Abstract
Activation of the phosphoinositide (PI) cycle generates the second messengers that control various aspects of cellular signaling. We have previously shown that two PI cycle enzymes, type II phosphatidylinositol 5-phosphate 4-kinase (PIPK IIα) and phosphoinositide 3-kinase (PI3K), are activated through light stimulation. In our earlier studies, we measured enzyme activities, instead of directly measuring the products, due to lack of sensitive analytical techniques. Cells have very low levels of PIs, compared to other lipids, so special techniques and sensitive analytical instruments are necessary for their identification and quantification. There are also other considerations, such as different responses in different cell types, which may complicate quantification of PIs. For example, although light activated PIPK IIα, there was no increase in PI-4,5-P2 measured by liquid chromatography–mass spectrometry (LC/MS) This discrepancy is due to the heterogeneous nature of the retina, which is composed of various cell types. In this study, we examined PI generation in situ using immunohistochemistry with specific PI antibodies. PIs were generated in specific retinal cell layers, suggesting that analyzing PIs from the total retina by LC/MS underscores the significance. This suggests that PI-specific antibodies are useful tools to study the cell-specific regulation of PIs in the retina.
Phosphatidylinositol, a component of phospholipid in the cell membrane, contains a D-myo-inositol head group, a glycerol backbone, and two fatty acids at the C1 and C2 acyl positions of glycerol1,3. Phosphorylation of multiple free hydroxyls in the inositol head group generates several phosphorylated PI derivatives. Differential phosphorylation at the 3-, 4-, and 5-positions allows for the generation of seven distinct phosphoinositides1,2. Activation of the phosphoinositide (PI) cycle generates the second messengers that control various aspects of cellular signaling3,4. The intracellular levels of phosphoinositides are controlled by PI-specific kinases and phosphatases that can rapidly convert one phosphoinositide into another2. PI signals regulate signal transduction, cytoskeletal assembly, membrane binding, and fusion that is spatially restricted to specific membrane domains2. The formation of all seven phosphoinositides has been demonstrated in mammalian cells5. We have also shown the formation of all PIs in intact photoreceptor outer segment membranes (ROS) prepared from fresh bovine retinas6,7,8,9.
Studies from our laboratory have shown that the retina and ROS have an active PI metabolism. Biochemical studies revealed that the ROS contain the enzymes necessary for phosphorylation of phosphoinositides. We showed that light stimulates various components of the PI cycle in the vertebrate ROS, including diacylglycerol kinase, PI synthetase, phosphatidylinositol phosphate kinase, phospholipase C, and phosphoinositide 3-kinase (PI3K)6,8,10,11,12.
We previously reported the light activated type II phosphatidylinositol 5-phosphate 4-kinase (PIPK IIα, which generates PI-4,5-P2) and class IA phosphoinositide 3-kinase (PI3K, which generates PI-3-P, PI-3,4-P2 and PI-3,4,5-P3) in the retina12,13. In these experiments, we measured enzyme activities, instead of measuring product formation. Phosphoinositides are minor components cells. Sensitive techniques are needed to determine the PI species and quantity in the cell. Liquid chromatography–mass spectrometry (LC/MS) has been shown to identify and quantify various species of PIs14. One of the unanswered questions from our earlier studies is the in vivo level of PIPK IIα and PI3K-generated phosphoinositides that form in response to light.
In the current study, we measured the PIP2 levels in dark- and light-adapted retinas by LC/MS and found no difference between dark- and light-adapted conditions. This contradicts our earlier observation on the light-induced activation of PIPK II α13. However, when we examined the generation of PI-4,5-P2 by immunohistochemistry in situ, we found increased PI-4,5-P2 in the rod outer segment membranes of light-adapted retina. We also examined other PIs and their generation in gene knockout mouse models using PI-specific antibodies. The LC/MS technique is sensitive and can determine the PI levels in relatively homogenous tissues, such as the heart, liver, kidney, lungs, and spleen. The retina is a highly organized structure made up of seven layers of heterogeneous cells. Each retinal cell type has its unique function. It is important to determine in which cell type the PIs are generated in response to light. Our studies suggest that PI-specific antibodies are useful tools to study the cell-specific regulation of PIs in the retina.
Methods
Materials
Purified mouse monoclonal anti-PI-3,4-P2, anti-PI-3,4,5-P3, anti-PI-4,5-P2, anti-PI-3-P and polyclonal anti-hVps34 (class III PI3K) antibodies were obtained from Echelon Biosciences, Inc. (Salt Lake City, UT). Polyclonal anti-transducin alpha (Tα) antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). DAPI stain used for nuclear staining and secondary antibodies were purchased from Invitrogen-Molecular Probes (Carlsbad, CA). Monoclonal anti-arrestin antibody was a kind gift from Dr. Paul Hargrave (University of Florida, Gainesville). All other reagents used for buffer preparations were of analytical grade and purchased from Sigma (St. Louis, MO).
Animals
All animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the NIH Guide for the Care and Use of Laboratory Animals. The protocols were approved by the IACUC at the University of Oklahoma Health Sciences Center and Dean McGee Eye Institute. Animals were born and raised in our vivarium and kept under dim cyclic light (40–60 lux, 12 h light/dark cycle). Photoreceptor-specific conditional insulin receptor knockout mice15 were born in the animal facility in 60-lux cyclic light (12 h on/off) and maintained under these lighting conditions until they were used in an experiment. The Nrl-/- mice were kindly provided by Dr. Anand Swaroop (NIH, Bethesda, MD). The Rpe65-/- mice were kindly provided by Dr. Jing-Xing Ma (University of Oklahoma Health Sciences Center, Oklahoma City). For experiments that required enucleating the eye or removing the retina, mice were killed by asphyxiation with CO2 followed by cervical dislocation. On the day of an experiment, animals were dark-adapted overnight and the next day morning around 9.00 AM, half of the animals were exposed to normal fluorescent room light (300 lux) for 30 min11 before the retinas were being subjected to either biochemistry or for an immunohistochemistry.
Preparation of tissue for paraffin sectioning using Prefer as a fixative
Mice were euthanized by CO2 asphyxiation and the eyeballs were placed in Prefer solution (Anatech Ltd, Battle Creek, MI) for 15 min at room temperature followed by 70% ethanol overnight. The tissue was paraffin-embedded and 5 μm thick sections were cut and mounted onto slides. Sections were deparaffinized in 2–3 changes of xylene (10 minutes each) and hydrated in 2 changes of 100% ethanol for 3 minutes each, 95% and 80% ethanol for 1 minute each, and then rinsed in distilled water. The slides were then subjected to antigen retrieval, boiled in 10 mM sodium citrate buffer pH 6.0, then in sub-boiling temperature for 10 min, and cooled down for 30 min. The slides were washed three times in 1X PBS containing 0.1% Triton-X 100, blocked with horse serum for 1 h, and primary antibody was added overnight at 4°C. For fluorescent detection, slides were incubated with a mixture of Texas-red-anti-mouse and FITC-anti-rabbit antibodies (Vector Laboratories, Burlingame, CA), each diluted 1:200 in PBS with 10% horse serum. Following incubation for 1 h at room temperature, the slides were washed with PBS and cover-slipped in 50% glycerol in PBS. Antibody-labeled complexes were examined on a Nikon Eclipse E800 microscope equipped with a digital camera. Images were captured using Metamorph (Universal Imaging, West Chester, PA) image analysis software. All images were captured using identical microscope and camera settings.
Quantitation of Polyphosphoinositides in the retina
Polyphosphoinositides were extracted using a modified Bligh-Dyer extraction16 and were derivatized using trimethylsilyl diazomethane as described17. Polyphosphoinositides were measured as their TMS-diazomethane derivatives using a Shimadzu UFLC equipped with a Vydac 214MS C4, 5 m, 4.66250 mm column, coupled with an ABI 4000-Qtrap hybrid linear ion trap triple quadrupole mass spectrometer in multiple reaction monitoring (MRM) mode. 17:0–20:4 PI-4,5-P2 was used as the internal standard.
Statistical analysis
One-way ANOVA and post-hoc statistical analysis using Bonferroni's pairwise comparisons were used to determine statistical significance (p<0.05).
Results
Light-dependent generation of PI-4,5-P2 (PIP2) in rod outer segment (ROS) membranes
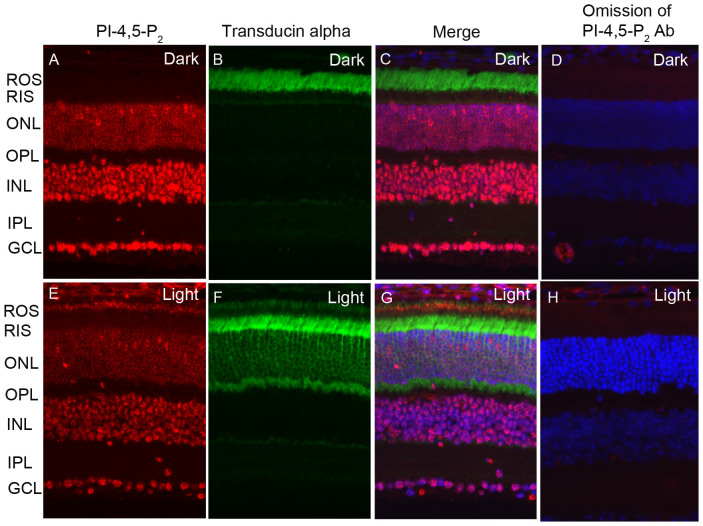
PIPKIIα catalyzes the synthesis of PI-4,5-P2. We previously reported significantly higher levels of PIPKIIα enzyme activity associated with ROS membranes prepared from light-adapted rats13. However, the endogenous PI-4,5-P2 lipid synthesis in retina in situ has never been reported. Retinal sections from dark- and light-adapted (300 lux for 30 min) mice were subjected to immunohistochemistry with PI-4,5-P2 and rod transducin antibodies. The adaptability of animals to dark and light conditions was examined with transducin immunolocalization. In dark-adapted retinas, transducin is localized to the rod outer segments (ROS; Fig. 1B). Upon light illumination, transducin is translocated to rod inner segment (RIS) and the outer plexiform layer (Fig. 1F). Immunolocalization studies suggest a strong PI-4,5-P2 immunoreactivity observed in light-adapted ROS (Fig. 1E, G), but not in dark-adapted ROS (Fig. 1A, C). The PI-4,5-P2 immunoreactivity was also observed in the outer nuclear layer (ONL), inner nuclear layer (INL), and ganglion cell layer (GCL). However, the localization was independent of either dark- or light-adaptation. This experiment suggests that PI-4-5-P2 generation in the ROS is light-dependent.
Figure 1. Immunofluorescence analysis of PI-4,5-P2 in mouse retina.
Prefer-fixed sections of dark- (A–D) and light-adapted (E–H) mouse retinas were stained for PI-4,5-P2 (A, E), transducin alpha (B, F), and DAPI (C, G). Immunofluorescence was analyzed by epifluorescence. Panels C and G represent the merged images of PI-4,5-P2 and transducin alpha. Panels D and H represent the omission of PI-4,5-P2 and transducin alpha antibodies. ROS, rod outer segments; RIS, rod inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
Light-dependent generation of PI-3-P in outer nuclear layer of rod photoreceptor cells
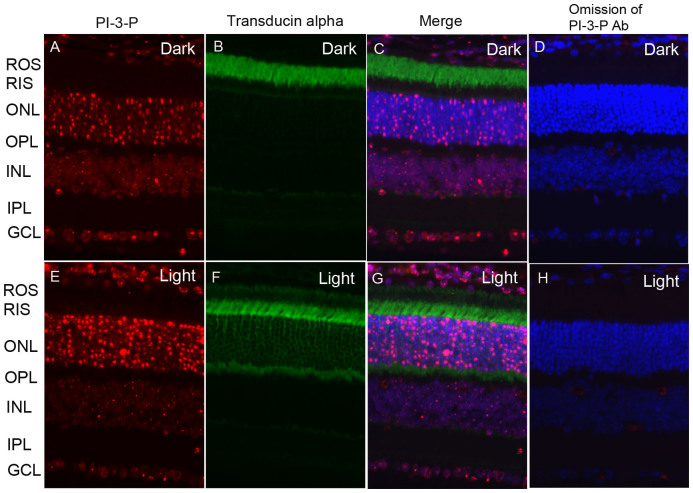
We previously reported a light-dependent activation of PI3K in the retina as well as in isolated outer segment membranes8,11,12. However, in these studies, we measured only the enzyme activity using exogenous substrates, not the actual PI3K-generated products. Retinal sections from dark- and light-adapted (300 lux for 30 min) mice were subjected to immunohistochemistry with PI-3-P and rod transducin antibodies. Immunolocalization studies suggest a strong PI-3-P immunoreactivity observed in the outer nuclear layer of rod photoreceptor cells from light-adapted mice (Fig. 2E, G) compared with dark- adapted mice (Fig. 2A, C). We also found PI-3-P in the INL layer and GCL. However, the localization was independent of either dark- or light-adaptation. This experiment suggests that light enhanced the generation of PI-3-P in the rod photoreceptor cells.
Figure 2. Immunofluorescence analysis of PI-3-P in mouse retina.
Prefer-fixed sections of dark- (A–D) and light-adapted (E–H) mouse retinas were stained for PI-3-P (A, E), transducin alpha (B, F), and DAPI (C, G). Immunofluorescence was analyzed by epifluorescence. Panels C and G represent the merged images of PI-3-P and transducin alpha. Panels D and H represent the omission of PI-3-P antibody. ROS, rod outer segments; RIS, rod inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
Light-dependent generation of PI-4,5-P2 and PI-3-P is signaled through the photoactivation of G-protein coupled receptor rhodopsin
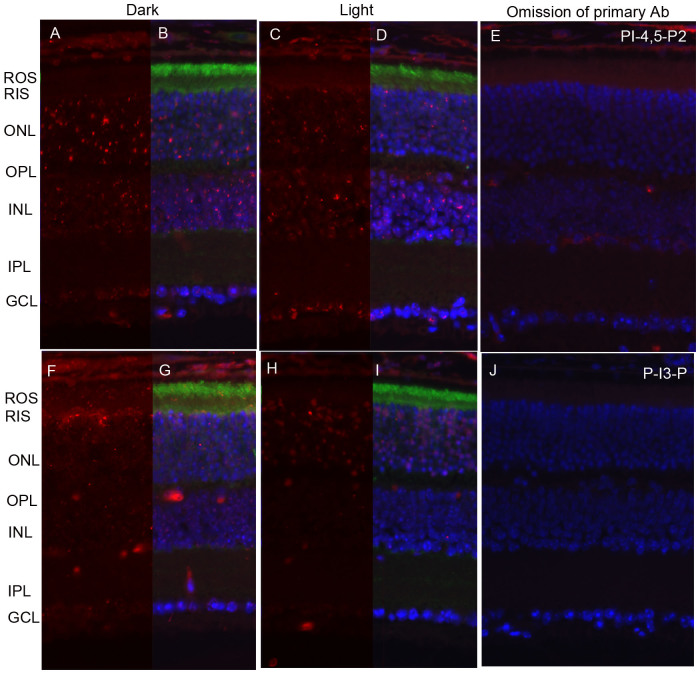
To determine whether the light-induced generation of PI-4,5-P2 and PI-3-P generation is signaled through bleachable rhodopsin, we examined the generation of PI-4,5-P2 and PI-3-P in retinal sections of dark- and light-adapted retinal pigment epithelium 65 knockout (Rpe65) mice. Rpe65-/- mice have opsin in their rod outer segments, but do not form photobleachable rhodopsin due to the absence of regeneration of chromophore 11-cis-retinal18. No light-dependent generation of PI-4,5-P2 (Fig. 3A–D) or PI-3-P (Fig. 3 F–I) was found in Rpe65-/- mouse retinas. This experiment suggests that activation of the enzyme responsible for the generation of PI-4,5-P2, PIPKIIα13, and the enzyme responsible for the generation of PI-3-P, the class IA phosphoinositide 3-kinase12, is controlled by photoactivation of rhodopsin in photoreceptor cells.
Figure 3. Generation of PI-4,5-P2 and PI-3-P is under the control G-protein coupled receptor rhodopsin activation.
Prefer-fixed sections of dark- (A–E) and light-adapted (F–J) Rpe65-/- mouse retinas were stained for PI-4,5-P2 (A, C), PI-3-P (F, H), transducin alpha (B, D, G, I), and DAPI (B, D, G, I). Immunofluorescence was analyzed by epifluorescence. Panels B, D, G, and I represent the merged images of either PI-4,5-P2 or PI-3-P with transducin alpha. Panels E and J represent the omission of primary antibodies. ROS, rod outer segments; RIS, rod inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
Light-dependent generation of PI-3-P is signaled through the insulin receptor in photoreceptors
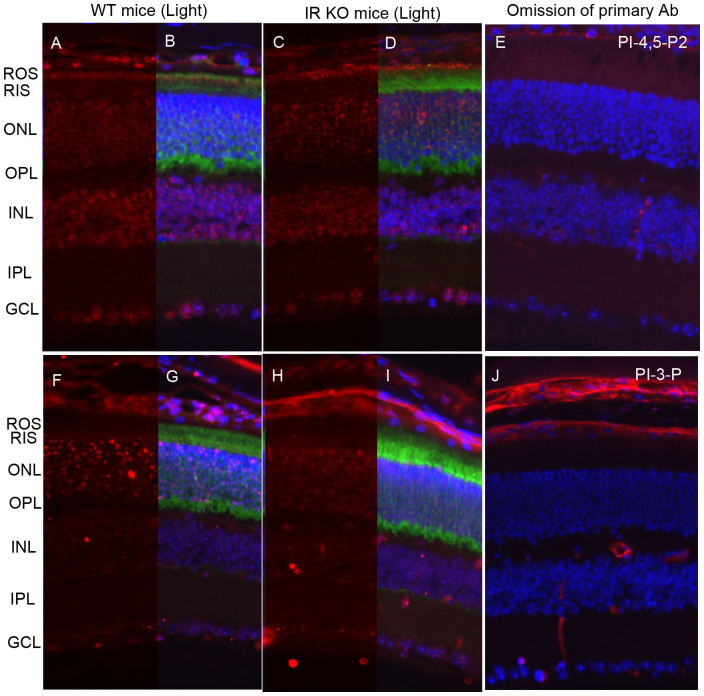
We previously reported an increased association of PI3K with the insulin receptor in light-adapted ROS membranes compared with dark-adapted ROS membranes12. To further validate our earlier observations, we examined the generation of PI-4,5-P2 and PI-3-P in retinal sections of light-adapted wild type and conditional rod photoreceptor-specific insulin receptor knockout mice15. No PI-3-P generation was found in the ONL of photoreceptors in the insulin receptor knockout mice compared with wild type mice (Fig. 4 H, I vs Fig. 4 F, G). These studies further confirm our earlier findings that the insulin receptor regulates PI3K activity in vivo. The generation of PI-4-5-P2 in the rod outer segment membranes is almost identical in wild type and insulin receptor knockout mouse retinas (Fig. 4 A–D), further attesting to the specificity of light-dependent IR/PI3K-mediated generation of PI-3-P in photoreceptor cells.
Figure 4. Generation of PI-3-P is under the control of insulin receptor activation.
Prefer-fixed sections of light-adapted wild type (A, B, F, G) and IR KO (C, D, H, I) mouse retinas were stained for PI-4,5-P2 (A–D), PI-3-P (F–I), transducin alpha, and DAPI (B, D, G, J). Immunofluorescence was analyzed by epifluorescence. Panels B, D, G, and I represent the merged images of either PI-4,5-P2 or PI-3-P with transducin alpha. Panels E and J represent the omission of primary antibodies. ROS, rod outer segments; RIS, rod inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
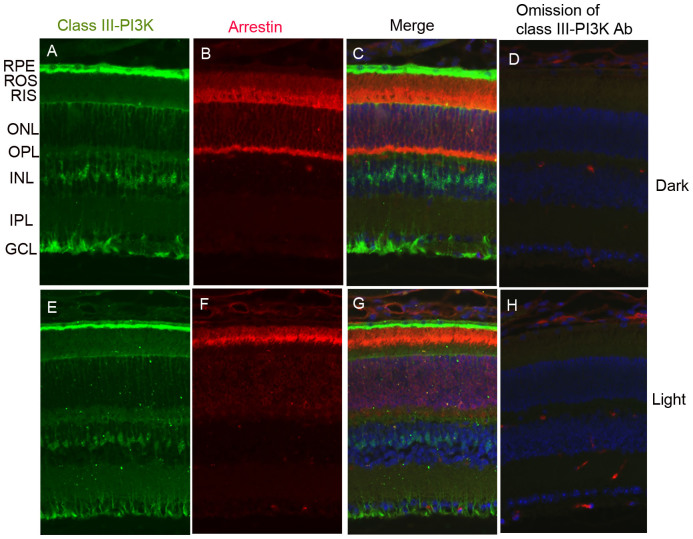
Localization of class III PI3K in the retina
PI-3-P can be generated by class IA/B, class II, and class III PI3K enzymes1. Class III enzymes will phosphorylate only PI to produce PI-3-P1. We examined the localization of class III PI3K in retinal sections from dark- and light-adapted mice. The adaptability of animals to dark and light conditions was observed with arrestin immunolocalization. In dark-adapted retinas, arrestin is localized to the rod inner segments and the outer plexiform layer (Fig. 5B). Upon light illumination, arrestin translocates to photoreceptor outer segments (Fig. 5F). Our immunohistochemical data suggest that class III PI3K is predominantly localized to retinal pigment epithelium and inner retinal layers, especially Müller cells, irrespective of dark or light adaptation (Fig. 5A, C, E, G). The class III PI3K immunoreactivity was absent in the photoreceptor layer (Fig. 5A and E). This experiment also suggests that light-dependent generation of PI-3-P as we observed in the ONL (Fig. 2) may be by class IA/B PI3K.
Figure 5. Immunofluorescence analysis of class III PI3K in mouse retina.
Prefer-fixed sections of dark- (A–D) and light-adapted (E–H) mouse retinas were stained for class III PI3K (A, E), arrestin (B, F), and DAPI (C, G). Immunofluorescence was analyzed by epifluorescence. Panels C and G represent the merged images of class III PI3K and arrestin. Panels D and H represent the omission of class III PI3K antibody. RPE, retinal pigment epithelium; ROS, rod outer segments; RIS, rod inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
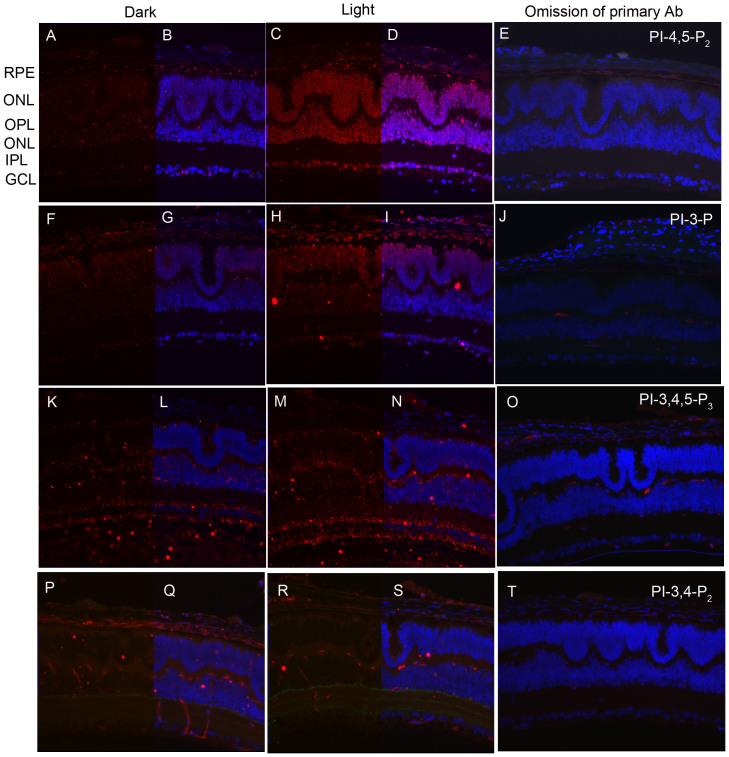
Light-dependent generation of phosphorylated phosphoinositides in cone-dominant retina
The data presented in Figures 1 through 5 were obtained from either wild type or knockout mouse retinas, which are rod-dominant (>95% rods and 3–5% cones). Although cones constitute a small percent of retinal photoreceptors in humans and rodents19,20, they are essential for optimal visual acuity, color vision, and visual perception under moderate to high light intensities in humans. To determine the light-dependent generation of phosphoinositides in cones, we used neural retina leucine zipper (Nrl) knockout mice21. Mice lacking the transcription factor Nrl experience a block in the differentiation of rod precursor cells, resulting in retinas containing a single class of photoreceptors that are indistinguishable from authentic cones21,22,23,24. The Nrl-/- retina is characterized by large undulations of the outer nuclear layer (ONL), commonly known as rosettes. These arise due to defects in the outer limiting membrane and delayed maturation of a subset of photoreceptors25. We stained the retinal sections from dark- and light-adapted Nrl-/- mice with antibodies against PI-4,5-P2, PI-3-P, PI-3,4,5-P3, and PI-3,4-P2. The results showed a light-dependent generation of PI-4,5-P2 (Fig. 6 C,D) and PI-3-P (Fig. 6 H,I), in Nrl-/- mouse retinas, but no difference in PI-3,4,5-P3 (Fig. 6 K, N) and PI-3,4-P2 generation between dark- and light-adaptation (Fig. 6 P,S). These experiments suggest that, similar to rods, cones have an active light-dependent PI generating system through a light-dependent activation of respective enzymes of the PI cycle (PIPK IIα- and PI3-kinases).
Figure 6. Immunofluorescence analysis of PI-4,5-P2, PI-3-P, PI-3,4,5-P3 and PI-3,4-P2 in cone dominant retina.
Prefer-fixed sections of dark- (A, F, K, P) and light-adapted (C, H, M, R) Nrl-/- mouse retinas were stained for PI-4,5-P2 (A, C), PI-3-P (F, H), PI-3,4,5-P3 (K, M), PI-3,4-P2 (P,R), and DAPI (B, D, G, I, L, N, Q, S). Immunofluorescence was analyzed by epifluorescence. Panels E, J, O, and T represent the omission of primary PI antibodies. RPE, retinal pigment epithelium; ROS, rod outer segments; RIS, rod inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
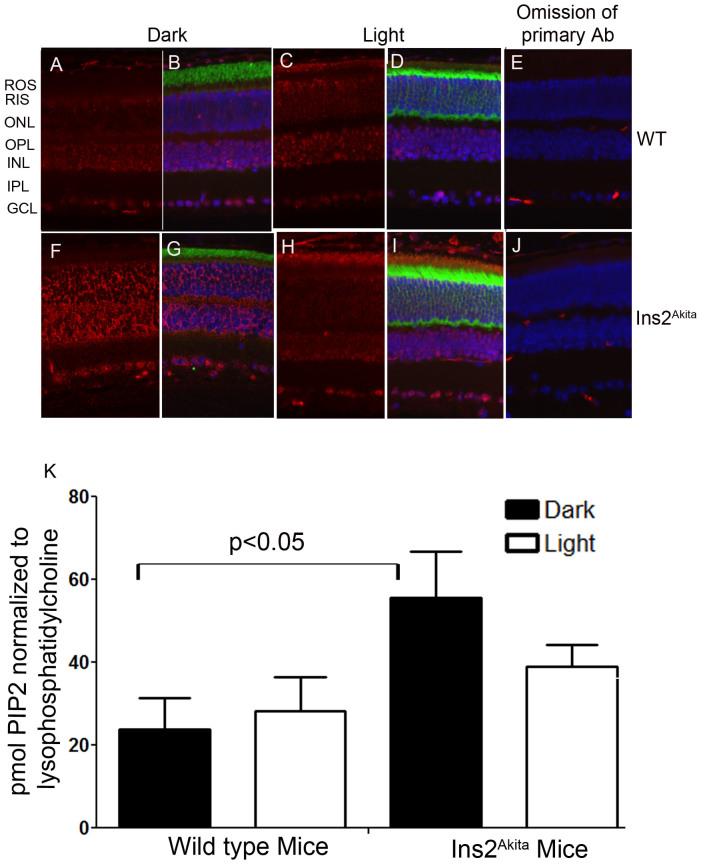
Increased generation of PI-4,5-P2 in dark-adapted type 1 diabetic Ins2Akita mouse retinas
In this experiment, we examined the generation of PI-4,5-P2 in retinal sections from dark- and light-adapted Ins2Akita mice. The Ins2Akita mutation results in a single amino acid substitution in the insulin 2 gene that causes misfolding of the insulin protein26. As early as 4 weeks of age, male mice heterozygous for this mutation show progressive loss of β-cell function, decreased pancreatic β-cell density, and significant hyperglycemia26. Immunolocalization studies suggest a strong PI-4,5-P2 immunoreactivity observed in ROS of light-adapted Ins2Akita mice (Fig. 7 H, I) compared with light-adapted wild type mice (Fig. 7 C, D). We also found an increased PI-4,5-P2 immunoreactivity in the outer nuclear layer, inner nuclear layer, and ganglion cell layers in dark-adapted Ins2Akita mouse retinas (Fig. 7 F, G) compared with dark-adapted wild type mouse retinas (Fig. 7A, B). The PIP2 levels were measured from dark- and light-adapted wild type and Ins2Akita mouse retinas as their TMZ-diazomethane derivatives17 using a Schimadzu UFLC equipped with a Vydac 214MS C4, 5µ, 4.6 × 250 mm column, coupled with an ABI 4000-Qtrap hybrid linear ion trap triple quadrupole LC-mass spectrometer in multiple reaction monitoring (MRM) mode14. In these studies, we measured lysophosphatidylcholine (lysoPC) to normalize the data (as the amount of retina material was too small to weigh or to do a lipid phosphate assay on) as this lipid level was not changed during light/dark conditions. Our data suggest an increased level of PIP2 formed in dark-adapted Akita mouse retinas compared with light-adapted conditions, further confirming our immunohistochemistry data (Fig. 7K). Our data also suggest that there was no difference in PIP2 levels between dark- and light-adapted wild type mouse retinas (Fig. 7K). It is interesting to note that even though there was an increase of PI-4,5-P2 immunoreactivity in the ROS layer of light-adapted wild type mice (Fig. 1E), we did not observe a significant difference when we subjected the total retinas to LC/MS analysis (Fig. 7K).
Figure 7. Immunofluorescence analysis of PI-4,5-P2 in type 1 diabetic Ins2Akita mouse retina.
Prefer-fixed sections of dark- (A–E) and light-adapted (F–J) wild type (A–E) and type 1 diabetic Ins2Akita (F–J) mouse retinas were stained for PI-4,5-P2 (A, C, F, H), transducin alpha, and DAPI (B, D, G, I). Immunofluorescence was analyzed by epifluorescence. Panels B, D, G, and I represent the merged images of PI-4,5-P2 and transducin alpha. Panels E and J represent the omission of PI-4,5-P2 antibody. ROS, rod outer segments; RIS, rod inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Quantification of PIP2 levels in the whole retina from dark- and light-adapted wild type and Ins2Akita mice. Polyphosphoinositides were extracted from the retina and derivatized using trimethylsilyl diazomethane, then were measured using mass spectrometry. Data are mean ± SEM, n = 6. The PIP2 levels were normalized to lysophosphatidylcholine. The significance between dark-adapted WT and type diabetic Ins2Akita shows p<0.05.
Discussion
Over the past two decades, we and others have shown that light stimulates various components of the PI cycle in the vertebrate retina6,7,11,27,28,29,30,31. We reported earlier that the activation of PIPK IIα and the enzyme class IA PI3K are light-dependent11,13. In our earlier studies, we indirectly measured the PIPK IIα and class IA PI3K activites in the presence of added exogenous PIPK IIα substrate PI-5-P and PI3K-substrate PI-4,5-P2, and quantified the radiolabelled phosphorylated products PI-4,5-P2 and PI-3,4,5-P311,12,13,15. Fluorescently-tagged protein domains (e.g., PH domains) have been used by a number of independent laboratories as phosphoinositide sensors to detect PIs in vivo32,33,34,35,36. We have also applied this approach to an in vivo model of transgenic Xenopus laevis to demonstrate the light-dependent generation of PIs specifically in photoreceptor cells, using the Akt1-AH domain as the cellular probe37. Identification of these phosphoinositide species in response to light in vivo would further advance our understanding of the role of individual PIs in the regulation of specific phospholipid-binding proteins.
One of the unanswered questions from our previous studies was whether the in vivo levels of PIPK IIα and PI3K-generated phosphoinositides increased in response to light. LC/MS is a sensitive analytical chemistry technique that has been used for the identification and quantification of less abundant phosphoinositides14. This technique has limitations, however, in its ability to analyze PI levels in heterogeneous areas, such as the retina. The retina is a highly organized structure made up of seven layers of cells. Seven types of neural cells make up the retina: two kinds of photoreceptor cells (rods and cones), retinal pigment epithelial cells (RPE), bipolar cells, amacrine cells, horizontal cells, and ganglion cells. Each retinal cell type has its unique function. It is desirable to know in which cell type the PIs are generated in response to light. LC/MS can measure PIP, PIP2, and PIP3. However, it cannot discriminate between the different positional enantiomers of PIP (PI-3-P, -4-P, and -5-P) and PIP2 (PI-4,5-P2 and PI-3,4-P2). This is because mass spectrometry measures ion masses, and the masses of the ions we measure for these lipids are the same. Treating the samples with a specific PI-phosphatase followed by measuring their levels via LC/MS would allow us to identify the specific PI.
When we measured PIP2 levels in the total retinas of dark and light-adapted animals, we did not observe any significant increase in the level of this lipid from light-adapted retinas compared with dark-adapted retinas, despite our previous finding of increased activity of PIPK IIα in rod outer segments isolated from light-adapted rats13. However, we did observe increased levels of PIP2 in dark-adapted type I diabetic Ins2Akita mouse retinas compared with light-adapted retinas. To understand the discrepancy between our biochemical and analytical results, we examined the generation of various phosphoinositides in situ employing immunohistochemistry with specific PI antibodies. We observed PI-4,5-P2 immunoreactivity in the retinal ONL, INL, and GCL in both dark- and light-adapted conditions. Rod outer segments also displayed PI-4,5-P2 immunoreactivity, but only under light-adapted condtions. This finding further confirms our earlier studies on the activation of PIPK IIα in ROS membranes isolated from light-adapted animals13. The LC/MS data from the total retina shows a marginal increase in the level of PIP2, because the ROS generated pool of PI-4,5-P2 is small compared with PI-4,5-P2 in other layers under both conditions. With a significant elevation of PI-4,5-P2, observed in dark-adapted type I Ins2Akita mice, we clearly detected that difference by LC/MS (Fig. 7).
Another technical limitation is that the LC/MS cannot differentiate between PI-4,5-P2 and PI-3,4-P2. Our studies suggest that immunohistochemistry coupled with LC/MS would be an ideal way to determine the levels and location of PIs in the retina. PI-specific antibodies have been previously used to demonstrate multiple, distinct cellular pools of PIs in mammalian cells38. The immunohistochemical analysis also indicates the generation of PI-4,5-P2 and PI-3-P. Presumably, the enzymes are controlled by GPCR rhodopsin activation. Collectively, our studies suggest that PI-specific antibodies are useful tools to study the cell-specific regulation of PIs in the retina.
Author Contributions
R.R. and R.E.A. designed research. R.R., A.R. and A.J.M. performed the research; R.R., A.R. and A.J.M. analyzed the data; and R.R. wrote the paper.
Acknowledgments
This study was supported by grants from the National Institutes of Health (EY016507, EY00871, and NEI Core grant EY12190); and an unrestricted grant from Research to Prevent Blindness, Inc., to the department of Ophthalmology. The authors also thank the NCRR COBRE grant (8P20GM103527-05) that provided partial support for these studies.
References
- Fruman D. A., Meyers R. E. & Cantley L. C. Phosphoinositide kinases. Annu. Rev. Biochem. 67, 481–507 (1998). [DOI] [PubMed] [Google Scholar]
- Martin T. F. Phosphoinositide lipids as signaling molecules: common themes for signal transduction, cytoskeletal regulation, and membrane trafficking. Annu. Rev. Cell Dev. Biol. 14, 231–264 (1998). [DOI] [PubMed] [Google Scholar]
- Balla T. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol Rev. 93, 1019–1137 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J. & Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature 306, 67–69 (1983). [DOI] [PubMed] [Google Scholar]
- Rameh L. E. & Cantley L. C. The role of phosphoinositide 3-kinase lipid products in cell function. J. Biol. Chem. 274, 8347–8350 (1999). [DOI] [PubMed] [Google Scholar]
- Ghalayini A. J. & Anderson R. E. Light adaptation of bovine retinas in situ stimulates phosphatidylinositol synthesis in rod outer segments in vitro. Curr. Eye Res. 14, 1025–1029 (1995). [DOI] [PubMed] [Google Scholar]
- Huang Z., Ghalayini A., Guo X. X., Alvarez K. M. & Anderson R. E. Light-mediated activation of diacylglycerol kinase in rat and bovine rod outer segments. J. Neurochem. 75, 355–362 (2000). [DOI] [PubMed] [Google Scholar]
- Guo X., Ghalayini A. J., Chen H. & Anderson R. E. Phosphatidylinositol 3-kinase in bovine photoreceptor rod outer segments. Invest Ophthalmol. Vis. Sci. 38, 1873–1882 (1997). [PubMed] [Google Scholar]
- Guo X. X., Huang Z., Bell M. W., Chen H. & Anderson R. E. Tyrosine phosphorylation is involved in phosphatidylinositol 3-kinase activation in bovine rod outer segments. Mol. Vis. 6, 216–221 (2000). [PubMed] [Google Scholar]
- Ghalayini A. J., Guo X. X., Koutz C. A. & Anderson R. E. Light stimulates tyrosine phosphorylation of rat rod outer segments In vivo. Exp. Eye Res. 66, 817–821 (1998). [DOI] [PubMed] [Google Scholar]
- Rajala R. V., McClellan M. E., Ash J. D. & Anderson R. E. In vivo regulation of phosphoinositide 3-kinase in retina through light-induced tyrosine phosphorylation of the insulin receptor beta-subunit. J. Biol. Chem. 277, 43319–43326 (2002). [DOI] [PubMed] [Google Scholar]
- Rajala A. et al. G-protein-coupled Receptor Rhodopsin Regulates the Phosphorylation of Retinal Insulin Receptor. J. Biol. Chem. 282, 9865–9873 (2007). [DOI] [PubMed] [Google Scholar]
- Huang Z., Anderson R. E., Cao W., Wiechmann A. F. & Rajala R. V. Light-Induced Tyrosine Phosphorylation of Rod Outer Segment Membrane Proteins Regulate the Translocation, Membrane Binding and Activation of Type II alpha Phosphatidylinositol-5-Phosphate 4-Kinase. Neurochem. Res. 36, 627–635 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z. et al. PIPKIgamma regulates focal adhesion dynamics and colon cancer cell invasion. PLoS. One. 6, e24775 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala A., Tanito M., Le Y. Z., Kahn C. R. & Rajala R. V. Loss of neuroprotective survival signal in mice lacking insulin receptor gene in rod photoreceptor cells. J. Biol. Chem. 283, 19781–19792 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeyman T. W., Strohsnitter W., Scheid C. R. & Schimmel R. J. Phosphatidic acid and phosphatidylinositol labelling in adipose tissue. Relationship to the metabolic effects of insulin and insulin-like agents. Biochem. J. 212, 489–498 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. et al. Quantification of PtdInsP3 molecular species in cells and tissues by mass spectrometry. Nat. Methods 8, 267–272 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond T. M. et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat. Genet. 20, 344–351 (1998). [DOI] [PubMed] [Google Scholar]
- Carter-Dawson L. D. & LaVail M. M. Rods and cones in the mouse retina. II. Autoradiographic analysis of cell generation using tritiated thymidine. J. Comp Neurol. 188, 263–272 (1979). [DOI] [PubMed] [Google Scholar]
- Carter-Dawson L. D. & LaVail M. M. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J. Comp Neurol. 188, 245–262 (1979). [DOI] [PubMed] [Google Scholar]
- Mears A. J. et al. Nrl is required for rod photoreceptor development. Nat. Genet. 29, 447–452 (2001). [DOI] [PubMed] [Google Scholar]
- Zhu X. et al. GRK1-dependent phosphorylation of S and M opsins and their binding to cone arrestin during cone phototransduction in the mouse retina. J. Neurosci. 23, 6152–6160 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov S. S. et al. Photoreceptors of Nrl -/- mice coexpress functional S- and M-cone opsins having distinct inactivation mechanisms. J. Gen. Physiol 125, 287–304 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele L. L. et al. Cone-like morphological, molecular, and electrophysiological features of the photoreceptors of the Nrl knockout mouse. Invest Ophthalmol. Vis. Sci. 46, 2156–2167 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuck M. W., Conley S. M. & Naash M. I. Defects in the outer limiting membrane are associated with rosette development in the Nrl-/- retina. PLoS. One. 7, e32484 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseyama T. et al. Complications of IgA nephropathy in a non-insulin-dependent diabetes model, the Akita mouse. Tohoku J. Exp. Med. 198, 233–244 (2002). [DOI] [PubMed] [Google Scholar]
- Das N. D., Yoshioka T., Samuelson D. & Shichi H. Immunocytochemical localization of phosphatidylinositol-4,5-bisphosphate in dark- and light-adapted rat retinas. Cell Struct. Funct. 11, 53–63 (1986). [DOI] [PubMed] [Google Scholar]
- Das N. D., Yoshioka T., Samuelson D., Cohen R. J. & Shichi H. Immunochemical evidence for the light-regulated modulation of phosphatidylinositol-4,5-bisphosphate in rat photoreceptor cells. Cell Struct. Funct. 12, 471–481 (1987). [DOI] [PubMed] [Google Scholar]
- Hayashi F. & Amakawa T. Light-mediated breakdown of phosphatidylinositol-4,5-bisphosphate in isolated rod outer segments of frog photoreceptor. Biochem. Biophys. Res. Commun. 128, 954–959 (1985). [DOI] [PubMed] [Google Scholar]
- Millar F. A., Fisher S. C., Muir C. A., Edwards E. & Hawthorne J. N. Polyphosphoinositide hydrolysis in response to light stimulation of rat and chick retina and retinal rod outer segments. Biochim. Biophys. Acta 970, 205–211 (1988). [DOI] [PubMed] [Google Scholar]
- Kapoor C. L., O'Brien P. J. & Chader G. J. Phorbol ester- and light-induced endogenous phosphorylation of rat rod outer-segment proteins. Exp. Eye Res. 45, 545–556 (1987). [DOI] [PubMed] [Google Scholar]
- Halet G. Imaging phosphoinositide dynamics using GFP-tagged protein domains. Biol. Cell 97, 501–518 (2005). [DOI] [PubMed] [Google Scholar]
- Lorenzo O., Urbe S. & Clague M. J. Analysis of phosphoinositide binding domain properties within the myotubularin-related protein MTMR3. J. Cell Sci. 118, 2005–2012 (2005). [DOI] [PubMed] [Google Scholar]
- Dormann D., Weijer G., Dowler S. & Weijer C. J. In vivo analysis of 3-phosphoinositide dynamics during Dictyostelium phagocytosis and chemotaxis. J. Cell Sci. 117, 6497–6509 (2004). [DOI] [PubMed] [Google Scholar]
- Pattni K., Jepson M., Stenmark H. & Banting G. A PtdIns(3)P-specific probe cycles on and off host cell membranes during Salmonella invasion of mammalian cells. Curr. Biol. 11, 1636–1642 (2001). [DOI] [PubMed] [Google Scholar]
- Rusten T. E. & Stenmark H. Analyzing phosphoinositides and their interacting proteins. Nat. Methods 3, 251–258 (2006). [DOI] [PubMed] [Google Scholar]
- Li G., Rajala A., Wiechmann A. F., Anderson R. E. & Rajala R. V. Activation and membrane binding of retinal protein kinase Balpha/Akt1 is regulated through light-dependent generation of phosphoinositides. J. Neurochem. 107, 1382–1397 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. R., Schiavo G. & Irvine R. F. Immunocytochemical techniques reveal multiple, distinct cellular pools of PtdIns4P and PtdIns(4,5)P(2). Biochem. J. 422, 23–35 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]