Abstract
Background
Schizophrenia is associated with immune system dysfunction, including aberrant cytokine levels. We performed a meta-analysis of these associations, considering effects of clinical status and antipsychotic treatment following an acute illness exacerbation.
Methods
We identified articles by searching PubMed, PsychInfo, and Institute for Scientific Information and the reference lists of identified studies.
Results
Forty studies met the inclusion criteria. Effect sizes were similar for studies of acutely relapsed inpatients (AR) and first-episode psychosis (FEP). Interleukin (IL)-1β, IL-6, and transforming growth factor-β (TGF-β) appeared to be state markers, as they were increased in AR and FEP (p.001 for each) and normalized with antipsychotic treatment (p.001, p.008, and p.005, respectively). In contrast, IL-12, interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and soluble IL-2 receptor (sIL-2R) appeared to be trait markers, as levels remained elevated in acute exacerbations and following antipsychotic treatment. There was no difference in IL-6 levels between stable medicated outpatients and control subjects (p.69). In the cerebrospinal fluid, IL-1β was significantly decreased in schizophrenia versus controls (p.01).
Conclusions
Similar effect sizes in AR and FEP suggest that the association between cytokine abnormalities and acute exacerbations of schizophrenia is independent of antipsychotic medications. While some cytokines (IL-1β, IL-6, and TGF-β) may be state markers for acute exacerbations, others (IL-12, IFN-γ, TNF-α, and sIL-2R) may be trait markers. Although these results could provide the basis for future hypothesis testing, most studies did not control for potential confounding factors such as body mass index and smoking.
Keywords: Cytokines, first-episode psychosis, meta-analysis, re lapse, schizophrenia
Replicated prenatal risk factors for schizophrenia—including prenatal maternal infections, low birth weight, and advanced paternal age—suggest that abnormalities in the disorder would be widespread and not just restricted to psychosis. In the brain, this is evidenced by widespread cognitive impairment (1). Schizophrenia is also associated with physiological abnormalities outside of the brain, including the immune system. An etiopatho-physiological role for immunologic abnormalities in schizophrenia was first hypothesized over 40 years ago (2-4), although interest in this field has waxed and waned. Recently, there has been exponential growth of the interface between immunology and chronic mental illness, including areas such as stress, neuroplasticity, genetics, and cytokines.
Cytokines are key signaling molecules of the immune system that exert effects in the periphery and the brain. They are produced by both immune and nonimmune cells and exert their effects by binding specific cytokine receptors on a variety of target cells. Cytokine receptors also exist in soluble forms, which can inhibit (e.g., soluble interleukin-2 receptor [sIL-2R]) or enhance (e.g., soluble interleukin [IL]-6 receptor) the biological activity of cytokines. There are also endogenous cytokine receptor antagonists (e.g., IL-1 receptor antagonist [IL-1RA]) that compete with cytokines for membrane receptors. Cytokines (and cytokine receptors or antagonists) are key regulators of inflammation—the complex response of blood vessels to injury—which involves activation and recruitment of immune cells and increased blood supply and vascular permeability. They coordinate both innate (e.g., granulocytes, monocytes/ macrophages, and natural killer cells) and adaptive (e.g., B-lymphocytes and T-lymphocytes) arms of the immune system.
There is an increased prevalence of aberrant cytokine levels in both patients with schizophrenia (5–7) and their first-degree relatives (8), suggesting that immune system abnormalities may be an endophenotype of the disorder. There is also converging evidence of immune system dysfunction and inflammation in patients with schizophrenia, which is briefly reviewed in Supplement 1. Clinical trials of antipsychotic augmentation with nonsteroidal anti-inflammatory agents also support an association between inflammation and psychosis. Four trials of adjunctive nonsteroidal anti-inflamma-tory agents in acutely relapsed patients with schizophrenia showed significant improvement in total symptoms (9–12). In one study, responders to celecoxib had significantly decreased soluble tumor necrosis factor-receptor levels (13) and aspirin was more efficacious in patients with a lower baseline in vitro interferon-γ (IFN-γ)/IL-4 ratio (10). These findings suggest the possibility that cytokine (and cytokine receptor) levels may be a biomarker of illness relapse and/or response to adjunctive anti-inflammatory treatment for a subset of patients with schizophrenia.
Several hypotheses regarding an immune-cytokine basis for schizophrenia have been postulated. Smith and Maes (14) proposed the macrophage-T-lymphocyte theory that IL-1, IL-2 tumor necrosis factor, interferon-α, and IFN-γ produced by chronically activated macrophages and T-lymphocytes are the fundamental mediators of schizophrenia. Schwarz et al. (15) proposed the Th2-hypothesis, which argues that a shift away from Th1-cell (cytotoxic) immune function and toward Th2-cell (antibody-dependent) immune responses predominates in schizophrenia. Lastly, Monji et al. (16) described the microglial hypothesis that activated central nervous system microglia release proinflammatory cytokines and free radicals that cause abnormal neurogenesis, neuronal degradation, and white matter abnormalities contributing to the pathophysiology of schizophrenia. The characteristic cytokine profiles of specific immune cells are described in Supplement 1.
A systematic, quantitative review of cross-sectional studies found a significant increase in blood levels of IL-1RA, sIL-2R, and IL-6 in patients with schizophrenia (5). However, there is considerable heterogeneity among these studies with respect to 1) illness duration, 2) treatment setting, 3) consideration of potential confounding factors (17), and 4) for acute exacerbations of psychosis, the timing of assays. Meta-analysis is one approach to bring increased clarity to an area of research with significant heterogeneity (18).
We performed a meta-analysis of blood and cerebrospinal fluid (CSF) cytokine (and cytokine receptor and antagonist) levels, considering the effects of clinical status, antipsychotic treatment following an acute exacerbation of psychosis, and correlations with clinical features. The primary aim of the meta-analysis was to establish the characteristic cytokine profile that emerges in schizophrenia and in doing so, further evaluate leading hypotheses for the immune-cytokine basis of schizophrenia.
Methods and Materials
Study Selection
Studies of blood and CSF cytokine (and cytokine receptor or antagonist) levels in schizophrenia were identified from two sources. First, supplementary material from Potvin et al. (5), which describes a systematic search of studies through the end of 2005, was accessed. Second, studies on cytokine (and cytokine receptor or antagonist) levels in schizophrenia published after 2005 were systematically searched using MEDLINE (National Center for Bio-technology Information, US National Library of Medicine, Bethesda, Maryland), PsychInfo (via Ovid, American Psychological Association, Washington, DC), and Science Citation Index and Social Sciences Citation Index (both Institute for Scientific Information Web of Knowledge, Thomson Reuters, Charlottesville, Virginia) in April 2010. The primary search strategy was schizophrenia and (inflammation or cytokine or interleukin or interferon or tumor necrosis factor). Limiting results to human studies in English published after 2005 identified 192 articles from PubMed, 103 from PsycInfo, and 334 from Science Citation Index and Social Sciences Citation Index, and the resulting matches were screened. From both sources, we identified 83 potential studies of cytokines (or cytokine receptors or antagonists) for inclusion (6–8,19–98), which are described in Supplement 1. The majority of initial matches were excluded because they 1) were review articles, 2) did not present cytokine (or cytokine receptor or antagonist) data, or 3) measured only in vitro cytokine production, or 4) were genetic studies related to cytokines. We excluded studies of in vitro cytokine production because most studies measured cytokine production in stimulated, separated monocytes, which does not necessarily reflect endogenous immune system functioning.
The inclusion criteria were 1) cross-sectional studies of patients with schizophrenia or related psychotic disorder (including schizophreniform disorder, brief psychotic disorder, psychotic disorder not otherwise specified, delusional disorder, and schizoaffective disorder) and healthy control subjects or studies assessing cytokines (or cytokine receptors or antagonists) in patients with an acute exacerbation of psychosis at baseline and again following a period of antipsychotic treatment; 2) studies assessing either blood or CSF cytokine (or cytokine receptor or antagonist) levels; and 3) studies published in English. For studies of blood levels, additional inclusion criteria were 1) clinical status of patients clearly defined as either acutely relapsed inpatients (AR), first-episode psychosis (FEP), stable medicated outpatients (SO), or treatment-resistant psychosis (TR); and 2) for the AR and FEP groups, blood samples were taken within 4 days of hospitalization. Because of small numbers, these two criteria were not applied to CSF studies. If the timing of assessments relative to admission was unclear, we attempted to contact study authors. For studies that included patients with different clinical statuses (e.g., both AR and FEP), if stratified data were not presented in the manuscript, we attempted to contact study authors. The exclusion criteria were 1) studies without a control group (except for studies with serial measurements of cytokines (or cytokine receptors or antagonists) in patients with an acute exacerbation of psychosis), 2) studies that did not present mean and standard deviations for cytokine (or cytokine receptor or antagonist) levels (after attempting to contact the study authors), 3) significant overlap in study population, and 4) genetic studies related to cytokines. Because of the potential for low concentrations of some cytokines (e.g., IL-1β, IL-2, and IL-4), the methods of the potential studies were reviewed to evaluate assay sensitivity. An individual cytokine (or cytokine receptor or antagonist) was excluded if 1) the mean concentration reported was less than the lower limit of detection of the assay, 2) concentrations were not detectable in <50% of subjects, or 3) either the intra-assay coefficient of variation was <10% or the interassay coefficient of variation was <15%.
After independent searches, review of study methods by two authors (B.J.M. and W.S.), and attempts to contact the authors, 40 studies met the inclusion criteria (33 studies of blood and 7 studies of CSF). There was universal agreement on the included studies. Studies of blood cytokines (and cytokine receptors or antagonists) included 10 studies of AR, 14 studies of FEP, 3 studies of SO, and 5 studies of TR. Additionally, 12 studies assessed blood cytokines (or cytokine receptors or antagonists) in patients with an acute illness exacerbation (defined as either AR or FEP) at baseline and again after antipsychotic treatment. The seven studies of CSF were all done on inpatients. Forty-three studies were excluded because of cytokines measured after hospital day 4 (n = 16), stratified data not available by clinical status (n = 8), group means and/or standard deviations not available (n = 4), no control group (n = 3), did not measure CSF concentrations (n = 3), timing of assessments unclear (n = 2), inadequate assay sensitivity (n = 2), clinical status not available (n = 1), significant study population overlap (n = 1), samples obtained postmortem (n = 1), unmedicated stable outpatients (n = 1), and article not in English (n = 1). A flow chart summarizing the study selection process is presented in Supplement 1.
Data Extraction and Meta-Analysis
Data were extracted (sample size, mean, and standard deviation for schizophrenia and control subjects) for every individual cytokine (or cytokine receptor or antagonist) assessed in each study, with blood and CSF considered separately. We excluded data on blood levels of IL-4 from three studies (44,49,69), IL-2 from one study (44), IFN-γ from one study (49), and CSF IL-1α levels from one study (34) because of assay sensitivity criteria. One author (B.J.M.) extracted all data, which was independently verified by another author (W.S.). We then calculated effect size estimates (Hedges’ g) for every individual cytokine (or cytokine receptor or antagonist) in each study, and these data are included in Supplement 1. Random effects pooled effect size estimates and 95% confidence intervals were calculated using the method of DerSimonian and Laird. The random effects model is more conservative than the fixed effects model, as it yields a lower type I error rate and wider confidence intervals, and its use was supported by significant heterogeneity between studies (99). Cytokines (or cytokine receptors or antagonists) assessed in only one study were not included. Separate meta-analyses were performed for blood levels by each clinical status (AR, FEP, SO, and TR), as well as for the difference between baseline and end point cytokine (or cytokine receptor or antagonist) levels following antipsychotic treatment for an acute exacerbation of psychosis. The p values were considered statistically significant at the α = .05 level. The statistical analyses were performed in Stata 10.0 (StataCorp LP, College Station, Texas).
For descriptive purposes, we also extracted descriptive data on statistical significance (yes/no) and direction (positive/negative) for correlations between individual cytokine (or cytokine receptor or antagonist) levels in each study and the following clinical features: age, age of onset of illness, duration of illness, and psychopathology scores.
Results
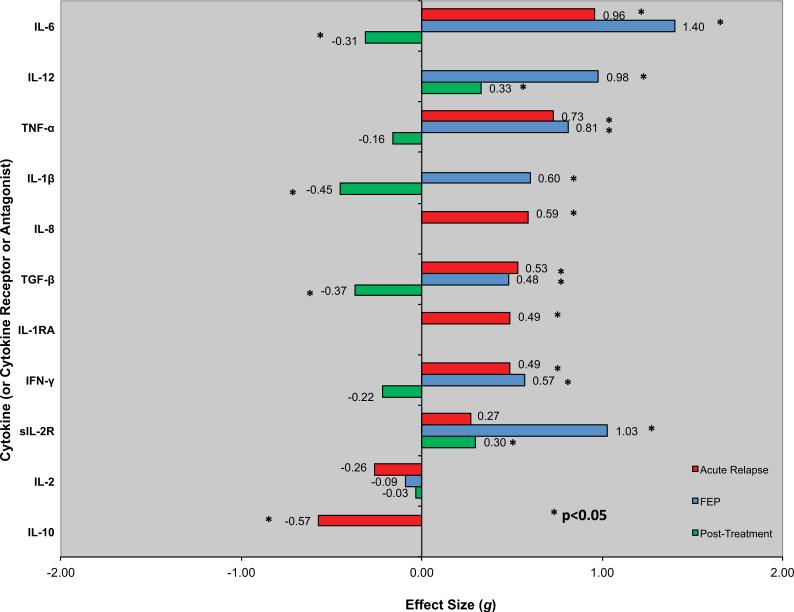
Figure 1 and Table 1 present effect size estimates with 95% confidence intervals by clinical status.
Figure 1.
Blood levels of cytokines (or cytokine receptors or antagonists) in schizophrenia by clinical status. Effect sizes for levels of individual cytokines (or cytokine receptors or antagonists) in acute relapse of psychosis (AR) and drug-naive first-episode psychosis (FEP) versus control subjects are represented by red and blue bars, respectively. For AR and FEP, positive effect sizes (bars going to the right) indicate that levels were higher in schizophrenia than control subjects; negative effect sizes (bars going to the left) indicate that levels were higher in control subjects than in patients with schizophrenia. Note that for IL-6, TNF-α, TGF-β, IFN-γ, and IL-2, effect sizes are very similar for both AR and FEP. Similarly, effect sizes for changes in levels of individual cytokines (or cytokine receptors or antagonists) following antipsychotic treatment for an acute illness exacerbation are represented by green bars. Positive effect sizes (bars going to the right) indicate that levels increased following antipsychotic treatment; negative effect sizes (bars going to the left) mean that levels decreased following antipsychotic treatment. IFN-γ, interferon-γ; IL, interleukin; IL-1RA, IL-1 receptor antagonist; sIL-2R, soluble IL-2 receptor; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α.
Table 1.
Meta-Analyses of Cytokine (and Cytokine Receptor or Antagonist) Levels by Clinical Status
| Cytokine | N Studies | Patients | Control Subjects | Mean ES | 95% CI | 95% CI | p Value | Heterogeneity |
I2 | References | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| χ 2 | p Value | |||||||||||
| Acutely Relapsed Inpatients | ||||||||||||
| IL-10 | 2 | 46 | 52 | –.57 | – .98 | – .17 | .006 | 3.40 | .07 | 70.6 | 44,69 | |
| IL-2 | 2 | 43 | 199 | –.26 | – .60 | .08 | .13 | 13.93 | < .01 | 92.8 | 46,50 | |
| sIL-2R | 2 | 32 | 94 | .27 | – .13 | .68 | .19 | 1.83 | .18 | 45.2 | 42,57 | |
| IFN-γ | 2 | 57 | 202 | .49 | .18 | .80 | .002 | 3.12 | .08 | 67.9 | 44,50 | |
| IL-1RA | 2 | 32 | 94 | .49 | .07 | .90 | .02 | 8.17 | < .01 | 87.8 | 42,57 | |
| TGF-β | 2 | 78 | 262 | .53 | .26 | .79 | < .001 | 4.94 | .03 | 79.8 | 49,50 | |
| IL-8 | 2 | 46 | 52 | .59 | .18 | 1.00 | .004 | 2.05 | .15 | 51.1 | 44,69 | |
| IL-1β | ||||||||||||
| TNF-α | 4 | 97 | 290 | .73 | .46 | .99 | .001 | 82.47 | < .01 | 96.4 | 42,44,50,69 | |
| IL-12 | ||||||||||||
| IL-6 | 6 | 156 | 373 | .96 | .74 | 1.18 | < .001 | 119.00 | < .01 | 95.8 | 3,39,44,46,50,69 | |
| Drug-Naïve First-Episode Psychosis | ||||||||||||
| IL-10 | ||||||||||||
| IL-2 | 4 | 116 | 276 | – .09 | – .32 | .14 | .44 | 11.16 | .01 | 73.1 | 41,46,50,87 | |
| sIL-2R | 3 | 30 | 97 | 1.03 | .55 | 1.52 | < .001 | 10.10 | < .01 | 80.2 | 23,38,75 | |
| IFN-γ | 2 | 48 | 189 | .57 | .24 | .90 | .001 | 2.52 | .11 | 60.3 | 41,50 | |
| IL-1RA | ||||||||||||
| TGF-β | 2 | 81 | 262 | .48 | .22 | .74 | < .001 | 9.86 | < .01 | 89.9 | 49,50 | |
| IL-8 | ||||||||||||
| IL-1β | 3 | 151 | 152 | .60 | .37 | .84 | < .001 | 11.27 | < .01 | 82.3 | 46,86,87 | |
| TNF-α | 4 | 200 | 323 | .81 | .61 | 1.01 | < .001 | 33.74 | < .01 | 91.1 | 36,50,86,87 | |
| IL-12 | 2 | 78 | 113 | .98 | .61 | 1.35 | < .001 | 47.31 | < .01 | 97.9 | 31,58 | |
| IL-6 | 4 | 117 | 275 | 1.40 | 1.14 | 1.65 | < .001 | 82.80 | < .01 | 96.4 | 12,36,46,80 | |
| CSF | ||||||||||||
| IL-10 | ||||||||||||
| IL-2 | 4 | 100 | 57 | – .01 | – .35 | .32 | .94 | 3.33 | .34 | 9.9 | 25,34,51,77 | |
| sIL-2R | ||||||||||||
| IFN-γ | ||||||||||||
| IL-1RA | ||||||||||||
| TGF-β | ||||||||||||
| IL-8 | ||||||||||||
| IL-1β | 2 | 13 | 15 | – .99 | –1.78 | – .20 | .01 | .22 | .64 | .0 | 25,45 | |
| TNF-α | ||||||||||||
| IL-12 | 2 | 40 | 24 | .29 | –.24 | .81 | .29 | 2.25 | .13 | 55.6 | 6,25 | |
| IL-6 | 2 | 42 | 31 | –.34 | –.81 | .14 | .16 | 2.21 | .14 | 54.7 | 51,77 | |
| Following Antipsychotic Treatment | ||||||||||||
| IL-10 | ||||||||||||
| IL-2 | 4 | 132 | 132 | 49 | –.03 | –.27 | .22 | .84 | 8.29 | .04 | 63.8 | 46,47,50,70 |
| sIL-2R | 3 | 90 | 90 | 69 | .30 | .01 | .60 | .04 | 2.19 | .34 | 8.6 | 63,81,84 |
| IFN-γ | 2 | 62 | 62 | 47 | –.22 | –.55 | .11 | .19 | .64 | .42 | .0 | 47,50 |
| IL-1RA | ||||||||||||
| TGF-β | 2 | 119 | 119 | 56 | –.37 | –.63 | –.11 | .005 | 7.38 | <.01 | 86.5 | 49,50 |
| IL-8 | ||||||||||||
| IL-1β | 3 | 127 | 127 | 37 | –.45 | –.70 | .20 | <.001 | .31 | .85 | .0 | 46,47,86 |
| TNF-α | 3 | 171 | 171 | 47 | –.16 | –.37 | .06 | .15 | .52 | .77 | .0 | 50,86 |
| IL-12 | 3 | 104 | 104 | 42 | .33 | .06 | .60 | .02 | 3.77 | .15 | 46.9 | 31,47,48 |
| IL-6 | 5 | 164 | 164 | 50 | –.31 | –.54 | .08 | .008 | 5.65 | .23 | 29.3 | 37,46,47,50,70 |
CI, confidence interval; CSF, cerebrospinal fluid; ES, effect size; I2 squared; IFN-γ, interferon-γ; IL, interleukin; IL-1RA, IL-1 receptor antagonist; sIL-2R, soluble IL-2 receptor; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α.
Acutely Relapsed Inpatients
Blood IL-10 levels were significantly decreased (p ≤ .006) and IL-6, IL-8, tumor necrosis factor-α (TNF-α), IFN-γ, transforming growth factor-β (TGF-β), and IL-1RA levels were significantly increased in AR compared with control subjects (p ≤ .02 for each). The most replicated finding was for IL-6, which was increased in five of six studies. By contrast, there were no significant differences in blood levels of IL-2 (p = .24) or sIL-2R (p = .19) between AR and control subjects. For a majority of cytokines (or cytokine receptors or antagonists) assessed, there was significant heterogeneity in effect size estimates. In sensitivity analyses, the heterogeneity was no longer significant, but the effect size estimate remained signifi-cant after removing two studies for IL-6 (50,69). The heterogeneity remainedsignificantinasensitivityanalysisforTNF-α.Othersensitivity analyses could not performed because of the small number of studies.
First-Episode Psychosis
Effect sizes for FEP were noted to be similar in direction and magnitude to those in AR. Blood IL-1β, IL-6, IL-12, IFN-γ, TNF-α, TGF-β, and sIL-2R levels were significantly increased in FEP versus control subjects (p ≤ .003 for each). The most replicated finding was for TNF-α, which was increased in all four studies. By contrast, there was no significant difference in blood IL-2 levels between FEP and control subjects (p = .44). There was significant heterogeneity in effect size estimates for all cytokines and sIL-2R. In sensitivity analyses for sIL-2R, IL-1β, TNF-α, and IL-6, the heterogeneity was no longer significant, but the effect size estimates remained significant after removing one study (Ganguli and Rabin [38] for sIL-2R, Theodoropoulou et al. [87] for IL-1β, and Kim et al. [50] for TNF-α and IL-6). In a sensitivity analysis for IL-2, the heterogeneity was no longer significant, and there was a nonsignificant trend (p = .07) for decreased IL-2 in FEP versus control subjects after removing one study (46). Sensitivity analyses could not be performed for either TGF-β or IL-12 because of the small number of studies.
Stable Medicated Outpatients and Treatment-Resistant Psychosis
There were no significant differences in blood IL-6 levels between SO and control subjects (p = .69), but there was significant heterogeneity. Thus, although IL-6 levels were measured in a relatively large number of SO (n = 178) and control subjects (n = 136), the results must be interpreted with caution in light of a small number of studies and high heterogeneity. A sensitivity analysis for IL-6 could not be performed because of the small number of studies.
Compared with control subjects, blood levels of sIL-2R (p = .001), but not IL-6 (p = .46), were significantly increased in patients with treatment-resistant psychosis. Sensitivity analyses could not be performed because of the small number of studies.
Cerebrospinal Fluid
Compared with control subjects, CSF IL-1β levels were significantly decreased in patients with schizophrenia (p = .01). By contrast, there was no significant difference in CSF levels of IL-2 (p = .94), IL-6 (p = .29), or IL-1α (p = .44) between schizophrenia and control subjects (p = .44). However, these results should be interpreted with caution in light of small numbers of studies and subjects.
Correlations with Clinical Features
Forty-three percent (n = 17) of studies provided data on correlations between cytokine (or cytokine receptor or antagonist) levels and clinical features. A descriptive summary of these correlations is included in Supplement 1. Blood IL-6 levels had a significant positive correlation with duration of illness in three studies (23,39,46), although there was a failure to replicate (86). There was also a significant positive correlation between baseline IL-6 levels and total psychopathology in two studies (37,70), although several studies did not find a significant correlation (23,53,59). There were no other replicated significant correlations between individual blood cytokines (or cytokine receptors or antagonists) and any clinical features. In the CSF, there was a trend for a negative correlation between IL-6 levels and positive symptoms in one study (6) but no significant correlations with any clinical features.
Cytokine Changes Following Antipsychotic Treatment
Figure 1 and Table 1 also present effect size estimates with 95% confidence intervals for cytokine (or cytokine receptor or antagonist) levels following antipsychotic treatment. Blood levels were measured at baseline and after a mean of 53 days of antipsychotic treatment in 488 patients following an acute exacerbation of psychosis. Antipsychotic medication was not standardized in seven studies (58%). There was a significant increase in sIL-2R (p = .04) and IL-12 (p = .02) levels and a significant decrease in three cytokines (IL-1β, IL-6, and TGF-β; p ≤ .005 for each) following antipsychotic treatment. There was significant heterogeneity in the effect size estimate for TGF-β, but sensitivity analyses could not performed because of the small number of studies. Furthermore, in the two studies that reported a significant positive correlation between IL-6 levels and total psychopathology scores at baseline (37,70), there was also a significant positive correlation between the change in IL-6 levels and change in total psychopathology scores with anti- psychotic treatment. However, these results should be interpreted with caution in light of nonstandardized antipsychotic treatment.
Discussion
Taken together, our findings suggest that cytokine alterations in schizophrenia may vary with clinical status. IL-1β, IL-6, and TGF-β appeared to be state-related markers, as they were increased during acute exacerbations (AR and/or FEP; p = .001 for each) and normalized with antipsychotic treatment (p = .001, p = .008, and p = .005, respectively). Furthermore, there was a significant positive correlation between the change in IL-6 levels and change in total psychopathology scores in two studies (37,70). In contrast, IL-12, IFN-γ, TNF-α, and sIL-2R appeared to be trait markers, as levels remained elevated in acute exacerbations and following antipsychotic treatment. The sIL-2R levels may be a marker for patients with treatment-resistant psychosis, as evidenced by our results and a recent longitudinal treatment study (28). However, our results should be interpreted with caution in light of significant heterogeneity across studies. The alterations in drug-naïve, first-episode psychosis suggest an association between cytokine (and cytokine receptor or antagonist) abnormalities and acute exacerbations of schizophrenia that is independent of antipsychotic medications.
An important strength of our study is that we considered the effects of clinical status and antipsychotic treatment following an acute illness exacerbation and correlations between cytokine (and cytokine receptor or antagonist) levels and clinical features. A previous meta-analysis found evidence for cytokine alterations in schizophrenia but did not find an effect of clinical status, which was classified as acute, nonacute, or mixed (5). Our analysis differed from this study in several ways. First, we considered drug-naive FEP separately from acute exacerbations of chronic psychosis, which eliminates potential confounding by antipsychotic medications. Second, we utilized a narrower definition of acute relapse than the previous meta-analysis. Third, we investigated changes in cytokine (and cytokine receptor or antagonist) levels with antipsychotic treatment following an acute exacerbation of psychosis. Lastly, we also considered stable medicated outpatients and treatment-resistant psychosis as separate clinical statuses.
There are several limitations of the present study. Many studies were excluded because either stratified data based on clinical status, clinical status of the patient population, or summary data on cytokine levels were not available. Many of these studies would have otherwise been included in the meta-analysis, and their influence on the results is uncertain. Furthermore, findings on individual cytokines (and cytokine receptors or antagonists) should be interpreted with caution in light of small numbers of studies, and replications are warranted. Most studies did not control for potential confounding factors known to influence cytokine levels (54). For example, effects of age and gender were considered in 97% of studies of blood cytokines. By contrast, blood was collected at a standardized time of day (within a 1–3 hour period) in 69% of studies. Even fewer studies considered potential effects of race (41%), body mass index (35%), and smoking (24%). One study that controlled for many potential confounders reported a significant difference in cytokine levels between patients and control subjects (36), whereas another did not (43).
Another limitation of the present study is that there were relatively few studies of stable medicated outpatients and treatment-resistant psychosis; thus, only a small number of cytokines (or cytokine receptors) were included in the meta-analyses of these patient populations. Only 43% (n = 17) of studies included data on correla tions between cytokine (and cytokine receptor or antagonist) levels and clinical features, making only a descriptive analysis of the correlative data possible. A major limitation of the analysis of cytokine (and cytokine receptor or antagonist) changes following antipsychotic treatment is that medications were not standardized in a majority of studies. Different antipsychotics may have different effects on the immune system. For example, clozapine has been shown to increase sIL-2R and IL-6 levels (54).
Although our definition of acute relapse excluded of a number of studies, we believe this approach is justified. In the previous meta-analysis (5), some studies classified as acute measured cytokine (or cytokine receptor or antagonist) levels at least 1 week (or longer) following inpatient admission or after a 1-week to 2-week washout period with no medications. By contrast, we found significant changes in cytokine (or cytokine receptor or antagonist) levels within weeks of antipsychotic treatment following an acute exacerbation of psychosis. One study found significant changes in cytokine levels after a mean of 4 weeks of antipsychotic treatment (86). Another study observed decreased IL-6 levels within 9 days of anti-psychotic treatment following an acute relapse, and by 8 weeks there was no significant difference between patients and control subjects (37). Taken together, these findings suggest that cytokine (or cytokine receptor or antagonist) levels may normalize to some extent shortly after treatment for an acute exacerbation of psychosis.
In diseases outside of psychosis, cytokine levels also vary by clinical status. For example, patients with active (vs. inactive) systemic lupus erythematosus (including central nervous system disease) have significantly higher blood IL-6 and IFN-γ levels (100). Another study found significant differences in cytokine levels in patients with active celiac disease versus patients on a gluten-free diet (101). Within schizophrenia, in a study of 36 patients who underwent weekly assessments for 1 year, in vitro IL-2 production plus antihippocampal immunoglobulin G levels from the previous week significantly predicted relapse in three of seven patients (102). Similarly, in a study of 64 male subjects with schizophrenia, increased CSF IL-2 levels following haloper-idol withdrawal were a significant predictor of acute psychotic relapse (78). Our finding of significant alterations in cytokine (and cytokine receptor and antagonist) levels in acute illness exacerbations, measured within days of admission, that start to normalize following antipsychotic treatment support the plausibility of cytokine levels as a putative state-related marker for acute psychosis. This finding is of importance, as acute relapse of psychosis is common and is associated with adverse outcomes, including increased treatment-resistant symptoms, cognitive decline, and functional disability (103–105).
Our results inform on the immune-cytokine hypotheses of schizophrenia. In support of the macrophage-T-lymphocyte theory, we found significant increases in macrophage-derived cytokines IL-1β, IL-6, and TNF-α, as well as the Th1-derived cytokines IFN-γ and IL-12 in AR and/or FEP. However, we found nonsignificant decreases in IL-2. One potential explanation for this discordant finding is increased binding of IL-2 by sIL-2R, which was increased in FEP. Thus, overall, our findings are largely consistent with this theory. In general, there was less consistent evidence in favor of Th2-hypothesis. The observed increases in IFN-γ, IL-12, and sIL-2R support a Th1 response in acute exacerbations of psychosis, and other findings—such as increased neopterin and kynurenic acid—are consistent with our findings. Furthermore, there was a significant decrease in the Th2 cytokine IL-10 in AR. The microglial hypothesis predicts an increase in proinflamma-tory cytokines such as IL-1β, IL-6, and TNF-α, which is also con sistent with our results. However, this theory does not consider abnormalities in cell-mediated immunity in schizophrenia, which is supported by our findings. We also found evidence for activation of the compensatory anti-inflammatory response syndrome—a counter-regulatory mechanism that inhibits the primary inflammatory response and involves an adaptive reprogramming of leukocytes (106)—in schizophrenia, including increased IL-1RA and TGF-β in acute exacerbations that decreased with antipsychotic treatment. Consistent with our findings, a flow-cytometric study in patients with recent-onset schizophrenia found activation of both pro- inflammatory and anti-inflammatory networks (107). However, our results do not enable us to firmly identify the cell sources of specific cytokines (or cytokine receptors or antagonists). For example, adipocytes are sources of pro-inflammatory cytokines, such as IL-6 and TNF-α. Given the potential for antipsychotic-induced weight gain, this does not exclude the possibility of confounding by factors such as increased body mass index, which was considered in a minority of studies. Thus, we underscore that our results should be interpreted with caution in light of small numbers of studies, high study heterogeneity, and inadequate consideration of potential confounding factors.
Future studies of cytokines (and cytokine receptors or antagonists) in schizophrenia should stratify patients by clinical status to evaluate if these abnormalities are specific to illness exacerbations or schizophrenia in general. More studies of stable outpatients and patients with treatment-resistant psychosis, as well as CSF studies, are needed. Should alterations in cytokines (or cytokine receptors or antagonists) be found to precede an acute psychotic relapse, this would support their utility as a biomarker to inform relapse prevention strategies. Future studies should also control for factors known to influence cytokine levels (17). Given that individual cytokines (or cytokine receptors or antagonists) may induce or inhibit production of other cytokines, consideration of cytokine networks, which are possible through multiplex assays that allow simultaneous quantification of many cytokines from a single serum sample, may suggest abnormalities in specific immune pathways, particularly when combined with techniques such as flow cytometry and gene expression analysis (107). Correlations between cytokine (and cytokine receptor or antagonist) levels and psychopathology might suggest novel immunomodulatory strategies for treatment in schizophrenia. Additionally, the signal-to-noise ratio of treatment trials of adjunctive anti-inflammatory agents in schizophrenia may be increased by stratifying patients based on cytokine (or cytokine receptor or antagonist) alterations. Taken together, cytokines (or cytokine receptors or antagonists) may serve as potential biomarkers and therapeutic targets in the etiopathophysiology and clinical course of schizophrenia.
Supplementary Material
Acknowledgments
We thank Drs. Gloria Arankowsky Sandoval, Roosmarijn Drexhage, Wagner Gattaz, Yong-Ku Kim, Norbert Muller, Jean Naudin, Sinead O'Brien, Mark Rapaport, and Matthias Rothermundt for sharing data and/or information about their studies.
Dr. Miller received grant support from the University of Oulu and Oy H. Lundbeck.
Dr. Buckley received grant/research support from the National Instituteof Mental Health, Janssen Pharmaceutica, Pfizer, and Sunovion, and is a consultant (honorarium/expenses) for the National Institute of Mental Health.
Dr. Seabolt has no biomedical financial interests or potential conflicts of interest to disclose.
Dr. Mellor received funding support from the National Institutes of Health (AI083005, AI075165), the Juvenile Diabetes Research Founda tion, and the Carlos and Marguerite Mason Trust. Dr. Mellor is a member of the Scientific Advisory Board of NewLink Genetics, Inc., and receives compensation for this service.
Dr. Kirkpatrick received consulting and/or speaking fees from Pfizer, Organon, AstraZeneca, Wyeth, Bristol-Myers Squibb, Solvay, Sunovion, Boehringer Ingelheim, Abbott, and Cephalon.
Footnotes
Supplementary material cited in this article is available online.
References
- 1.Dickinson D, Ragland JD, Gold JM, Gur RC. General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol Psychiatry. 2008;64:823–827. doi: 10.1016/j.biopsych.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heath RG, Krupp IM. Schizophrenia as an immunologic disorder. I. Demonstration of antibrain globulins by fluorescent antibody techniques. Arch Gen Psychiatry. 1967;16:1–9. doi: 10.1001/archpsyc.1967.01730190003001. [DOI] [PubMed] [Google Scholar]
- 3.Heath RG, Krupp IM, Byers LW, Liljekvist JI. Schizophrenia as an immunologic disorder. II. Effects of serum protein fractions on brain function. Arch Gen Psychiatry. 1967;16:10–23. doi: 10.1001/archpsyc.1967.01730190012002. [DOI] [PubMed] [Google Scholar]
- 4.Heath RG, Krupp IM, Byers LW, Lijekvist JI. Schizophrenia as an immunologic disorder. 3. Effects of antimonkey and antihuman brain antibody on brain function. Arch Gen Psychiatry. 1967;16:24–33. doi: 10.1001/archpsyc.1967.01730190026003. [DOI] [PubMed] [Google Scholar]
- 5.Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: A systematic quantitative review. Biol Psychiatry. 2008;63:801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 6.Garver DL, Tamas RL, Holcomb JA. Elevated interleukin-6 in the cerebrospinal fluid of a previously delineated schizophrenia subtype. Neuropsychopharmacology. 2003;28:1515–1520. doi: 10.1038/sj.npp.1300217. [DOI] [PubMed] [Google Scholar]
- 7.van Kammen DP, McAllister-Sistilli CG, Kelley ME, Gurklis JA, Yao JK. Elevated interleukin-6 in schizophrenia. Psychiatry Res. 1999;87:129–136. doi: 10.1016/s0165-1781(99)00053-0. [DOI] [PubMed] [Google Scholar]
- 8.Nunes SO, Matsuo T, Kaminami MS, Watanabe MA, Reiche EM, Itano EN. An autoimmune or an inflammatory process in patients with schizophrenia, schizoaffective disorder, and in their biological relatives. Schizophr Res. 2006;84:180–182. doi: 10.1016/j.schres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Akhondzadeh S, Tabatabaee M, Amini H, et al. Celecoxib as adjunctive therapy in schizophrenia: A double-blind, randomized and placebo-controlled trial. Schizophr Res. 2007;90:179–185. doi: 10.1016/j.schres.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Laan W, Grobbee DE, Selten JP, Heijnen CJ, Kahn RS, Burger H. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: Results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71:520–527. doi: 10.4088/JCP.09m05117yel. [DOI] [PubMed] [Google Scholar]
- 11.Müller N, Riedel M, Scheppach C, Brandstätter B, Sokullu S, Krampe K, et al. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am J Psychiatry. 2002;159:1029–1034. doi: 10.1176/appi.ajp.159.6.1029. [DOI] [PubMed] [Google Scholar]
- 12.Müller N, Krause D, Dehning S, Musil R, Schennach-Wolff R, Obermeier M, et al. Celecoxib treatment in an early stage of schizophrenia: Results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophr Res. 2010;121:118–124. doi: 10.1016/j.schres.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Müller N, Ulmschneider M, Scheppach C, Schwarz MJ, Ackenheil M, Möller HJ, et al. COX-2 inhibition as a treatment approach in schizophrenia: Immunological considerations and clinical effects of celecoxib add-on therapy. Eur Arch Psychiatry Clin Neurosci. 2004;254:14–22. doi: 10.1007/s00406-004-0478-1. [DOI] [PubMed] [Google Scholar]
- 14.Smith RS, Maes M. The macrophage-T-lymphocyte theory of schizophrenia: Additional evidence. Med Hypotheses. 1995;45:135–141. doi: 10.1016/0306-9877(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz MJ, Müller N, Riedel M, Ackenheil M. The Th2-hypothesis of schizophrenia: A strategy to identify a subgroup of schizophrenia caused by immune mechanisms. Med Hypotheses. 2001;56:483–486. doi: 10.1054/mehy.2000.1203. [DOI] [PubMed] [Google Scholar]
- 16.Monji A, Kato T, Kanba S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin Neurosci. 2009;63:257–265. doi: 10.1111/j.1440-1819.2009.01945.x. [DOI] [PubMed] [Google Scholar]
- 17.O'Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, et al. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: Evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 19.Kowalski J, Blada P, Kucia K, Madej A, Herman ZS. Neuroleptics normalize increased release of interleukin-1 beta and tumor necrosis factor-alpha from monocytes in schizophrenia. Schizophr Res. 2001;50:169–175. doi: 10.1016/s0920-9964(00)00156-0. [DOI] [PubMed] [Google Scholar]
- 20.Wilke I, Arolt V, Rothermundt M, Weitzsch C, Hornberg M, Kirchner H. Investigations of cytokine production in whole blood cultures of paranoid and residual schizophrenic patients. Eur Arch Psychiatry Clin Neurosci. 1996;246:279–284. doi: 10.1007/BF02190280. [DOI] [PubMed] [Google Scholar]
- 21.Schwarz MJ, Riedel M, Gruber R, Müller N, Ackenheil M. Autoantibodies against 60-kDa heat shock protein in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 1998;248:282–288. doi: 10.1007/s004060050051. [DOI] [PubMed] [Google Scholar]
- 22.Barry S, Clarke G, Scully P, Dinan TG. Kynurenine pathway in psychosis: Evidence of increased tryptophan degradation. J Psychopharmacol. 2009;23:287–294. doi: 10.1177/0269881108089583. [DOI] [PubMed] [Google Scholar]
- 23.Akiyama K. Serum levels of soluble IL-2 receptor alpha, IL-6 and IL-1 receptor antagonist in schizophrenia before and during neuroleptic administration. Schizophr Res. 1999;37:97–106. doi: 10.1016/s0920-9964(98)00140-6. [DOI] [PubMed] [Google Scholar]
- 24.Baker I, Masserano J, Wyatt RJ. Serum cytokine concentrations in patients with schizophrenia. Schizophr Res. 1996;20:199–203. doi: 10.1016/0920-9964(95)00089-5. [DOI] [PubMed] [Google Scholar]
- 25.Barak V, Barak Y, Levine J, Nisman B, Roisman I. Changes in interleukin-1 beta and soluble interleukin-2 receptor levels in CSF and serum of schizophrenic patients. J Basic Clin Physiol Pharmacol. 1995;6:61–69. doi: 10.1515/jbcpp.1995.6.1.61. [DOI] [PubMed] [Google Scholar]
- 26.Bechter K, Reiber H, Herzog S, Fuchs D, Tumani H, Maxeiner HG. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: Identification of subgroups with immune responses and blood-CSF barrier dysfunction. J Psychiatr Res. 2010;44:321–330. doi: 10.1016/j.jpsychires.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Becker D, Kritschmann E, Floru S, Shlomo-David Y, Gotlieb-Stematsky T. Serum interferon in first psychotic attack. Br J Psychiatry. 1990;157:136–138. doi: 10.1192/bjp.157.1.136. [DOI] [PubMed] [Google Scholar]
- 28.Bresee C, Rapaport MH. Persistently increased serum soluble interleukin-2 receptors in continuously ill patients with schizophrenia. Int J Neuropsychopharmacol. 2009;12:861–865. doi: 10.1017/S1461145709000315. [DOI] [PubMed] [Google Scholar]
- 29.Cazzullo CL, Sacchetti E, Galluzzo A, Panariello A, Adorni A, Pegoraro M, et al. Cytokine profiles in schizophrenic patients treated with risperidone: A 3-month follow-up study. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:33–39. doi: 10.1016/s0278-5846(01)00221-4. [DOI] [PubMed] [Google Scholar]
- 30.Coelho FM, Reis HJ, Nicolato R, Romano-Silva MA, Teixeira MM, Bauer ME, Teixeira AL. Increased serum levels of inflammatory markers in chronic institutionalized patients with schizophrenia. Neuroimmunomodulation. 2008;15:140–144. doi: 10.1159/000148197. [DOI] [PubMed] [Google Scholar]
- 31.Crespo-Facorro B, Carrasco-Marín E, Pérez-Iglesias R, Pelayo-Terán JM, Fernandez-Prieto L, Leyva-Cobián F, Vázquez-Barquero JL. Interleukin-12 plasma levels in drug-naïve patients with a first episode of psychosis: Effects of antipsychotic drugs. Psychiatry Res. 2008;158:206–216. doi: 10.1016/j.psychres.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Drexhage RC, Padmos RC, de Wit H, Versnel MA, Hooijkaas H, van der Lely AJ, et al. Patients with schizophrenia show raised serum levels of the pro-inflammatory chemokine CCL2: Association with the metabolic syndrome in patients? Schizophr Res. 2008;102:352–355. doi: 10.1016/j.schres.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Ebrinç S, Top C, Oncül O, Başogğlu C, Cavuşlu S, Cetin M. Serum interleukin 1 alpha and interleukin 2 levels in patients with schizophrenia. J Int Med Res. 2002;30:314–317. doi: 10.1177/147323000203000313. [DOI] [PubMed] [Google Scholar]
- 34.el-Mallakh RS, Suddath RL, Wyatt RJ. Interleukin-1 alpha and interleukin-2 in cerebrospinal fluid of schizophrenic subjects. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:383–391. doi: 10.1016/0278-5846(93)90072-z. [DOI] [PubMed] [Google Scholar]
- 35.Erbağci AB, Herken H, Köylüoglu O, Yilmaz N, Tarakçioglu M. Serum IL-1beta, sIL-2R, IL-6, IL-8 and TNF-alpha in schizophrenic patients, relation with symptomatology and responsiveness to risperidone treatment. Mediators Inflamm. 2001;10:109–115. doi: 10.1080/09629350123895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez-Egea E, Bernardo M, Donner T, Congent I, Parellada E, Justicia A, et al. Metabolic profile of antipsychotic-naïve patients with nonaffective psychosis. Br J Psychiatry. 2009;194:434–438. doi: 10.1192/bjp.bp.108.052605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frommberger UH, Bauer J, Haselbauer P, Fräulin A, Riemann D, Berger M. Interleukin-6-(IL-6) plasma levels in depression and schizophrenia: Comparison between the acute state and after remission. Eur Arch Psychiatry Clin Neurosci. 1997;247:228–233. doi: 10.1007/BF02900219. [DOI] [PubMed] [Google Scholar]
- 38.Ganguli R, Rabin BS. Increased serum interleukin 2 receptor concentration in schizophrenic and brain-damaged subjects. Arch Gen Psychiatry. 1989;46:292. doi: 10.1001/archpsyc.1989.01810030098018. [DOI] [PubMed] [Google Scholar]
- 39.Ganguli R, Yang Z, Shurin G, Chengappa KN, Brar JS, Gubbi AV, Rabin BS. Serum interleukin-6 concentration in schizophrenia: Elevation associated with duration of illness. Psychiatry Res. 1994;51:1–10. doi: 10.1016/0165-1781(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 40.García-Miss Mdel R, Pérez-Mutul J, López-Canul B, Solís-Rodríguez F, Puga-Machado L, Oxté-Cabrera A, et al. Folate, homocysteine, interleukin-6, and tumor necrosis factor alfa levels, but not the methylenetetrahydrofolate reductase C677T polymorphism, are risk factors for schizophrenia. J Psychiatr Res. 2010;44:441–446. doi: 10.1016/j.jpsychires.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Gattaz WF, Dalgalarrondo P, Schröder HC. Abnormalities in serum concentrations of interleukin-2, interferon-alpha and interferon-gamma in schizophrenia not detected. Schizophr Res. 1992;6:237–241. doi: 10.1016/0920-9964(92)90006-q. [DOI] [PubMed] [Google Scholar]
- 42.Haack M, Hinze-Selch D, Fenzel T, Kraus T, Kühn M, Schuld A, et al. Plasma levels of cytokines and soluble cytokine receptors in psychiatric patients upon hospital admission: Effects of confounding factors and diagnosis. J Psychiatr Res. 1999;33:407–418. doi: 10.1016/s0022-3956(99)00021-7. [DOI] [PubMed] [Google Scholar]
- 43.Hope S, Melle I, Aukrust P, Steen NE, Birkenaes AB, Lorentzen S, et al. Similar immune profile in bipolar disorder and schizophrenia: Selective increase in soluble tumor necrosis factor receptor I and von Willebrand factor. Bipolar Disord. 2009;11:726–734. doi: 10.1111/j.1399-5618.2009.00757.x. [DOI] [PubMed] [Google Scholar]
- 44.Kaminska T, Wysocka A, Marmurowska-Michalowska H, Dubas-Slemp H Kandefer-Szerszen M. Investigation of serum cytokine levels and cytokine production in whole blood cultures of paranoid schizophrenic patients. Arch Immunol. Ther Exp (Warsz) 2001;49:439–445. [PubMed] [Google Scholar]
- 45.Katila H, Hurme M, Wahlbeck K, Appelberg B, Rimón R. Plasma and cerebrospinal fluid interleukin-1 beta and interleukin-6 in hospitalized schizophrenic patients. Neuropsychobiology. 1994;30:20–23. doi: 10.1159/000119130. [DOI] [PubMed] [Google Scholar]
- 46.Kim YK, Kim L, Lee MS. Relationships between interleukins, neurotransmitters and psychopathology in drug-free male schizophrenics. Schizophr Res. 2000;44:165–175. doi: 10.1016/s0920-9964(99)00171-1. [DOI] [PubMed] [Google Scholar]
- 47.Kim DJ, Kim W, Yoon SJ, Go HJ, Choi BM, Jun TY, Kim YK. Effect of risperidone on serum cytokines. Int J Neurosci. 2001;111:11–19. doi: 10.3109/00207450108986549. [DOI] [PubMed] [Google Scholar]
- 48.Kim YK, Suh IB, Kim H, Han CS, Lim CS, Choi SH, Licinio J. The plasma levels of interleukin-12 in schizophrenia, major depression, and bipolar mania: Effects of psychotropic drugs. Mol Psychiatry. 2002;7:1107–1114. doi: 10.1038/sj.mp.4001084. [DOI] [PubMed] [Google Scholar]
- 49.Kim YK, Myint AM, Lee BH, Han CS, Lee HJ, Kim DJ, Leonard BE. Th1, Th2 and Th3 cytokine alteration in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1129–1134. doi: 10.1016/j.pnpbp.2004.05.047. [DOI] [PubMed] [Google Scholar]
- 50.Kim YK, Myint AM, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Cytokine changes and tryptophan metabolites in medicationnaïve and medication-free schizophrenic patients. Neuropsychobiology. 2009;59:123–129. doi: 10.1159/000213565. [DOI] [PubMed] [Google Scholar]
- 51.Licinio J, Seibyl JP, Altemus M, Charney DS, Krystal JH. Elevated CSF levels of interleukin-2 in neuroleptic-free schizophrenic patients. Am J Psychiatry. 1993;150:1408–1410. doi: 10.1176/ajp.150.9.1408. [DOI] [PubMed] [Google Scholar]
- 52.Lin A, Kenis G, Bignotti S, Tura GJ, De Jong R, Bosmans E, et al. The inflammatory response system in treatment-resistant schizophrenia: Increased serum interleukin-6. Schizophr Res. 1998;32:9–15. doi: 10.1016/s0920-9964(98)00034-6. [DOI] [PubMed] [Google Scholar]
- 53.Lu S, Liu G, Kong X, Shen Chen, Chen J, Yao Y. Interleukin level in CSF of patients with first episode schizophrenia. Chinese Mental Health Journal. 2003;17:206–208. [Google Scholar]
- 54.Maes M, Meltzer HY, Bosmans E. Immune-inflammatory markers in schizophrenia: Comparison to normal controls and effects of clozapine. Acta Psychiatr Scand. 1994;89:346–351. doi: 10.1111/j.1600-0447.1994.tb01527.x. [DOI] [PubMed] [Google Scholar]
- 55.Maes M, Meltzer HY, Buckley P, Bosmans E. Plasma-soluble interleukin-2 and transferrin receptor in schizophrenia and major depression. Eur Arch Psychiatry Clin Neurosci. 1995;244:325–329. doi: 10.1007/BF02190412. [DOI] [PubMed] [Google Scholar]
- 56.Maes M, Bosmans E, Calabrese J, Smith R, Meltzer HY. Interleukin-2 and interleukin-6 in schizophrenia and mania: Effects of neuroleptics and mood stabilizers. J Psychiatr Res. 1995;29:141–152. doi: 10.1016/0022-3956(94)00049-w. [DOI] [PubMed] [Google Scholar]
- 57.Maes M, Bosmans E, Ranjan R, Vandoolaeghe E, Meltzer HY, De Ley M, et al. Lower plasma CC16, a natural anti-inflammatory protein, and increased plasma interleukin-1 receptor antagonist in schizophrenia: Effects of antipsychotic drugs. Schizophr Res. 1996;21:39–50. doi: 10.1016/0920-9964(96)00029-1. [DOI] [PubMed] [Google Scholar]
- 58.Maes M, Bosmans E, Kenis G, De Jong R, Smith RS, Meltzer HY. In vivo immunomodulatory effects of clozapine in schizophrenia. Schizophr Res. 1997;26:221–225. doi: 10.1016/s0920-9964(97)00057-1. [DOI] [PubMed] [Google Scholar]
- 59.Maes M, Bocchio Chiavetto L, Bignotti S, Battisa Tura G, Pioli R, Boin F, et al. Effects of atypical antipsychotics on the inflammatory response system in schizophrenic patients resistant to treatment with typical neuroleptics. Eur Neuropsychopharmacol. 2000;10:119–124. doi: 10.1016/s0924-977x(99)00062-0. [DOI] [PubMed] [Google Scholar]
- 60.Maes M, Bocchio Chiavetto L, Bignotti S, Battisa Tura GJ, Pioli R, Boin F, et al. Increased serum interleukin-8 and interleukin-10 in schizophrenic patients resistant to treatment with neuroleptics and the stimulatory effects of clozapine on serum leukemia inhibitory factor receptor. Schizophr Res. 2002;54:281–291. doi: 10.1016/s0920-9964(00)00094-3. [DOI] [PubMed] [Google Scholar]
- 61.Monteleone P, Fabrazzo M, Tortorella A, Maj M. Plasma levels of interleukin-6 and tumor necrosis factor alpha in chronic schizophrenia: Effects of clozapine treatment. Psychiatry Res. 1997;71:11–17. doi: 10.1016/s0165-1781(97)00036-x. [DOI] [PubMed] [Google Scholar]
- 62.Müller N, Dobmeier P, Empl M, Riedel M, Schwarz M, Ackenheil M. Soluble IL-6 receptors in the serum and cerebrospinal fluid of paranoid schizophrenic patients. Eur Psychiatry. 1997;12:294–299. doi: 10.1016/S0924-9338(97)84789-X. [DOI] [PubMed] [Google Scholar]
- 63.Müller N, Empl M, Riedel M, Schwarz M, Ackenheil M. Neuroleptic treatment increases soluble IL-2 receptors and decreases soluble IL-6 receptors in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 1997;247:308–313. doi: 10.1007/BF02922260. [DOI] [PubMed] [Google Scholar]
- 64.Naudin J, Mège JL, Azorin JM, Dassa D. Elevated circulating levels of IL-6 in schizophrenia. Schizophr Res. 1996;20:269–273. doi: 10.1016/0920-9964(96)00014-x. [DOI] [PubMed] [Google Scholar]
- 65.Naudin J, Capo C, Giusano B, Mege JL, Azorin JM. A differential role for interleukin-6 and tumor necrosis factor-alpha in schizophrenia? Schizophr Res. 1997;26:27–233. doi: 10.1016/s0920-9964(97)00059-5. [DOI] [PubMed] [Google Scholar]
- 66.Nikkilä HV, Müller K, Ahokas A, Miettinen K, Rimón R, Andersson LC. Accumulation of macrophages in the CSF of schizophrenic patients during acute psychotic episodes. Am J Psychiatry. 1999;156:1725–1729. doi: 10.1176/ajp.156.11.1725. [DOI] [PubMed] [Google Scholar]
- 67.Nikkilä HV, Ahokas A, Wahlbeck K, Rimón R, Andersson LC. Neopterin and macrophage inflammatory protein-1alpha in the cerebrospinal fluid of schizophrenic patients: No evidence of intrathecal inflammation. Neuropsychobiology. 2002;46:169–172. doi: 10.1159/000067805. [DOI] [PubMed] [Google Scholar]
- 68.Nimgaonkar VL, Yang ZW, Zhang XR, Brar JS, Chakravarti A, Ganguli R. Association study of schizophrenia and the IL-2 receptor beta chain gene. Am J Med Genet. 1995;60:448–451. doi: 10.1002/ajmg.1320600517. [DOI] [PubMed] [Google Scholar]
- 69.O'Brien SM, Scully P, Dinan TG. Increased tumor necrosis factor-alpha concentrations with interleukin-4 concentrations in exacerbations of schizophrenia. Psychiatry Res. 2008;160:256–262. doi: 10.1016/j.psychres.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 70.Pae CU, Yoon CH, Kim TS, Kim JJ, Park SH, Lee CU, et al. Antipsychotic treatment may alter T-helper (TH) 2 arm cytokines. Int Immunopharmacol. 2006;6:666–671. doi: 10.1016/j.intimp.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 71.Preble OT, Torrey EF. Serum interferon in patients with psychosis. Am J Psychiatry. 1985;142:1184–1186. doi: 10.1176/ajp.142.10.1184. [DOI] [PubMed] [Google Scholar]
- 72.Rapaport MH, McAllister CG, Pickar D, Nelson DL, Paul SM. Elevated levels of soluble interleukin 2 receptors in schizophrenia. Arch Gen Psychiatry. 1989;46:291–292. doi: 10.1001/archpsyc.1989.01810030097017. [DOI] [PubMed] [Google Scholar]
- 73.Rapaport MH, Torrey EF, McAllister CG, Nelson DL, Pickar D, Paul SM. Increased serum soluble interleukin-2 receptors in schizophrenic monozygotic twins. Eur Arch Psychiatry Clin Neurosci. 1993;243:7–10. doi: 10.1007/BF02191517. [DOI] [PubMed] [Google Scholar]
- 74.Rapaport MH, McAllister CG, Kim YS, Han JH, Pickar D, Nelson DL, et al. Increased serum soluble interleukin-2 receptors in Caucasian and Korean schizophrenic patients. Biol Psychiatry. 1994;35:767–771. doi: 10.1016/0006-3223(94)91137-1. [DOI] [PubMed] [Google Scholar]
- 75.Rapaport MH, Lohr JB. Serum-soluble interleukin-2 receptors in neuroleptic-naive schizophrenic subjects and in medicated schizophrenic subjects with and without tardive dyskinesia. Acta Psychiatr Scand. 1994;90:311–315. doi: 10.1111/j.1600-0447.1994.tb01599.x. [DOI] [PubMed] [Google Scholar]
- 76.Rapaport MH, Caligiuri MP, Lohr JB. An association between increased serum-soluble interleukin-2 receptors and a disturbance in muscle force in schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:817–827. doi: 10.1016/s0278-5846(97)00082-1. [DOI] [PubMed] [Google Scholar]
- 77.Rapaport MH, McAllister CG, Pickar D, Tamarkin L, Kirch DG, Paul SM. CSF IL-1 and IL-2 in medicated schizophrenic patients and normal volunteers. Schizophr Res. 1997;25:123–129. doi: 10.1016/S0920-9964(97)00008-X. [DOI] [PubMed] [Google Scholar]
- 78.McAllister CG, van Kammen DP, Rehn TJ, Miller AL, Gurklis J, Kelley ME, et al. Increases in CSF levels of interleukin-2 in schizophrenia: Effects of recurrence of psychosis and medication status. Am J Psychiatry. 1995;152:1291–1297. doi: 10.1176/ajp.152.9.1291. [DOI] [PubMed] [Google Scholar]
- 79.Sarandol A, Kirli S, Akkaya C, Ocak N, Eroz E, Sarandol E. Coronary artery disease risk factors in patients with schizophrenia: Effects of short term antipsychotic treatment. J Psychopharmacol. 2007;21:857–863. doi: 10.1177/0269881107077609. [DOI] [PubMed] [Google Scholar]
- 80.Schattner A, Cori Y, Hahn T, Sirota P. No evidence for autoimmunity in schizophrenia. J Autoimmun. 1996;9:661–666. doi: 10.1006/jaut.1996.0086. [DOI] [PubMed] [Google Scholar]
- 81.Schwarz MJ, Riedel M, Gruber R, Müller N, Ackenheil M. Autoantibodies against 60-kDa heat shock protein in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 1998;248:282–288. doi: 10.1007/s004060050051. [DOI] [PubMed] [Google Scholar]
- 82.Shintani F, Kanba S, Maruo N, Nakaki T, Nibuya M, Suzuki E, et al. Serum interleukin-6 in schizophrenic patients. Life Sci. 1991;49:661–664. doi: 10.1016/0024-3205(91)90112-o. [DOI] [PubMed] [Google Scholar]
- 83.Singh B, Bera NK, Nayak CR, Chaudhuri TK. Decreased serum levels of interleukin-2 and interleukin-6 in Indian Bengalee schizophrenic patients. Cytokine. 2009;47:1–5. doi: 10.1016/j.cyto.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 84.Sirota P, Meiman M, Herschko R, Bessler H. Effect of neuroleptic administration on serum levels of soluble IL-2 receptor-alpha and IL-1 receptor antagonist in schizophrenic patients. Psychiatry Res. 2005;134:151–159. doi: 10.1016/j.psychres.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 85.Söderlund J, Schröder J, Nordin C, Samuelsson M, Walther-Jallow L, Karlsson H, et al. Activation of brain interleukin-1beta in schizophrenia. Mol Psychiatry. 2009;14:1069–1071. doi: 10.1038/mp.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song XQ, Lv LX, Li WQ, Hao YH, Zhao JP. The interaction of nuclear factor-kappa B and cytokines is associated with schizophrenia. Biol Psychiatry. 2009;65:481–488. doi: 10.1016/j.biopsych.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 87.Theodoropoulou S, Spanakos G, Baxevanis CN, Economou M, Gritzapis AD, Papamichail MP, Stefanis CN. Cytokine serum levels, autologous mixed lymphocyte reaction and surface marker analysis in never medicated and chronically medicated schizophrenic patients. Schizophr Res. 2001;47:13–25. doi: 10.1016/s0920-9964(00)00007-4. [DOI] [PubMed] [Google Scholar]
- 88.Vawter MP, Dillon-Carter O, Tourtellotte WW, Carvey P, Freed WJ. TGFbeta1 and TGFbeta2 concentrations are elevated in Parkinson's disease in ventricular cerebrospinal fluid. Exp Neurol. 1996;142:313–322. doi: 10.1006/exnr.1996.0200. [DOI] [PubMed] [Google Scholar]
- 89.Vawter MP, Dillon-Carter O, Issa F, Wyatt RJ, Freed WJ. Transforming growth factors beta 1 and beta 2 in the cerebrospinal fluid of chronic schizophrenic patients. Neuropsychopharmacology. 1997;16:83–87. doi: 10.1016/S0893-133X(96)00143-1. [DOI] [PubMed] [Google Scholar]
- 90.Xiu MH, Chen S, Wang F, Cao LY, Qi LY, Chen da C, et al. Altered interleukin-3 serum levels in drug-naïve and neuroleptic-treated schizophrenic patients. Schizophr Res. 2008;106:369–370. doi: 10.1016/j.schres.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 91.Yang ZW, Chengappa KN, Shurin G, Brar JS, Rabin BS, Gubbi AV, Ganguli R. An association between anti-hippocampal antibody concentration and lymphocyte production of IL-2 in patients with schizophrenia. Psychol Med. 1994;24:449–455. doi: 10.1017/s0033291700027410. [DOI] [PubMed] [Google Scholar]
- 92.Yao JK, Sistilli CG, van Kammen DP. Membrane polyunsaturated fatty acids and CSF cytokines in patients with schizophrenia. Prostaglandins Leukot Essent Fatty Acids. 2003;69:429–436. doi: 10.1016/j.plefa.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 93.Zhang XY, Zhou DF, Zhang PY, Wu GY, Cao LY, Shen YC. Elevated interleukin-2, interleukin-6 and interleukin-8 serum levels in neuroleptic-free schizophrenia: Association with psychopathology. Schizophr Res. 2002;57:247–258. doi: 10.1016/s0920-9964(01)00296-1. [DOI] [PubMed] [Google Scholar]
- 94.Xu HM, Wei J, Hemmings GP. Changes of plasma concentrations of interleukin-1 alpha and interleukin-6 with neuroleptic treatment for schizophrenia. Br J Psychiatry. 1994;164:251–253. doi: 10.1192/bjp.164.2.251. [DOI] [PubMed] [Google Scholar]
- 95.Zhang XY, Zhou DF, Cao LY, Zhang PY, Wu GY, Shen YC. Changes in serum interleukin-2, -6, and -8 levels before and during treatment with risperidone and haloperidol: Relationship to outcome in schizophrenia. J Clin Psychiatry. 2004;65:940–947. doi: 10.4088/jcp.v65n0710. [DOI] [PubMed] [Google Scholar]
- 96.Zhang XY, Zhou DF, Cao LY, Wu GY, Shen YC. Cortisol and cytokines in chronic and treatment-resistant patients with schizophrenia: Association with psychopathology and response to antipsychotics. Neuropsychopharmacology. 2005;30:1532–1538. doi: 10.1038/sj.npp.1300756. [DOI] [PubMed] [Google Scholar]
- 97.Zhang XY, Cao LY, Song C, Wu GY, Chen da C, Qi LY, et al. Lower serum cytokine levels in smokers than nonsmokers with chronic schizophrenia on long-term treatment with antipsychotics. Psychopharmacology (Berl) 2008;201:383–389. doi: 10.1007/s00213-008-1295-4. [DOI] [PubMed] [Google Scholar]
- 98.Zhang XY, Zhou DF, Qi LY, Chen S, Cao LY, Chen da C, et al. Superoxide dismutase and cytokines in chronic patients with schizophrenia: Association with psychopathology and response to antipsychotics. Psychopharmacology (Berl) 2009;204:177–184. doi: 10.1007/s00213-008-1447-6. [DOI] [PubMed] [Google Scholar]
- 99.Hunter J, Schmidt F. Fixed effects vs. random effects meta-analysis models: Implications for cumulative research knowledge. Int J Sel Assess. 2000;8:275–292. [Google Scholar]
- 100.al-Janadi M, al-Balla S, al-Dalaan A, Raziuddin S. Cytokine profile in systemic lupus erythematosus, rheumatoid arthritis, and other rheumatic diseases. J Clin Immunol. 1993;13:58–67. doi: 10.1007/BF00920636. [DOI] [PubMed] [Google Scholar]
- 101.Manavalan JS, Hernandez L, Shah JG, Konikkara J, Naiyer AJ, Lee AR, et al. Serum cytokine elevations in celiac disease: Association with disease presentation. Hum Immunol. 2010;71:50–57. doi: 10.1016/j.humimm.2009.09.351. [DOI] [PubMed] [Google Scholar]
- 102.Ganguli R, Gubbi A. Clinical and immunological characteristics of a subgroup of patients suffering from schizophrenia. In: Henneber AE, Kaschka WP, editors. Immunological Alterations in Psychiatric Diseases. Adv Biol Psychiatry. Vol. 18. Karger; Basle, Switzerland: 1997. pp. 35–43. [Google Scholar]
- 103.Müller N. Mechanisms of relapse prevention in schizophrenia. Pharmacopsychiatry. 2004;37(suppl 2):S141–S147. doi: 10.1055/s-2004-832668. [DOI] [PubMed] [Google Scholar]
- 104.Shepherd M, Watt D, Falloon I, Smeeton N. The natural history of schizophrenia: A five-year follow-up study of outcome and prediction in a representative sample of schizophrenics. Psychol Med.Monogr Suppl. 1989;15:1–46. doi: 10.1017/s026418010000059x. [DOI] [PubMed] [Google Scholar]
- 105.Wyatt RJ. Early intervention with neuroleptics may decrease the long-term morbidity of schizophrenia. Schizophr Res. 1991;5:201–202. doi: 10.1016/0920-9964(91)90073-z. [DOI] [PubMed] [Google Scholar]
- 106.Adib-Conquy M, Cavaillon JM. Compensatory anti-inflammatory response syndrome. Thromb Haemost. 2009;101:36–47. [PubMed] [Google Scholar]
- 107.Drexhage RC, Hoogenboezem TA, Cohen D, Versnel MA, Nolen WA, van Beveren NJ, Drexhage HA. An activated set point of T-cell and monocyte inflammatory networks in recent-onset schizophrenia patients involves both pro- and anti-inflammatory forces. Int J Neuropsychopharmacol. 2011;24:1–10. doi: 10.1017/S1461145710001653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.