher SES) and later among other subgroups, similar to what was observed for mammographic screening (39). Some of the observed temporal patterns for thyroid cancer are consistent with a role of diagnostic technology or screening, but most are not. While our study and others (2, 8, 11) observed significant temporal increases in thyroid cancer among both men and women, the magnitude of the increase in women is greater than in men in our study and others (8) but not in all studies(11). In addition, the temporality of that increase is earlier in women than men in the California data presented here but not in the SEER data as a whole (11). When examined by race/ethnicity and as observed in our population, significant increases of a similar magnitude and temporality are generally observed within and across all racial/ethnic groups in the California data (32), whereas within the SEER data, the magnitude of the increase is similar among whites and blacks but greater in those groups than other racial/ethnic groups (1, 8, 19). The observed patterns also differ by age. Similar to previously reported analyses using SEER data (8, 11), in our study, the magnitude of the increased incidence was greater among persons age 50 years and older, compared with younger men and women, although the temporality was similar. Those persons age 65 years and older, who would be eligible for Medicare coverage, experience rate increases similar to those age 50–64. Women age 20–34, who may have greater contact with the medical care system due to childbearing, experience rate increases that are less than women age 35–49 or older. Contrary to a previous report of SEER data that examined neighborhood SES based on county-level data and found the increase in thyroid cancer to be greater among those in the highest 75% of SES compared to the lowest 25% (21), our analysis of neighborhood SES, based on census tract data (i.e., a more cohesive measure of neighborhood) suggests no differences in the magnitude of the rate increase by SES, with the exception of a slightly higher rate of increase of small tumors among those in the lower 60% of SES. Finally, we examined the impact of acculturation on the trends in papillary thyroid cancer incidence. We observed no differences by nativity, an individual-level proxy for acculturation. Hispanic and API women have experienced significantly increased rates of thyroid cancer, regardless of whether they are US-born or foreign-born; although the rate of increase for foreign-born API women is small. For men, among whom thyroid cancer is less common, the changes generally do not reach statistical significance. For Hispanics and APIs, we also examined the impact of residence in an ethnic enclave, a neighborhood-level proxy for acculturation. This composite variable is based on census data at the tract- and block-levels which combines data on nativity, recent immigration, poverty, language proficiency, and linguistic isolation (23). Residents of more dense ethnic enclaves would be less acculturated and less likely to benefit from new diagnostic technology. As with nativity, the incidence of papillary thyroid cancer increased to a similar degree at all levels of ethnicity density whether examined alone or in conjunction with SES. Thus, as with patterns examined by tumor characteristics, patterns by patient and contextual characteristics suggest that factors beyond changes in diagnostic technology are likely to be responsible for a significant portion of the temporal increase in thyroid cancer incidence. Such factors may include exposure to various types of radiation (10, 16, 18, 40) or environmental endocrine disruptors (2, 16), consequences of the obesity epidemic, or the effects on immune function of the increasingly sterile environment in which we live today.
Several strengths and limitations of the current analysis should be noted. First, in 1988, the World Health Organization made modifications to their histologic classification scheme for thyroid cancer, resulting in tumors which would have previously been classified as follicular to now be classified as follicular variants of papillary, and thus, be included in our papillary group (2). While it is not known how long it took for this new standard to diffuse across all California hospitals, our analyses start with cases diagnosed concurrently with this classification change and show significant increases in both types of thyroid cancer in women, where we had sufficient statistical power. Thus, it is unlikely that this change in classification had a major impact on the trends reported here. Second, the large and diverse California population, and our ability to look at the effects of SES and acculturation based on our enhanced surveillance data (23, 40), provides a powerful resource for examining trends not only at the individual and tumor level but also on the social/contextual level. Nonetheless, despite our large US- and foreign-born Hispanic and API populations, we did suffer from imprecision in some of our estimates due to small numbers of cases in some subgroups, particularly among men. In addition, despite the use of our validated imputation method for determining nativity, there is the possibility of misclassification (32, 33). We are also aware that there is misclassification of Hispanic ethnicity in cancer registry records; however, accuracy of ethnicity-reporting has improved over time and the relative bias when calculating age-adjusted incidence rates is minimal (<1%) (41, 42). There also may be errors associated with the inter- and post-censal annual population estimates; this is a particular concern for the extrapolated post-2000 estimates (43). Finally, a major strength of our analysis is the conduct of a sensitivity analysis to address the impact that the temporal trends in unknown tumor size had on the tumor size-specific trends in thyroid cancer rates. This is particularly important since it impacts the interpretation of the relative contribution of diagnostic technology, versus other causes, in the observed temporal trends.
In summary, we found that papillary thyroid cancer rates continue to increase and while there was some variation in the magnitude of the increasing trends, there were statistically significant increases in just about every subgroup examined. We conclude that while diagnostic technology may account for a moderate portion of this increase, it is likely that widespread environmental or common population trends are responsible for a substantial portion of the continuing increase in thyroid cancer incidence. Furthermore, it is likely that no one factor alone accounts for the temporal increase but that multiple factors or interactions between host and environmental factors are involved. Further research that directly addresses the causes of these temporal trends is critical.
Acknowledgments
Financial Support:
The collection of cancer incidence data used in this study was supported by the California Department of Public Health (CDPH) as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; by the National Cancer Institute’s (NCI) Surveillance, Epidemiology and End Results Program under contracts N01-PC-35136 and HHSN261201000140C awarded to the Cancer Prevention Institute of California, N02-PC-15105 and HHSN261201000034C awarded to the Public Health Institute (PHI), HHSN261201000035C University of Southern California; and the Centers for Disease Control and Prevention’s (CDCP) National Program of Cancer Registries, under agreements U55/CCR921930-02 awarded to PHI and U58DP003862-01 awarded to the CDPH. The ideas and opinions expressed herein are those of the author(s) and endorsement by the CDPH, NCI, and CDCP or their contractors and subcontractors is not intended nor should be inferred.
Footnotes
Conflict of interest: None of the authors report a conflict of interest.
References
- 1.Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LAG, et al. Annual Report to the Nation on the Status of Cancer, 1975–2002, featuring population-based trends in cancer treatment. J Nat’l Cancer Inst. 2005;97:1407–27. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 2.Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, et al. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control. 2009;20:525–31. doi: 10.1007/s10552-008-9260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lubina A, Cohen O, Barchana M, Liphshiz I, Vered I, Sadetzki S, et al. Time trends of incidence rates of thyroid cancer in Israel: what might explain the sharp increase. Thyroid. 2006;16:1033–40. doi: 10.1089/thy.2006.16.1033. [DOI] [PubMed] [Google Scholar]
- 4.Burgess JR. Temporal trends for thyroid carcinoma in Australia: an increasing incidence of papillary thyroid carcinoma (1982–1997) Thyroid. 2002;12:141–9. doi: 10.1089/105072502753522374. [DOI] [PubMed] [Google Scholar]
- 5.Rego-Iraeta A, Perez-Mendez LF, Mantinan B, Garcia-Mayor RV. Time trends for thyroid cancer in northwestern Spain: true rise in the incidence of micro and larger forms of papillary thyroid cancer. Thyroid. 2009;19:333–40. doi: 10.1089/thy.2008.0210. [DOI] [PubMed] [Google Scholar]
- 6.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) 2012 Apr; Available from: http://seer.cancer.gov/csr/1975_2009_pops09/, based on November 2011 SEER data submission, posted to the SEER web site.
- 7.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. J Am Med Assoc. 2006;295:2164–7. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 8.Kilfoy BA, Devesa SS, Ward MH, Zhang Y, Rosenberg PS, Holford TR, et al. Gender is an age-specific effect modifier for papillary cancers of the thyroid gland. Cancer Epidemiol Biomarkers Prev. 2009;18:1092–100. doi: 10.1158/1055-9965.EPI-08-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montanaro F, Pury P, Bordoni A, Lutz J-M. Unexpected additional increase in the incidence of thyroid cancer among a recent birth cohort in Switzerland. Eur J Cancer Prev. 2006;15:178–86. doi: 10.1097/01.cej.0000197450.94980.36. [DOI] [PubMed] [Google Scholar]
- 10.Zheng T, Holford TR, Chen Y, Ma JZ, Flannery J, Liu W, et al. Time trend and age-period-cohort effect on incidence of thyroid cancer in Connecticut, 1935–1992. Int J Cancer. 1996;67:504–9. doi: 10.1002/(SICI)1097-0215(19960807)67:4<504::AID-IJC7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 11.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–7. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 12.Sprague BL, Andersen SW, Trentham-Dietz A. Thyroid cancer incidence and socioeconomic indicators of health care access. Cancer Causes Control. 2008;19:585–93. doi: 10.1007/s10552-008-9122-0. [DOI] [PubMed] [Google Scholar]
- 13.Kent WDT, Hall SF, Isotalo PA, Houlden RL, George RL, Groome PA. Increased incidence of differentiated thyroid carcinoma and deteciton of subclinical disease. Canadian Med Assoc J. 2007;177:1357–61. doi: 10.1503/cmaj.061730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito Y, Nikiforov YE, Schlumberger M, Vigneri R. Increasing incidence of thyroid cancer: controversies explored. Nat Rev Endocrinol. 2013;9:178–84. doi: 10.1038/nrendo.2012.257. [DOI] [PubMed] [Google Scholar]
- 15.Morris LGT, Sikora AG, Tosteson TD, Davies L. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid. 2013;23:885–91. doi: 10.1089/thy.2013.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu C, Zheng T, Kilfoy BA, Han X, Ma S, Ba Y, et al. A birth cohort analysis of the incidence of papillary thyroid cacner in the United States, 1973–2004. Thyroid. 2009;19:1061–6. doi: 10.1089/thy.2008.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enewold L, Zhou J, Devesa S, Berrington de Gonzalez A, Anderson WF, Zahm SH, et al. Thyroid cancer incidence among active duty US military personnel, 1990–2004. Cancer Epidemiol Biomarkers Prev. 2011;20:2369–76. doi: 10.1158/1055-9965.EPI-11-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S, Semenciw R, Ugnat A-M, Mao Y. Increasing thyroid cancer incidence in Canada, 1970–1996: time trends and age-period-cohort effects. Br J Cancer. 2001;86:1335–9. doi: 10.1054/bjoc.2001.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu G-P, Li JC-L, Branovan D, McCormick S, Schantz SP. Thyroid cancer incidence and survival in the National Cancer Institute Surveillance, Epidemiology, and End Results race/ethnicity groups. Thyroid. 2010;20:465–73. doi: 10.1089/thy.2008.0281. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Zhu Y, Risch HA. Changing incidence of thyroid cancer. J Am Med Assoc. 2006;296:1350. doi: 10.1001/jama.296.11.1350-a. [DOI] [PubMed] [Google Scholar]
- 21.Li N, Du XL, Reitzel LR, Xu L, Sturgis EM. Impact of enhanced detection on the increase in thyroid cancer incidence in the United States: review of incidence trends by socioeconomic status within the Surveillance, Epidemiology, and End Results Registry, 1980–2008. Thyroid. 2013;23:103–10. doi: 10.1089/thy.2012.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aschebrook-Kilfoy B, Kaplan EL, Chiu BC-H, Angelos P, Grogan RH. The acceleration in papillary thyroid cancer incidence rates is similar among racial and ethnic groups in the United States. Ann Surg Onocol. 2013;20:2746–53. doi: 10.1245/s10434-013-2892-y. [DOI] [PubMed] [Google Scholar]
- 23.Gomez SL, Glaser SL, McClure LA, Shema SJ, Kealey M, Keegan THM, et al. The California Neighborhood Data System: a new resource for examining the impact of neighborhood characteristics on cancer incidence and outcomes in populations. Cancer Causes Control. 2011;22:631–47. doi: 10.1007/s10552-011-9736-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keegan THM, John EM, Fish K, Alfaro-Velcamp T, Clarke C, Gomez SL. Breast cancer incidence patterns among California Hispanic women: Differences by nativity and residence in an enclave. Cancer Epidemiol Biomarkers Prev. 2010;19:1208–18. doi: 10.1158/1055-9965.EPI-10-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.California Cancer Registry. Available from: http://www.ccrcal.org/
- 26.NAACCR Latino Research Work Group. NAACCR Guideline for Enhancing Hispanic/Latino Identification Algorithm [NHIA v2] Springfield, IL: North American Association of Cancer Registries (NAACCR); Sep, 2005. [Google Scholar]
- 27.Asian/Pacific Islander Work Group. NAACR Asian Pacific Islander Identification Algorithm [NAPIIA version 1.2] Springfield, IL: North American Association of Central Cancer Registries; 2008. [Google Scholar]
- 28.Gomez SL, Le GM, Miller T, et al. Cancer Incidence Among Asians in the Greater Bay Area, 1990–2002. Fremont, CA: Northern California Cancer Center; 2005. [Google Scholar]
- 29.Bates D, Chambers J, Dalgaard P, et al. R Program [R] 2.8.0. The R Foundation for Statistical Computing; [Google Scholar]
- 30.Gomez SL, Glaser SL. Quality of birthplace information obtained from death certificates for Hispanics, Asians, and Pacific Islanders. Ethn Dis. 2004;14:292–5. [PubMed] [Google Scholar]
- 31.Gomez SL, Glaser SL. Quality of cancer registry birthplace data for Hispanics living in the United States. Cancer Causes Control. 2005;16:713–23. doi: 10.1007/s10552-005-0694-7. [DOI] [PubMed] [Google Scholar]
- 32.Horn-Ross PL, Chang ET, Clarke CA, Keegan THM, Rull RP, Quach T, et al. Nativity and papillary thyroid cancer incidence rates among Hispanic women in California. Cancer. 2012;118:216–22. doi: 10.1002/cncr.26223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horn-Ross PL, McClure LA, Chang ET, Clarke CA, Keegan THM, Rull RP, et al. Papillary thyroid cancer incidence rates vary significantly by birthplace in Asian American women. Cancer Causes Control. 2011;22:479–85. doi: 10.1007/s10552-010-9720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12:703–11. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 35.Gomez SL, Clarke CA, Shema SJ, Chang ET, Keegan TH, Glaser SL. Disparities in breast cancer survival among Asian women by ethnicity and immigrant status: a population-based study. Am J Public Health. 2010;100:861–9. doi: 10.2105/AJPH.2009.176651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Surveillance Research Program. 6.1.4. SEER*Stat Software; 2005. [Google Scholar]
- 37.Joinpoint Regression Program. http://surveillance.cancer.gov/joinpoint/. version 3.4.3.
- 38.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 39.Swanson GM, Ragheb NE, Lin C-S, Hankey BF, Miller B, Horn-Ross P, et al. Breast cancer among black and white women in the 1980s: changing patterns in the United States by race, age, and extent of disease. Cancer. 1993;72:788–98. doi: 10.1002/1097-0142(19930801)72:3<788::aid-cncr2820720326>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 40.Gomez SL, Glaser SL, Kelsey JL, Lee MM. Bias in completeness of birthplace data for Asian groups in a population-based cancer registry (United States) Cancer Causes Control. 2004;15:243–53. doi: 10.1023/B:CACO.0000024244.91775.64. [DOI] [PubMed] [Google Scholar]
- 41.Clegg LX, Reichman ME, Hankey BF, Miller BA, Lin YD, Johnson NJ, et al. Quality of race, Hispanic ethnicity, and immigrant status, in population-based cancer registry data: implications for health disparity studies. Cancer Causes Control. 2007;18:177–87. doi: 10.1007/s10552-006-0089-4. [DOI] [PubMed] [Google Scholar]
- 42.Gomez SL, Glaser SL. Misclassification of race/ethnicity in a population-based cancer registry (United States) Cancer Causes Control. 2006;17:771–81. doi: 10.1007/s10552-006-0013-y. [DOI] [PubMed] [Google Scholar]
- 43.Boscoe FP, Miller AB. Population estimation error and its impact on 1991–1999 cancer rates. The Professional Geographer. 2004;54:516–29. [Google Scholar]