Summary
The ability of platelet-derived growth factor (PDGF), insulin-like growth factor-1 (IGF-1) and platelet-rich plasma (PRP) to increase the rate of osseointegration of endosseous implants and to improve the quality of bone remodeling on the surface of titanium, has been investigated in an experimental intraosseous defect model by an histological and immunohistochemical evaluation. The results from this study demonstrate that rabbits treated with the combination PDGF/IGF-1 showed a higher positive effect on bone regeneration than PRP-treated or controls.
Keywords: PDGF, IGF-1, PRP, growth factors, bone formation, dental implants
Introduction
The release of growth factors by platelets can contribute to cell proliferation and healing of damaged tissues. In fact, platelets play an important role in many processes by releasing many factors, such as platelet-derived growth factor (PDGF), insulin-like growth factor-1 (IGF-1), transforming growth factor (TFG) and vascular endothelial growth factor (VEGF), that control cell proliferation, differentiation, chemotaxis and tissue morphogenesis. The clinical use of platelet-rich plasma (PRP) has been shown to enhance soft and bone tissue healing and PRP has been used in implantology to supply growth factors to sites of surgery, in order to increase the rate of bone deposition and quality of bone regeneration (1,2).
Once it is released from platelets, PDGF can improve new bone deposition by promoting collagen synthesis and increasing the deposition of bone matrix (3,4). It has been shown that PDGF, which binds to specific surface receptors, has important functions in many physiological processes including wound healing and angiogenesis, also playing a synergistic role with other factors, such as IGF-1 (5,6). In the present work we carried out a study in an experimental animal model to evaluate the effect of PDGF, IGF-1 and PRP on the integration of bone implants placed in the femurs of rabbits. The influence of the treatment on the process of implant osseointegration was evaluated by histological and immunohistochemical examination performed on bone samples taken at different time after implantation.
Materials and methods
All procedures were in accord with the Italian laws (DL 27/1/92 n.116) and the directives of the Council of European Communities (886/609 CE). The study was performed on eight healthy male breeding rabbits “Fauve de Bourgogne”, weighing 2.5 to 3 Kg. Experimental subjects were randomly assigned to 2 groups for evaluating osseointegration and bone regeneration capability around dental implants: one group treated with PDGF/IGF-1, and the other one treated with PRP. The animals were anesthetized with an intravenous injection of penthobarbital (30 mg/kg). Anesthesia was maintained, when needed, with additional penthobarbital. Additionally, a local anesthetic (lidocaine 0.5 ml/adrenaline) was applied subcutaneously. After the incision of the skin and the subcutaneous tissues, planes were dissected in order to expose the femur. Then, using a low speed drill with continuous irrigation, bone defects were performed. Before implantation, bone defects were filled with PDGF/IGF-1 combination or with PRP. In each animal one intraosseous defect performed in the controlateral femur was simultaneously filled with methylcellulose gel vehicle to serve as control.
The combination of PDGF and IGF-1 (both supplied by Sigma Chemicals, Milan, Italy, and diluted at a concentration of 4 μg/μL) was added immediately before use to 30 μl of vehicle, which consisted of a 2% methylcellulose gel solution (Methocel, Fluka, Milan, Italy). To obtain PRP, an average 5 mL autologous blood sample was retrieved, and 3,8 % sodium citrate was immediately added to blood as anticoagulant. Then, the whole blood was centrifuged for 15 minutes at 100 g. The supernatant was carefully removed and 30 μl of PRP (containing an average of 50000–70000 platelets) were added to 20 μl of methylcellulose gel vehicle (Methocel).
A total volume of 50 μl of methylcellulose gel containing PDGF-IGF1 or PRP was applied in the hole, and the implant placement followed. The dental implants, measuring 3.3 mm in diameter and 8 mm in length, were positioned in each femoral metaphysis. After this procedure, the muscle planes and the superficial tissues were sutured. For preventing infection, and antibiotic therapy (rocefin, 20 mg/kg) was administered via intramuscular injection after surgery.
After 4, 7 and 12 days from implant insertion, the rabbits were euthanized by an intravenous injection of penthobarbital solution. Their femurs were dissected, and a segment of metaphysis about 1.5 cm in length comprising the implant was obtained for histological and immunohistochemical study.
After 24 hours of fixation in buffered formalin and additional 36 hours of decalcification with a commercial EDTA-hydrochloric acid mixture (Surgipath Decalcifier II, Leica Biosystem, USA) the implant was “gently” removed and the bone segment was cut longitudinally with a plane passing through the hole. The bone fragments were embedded in paraffin and 4 μm consecutive sections were cut and numbered.
For conventional histology staining with hematoxylineosin or Van Gieson to assess the morphological detail of osseointegration was performed. For immunohistochemistry, deparaffinized sections were incubated for 10 minutes with methanol containing 10% H2O2 to block the activity of endogenous peroxidases. The sections were then incubated with polyclonal rabbit anti-PDGFR-α and monoclonal mouse anti PDGFR-β antibodies (Santa Cruz Biotechnology, at cat. sc-338 and sc-6252 dilution of 1:200), anti-VEGF and anti-VEGFR antibodies.
After washing, the sections were incubated with streptavidin-biotin detection system (LSAB, Dako). Finally, the reaction was developed with diaminobenzidine solution.
Results
4th day
The implant sites treated with PRP or PDGF/IGF1 showed abundant cap-like deposition of fibroblastic tissue around the implants. In contrast, the control sites showed only a focal and discontinuous deposition of fibroblasts around the implants.
This fibrous tissue was composed of spindle cells with elongated or oval nuclei and numerous mitotic figures (indicating high proliferative activity) (Fig. 1a).
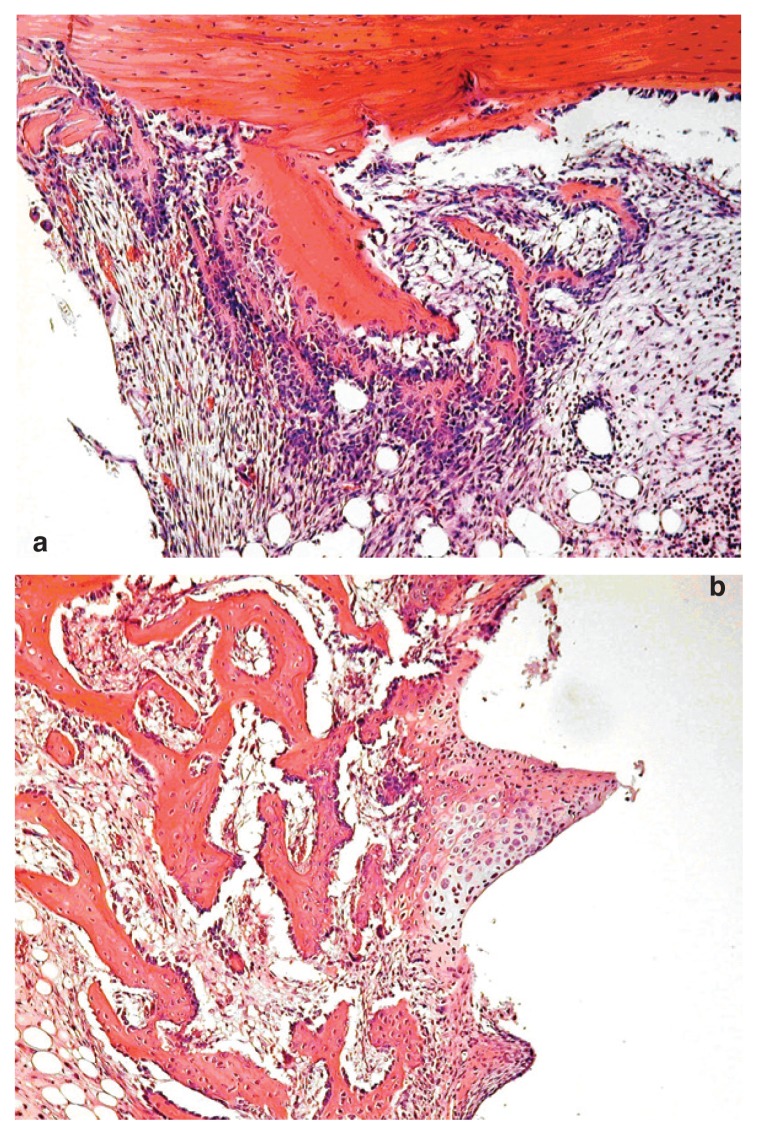
Figure 1.
Histological analysis of the implant site treated with PDGF and IGF-1. 1a) At 4th day, the implant sites treated with PRP or PDGF/IGF1 showed abundant cap-like deposition of fibroblastic tissue around the implants; this fibrous tissue was composed of spindle cells with elongated or oval nuclei and numerous mitotic figures. 1b) At the 12th day, a substantial amount of well formed, significantly more abundant woven bone in subjects treated with PDGF/IGF1 than in animals treated with PRP and controls, was found.
In this phase, the first deposition of bone was observed below the cortical bone in proximity of the neck of the hole, consisting of osteoid matrix trabeculae, bordered by osteoblasts. These trabeculae of osteoid appear not yet joined neither to the cortical bone adjacent neither to the implant.
7th to 12th day
All histological samples taken from the animals treated with PDGF/IGF-1 exhibited significant bone regeneration in the neck hole adherent to the cortical bone, through a process of endosteal bone formation. On the other hand, bone formation appeared uneven in animals PRP-treated. Scarce bone deposition was observed in control sites. In the animals treated with PDGF/IGF1 numerous islands of cartilage leading to endochondral ossification were observed around implants, and by the 12th day, in the same group of animals, a substantial amount of well formed, woven bone significantly more abundant than in animals treated with PRP and controls, was found (Fig. 1b).
Immunohistochemistry
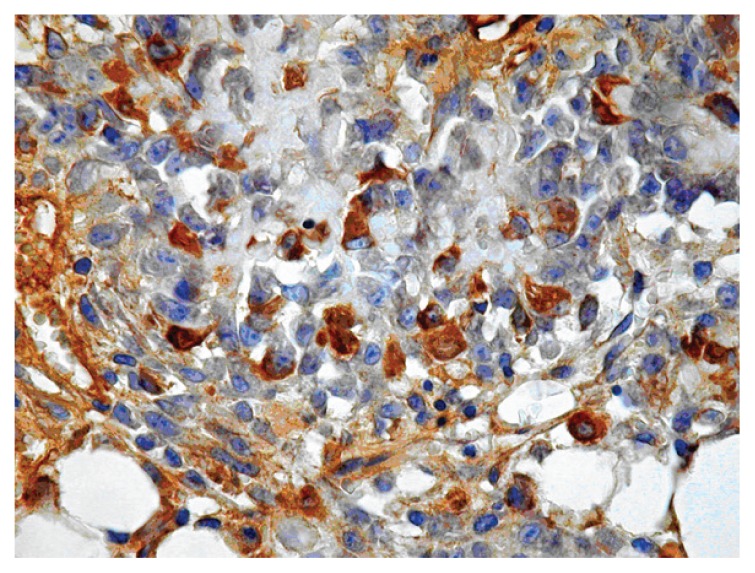
After immunohistochemistry with antibodies against PDGFR-β, presence of scattered positive blast-like cells was observed in both treated and untreated animals (Fig. 2). Also megakaryocytes appeared to be labelled, but lymphocytes were generally negative. In addition, a fraction of chondroblasts and osteoblasts in osteoid matrix were also positive.
Figure 2.
Near the implant site at 4th day from surgery, immunohistochemical staining for PDGFR-β showed scattered cells in the reparative tissue rich in fibroblast.
After immunohistochemistry with antibodies against PDGFR-α, no significative results were seen.
A significant expression of VEGF and VEGFR-2 in animals sacrificed at 4th and 7th day was observed, with a strong expression present in the cytoplasm of osteoblasts bordering the osteoid matrix. Then, the expression rapidly decreased in the animals sacrificed at 12th day. A widespread, although weaker positivity was observed in the cytoplasm of fibroblast-like cells forming the fibrous callus. A similar, although more discrete positivity, was observed after immunohistochemistry with anti-VEGFR2 antibody.
Discussion and Conclusions
Recent studies have suggested that a number of growth factors, including PDGF, promote tissue repair and regeneration, including bone formation; moreover, the efficacy of growth factor therapies in periodontology and implantology has been well characterized in a variety of in vitro and in vivo studies (7,8). The present study, focused on the histological characteristics of the early osseointegration of implants, with or without addition of PRP and PDGF/IGF-1 in combination, in an animal model, demonstrate a higher bone regeneration rate in growth factors-treated group than in PRP or control group. Our results show a greater new bone deposition in animals in which PDGF/IGF-1 was added to bone defect, when compared to animals treated with PRP or controls.
Interestingly, our immunohistochemical demonstration that various types of cells present in reparative foci express receptors for these molecules, in particular PDGFR-β, support the experimental evidence of positive therapeutic effects of PDGF on osseointegration. In normal bone marrow around the implant, many blast cells, also including osteoprogenitors, chondrocytes and osteoblasts involved in tissue healing and bone formation, expressed receptors for PDGF-β. Therefore, from our data, showing presence of cells expressing PDGF receptors in foci of osteointegration, add further evidence for usefulness of growth factors addition in site of implant surgery.
References
- 1.Torres J, Tamimi F, Alkhraisat MH, et al. Platelet-rich plasma may prevent titanium-mesh exposure in alveolar ridge augmentation with anorganic bovine bone. J Clin Periodontol. 2010;37:943–951. doi: 10.1111/j.1600-051X.2010.01615.x. [DOI] [PubMed] [Google Scholar]
- 2.Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:E37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Chang PC, Seol YJ, Cirelli JA, et al. PDGF-B gene therapy accelerates bone engineering and oral iplant osseointegration. Gene Ther. 2010;17:95–104. doi: 10.1038/gt.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollinger JO, Hart CE, Hirsch SN, Lynch S, Friedlaender GE. Recombinant human platelet-derived growthfactor: Biology and clinical applications. J Bone Joint Surg Am. 2008;90 (suppl 1):48–54. doi: 10.2106/JBJS.G.01231. [DOI] [PubMed] [Google Scholar]
- 5.Lynch SE, Nixon JC, Colvin RB, Antoniades HN. Role of platelet-derived growth factor in wound healing: Synergistic effects with other growth factors. Proc Natl Acad Sci U S A. 1987;84:7696–7700. doi: 10.1073/pnas.84.21.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tokunaga A, Oya T, Ishii Y, et al. PDGF receptor beta is a potent regulator of mesenchymal stromal cell function. J Bone Miner Res. 2008;23:1519–1528. doi: 10.1359/jbmr.080409. [DOI] [PubMed] [Google Scholar]
- 7.Yun JH, Han SH, Choi SH, et al. Effects of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on bone regeneration for osseointegration of dental implants: Preliminary study in canine three-wall intrabony defects. J Biomed Mater Res B Appl Biomater. 2013 Dec 5; doi: 10.1002/jbm.b.33084. [DOI] [PubMed] [Google Scholar]
- 8.Kaigler D, Avila G, Wisner-Lynch L, et al. Platelet-derived growth factor applications in periodontal and peri-implant bone regeneration. Expert Opin Biol Ther. 2011;11:375–85. doi: 10.1517/14712598.2011.554814. [DOI] [PMC free article] [PubMed] [Google Scholar]