Abstract
Cardiac fibroblasts have been long recognized as active participants in heart disease; however, their exact physiological and pathological roles remain elusive, mainly due to the lack of specific markers. In this issue of the JCI, Moore-Morris and colleagues used a fibroblast-specific collagen1a1-GFP reporter to demonstrate that fibroblast accumulation after aortic banding in murine hearts arises almost exclusively from proliferation of resident fibroblasts originating from both the epicardium and a previously unrecognized source, the endocardium. Further characterization of fibroblast origin and function in different types and stages of heart disease could lead to development of improved fibroblast-targeted cardiac therapies.
Lack of specific cardiac fibroblast markers
Cardiac fibroblasts comprise 30% to 70% of all the cells in the healthy adult heart (1). The number of fibroblasts in the heart is not constant and changes dynamically with development, disease, and aging (2, 3). Traditionally, cardiac fibroblasts have been thought to play passive roles in the heart and to be solely responsible for maintaining homeostasis of extracellular matrix proteins, including type I and III collagens and fibronectin. Due to their ubiquitous presence in the heart, fibroblasts are well poised to actively regulate and modify cardiac function through their direct contacts with other cardiac cells and matrix as well as through secretion of different cytokines, matrix proteins, and proteases. Over the last decade, the pleiotropic roles of fibroblasts in cardiac biology and disease have been studied extensively (reviewed in refs. 4, 5); however, the lack of specific and comprehensive markers of fibroblast phenotypes has hampered the progress in this important research field. In particular, vimentin, the most inclusive marker of cardiac fibroblasts, also labels all other mesoderm-derived cells in the heart. Thymus cell antigen-1 (Thy-1, also known as CD90), discoidin domain receptor-2 (DDR2), prolyl-4-hydroxylase (P4H), transcription factor 21 (TCF21, also known as epicardin, Pod1, and capsulin), periostin, cadherin-11, and fibroblast-specific protein-1 (FSP1, also known as S100A4) have all been used to study cardiac fibroblasts, but all of these markers label only a subset of fibroblasts, have poor expression in the healthy adult heart, or nonselectively label endothelial, smooth muscle, or immune/inflammatory cells (6). Because the pathological tissue remodeling that is secondary to cardiac injury and inflammation involves contributions from both resident and extracardiac cells, the lack of adequate markers for cardiac fibroblasts may lead to erroneous conclusions about their origin, roles, and potential to be therapeutically targeted in fibrotic heart disease.
Collagen1a1-GFP, but not FSP1, is a robust cardiac fibroblast marker
In this issue of the JCI, Moore-Morris and colleagues (7) used mice in which GFP expression is driven by a collagen1a1 enhancer (8) to investigate the origin and fate of cardiac fibroblasts in healthy hearts and in pressure overload hypertrophy induced by transverse aortic constriction (TAC). In the healthy ventricles, collagen1a1-GFP was expressed robustly in interstitial noncardiomyocytes that were also positive for vimentin and PDGFRα, a mesenchymal marker shown recently to label cardiac fibroblasts (9). This population of GFP+ cells also expressed Thy-1 (in 70% of cells) but did not express markers of endothelial cells (PECAM1), pericytes (PDGRFβ), leukocytes (CD45), or smooth muscle cells (αSMA). After TAC, GFP+ cells maintained PDGFRα expression, did not express other cell type–specific markers, and accumulated in both interstitial and perivascular areas rich in collagen I. On the other hand, FSP1, which is frequently used as a cardiac fibroblast marker (10), was almost exclusively expressed in CD45+ cells, with no expression detected in interstitial GFP+ fibroblasts and a limited presence in perivascular GFP+ fibroblasts; therefore, similar to previous studies (11, 12), FSP1 preferentially labeled immune cells. Hence, Moore-Morris et al. have identified collagen1a1-GFP as a robust and specific marker of cardiac fibroblasts in both healthy and pressure-overloaded hearts. It remains to be determined whether all fibroblasts in the heart are labeled with this marker.
Fibroblast origin in developing, adult, and pressure-overloaded hearts
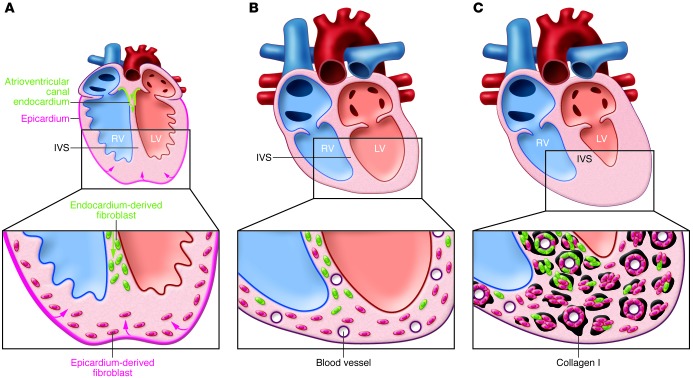
By using the collagen1a1-GFP reporter along with constitutive and inducible Cre-based lineage tracing of epicardial (Wt1 and Tbx18), endocardial/endothelial (Tie2, VE-cadherin, Nfatc1), and hematopoietic (Vav) populations, Moore-Morris and colleagues have further explored the origin of cardiac fibroblasts in developing, adult, and pressure-overloaded hearts. Traditionally, all nonvalvular fibroblasts in the healthy heart have been thought to derive from epicardium (2). Thus, it was surprising that epicardial-specific Cre drivers labeled only 30% of GFP+ cells in the interventricular septum (IVS), while 92% of GFP+ cells in the LV and RV were of epicardial origin. Even more surprising, complementary numbers of nonepicardium-derived fibroblasts (64% in the IVS and 12% in the LV and RV) were found to be of endocardial/endothelial origin, characteristic of valvular interstitial cells (13). Together, in healthy ventricles, 95% of all GFP+ fibroblasts were of endocardial or epicardial origin, and none of them were hematopoietically derived, as the Vav-Cre driver exclusively labeled CD45+ or PECAM1+ cells. After aortic bending, both epicardial- and endocardial-derived fibroblasts underwent transient proliferation while maintaining their relative proportions within the GFP+ population and spatial distributions in the LV, RV, IVS, interstitial and perivascular regions. Furthermore, the resulting fibroblast accumulation was not the result of de novo activation of the epicardium or endothelium/endocardium (in contrast to ref. 10) or caused by differentiation of hematopoietic cells. By performing additional lineage tracing, Moore-Morris and colleagues showed that the newly identified endocardial fibroblast lineage in the adult myocardium is created in early embryos by endothelial-to-mesenchymal transition of atrioventricular canal endocardium during cushion formation (Figure 1A).
Figure 1. Cardiac fibroblasts in developing, adult, and pressure overloaded hearts.
(A) In the embryonic heart, most fibroblasts are derived from epicardium (pink). The fibroblast lineage described by Moore-Morris et al. is generated from the atrioventricular canal endocardium (green) and migrates down the IVS. (B) In the adult heart, the dominant fraction of fibroblasts (∼90%) in the RV and LV are of epicardial origin, while 64% of fibroblasts in the IVS derive from the endocardium. (C) In response to pressure overload, the heart becomes hypertrophic and undergoes fibrosis. In response to pressure overload, both epicardium-derived and endocardium-derived fibroblast lineages proliferate and accumulate in interstitial and perivascular regions along with collagen I (black). The LV undergoes more fibrosis than the RV. Endocardium-derived fibroblasts participate in perivascular fibrosis in the IVS but not in the LV or RV. These two resident lineages account for approximately 95% of fibroblasts in the healthy and pressure-overloaded ventricles.
Taken together, these results strongly suggest that the vast majority of fibroblasts in the adult mouse heart derive from two resident lineages, a previously characterized epicardial-derived population and a newly described endocardial-derived population (Figure 1B). These two fibroblast lineages have a specific spatial distribution in different regions of the heart as well as in perivascular and interstitial space. During pressure overload (Figure 1C), localized proliferation of these fibroblasts (without substantial migration) and their persistence, rather than de novo epicardial and endothelial activation or differentiation of hematopoietic cells is the main driver of fibrosis and thus has potential as a therapeutic target. The origin of the remaining GFP+ fibroblasts (5%–6% of fibroblasts) that are not derived from the epicardium or endocardium is currently unknown, and, as suggested by Moore-Morris et al., these cells could arise from other resident or nonhematopoietic circulating progenitors and may also contribute to fibrosis. An inducible collagen1a1-Cre mouse line, when available, could further help elucidate the fibroblast fate in cardiac pathology.
Future studies and therapeutic implications
As with any good study, the findings by Moore-Morris et al. open multiple avenues for future investigation. Surprisingly, even after 4 weeks of TAC, none of the perivascular fibroblasts and only 15% of interstitial fibroblasts expressed the myofibroblast marker αSMA, suggesting that, unlike in healing myocardial infarcts (14), myofibroblasts in pressure overload hypertrophy are not abundant or associated with the collagen accumulation. Furthermore, as recently shown by Braitsch et al. (15) and confirmed in the study by Moore-Morris and colleagues, pressure overload does not induce epicardial activation and thickening observed in myocardial infarction. Together, these results suggest that the specific fibrogenic roles of resident fibroblasts and myofibroblasts (through proliferation and/or migration), epicardial and endothelial activation, and pericyte- or bone marrow–derived progenitors may differ depending on the underlying heart pathology (e.g., myocardial infarction, congenital cardiomyopathy, diabetes, hypertension, etc.). While disease-specific differences in the mechanical environment (16) or the level of inflammation in the heart may favor a particular fibrotic response, detailed studies in the mouse lines used by Moore-Morris et al. are warranted and will help to answer these important questions.
Curiously, Moore-Morris and colleagues found that fibroblast proliferation peaks at 4 to 7 days after TAC and ceases thereafter, as previously reported (17). Simultaneously, the degree of fibrosis remained steady, and while ventricles developed hypertrophy, cardiac function decreased only slightly, without signs of cardiomyocyte apoptosis. Taken together, the 4-week remodeling after aortic banding in the study by Moore-Morris et al. appears to be mostly adaptive rather than maladaptive. On the other hand, molecular changes in cardiac fibroblasts associated with irreversible maladaptive remodeling may represent the most appropriate targets for fibroblast-specific therapies. It will be important for future investigations to examine the fate of cardiac fibroblasts in the later phases of hypertrophic disease, including accelerated pathological remodeling and subsequent heart failure. Furthermore, transcriptional profiling at 7 days after TAC revealed that both the epicardial and endocardial fibroblast lineages underwent similar gene expression changes in response to pressure overload, including upregulation of anti-death–associated genes. This observation implies that timely proapoptotic targeting of all resident cardiac fibroblasts, by inhibition of miR-21 or activation of miR-29 (18) for example, has potential as a promising strategy to alleviate fibrosis in the heart. It should be noted that, despite the global nature of pressure overload, the resulting fibrotic sequela occurs in distinct interstitial and perivascular lesions. Why these particular regions of the myocardium are especially prone to fibrogenesis remains unknown. In fact, one could even imagine the existence of “fibrogenic niches” throughout the heart in which certain highly responsive fibroblasts and/or surrounding biochemical or biomechanical cues locally drive cardiac fibrosis.
Most importantly, the relevance of the findings by Moore-Morris et al. for human heart disease and therapy remains to be determined (19, 20). Undoubtedly, an improved understanding of cardiac fibroblast origin, fate, and interaction with other cell types in the heart as well as the complex roles of cardiac fibroblasts in cardiac development, function, and disease will be fundamental to our ability to use these cells as a future therapeutic tool. The study by Moore-Morris et al. is a step in the right direction.
Acknowledgments
This work is supported by NIH/National Heart, Lung, and Blood Institute grant HL104326. The content of this commentary is solely the responsibility of the author and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article:J Clin Invest. 2014;124(7):2850–2853. doi:10.1172/JCI76628.
See the related article beginning on page 2921.
References
- 1.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105(12):1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snider P, Standley KN, Wang J, Azhar M, Doetschman T, Conway SJ. Origin of cardiac fibroblasts and the role of periostin. Circ Res. 2009;105(10):934–947. doi: 10.1161/CIRCRESAHA.109.201400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biernacka A, Frangogiannis NG. Aging and cardiac fibrosis. Aging Dis. 2011;2(2):158–173. [PMC free article] [PubMed] [Google Scholar]
- 4.Hirsch E, Nagai R, Thum T. Heterocellular signalling and crosstalk in the heart in ischaemia and heart failure. Cardiovasc Res. 2014;102(2):191–193. doi: 10.1093/cvr/cvu073. [DOI] [PubMed] [Google Scholar]
- 5.Sadoshima J, Weiss JN. Cardiac fibroblasts: The good, the bad, the ugly, the beautiful. J Mol Cell Cardiol. 2014;70:1. doi: 10.1016/j.yjmcc.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krenning G, Zeisberg EM, Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol. 2010;225(3):631–637. doi: 10.1002/jcp.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore-Morris T, et al. Resident fibroblast lineages mediate pressure overload–induced cardiac fibrosis. J Clin Invest. 2014;124(7):2921–2934. doi: 10.1172/JCI74783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yata Y, et al. DNase I-hypersensitive sites enhance alpha1(I) collagen gene expression in hepatic stellate cells. Hepatology. 2003;37(2):267–276. doi: 10.1053/jhep.2003.50067. [DOI] [PubMed] [Google Scholar]
- 9.Smith CL, Baek ST, Sung CY, Tallquist MD. Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require PDGF receptor signaling. Circ Res. 2011;108(12):e15–e26. doi: 10.1161/CIRCRESAHA.110.235531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeisberg EM, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13(8):952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 11.Osterreicher CH, et al. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc Natl Acad Sci U S A. 2011;108(1):308–313. doi: 10.1073/pnas.1017547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong P, Christia P, Saxena A, Su Y, Frangogiannis NG. Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis. Am J Physiol Heart Circ Physiol. 2013;305(9):H1363–H1372. doi: 10.1152/ajpheart.00395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenberg LM, Markwald RR. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ Res. 1995;77(1):1–6. doi: 10.1161/01.RES.77.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Shinde AV, Frangogiannis NG. Fibroblasts in myocardial infarction: A role in inflammation and repair. J Mol Cell Cardiol. 2014;70C:74–82. doi: 10.1016/j.yjmcc.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braitsch CM, Kanisicak O, van Berlo JH, Molkentin JD, Yutzey KE. Differential expression of embryonic epicardial progenitor markers and localization of cardiac fibrosis in adult ischemic injury and hypertensive heart disease. J Mol Cell Cardiol. 2013;65:108–119. doi: 10.1016/j.yjmcc.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43(1):146–155. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Souders CA, Borg TK, Banerjee I, Baudino TA. Pressure overload induces early morphological changes in the heart. Am J Pathol. 2012;181(4):1226–1235. doi: 10.1016/j.ajpath.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu H, Fan GC. Role of microRNAs in the reperfused myocardium towards post-infarct remodelling. Cardiovasc Res. 2012;94(2):284–292. doi: 10.1093/cvr/cvr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasenfuss G. Animal models of human cardiovascular disease, heart failure and hypertrophy. Cardiovasc Res. 1998;39(1):60–76. doi: 10.1016/S0008-6363(98)00110-2. [DOI] [PubMed] [Google Scholar]
- 20.Masci PG, et al. Incremental prognostic value of myocardial fibrosis in patients with non-ischemic cardiomyopathy without congestive heart failure. Circ Heart Fail. 2014;7(3):448–456. doi: 10.1161/CIRCHEARTFAILURE.113.000996. [DOI] [PubMed] [Google Scholar]