Abstract
Step length variability (SLV) increases with age in those without overt neurologic disease, is higher in neurologic patients, is associated with falls, and predicts dementia. Whether higher SLV in older adults without neurologic disease indicates presence of neurologic abnormalities is unknown. Our objective was to identify whether SLV in older adults without overt disease is associated with findings from multimodal neuroimaging. A well-characterized cohort of 265 adults (79–90 years) was concurrently assessed by gait mat, magnetic resonance imaging with diffusion tensor, and neurological exam. Linear regression models adjusted for gait speed, demographic, health, and functional covariates assessed associations of MRI measures (grey matter volume, white matter hyperintensity volume, mean diffusivity, fractional anisotropy) with SLV. Regional distribution of associations was assessed by sparse partial least squares analyses. Higher SLV (mean: 8.4, SD: 3.3) was significantly associated with older age, slower gait speed, and poorer executive function and also with lower grey matter integrity measured by mean diffusivity (standardized beta=0.16; p=0.02). Associations between SLV and grey matter integrity were strongest for the hippocampus and anterior cingulate gyrus (both β=0.18) as compared to other regions. Associations of SLV with other neuroimaging markers were not significant. Lower integrity of normal-appearing grey matter may underlie higher SLV in older adults. Our results highlighted the hippocampus and anterior cingulate gyrus, regions involved in memory and executive function. These findings support previous research indicating a role for cognitive function in motor control. Higher SLV may indicate focal neuropathology in those without diagnosed neurologic disease.
Keywords: gait disorders, diffusion tensor imaging, aging, brain
INTRODUCTION
Higher step length variability is present in those with neurologic diseases and may precede dementia onset1. Variability in step length has been related to gait instability, loss of postural control, and increased fall risk2. Step length at preferred speeds is quite constant in healthy adults, but higher step length variability can be present in older adults without overt neurologic disease3. Previous research has demonstrated that the range of values observed for step length variability is greater in older age, indicating that variability can be particularly high in a subset of older adults4. Slower gait is common in older adults and is associated with higher gait variability, but age-related increases in variability are independent of gait slowing5.
Step control is a multifactorial process with involvement from the musculoskeletal system, peripheral nervous system, and central nervous system (CNS). A healthy neural system is needed to minimize stride-to-stride fluctuations in walking2. Declines in automatic motor control may lead to greater cortical involvement in walking and increased step variability. However, the neural contributors to higher step length variability in the older population without overt neurologic disease are unknown. An emerging understanding of disease-related pathologies in the brain has revealed that subclinical pathology, both neurodegenerative and cerebrovascular, is quite common in older adults without conventionally defined neurologic diseases. Furthermore, these pathologies can adversely affect motor function and gait in older adults6.
Recent implementation of advanced neuroimaging techniques in gait studies has revealed that lower total brain volume and lower integrity of the white matter are associated with higher step length variability7. However, there is limited evidence regarding the regional distribution of CNS abnormalities related to step length variability in older adults without overt neurologic disease. Initial results have demonstrated associations for three general regions: the basal ganglia8, the hippocampus9,10, and motor areas10. In addition, executive function and attention may be related to step length variability11,12.
Determining the neuroanatomical correlates of step length variability in older adults is important to developing appropriate and effective interventions to improve gait and prevent falls. Gait variability is amenable to pharmacologic intervention and behavioral training in PD and stroke patients13,14, but evidence is lacking for older adults without overt disease. Part of the barrier to development of interventions in those without overt disease is the lack of evidence for the pathophysiology or regional distribution of brain abnormalities underlying gait variability in this population. Mean diffusivity (MD) measured from magnetic resonance imaging (MRI) with diffusion tensor imaging (DTI) may indicate abnormalities of the brain parenchyma that precede measurable changes to grey matter macrostructure15 and may provide evidence for the early brain changes associated with step variability.
We explored the regional distribution of differences in neuroanatomy related to step length variability as determined by DTI in a cohort of community-dwelling older adults free from neurologic disease. Grey matter regions related to memory, executive function, and motor function were selected based on previous research indicating associations between gait and these neurologic domains16,17. We hypothesized that lower integrity of these regions, indicated by higher mean diffusivity, would be associated with higher step length variability.
METHODS
Study Subjects
Participants were from the Healthy Brain Project ancillary to the Health, Aging, and Body Composition (Health ABC) study. Health ABC is a cohort of 3,075 well-functioning, white and black, men and women, aged 70–79 years from Pittsburgh, PA and Memphis, TN enrolled 1997–1998. In 2006–2007, 314 of the eligible 652 Health ABC participants at the Pittsburgh site were interested and eligible for MRI of the brain and were able to walk 20 meters. Medical histories were reviewed to rule out endocrinal, neurological and psychological illnesses. Participants in the Healthy Brain Project were similar to the Pittsburgh cohort of the Health ABC study as previously reported18. All subjects provided written informed consent and the protocol was approved by the University of Pittsburgh institutional review board.
Image acquisition
Details of the image acquisition protocol have been previously published19. Images were obtained with a Siemens 12-channel head coil and 3T Siemens Tim Trio MR scanner at the Magnetic Resonance Research Center, University of Pittsburgh. T1-weighted, fluid-attenuated inversion recovery (FLAIR), and diffusion-weighted images were collected. Diffusion-weighted images were acquired using a single short spin-echo sequence (TR=5,300 ms, TE=88 ms, TI=2,500 ms, 90° flip angle, 256×256 mm FOV, two diffusion values of b=0 and 1,000 s/mm, 12 diffusion directions, four repeats, 40 slices, 3 mm thick, 128×128 matrix size, 2×2×3 mm voxel size, and GRAPPA=2). A neuroradiologist examined each MRI for neurologic abnormalities.
Image Processing
Macro-structural measures (grey matter (GM) volume and white matter hyperintensity (WMH) volume) and micro-structural measures (MD and fractional anisotropy (FA)) were obtained using previously published methods19, briefly described below.
Volumes for GM, white matter (WM), and cerebrospinal fluid (CSF), were calculated by segmenting the skull-stripped T1-weighted image in native anatomical space. Volumes were estimated in cubic millimeters by summing tissue-specific voxels. Intracranial volume was contained within the inner skull. Atrophy was calculated as 1-GM volume/intracranial volume. WMH volume was obtained from T2-weighted FLAIR image and was normalized to brain volume.
DTI estimates the microstructural integrity of brain tissues using the molecular diffusion of water which is influenced by the characteristics of the surrounding tissue15. MD estimates an average magnitude of water diffusion and in grey matter likely represents the density of the molecular structure. Greater structural density results in greater restriction of water diffusion and a lower MD value. FA is an index of white matter tract integrity with higher values indicating greater integrity15. The diffusion-weighted images were pre-processed to remove eddy current distortions and the tensor were computed and diagonalized to compute the FA and MD maps. Mean FA and MD were calculated for normal-appearing WM and GM only. Due to poor segmentation between WM and CSF, two individuals were excluded from analyses of WM.
Gait Analysis
Gait measures were obtained from GaitMat™ II, an instrumented, computerized eight meter walkway. The first and last two meters were inactive for acceleration and deceleration. Participants were asked to walk at their usual pace and made at least four passes. Only passes with at least four valid steps were included. Gait speed was distance divided by time in seconds. Step length was defined as the distance between the heel of one footprint and the heel of the next footprint from the opposite foot. Step length variability was calculated from both left and right steps as the coefficient of variation (CoV) using the formula (standard deviation/mean)*1008. Results using the standard deviation were qualitatively similar to those using CoV; only results with CoV are reported here. The coefficient of variation was based on no fewer than 16 steps made over 16 meters of walking. Reliability of this measure over similar distances has been previously reported20.
Covariates
Variables known to be associated with brain health and gait were included as covariates. Age, gender, and race were self-reported. Body mass index (BMI) was calculated by the standard formula (weight in kilograms)/(height in meters)2 and obesity was defined as ≥30. Diabetes was determined by self-report, use of hypoglycemia medication, a fasting glucose of ≥126 mg/dL, or a two hour glucose tolerance test >200 mg/dL at baseline or during follow-up until time of MRI. Prevalent hypertension was defined by self-report or current medication use. Cardiovascular disease was a composite measure of self-reported or medicated cerebrovascular disease, coronary heart disease, myocardial infarction, or peripheral arterial disease. Recurrent falls were two or more self-reported in the past twelve months. Muscle strength was measured as the peak torque from isokinetic knee extension on a dynamometer (model 125 AP, Kin-Com, Chattanooga, TN). The right leg was measured unless contraindicated due to prior surgery, injury or pain.
A detailed neurologic exam was conducted by a trained physician. Balance was abnormal by the Romberg test if the participant was unable to stand with eyes closed for at least 30 seconds. Peripheral nerve function was tested by a vibration fork applied to the big toe and was considered abnormal if the individual was unable to feel vibration for at least 10 seconds.
Depressive symptoms were assessed by the short form Center for Epidemiologic Studies – Depression (CES-D) scale and reported as number of symptoms endorsed21. Global cognitive function was tested by the Modified Mini Mental Status Exam (3MS) with a cut-off of 80 indicating poor cognitive function22. Executive function was assessed by the Digit Symbol Substitution Test (DSST)23.
Statistical Analysis
To compare step length variability with MRI measures and covariates we used t-tests and Spearman’s correlation coefficients. Age-adjusted associations were determined by partial correlations for continuous variables and linear regression for categorical ones. Step length variability and WMH were skewed with the majority of participants having low variability and low WMH volume, so all analyses used log transformed variables. Linear regression was used to determine the association of whole brain MD and FA with step length variability after adjustment for covariates. Four models were developed with inclusion of covariates based on a p-value ≤ 0.1 in bivariate associations: 1) DTI measure alone, 2) model 1 plus age, gender, obesity status, diabetes status, and muscle strength, 3) model 2 plus gait speed, and 4) model 3 plus CES-D and DSST. Analyses used SAS 9.3.
To explore the spatial distribution of DTI measures by step length variability, we utilized a sparse partial least squares (SPLS) analysis. SPLS is a variable selection technique that addresses collinearity, multiple comparisons, and over-fitting24. As FA was not significantly associated with step length variability in adjusted whole brain analyses, regional analysis was not completed. For MD of the GM, 17 brain regions were selected a priori to include sensorimotor function (precentral gyrus, putamen, caudate, thalamus, supplementary motor, precuneus, postcentral gyrus, inferior parietal, pallidum), executive function (anterior cingulate, middle frontal gyrus, superior parietal), and memory (hippocampus, entorhinal cortex, parahippocampus, amygdala, posterior cingulate)16,17. MD of the left and right hemispheres were combined by a weighted average based on total volume of each region. MD for the pallidum was skewed right with multiple zero values. Therefore, a small constant (0.0001) was added to all observations and the log value was used. Outcome and predictors were standardized prior to SPLS analysis. Leave-one-out cross-validation based on K={1,2,…,17} and η from 0.025 to 0.975 by increments of 0.025 was used to select optimal tuning parameters (K=2 and η=0.925) for the model. As this was an exploratory analysis, these were not adjusted for covariates. SPLS analyses used R version 2.15.1, and ‘spls’ package version 2.1–2.
Due to the sensitivity of SPLS methods to outliers/influential points, analyses were conducted to determine the effect of these observations on both regression and SPLS results. Linear regression of MRI measures by step length variability was used to identify observations with a residual greater than 2.5 or Cook’s D value greater than 1 (number of observations removed: MD: four, FA: five; optimal tuning parameters with observations removed: K=1 and η=0.925).
RESULTS
Of the 314 participants, 41 did not have complete DTI measures and eight were missing gait or covariate data. This resulted in an analytic sample of 265 individuals. There were no significant differences between included and excluded individuals on demographics or health-related characteristics except that those included in these analyses were less likely to have diabetes (24% vs 39%; p=0.03). The analytic sample had an average age of 82.9 years and was 57.4% female (Table 1). The sample had a mean log step length variability of 8.4 (SD=3.3) and a median of 7.9 (range=3.1–18.7).
Table 1.
Characteristics of 265 older adults in a subsample of the Health, Aging, and Body Composition study with diffusion tensor imaging at time of MRI. Unadjusted and age-adjusted associations with step length variability (SLV) are shown.
| Total | Association with SLV* | p-value | Age-Adjusted | p-value | |
|---|---|---|---|---|---|
| Sample (n=265) |
Association with SLV* | ||||
| Step Length Variability, mean (SD) | 8.4 (3.3) | — | — | — | — |
| Demographics | |||||
| Age, mean (SD) | 82.9 (2.7) | 0.16 | 0.008 | — | — |
| Female Gender, n (%) | 152 (57.4%) | 0.14 (0.41) | 0.7 | 0.19 (0.40) | 0.6 |
| Black Race, n (%) | 107 (40.4%) | 0.33 (0.41) | 0.4 | 0.43(0.40) | 0.3 |
| Health and Physical Function | |||||
| Diabetic, n (%) | 64 (24.2%) | 1.48 (0.46) | 0.001 | 1.53 (0.45) | 0.001 |
| Obese, n (%) | 68 (26.1%) | 0.99 (0.50) | 0.05 | 1.04 (0.45) | 0.02 |
| Hypertension, n (%) | 217 (69.3%) | −0.27 (0.20) | 0.5 | −0.21 (0.42) | 0.6 |
| Cardiovascular Disease, n (%) | 91 (29.0%) | 0.38 (0.20) | 0.4 | 0.38 (0.44) | 0.4 |
| Recurrent Falls in Past 12 Months, n (%) | 48 (15.4%) | −1.12 (0.20) | 0.05 | −0.56 | 0.08 |
| Muscle Strength (N*m), mean (SD) | 82.1 (30.6) | −0.14 | 0.03 | −0.13 | 0.06 |
| Gait Speed (m/sec), mean (SD) | 0.91 (0.19) | −0.44 | <0.001 | −0.46 | <0.001 |
| Abnormal Romberg, n (%) | 41 (16.0%) | 0.89 (0.56) | 0.1 | 0.84 (0.55) | 0.1 |
| Abnormal Vibration Sensitivity, n (%) | 71 (29.3%) | 0.71 (0.46) | 0.1 | 0.52 (0.46) | 0.3 |
| Psychological and Cognitive Function | |||||
| CES-D Score, mean (SD) | 6.9 (6.4) | 0.14 | 0.02 | 0.10 | 0.1 |
| Digit Symbol Score, mean (SD) | 37.3 (13.2) | −0.21 | 0.001 | −0.23 | <0.001 |
| 3MSE Score <80, n (%) | 16.0 (6.1%) | 0.25 (0.84) | 0.8 | 0.29 (0.85) | 0.7 |
| MRI Measures | |||||
| Atrophy, mean (SD) | 0.72 (0.02) | −0.12 | 0.05 | −0.11 | 0.1 |
| Log WMH, mean (SD) | −2.56 (0.62) | 0.14 | 0.03 | 0.09 | 0.2 |
| Mean diffusivity, mean (SD) | 0.0013 (0.0001) | 0.25 | <0.001 | 0.20 | 0.002 |
| Fractional Anisotropy, mean (SD) | 0.36 (0.01) | −0.14 | 0.02 | −0.19 | 0.004 |
for continuous variables, Spearman’s rho for correlation with SLV reported; for categorical variables, mean difference and standard error of SLV are reported.
SLV = step length variability
CES-D = Center for Epidemiologic Studies – Depression scale
3MSE = Modified Mini Mental Status Exam
WMH = White matter hyperintensities
Higher step length variability was significantly associated with older age, lower muscle strength, slower gait speed, being diabetic, and being obese (Table 1). Higher step length variability was associated with poorer brain integrity by all measures (Table 1). Of the MRI variables, only MD and FA remained significantly associated with step length variability after adjustment for age (Table 1). FA was not significantly associated with step length variability after adjustment for age and gait speed (standardized beta = −0.11; p = 0.06) or in fully adjusted models (standardized beta = −0.09; p = 0.16; Table 2); no further analyses were performed for FA.
Table 2.
Linear regression of step length variability by mean diffusivity and covariates in a subsample (n=265) of the Health, Aging, and Body Composition study with diffusion tensor imaging.
| Unadjusted | Model 1a | Model 2b | Model 3c | |||||
|---|---|---|---|---|---|---|---|---|
| Standardized beta |
p-value | Standardized beta |
p-value | Standardized beta |
p-value | Standardized beta |
p-value | |
| Mean diffusivity (n=265) |
0.25 | <0.001 | 0.19 | 0.007 | 0.15 | 0.03 | 0.15 | 0.03 |
| r2 | 0.06 | 0.13 | 0.13 | 0.26 | ||||
| Fractional Anisotropy (n=263) |
−0.18 | 0.004 | −0.12 | 0.08 | −0.10 | 0.11 | −0.09 | 0.16 |
| r2 | 0.03 | 0.11 | 0.12 | 0.26 | ||||
adjusted for age, gender, muscle strength, diabetes, and obesity status
model 1 + gait speed
model 2 + digit symbol substitution test and Center for Epidemiologic Studies – Depression scale
MD for the whole brain remained significantly associated with step length variability after adjustment for age, gait speed, cognitive performance, gender, muscle strength and health status (standardized beta = 0.15; p = 0.03; Table 2). Exclusion of outliers (n=4) did not change the results (fully adjusted model: standardized beta = 0.15; p = 0.03).
SPLS analysis identified two regions for which MD was significantly associated with step length variability (Figure 1). Higher MD in the hippocampus (β= 0.20) and anterior cingulate cortex (β=0.21) was associated with greater step length variability in these analyses. In contrast, lower MD, indicating higher integrity, of the superior parietal lobe was associated with higher step length variability (β= −0.12). Analyses that excluded all outliers (n=4) revealed similar associations for the hippocampus (β= 0.18) and anterior cingulate (β= 0.18), but no longer identified the superior parietal lobe.
Figure 1.
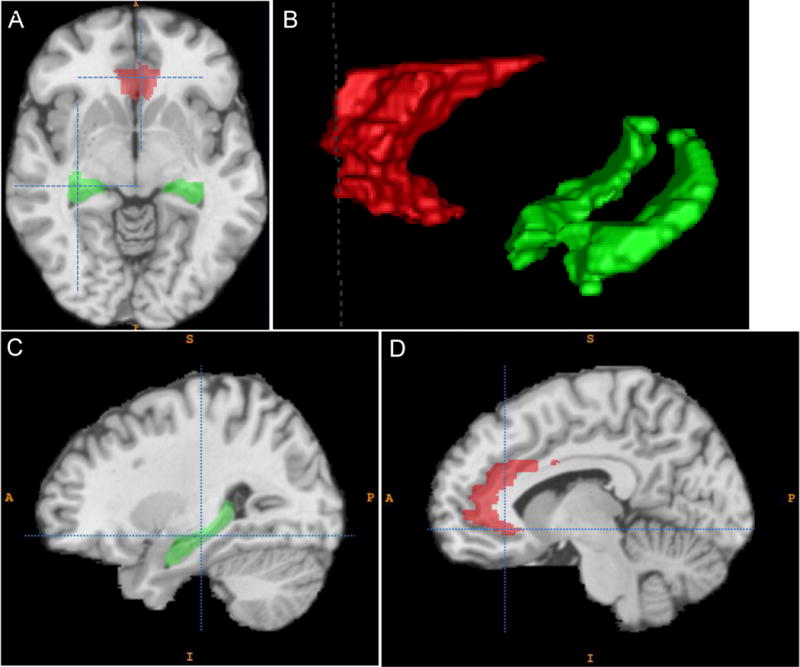
Highlighted regions indicate where higher mean diffusivity of the grey matter was most strongly associated with higher step length variability of older adults without neurologic disease. Hippocampus is shown in green, anterior cingulate gyrus in red. A. Axial orientation. B. 3D reconstruction of regions of interest. C. Sagittal orientation at the temporal lobe showing the hippocampus. D. Sagittal orientation near the midline showing the anterior cingulate gyrus.
DISCUSSION
In this sample of community-dwelling, older adults without overt neurologic disease, we found that lower integrity of the grey matter was associated with greater step length variability even after adjustment for gait speed, demographics, health status, and cognitive function. In contrast, GM volume, WMH, and WM integrity were not significantly associated. In addition, we identified specific grey matter regions where higher MD was significantly related to greater step length variability.
MD is a measure of water diffusion, with higher values indicating less resistance to diffusion by cellular structures15. Higher MD in the grey matter may indicate cortical thinning, lower tissue density, lower geometrical complexity, and possible neurodegeneration25. MD increases in grey matter with aging26 and in neurologic diseases including multiple sclerosis27 and Parkinson’s28. Studies that explore the underlying mechanisms of increased MD of the grey matter are currently lacking and are needed to better understand this potentially important CNS marker. Measures of WM were not associated with higher step length variability in this sample, indicating that the underlying pathology is likely related to cellular degeneration in the grey matter rather than degradation of the WM tracts.
Two regions, the hippocampus and anterior cingulate cortex, were identified here as having lower integrity in relation to higher step length variability. The hippocampus is involved in memory and spatial navigation and previous studies have associated lower metabolic activity in the hippocampus with higher step length variability9,10. The anterior cingulate gyrus is connected with the motor system and is important for premotor functions, attention, error detection, and executive function29. Previous research has identified executive function as an important correlate of step length variability in healthy older adults11,12. Interestingly, information from the hippocampus is transmitted to neocortical association areas by way of the cingulate cortex, allowing the hippocampus to influence spatial planning and motor adaptation30. No sensorimotor regions were identified in this analysis.
Analyses that did not exclude outliers also found higher integrity of the superior parietal lobe to be associated with higher step length variability. The superior parietal lobe works in conjunction with the anterior cingulate for spatial orientation and executive function29. The finding that higher integrity was associated with greater variability may indicate compensation in specific regions of the brain when deficits occur in related areas. However, these results must be interpreted with caution as SPLS can be sensitive to outliers and analyses excluding outliers found no association in this region.
Integrity of the WM was not related to step length variability in our analyses. This is consistent with previous findings indicating a stronger role for grey matter integrity in step length variability and in contrast to findings for measures of step timing variability which appear to be more related to WM integrity7,17. Of note, De Laat7 found significant associations for the WM but they did not control for gait speed which we found to be a strong confounder in our study. We also did not find an association between step length variability and WMH after adjustment for age, in contrast to a previous study8. The primary difference between these analyses was the use of continuous measures of WMH and step length variability here and categorized ones previously. The continuous measure used here provides a more accurate measure of WMH burden than do the crude ratings used previously. On the other hand, the significant findings using categorical measures may indicate a non-linear relation between step length variability and WMH; this was not explored here.
There are several limitations to consider. Most notably, this was a cross-sectional study that did not allow for assessment of the temporal direction of associations. In addition, we did not assess integrity of the cerebellum, which may play an important role in gait adaptation.
Our analysis benefitted from being conducted in a well-characterized cohort of older adults who underwent 3T MRI with DTI. This allowed adjustment for a number of potential confounders. By using DTI in addition to standard MRI measures, we were able to distinguish differences in the micro-as well as macro-structure of the brain15. Finally, use of the SPLS method allowed for assessment of the specific spatial distribution of these associations across multiple brain regions while addressing issues of collinearity, multiple comparisons, and over-fitting24.
CONCLUSIONS
Higher integrity of the GM of brain regions specific to memory and executive function may underlie increased step length variability in older adults. This may imply that specific neuropathology underlies observed increases in step length variability even in those without diagnosed neurologic disease. Further, these findings may indicate that increases in step length variability result from changes in the grey, rather than white, matter of the brain. It is unclear whether this pathology indicates a pre-or sub-clinical state of a clinically-recognized neurologic disease or is a discrete phenomenon. However, there is evidence that high step length variability may precede overt neurodegenerative disease1. This distinction needs further exploration, as it can have important implications for prevention and treatment. By identifying the spatial distribution and underlying pathology of increased step length variability in older adults, we can begin to explore targeted intervention strategies, possibly including behavioral training, pharmacologic interventions, or transcranial magnetic stimulation.
Research Highlights.
We assess neurologic correlates of step length variability in older adults.
We use diffusion tensor imaging to assess brain microstructure.
We examine regional distribution of microstructure differences in step variability.
Lower grey matter integrity is associated with higher step variability.
Associations with variability are specific to hippocampus and anterior cingulate.
Acknowledgments
Health ABC was supported by National Institute on Aging (NIA) Contracts (N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106, NIA grant R01-AG-028050, and NINR grant R01-NR-012459). This research was supported in part by the Intramural Research Program of the NIA (K23-AG-028966, R01-AG-029232), the University of Pittsburgh Claude D. Pepper Older Americans Independence Center P30-AG-024827-07, and a training grant from the NIA (T32-AG-000181). The study sponsor had no role in the design, analysis, or writing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: None of the authors has a conflict of interest to report.
References
- 1.Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. Journal of neurology, neurosurgery, and psychiatry. 2007 Sep;78(9):929–935. doi: 10.1136/jnnp.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hausdorff JM. Gait variability: methods, modeling and meaning. Journal of neuroengineering and rehabilitation. 2005;2:19. doi: 10.1186/1743-0003-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callisaya ML, Blizzard L, Schmidt MD, McGinley JL, Srikanth VK. Ageing and gait variability—a population-based study of older people. Age and ageing. 2010 Mar;39(2):191–197. doi: 10.1093/ageing/afp250. [DOI] [PubMed] [Google Scholar]
- 4.Gabell A, Nayak US. The effect of age on variability in gait. J Gerontol. 1984 Nov;39(6):662–666. doi: 10.1093/geronj/39.6.662. [DOI] [PubMed] [Google Scholar]
- 5.Kang HG, Dingwell JB. Separating the effects of age and walking speed on gait variability. Gait & posture. 2008 May;27(4):572–577. doi: 10.1016/j.gaitpost.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Rosso AL, Studenski SA, Chen WG, et al. The journals of gerontology. Jul 10, 2013. Aging, the Central Nervous System, and Mobility. (Series A, Biological sciences and medical sciences). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Laat KF, van Norden AG, Gons RA, et al. Diffusion tensor imaging and gait in elderly persons with cerebral small vessel disease. Stroke; a journal of cerebral circulation. 2011 Feb;42(2):373–379. doi: 10.1161/STROKEAHA.110.596502. [DOI] [PubMed] [Google Scholar]
- 8.Rosano C, Brach J, Studenski S, Longstreth WT, Jr, Newman AB. Gait variability is associated with subclinical brain vascular abnormalities in high-functioning older adults. Neuroepidemiology. 2007;29(3–4):193–200. doi: 10.1159/000111582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmerman ME, Lipton RB, Pan JW, Hetherington HP, Verghese J. MRI- and MRS-derived hippocampal correlates of quantitative locomotor function in older adults. Brain research. 2009 Sep 29;1291:73–81. doi: 10.1016/j.brainres.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimada H, Ishii K, Ishiwata K, et al. Gait adaptability and brain activity during unaccustomed treadmill walking in healthy elderly females. Gait & posture. 2012 Dec 19; doi: 10.1016/j.gaitpost.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Brach JS, Studenski S, Perera S, VanSwearingen JM, Newman AB. Stance time and step width variability have unique contributing impairments in older persons. Gait & posture. 2008 Apr;27(3):431–439. doi: 10.1016/j.gaitpost.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holtzer R, Wang C, Verghese J. The relationship between attention and gait in aging: facts and fallacies. Motor control. 2012 Jan;16(1):64–80. doi: 10.1123/mcj.16.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryant MS, Rintala DH, Hou JG, et al. Gait variability in Parkinson’s disease: influence of walking speed and dopaminergic treatment. Neurological research. 2011 Nov;33(9):959–964. doi: 10.1179/1743132811Y.0000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reisman DS, McLean H, Keller J, Danks KA, Bastian AJ. Repeated Split-Belt Treadmill Training Improves Poststroke Step Length Asymmetry. Neurorehabilitation and neural repair. 2013 Feb 7; doi: 10.1177/1545968312474118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2007 Jul;4(3):316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seidler RD, Bernard JA, Burutolu TB, et al. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neuroscience and biobehavioral reviews. 2010 Apr;34(5):721–733. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosano C, Aizenstein H, Brach J, Longenberger A, Studenski S, Newman AB. Special article: gait measures indicate underlying focal gray matter atrophy in the brain of older adults. (Series A, Biological sciences and medical sciences).The journals of gerontology. 2008 Dec;63(12):1380–1388. doi: 10.1093/gerona/63.12.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venkatraman VK, Aizenstein HJ, Newman AB, et al. Lower Digit Symbol Substitution Score in the Oldest Old is Related to Magnetization Transfer and Diffusion Tensor Imaging of the White Matter. Frontiers in aging neuroscience. 2011;3:11. doi: 10.3389/fnagi.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosano C, Aizenstein HJ, Newman AB, et al. Neuroimaging differences between older adults with maintained versus declining cognition over a 10-year period. NeuroImage. 2012 Aug 1;62(1):307–313. doi: 10.1016/j.neuroimage.2012.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brach JS, Perera S, Studenski S, Newman AB. The reliability and validity of measures of gait variability in community-dwelling older adults. Archives of physical medicine and rehabilitation. 2008 Dec;89(12):2293–2296. doi: 10.1016/j.apmr.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) American journal of preventive medicine. 1994 Mar-Apr;10(2):77–84. [PubMed] [Google Scholar]
- 22.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. The Journal of clinical psychiatry. 1987 Aug;48(8):314–318. [PubMed] [Google Scholar]
- 23.Wechsler D. Manual for the Wechsler Adult Intelligence Scale–Revised. New York, NY: Psychological Corp; 1981. [Google Scholar]
- 24.Chun H, Keles S. Sparse partial least squares regression for simultaneous dimension reduction and variable selection. (Series B, Statistical methodology).Journal of the Royal Statistical Society. 2010 Jan;72(1):3–25. doi: 10.1111/j.1467-9868.2009.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koo BB, Hua N, Choi CH, Ronen I, Lee JM, Kim DS. A framework to analyze partial volume effect on gray matter mean diffusivity measurements. NeuroImage. 2009 Jan 1;44(1):136–144. doi: 10.1016/j.neuroimage.2008.07.064. [DOI] [PubMed] [Google Scholar]
- 26.Abe O, Yamasue H, Aoki S, et al. Aging in the CNS: comparison of gray/white matter volume and diffusion tensor data. Neurobiology of aging. 2008 Jan;29(1):102–116. doi: 10.1016/j.neurobiolaging.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Rovaris M, Bozzali M, Iannucci G, et al. Assessment of normal-appearing white and gray matter in patients with primary progressive multiple sclerosis: a diffusion-tensor magnetic resonance imaging study. Archives of neurology. 2002 Sep;59(9):1406–1412. doi: 10.1001/archneur.59.9.1406. [DOI] [PubMed] [Google Scholar]
- 28.Kim HJ, Kim SJ, Kim HS, et al. Alterations of mean diffusivity in brain white matter and deep gray matter in Parkinson’s disease. Neuroscience letters. 2013 Aug 29;550:64–68. doi: 10.1016/j.neulet.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 29.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in cognitive sciences. 2000 Jun;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 30.Scheidt RA, Zimbelman JL, Salowitz NM, et al. Remembering forward: neural correlates of memory and prediction in human motor adaptation. NeuroImage. 2012 Jan 2;59(1):582–600. doi: 10.1016/j.neuroimage.2011.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]


