Abstract
Aromatic organosulfates are identified and quantified in fine particulate matter (PM2.5) from Lahore, Pakistan, Godavari, Nepal, and Pasadena, California. To support detection and quantification, authentic standards of phenyl sulfate, benzyl sulfate, 3-and 4-methylphenyl sulfate and 2-, 3-, and 4-methylbenzyl sulfate were synthesized. Authentic standards and aerosol samples were analyzed by ultra-performance liquid chromatography (UPLC) coupled to negative electrospray ionization (ESI) quadrupole time-of-flight (ToF) mass spectrometry. Benzyl sulfate was present in all three locations at concentrations ranging from 4 – 90 pg m−3. Phenyl sulfate, methylphenyl sulfates and methylbenzyl sulfates were observed intermittently with abundances of 4 pg m−3, 2-31 pg m−3, 109 pg m−3, respectively. Characteristic fragment ions of aromatic organosulfates include the sulfite radical (•SO3−, m/z 80) and the sulfate radical (•SO4−,m/z 96). Instrumental response factors of phenyl and benzyl sulfates varied by a factor of 4.3, indicating that structurally-similar organosulfates may have significantly different instrumental responses and highlighting the need to develop authentic standards for absolute quantitation organosulfates. In an effort to better understand the sources of aromatic organosulfates to the atmosphere, chamber experiments with the precursor toluene were conducted under conditions that form biogenic organosulfates. Aromatic organosulfates were not detected in the chamber samples, suggesting that they form through different pathways, have different precursors (e.g. naphthalene or methylnaphthalene), or are emitted from primary sources.
Keywords: sulfate ester synthesis, atmospheric aerosol, mass spectrometry, toluene
1. Introduction
Sulfate esters (a.k.a. organosulfates) are ubiquitous in atmospheric aerosols, having been detected in remote, urban, forested, marine, and arctic locations worldwide (Romero et al. 2005; Surratt et al. 2007; Frossard et al. 2011; Kristensen et al. 2011; Mazzoleni et al. 2012; Stone et al. 2012). Monosulfate esters are strong acids with pKa values estimated below -3 (Guthrie 1978), so they are deprotonated (R-O-SO -3) in environmental systems. These negatively charged molecules are highly water-soluble and non-volatile, such that they remain in the particle phase in the atmosphere. Organosulfates may play a role in climate forcing, by direct light absorption (Nguyen et al. 2012) and/or by affecting aerosol hygroscopicity due to their acidic and amphiphilic nature. They also help to reconcile the under-predictions of particle-phase organic carbon in atmospheric models by providing a means to account for acid-catalyzed secondary organic aerosol (SOA) formation (Iinuma et al. 2013; Pye et al. 2013).
The total atmospheric abundance of organosulfates has been estimated to range from 4-30% of fine particle organic mass (Surratt et al. 2008; Frossard et al. 2011; Stone et al. 2012). These estimates remain highly uncertain due to the lack of quantitation standards and direct measurement techniques. Tolocka and Turpin (2012) calculate the upper limit of organosulfate contributions to fine particle (PM2.5) organic mass at 5-10% for background locations in the United States. Meanwhile, individual organosulfate species account for less than 1% of organic mass (Iinuma et al. 2009; Olson et al. 2011; Kundu et al. 2013), indicating that organosulfate concentrations are not dominated by a few highly abundant species and, instead, are present in a wide range of chemical forms.
The chemical structures of organosulfates range from small molecules to high-molecular weight organic matter (Stone et al. 2009), with a high oxygen-to-carbon ratio (Mazzoleni et al. 2012; Stone et al. 2012). Qualitative surveys of organosulfates have revealed that they are largely aliphatic in structure and also contain hydroxy, carboxylic acid, and nitrooxy functional groups (Lin et al. 2012). Among the most abundant and ubiquitous organosulfates are those derived from isoprene and isoprene oxidation products (Zhang et al. 2012; Lin et al. 2013b); these small, multi-functional organosulfates have been widely observed in ambient aerosol (Surratt et al. 2007; Kristensen et al. 2011; Stone et al. 2012), and used as markers of isoprene-derived SOA in field experiments (Zhang et al. 2012; Lin et al. 2013b).
Aromatic organosulfates, containing an intact aromatic ring, have recently been observed in ambient aerosol in Lahore, Pakistan (Stone et al. 2012; Kundu et al. 2013) and in urban sites in East Asia in low abundance (Lin et al. 2012). Kundu et al. (2013) unequivocally identified benzyl sulfate against a synthesized authentic standard, quantified its monthly-average concentration in PM2.5 (0.05 – 0.50 ng m−3).
The current study builds on the tentative identification of homologous series of phenyl and benzyl sulfates with methyl substituents by Kundu et al. (2013). Series of phenyl and benzyl sulfates were synthesized and confirmed as constituents of fine particulate matter. These standards were used to study their mass spectra and response factors under negative ESI, and to evaluate their abundance in atmospheric aerosols from three distinct urban locations: Lahore, Pakistan, Godavari, Nepal, and Pasadena, California, USA. The potential for secondary formation of aromatic organosulfates from toluene was also examined (Zhang et al. 2012).
2. Materials and Methods
2.1. Synthesized product characterization
Aromatic organosulfates standards were characterized by high resolution mass spectrometry (HRMS, Micromass Q-ToF Premier, Waters) with negative ESI and 1H nuclear magnetic resonance (NMR, Bruker DRX, 400 MHz). Phenyl sulfates were also characterized by 13C NMR. Standardization of the aromatic organosulfates was performed in D O using 12 H-NMR (Varian 300 MHz). For quantification purposes, dichloroacetic acid (DCA) was used as an internal standard, following Olson et al. (2011). Phenols and benzyl alcohols were obtained from Acros Organics or Sigma Aldrich and were used without further purification.
2.2. General procedure for the synthesis of phenyl sulfates
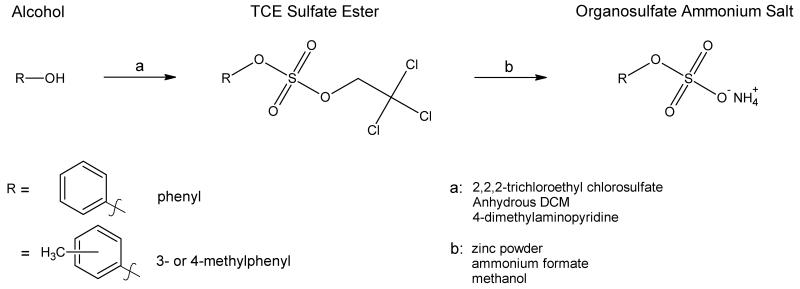
The reagent 2,2,2-trichloroethyl chlorosulfate (TCE) was prepared from trichloroethanol and sulfuryl chloride following Hedayatu et al. (1971). A solution of TCE in dry tetrahydrofuran (THF, 10 mL) was added dropwise to phenols (1.0 equiv), triethylamine (1.2 equiv), 4-dimethylaminopyridine (DMAP, 1.2 equiv), in dry THF (40 mL) and was stirred for 2 h (Liu et al. 2004; Li et al. 2010). The reaction mixture was then extracted with ethyl acetate and washed with H2O, 1.0 N HCl, and saturated brine. The organic layer was then dried with Na2SO4 and solvent removed under vacuum. The resulting white residue was purified by silica gel column chromatography with hexane and ethyl acetate (10:1, v/v) as mobile phase. The TCE-sulfate ester was then dissolved in methanol and excess ammonium formate and zinc powder were added to generate the free sulfate ester. Reaction progress was monitored with thin-layer chromatography. The product was purified with column chromatography on silica gel with dichloromethane, methanol, and ammonium hydroxide (30:6:1, v/v/v) as mobile phase. Following vacuum filtration, excess solvent was removed under reduced pressure and the product dried with light heating.
2.2.1. Phenyl sulfate, ammonium salt
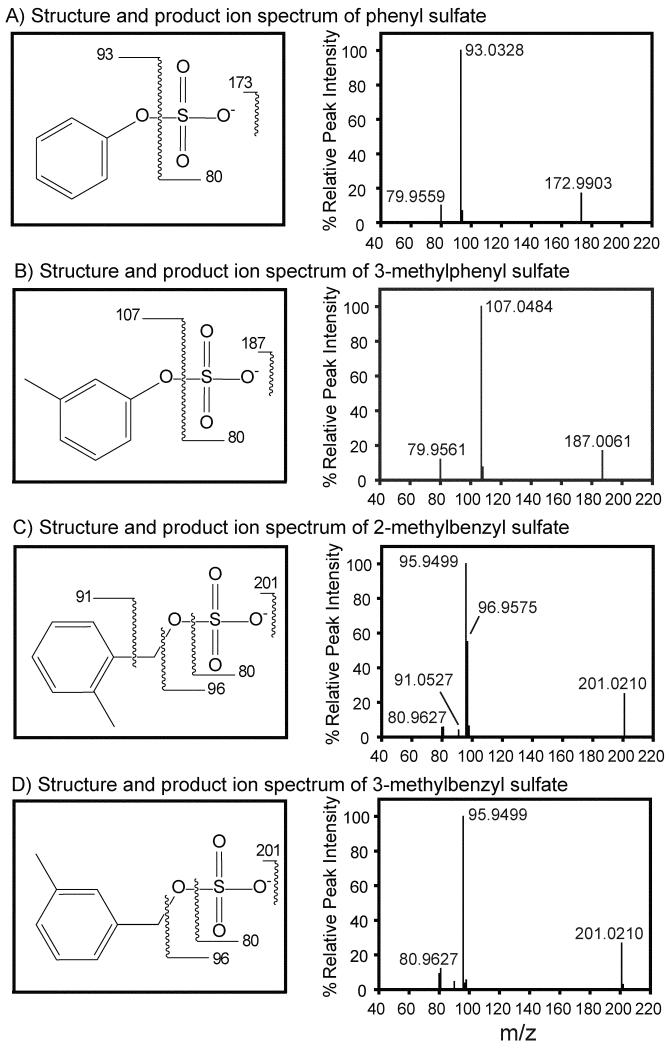
Yield: 31 %; purity: 93 %; white solid; 1H NMR (400 MHz, CD3OD): δ/ppm 4.60 (s, 4H), 7.12-7.38 (m, 5H); 13C-NMR (400 MHz, CD3OD): δ/ppm 122.2, 125.5, 129.8, 153.7; HR-MS (ESI, negative) m/z (relative intensity, %): 172.9903 (17, C6H5O4S−), 93.0328 (100), 79.9559 (10).
2.2.2. 3-Methylphenyl sulfate, ammonium salt
Yield: 64 %; purity 85 %; white solid; 1H NMR (400 MHz, CD3OD): δ/ppm 2.32 (s, 3H), 6.97-7.21 (m, 4H); 13C-NMR (400 MHz, CD3OD): δ/ppm 21.3, 119.3, 122.9, 126.8, 129.9, 140.4, 153.6; HR-MS (ESI, negative) m/z (relative intensity, %): 187.0061 (17, C7H7O4S−), 107.0484 (100), 79.9561 (10).
2.2.3. 4-Methylphenyl sulfate, ammonium salt
Yield: 39 %; purity: 85%; white solid; 1H NMR (400 MHz, CD3OD): δ/ppm 2.24 (s, 3H), 7.05-7.10 (m, 4H); 13C-NMR (400 MHz, CD3OD): δ/ppm 20.9, 122.5, 130.7, 135.7, 151.8; HR-MS (ESI, negative) m/z (relative intensity, %): 187.0061 (19, C7H7O4S−), 107.0486 (100), 79.9560 (17).
2.3. General procedure for the synthesis of benzyl sulfates
Benzyl and methylbenzyl sulfates were synthesized following Olson et al. (2011), wherein benzyl alcohol, 2-, 3-, and 4-methylbenzyl alcohols were dissolved in acetonitrile with N,N-diisopropylethylamine (DIEA). The solution was cooled to 0 °C before slow addition of chlorosulfonic acid. The reaction was allowed to proceed in an ice bath for 3 hours, prior to removal of acetonitrile under reduced pressure. The resulting product mixture was liquid and was not subjected to further purification.
2.3.1. Benzyl sulfate
Purity: 44 %; 1H NMR (400 MHz, D2O): δ/ppm 5.03 (s, 2H), 7.37-7.44 (m, 4H); HR-MS (ESI, negative) m/z (relative intensity, %): 187.0049 (58, C7H7O4S), 107.0483 (4), 95.9507 (100), 80.9639 (21), 79.9553 (11).
2.3.1. 2-Methylbenzyl sulfate
Purity: 42 %; 1H NMR (400 MHz, D2O): δ/ppm 2.33 (s, 3H), 5.05 (s, 2H), 7.18-7.38 (m, 4H); HR-MS (ESI, negative) m/z (relative intensity, %): 201.0210 (25, C8H9O4S−), 96.9575 (56), 95.9499 (100), 91.0527 (4), 80.9628 (6), 79.9549 (6).
2.3.1. 3-Methylbenzyl sulfate
Purity: 33 %; 1H NMR (400 MHz, D2O): δ/ppm 2.30 (s, 3H), 4.98 (s, 2H), 7.13-7.32 (m, 4H); HR-MS (ESI, negative) m/z (relative intensity, %): 201.0210 (25, C8H9O4S−), 96.9575 (55), 95.9499 (100), 91.0525 (4) 80.9627 (6), 79.9550 (6).
2.3.1. 4-Methylbenzyl sulfate
Purity: 32 %; 1H NMR (400 MHz, D2O): δ/ppm 2.21 (s, 3H), 4.98 (s, 2H), 7.12-7.25 (m, 4H); HR-MS (ESI, negative) m/z (relative intensity, %): 201.0208 (25, C8H9O4S−), 96.9559 (4), 95.9498 (100), 80.9627 (9), 79.9550 (7).
2.4 Aerosol Sample Collection
2.4.2. Lahore, Pakistan
PM2.5samples in Lahore were collected onto prebaked quartz fiber filters (QFF, Pall Life Sciences, Tissuquartz, 47mm diameter) using a medium-volume sampling apparatus (URG-3000, Chapel Hill, NC, USA). The sampler was located on the rooftop of the Institute for Environmental Engineering on University of Engineering and Technology campus (Stone et al. 2010a). Samples were collected from January 2007 – January 2008 following the one-in-six sampling schedule. A sample collected on December 26, 2007 was used for qualitative identification of aromatic organosulfates and a composite of sub-samples from March 2007 was used for quantitation.
2.4.2. Godavari, Nepal
PM2.5samples were collected onto prebaked 47mm QFF using a medium-volume sampling apparatus (URG-3000, Chapel Hill, NC, USA) as part of the Atmospheric Brown Cloud monitoring network. The sampler was located at the International Center for Integrated Mountain Development (ICIMOD) Training and Demonstration site in the southeastern foothills of the Kathmandu Valley. Samples were collected daily during 2007; sub-samples from twenty-eight filter samples from February were composited and analyzed.
2.4.3 Pasadena, California, USA
PM2.5samples were collected onto prebaked QFF (Pall Life Sciences, Tissuquartz filters, 20.3cm × 25.4 cm) using high-volume PM2.5 samplers (Tisch Environmental) at the Pasadena ground site during the 2010 California Research at the Nexus of Air Quality and Climate Change (CalNex) field study Ryerson et al. (2013). PM2.5 filter samples collected on 5 June and 6 June were analyzed in this study. PM2.5mass was estimated as the sum of measured PM1 components and the product of the PM2.5-1 volume concentration and an assumed aerosol density of 1.49 (Hayes et al. 2013).
2.5 Elemental and Organic Carbon Analysis
PM2.5organic and elemental carbon (OC and EC) measurements were made from QFF using a Sunset Laboratories OCEC analyzer (Model 3F, Forest Grove, OR). Pasadena filter samples were analyzed following the NIOSH thermal-optical transmittance protocol (Hayes et al. 2013), whereas the Lahore and Godavari samples were analyzed by the ACE-Asia protocol (Schauer et al. 2003).
2.6 Organosulfate Extraction
Sub-samples of QFF were combined in pre-baked and solvent-rinsed jars and were extracted into high-purity methanol (99.9%, Fisher Scientific) by 40 minutes of sonication (Branson 5510). Extracts were then filtered with a pre-rinsed polytetrafluoroethylene (PTFE) syringe filter (13 mm, 0.2 μm pore size, Whatman) and reduced in volume under high-purity nitrogen (5 psi) and gentle 50 °C heating. Once dry, samples were reconstituted in 2:1 water-methanol (v/v) to 200 μL. This extraction procedure gave average recoveries of 84% for benzyl sulfate, 76% for phenyl sulfate, and 92% for 3-methylphenyl sulfate.
2.7 Instrumental Analysis of Aromatic Organosulfates
Synthesized standards and aerosol samples were analyzed by UPLC-HRMS following the method described by Kundu et al. (2013). Briefly, UPLC separation occurred on a high-strength silica (HSS) reversed-phase octadecyl (C18) column (ACQUITY UPLC® HSS T3, 2.1 mm ID × 75 mm length, 1.8 μm particle size). Aqueous (high-purity water with resistivity > 18.2 Ω cm) and methanol mobile phases each contained 0.1% glacial acetic acid and followed the gradient elution program described by Surratt et al. (2008), with re-equilibration prior to the next injection. The column temperature was maintained at 45 °C, mobile phase flow rate at 0.3 mL min-1, and injection volume at 5.0 μL.
UPLC eluent was directed to the Q-ToF HRMS operating in negative ESI mode. Mass spectra were collected from m/z 40 to 400 in reflectron mode with V geometry. Val-Tyr-Val (Sigma-Aldrich, m/z 379.2029) was used for lock mass correction. Tandem mass spectrometry (MS2) utilized 15 V collision energy. All sample and standard spectra were background subtracted. Calibration standards of phenyl sulfate, 3-methylphenyl sulfate, benzyl sulfate, 2-methylbenzyl sulfate, and 3-methylbenzyl sulfate with concentrations ranging from approximately 0.5 to 25 ng mL−1were analyzed to construct six-point linear calibration curves (R2 > 0.995) based on peak area molecular ion chromatograms. The method detection limit was 0.35 ng mL−1. Aromatic organosulfates were not detected in laboratory or field blank samples.
2.8 Smog Chamber Experiments
Toluene photooxidation experiments were conducted to determine if aromatic organosulfates form under the same conditions as biogenic organosulfates and if aerosol acidity affects this formation. Toluene-derived SOA was generated in the University of North Carolina 274 m3 dual outdoor smog chamber facility located in Pittsboro, NC, under clear natural sunlight (Kamens et al. 2011; Zhang et al. 2011). Chambers were vented with rural North Carolina background air (with < 8-10 μg m−3 aerosol mass) for at least 12 hours before each experiment.
Experimental conditions used to generate toluene-derived SOA are summarized in Table 1. Prior to the start of each experiment, neutral or acidic seed aerosol was introduced into each side of the chamber by atomizing a 0.06 M ammonium sulfate (aq) or 0.06 M magnesium sulfate plus 0.06 M sulfuric acid (aq), respectively, to mass concentrations of 35–38 μg m−3. Seed aerosol volume concentrations and size distributions were measured by a scanning mobility particle sizer (TSI 3080, Shoreview, MN) with a condensation particle counter (TSI 3022A, Shoreview, MN) as described in Zhang et al. (2011). Background aerosol filter samples of seed aerosol only (totaling ~ 2.6 m3), were collected on Teflo® substrates (Pall Life Sciences, 47-mm, 1-μm pore size). Nitric oxide (NO) was injected into the chamber from a high-pressure gas cylinder. Ozone and nitrogen oxides (NOx = NO + NO2) were measured by UV photometric (Thermo-Environmental 49P) and chemiluminescent (Bendix Model 8101B) analyzers. High-purity liquid toluene (99.8%, Sigma-Aldrich) was heated in a U-tube and flushed into the chamber with high-purity N2. Toluene was measured by gas chromatograph/flame ionization detector. Filter samples of the toluene-derived SOA (totaling ~ 10.9 m3) were collected after the aerosol volume concentration peaked. Backup Teflon filters were also collected to evaluate evaporative losses. Figure S1 (supporting information) shows that the experimental profile for gases and aerosol mass concentrations were similar between the neutral and acidic experiments.
Table 1.
Experimental conditions and results for toluene/NOx experiments conducted in UNC dual outdoor smog chamber.
| Experimenta 1 IDa |
Initial [toluene] (ppb) |
Initial [NO2] (ppb) |
Initial [NO2] (ppb) |
Initial O3 (ppb)b |
Initial toluene/ NOx |
RH (%)c | Max O3 (ppb) |
Max SOA concentration (μg m−3) |
|---|---|---|---|---|---|---|---|---|
| Acidic | 189 | 99 | 4 | b.d.l. | 1.8 | 63 | 161 | 15.9 |
| Neutral | 189 | 99 | 3 | b.d.l. | 1.9 | 63 | 151 | 19.4 |
Acidic seed aerosol was generated from atomizing 0.06 M MgSO4 + 0.06 M H2SO4 (aq) solution into north chamber; neutral seed aerosol was generated from atomizing 0.06 M (NH4)2SO4 (aq) solution into south chamber.
b.d.l. = below detection limits.
These are average values from the experiments.
3. Results and Discussion
3.1 Mass Spectral Fragmentation of Aromatic Organosulfate Standards
Aromatic organosulfate standards were analyzed by high-resolution MS2; resulting product-ion mass spectra; Figure 2 shows the corresponding mass fragmentation patterns. The molecular ion for phenyl sulfate (Figure 2A) was observed at m/z 172.9903 (with molecular formula of C6H5O4S− and error of −3.5 ppm); major fragments included the sulfite radical at m/z 79.9559 (•SO3−, −11.3 ppm) and the phenolate anion at m/z 93.0328 (C6H5O−, −12.9 ppm) formed by the neutral loss of SO3. The errors in observed m/z relative to their theoretical m/z are expressed in parts per million and consequently errors increase as m/z decreases. The observed phenyl sulfate spectrum was consistent with prior studies (Attygalle et al. 2001). 3-Methylphenyl sulfate (Figure 2B) produced a molecular ion at m/z 187.0061 (C7H7O4S−, −2.1 ppm) and major fragments included the sulfite radical at m/z 79.9561 (•SO3−, −8.8 ppm) and the 3-methylphenolate anion at m/z 107.0484 (C7H7O−, −12.1 ppm) formed by the neutral loss of SO3. The product ion mass spectrum for 4-methylphenyl sulfate was not different from 3-methylphenyl sulfate, and is not shown.
Figure 2.
Molecular structures, fragmentation patterns, and MS2 spectra of A) phenyl sulfate, B) 3-methylphenyl sulfate, C) 2-methylbenzyl sulfate and D) 3-methylbenzyl sulfate.
The characteristic mass spectral features of phenyl sulfate and methylphenyl sulfates are the sulfite radical (m/z 80) and neutral loss of SO3 (also 80). The sulfite radical at m/z 80 has previously been observed for organosulfates with aliphatic, aromatic, and allylic structures (Attygalle et al. 2001; Surratt et al. 2007). To form the sulfite radical the organosulfate S-O bond undergoes homolytic cleavage as described by Attygalle et al. (2001). The neutral loss of SO3 (m/z) by heterolytic cleavage from phenyl and methylphenyl sulfates gives rise to even-electron phenolate and methylphenolate anions. These anions are resonance stabilized and have the greatest intensity in product ion spectra. The spectra shown in Figure 2A and 2B are notably void of the bisulfate anion at m/z 97, which is commonly observed for organosulfates of aliphatic structure (Attygalle et al. 2001; Romero et al. 2005; Surratt et al. 2007; Stone et al. 2009). The bisulfate anion (HSO −4) at m/z 97 forms by concerted syn-elimination of a hydrogen atom from a carbon atom in the C2 position from the sulfate moiety; such elimination is not observed in aryl or vinyl sulfate esters (Attygalle et al. 2001) such as these.
The MS fragmentation of benzyl sulfate are consistent with prior work by Kundu et al. (2013) and Attygalle et al. (2001). Notably, benzyl sulfate is a conformational isomer of methylphenyl sulfates and its molecular ion was detected at m/z 187.0049 (C7H7O4S−, −8.6 ppm). Major fragments of benzyl sulfate include the sulfite radical 79.9553 (•SO3−, −,18.8 ppm) and sulfate radical 95.9507 (•SO4−, −10.4 ppm). The formation of the sulfate radical occurs by homolytic fission of the C-O bond and is characteristic of allylic and aryl organosulfates, which can resonance-stabilize the simultaneously forming allylic and benzyl radicals (Attygalle et al. 2001).
Product ion spectra for 2-methylbenzyl sulfate and the 3- methylbenzyl sulfate are shown in Figure 2C and 2D, respectively. The molecular ion for 2-methylbenzyl sulfate was observed at m/z 201.0210 (C8H9O4S− , −6.0 ppm) with major fragments including the sulfite radical at m/z 79.9549 (•SO3−, −23.8 ppm), the bisulfite anion at m/z 80.9628 (HSO3−, 22.2 ppm), the 2-methylbenzenide anion at m/z 91.0527 (C7H7−, −23.7 ppm), the sulfate radical at m/z 95.9499 (•SO4−, −18.8 ppm), and the bisulfate anion at m/z 96.9575 (HSO4−, −20.6 ppm). The molecular ion for 3-methylbenzyl sulfate was observed at m/z 201.0210 (C8H9 O4S− , −6.0 ppm) with major fragments including the sulfite radical at m/z 79.9550 (•SO3−, −22.5 ppm), the bisulfite anion at m/z 80.9627 (HSO3−, −23.5 ppm), and the sulfate radical 95.9499 (•SO4−, −18.8 ppm). The mass spectra for 3-methylbenzyl sulfate and 4-methylbenzyl sulfate were sufficiently similar that the latter is not shown.
The characteristic fragments benzyl sulfate and methylbenzyl sulfates are the sulfite radical (•SO3−, m/z 80) and the sulfate radical (•SO4−, m/z 96). The bisulfite anion (HSO3−, m/z 81) was detected only for the three methylbenzyl sulfate isomers. The bisulfate anion (HSO4−, m/z 97) was detected for 2-methylbenzyl sulfate only, because of the proximity of the methyl group to the sulfate moiety. In the case of 2-methylbenzyl sulfate, the bisulfate anion is not formed by syn elimination, as described previously, but by proton transfer from the nearby methyl group (Attygalle et al. 2001). The identification of characteristic fragment ions of aromatic organosulfates will facilitate their identification and quantification in atmospheric aerosol samples.
3.3 Identification and Quantification of Aromatic Organosulfates in Ambient Aerosol
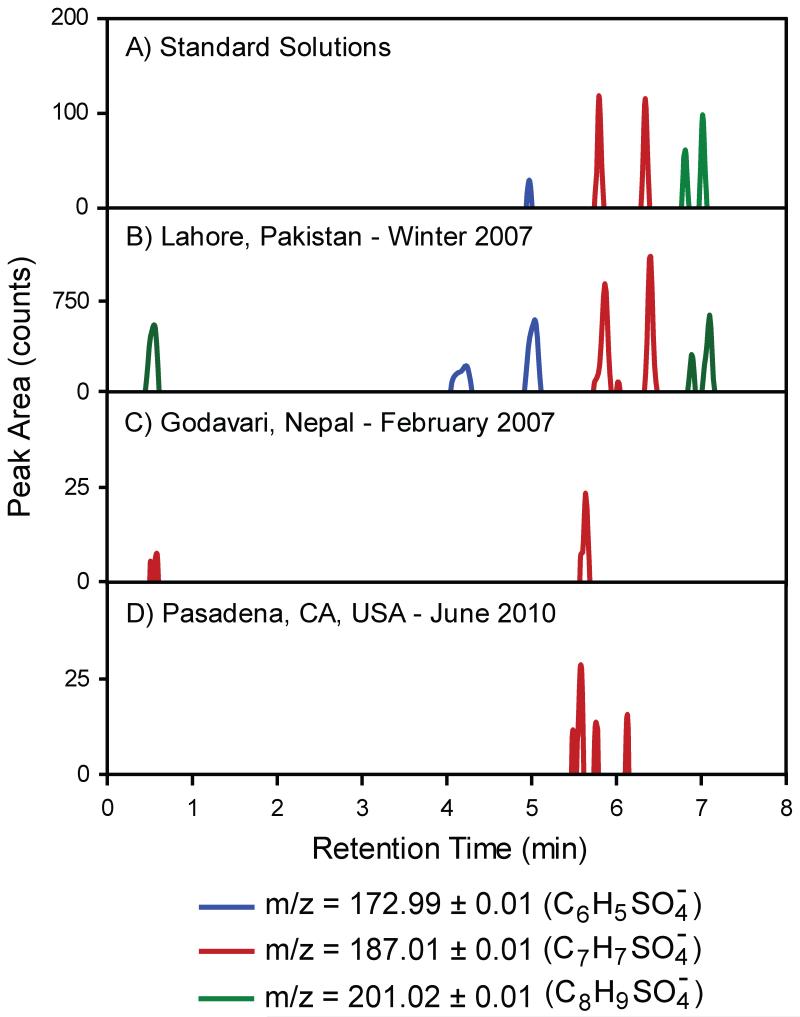
Aromatic organosulfate standards were analyzed by UPLC-ToF MS; extracted ion chromatograms (EIC) for the molecular ions 172.99 ± 0.01 (C6H5SO4−, phenyl sulfate), 187.00 ± 0.01 (C7H7SO4−, benzyl sulfate and methylphenyl sulfates), and 201.02 ± 0.01 (C8H9SO4−, methylbenzyl sulfates) are shown in Figure 3A. Retention times of aromatic organosulfate standards on the reversed-phase UPLC column were 4.9 minutes for phenyl sulfate, 5.8 minutes for benzyl sulfate, 6.3 for 3- and 4-methylphenyl sulfates, which coeluted, 6.8 minutes for 2-methylbenzyl sulfate, and 7.0 minutes for 3- and 4-methylbenzyl sulfates, which also co-eluted. For the homologous series of phenyl and benzyl sulfates, retention on the UPLC column increased with increasing methyl substitution. In reversed-phase LC solute retention is caused by hydrophobic interactions between the nonpolar portion of the molecule and the octadecyl stationary phase. Analytes with increasingly hydrophobic characteristics (i.e. additional methyl groups) have stronger interactions with the stationary phase and longer retention times.
Figure 3.
Overlaid extracted ion chromatograms for aromatic organosulfate molecular ions. A) Standard solutions at 5 ng mL−1, and PM2.5 samples from B) Lahore, Pakistan, C) Godavari, Nepal, and D) Pasadena, California.
Ambient PM2.5 samples, extracted into methanol, from Lahore, Pakistan, Godavari, Nepal, and Pasadena, CA, USA were analyzed by the same analytical method as the standard solutions and resulting EIC are shown in Figure 3B-D. Aromatic organosulfates were identified using high-resolution MS data (described in Section 3.1) and UPLC retention times. The aerosol sample from Lahore (Figure 3B) contained phenyl sulfate, benzyl sulfate, methylphenyl, and methylbenzyl sulfates. Of the aromatic organosulfates, only benzyl sulfate was detected in the Godavari, Nepal sample and benzyl sulfate and methylphenyl sulfates were detected in the Pasadena, CA samples.
Ambient concentrations of aromatic organosulfates are summarized in Table 2 with ambient OC, EC, and PM2.5 mass concentrations. Concentrations of aromatic organosulfates were greatest in Lahore; the sum of three methylbenzyl sulfate isomers had the greatest abundance with 109 pg m−3 followed by benzyl sulfate at 90 pg m−3, the three methylphenyl sulfate isomers at 31 pg m−3, and phenyl sulfate with 4.4 pg m−3. With sub-nanogram per cubic meter concentrations, aromatic organosulfates are significant contributors neither to PM2.5 mass nor OC nor total organosulfates.
Table 2.
Concentrations of PM2.5, organic carbon, elemental carbon, and aromatic organosulfates at the study sites.
| Site Name | Date | Sampling Rate (n) |
PM2.5 3 (μg m−3) | Organic Carbon (μgC m−3) |
Elemental Carbon (μgC m−3) |
Aromatic Sulfate Concentration (pg m−3) |
|||
|---|---|---|---|---|---|---|---|---|---|
| Phenyl Sulfate |
Benzyl Sulfate |
Methylphenyl Sulfatesa |
Methylbenzyl Sulfatesb |
||||||
| Lahore, Pakistan | March, 2007 | 1-in-6 day (6) | 177.1 | 44.6 | 11.1 | 4.4 | 90 | 31 | 109 |
| Godavari, Nepal | February, 2007 | Daily (28) | 42.0 | 4.7 | 1.3 | ND | 3.9 | ND | ND |
| Pasadena, USA | 5 June, 2010 | 23-hour (1) | 44.1 | 7.3 | 0.6 | ND | 6.3 | 2.3 | ND |
| Pasadena, USA | 6 June, 2010 | 23-hour (1) | 41.8 | 7.6 | 0.5 | ND | 6.8 | 2.3 | ND |
sum of 2-, 3-, and 4- methyl phenyl sulfate.
sum of 2-, 3-, and 4- methyl benzyl sulfate.
ND - not detected
The ambient concentrations of aromatic organosulfates were approximately an order of magnitude lower at Godavari and Pasadena, which also had substantially lower OC and EC concentrations. Prior studies have documented the contributions of motor vehicles, fossil fuel use, and biomass burning to these three study sites (Stone et al. 2010a; Stone et al. 2010b; Hayes et al. 2013). In addition to having OC loadings 6-9 times greater than Godavari and Pasadena, the coal combustion impact at Lahore was an order of magnitude greater than the other sites. Coal burning serves as a source of atmospheric sulfur and acidity, which may promote aromatic organosulfate formation. Biomass burning was suggested to be a source of aromatic organosulfates in Lahore by Kundu et al. (2013) following the correlation of benzyl sulfate with levoglucosan (R2 = 0.82), suggesting aromatic organosulfates may derive from cresols or lignin-breakdown products emitted by biomass burning (Schauer et al. 2001). Likewise, fossil fuel use is a source of aromatic VOC to the atmosphere and may be a potential precursor to organosulfates.
3.4 Mass Spectral Response of Aromatic Organosulfate Standards
Due to the lack of authentic standards for atmospherically-relevant organosulfates, researchers have used surrogate standards for semi-quantitation. For organosulfate quantification in chamber experiments and ambient aerosols, camphor-10- sulfonic acid (Iinuma et al. 2007), sodium propylsulfate (Lin et al. 2013a), sodium 2-ethylhexyl sulfate (Stone et al. 2009), sodium galactose sulfate (Stone et al. 2012), sodium octyl sulfate and sodium decyl sulfate (Kahnt et al. 2013) have been used, which have different organic and ionic functional groups. Surrogates provide only a rough approximation of organosulfate concentrations, because they cannot represent the behavior of the analytes in the MS detector.
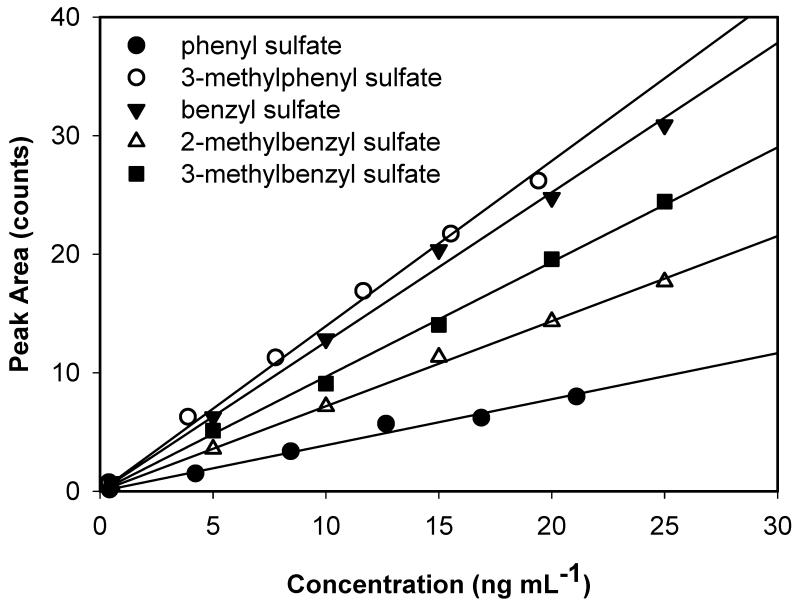
The homologous series of phenyl and benzyl sulfates synthesized in this study were used to investigate how organosulfates of similar molecular structure respond under ESI-MS conditions. Calibration curves for aromatic organosulfate standards based on peak area of molecular ions were developed over the concentration range of 1.0 – 25.0 ng mL−1 and are shown in Figure 4. The response factors (i.e. slope of the calibration curves with units of counts per ng mL−1) ranged from a low of 0.32 ± 0.03 for phenyl sulfate to 1.39 ± 0.06 for 3-methylphenyl sulfate, which differ by a factor of 4.3. Statistical analysis of least-squares linear regression parameters indicated significant differences (p = 0.01) in the response of phenyl sulfate from the four other standards. Benzyl sulfate and the structural isomers, 2- and 3-methyl benzyl sulfate did not have significantly different responses at the 95% confidence interval. These results demonstrate that significant biases may be introduced in to absolute quantitation of organosulfates when using a surrogate standard. Even when surrogate standards are structurally similar (i.e. phenyl sulfate and 3-methylphenyl sulfate), response factors may differ by more than a factor of four. Thus, future developments in detection and quantification of organosulfates should proceed in parallel with authentic standard development.
Figure 4.
Comparison of response factors of synthesized aromatic organosulfates measured by UPLC-ToF.
3.5 Toluene Chamber Samples
The photooxidation of toluene under conditions that form biogenic organosulfates (Zhang et al. 2012) did not produce detectable amounts of aromatic organosulfates. From this result, we conclude that aromatic organosulfates form under conditions and perhaps chemical pathways that are different from biogenic organosulfates. Primary combustion of biomass burning or fuels with high sulfur content are also suggested sources (Kundu et al. 2013).
Other volatile organic compound (VOC) precursors, such as styrene or polycyclic aromatic hydrocarbons (PAH), form aromatic ring-retaining SOA products (Shakya et al. 2010). Notably, in the study of SOA produced from the photooxidation of naphthalene in the presence of ammonium sulfate seed aerosol using ESI-HRMS, Kautzman et al. (2010) observed the molecular ion at m/z 172.9903 (C6H5O4S−) at 4.1 minutes which accounted for 0.5% of the signal. This peak was tentatively assigned as an isomer of hydroxybenzene sulfonic acid due to the similar MS2 spectra of an authentic standard; however the observed retention times did not match the standard (0.95 min). Instead, there is a closer match to the phenyl sulfate retention time reported in this study (at 4.9 minutes). These data support that phenyl sulfate could be produced from the photooxidation of naphthalene in the presence of sulfate aerosol. Likewise, methylphenyl sulfates could be produced from the photooxidation of methylnapthlene. Future work should examine the chemical composition of SOA produced from naphthalene and methylnapthalene in the presence of sulfate aerosol in order to evaluate the extent to which they contribute to aromatic organosulfates in the atmosphere.
4. Conclusions
Aromatic organosulfates are ubiquitous components of atmospheric aerosol, though observed at low atmospheric abundance (< 0.1 ng m−3). These molecules are unique from other organosulfates reported in the literature, in that they contain intact aromatic rings and sulfate moieties in the phenyl and benzyl positions. Through authentic standard development, we have identified the characteristic fragment ions of aromatic organosulfates, which include the sulfite radical (•SO3−, m/z 80) and the sulfate radical (•SO4−, m/z 96), but largely exclude the bisulfite anion (HSO3−, m/z 81) and bisulfate anion (HSO4−, m/z 97), which are characteristic of aliphatic organosulfates. The importance of using authentic standards for absolute quantitation has been demonstrated, while the use of surrogate standards may significantly bias quantitative results. While the sources of aromatic organosulfates in the atmosphere have not yet been fully elucidated, the toluene photooxidation experiments described herein show that they form under conditions different from biogenic organosulfates or have other VOC precursors. This work increases the breadth of understanding the composition and abundance of organosulfates in the atmosphere, and highlights the important role of authentic standard development in organosulfate identification and quantitation.
Supplementary Material
Highlights.
Homologous series of benzyl and phenyl sulfates were synthesized.
Aromatic organosulfates were identified and quantified in ambient aerosol.
Major ESI fragments of aromatic organosulfates include the sulfite and sulfate radicals.
Toluene chamber experiments did not generate aromatic organosulfates.
Figure 1.
The reaction scheme used in the synthesis of phenyl sulfate ammonium salts, via a trichloroethylester sulfate intermediate.
Acknowledgements
We thank Frank Keutsch, Ge Yu, and Jean C. Rivera at the University of Wisconsin-Madison for synthesizing benzyl and methylbenzyl sulfates, Lynn Teesch and Vic Parcell at the University of Iowa High Resolution Mass Spectrometry Facility for assistance with instrumental analysis, Patrick Hayes and Jose Jimenez for useful discussions on the estimation of PM2.5 mass during CalNex, Bidya Banmali Pradhan and Pradeep Dangol at ICIMOD for collection of samples at Godavari, Tauseef Quraishi (retired) from the University of Engineering and Technology for collection of samples in Lahore, Pakistan, and Haofei Zhang for useful discussions, and James Schauer at the University of Wisconsin-Madison for access to filter samples. Research at the University of Iowa was funded by the Office of the Vice President for Research Mathematical and Physical Sciences Program. The synthesis of the phenol sulfate standards was in part supported by NIH grants ES05605 and ES013661 from the National Institute of Environmental Health Sciences. The University of North Carolina researchers thank Alion Science and Technology (subcontract EP-D-05-065) for supporting filter collection efforts during the 2010 CalNex field campaign.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Attygalle AB, Garcia-Rubio S, Ta J, Meinwald J. Collisionally-induced dissociation mass spectra of organic sulfate anions. Journal of the Chemical Society-Perkin Transactions. 2001;2(4):498–506. [Google Scholar]
- Frossard AA, Shaw PM, Russell LM, Kroll JH, Canagaratna MR, Worsnop DR, Quinn PK, Bates TS. Springtime Arctic haze contributions of submicron organic particles from European and Asian combustion sources. Journal of Geophysical Research-Atmospheres. 2011:116. [Google Scholar]
- Guthrie JP. Hydrolysis of esters of oxy acids - pKa values for strong acids. Canadian Journal of Chemistry. 1978;56(17):2342–2354. [Google Scholar]
- Hayes PL, Ortega AM, Cubison MJ, Froyd KD, Zhao Y, Cliff SS, Hu WW, Toohey DW, Flynn JH, Lefer BL, Grossberg N, Alvarez S, Rappenglueck B, Taylor JW, Allan JD, Holloway JS, Gilman JB, Kuster WC, De Gouw JA, Massoli P, Zhang X, Liu J, Weber RJ, Corrigan AL, Russell LM, Isaacman G, Worton DR, Kreisberg NM, Goldstein AH, Thalman R, Waxman EM, Volkamer R, Lin YH, Surratt JD, Kleindienst TE, Offenberg JH, Dusanter S, Griffith S, Stevens PS, Brioude J, Angevine WM, Jimenez JL. Organic aerosol composition and sources in Pasadena, California, during the 2010 CalNex campaign. Journal of Geophysical Research-Atmospheres. 2013;118(16):9233–9257. [Google Scholar]
- Hedayatu M, Leveque JC. Action of Sulfuryl Chloride on some Phenols and Alcohols - Alkylated and Arylated Phenol Chlorosulfates. Comptes Rendus Hebdomadaires Des Seances De L Academie Des Sciences Serie C. 1971;273(21):1444. D. L. [Google Scholar]
- Iinuma Y, Boge O, Kahnt A, Herrmann H. Laboratory chamber studies on the formation of organosulfates from reactive uptake of monoterpene oxides. Physical Chemistry Chemical Physics. 2009;11(36):7985–7997. doi: 10.1039/b904025k. [DOI] [PubMed] [Google Scholar]
- Iinuma Y, Kahnt A, Mutzel A, Boge O, Herrmann H. Ozone-Driven Secondary Organic Aerosol Production Chain. Environmental Science & Technology. 2013;47(8):3639–3647. doi: 10.1021/es305156z. [DOI] [PubMed] [Google Scholar]
- Iinuma Y, Muller C, Berndt T, Boge O, Claeys M, Herrmann H. Evidence for the existence of organosulfates from beta-pinene ozonolysis in ambient secondary organic aerosol. Environmental Science & Technology. 2007;41(19):6678–6683. doi: 10.1021/es070938t. [DOI] [PubMed] [Google Scholar]
- Kahnt A, Behrouzi S, Vermeylen R, Shalamzari MS, Vercauteren J, Roekens E, Claeys M, Maenhaut W. One-year study of nitro-organic compounds and their relation to wood burning in PM10 aerosol from a rural site in Belgium. Atmospheric Environment. 2013;81:561–568. [Google Scholar]
- Kamens RM, Zhang H, Chen EH, Zhou Y, Parikh HM, Wilson RL, Galloway KE, Rosen EP. Secondary organic aerosol formation from toluene in an atmospheric hydrocarbon mixture: Water and particle seed effects. Atmospheric Environment. 2011;45(13):2324–2334. [Google Scholar]
- Kautzman KE, Surratt JD, Chan MN, Chan AWH, Hersey SP, Chhabra PS, Dalleska NF, Wennberg PO, Flagan RC, Seinfeld JH. Chemical Composition of Gas- and Aerosol-Phase Products from the Photooxidation of Naphthalene. Journal of Physical Chemistry A. 2010;114(2):913–934. doi: 10.1021/jp908530s. [DOI] [PubMed] [Google Scholar]
- Kristensen K, Glasius M. Organosulfates and oxidation products from biogenic hydrocarbons in fine aerosols from a forest in North West Europe during spring. Atmospheric Environment. 2011;45(27):4546–4556. [Google Scholar]
- Kundu S, Quraishi TA, Yu G, Suarez C, Keutsch FN, Stone EA. Evidence and quantitation of aromatic organosulfates in ambient aerosols in Lahore, Pakistan. Atmospheric Chemistry and Physics. 2013;13(9):4865–4875. [Google Scholar]
- Li XS, Parkin S, Duffel MW, Robertson LW, Lehmler HJ. An efficient approach to sulfate metabolites of polychlorinated biphenyls. Environment International. 2010;36(8):843–848. doi: 10.1016/j.envint.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P, Yu JZ, Engling G, Kalberer M. Organosulfates in Humic-like Substance Fraction Isolated from Aerosols at Seven Locations in East Asia: A Study by Ultra-High-Resolution Mass Spectrometry. Environmental Science & Technology. 2012;46(24):13118–13127. doi: 10.1021/es303570v. [DOI] [PubMed] [Google Scholar]
- Lin YH, Knipping EM, Edgerton ES, Shaw SL, Surratt JD. Investigating the influences of SO2 and NH3 levels on isoprene-derived secondary organic aerosol formation using conditional sampling approaches. Atmospheric Chemistry and Physics. 2013a;13(16):8457–8470. [Google Scholar]
- Lin YH, Zhang HF, Pye HOT, Zhang ZF, Marth WJ, Park S, Arashiro M, Cui TQ, Budisulistiorini H, Sexton KG, Vizuete W, Xie Y, Luecken DJ, Piletic IR, Edney EO, Bartolotti LJ, Gold A, Surratt JD. Epoxide as a precursor to secondary organic aerosol formation from isoprene photooxidation in the presence of nitrogen oxides. Proceedings of the National Academy of Sciences of the United States of America. 2013b;110(17):6718–6723. doi: 10.1073/pnas.1221150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lien IFF, Ruttgaizer S, Dove P, Taylor SD. Synthesis and protection of aryl sulfates using the 2,2,2-trichloroethyl moiety. Organic Letters. 2004;6(2):209–212. doi: 10.1021/ol036157o. [DOI] [PubMed] [Google Scholar]
- Mazzoleni LR, Saranjampour P, Dalbec MM, Samburova V, Hallar AG, Zielinska B, Lowenthal DH, Kohl S. Identification of water-soluble organic carbon in non-urban aerosols using ultrahigh-resolution FT-ICR mass spectrometry: organic anions. Environmental Chemistry. 2012;9(3):285–297. [Google Scholar]
- Nguyen TB, Lee PB, Updyke KM, Bones DL, Laskin J, Laskin A, Nizkorodov SA. Formation of nitrogen- and sulfur-containing light-absorbing compounds accelerated by evaporation of water from secondary organic aerosols. Journal of Geophysical Research-Atmospheres. 2012:117. [Google Scholar]
- Olson CN, Galloway MM, Yu G, Hedman CJ, Lockett MR, Yoon T, Stone EA, Smith LM, Keutsch FN. Hydroxycarboxylic Acid-Derived Organosulfates: Synthesis, Stability, and Quantification in Ambient Aerosol. Environmental Science & Technology. 2011;45(15):6468–6474. doi: 10.1021/es201039p. [DOI] [PubMed] [Google Scholar]
- Pye HOT, Pinder RW, Piletic IR, Xie Y, Capps SL, Lin Y-H, Surratt JD, Zhang Z, Gold A, Luecken DJ, Hutzell WT, Jaoui M, Offenberg JH, Kleindienst TE, Lewandowski M, Edney EO. Epoxide pathways improve model predictions of isoprene markers and reveal key role of acidity in aerosol formation. Environmental Science & Technology. 2013;47(19):11056–11064. doi: 10.1021/es402106h. [DOI] [PubMed] [Google Scholar]
- Romero F, Oehme M. Organosulfates - A new component of humic-like substances in atmospheric aerosols? Journal Of Atmospheric Chemistry. 2005;52(3):283–294. [Google Scholar]
- Ryerson TB, Andrews AE, Angevine WM, Bates TS, Brock CA, Cairns B, Cohen RC, Cooper OR, de Gouw JA, Fehsenfeld FC, Ferrare RA, Fischer ML, Flagan RC, Goldstein AH, Hair JW, Hardesty RM, Hostetler CA, Jimenez JL, Langford AO, McCauley E, McKeen SA, Molina LT, Nenes A, Oltmans SJ, Parrish DD, Pederson JR, Pierce RB, Prather K, Quinn PK, Seinfeld JH, Senff CJ, Sorooshian A, Stutz J, Surratt JD, Trainer M, Volkamer R, Williams EJ, Wofsy SC. The 2010 California Research at the Nexus of Air Quality and Climate Change (CalNex) field study. Journal of Geophysical Research-Atmospheres. 2013;118(11):5830–5866. [Google Scholar]
- Schauer JJ, Kleeman MJ, Cass GR, Simoneit BRT. Measurement of emissions from air pollution sources. 3. C-1-C-29 organic compounds from fireplace combustion of wood. Environmental Science & Technology. 2001;35(9):1716–1728. doi: 10.1021/es001331e. [DOI] [PubMed] [Google Scholar]
- Schauer JJ, Mader BT, Deminter JT, Heidemann G, Bae MS, Seinfeld JH, Flagan RC, Cary RA, Smith D, Huebert BJ, Bertram T, Howell S, Kline JT, Quinn P, Bates T, Turpin B, Lim HJ, Yu JZ, Yang H, Keywood MD. ACE-Asia intercomparison of a thermal-optical method for the determination of particle-phase organic and elemental carbon. Environmental Science & Technology. 2003;37(5):993–1001. doi: 10.1021/es020622f. [DOI] [PubMed] [Google Scholar]
- Shakya KM, Griffin RJ. Secondary Organic Aerosol from Photooxidation of Polycyclic Aromatic Hydrocarbons. Environmental Science & Technology. 2010;44(21):8134–8139. doi: 10.1021/es1019417. [DOI] [PubMed] [Google Scholar]
- Stone E, Schauer J, Quraishi TA, Mahmood A. Chemical characterization and source apportionment of fine and coarse particulate matter in Lahore, Pakistan. Atmospheric Environment. 2010a;44(8):1062–1070. [Google Scholar]
- Stone EA, Hedman CJ, Sheesley RJ, Shafer MM, Schauer JJ. Investigating the chemical nature of humic-like substances (HULIS) in North American atmospheric aerosols by liquid chromatography tandem mass spectrometry. Atmospheric Environment. 2009;43(27):4205–4213. [Google Scholar]
- Stone EA, Schauer JJ, Pradhan BB, Dangol PM, Habib G, Venkataraman C, Ramanathan V. Characterization of emissions from South Asian biofuels and application to source apportionment of carbonaceous aerosol in the Himalayas. Journal of Geophysical Research-Atmospheres. 2010b:115. [Google Scholar]
- Stone EA, Yang L, Yu LE, Rupakheti M. Characterization of organosulfates in atmospheric aerosols at Four Asian locations. Atmospheric Environment. 2012;47:323–329. [Google Scholar]
- Surratt JD, Gomez-Gonzalez Y, Chan AWH, Vermeylen R, Shahgholi M, Kleindienst TE, Edney EO, Offenberg JH, Lewandowski M, Jaoui M, Maenhaut W, Claeys M, Flagan RC, Seinfeld JH. Organosulfate formation in biogenic secondary organic aerosol. Journal of Physical Chemistry A. 2008;112(36):8345–8378. doi: 10.1021/jp802310p. [DOI] [PubMed] [Google Scholar]
- Surratt JD, Kroll JH, Kleindienst TE, Edney EO, Claeys M, Sorooshian A, Ng NL, Offenberg JH, Lewandowski M, Jaoui M, Flagan RC, Seinfeld JH. Evidence for organosulfates in secondary organic aerosol. Environmental Science & Technology. 2007;41(2):517–527. doi: 10.1021/es062081q. [DOI] [PubMed] [Google Scholar]
- Tolocka MP, Turpin B. Contribution of Organosulfur Compounds to Organic Aerosol Mass. Environmental Science & Technology. 2012;46(15):7978–7983. doi: 10.1021/es300651v. [DOI] [PubMed] [Google Scholar]
- Zhang H, Lin Y-H, Zhang Z, Zhang X, Shaw SL, Knipping EM, Weber RJ, Gold A, Kamens RM, Surratt JD. Secondary organic aerosol formation from methacrolein photooxidation: roles of NOx level, relative humidity and aerosol acidity. Environmental Chemistry. 2012;9(3):247–262. [Google Scholar]
- Zhang H, Surratt JD, Lin YH, Bapat J, Kamens RM. Effect of relative humidity on SOA formation from isoprene/NO photooxidation: enhancement of 2-methylglyceric acid and its corresponding oligoesters under dry conditions. Atmospheric Chemistry and Physics. 2011;11(13):6411–6424. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.