Abstract
Acute exposure to particulate matter (PM) air pollution causes thrombotic cardiovascular events, leading to increased mortality rates; however, the link between PM and cardiovascular dysfunction is not completely understood. We have previously shown that the release of IL-6 from alveolar macrophages is required for a prothrombotic state and acceleration of thrombosis following exposure to PM. Here, we determined that PM exposure results in the systemic release of catecholamines, which engage the β2-adrenergic receptor (β2AR) on murine alveolar macrophages and augment the release of IL-6. In mice, β2AR signaling promoted the development of a prothrombotic state that was sufficient to accelerate arterial thrombosis. In primary human alveolar macrophages, administration of a β2AR agonist augmented IL-6 release, while the addition of a beta blocker inhibited PM-induced IL-6 release. Genetic loss or pharmacologic inhibition of the β2AR on murine alveolar macrophages attenuated PM-induced IL-6 release and prothrombotic state. Furthermore, exogenous β2AR agonist therapy further augmented these responses in alveolar macrophages through generation of mitochondrial ROS and subsequent increase of adenylyl cyclase activity. Together, these results link the activation of the sympathetic nervous system by β2AR signaling with metabolism, lung inflammation, and an enhanced susceptibility to thrombotic cardiovascular events.
Introduction
Based on air pollution exposure estimates in the US from the late 1970s to the early 2000s, Pope et al. projected that a 10 μg/m3 fall in the mean levels of fine particulate air pollution (PM2.5) would increase life expectancy by 0.61 years (1). At current levels in the developed world, these data suggest that PM exposure is associated with an average loss of 0.7 to 1.6 years of life, with a larger burden on urban dwellers. The public health impact of PM2.5 exposure is greater in the developing world, where particle levels are often 10-fold higher than those observed in the US and Western Europe (2).
The mortality associated with acute exposure to ambient PM is largely due to an increased incidence of ischemic cardiovascular events; however, the mechanisms explaining this association are incompletely understood (3). We and others have suggested that exposure to PM induces a local inflammatory response in the lung, resulting in the release of proinflammatory cytokines, which enhance the systemic tendency toward thrombosis (3–9). Specifically, we discovered that exposure of mice to PM causes a prothrombotic state and accelerates vascular thrombosis by inducing the release of IL-6 from alveolar macrophages (4, 10). An additional mechanism linking PM exposure with cardiovascular events has been described by several groups of investigators who have observed changes in heart rate variability or peripheral vasoreactivity following exposure to PM and inferred from these data that PM-induced activation of the sympathetic nervous system might induce coronary vasoconstriction or arrhythmias (3, 11–15). However, the effect of PM on the sympathetic nervous system has not been directly investigated, and the consequences of sympathetic nervous system activation on lung inflammation and thrombosis are not known.
Here, we report that exposure to concentrated ambient PM at levels similar to those observed in the developing world induces the systemic and local release of catecholamines, which activate β2-adrenergic receptors (β2ARs) on human and murine alveolar macrophages to enhance PM-induced release of IL-6 and resulting thrombosis. In alveolar macrophages, exposure to PM induced the generation of ROS from the mitochondria, which primed adenylyl cyclase and consequently enhanced β2AR-mediated generation of cAMP and phosphorylation of cAMP response element-binding protein (CREB) to augment IL6 transcription. Consistent with these findings, the administration of formoterol, a selective β2AR agonist widely used in clinical practice, augmented PM-induced IL-6 release and the resulting prothrombotic state and accelerated arterial thrombosis. These findings reveal an important adaptive mechanism by which systemic stress acting through catecholamines can enhance inflammation to promote thrombosis.
Results
Exposure to PM causes the release of catecholamines, which are required for PM-induced release of IL-6 and thrombosis.
To directly evaluate the effects of PM on sympathetic nervous system activity, we measured tissue catecholamine levels in mice exposed to concentrated ambient fine PM (<2.5 μm in diameter, PM2.5) or filtered air, using a versatile aerosol concentration enrichment system (VACES) (ref. 16 and Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI75157DS1). In mice exposed to concentrated ambient particles (CAPs) for 8 hours daily on 3 consecutive days, we observed a significant increase in systemic and lung levels of catecholamines compared with mice exposed to filtered air (Figure 1, A and B). We observed similar changes in mice exposed to a well-characterized urban PM collected from the ambient air in Washington, DC (refs. 4, 10, and Figure 1, C–E). Both basal and PM-induced catecholamine levels were reduced after we induced a chemical sympathectomy by pretreating the mice with the vesicular monoamine transporter reserpine prior to the intratracheal instillation of PM (Figure 1, C–E, and ref. 17). We have previously shown that exposure of mice to PM via inhalation or intratracheal instillation results in an IL-6–dependent increase in thrombin-antithrombin (TAT) complexes in the plasma (4, 10). In mice treated with reserpine, we found that the PM-induced increases in BAL fluid (BALF) levels of IL-6 and plasma levels of TAT complexes were reduced (Figure 1, F and G). These data demonstrate that inhalational exposure of PM at levels seen in many world cities is sufficient to trigger the systemic release of catecholamines and suggest that these catecholamines contribute to PM-induced IL-6 release and thrombosis.
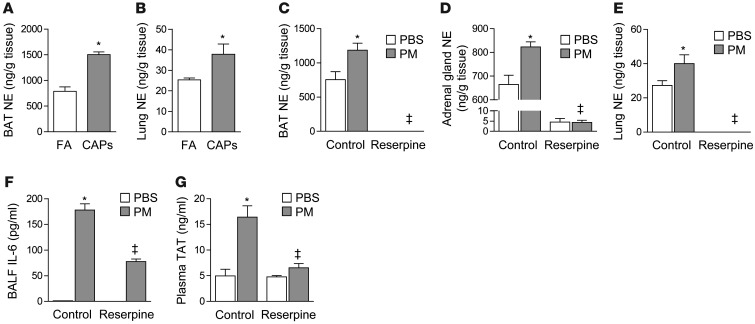
Figure 1. Catecholamines are required for PM-induced inflammation and thrombosis.
We exposed wild-type (C57BL/6) mice contemporaneously to either CAPs (PM2.5) or filtered air (FA) for 8 hours daily on 3 consecutive days and measured levels of norepinephrine (NE) in the (A) BAT and (B) lung tissue. We also performed intratracheal instillations of PM (200 μg/mouse) or control (PBS) in wild-type mice and gave reserpine (5 mg/kg 100 μl) or vehicle (20% ascorbic acid 100 μl) by gavage once 4 hours before treatment with PM or control (PBS). Twenty-four hours later, we measured levels of norepinephrine in the (C) BAT, (D) adrenal gland, and (E) lung. In identically treated mice, we measured (F) IL-6 in the BALF and (G) TAT complexes in the plasma. *P < 0.05, CAPs vs. FA, PM vs. PBS; ‡ P < 0.05, reserpine vs. control.
β2ARs are required for catecholamine-induced upregulation of IL-6 and the resulting thrombosis after PM exposure. Signaling by catecholamines is mediated by αARs and/or βARs, which are variably expressed in different tissues and cell types, including the lung (18–20) and alveolar macrophages (21, 22). We treated mice with a nonselective βAR antagonist (propranolol 3 mg/kg i.p. q 8 hours for 48 hours) prior to the administration of PM and measured IL-6 release and thrombosis 24 hours later. Treatment with propranolol reduced the PM-induced increase in IL-6 levels in BALF (Figure 2A). We have previously reported that IL-6 is released from alveolar macrophages in response to PM and acts to increase the synthesis of clotting factors in the liver. We found that IL-6 release from macrophages is required for the PM-induced generation of intravascular thrombin and the reduced time to thrombosis after FeCl3-induced carotid artery injury, a widely used murine model of stroke, following PM exposure (Supplemental Video 1, Supplemental Figure 2, and refs. 4, 10, 23). All of these effects were reduced in mice treated with propranolol (Figure 2, B–D).
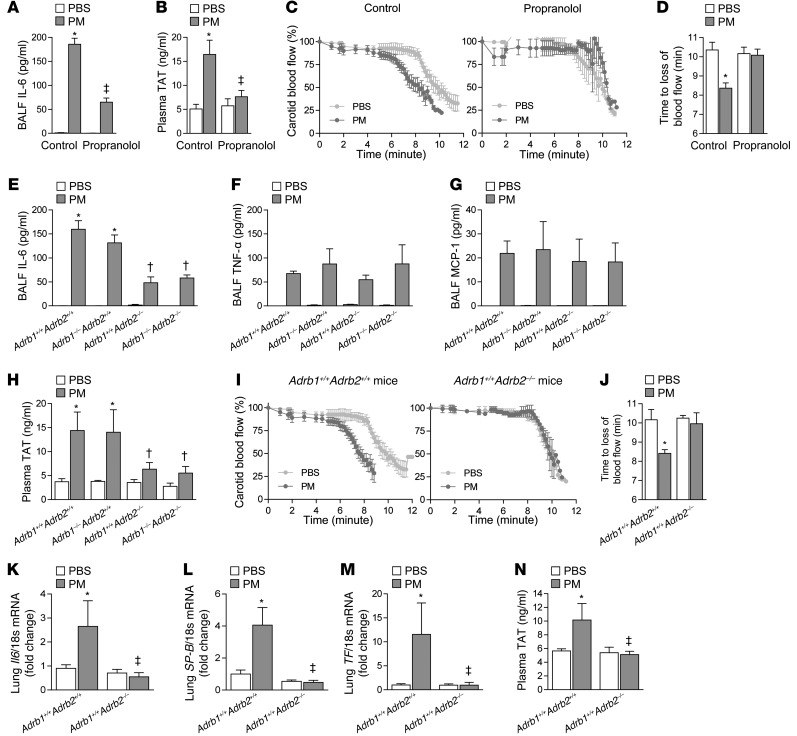
Figure 2. β2ARs are required for the catecholamine-induced upregulation of IL-6 and the resulting thrombosis after PM exposure.
We pretreated mice with propranolol (3 mg/kg in 100 μl i.p.) or vehicle (saline 100 μl i.p.) every 8 hours, followed 24 hours later by PM (200 μg/mouse) or control (PBS) and measured (A) the levels of IL-6 in the BALF, (B) the plasma levels of TAT complexes, and (C) the time to loss of blood flow after FeCl3-induced injury to the carotid artery. (D) The time to complete (>75% reduction) loss of blood flow is shown. We performed intratracheal instillations in mice lacking either β1AR (Adrb1–/–Adrb2+/+), β2AR (Adrb1+/+Adrb2–/–), or both β1AR and β2AR (Adrb1–/–Adrb2–/–) and their wild-type littermate controls (Adrb1+/+Adrb2+/+) with PM or PBS and, 24 hours later, measured the levels of (E) IL-6, (F) TNF-α, and (G) MCP-1 in BALF, (H) the plasma levels of TAT complexes in the plasma, and (I and J) the time to loss of blood flow after FeCl3-induced injury to the carotid artery. We exposed β2AR deficient mice (Adrb1+/+Adrb2–/–) and wild-type littermate controls (Adrb1+/+Adrb2+/+) contemporaneously to either CAPs (PM2.5) or filtered air for 8 hours daily on 3 consecutive days and measured levels of (K) Il6, (L) SP-B, and (M) TF mRNA in the lung tissue and (N) TAT complexes in the plasma. *P < 0.05, PM vs. PBS or CAPs vs. FA; ‡P < 0.05, propranolol vs. control, Adrb1+/+Adrb2–/– and Adrb1–/–Adrb2–/– vs. Adrb1–/–Adrb2+/+ and Adrb1+/+Adrb2+/+.
Of the 3 subtypes of βARs, β1AR and β2AR are widely expressed, while expression of the β3AR is largely restricted to the adipose tissue (24). To determine which βAR subtype is responsible for the catecholamine-induced augmentation of IL-6 release and thrombosis induced by PM, we performed intratracheal instillations of PM or PBS in global knockout mice singly deficient in β1AR or β2AR or doubly deficient in β1AR and β2AR and their wild-type littermate controls. Mice deficient in β2AR or doubly deficient in β1AR and β2AR released less IL-6 into the BALF (Figure 2E). The effect of catecholamines and β2AR was specific to IL-6, as the loss of β2AR did not have an effect on the BALF levels of other cytokines induced by PM exposure (Figure 2, F and G; only TNF-α and MCP-1 are shown).
In addition to a reduction in IL-6, mice deficient in β2AR or doubly deficient in β1AR and β2AR generated less intravascular thrombin (Figure 2H) and showed no acceleration of arterial thrombosis (Figure 2, I and J) in response to PM. We observed a similar reduction in IL-6 release and plasma TAT levels in β2AR knockout mice (Adrb2–/–) compared with their littermate controls 24 hours after the intratracheal administration of LPS (Supplemental Figure 3), suggesting that the role of catecholamines and β2AR-mediated upregulation of IL-6 may not be limited to PM, but may represent a general response during lung inflammation and injury. In wild-type mice exposed to concentrated ambient PM2.5 via inhalation, we found an increase in the mRNA encoding Il6 and its transcriptional targets surfactant protein B (SP-B) and tissue factor (TF) in whole-lung homogenates as well as an increase in plasma thrombin generation. None of these changes were observed in mice lacking β2AR (Figure 2, K–N).
In human alveolar macrophages, stimulation of β2ARs increases and βAR blockade inhibits the release of IL-6 in response to PM.
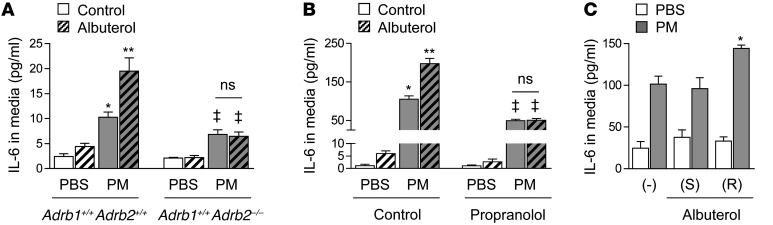
We have previously shown that the IL-6 release from alveolar macrophages is required for the development of a PM-induced prothrombotic state sufficient to accelerate arterial thrombosis (4, 10). We cultured primary human macrophages from intubated patients undergoing nonbronchoscopic alveolar lavage in the intensive care unit. After 24 hours in culture, we found that these cells released IL-6 into the medium in response to PM (Figure 3). To determine whether the stimulation of adrenergic receptors on the alveolar macrophages affects PM-induced IL-6 release, we treated primary human macrophages from the same patients with PM or control in the presence or absence of a β2AR agonist, albuterol, or a βAR antagonist (beta blocker), propranolol, and measured the concentration of IL-6 released into the medium 24 hours later. Albuterol caused a doubling of IL-6 in the medium, whereas propranolol inhibited the PM-induced release of IL-6 into the medium (Figure 3).
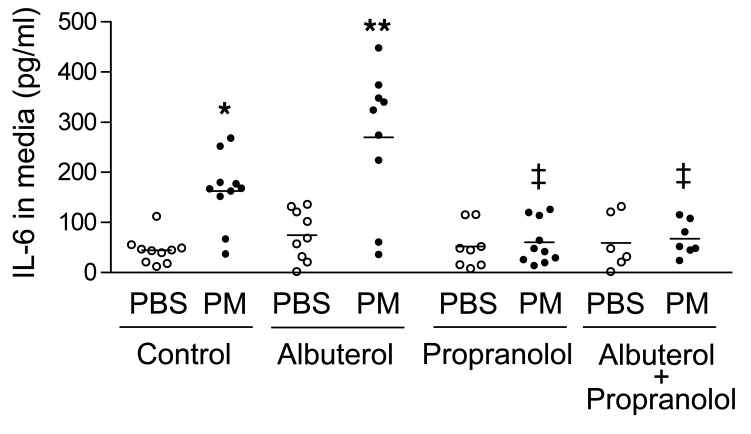
Figure 3. In human alveolar macrophages, stimulation of β2AR augments and inhibition of βAR inhibits PM-induced IL-6 release.
We treated primary human alveolar macrophages with PM (10 μg/cm2) or control (medium) and with albuterol (10–7M) or control (medium) in the presence or absence of propranolol (10 μM) or control (saline) and measured IL-6 levels in the medium 24 hours later. *P < 0.05, PM vs. PBS; ** P < 0.05, albuterol vs. control; ‡P < 0.05, propranolol, vs. control.
β2ARs on alveolar macrophages are required for catecholamine-induced upregulation of IL-6 and resulting thrombosis after PM exposure. Using intratracheal liposomal clodronate, we have previously reported that the majority of IL-6 released in response to PM originates from lung macrophages (4). Therefore, we sought to determine whether the activation of β2ARs on these cells was directly responsible for the catecholamine-mediated augmentation of IL-6 release in response to PM. After confirming the expression of the β2AR in primary alveolar macrophages from wild-type mice (Supplemental Figure 4 and ref. 25–27), we generated Lysm-Cre Adrb2flox/flox mice and validated macrophage-specific deletion of β2AR in cells isolated from homogenized lungs (Figure 4, A and B). We then exposed Lysm-Cre Adrb2flox/flox and control mice (Adrb2flox/flox) to concentrated ambient PM2.5 via inhalation. Compared with the control mice, the Lysm-Cre Adrb2flox/flox mice had reduced levels of IL-6, generated fewer TAT complexes in the plasma, and exhibited slower thrombosis after carotid injury 24 hours after PM exposure (Figure 4, C–F).
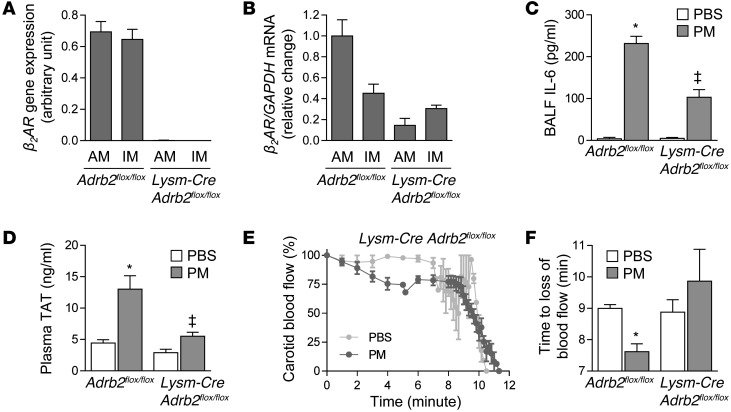
Figure 4. β2ARs on alveolar macrophages are required for catecholamine-induced upregulation of IL-6 and resulting thrombosis after PM exposure.
(A and B) Mice deficient in β2AR specifically in monocytes and macrophages were generated by crossing Lysm-Cre mice with Adrb2flox/flox mice. Inflammatory cells from lung homogenates of Adrb2flox/flox and Lysm-Cre Adrb2flox/flox mice were sorted, and deletion of the Adrb2 gene was confirmed by assessment of (A) gene expression and (B) mRNA. We performed intratracheal instillations in Adrb2flox/flox and Lysm-Cre Adrb2flox/flox mice with PM (200 μg/mouse) or vehicle (PBS) and, 24 hours later, measured (C) the levels of IL-6 in BALF, (D) TAT complexes in the plasma, and (E and F) the time to loss of blood flow after FeCl3-induced injury to the carotid artery (the time to complete loss of blood flow [>75% reduction]). *P < 0.05, PM vs. PBS or CAPs vs. FA; ‡P < 0.05, Adrb2flox/flox vs. Lysm-Cre Adrb2flox/flox.
Treatment with β2 agonists worsens PM-induced release of IL-6 and resulting thrombosis.
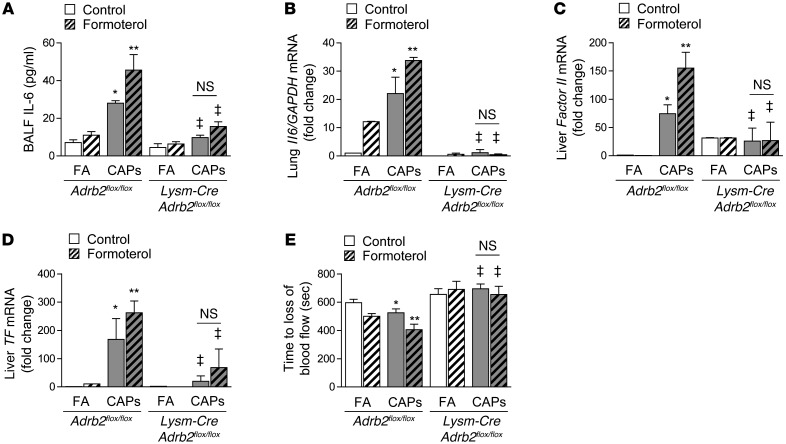
To determine whether activation of β2AR is sufficient to augment PM-induced IL-6 release and thrombosis, we treated wild-type mice and Lysm-Cre Adrb2flox/flox mice with a long-acting β2AR agonist, formoterol (1 × 10–5 M via inhalation, every 12 hours), beginning 24 hours before exposure to concentrated ambient PM. In wild-type mice treated with formoterol, the levels of IL-6 in the BALF were twice that of the sham-treated controls (Figure 5A), and the levels of Il6 mRNA in whole-lung homogenates were increased after inhalational exposure to concentrated ambient PM (Figure 5B). In contrast, formoterol did not affect the IL-6 levels in Lysm-Cre Adrb2flox/flox mice (Figure 5, A and B). The enhanced PM-induced release of IL-6 in response to formoterol in wild-type (Adrb2flox/flox) mice was associated with increased expression of prothrombin (factor II) and TF mRNA in the liver (Figure 5, C and D) and a further acceleration of carotid artery thrombosis after FeCl3-induced injury (Figure 5E). These effects were absent in Lysm-Cre Adrb2flox/flox mice (Figure 5, C–E). Similar effects of formoterol were seen after the intratracheal administration of PM (Supplemental Figure 5). Exposure to concentrated ambient PM with or without formoterol did not cause a significant change in the number or differential of immune cells in the lungs in Adrb2flox/flox and Lysm-Cre Adrb2flox/flox mice (Supplemental Figure 6).
Figure 5. Treatment with β2 agonists worsens PM-induced release of IL-6 and resulting thrombosis.
We treated wild-type mice with formoterol (10–5M) or vehicle control (ethanol 2%), both with 1 ml via inhalation over 30 minutes every 12 hours, and exposed them contemporaneously to CAPs (PM2.5) or filtered air for 8 hours daily on 3 consecutive days. We measured levels of (A) IL-6 in BALF, (B) Il6 mRNA in lung tissue, (C) prothrombin (factor II), and (D) TF mRNA in liver and (E) the time to loss of blood flow after FeCl3-induced injury to the carotid artery (the time to complete loss of blood flow [>75% reduction]). *P < 0.05, PM vs. PBS or CAPs vs. FA; **P < 0.05, formoterol vs. control; ‡P < 0.05, Adrb2flox/flox vs. Lysm-Cre Adrb2flox/flox.
Engagement of β2AR augments PM-induced release of IL-6 from alveolar macrophages in vitro.
We conducted a series of experiments to determine the mechanism by which the activation of β2ARs on macrophages augments PM-induced IL-6 release and thrombosis. We isolated primary alveolar macrophages from Adrb2–/– mice and their wild-type littermate controls and compared the release of IL-6 in response to PM. Medium levels of IL-6 measured 24 hours after treatment with PM were lower in the Adrb2–/– compared with the wild-type macrophages. Treatment of primary alveolar macrophages from wild-type, but not Adrb2–/–, animals with the β2AR agonist albuterol (10–7 M) augmented the PM-induced release of IL-6 (Figure 6A). To examine the molecular mechanisms of this response, we used an alveolar macrophage cell line (MH-S), which expresses many of the markers seen in wild-type macrophages (Supplemental Figure 7). MH-S cells treated with PM 1 hour earlier showed augmented IL-6 release in response to treatment with epinephrine, and the (R), but not the (S), enantiomer of albuterol (Figure 6, B and C, and Supplemental Figure 8).
Figure 6. Engagement of β2AR augments PM-induced release of IL-6 from alveolar macrophages in vitro.
(A) We treated primary alveolar macrophages isolated from Adrb2–/– mice (Adrb1+/+Adrb2–/–) and their wild-type littermate controls (Adrb1+/+Adrb2+/+) with PM (10 μg/cm2) or control (medium) and measured IL-6 levels in the medium 24 hours later. (B) We treated murine alveolar macrophages (MH-S cells) with PM (10 μg/cm2) or control (medium) and with albuterol (10–7M) or control (medium) in the presence or absence of propranolol (10 μM) (or control [PBS]) and measured IL-6 levels in the medium 24 hours later. (C) We treated MH-S cells with PM (10 μg/cm2) or control (medium) and with the (S) or (R) enantiomer of albuterol (10–7M) or control. *P < 0.05 for comparison between PM-treated cells with or without albuterol or forskolin; *P < 0.05, PM vs. PBS; **P < 0.05, albuterol vs. control, and simultaneous vs. control; ‡P < 0.05, Adrb1+/+Adrb2–/– vs. Adrb1+/+Adrb2+/+, propranolol vs. control.
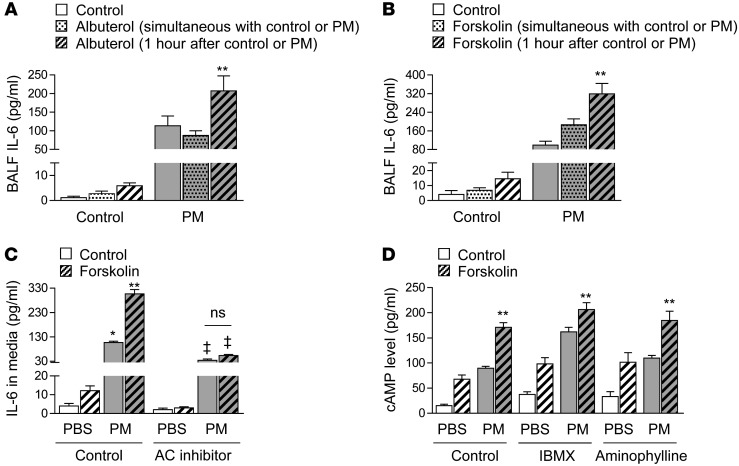
β2 agonist therapy and forskolin augment PM-induced release of IL-6 only when they are administered following exposure to PM. We treated MH-S cells with PM or control in the presence or absence of a β2AR agonist, albuterol (10–7 M), or a direct activator of adenylyl cyclase, forskolin (50 μM), administered either simultaneously or 1 hour after treatment with PM, and measured IL-6 release into the medium. While albuterol did not have an effect on IL-6 when it was administered simultaneously with PM, it doubled the PM-induced IL-6 release when it was administered 1 hour after the PM (Figure 7A). Similarly, forskolin induced a modest increase in PM-induced IL-6 release, but doubled the IL-6 release when it was administered 1 hour after PM treatment (Figure 7B).
Figure 7. β2 Agonist therapy and forskolin augment PM-induced release of IL-6 only when administered following exposure to PM.
We treated MH-S cells with PM (10 μg/cm2) or control (medium) and with (A) albuterol (10–7M) or (B) forskolin (50 μM) and control administered simultaneously with the PM or 1 hour after the PM. (C) We treated MH-S cells with PM (10 μg/cm2) or control (medium) and with forskolin (50 μM) or control (saline) 1 hour after PM treatment in the absence of presence of an adenylyl cyclase (AC) inhibitor (SQ22536, 300 μM) (or control [H2O]) and measured IL-6 levels in the medium 24 hours later. (D) We treated MH-S cells with PM (10 μg/cm2) or control (medium) and with forskolin (50 μM) or control 1 hour after PM treatment in the absence or presence of IBMX (1 μM) and aminophylline (10 μM) and measured cAMP levels in cell lysates 1 minute later. *P < 0.05, PM vs. PBS; **P < 0.05, albuterol or forskolin (1 hour after PM) vs. control and albuterol or forskolin (simultaneous with PM ); ‡ P < 0.05, AC inhibitor vs. control.
PM-induced mitochondrial ROS release primes adenylyl cyclase to augment the release of IL-6 in response to β2 agonists.
Stimulation of β2AR initiates a signaling pathway that increases the activity of adenylyl cyclase, which converts ATP to 3′,5′cAMP (21). When we directly activated adenylyl cyclase with forskolin or inhibited it with SQ2253, the augmentation and inhibition of PM-induced IL-6 release in MH-S cells was similar to that observed after treatment with albuterol or propranolol, respectively (Figure 7C). Consistent with our findings using albuterol, the PM-induced augmentation of cAMP levels after forskolin administration was only observed when the PM was administered 1 hour before the forskolin (Figure 7, A and B). We observed a similar augmentation of forskolin-induced cAMP by PM in the presence of 2 phosphodiesterase inhibitors (3-isobutyl-1-methylxanthine [IBMX] and aminophylline), suggesting the increase in cAMP did not result from an inhibition of phosphodiesterases (Figure 7D).
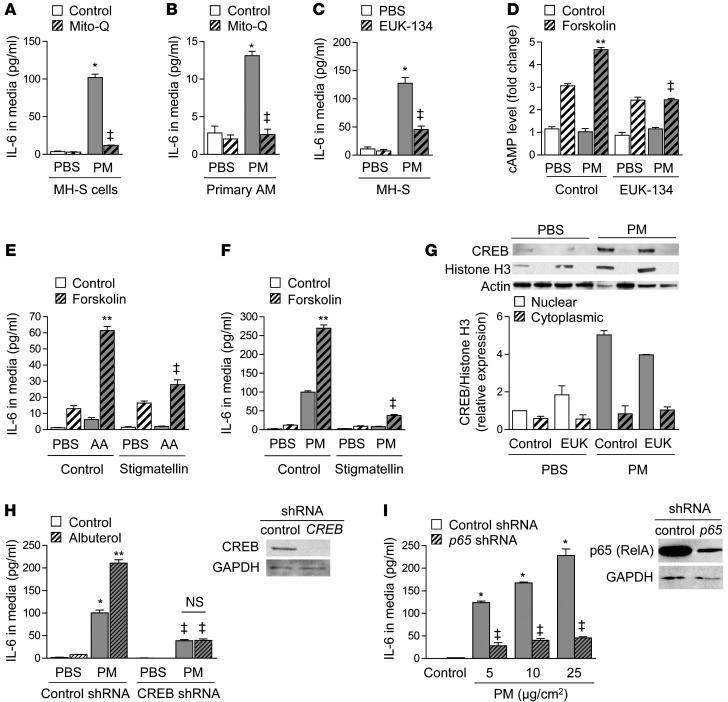
To explore the mechanism by which PM exposure enhances the activity of adenylyl cyclase to augment IL-6 release, we treated MH-S cells and primary alveolar macrophages isolated from wild-type mice with the mitochondrially targeted antioxidant Mito-Q (Figure 8, A and B) or the nonselective combined superoxide dismutase/catalase mimetic Eukarion 134 (EUK-134) (Figure 8C). Inhibition of mitochondrial oxidant generation with these compounds prevented the PM-induced augmentation of cAMP after forskolin treatment (Figure 8D), suggesting that PM-induced ROS are required for increased adenylyl cyclase activity. To determine whether mitochondrial ROS alone were sufficient to augment adenylyl cyclase activity, we measured forskolin-induced cAMP generation in cells treated 1 hour earlier with antimycin A, an electron transport chain inhibitor that increases mitochondrial ROS generation (Supplemental Figure 9 and Figure 8E). The addition of stigmatellin, which also blocks electron transport but inhibits mitochondrial ROS generation (Supplemental Figure 9), prevented the augmentation of IL-6 release by PM or antimycin A (Figure 8, E and F). These results demonstrate an unexpected link between mitochondrial metabolism and the stress response mediated by catecholamines.
Figure 8. PM-induced ROS generation and priming of adenylyl cyclase are required for β2 agonist–mediated worsening of IL-6 release.
We treated (A) MH-S cells and (B) primary alveolar macrophages (AM) with PM (10 μg/cm2) or control (medium) and measured IL-6 levels in the absence or presence of a mitochondrially targeted antioxidant (Mito-Q or control [TPP]) or (C) a nontargeted antioxidant (EUK-134) (20 μM). (D) We treated MH-S cells with PM or control and with forskolin (50 μM) or control and measured cAMP levels 1 minute after forskolin treatment. (E) We treated MH-S cells with antimycin A (AA) (1 μM) or vehicle and with forskolin (50 μM) and measured IL-6 in the medium 24 hours later in the absence or presence of stigmatellin (1 μM). (F) We treated MH-S cells with PM or control and with forskolin (50 μM) or control and measured IL-6 levels 24 hours later in the absence or presence of stigmatellin. (G) We treated MH-S cells with PM or control and with EUK-134 or control and performed immunoblotting against cAMP CREB in the nuclear and cytoplasmic fractions 4 hours later. (H) We measured IL-6 levels in control and CREB shRNA–transfected MH-S cells after treatment with PM or PBS. (I) We treated control and p65-shRNA–transfected cells with PM or control and with albuterol or control and measured IL-6 levels. *P < 0.05, PM vs. PBS; **P < 0.05, albuterol or forskolin vs. control; ‡P < 0.05, Adrb1+/+Adrb2–/– vs. Adrb1+/+Adrb2+/+, AC inhibitor, mito-Q, EUK-134, or stigmatellin vs. control, CREB or p65 vs. control shRNA.
We then examined the consequences of the enhanced cAMP production in response to ligation of the β2AR on IL-6 release. Increases in cAMP activate PKA to induce the phosphorylation and nuclear translocation of the transcription factor CREB (21). We observed an oxidant-dependent increase in nuclear levels of CREB following forskolin treatment in cells treated 1 hour earlier with PM as compared with vehicle (Figure 8G). Similar to our findings in cells with genetic loss or pharmacologic inhibition of β2AR, the administration of albuterol 1 hour after PM failed to augment PM-induced IL-6 release in MH-S cells with a stable knockdown of CREB (Figure 8H). Consistent with reports that CREB augments the NF-κB–mediated transcription of Il6 (28), we found that PM-induced IL-6 release was completely inhibited in MH-S cells with a stable knockdown of p65 (Figure 8I) (29). Collectively, these results show that activation of β2AR following exposure to PM augments adenylyl cyclase activity, which acts through CREB to augment NF-κB–mediated transcription of Il6.
Discussion
We describe an important mechanism linking PM air pollution to the activation of the sympathetic nervous system and consequently the release of catecholamines, which act through β2ARs on macrophages to augment IL-6 release and accelerate thrombosis. Our findings reveal an elegant system for augmenting inflammation-induced thrombosis in response to systemic stress exclusively in the presence of a concomitant inflammatory stimulus. In the absence of an inflammation-induced metabolic signal (mitochondrial ROS generation), catecholamines have no effect on IL-6 release from murine or human lung macrophages or the resulting thrombosis in mice. Similarly, in the absence of catecholamines, IL-6 release in response to particles is not sufficient to augment thrombosis. However, mitochondrial ROS released in response to PM primed adenylyl cyclase such that a subsequent catecholamine signal enhances IL-6 release to levels sufficient to promote thrombosis. The excess cardiovascular mortality associated with urban PM air pollution exposure, which is not seen with other pollutants, may be explained by their ability to activate both pathways simultaneously.
IL-6 is unique among the cytokines in its ability to induce an acute phase response and enhance the tendency toward thrombosis (30). Exposure of normal humans to ambient PM has often been associated with increases in IL-6 and its transcriptional targets fibrinogen, factor VIII, and C-reactive protein as well as alterations in the levels of platelet activation and coagulation factors (7, 8, 31). For example, in a recent observational study of healthy young people exposed to marked reductions in the levels of ambient PM during the Beijing Olympics, the number of subjects with detectable levels of C-reactive protein, which is transcriptionally regulated by IL-6, decreased from 55% in the pre-Olympic period to 36% in the post-Olympic period (31). The relevance of our findings to human exposure is supported by our observation that the release of IL-6 from human alveolar macrophages was augmented by the β2 agonist albuterol and inhibited by the nonselective βAR antagonist propranolol.
We found that activation of β2AR in alveolar macrophages augmented the NF-κB–mediated transcription of Il6 via a mechanism that required adenylyl cyclase and CREB. However, in the absence of an inflammatory stimulus (PM), activation of β2AR had no detectable effect on Il6 transcription. These data reveal an important mechanism by which activation of the systemic nervous system can augment the innate immune response. The selectivity of this system is explained by a common requirement for mitochondrial ROS generation in the signaling pathways linking PM with the activation of NF-κB and the activation of CREB via β2ARs and adenylyl cyclase. These findings suggest that mitochondrial ROS not only participate directly in the activation of NF-κB but also “prime” the cell to respond to catecholamines with enhanced inflammation. This mechanism is attractive, as the IL-6–mediated activation of coagulation will occur when stress and injury coexist, but will not occur in response to stress in the absence of injury. This mechanism also explains why this effect may have been missed in other studies examining the effect of β2AR agonists on inflammatory cytokine levels; we only observed an effect of β2AR agonists on IL-6 release when they were administered after PM exposure, allowing time for priming of adenylyl cyclase (22).
In our studies, we limited our examination to the effects of β2AR agonists on PM-induced IL-6 release. However, our limited studies using LPS suggest this mechanism might be generalized to other stimuli that result in lung inflammation. If so, our findings suggest a potential mechanism for explaining the excess mortality observed in patients with inflammatory lung diseases treated with β2AR agonists. In observational studies conducted in patients with asthma, investigators reported an association between the use of these drugs and a 4-fold increase in the risk of asthma-related mortality, prompting the US FDA to issue a black box warning regarding the safety of these agents (32–38). In contrast, the use of βAR blockers in patients with chronic obstructive pulmonary disease has been associated with a reduced risk of exacerbation (∼30%–50%) and improved survival (39–42), despite the prediction that these agents might worsen bronchoconstriction through their effects on airway smooth muscle cells. In 2 large randomized trials of β2 agonist therapy for patients with acute lung injury, treatment with the β2 agonist of acute lung injury showed no benefit and a trend toward harm (43–45).
Using a murine model of acid aspiration and influenza A–induced lung injury, Imai and colleagues found that the release of IL-6 required the oxidation of plasma membrane phospholipids, which activated TLR4 to induce the TIR domain–containing adapter-inducing IFN-β–mediated (TRIF-mediated) and TNF receptor–associated factor–mediated (TRAF-mediated) activation of NF-κB (46). We also found that the release of IL-6 in response to PM required mitochondrial ROS generation and NF-κB, suggesting it might occur via a similar pathway. An important finding of our study is that the NF-κB–mediated increase in IL-6 was not sufficient to activate systemic coagulation in the absence of a parallel activation of macrophage β2ARs.
There are some limitations to our study. The Lysm-Cre system results in the expression of Cre recombinase in neutrophils in addition to monocytes and macrophages (47). As we and others have reported that PM exposure is associated with an influx of neutrophils into the lung, we cannot exclude the possibility that the loss of β2ARs in neutrophils contributes to the diminished thrombosis we observed in these animals (4, 48). In addition, our in vitro work raises important questions regarding the mechanisms by which particle exposure increases the generation of ROS from the mitochondria and the molecular targets of these ROS, which are responsible for the priming of adenylyl cyclase. In addition, we have previously shown that PM exposure increases the number of platelets via a mechanism that requires the release of IL-6 from alveolar macrophages. (4) While other groups have shown that PM can cause the activation of platelets (49), we have not been able to detect this in our model (4). In mice, epinephrine alone is unable to promote platelet shape change or aggregation (50–53), but it may potentiate the effect of known activators of platelets such as adenosine diphosphate (ADP) (51, 54, 55). Therefore, while a PM-induced increase in platelet number or activity may contribute to the development of ischemic cardiovascular events in humans, catecholamine-induced changes in platelet numbers or activation alone are unlikely to explain the effects of catecholamines in our model (56).
We did not investigate the mechanism or mechanisms by which PM leads to the activation and the release of catecholamines. In this regard, nerve fibers in the lung have been shown to be activated in response to acrolein (a component of cigarette smoke) or capsaicin through the activation of transient receptor potential (TRP) channels, a superfamily of cation channels that are present on the plasma membrane of many cells including nerve fibers (57, 58). Over 26 TRP channels have been described, and at least 2 of these channels, TRPA1 and TRPV1, have been reported to be activated by PM and play a role in PM-induced lung inflammation; however, it is not known whether they play a role in the PM-induced activation of the sympathetic nervous system (59, 60).
In conclusion, we have discovered a new molecular link between activation of the sympathetic nervous system and the innate immune response in the lung following exposure to PM air pollution. The systemic increase in catecholamines induced by PM air pollution exposure augments the release of IL-6 from lung macrophages via a pathway that requires mitochondrial ROS, adenylyl cyclase, and CREB. The resulting increase in IL-6 release contributes to the development of a hypercoagulable state. These results provide an important mechanism by which activation of the sympathetic nervous system following PM air pollution exposure can increase the risk of thrombotic cardiovascular events. Furthermore, these data provide a possible mechanism explaining the observed association between the use of β2AR agonists and mortality in patients with inflammatory lung diseases and provide a rationale for the use of βAR blocking agents in these patients.
Methods
Human subjects
Nonbronchoscopic BALF was obtained for analysis as part of the clinical care of patients in an intensive care unit. Nonbronchoscopic BALF specimens were collected by trained respiratory therapists using a BALCath (Kimberly-Clark Corporation). The therapist instilled 60 ml of nonbacteriostatic into the lung via the wedged catheter followed by aspiration of returned fluid (typically 10–30 ml). The intensivist caring for the patient chose the lung to be sampled, and the design of the catheter directed it to the dependent lobes of the lung. After the fluid was sent for clinical testing, 1 ml of the residual fluid was placed on ice and brought to the laboratory. The fluid was centrifuged at 600 g for 10 minutes, then washed and resuspended in 10 ml of RPMI medium with 10% FBS supplemented with penicillin, streptomycin, and amphotericin B. The cells were then counted (hemacytometer; Trypan Blue), and 100,000 cells were plated on Primaria Cell Culture 12-well plates (Corning). The cells were used 24 hours after plating.
Animals
We used 8- to 12-week-old male mice lacking either Adrb1 or Adrb2 or both Adrb1 and Adrb2 and their wild-type littermates as controls. Conditional deletion of Adrb2 was achieved by crossing Adrb2flox/flox mice (a gift of Florent Elefteriou, Vanderbilt University, Nashville, Tennessee, USA, and Gerard Karsenty, Columbia University, New York, New York, USA) (61, 62) with Lysm-Cre mice (Jackson Laboratories strain B6.129P2-Lyz2tm1(cre)Ifo/J), both on the C57BL/6 background.
Antibodies and reagents
Propranolol was purchased from Hikma Farmaceutica/West Ward Pharmaceuticals. Albuterol was purchased from Nephron Pharmaceuticals. EUK-134 was purchased from Eukarion Inc. TRIzol was purchased from Life Technologies. CREB and p65 antibodies were purchased from Cell Signaling. All other chemicals were purchased from Sigma-Aldrich.
Exposure of mice to PM
Inhalational exposure to CAPs.
We exposed mice to PM2.5 CAPs (concentrated from ambient air in downtown Chicago) 8 hours per day for 3 days in a chamber connected to VACES (refs. 10, 63–65, and Supplemental Figure 1). The VACES system draws approximately 100 l/min of ambient air from an elevation of 9 m from which the PM is condensed and then resuspended for delivery to a chamber designed specifically to ensure uniform distribution of the particles. We exposed control mice to filtered air in an identical chamber connected to the VACES in which a Teflon filter was placed on the inlet valve to remove all particles. We estimated ambient PM2.5 concentrations as the mean of reported values from the 4 EPA monitoring locations closest to our location (66). Particle counts in the chamber were measured with a TSI 3775 particle counter (Shoreview) and used to determine the enrichment in the chamber compared with the ambient air as previously described (10). The mean daily ambient PM2.5 concentration in Chicago was 11.99 ± 0.68 μg/m3 during the study period, and the mean concentration in the PM exposure chamber was 109.1 ± 6.18 μg/m3. Chicago is the third largest city in the US, with approximately 2.7 million and 9.5 million residents in the city and metropolitan area, respectively. Interstate highways, railroads, and 2 major airports connect the city to other urban areas in the region. Major point sources of particulate air pollution include 2 coal-fired power plants and metal processing, paint, and solvent factories (the last being in the southern and southeast parts of the city) (67). Mobile source emissions account for a majority of atmospheric nitrogen compounds, while refineries, coal burning, and steel manufacturing are responsible for sulfur compounds (67). The composition of airborne PM is primarily sulfate and organic carbons and secondary nitrates (68). Particulate NO3–, SO42–, and elemental carbon concentrations (2.5, 2.9, and 1.5 μg/m3, respectively) approximate those in other major American cities (69).
Intratracheal instillation of PM.
For intratracheal exposure experiments in mice, we used an urban PM collected from ambient air in Washington, DC (National Institute of Standards and Technology standard reference material, SRM 1649a). The characteristics of PM have been previously described (70, 71). We anesthetized the mice with isoflurane and intubated them orally with a 20-gauge angiocath (4, 10, 72). We instilled either PM suspended in 50 μl of sterile PBS (vortexed prior to instillation) or PBS (control).
Collection of BALF and plasma and measurement of IL-6 and plasma TAT levels
We collected BALF and plasma 24 hours after exposure to PM or PBS or 3 days after exposure to CAPs or filtered air, as we have previously described (4, 10, 72). A 200 μl aliquot of the BALF was placed in a cytospin and centrifuged at 1,200 g for 5 minutes at 4°C. The supernatant was used to measure IL-6 and other cytokine levels using the Mouse Inflammation Kit and BD Cytometric Bead Array (BD Biosciences) as we previously described (4). We measured plasma TAT levels as previously described (1, 2).
FeCl3 model of arterial thrombosis
After mice were anesthetized, the left carotid artery was dissected and isolated from the surrounding tissue with paraffin; the adventitia of the artery was treated with Whatman filter paper precisely cut to 1 mm in diameter and soaked in 10% FeCl3. The carotid blood flow was continuously measured using Transonic TS420 Transit-Time Perivascular Flowmeter (Transonic Systems) until blood flow was reduced by more than 75% from baseline, which was used to define the time to loss of blood flow. The application of FeCl3 led to a 2- to 3 mm-long carotid thrombus (Supplemental Figure 2 and Supplemental Video 1).
Cell culture and generation of MH-S cells with stable knockdown of CREB
The murine alveolar macrophage cell line (MH-S, ATCC) was cultured in RPMI medium in 10% FBS. Stably transfected cell lines were generated by infection with lentiviruses encoding a scrambled shRNA or shRNA constructs against CREB and selected with puromycin (5 μg/ml). The lentiviruses were generated by cotransfecting HEK293 cells with pLKO.1-puro plasmids using the Mission Lentiviral Packaging DNA according to the manufacturer’s recommendations (SHP001, SHCLNG NM_1333828 [CREB], and SHC312 [scrambled control]; Sigma-Aldrich). Primary alveolar macrophages were obtained as previously described by repeated bronchoalveolar lavage with PBS containing 0.6 mM ethylenediaminetetraacetic acid (4). The primary alveolar macrophages were plated and cultured in RPMI medium in 10% FBS.
Tissue homogenization
The lung, heart, adrenal glands, and uterus were removed after the right ventricle was perfused in situ with sterile PBS until the lungs and liver were clear. Brown fat was isolated from the midscapular region of the back. All organs were kept on ice prior to and during homogenization (Tissue Tearor, 30 s) in a flow cytometry tube with 1 ml of PBS. An additional 2 ml of PBS was added to the resulting homogenate and subjected to Dounce homogenization (20 strokes).
Assessment of CREB nuclear translocation
Approximately 107 cells per condition (10 cm dishes) were removed from the plate with trypsin and washed twice in PBS (200 g, 10 minutes). The cell pellet was suspended in 2 ml of NP-40 buffer (100 ml of Chelsky buffer [0.01M Tris-HCl, 0.01M NaCl, 0.003 M MgCl2, 0.03 M sucrose, pH 7.0] and 500 μl NP-40), then centrifuged twice (HB-40 rotor, 1500 g, 10 minutes). The supernatants from the washes were used for immunoblotting (cytosolic fraction). The nuclear pellet was washed twice in 2 ml CaCl2 buffer (100 ml Chelsky buffer, 1 ml 10 mM CaCl2) and centrifuged (HB-40 rotor, 1500 g, 10 minutes). The resulting pellet was resuspended in 1 ml of buffer (20 mM Tris-HCl, pH 7.9, 20% glycerol, 0.1 M KCl, 0.2 M EDTA, pH 7.9) and centrifuged (SS43 rotor, 25,000 g, 30 minutes), and the supernatant (nucleoplasm) was used for immunoblotting (nuclear fraction).
Measurement of IL-6
Cell supernatants or BALF was centrifuged at 300 g for 10 minutes, and IL-6 levels were measured using a commercially available ELISA according to the manufacturer’s instructions (Invitrogen).
Quantitative real-time RT-PCR (qRT-PCR)
We isolated total RNA from mouse lung using a commercially available system (TRIzol; Invitrogen) and performed qRT-PCR reactions for Il6, SP-B, TF, Creb, and Adrb2 using IQ SYBR Green superscriptΠ analyzedΠon a Bio-Rad IQ5 Real-Time PCR Detection System using previously described primer sequences and the Pfaffl method (10). The following primer sequences were used: IL-6, forward: CCGGAGAACCTGCTGGCAATCC, IL-6, reverse: TTCCATCCAGTTGCCTTCTTGG; SP-B, forward: GCCTTGTCCTCGGATGTTC, SP-B, reverse: TAGCCTGTTCACTGGTGTTC; β2AR, forward: ATCTGAAGGAAGATTCCACGCCCA, β2AR, reverse: AGAGGGTGAATGTGCCCATGATGA.
Measurement of cAMP
cAMP levels in cell lysates were measured using a cAMP immunoassay kit according to the manufacturer’s directions (Assay Designs). Treatment with forskolin, a direct activator of adenylyl cyclase (100 μM, 1 minute) (Ascent Scientific) or control vehicle (2% ethanol) was used as a positive control.
Measurement of catecholamine levels
Catecholamine levels in plasma, BALF, homogenized lung, brown adipose tissue (BAT), and adrenal gland were measured using high-pressure liquid chromatography with an electrochemical detector (LC-4C Detector; Bioanalytical Systems Inc.) as we previously described (73, 74).
Measurement of inflammatory cell populations in dispase-digested lungs
After the mice were euthanized, lungs were perfused through the right ventricle with 5 ml of PBS. The lungs were removed, and the large airways were dissected from the peripheral lung tissue. The peripheral lung tissue was cut into small pieces with scissors, transferred into C-tubes (Miltenyi Biotec), and processed in digestion buffer (1 mg/ml of collagenase D and 0.1 mg/ml DNase I, both from Roche in HBSS) and a gentleMACS dissociator (Miltenyi Biotec), as we have recently described (75).
Cells were stained with viability dye Aqua (Invitrogen) or eFluor 506 (eBioscience), incubated with FcBlock (BD Biosciences), and stained with a mixture of fluorochrome-conjugated antibodies. Data were acquired on a BD LSR II Flow Cytometer using BD FACSDiva software (BD Biosciences), and compensation and data analyses were performed offline using FlowJo software (TreeStar). Cell sorting was performed on a FACSAria II instrument (BD Biosciences) with the same configuration as the LSR II. Cell populations were identified using sequential gating strategy (see Results), and the percentage of cells in the live/singlets gate was multiplied by the number of live cells (after Trypan blue exclusion) to obtain an absolute live-cell count.
Statistics
We report all data as mean ± SEM. We subjected all data to 1-way ANOVA. When ANOVA indicated a significant difference, we explored individual differences with 2-tailed Student’s t test using Bonferroni’s correction for multiple comparisons (Prism 6; Graphpad). Statistical significance was defined as P < 0.05.
Study approval
All animal experiments and procedures were performed according to protocols approved by the Institutional Animal Care and Use Committee at Northwestern University. All studies using samples obtained from human subjects were approved by the Northwestern University Institutional Review Board with a waiver of consent.
Supplementary Material
Acknowledgments
This work was supported by NIH ES015024, ES013995, HL071643, the Northwestern University Clinical and Translational Sciences Institute (NUCATS) Center for Translational Innovation (CTI) Pilot Award (NCCR UL1 RR025741), and the Veterans Administration. The authors would like to thank Yiu-Kuen Chow and Nancy Foiles for their assistance with the measurement of catecholamines and plasma TAT levels, respectively, and Robert Schleimer for his careful review of the data.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article:J Clin Invest. 2014;124(7):2935–2946. doi:10.1172/JCI75157.
Gökhan M. Mutlu’s present address is: Section of Pulmonary and Critical Care Medicine, University of Chicago, Chicago, Illinois, USA.
References
- 1.Pope CA, 3rd, Ezzati M, Dockery DW. Fine-particulate air pollution and life expectancy in the United States. N Engl J Med. 2009;360(4):376–386. doi: 10.1056/NEJMsa0805646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. UN Environment Program World Health Organization. Air Pollution In The World’s Megacities: A Report From The UN Environment Programme And Who Environment. New York, New York, USA: United Nations; 1994;35–37. [Google Scholar]
- 3.Brook RD, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 4.Mutlu GM, et al. Ambient particulate matter accelerates coagulation via an IL-6-dependent pathway. J Clin Invest. 2007;117(10):2952–2961. doi: 10.1172/JCI30639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baccarelli A, et al. Effects of exposure to air pollution on blood coagulation. J Thromb Haemost. 2007;5(2):252–260. doi: 10.1111/j.1538-7836.2007.02300.x. [DOI] [PubMed] [Google Scholar]
- 6.Baccarelli A, et al. Exposure to particulate air pollution and risk of deep vein thrombosis. Arch Intern Med. 2008;168(9):920–927. doi: 10.1001/archinte.168.9.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghio AJ, Kim C, Devlin RB. Concentrated ambient air particles induce mild pulmonary inflammation in healthy human volunteers. Am J Respir Crit Care Med. 2000;162(3 pt 1):981–988. doi: 10.1164/ajrccm.162.3.9911115. [DOI] [PubMed] [Google Scholar]
- 8.Riediker M, et al. Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am J Respir Crit Care Med. 2004;169(8):934–940. doi: 10.1164/rccm.200310-1463OC. [DOI] [PubMed] [Google Scholar]
- 9.Mills NL, et al. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 2007;357(11):1075–1082. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- 10.Budinger GR, et al. Particulate matter-induced lung inflammation increases systemic levels of PAI-1 and activates coagulation through distinct mechanisms. PLoS One. 2011;6(4):e18525. doi: 10.1371/journal.pone.0018525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold DR, et al. Ambient pollution and heart rate variability. Circulation. 2000;101(11):1267–1273. doi: 10.1161/01.CIR.101.11.1267. [DOI] [PubMed] [Google Scholar]
- 12.Pope CA, 3rd, et al. Heart rate variability associated with particulate air pollution. Am Heart J. 1999;138(5 pt 1):890–899. doi: 10.1016/s0002-8703(99)70014-1. [DOI] [PubMed] [Google Scholar]
- 13.Watkinson WP, Campen MJ, Costa DL. Cardiac arrhythmia induction after exposure to residual oil fly ash particles in a rodent model of pulmonary hypertension. Toxicol Sci. 1998;41(2):209–216. doi: 10.1093/toxsci/41.2.209. [DOI] [PubMed] [Google Scholar]
- 14.Bartoli CR, et al. Mechanisms of inhaled fine particulate air pollution-induced arterial blood pressure changes. Environ Health Perspect. 2009;117(3):361–366. doi: 10.1289/ehp.11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niwa Y, Hiura Y, Sawamura H, Iwai N. Inhalation exposure to carbon black induces inflammatory response in rats. Circ J. 2008;72(1):144–149. doi: 10.1253/circj.72.144. [DOI] [PubMed] [Google Scholar]
- 16.Maciejczyk P, Zhong M, Li Q, Xiong J, Nadziejko C, Chen LC. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. II. The design of a CAPs exposure system for biometric telemetry monitoring. Inhal Toxicol. 2005;17(4–5):189–197. doi: 10.1080/08958370590912743. [DOI] [PubMed] [Google Scholar]
- 17.Erickson JD, Schafer MK, Bonner TI, Eiden LE, Weihe E. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1996;93(10):5166–5171. doi: 10.1073/pnas.93.10.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mutlu GM, et al. Upregulation of alveolar epithelial active Na+ transport is dependent on β2-adrenergic receptor signaling. Circ Res. 2004;94(8):1091–1100. doi: 10.1161/01.RES.0000125623.56442.20. [DOI] [PubMed] [Google Scholar]
- 19.Mutlu GM, Koch WJ, Factor P. Alveolar epithelial β2-adrenergic receptors: their role in regulation of alveolar active sodium transport. Am J Respir Crit Care Med. 2004;170(12):1270–1275. doi: 10.1164/rccm.200404-470CP. [DOI] [PubMed] [Google Scholar]
- 20.Mutlu GM, Factor P. Alveolar epithelial β2-adrenergic receptors. Am J Respir Cell Mol Biol. 2008;38(2):127–134. doi: 10.1165/rcmb.2007-0198TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stiles GL, Caron MG, Lefkowitz RJ. β-Adrenergic receptors: biochemical mechanisms of physiological regulation. Physiol Rev. 1984;64(2):661–743. doi: 10.1152/physrev.1984.64.2.661. [DOI] [PubMed] [Google Scholar]
- 22.Bosmann M, et al. Anti-inflammatory effects of β2 adrenergic receptor agonists in experimental acute lung injury. FASEB J. 2012;26(5):2137–2144. doi: 10.1096/fj.11-201640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konstantinides S, Schafer K, Thinnes T, Loskutoff DJ. Plasminogen activator inhibitor-1 and its cofactor vitronectin stabilize arterial thrombi after vascular injury in mice. Circulation. 2001;103(4):576–583. doi: 10.1161/01.CIR.103.4.576. [DOI] [PubMed] [Google Scholar]
- 24.Krief S, et al. Tissue distribution of β3-adrenergic receptor mRNA in man. J Clin Invest. 1993;91(1):344–349. doi: 10.1172/JCI116191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hjemdahl P, Larsson K, Johansson MC, Zetterlund A, Eklund A. β-Adrenoceptors in human alveolar macrophages isolated by elutriation. Br J Clin Pharmacol. 1990;30(5):673–682. doi: 10.1111/j.1365-2125.1990.tb03835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Broug-Holub E, Persoons JH, Schornagel K, Mastbergen SC, Kraal G. Effects of stress on alveolar macrophages: a role for the sympathetic nervous system. Am J Respir Cell Mol Biol. 1998;19(5):842–848. doi: 10.1165/ajrcmb.19.5.3103. [DOI] [PubMed] [Google Scholar]
- 27.Liggett SB. Identification and characterization of a homogeneous population of β2-adrenergic receptors on human alveolar macrophages. Am Rev Respir Dis. 1989;139(2):552–555. doi: 10.1164/ajrccm/139.2.552. [DOI] [PubMed] [Google Scholar]
- 28.Delghandi MP, Johannessen M, Moens U. The cAMP signalling pathway activates CREB through PKA, p38 and MSK1 in NIH 3T3 cells. Cell Signal. 2005;17(11):1343–1351. doi: 10.1016/j.cellsig.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Soberanes S, et al. Mitochondrial complex III-generated oxidants activate ASK1 and JNK to induce alveolar epithelial cell death following exposure to particulate matter air pollution. J Biol Chem. 2009;284(4):2176–2186. doi: 10.1074/jbc.M808844200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerr R, Stirling D, Ludlam CA. Interleukin 6 and haemostasis. Br J Haematol. 2001;115(1):3–12. doi: 10.1046/j.1365-2141.2001.03061.x. [DOI] [PubMed] [Google Scholar]
- 31.Rich DQ, et al. Association between changes in air pollution levels during the Beijing Olympics and biomarkers of inflammation and thrombosis in healthy young adults. JAMA. 2012;307(19):2068–2078. doi: 10.1001/jama.2012.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kramer JM. Balancing the benefits and risks of inhaled long-acting β-agonists — the influence of values. N Engl J Med. 2009;360(16):1592–1595. doi: 10.1056/NEJMp0810561. [DOI] [PubMed] [Google Scholar]
- 33.Drazen JM, O’Byrne PM. Risks of long-acting β-agonists in achieving asthma control. N Engl J Med. 2009;360(16):1671–1672. doi: 10.1056/NEJMe0902057. [DOI] [PubMed] [Google Scholar]
- 34.Martinez FD. Safety of long-acting β-agonists — an urgent need to clear the air. N Engl J Med. 2005;353(25):2637–2639. doi: 10.1056/NEJMp058299. [DOI] [PubMed] [Google Scholar]
- 35. FDA. Long-Acting Beta-Agonists (LABAs): New Safe Use Requirements. FDA Web site. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm201003.htm. Updated April 15, 2011. Accessed April 23, 2014.
- 36.Salpeter SR, Buckley NS, Salpeter EE. Meta-analysis: anticholinergics, but not β-agonists, reduce severe exacerbations and respiratory mortality in COPD. J Gen Intern Med. 2006;21(10):1011–1019. doi: 10.1111/j.1525-1497.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chowdhury BA, Dal Pan G. The FDA and safe use of long-acting β-agonists in the treatment of asthma. N Engl J Med. 2010;362(13):1169–1171. doi: 10.1056/NEJMp1002074. [DOI] [PubMed] [Google Scholar]
- 38.Spitzer WO, et al. The use of β-agonists and the risk of death and near death from asthma. N Engl J Med. 1992;326(8):501–506. doi: 10.1056/NEJM199202203260801. [DOI] [PubMed] [Google Scholar]
- 39.Rutten FH, Zuithoff NP, Hak E, Grobbee DE, Hoes AW. Beta-blockers may reduce mortality and risk of exacerbations in patients with chronic obstructive pulmonary disease. Arch Intern Med. 2010;170(10):880–887. doi: 10.1001/archinternmed.2010.112. [DOI] [PubMed] [Google Scholar]
- 40.Sin DD, Man SF. A curious case of beta-blockers in chronic obstructive pulmonary disease. Arch Intern Med. 2010;170(10):849–850. doi: 10.1001/archinternmed.2010.97. [DOI] [PubMed] [Google Scholar]
- 41.Dransfield MT, Rowe SM, Johnson JE, Bailey WC, Gerald LB. Use of beta blockers and the risk of death in hospitalised patients with acute exacerbations of COPD. Thorax. 2008;63(4):301–305. doi: 10.1136/thx.2007.081893. [DOI] [PubMed] [Google Scholar]
- 42.Au DH, et al. Beta-blockers as single-agent therapy for hypertension and the risk of mortality among patients with chronic obstructive pulmonary disease. Am J Med. 2004;117(12):925–931. doi: 10.1016/j.amjmed.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 43.National Heart, Lung, Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, et al. Randomized, placebo-controlled clinical trial of an aerosolized β2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184(5):561–568. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao Smith F, et al. Effect of intravenous β-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet. 2012;379(9812):229–235. doi: 10.1016/S0140-6736(11)61623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Budinger GR, Mutlu GM. β2-agonists and acute respiratory distress syndrome. Am J Respir Crit Care Med. 2014;189(6):624–625. doi: 10.1164/rccm.201401-0170ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imai Y, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133(2):235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hume DA. Applications of myeloid-specific promoters in transgenic mice support in vivo imaging and functional genomics but do not support the concept of distinct macrophage and dendritic cell lineages or roles in immunity. J Leukoc Biol. 2011;89(4):525–538. doi: 10.1189/jlb.0810472. [DOI] [PubMed] [Google Scholar]
- 48.Nemmar A, Hoet PH, Dinsdale D, Vermylen J, Hoylaerts MF, Nemery B. Diesel exhaust particles in lung acutely enhance experimental peripheral thrombosis. Circulation. 2003;107(8):1202–1208. doi: 10.1161/01.CIR.0000053568.13058.67. [DOI] [PubMed] [Google Scholar]
- 49.Wilson DW, et al. Exposure of mice to concentrated ambient particulate matter results in platelet and systemic cytokine activation. Inhal Toxicol. 2010;22(4):267–276. doi: 10.3109/08958370903278069. [DOI] [PubMed] [Google Scholar]
- 50.Nunn B. Some characteristics of mouse platelet aggregation and a comparison of the activity of a range of compounds in mouse and human platelet-rich plasma in vitro. Thromb Haemost. 1981;45(1):1–5. [PubMed] [Google Scholar]
- 51.Pozgajova M, Sachs UJ, Hein L, Nieswandt B. Reduced thrombus stability in mice lacking the α2A-adrenergic receptor. Blood. 2006;108(2):510–514. doi: 10.1182/blood-2005-12-4835. [DOI] [PubMed] [Google Scholar]
- 52.Lanza F, Cazenave JP. Studies of α2-adrenergic receptors of intact and functional washed human platelets by binding of 3H-dihydroergocryptine and 3H-yohimbine — correlation of 3H-yohimbine binding with the potentiation by adrenaline of ADP-induced aggregation. Thromb Haemost. 1985;54(2):402–408. [PubMed] [Google Scholar]
- 53.Nieswandt B, et al. Evidence for cross-talk between glycoprotein VI and Gi-coupled receptors during collagen-induced platelet aggregation. Blood. 2001;97(12):3829–3835. doi: 10.1182/blood.V97.12.3829. [DOI] [PubMed] [Google Scholar]
- 54.Larson MK, et al. Identification of P2Y12-dependent and -independent mechanisms of glycoprotein VI-mediated Rap1 activation in platelets. Blood. 2003;101(4):1409–1415. doi: 10.1182/blood-2002-05-1533. [DOI] [PubMed] [Google Scholar]
- 55.Woulfe D, Jiang H, Mortensen R, Yang J, Brass LF. Activation of Rap1B by G(i) family members in platelets. J Biol Chem. 2002;277(26):23382–23390. doi: 10.1074/jbc.M202212200. [DOI] [PubMed] [Google Scholar]
- 56.Kjeldsen SE, Weder AB, Egan B, Neubig R, Zweifler AJ, Julius S. Effect of circulating epinephrine on platelet function and hematocrit. Hypertension. 1995;25(5):1096–1105. doi: 10.1161/01.HYP.25.5.1096. [DOI] [PubMed] [Google Scholar]
- 57.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426(6966):517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 58.Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82(2):429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- 59.Deering-Rice CE, et al. Transient receptor potential vanilloid-1 (TRPV1) is a mediator of lung toxicity for coal fly ash particulate material. Mol Pharmacol. 2012;81(3):411–419. doi: 10.1124/mol.111.076067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deering-Rice CE, et al. Electrophilic components of diesel exhaust particles (DEP) activate transient receptor potential ankyrin-1 (TRPA1): a probable mechanism of acute pulmonary toxicity for DEP. Chem Res Toxicol. 2011;24(6):950–959. doi: 10.1021/tx200123z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hinoi E, et al. The sympathetic tone mediates leptin’s inhibition of insulin secretion by modulating osteocalcin bioactivity. J Cell Biol. 2008;183(7):1235–1242. doi: 10.1083/jcb.200809113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kajimura D, et al. Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual. J Exp Med. 2011;208(4):841–851. doi: 10.1084/jem.20102608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sioutas C, Koutrakis P, Burton RM. A technique to expose animals to concentrated fine ambient aerosols. Environ Health Perspect. 1995;103(2):172–177. doi: 10.1289/ehp.95103172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maciejczyk P, Chen LC. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. VIII. Source-related daily variations in in vitro responses to CAPs. Inhal Toxicol. 2005;17(4–5):243–253. doi: 10.1080/08958370590912914. [DOI] [PubMed] [Google Scholar]
- 65.Sioutas C, Seongheon K, Chang M. Development and evaluation of a prototype ultrafine particle concentrator. J Aerosol Sci. 1999;30(8):1001–1017. doi: 10.1016/S0021-8502(98)00769-1. [DOI] [Google Scholar]
- 66.State of Illinois Environmental Protection Agency. Illinois Annual Air Quality Report 2012. State of Illinois Web site. http://www.epa.state.il.us/air/air-quality-report/2012/air-quality-report-2012.pdf Accessed April 23, 2014.
- 67.Binaku K, O’Brien T, Schmeling M, Fosco T. Statistical analysis of aerosol species, trace gasses, and meteorology in Chicago. Environ Monit Assess. 2013;185(9):7295–7308. doi: 10.1007/s10661-013-3101-y. [DOI] [PubMed] [Google Scholar]
- 68. EPA. Our Nation’s Air: Status and Trends through 2008. EPA Web site. http://www.epa.gov/airtrends/2010/report/fullreport.pdf. Accessed April 23, 2014.
- 69.Babich P, Davey M, Allen G, Koutrakis P. Method comparisons for particulate nitrate, elemental carbon, and PM2.5 mass in seven U.S. cities. J Air Waste Manag Assoc. 2000;50(7):1095–1105. doi: 10.1080/10473289.2000.10464152. [DOI] [PubMed] [Google Scholar]
- 70.Currie LA, et al. A critical evaluation of interlaboratory data on total, elemental, and isotopic carbon in the carbonaceous particle reference material, NIST SRM 1649a. J Res NIST. 2002;107(3):279–298. doi: 10.6028/jres.107.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. National Institute of Standards and Technology. SRM 1649a: Urban Dust/Organics. US Department of Commerce Web site. https://www-s.nist.gov/srmors/view_detail.cfm?srm=1649A. Updated August 28, 2013. Accessed April 23, 2014.
- 72.Mutlu GM, et al. Airborne particulate matter inhibits alveolar fluid reabsorption in mice via oxidant generation. Am J Respir Cell Mol Biol. 2006;34(6):670–676. doi: 10.1165/rcmb.2005-0329OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Williams M, Young JB, Rosa RM, Gunn S, Epstein FH, Landsberg L. Effect of protein ingestion on urinary dopamine excretion. Evidence for the functional importance of renal decarboxylation of circulating 3,4-dihydroxyphenylalanine in man. J Clin Invest. 1986;78(6):1687–1693. doi: 10.1172/JCI112763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adir Y, et al. Augmentation of endogenous dopamine production increases lung liquid clearance. Am J Respir Crit Care Med. 2004;169(6):757–763. doi: 10.1164/rccm.200207-744OC. [DOI] [PubMed] [Google Scholar]
- 75.Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol. 2013;49(4):503–510. doi: 10.1165/rcmb.2013-0086MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.