Abstract
Why does a constant barrage of DNA damage lead to disease in some individuals, while others remain healthy? This article surveys current work addressing the implications of inter-individual variation in DNA repair capacity for human health, and discusses the status of DNA repair assays as potential clinical tools for personalized prevention or treatment of disease. In particular, we highlight research showing that there are significant inter-individual variations in DNA Repair Capacity (DRC), and that measuring these differences provides important biological insight regarding disease susceptibility and cancer treatment efficacy. We emphasize work showing that it is important to measure repair capacity in multiple pathways, and that functional assays are required to fill a gap left by genome wide association studies, global gene expression and proteomics. Finally, we discuss research that will be needed to overcome barriers that currently limit the use of DNA repair assays in the clinic.
Keywords: DNA repair capacity, multiplex assays, personalized disease prevention and treatment
1. Introduction
During the time it takes to read this sentence, it can be estimated that the reader's DNA will incur on the order of 10 trillion DNA lesions. Left unrepaired DNA damage has the potential to lead to mutant cells, dead cells and ensuing disease (Figure 1). The precise number and type of DNA lesions formed varies from one individual to the next in part because of differences in exposure and lifestyle, and also because of variation in metabolism and other cellular processes. Many types of DNA damage, such as abasic sites, alkylation damage, oxidative damage, mismatches, single and double strand breaks, result from normal metabolic processes. Others are induced upon exposure to environmental agents. Among the environmentally induced lesions are bulky DNA adducts, including heterocyclic amines induced by compounds in cooked foods, cyclobutane pyrimidine dimers induced by sunlight, alkylation damage from nitroso compounds in combustion products, and oxidative damage and DNA strand breaks induced by ionizing radiation from cosmic rays and radionuclides such as Radon gas. In addition, some environmental exposures such as arsenic do not directly induce DNA damage, but are thought to increase DNA damage levels both by inducing inflammation and by disrupting DNA repair (1-3) (4, 5).
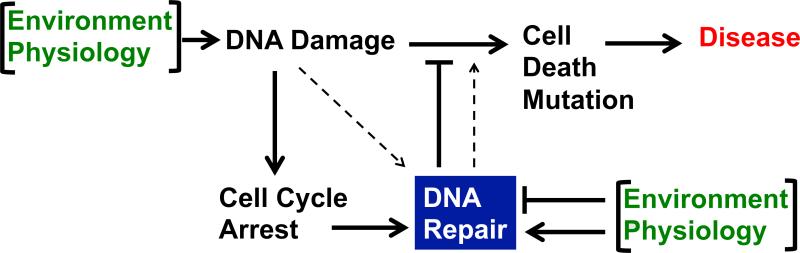
Figure 1.
DNA damage, DNA repair, and disease. The canonical role of DNA repair is to protect cells from death, mutation, and the inception of disease. As discussed in the main text, increasing DNA repair can also have the opposite effect, inducing cell death because of the potential accumulation of toxic repair intermediates. The environment and the physiology of the individual enter this diagram at two points; both factors may may increase DNA damage, or they may affect DRC either positively or negatively.
Fortunately, human cells mount a robust response to DNA damage that includes at least 7 major DNA repair pathways that specialize in the repair of subsets of DNA lesions, namely direct reversal (DR), mismatch repair (MMR), nucleotide excision repair (NER), homologous recombination (HR), base excision repair (BER), single strand break repair (SSBR), ,non-homologous end joining (NHEJ), and Fanconi Anemia DNA crosslink repair (FANC) (Table 1). The relationship between DNA damage and DNA repair is complex; no single pathway efficiently repairs all types of DNA lesions, some lesions are substrates for more than one pathway, and evidence for extensive interactions among proteins involved in distinct pathways continues to emerge (6-11). Mutations in DNA repair genes can have profound consequences for disease risk. The classic example is that individuals with the disease Xeroderma Pigmentosum (XP) are highly prone to skin cancer because they have mutations in genes required for nucleotide excision repair (NER), which repairs bulky lesions such as those induced by UV light. These individuals are at a 2000-fold higher risk of skin cancer in sun-exposed skin (12). A variety of other diseases including neurological, developmental and immunological disorders, as well as premature aging, are associated with aberrant DNA repair in humans (Table 1) (13). Thus, it is clear that defective DNA repair caused by mutations in repair genes represents a major disease risk factor, and genetic tests are now available for the most common disease-associated mutations in DNA repair genes (14).
Table 1.
Human diseases associated with DNA repair deficiencies categorized by DNA repair pathway.
| Repair pathway | Primary Lesions | Genes Associated with Disease | Diseases Associated | References |
|---|---|---|---|---|
| DR | O6-meG | MGMT | - Esophageal, Lung Cancer | (216) |
| MMR | Mismatches, loops | MSH2, MLH1, MSH6, PMS2 | -Hereditary Non-Polyposis Colon Cancer (HNPCC) -Class Switch Recombination (CSR) Defects -T-cell non-Hodgkin's lymphoma (T-NHL) -Diffuse large B-cell lymphoma (DLBCL) |
(217) (218) (219) (220) (221)(178) (222)(223) |
| NER | Bulky adducts | XPA, XPB (ERCC3), XPC, XPD (ERCC2), XPE (DDB1 & DDB2), XPF (ERCC4), XPG (ERCC5), ERCC1, CSA, CSB, TTDA | -Xeroderma Pigmentosum (XP) -Cockayne Syndrome (CS) -Trichothiodystrophy (TTD) |
(12) (224) |
| HR | DSB | BRCA1, BRCA2, NBS1 | -Breast, Prostate Cancer -Nijmegen Breakage Syndrome (NBS) |
(225) (226) |
| BER & SSBR | Damaged bases, SSB | MUTYH, UNG, OGG1, AAG, APE1, TDP1, APTX | -MUTYH-Associated, Polyposis (MAP) -Hyper-IgM syndrome (HIGM) type V -Lung Cancer -Spinocerebellar ataxia with axonal neuropathy 1 (SCAN1) -Ataxia-oculomotor apraxia 1 (AOA1) |
(227)(177) (228)(157) (143) |
| NHEJ | DSB | DNA-PKcs, Artemis, LigIV, NHEJ1/XLF/Cernunnos | - Severe Combined Immunodeficiency (SCID) | (170)(172) (171)(173) (169) |
| FANC | Cross-links | FANCA, FANCB, FANCC, FANCD1/BRCA2, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCJ/BRIP1, FANCL, FANCM, FANCN/PALB2, FANCO/RAD51C, FANCP/SLX4, NBS1 | -Fanconi Anemia (FA) -Nijmegen Breakage Syndrome (NBS) |
(182) (226) (8) |
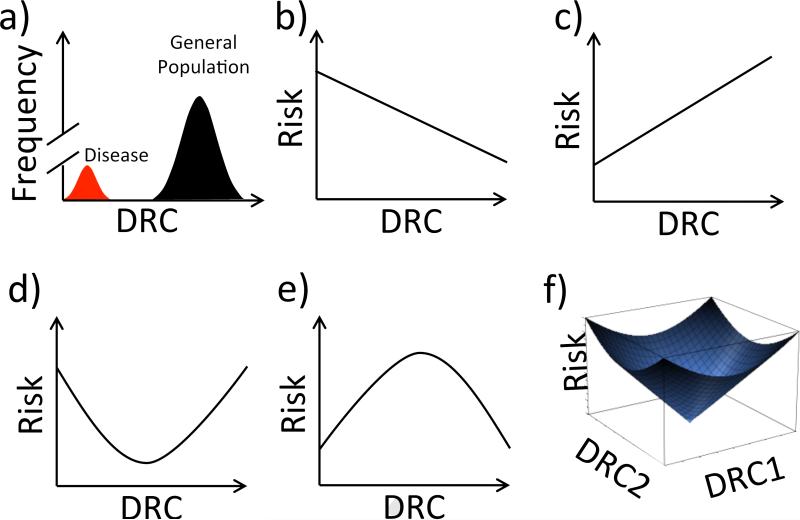
Intuitively, one might expect that DRC in a given pathway should vary even among individuals who do not have rare disease-associated mutations in key DNA repair genes, perhaps due to common sequence variants and epigenetic heterogeneity across populations. DRC might thus adopt a normal distribution among individuals with disease-associated DNA repair defects (red curve in Figure 2A), as well as among apparently healthy individuals in the general population (black curve in Figure 2A) (15). One might further hypothesize, based simply on interpolation (Figure 2B), that those in the general population falling to the left of the distribution would be at higher than average risk for disease, and that they might be candidates for personalized prevention schemes. It has been over two decades since these ideas were articulated by Hsu (16), and by Grossman and Wei (15), and although a wealth of evidence for interindividual DRC differences has since emerged from multiple laboratories using various methods (Table 2), it seems fair to say that the intervening studies have not yet resulted in personalized prevention efforts. Other possible relationships between DRC and risk of disease must also be considered (Fig 2C-2F), and these will be discussed below. Inter-individual variation in DRC might also account for differing tolerance among cancer patients for cancer therapy with DNA damaging agents. Moreover, the sometimes dramatic changes in DRC in cancer cells versus non-cancer cells might be exploited for individualized treatment.
Figure 2.
Potential consequences of DRC variability. a) Both intuition and experimental data point to a distribution in DRC, shown here for a single pathway among healthy individuals (black curve), and a multimodal distribution when disease states (red curve) are included; this panel is inspired by a similar figure originally published by Grossman and Wei (15). b) The simplest assumption is that a generic representation of risk (cell death, disease diagnosis or mortality) will decrease as DRC increases. c) In some cases, such as when a level of glycosylase initiates BER leading to an accumulation of intermediates that are more toxic than the initial DNA damage, elevated DRC may be deleterious. d and e) In principle the combined influence of factors driving the relationships in panels b,c could lead to more complicated relationships between risk and DRC. f) The relationship between DRC and risk may be represented as a complex landscape that depends on DRC in more than one pathway. As discussed in section 5, multiple DRC defects can act synergistically, but can also produce surprising and counterintuitive phenotypes.
Table 2.
Studies in which DNA repair assays have been used to evaluate human inter-individual and/or tumor-specific variability in DRC. Publications are categorized by DNA repair pathway studied and the type of assay used. Black corresponds to studies in which a single repair pathway was assayed. Blue corresponds to studies in which two or more pathways were assayed as a consequence of a lesion being repaired by more than one pathway or a protein being involved in more than one pathway. Red corresponds to studies in which two or more pathways were assayed simultaneously either through sequencing of two or more genes or by the use of separate repair measurements.
| Repair pathway | Evidence of inter-individual/tumor differences in DRC | |||||||
|---|---|---|---|---|---|---|---|---|
|
Indirect measurements
|
Mutagen Sensitivity |
Direct measurements
|
||||||
| SNP/GWAS | Gene Methylation | mRNA | Protein | Cell free extract | Comet | HCR | ||
| DR | (32) (229) (20) (216) |
(54) (55) (56) (57) (43) (58) |
(48) (230) (231) (58) |
(231) | (64) (66) (48) (42) (231) (131) (7) (52) (53) |
(59) (90) (229) |
||
| MMR | (232) (233) (20) (180) |
(232) (63) (233) |
(230) (57) |
(63) (191) |
(63) (234) (235) |
|||
| NER | (236) (237) (25) (26) (120) (28) (20) (29) (30) (31) |
(70) (72) | (238) (239) (70) (230) (72) |
(240) (70) (72) |
(96) (241) | (106) (128) (238) (110) (242) (107) (131) (243) (108) (120) (129) (118) (121) (7) |
||
| HR | (237) (20) | (244) (66) (69) (245) |
(66) (69) (230) |
(69) | (89) | (98) (99) (101) (237) (97) |
||
| BER & SSBR | (246) (237) (42) (82) (20) |
(68) (71) | (78) (48) (68) (230) (42) (71) (126) |
(71) | (79) (246) (78) (247) (82) (91) (142) (92) (143) |
(237) (96) (100) |
(131) (126) | |
| NHEJ | (237) (248) (20) (180) |
(69) | (69) (230) |
(69) | (87) | (98) (99) (101) (237) (97) |
(243) (122) | |
| FANC | (67) (249) | (244) (64) (65) (66) (245) |
(65) (66) | (64) (67) |
(88) | (128) (129) (7) |
||
Over the last two decades, significant efforts have focused on testing the idea that measuring DRC has the potential to inform medical and clinical practice. In this review, we discuss major lines of evidence supporting the notion that there are significant interindividual differences in DRC, and further, supporting the claim that such variations are indeed associated with disease risk. We survey the experimental approaches used to measure DRC, and discuss future work that will be needed for clinical translation of functional DRC measurements.
2. Evidence for inter-individual differences in DRC from indirect measurements
Genetics
XP was the first human cancer-susceptibility disease found to be associated with a DNA repair defect, namely NER; direct in vivo DRC measurements in cells isolated from XP patients provided the critical insight that a DNA repair defect was the cause of the disease (17). Complementation studies identified numerous genes responsible for XP, providing the foundation for predicting NER defects and associated disease indirectly from genotype analysis. Thus DNA sequencing of well-characterized mutations in XP genes can be used to predict impaired DRC and increased disease susceptibility. Subsequent research has identified numerous other disease-associated rare gene mutations that cause severe defects in the MMR, NER, HR, BER, SSBR, NHEJ, and FANC pathways (Table 1), as well as defects in DNA damage surveillance (18) and tolerance pathways (19).
Common single nucleotide polymorphisms (SNPs) that are associated with disease have been identified in genes in the DR, BER, MMR, NER, HR, and NHEJ pathways (recently reviewed in (20)). In candidate gene association studies, SNPs in DNA repair genes have been associated with increased or decreased risk of many cancers including lung, colorectal, gallbladder, oral, breast, prostate, liver, ovarian, and laryngeal cancer, as well as lymphoma and squamous cell carcinoma (20). Genome wide association studies (GWAS) have revealed many additional lower penetrance disease-associated sequence variants using unbiased computational approaches (21, 22), but surprisingly few of these turn out to be DNA repair genes. This may be explained in part by the observation that the variants identified so far explain only a small portion of disease heritability. As yet unidentified DNA repair variants may contribute to the missing heritability if they are relatively rare but confer a relatively large risk increment. Variants in DNA repair genes that confer risk could also be missed if they represent copy number variants or they have relatively small effects; further, gene-gene interactions involving DNA repair gene variants may also be missed in GWAS studies due to low statistical power (23). Moreover, most GWAS-identified variants are not located in genic regions, but rather in intergenic regions that are presumably involved in gene regulation. Increased sample sizes,better accounting for rare variants and structural variants, and better understanding of the role of regulatory variants will likely increase the ability of DNA sequence-based assessments to identify individuals with elevated disease risk. In section 3, we will discuss in detail functional assays that may complement DNA sequence-based predictors of DRC defects.
In addition to disease prevention, genome profiling for sequence variants in DNA repair genes has the potential to enable personalized disease treatment (24); it is already clear that SNPs in DNA repair genes can play a role in assessing a prognosis for patients being treated for melanoma, pancreatic, esophageal, or non-small cell lung cancer. SNPs in the following DNA repair genes have been associated with the response of patients to cancer therapy: MGMT, XPA, XPC, XPD, XPE, XPG, ERCC1, ERCC3, XRCC1, XRCC2, and XRCC3 (25-32). Polymorphisms in some DNA repair genes, such as ERCC1 and XPD, have also been associated with increased cancer therapy toxicity (33), and MGMT polymorphisms are associated with increased risk of myelodysplastic syndromes following treatment with alkylating agents (34).
Major advantages of genomic profiling include the breadth of data that can be obtained for relatively small (and steadily decreasing) investment of resources using next generation sequencing (DNAseq), conceptual simplicity, and the universality of the approach; standardized sequencing procedures foster high inter-laboratory reproducibility (35). An important limitation of studies that aim to make predictions based on DNA sequence is that, with the possible exception of CpG methylation specific PCR (MSP), one cannot know a priori how well the gene with which the sequence variant has been associated is actually expressed; indeed differential allelic gene expression is common (36). In this regard genome sequence based assays may be regarded as the least direct means of measuring function (Figure 3).
Figure 3.
Methods of assessing DRC and their limitations. The biological trajectory that runs from genes to function includes numerous intermediate steps at which a given assay may fail to predict the functional endpoint. The point along this trajectory at which a particular method may fail is indicated by color-coded bars running from left to right. Although any of the assays may accurately predict function, only functional (Comet and HCR) assays integrate the complexity of this entire trajectory into their readout.
Transcriptional profiling
Transcriptional profiling has revealed that DNA repair gene expression has important consequences for disease biology. For example, studies have identified prognostic gene expression signatures in cancer cells that correlate with breast cancer survival (37, 38), breast cancer recurrence (39), and lung cancer survival (40). Tumor gene expression profiles that include DNA repair genes and correlate with cancer therapy response have also been identified (41), and in some cases tumor expression of a single DNA repair gene correlates with treatment efficacy (42-44). Moreover, gene expression profiling has been used to identify bleomycin-induced changes in DNA repair gene expression that predict bleomycin sensitivity, and low level radiation induced changes in DNA repair gene expression in (non-cancerous) human lymphocytes that can be used as biomarkers for occupational exposure to ionizing radiation (45, 46). An additional study in human lymphocytes demonstrated an inverse correlation between radiosensitivity measured by a G2 challenge assay and expression of the NFKB gene; this study also showed an association between radiosensitivity and breast cancer risk (47).
One study with human lymphoblastoid cell lines revealed that expression of a set of just 48 genes was sufficient to predict sensitivity to MNNG (48), an alkylating agent that generates the same spectrum of DNA lesions as the chemotherapeutic drugs temozolomide, dacarbazine, procarbazine and streptozotocin (49). These findings were important in several respects; first it was possible to predict MNNG sensitivity in cells derived from apparently healthy individuals using a small set of basally expressed genes, supporting the notion that gene expression patterns could be used to help predict how tumor cells will respond to therapy. Second, there were large variations in sensitivity to two different DNA kinds of alkylating agents, and it was subsequently shown that sensitivity to one agent did not accurately predict sensitivity to a second DNA damaging agent, indicating a unique response to each agent (50). One of the predictive transcripts for MNNG sensitivity encodes the methylguanine DNA methyltransferase (MGMT) protein that repairs O6-alkylguanine DNA lesions by DR. This might be expected, since O6-methylguanine is one of the most toxic lesions generated by MNNG. However, MGMT expression alone was a much weaker predictor than expression of the combined set of 48 genes, indicating that sensitivity to DNA damaging agents reflects the integration of numerous biological pathways. The other 47 genes included at least one other known DNA repair gene (MUTYH), and it is possible that the other genes identified in this study affect DRC in ways that have yet to be established.
A major strength of gene expression profiling is the relatively new ability to complement or replace quantitative real time PCR (qPCR) and microarray gene expression assays with next generation RNA sequencing (RNAseq) to generate very accurate data assessing genome wide expression levels, along with splicing information, at steadily falling costs. However, as with genomic DNA sequencing analysis, gene expression profiling is limited because it remains a relatively indirect measure of function; the presence of a transcript does not guarantee that the translation product will be correctly folded, active, appropriately modified and localized to the correct cellular compartment.
Mutagen sensitivity assays
Mutagen sensitivity assays, using mutagen-induced chromosome and chromatid breaks, can also provide an indirect assessment of DRC phenotype (16, 20, 51). Epidemiological studies comparing the response of lymphocytes to mutagens including bleomycin, UV-light, and benzopyrene diol epoxide (BPDE) have revealed increased sensitivity to mutagen-induced chromosomal aberrations in lymphocytes from individuals with cancer versus lymphocytes from healthy individuals. This has been borne out in patients with cutaneous melanoma, basal cell carcinoma, squamous cell carcinoma of the head and neck, plus patients with lung, breast and bladder cancers (51). Of particular interest, a prospective study found that higher bleomycin sensitivity of lymphocytes (>0.5 chromatid breaks per cell) from patients with head and neck cancer was associated with an elevated risk (hazard ratio of 1.38) of developing second, unrelated primary tumors as well as recurrence of the original cancer (52); importantly the blood cells were drawn before development of second primary or recurrent tumors. A second prospective study found a significant association between bleomycin sensitivity in lymphoblastoid cell lines and combined risk of prostate, lung, colorectal and ovarian cancers in the patients from whom the cells were derived (53). However, this study found no significant associations between cancer risk and several other measures of DRC, including BPDE sensitivity, endogenous DNA damage levels measured by comet assays, or host cell reactivation of UV-irradiated plasmids (comet and host cell reactivation assays are discussed in detail below). These negative results may reflect the small sample size and the focus on a limited number of DNA repair pathways.
A major advantage of mutagen sensitivity assays is that, by measuring the response of whole cells to specific mutagens of interest, they integrate biological complexity, such as SNPs, gene expression, epigenetics, protein folding and cell cycle checkpoint activation pathways that may not be accounted for by other methods of measuring DRC. On the other hand, it should be noted that a shortcoming of mutagen sensitivity assays is that they do not provide specific mechanistic information with regard to the identity of the genotoxic lesion or the pathways responsible for potentially defective repair, and they are relatively labor intensive.
3. Complications associated with indirect measurements of DRC exemplified by a simple repair pathway
The challenges associated with making accurate indirect DRC measurements can be illustrated by considering the performance of available methods for estimating MGMT activity; this protein essentially represents a one-protein DNA repair pathway. SNPs that may lead to MGMT defects have been associated with increased risk of some cancers, and better prognosis following chemotherapy with alkylating agents, as might be expected if the SNP leads to inefficient DNA repair (32). However, there are many examples of cancer cells in which MGMT is epigenetically silenced due to promoter hypermethylation (43, 54, 55); in these cases the sequence of the MGMT gene would be irrelevant to prognosis because the gene is not expressed. Thus, information about promoter methylation and/or gene expression may be needed to complement information obtained from DNA sequencing.
Epigenetic MGMT silencing due to promoter CpG hypermethylation in tumors has been detected by methylation-specific PCR methods and shown to correlate with the efficacy of cancer treatment with O6-MeG generating chemotherapeutic agents such as temozolomide or dacarbazine (56). Nevertheless, even a combination of SNP data and promoter methylation status may fail to predict function (and therefore clinical outcome) for several reasons. MGMT methylation status is sometimes not predictive of transcript levels (57, 58), and transcript levels are not informative unless the transcripts are translated and the protein stably folded and localized to the nucleus. For example, a significant fraction of the human MGMT protein is inactive in some cells (59), possibly due to posttranslational modifications (60). Furthermore, environmental exposures can alter the activity of DNA repair proteins, including MGMT (1, 2, 61). These phenomena may not be detected by the available indirect measurements of MGMT activity.
A defect in protein localization could also confound DRC assays. Mitochondria-associated OGG1 protein and activity levels are higher in the livers of old mice and in presenescent human fibroblasts compared to young mice and replicating human fibroblasts, respectively. However, a significant fraction of OGG1 remains inactive and sequestered in the mitochondrial outer membrane and intermembrane space, leading to accumulation of unrepaired oxidized bases in the mitochondrial DNA (62). An age-related localization defect was also observed for the mitochondrial uracil-DNA glycosylase (UNG) (62). A potential analogous localization defect for MGMT (or any other DNA repair protein) could be difficult to predict from the DNA sequence, and would not be detected by in vitro activity assays performed on whole cell lysates. In general, relatively laborious immunohistochemistry and subcellular fractionation techniques are required to detect protein localization defects.
In this section we have principally highlighted some of the pitfalls associated with making indirect measurements of DRC in the context of the simple one-protein MGMT pathway, and one might anticipate even more complex challenges for indirect measures of DRC in pathways that involve multi-protein complexes and multiple enzymatic steps (63-72). A major strength of in vivo functional DNA repair assays is the ability to integrate the complexity described above to reflect, as closely as possible, repair of genomic DNA damage. The next section discusses recent technological advances for making direct DRC measurements.
4. Evidence of inter-individual DRC differences from direct (functional) measurements
There are numerous methods for measuring DNA repair directly, and each has its strengths and weaknesses (Table 2). Some of the earliest protocols for measuring DRC, such as unscheduled DNA synthesis, removal of radiolabeled alkylation damage from genomic DNA (73, 74), and methods using antibodies specific for DNA lesions including BPDE, 8-oxoG, O6-MeG, pyrimidine dimers and cisplatin adducts (75), hold the advantage of measuring repair of genomic DNA in intact cells. While these assays have been used to detect a 3-5 fold range in inter-individual DRC (76, 77), they are relatively labor-intensive thus inhibiting their application to large-scale studies.
Activity assays with cell lysates
Pathway-specific DNA repair activity analyses in cell lysates have yielded considerable insight into inter-individual variation in DRC. An advantage of these assays is that because they measure levels of functional protein, they integrate much of the biological complexity that might confound indirect measures of DRC (Fig. 3); indeed a low correlation between enzymatic activity and mRNA levels has been documented in some cases (78). Quantitative in vitro functional assays have been developed for various steps of BER (79-83), MMR (84), DR of alkylation damage by MGMT (85), NER (86), NHEJ (87), cross-link repair (88), and HR (89).
In vitro assays with cell-free extracts prepared from human lymphocytes have so far been used to measure inter-individual differences in MGMT activity and in the efficiency of several key steps in the BER pathway. These studies revealed an approximately 10-fold variation in MGMT activity (90), a 10-fold variation in activity of alkyladenine DNA glycosylase (AAG, a.k.a. MPG) (49, 78, 91, 92) that initiates BER of several types of alkylation damage, and a 3-fold interindividual variation in activity of 8-oxoguanine DNA glycosylase (OGG1) (78) that initiates repair of oxidative DNA damage. Measurements of the subsequent BER steps has revealed a 1.9-fold to 2.5-fold variation in AP endonuclease activity (79, 82), 1.3-fold variation in subsequent polymerase beta dependent gap filling (82), and 3.4-fold variation in DNA nick ligation (82).
Although cell-free assays are quantitative and permit specificity with regard to the type of lesion being repaired, they have some limitations. The disruption of cells necessary for cell-free analyses can lead to dissociation of protein complexes and protein unfolding, or can mask defects in protein localization that would be detected using an in vivo assay. Similarly, the assay buffers may fail to reflect the in vivo intracellular environment. In addition, the substrates, typically naked short oligonucleotides, may not fully represent the complexity of repair in chromatinized DNA.
Comet Assays
Comet assays provide a powerful means of measuring endogenous DNA damage, induced DNA damage, and repair of DNA damage in genomic DNA in live cells (93). The assay is named for the comet-like appearance of DNA after single cell gel electrophoresis, before and after treatment with DNA damaging agents; measuring the disappearance of DNA damage following DNA damage induction enables estimates of DNA repair kinetics. Double strand breaks are measured at neutral pH, whereas single strand breaks are measured under alkaline conditions that dissociate DNA strands. Cells may be treated with ionizing radiation or bleomycin, which directly induce strand breaks, or agents such as UV-light, BPDE, peroxides, and alkylating agents that induce DNA lesions that can be converted into strand breaks upon processing in vivo by DNA repair machinery (94, 95). Genomic DNA base damage levels can also be measured using the alkaline comet assay following treatment of permeabilized gel-embedded cells with purified lesion-specific enzymes such as Endonuclease III (thymine glycol), FPG and OGG1 (8-oxoG), T4 endonuclease V (pyrimidine dimers), AlkA (alkylation damage such as 3-methyladenine), and UNG (uracil) that convert their respective substrates to alkalai-labile abasic sites or to single strand breaks (93).
Comet assays have been used in several studies with human lymphocytes to measure inter-individual differences in DRC. One study demonstrated a 4-fold inter-individual variation in BER of 8-oxoG and a 10-fold variation in NER of UV-induced damage (96). Differences in DRC provide biological insight; reduced DRC relative to healthy individuals has been associated with cancer risk in a number of studies (Table 2). An approximate 2-fold increase in endogenous DNA damage (suggestive of reduced DRC) has been observed in lymphocytes from brain cancer patients, relative to healthy individuals (97); up to 2-fold higher levels of bleomycin induced DNA damage have been observed in lymphocytes from breast cancer patients (98); and modestly reduced repair of bleomycin-induced DNA damage has been observed in lymphocytes from both breast cancer patients and non small cell lung cancer patients relative to healthy controls (98, 99). Furthermore, reduced repair of hydrogen peroxide-induced DNA damage was found in lymphocytes from lung cancer patients (100), and a 4-fold variation in the rate of repair for ionizing radiation induced DNA damage was found, wherein lymphocytes from head and neck cancer patients were more likely to exhibit slow repair (101). The aggregate data from these studies raise the possibility that individuals with lower DRC are more prone to cancer, and might be candidates for more aggressive cancer screening.
Finally, comet assays have also been used to show that lymphocytes from patients with extreme reactions to radiation treatment, defined as grade 4 (102), repair ionizing radiation induced DNA damage with slower kinetics than lymphocytes from normal responders (103). This work suggests that it may be possible to use DRC assays to predict radiation sensitivity, and to tailor treatment based on individual tolerance.
Until recently, the labor-intensive nature and large inter-laboratory variation in analysis of comet assay data constituted a significant barrier to the application of the comet assay in large studies. However, a chip-based comet assay with automated image analysis has opened the door to such studies (104); the comet chip assay provides a high throughput platform that significantly reduces the inherent noise in the conventional comet assay. The ability to measure repair of a wide variety of types of genomic DNA damage on a single comet chip represents a major step forward. A limitation of the assay is that while there are strategies for extending the comet assay to measure many types of DNA damage, the methodology is limited to the subset of DNA lesions that either induce strand breaks or induce damage that can be converted to strand breaks.
Host Cell Reactivation Assays
Host cell reactivation (HCR) assays offer a powerful way to measure DRC in living cells. The foundation of the assay lies in the ability of transcription blocking DNA damage to impede expression of a transiently transfected reporter gene; repair restores transcription of the reporter gene, which may encode enzymes such as chloramphenicol acetyltransferase (CAT) and luciferase, or a fluorescent protein (105). A major strength of HCR assays, stemming from the in vitro generation of damaged reporter plasmid DNA, is the ability to measure the in vivo repair of specific DNA lesions in intact cells.
The same association between reduced DRC and cancer risk found using mutagen sensitivity, comet and cell free assays, was also demonstrated in several epidemiological studies using HCR assays. For most of these studies, reporter protein activity was measured in cell lysates prepared from transiently transfected human lymphocytes. Early HCR assays using UV-irradiated CAT reporter plasmids showed approximately a 10-fold range of inter-individual differences in NER capacity, with a significantly lower average DRC in lymphocytes from basal cell carcinoma patients compared to those from controls (106). HCR assays making use of UV-irradiated CAT or luciferase reporters have revealed 10-20% reduced DRC (relative to control) in lymphocytes from patients with either melanoma or non-melanoma skin cancer (107, 108). In other studies, repair of plasmids damaged with BPDE or the nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) was reduced between 10 and 60% in lymphocytes from patients with lung cancer (109-112), non-small cell lung cancer (113), breast cancer (114-116), squamous cell carcinoma of the head and neck (117, 118), and lung adenocarcinoma (112). Furthermore, DRC below the control median was associated with an increased risk of cancer, with odds ratios ranging from 1.5 to 5.7 (51). In further studies, lymphocytes from bladder cancer patients repaired plasmids damaged with 4-aminobiphenyl with ~10% reduced efficiency (119), and ~10% reduction in repair of plasmids alkylated with dimethyl sulfate was observed in lymphocytes from patients with lung adenocarcinoma (112). Assessment of NER capacity from apparently healthy individuals has also shown a 5.6 to 11-fold range of inter-individual variation and an inverse correlation with age and adiposity (120, 121). A plasmid end-joining assay found a statistically insignificant ~6% reduced average DRC in lymphocytes from breast cancer patients, but the lowest DRC quartile was positively associated with increased cancer risk (odds ratio 2.2) (122).
Additional HCR assays, including a few with fluorescent reporters that do not require cell lysates for analysis have been developed for measuring HR (123, 124), MMR (125), BER (126), NHEJ (127), inter-strand cross link repair (7, 128, 129) and repair of oxidative damage (130, 131). A multiplexed fluorescence-based flow cytometric HCR assay (FM-HCR) that uses different colored fluorescent reporter plasmids to measure repair of multiple doses or multiple types of DNA damage in a single assay was recently developed (132). FM-HCR is less labor intensive than HCR assays that require cell lysate preparation, and uses DNA lesion-induced transcriptional mutagenesis to measure repair of specific DNA lesions, such as O6-methylguanine and 8-oxoguanine, that are bypassed by RNA polymerase and thus refractory to conventional HCR assays. An even higher throughput HCR assay that uses deep sequencing to measure and sequence reporter transcripts (HCR-seq) was also developed (132). While the epidemiological studies have so far been dominated by NER reporters, the availability of reporters for additional pathways and high throughput HCR assays should encourage future studies to examine multiple repair pathways.
HCR assays face some potential limitations. The repair of constitutively transcribed plasmid DNA measured by HCR may not accurately reflect repair of genomic DNA. However, the assays have been validated using a variety of cell lines and in primary human blood cells with DNA repair defects caused by mutated and inactivated DNA repair genes. Importantly, numerous epidemiological studies (discussed above) have confirmed that HCR assays can reproducibly measure small DRC differences in primary human tissues that are associated with disease. In further support of the notion that plasmid DNA transactions can be reflective of genomic DNA transactions, it appears that plasmids are readily complexed into a nucleosomal structure in human cells, i.e. they become chromatinized (133-136). Moreover, plasmid DNA damage induces histone modifications that affect expression of plasmid DNA (137), indicating a functional plasmid-chromatin structure.
5. The need for assays that measure DRC in more than one pathway
The majority of epidemiological studies that apply functional DRC assays have focused on a single DNA repair pathway, namely NER. However, data continue to emerge in support of the notion that DRC for more than one pathway will be required to gain maximal biological insight. Here we consider several contexts in which multiple DNA repair pathways, or the multiple steps within a single pathway, interact to influence disease risk or the sensitivity of cells and animals to DNA damaging agents.
Multiple repair defects and cancer
Treatment with SN1 type alkylating agents such as temozolomide and decarbazine generates toxic O6-MeG lesions that are repaired by MGMT. MGMT deficient cells are thus generally very sensitive to SN1 alkylating agents. However because the toxicity of O6-MeG lesions is mediated by MMR (138, 139), MGMT deficient cells can become resistant to alkylating agents if they acquire a MMR deficiency (140). This chemoresistance or tolerance mechanism would confound efforts to predict treatment efficacy based on tumor MGMT status alone, and suggests a need to measure both pathways for improved prognosis. A second example where the status of two or more pathways determines the sensitivity of cells is seen in the context of poly(ADP-ribose) polymerase (PARP) inhibitors. PARP is involved in DNA single strand break repair, and PARP inhibition potentiates DNA damage-induced cell death (141). In the absence of DNA damaging agents, cells can generally tolerate either PARP inhibition or a defect in HR. However, because HR rescues the collapsed replication forks generated when the replication machinery encounters a single strand break, treatment of HR-deficient cells with PARP inhibitors leads to a synthetic lethality (141).
Polymorphisms in multiple DNA repair pathways or multiple steps within a pathway are associated with elevated cancer risk. A study of non-small cell lung cancer patients revealed small individual hazard ratios (up to ~1.4) for polymorphisms in genes involved in NER (XPA, XPD, XPG), BER (XRCC1), and HR (XRCC2, XRCC3), but a larger hazard ratio for patients with any combination of 4 or more polymorphisms in different genes within a pathway or in different pathways (hazard ratio 1.8) (28). For pathways that involve more than one repair protein, such as NER, there is potential for an additive or synergistic effect of combining modest functional defects in multiple steps along the pathway. A recent candidate gene association study revealed that SNPs in the NER genes XPG, XPD, XPA, and XPE are associated with worse prognosis following skin cancer diagnosis; a hazard ratio of 1.26 was calculated for individuals with a variant genotype in one of the genes, but the hazard ratio increased dramatically for individuals with variants in 2 or 3 of these genes to 3.90 and 34.3, respectively (30).
Functional DRC measurements in multiple DNA repair pathways have also revealed higher risk factors than measurements in any single pathway would indicate. A breast cancer study found that combined NER deficiency (measured by an immunohistochemical assay for BPDE repair) and NHEJ deficiency (measured by a plasmid repair assay) represented a greater cancer risk factor (odds ratio 4.92) than deficiency in either pathway alone (odds ratio 1.16) (77, 122). A second case control study of both OGG1 and AAG activities showed that reduced OGG1 activity and elevated AAG activity were associated with a higher risk of lung cancer, and most important, that a combined score for the two enzyme activities was more strongly associated with cancer risk than either OGG1 or AAG activity alone (142). Recently, this study has been extended to incorporate APE1 into an integrated DNA repair score, termed “OMA” for OGG1, MPG (a.k.a AAG) and APE1; the OMA score varies over a 20-fold range and associates even more strongly with risk of lung cancer (odds ratio 5.6 comparing individuals with the lowest to highest tertile OMA scores) (143). These results emphasize that measuring repair capacity in more than one pathway has the potential to increase biological insight and reveal stronger correlations between DRC and disease risk. It should be noted that the lower OMA scores correspond to lower levels of OGG1 and APE1 activity, but higher AAG activity, underscoring the fact that for some pathways high levels of DRC are not always protective.
Imbalanced repair and toxic repair intermediates
Evidence for potentially harmful and tissue dependent effects from higher DRC levels has emerged from the characterization of Aag-dependent alkylation sensitivity in cells and animals. Aag deficiency has been associated with sensitivity to alkylating agents in mouse embryonic stem cells, consistent with a relationship wherein risk (defined for this specific example as the risk of cell death upon exposure to an alkylating agent) in one tissue decreases with increasing DRC (Fig. 2b). However an unexpected phenotype was observed in mouse models, wherein Aag deficiency leads to extreme alkylation resistance in certain tissues (49), indicating, that for some tissues risk increases with increasing DRC (Fig. 2c). Accordingly, overexpression of Aag leads to tissue-specific alkylation sensitivity in mice (92).
A lack of proper coordination among multiple DNA repair steps (repair imbalance) has been invoked to explain increased alkylation sensitivity in cells that overexpress Aag. Aag overexpression leads to the accumulation of DNA repair intermediates (49, 144), which include 5’-deoxyribose phosphate containing single strand breaks that can trigger hyperactivation of PARP. This enzyme modifies numerous other proteins, including several in DNA repair pathways (141, 145). PARP also facilitates both repair (SSBR and BER) as well as a cell death pathway involving NAD and ATP depletion, and an energetic crisis followed by cell death (146-148). Cells and whole animals that overexpress Aag but are genetically deficient for PARP show a complete rescue of wild type sensitivity to alkylating agents, confirming that SSB-stimulated PARP hyperactivation is responsible for hypersensitivity (92).
It should be noted that this situation contrasts with pharmacological PARP inhibition that generally leads to DNA alkylating agent sensitivity. The consequences pharmacological PARP inactivation using inhibitors may differ from the consequences of genetic depletion because inhibitors can induce formation of a stable 5’dRP:PARP:Inhibitor complex at SSBs that inhibits DNA repair and blocks replication (149-151), potentially leading to double strand breaks (152, 153). Thus PARP inhibition leads to alkylation hypersensitivity in cells that accumulate SSBs, including cells that overexpress AAG (154), and polymerase beta or ligase III deficient cells (155, 156).
SSBs also accumulate in cells from individuals with the neurodegenerative diseases spinocerebellar ataxia with axonal neuropathy-1 (SCAN1) and ataxia oculomotor apraxia-1 (AOA1) (157). For SCAN1, abortive topoisomerase-I reactions lead to SSBs covalently linked to the enzyme; SCAN1 patients are deficient for the TDP1 enzyme that hydrolyzes the 3’-phosphotyrosyl bond between stalled topoisomerase-I and a SSB to facilitate repair. A related enzyme, TDP2, hydrolyzes 5’-phosphotyrosyl bonds between topoisomerase-II and DNA at DSBs to facilitate NHEJ-dependent repair (158), and exhibits weak 3’-tyrosyl phosphodiesterase activity (159), however TDP2 has not as yet been associated with disease. AOA1 patients are deficient for APTX, an enzyme that catalyzes reversal of premature 5’-adenylation at SSBs; although 5’adenylation is required for ligation of SSBs, if this modification occurs in the absence of a free 3’ hydroxyl, ligation cannot be completed. It is of particular interest that SCAN1 and AOA1 manifest as neurodegenerative diseases, but do not predispose to cancer. To explain this disproportionate effect on terminally differentiated neurons, it has been proposed that TDP1 and APTX may be redundant in proliferating cells because alternative end processing factors and the HR pathway can resolve SSBs during replication (157).
An additional example of a phenotype caused by the accumulation of repair intermediates that depends on multiple DNA repair proteins, comes from a recent study implicating the DSB repair protein WRN in long patch BER of adenine opposite 8-oxoguanine (A:8-oxoG) (160). Cells deficient for WRN or polymerase λ are more sensitive than wild type to oxidizing agents such as hydrogen peroxide, due to inefficient BER of oxidative damage. However, WRN deficient cells and polymerase λ deficient cells that are also deficient for MUTYH exhibit wild type sensitivity to oxidizing agents, suggesting that MUTYH leads to toxic repair intermediates . Indeed, glycosylase mediated accumulation of toxic BER intermediates has also been invoked to explain sensitivity to a variety agents including alkylating agents (49, 144), ionizing radiation (161), and 5-fluorouracil (162).
Repair competition
Some DNA lesions are repaired by proteins from more than one of the canonical DNA repair pathways shown in Table 1 and Table 2. Multiple pathways may either complement or interfere with one another. For example, BER of 8-oxoG opposite cytosine is initiated by one of several DNA glycosylases (OGG1, NEIL1 and NIEL2) (163). Moreover, it was recently reported that proteins involved in transcription coupled NER (e.g. XPA, CSB and RNA polymerase II), also participate in an 8-oxoG repair in actively transcribed DNA (9). Another example of distinct repair proteins competing for the same lesions arises for highly mutagenic etheno base lesions (164-166). Ethenocytosine (εC) can be bound by AAG but not excised by it, and this binding interaction prevents repair by ALKBH2 (166) and possibly TDG glycosylase. As a result, individual activity levels for AAG, TDG or ALKBH2 would not provide a complete picture for the repair of εC base lesions. Finally, although agents that form inter-strand cross-links (ICL) have been widely used as chemotherapeutics, the detailed mechanisms of ICL repair are only now beginning to be understood (167). Evidence exists for replication-dependent and replication-independent ICL repair involving proteins from several DNA repair pathways including the FA, HR, and NER pathways, as well as TLS polymerases (7, 8). Recent work shows that BER and MMR play an epistatic role in mediating cisplatin sensitivity, implicating proteins from these pathways in ICL repair as well (6, 10).
Immune dysfunction
Because immune function involves programmed induction of multiple types of DNA damage, DNA repair proteins from multiple pathways also play a critical role in the immune system, and some DRC defects are associated with immunodeficiency (Table 1). Numerous DSB repair proteins are required for V(D)J recombination, which takes place in both T and B lymphocytes and is essential for the development of specialized antigenic receptors known as T-cell receptors (TCR) and B-cell receptors (BCR), composed of an immunoglobulin molecule and a CD79 moiety. The process is initiated by the Rag1 and Rag2 recombinase enzymes that induce DSBs in specific recombination signal sequences flanking V, D, and J gene units. These DSBs are then repaired by NHEJ. Consequently, many human patients with NHEJ deficiencies also have V(D)J recombination defects and suffer from a particular group of diseases known as severe combined immunodeficiencies (SCID), in particular, radiosensitive SCID. SCID is characterized by impaired T and B lymphocyte differentiation that is sometimes accompanied by deficiencies in other lineages (168). Individuals with deficiencies in NHEJ proteins (DNA-PKcs (169), Artemis (170, 171), LigIV (172) and NHEJ1/XLF/Cernunnos (173)) consistently present some degree of SCID.
Proteins from several DNA repair pathways are also involved in the terminal maturation of B lymphocytes during which two additional stages of DNA modification take place after VDJ recombination in order to increase the efficiency of the humoral response (174, 175). First, class switch recombination (CSR) exchanges the immunoglobulin (Ig) constant region to modify the Ig isotype (from IgM to IgG, IgA, etc.). In the second step, somatic hypermutation (SHM) introduces sequence diversity into the Ig variable domain to provide the potential for increased antigen affinity. Both CSR and SHM are initiated by the action of activation-induced cytidine deaminase (AID). Within hotspots, AID deaminates cytosine to uracil, creating U:G mismatches. CSR is induced when the BER protein UNG excises closely opposed uracils that are further processed by APE1 to generate a DSB that triggers processing by the HR machinery. U:G pairs escaping UNG recognition can go on to be processed by the MMR machinery and subsequently form DSB in a yet unidentified manner (176). The distinct process of SHM occurs by replication bypass of uracil (in the absence of repair by MMR or BER) inducing C to A transversions, or by translesion polymerase bypass of AP sites and gaps (generated by UNG and MMR proteins, respectively). As might be expected from their involvement in the immune response, deficiencies in both BER and MMR have been implicated in improper B-cell maturation; one of the autosomal forms of CSR deficiency known as hyper-IgM (HIGM) syndromes has been ascribed to mutations in the UNG gene (177). Similarly, three patients with deficiencies in the MMR protein PMS2 were shown to be deficient in CSR (178). Immunodeficiency syndromes are associated with increased risk of cancer (179), it has been recently hypothesized that germline mutations in genes involved in V(D)J recombination, SHM and CSR play a role in the lymphomagenesis of diffuse large B cell lymphomas (180).
Our understanding of the role that DNA repair plays during normal lymphocyte maturation continues to evolve as additional interactions between DNA repair pathways are discovered. For example, recent work suggests that interactions between MMR proteins and the MBD4 DNA glycosylase may be important for efficient CSR (181). An intriguing possibility is that the pronounced DNA repair defects associated with some severe immune disorders may presage discovery of milder immunodeficiency that can be attributed to modest defects in multiple DNA repair pathways.
The diversity of disease states and sensitivity phenotypes associated with inefficient DNA repair in more than one pathway, or in more than one step within a pathway, calls for more studies that explore multiple repair activities. Recently accumulating data discussed above suggest subtle defects in multiple DNA repair pathways might promote disease, raising the prospect that multiplexed DRC assays could be of value in clinical diagnosis, prevention and treatment of disease.
6. Current status of DRC measurements for prevention and treatment of disease
A long-term goal that motivates many of the epidemiological studies discussed herein is to eventually apply direct or indirect estimates of DRC to the personalized treatment or prevention of disease. However current clinical practice is limited to diagnostics. Genetic testing is available for known mutations in many of the genes associated with DRC defects and disease, including BRCA1/2, MLH1, MSH2/6, p53, and MUTYH (14); individuals with these mutations are advised to undergo more aggressive screening, and in some cases prophylactic surgery. T-cell chromosome breakage or aberrations following treatment with DNA damaging agents such as mitomycin C have been used as a diagnostic for Fanconi Anemia (182), and UV-induced unscheduled DNA synthesis and sister chromatid exchange assays have been used for the molecular diagnosis of XP and Blooms syndrome, respectively (183). However, because they are labor intensive and/or expensive, both genetic testing and cell-based assays are typically used only in cases where the disease is already suspected either because of pronounced symptoms or a family history of disease.
DRC Estimates may be close to finding application in cancer treatment. Relationships between DRC and improved tumor response to anticancer drugs have been reported for MMR proficiency in cisplatin, alkylating agents, and 5-fluorouracil cancer chemotherapy (140, 184-186). XRCC1 (BER) deficiency in tumors has been linked to cisplatin sensitivity (44, 187); MGMT deficiency has been linked to temozolomide and BCNU sensitivity (188, 189), and HR deficiency to PARP inhibitor sensitivity (141). A limited study suggests that functional assays could be useful for determining the maximum radiation dose that will be tolerated by a patient (103). Thus, if individuals could be pre-identified as therapy resistant, it may be possible to raise the treatment dose to improve efficacy. Despite these examples of potential clinical applications, enthusiasm for the use of DRC measurements to guide treatment decisions are dampened by concerns about assay standardization, assay reproducibility, and the lack of prospective, randomized studies. As a result, although DRC assays are used to retrospectively classify patients into good versus poor responders to cancer therapy (28, 43, 53, 187, 190, 191), these assays are not used currently to influence cancer patient management (20, 58, 192-197).
7. What is needed going forward
To speed the translation of functional DRC assays from a laboratory tool to biomedical applications, advances are needed in several areas. (i) Excepting a small number of prospective studies (52, 53, 198), virtually all studies associating DRC defects with cancer susceptibility have been retrospective, raising concerns that the observed DRC differences may reflect changes subsequent to cancer diagnosis, or to cancer treatment. Similar levels of DNA damage have been observed in lymphoblastoid cell lines derived before versus after cancer diagnosis (198), suggesting that cancer development may not alter DRC. Moreover, one line of evidence suggests that lymphocyte DRC may be altered in cancer patients due to a systemic inflammatory response to the disease (199-203), and it is also possible that cancer treatment affects DRC. There is thus a need for additional large prospective studies to confirm that the DRC was a cause, rather than an effect, of the disease, and that subsequent treatment did not cause long-term DRC changes. We advocate that as new studies are initiated, cells from patients and their tumors should be cryopreserved such that live cells can be recovered for the purpose of functional DRC assays. Currently such samples are most often preserved for DNA, RNA and protein analyses, under conditions incompatible with in vivo functional assays. (ii) To carry out large studies, high throughput quantitative assays measuring DRC in multiple pathways are needed to maximize biological insight and prognostic potential. Standardized quantitative assays will help overcome concerns about reproducibility, an issue that is especially acute for assessments that are more subjective and less quantitative DRC indicators, such as microsatellite instability (MSI), where labs use different thresholds to distinguish MSI from microsatellite stability (204). (iii) The complexity of the relationships between DRC, other pathways, genetics, epigenetics and environmental exposure suggest that complementary approaches combining several of the techniques described above and in Fig. 3 may be needed to provide a comprehensive assessment of DRC. Collaborative projects using multiple approaches would be helpful for determining which approach or combination of approaches yields the most robust DRC-based disease prediction and diagnostic potential. (iv) The suitability of lymphocytes as a surrogate tissue for DRC in other tissues requires additional testing. The numerous epidemiological studies referenced above support the utility of measuring DRC in lymphocytes to predict disease susceptibility, and some investigators have found strong correlations between DRC in lymphocytes and other tissues (205), while others have not (90). (v) DRC variation among tissues is underexplored. In both humans and mice, large tissue-specific DRC variation has been measured for some pathways, indicating that it may be necessary to measure DRC directly in the tissue of interest (92, 206, 207). For example, DRC in liver cells might be most useful in assessing liver cancer risk; however the need for an invasive biopsy represents a major barrier to tissue-specific DRC screening. A particularly promising solution to this problem would be to generate cells representative of various human tissues by differentiating induced pluripotent stem cells (iPS cells) generated from skin fibroblasts that can be obtained from a single, relatively less invasive biopsy. The commercial availability of multiple cell types from a single individual (including iPS cells) together with advancing methods of generating iPS cells from primary tissues (208), make an initial test this approach experimentally feasible. A long-term goal would be to develop methods of measuring tissue-specific DRC variation in a variety of human cell types derived from a skin biopsy from each individual. (vi) Finally, mitochondrial DNA repair represents a relatively understudied area, and given the severe health consequences of mitochondrial DNA depletion (209), as well as accumulating evidence of relationships among mitochondrial DNA damage, mitochondrial dysfunction and disease (210-215), it appears likely that defects in mitochondrial DRC can also be linked to disease susceptibility.
8. Conclusions
The aggregate data from several decades of molecular epidemiology indicate that DRC varies significantly among individuals, and that these variations associate with disease risk. No single DNA repair pathway is universally representative of DRC in general, and the effects of variation in repair efficiency at distinct steps or in separate pathways can combine to produce surprising and sometimes counterintuitive phenotypes. There are many ways to measure DRC. However each has its strengths and weaknesses. So far a lack of standardization and clinical validation, together with the relatively low throughput and labor-intensive nature of most methods of measuring DRC have precluded the application of functional DRC assays for personalized disease prevention and treatment. However a recent burst of technical advances, including the highly automated comet chip (104), a proof of concept for integrating DRC in multiple pathways to calculate disease risk (143), and highly multiplexed HCR assays (132), support the notion that measuring DRC could become common clinical practice. To promote this transition, these emerging technologies should be further developed, standardized and validated across multiple laboratories in large (ideally prospective) epidemiological studies employing measurements of multiple DNA repair pathways.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hartwig A, Blessing H, Schwerdtle T, Walter I. Modulation of DNA repair processes by arsenic and selenium compounds. Toxicology. 2003;193(1-2):161–9. doi: 10.1016/j.tox.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Andrew AS, Burgess JL, Meza MM, Demidenko E, Waugh MG, Hamilton JW, Karagas MR. Arsenic exposure is associated with decreased DNA repair in vitro and in individuals exposed to drinking water arsenic. Environ Health Perspect. 2006;114(8):1193–8. doi: 10.1289/ehp.9008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed S, Khoda SME, Rekha RS, Gardner RM, Ameer SS, Moore S, Ekstrom EC, Vahter M, Raqib R. Arsenic-Associated Oxidative Stress, Inflammation, and Immune Disruption in Human Placenta and Cord Blood. Environ Health Perspect. 2011;119(2):258–64. doi: 10.1289/ehp.1002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hengstler JG, Bolm-Audorff U, Faldum A, Janssen K, Reifenrath M, Gotte W, Jung DL, Mayer-Popken O, Fuchs J, Gebhard S, Bienfait HG, Schlink K, Dietrich C, Faust D, Epe B, Oesch F. Occupational exposure to heavy metals: DNA damage induction and DNA repair inhibition prove co-exposures to cadmium, cobalt and lead as more dangerous than hitherto expected. Carcinogenesis. 2003;24(1):63–73. doi: 10.1093/carcin/24.1.63. [DOI] [PubMed] [Google Scholar]
- 5.Mattern J, Koomagi R, Volm M. Smoking-related increase of O-6-methylguanine-DNA methyltransferase expression in human lung carcinomas. Carcinogenesis. 1998;19(7):1247–50. doi: 10.1093/carcin/19.7.1247. [DOI] [PubMed] [Google Scholar]
- 6.Kothandapani A, Dangeti VSMN, Brown AR, Banze LA, Wang X-H, Sobol RW, Patrick SM. Novel role of base excision repair in mediating cisplatin cytotoxicity. J Biol Chem. 2011;286(16):14564–74. doi: 10.1074/jbc.M111.225375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enoiu M, Jiricny J, Schärer OD. Repair of cisplatin-induced DNA interstrand crosslinks by a replication-independent pathway involving transcription-coupled repair and translesion synthesis. Nucleic Acids Res. 2012;40(18):8953–64. doi: 10.1093/nar/gks670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim H, D'Andrea AD. Regulation of DNA cross-link repair by the Fanconi anemia/BRCA pathway. Genes Dev. 2012;26(13):1393–408. doi: 10.1101/gad.195248.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo J, Hanawalt PC, Spivak G. Comet-FISH with strand-specific probes reveals transcription-coupled repair of 8-oxoGuanine in human cells. Nucleic Acids Res. 2013 doi: 10.1093/nar/gkt524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kothandapani A, Sawant A, Dangeti VSMN, Sobol RW, Patrick SM. Epistatic role of base excision repair and mismatch repair pathways in mediating cisplatin cytotoxicity. Nucleic Acids Res. 2013 doi: 10.1093/nar/gkt479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lv L, Wang F, Ma X, Yang Y, Wang Z, Liu H, Li X, Liu Z, Zhang T, Huang M, Friedberg EC, Tang T-S, Guo C. Mismatch repair protein MSH2 regulates translesion DNA synthesis following exposure of cells to UV radiation. Nucleic Acids Res. 2013;41(22):10312–22. doi: 10.1093/nar/gkt793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraemer KH, Lee MM, Scotto J. DNA repair protects against cutaneous and internal neoplasia - evidence from xeroderma pigmentosum. Carcinogenesis. 1984;5(4):511–4. doi: 10.1093/carcin/5.4.511. [DOI] [PubMed] [Google Scholar]
- 13.O'Driscoll M. Diseases Associated with Defective Responses to DNA Damage. Cold Spring Harbor Perspectives in Biology. 2012;4(12) doi: 10.1101/cshperspect.a012773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellis NC. In: Obtaining and Using Genetic Information. Ellis NC, editor. Springer; New York: 2003. [Google Scholar]
- 15.Grossman L, Wei Q. DNA repair and epidemiology of basal cell carcinoma. Clinical chemistry. 1995;41(12):1854–63. [PubMed] [Google Scholar]
- 16.Hsu TC. Genetic instability in the human population - a working hypothesis. Hereditas. 1983;98(1):1–9. doi: 10.1111/j.1601-5223.1983.tb00574.x. [DOI] [PubMed] [Google Scholar]
- 17.Cleaver JE. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968;218(5142):652. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- 18.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461(7267):1071–8. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waters LS, Minesinger BK, Wiltrout ME, D'Souza S, Woodruff RV, Walker GC. Eukaryotic translesion polymerases and their roles and regulation in DNA damage tolerance. Microbiology and Molecular Biology Reviews. 2009;73(1):134–54. doi: 10.1128/MMBR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jalal S, Earley JN, Turchi JJ. DNA Repair: From Genome Maintenance to Biomarker and Therapeutic Target. Clinical Cancer Research. 2011;17(22):6973–84. doi: 10.1158/1078-0432.CCR-11-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Easton DF, Eeles RA. Genome-wide association studies in cancer. Human Molecular Genetics. 2008;17:R109–R15. doi: 10.1093/hmg/ddn287. [DOI] [PubMed] [Google Scholar]
- 22.Varghese JS, Easton DF. Genome-wide association studies in common cancers what have we learnt? Curr Opin Genet Dev. 2010;20(3):201–9. doi: 10.1016/j.gde.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, Cho JH, Guttmacher AE, Kong A, Kruglyak L, Mardis E, Rotimi CN, Slatkin M, Valle D, Whittemore AS, Boehnke M, Clark AG, Eichler EE, Gibson G, Haines JL, Mackay TFC, McCarroll SA, Visscher PM. Finding the missing heritability of complex diseases. Nature. 2009;461(7265):747–53. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gossage L, Madhusudan S. Cancer pharmacogenomics - Role of DNA repair genetic Polymorphisms in individualizing cancer therapy. Mol Diagn Ther. 2007;11(6):361–80. doi: 10.1007/BF03256260. [DOI] [PubMed] [Google Scholar]
- 25.Kamikozuru H, Kuramochi H, Hayashi K, Nakajima G, Yamamoto M. ERCC1 codon 118 polymorphism is a useful prognostic marker in patients with pancreatic cancer treated with platinum-based chemotherapy. Int J Oncol. 2008;32(5):1091–6. [PubMed] [Google Scholar]
- 26.Kalikaki A, Kanaki M, Vassalou H, Souglakos J, Voutsina A, Georgoulias V, Mavroudis D. DNA Repair Gene Polymorphisms Predict Favorable Clinical Outcome in Advanced Non-Small-Cell Lung Cancer. Clin Lung Cancer. 2009;10(2):118–23. doi: 10.3816/CLC.2009.n.015. [DOI] [PubMed] [Google Scholar]
- 27.Gangawar R, Ahirwar D, Mandhani A, Mittal RD. Impact of nucleotide excision repair ERCC2 and base excision repair APEX1 genes polymorphism and its association with recurrence after adjuvant BCG immunotherapy in bladder cancer patients of North India. Med Oncol. 2010;27(2):159–66. doi: 10.1007/s12032-009-9187-y. [DOI] [PubMed] [Google Scholar]
- 28.Butkiewicz D, Rusin M, Sikora B, Lach A, Chorazy M. An association between DNA repair gene polymorphisms and survival in patients with resected non-small cell lung cancer. Mol Biol Rep. 2011;38(8):5231–41. doi: 10.1007/s11033-010-0674-1. [DOI] [PubMed] [Google Scholar]
- 29.He CY, Duan ZP, Li P, Xu Q, Yuan Y. Role of ERCC5 promoter polymorphisms in response to platinum-based chemotherapy in patients with advanced non-small-cell lung cancer. Anti-Cancer Drugs. 2013;24(3):300–5. doi: 10.1097/CAD.0b013e32835bd6ce. [DOI] [PubMed] [Google Scholar]
- 30.Li CY, Yin M, Wang LE, Amos CI, Zhu DK, Lee JE, Gershenwald JE, Grimm EA, Wei QY. Polymorphisms of Nucleotide Excision Repair Genes Predict Melanoma Survival. Journal of Investigative Dermatology. 2013;133(7):1813–21. doi: 10.1038/jid.2012.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rumiato E, Cavallin F, Boldrin E, Cagol M, Alfieri R, Basso D, Castoro C, Ancona E, Amadori A, Ruol A, Saggioro D. ERCC1 C8092A (rs3212986) polymorphism as a predictive marker in esophageal cancer patients treated with cisplatin/5-FU-based neoadjuvant therapy. Pharmacogenet Genomics. 2013;23(11):597–604. doi: 10.1097/FPC.0b013e3283653afc. [DOI] [PubMed] [Google Scholar]
- 32.Pegg AE, Fang Q, Loktionova NA. Human variants of O6-alkylguanine-DNA alkyltransferase. DNA Repair. 2007;6(8):1071–8. doi: 10.1016/j.dnarep.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khrunin AV, Moisseev A, Gorbunova V, Limborska S. Genetic polymorphisms and the efficacy and toxicity of cisplatin-based chemotherapy in ovarian cancer patients. Pharmacogenomics J. 2010;10(1):54–61. doi: 10.1038/tpj.2009.45. [DOI] [PubMed] [Google Scholar]
- 34.Dubois J, Etienne G, Laroche-Clary A, Lascaux A, Bidet A, Lippert E, Aitouferoukh A, Saada V, Micol J, Bouabdallah K, Robert J. Identification of methylguanine methyltransferase polymorphisms as genetic markers of individual susceptibility to therapy-related myeloid neoplasms. European Journal of Cancer. 2014;50:7. doi: 10.1016/j.ejca.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Kohlmann A, Klein HU, Weissmann S, Bresolin S, Chaplin T, Cuppens H, Haschke-Becher E, Garicochea B, Grossmann V, Hanczaruk B, Hebestreit K, Gabriel C, Iacobucci I, Jansen JH, Kronnie GT, van de Locht L, Martinelli G, McGowan K, Schweiger MR, Timmermann B, Vandenberghe P, Young BD, Dugas M, Haferlach T. The Interlaboratory RObustness of Next-generation sequencing (IRON) study: a deep sequencing investigation of TET2, CBL and KRAS mutations by an international consortium involving 10 laboratories. Leukemia. 2011;25(12):1840–8. doi: 10.1038/leu.2011.155. [DOI] [PubMed] [Google Scholar]
- 36.Lo HS, Wang ZN, Hu Y, Yang HH, Gere S, Buetow KH, Lee MP. Allelic variation in gene expression is common in the human genome. Genome Res. 2003;13(8):1855–62. doi: 10.1101/gr.1006603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van de Vijver MJ, He YD, van 't Veer LJ, Dai H, Hart AAM, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R. A gene-expression signature as a predictor of survival in breast cancer. New England Journal of Medicine. 2002;347(25):1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 38.Wan YW, Qian Y, Rathnagiriswaran S, Castranova V, Guo NL. A breast cancer prognostic signature predicts clinical outcomes in multiple tumor types. Oncol Rep. 2010;24(2):489–94. doi: 10.3892/or_00000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma Y, Qian Y, Wei L, Abraham J, Shi XL, Castranova V, Harner EJ, Flynn DC, Guo L. Population-based molecular prognosis of breast cancer by transcriptional profiling. Clinical Cancer Research. 2007;13(7):2014–22. doi: 10.1158/1078-0432.CCR-06-2222. [DOI] [PubMed] [Google Scholar]
- 40.Shedden K, Taylor JMG, Enkemann SA, Tsao MS, Yeatman TJ, Gerald WL, Eschrich S, Jurisica I, Giordano TJ, Misek DE, Chang AC, Zhu CQ, Strumpf D, Hanash S, Shepherd FA, Ding K, Seymour L, Naoki K, Pennell N, Weir B, Verhaak R, Ladd-Acosta C, Golub T, Gruidl M, Sharma A, Szoke J, Zakowski M, Rusch V, Kris M, Viale A, Motoi N, Travis W, Conley B, Seshan VE, Meyerson M, Kuick R, Dobbin KK, Lively T, Jacobson JW, Beer DG, Director's Challenge Consortium M. Gene expression-based survival prediction in lung adenocarcinoma: a multi-site, blinded validation study. Nat Med. 2008;14(8):822–7. doi: 10.1038/nm.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bild AH, Yao G, Chang JT, Wang QL, Potti A, Chasse D, Joshi MB, Harpole D, Lancaster JM, Berchuck A, Olson JA, Marks JR, Dressman HK, West M, Nevins JR. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2006;439(7074):353–7. doi: 10.1038/nature04296. [DOI] [PubMed] [Google Scholar]
- 42.Dopeso H, Mateo-Lozano S, Elez E, Landolfi S, Pascual FJR, Hernández-Losa J, Mazzolini R, Rodrigues P, Bazzocco S, Carreras MJ. Aprataxin tumor levels predict response of colorectal cancer patients to irinotecan-based treatment. Clinical Cancer Research. 2010;16(8):2375–82. doi: 10.1158/1078-0432.CCR-09-3275. [DOI] [PubMed] [Google Scholar]
- 43.Amatu A, Sartore-Bianchi A, Moutinho C, Belotti A, Bencardino K, Chirico G, Cassingena A, Rusconi F, Esposito A, Nichelatti M. Promoter CpG Island Hypermethylation of the DNA Repair Enzyme MGMT Predicts Clinical Response to Dacarbazine in a Phase II Study for Metastatic Colorectal Cancer. Clinical Cancer Research. 2013;19(8):2265–72. doi: 10.1158/1078-0432.CCR-12-3518. [DOI] [PubMed] [Google Scholar]
- 44.Olaussen KA, Mountzios G, Soria JC. ERCC1 as a risk stratifier in platinum-based chemotharapy for non-small-cell lung cancer. Current Opinion in Pulmonary Medicine. 2007;13(4):284–9. doi: 10.1097/MCP.0b013e32816b5c63. [DOI] [PubMed] [Google Scholar]
- 45.Cloos J, de Boer WPH, Snel MHJ, van den Ijssel P, Ylstra B, Leemans CR, Brakenhoff RH, Braakhuis BJM. Microarray analysis of bleomycin-exposed lymphoblastoid cells for identifying cancer susceptibility genes. Molecular Cancer Research. 2006;4(2):71–7. doi: 10.1158/1541-7786.MCR-05-0196. [DOI] [PubMed] [Google Scholar]
- 46.Fachin AL, Mello SS, Sandrin-Garcia P, Junta CM, Ghilardi-Netto T, Donadi EA, Passos GAD, Sakamoto-Hojo ET. Gene Expression Profiles in Radiation Workers Occupationally Exposed to Ionizing Radiation. Journal of Radiation Research. 2009;50(1):61–71. doi: 10.1269/jrr.08034. [DOI] [PubMed] [Google Scholar]
- 47.Sims AH, Finnon P, Miller CJ, Bouffler SD, Howell A, Scott D, Clarke RB. TPD52 and NFKB1 gene expression levels correlate with G2 chromosomal radiosensitivity in lymphocytes of women with and at risk of hereditary breast cancer. Int J Radiat Biol. 2007;83(6):409–20. doi: 10.1080/09553000701317366. [DOI] [PubMed] [Google Scholar]
- 48.Fry RC, Svensson JP, Valiathan C, Wang E, Hogan BJ, Bhattacharya S, Bugni JM, Whittaker CA, Samson LD. Genomic predictors of interindividual differences in response to DNA damaging agents. Genes Dev. 2008;22(19):2621–6. doi: 10.1101/gad.1688508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu D, Calvo JA, Samson LD. Balancing repair and tolerance of DNA damage caused by alkylating agents. Nat Rev Cancer. 2012;12(2):104–20. doi: 10.1038/nrc3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valiathan C, McFaline JL, Samson LD. A rapid survival assay to measure drug-induced cytotoxicity and cell cycle effects. DNA Repair. 2012;11(1):92–8. doi: 10.1016/j.dnarep.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li C, Wang L-E, Wei Q. DNA repair phenotype and cancer susceptibility-A mini review. Int J Cancer. 2009;124(5):999–1007. doi: 10.1002/ijc.24126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu XF, Gu J, Dong Q, Huang MS, Do KA, Hong WK, Spitz MR. Joint effect of mutagen sensitivity and insulin-like growth factors in predicting the risk of developing secondary primary tumors and tumor recurrence in patients with head and neck cancer. Clinical Cancer Research. 2006;12(23):7194–201. doi: 10.1158/1078-0432.CCR-06-0671. [DOI] [PubMed] [Google Scholar]
- 53.Sigurdson AJ, Jones IM, Wei QY, Wu XF, Spitz MR, Stram DA, Gross MD, Huang WY, Wang LE, Gu JA, Thomas CB, Reding DJ, Hayes RB, Caporaso NE. Prospective analysis of DNA damage and repair markers of lung cancer risk from the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Carcinogenesis. 2011;32(1):69–73. doi: 10.1093/carcin/bgq204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watts GS, Pieper RO, Costello JF, Peng YM, Dalton WS, Futscher BW. Methylation of discrete regions of the O-6-methylguanine DNA methyltransferase (MGMT) CpG island is associated with heterochromatinization of the MGMT transcription start site and silencing of the gene. Molecular and Cellular Biology. 1997;17(9):5612–9. doi: 10.1128/mcb.17.9.5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O-6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Research. 1999;59(4):793–7. [PubMed] [Google Scholar]
- 56.Hegi ME, Diserens A, Gorlia T, Hamou M, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JEC, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. New England Journal of Medicine. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 57.Kreth S, Thon N, Eigenbrod S, Lutz J, Ledderose C, Egensperger R, Tonn JC, Kretzschmar HA, Hinske LC, Kreth FW. O6-methylguanine-DNA methyltransferase (MGMT) mRNA expression predicts outcome in malignant glioma independent of MGMT promoter methylation. PLoS One. 2011;6(2):e17156. doi: 10.1371/journal.pone.0017156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cankovic M, Nikiforova MN, Snuderl M, Adesina AM, Lindeman N, Wen PY, Lee EQ. The Role of MGMT Testing in Clinical Practice A Report of the Association for Molecular Pathology. J Mol Diagn. 2013;15(5):539–55. doi: 10.1016/j.jmoldx.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 59.Zhukovskaya N, Rydberg B, Karran P. Inactive O6-methylguanine-DNA methyl transferase in human cells. Nucleic Acids Res. 1992;20(22):6081–90. doi: 10.1093/nar/20.22.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ishiguro K, Shyam K, Penketh PG, Bauman RP, Sartorelli AC, Rutherford TJ, Ratner ES. Expression of O6-Methylguanine-DNA Methyltransferase Examined by Alkyl-Transfer Assays, Methylation-Specific PCR, and Western Blots in Tumors, and Matched Normal Tissue. Journal of Cancer Therapy. 2013;4(4):14. doi: 10.4236/jct.2013.44103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iwitzki F, Schlepegrell R, Eichhorn U, Kaina B, Beyersmann D, Hartwig A. Nickel(II) inhibits the repair of O-6-methylguanine in mammalian cells. Archives of Toxicology. 1998;72(11):681–9. doi: 10.1007/s002040050561. [DOI] [PubMed] [Google Scholar]
- 62.Szczesny B, Hazra TK, Papaconstantinou J, Mitra S, Boldogh I. Age-dependent deficiency in import of mitochondrial DNA glycosylases required for repair of oxidatively damaged bases. Proceedings of the National Academy of Sciences. 2003;100(19):10670–5. doi: 10.1073/pnas.1932854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herman JG, Umar A, Polyak K, Graff JR, Ahuja N, Issa J-PJ, Markowitz S, Willson JK, Hamilton SR, Kinzler KW. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proceedings of the National Academy of Sciences. 1998;95(12):6870–5. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taniguchi T, Tischkowitz M, Ameziane N, Hodgson SV, Mathew CG, Joenje H, Mok SC, D'Andrea AD. Disruption of the Fanconi anemia-BRCA pathway in cisplatin-sensitive ovarian tumors. Nat Med. 2003;9(5):568–74. doi: 10.1038/nm852. [DOI] [PubMed] [Google Scholar]
- 65.Gasco M, Sullivan A, Smith P, Farrell P, Numico G, Colantonio I, Merlano M, Crook T. Transcriptional silencing of Fanconi anaemia genes and clinical outcome in head and neck cancer. J Clin Oncol. 2004;22:9546. [Google Scholar]
- 66.Narayan G, Arias-Pulido H, Nandula SV, Basso K, Sugirtharaj DD, Vargas H, Mansukhani M, Villella J, Meyer L, Schneider A. Promoter hypermethylation of FANCF disruption of Fanconi Anemia-BRCA pathway in cervical cancer. Cancer research. 2004;64(9):2994–7. doi: 10.1158/0008-5472.can-04-0245. [DOI] [PubMed] [Google Scholar]
- 67.Levran O, Attwooll C, Henry RT, Milton KL, Neveling K, Rio P, Batish SD, Kalb R, Velleuer E, Barral S. The BRCA1-interacting helicase BRIP1 is deficient in Fanconi anemia. Nature genetics. 2005;37(9):931–3. doi: 10.1038/ng1624. [DOI] [PubMed] [Google Scholar]
- 68.Peng B, Hurt EM, Hodge DR, Thomas SB, Farrar WL. DNA hypermethylation and partial gene silencing of human thymine-DNA glycosylase in multiple myeloma cell lines. Epigenetics. 2006;1(3):138–45. doi: 10.4161/epi.1.3.2938. [DOI] [PubMed] [Google Scholar]
- 69.Lee M-N, Tseng R-C, Hsu H-S, Chen J-Y, Tzao C, Ho WL, Wang Y-C. Epigenetic inactivation of the chromosomal stability control genes BRCA1, BRCA2, and XRCC5 in non-small cell lung cancer. Clinical cancer research. 2007;13(3):832–8. doi: 10.1158/1078-0432.CCR-05-2694. [DOI] [PubMed] [Google Scholar]
- 70.Wu Y, Chang JT, Cheng Y, Wu T, Chen C, Lee H. Xeroderma pigmentosum group C gene expression is predominantly regulated by promoter hypermethylation and contributes to p53 mutation in lung cancers. Oncogene. 2007;26(33):4761–73. doi: 10.1038/sj.onc.1210284. [DOI] [PubMed] [Google Scholar]
- 71.Wang P, Tang JT, Peng YS, Chen XY, Zhang YJ, Fang JY. XRCC1 downregulated through promoter hypermethylation is involved in human gastric carcinogenesis. Journal of Digestive Diseases. 2010;11(6):343–51. doi: 10.1111/j.1751-2980.2010.00459.x. [DOI] [PubMed] [Google Scholar]
- 72.Yang J, Xu Z, Li J, Zhang R, Zhang G, Ji H, Song B, Chen Z. XPC epigenetic silence coupled with p53 alteration has a significant impact on bladder cancer outcome. The Journal of urology. 2010;184(1):336–43. doi: 10.1016/j.juro.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 73.Rasmussen RE, Painter RB. Evidence for repair of ultra-violet damaged deoxyribonucleic acid in cultured mammalian cells. Nature. 1964;203(495):1360. doi: 10.1038/2031360a0. [DOI] [PubMed] [Google Scholar]
- 74.Pegg AE, Hui G. Formation and subsequent removal of O6-methylguanine from deoxyribonucleic acid in rat liver and kidney after small doses of dimethylnitrosamine. Biochem J. 1978;173(3):739–48. doi: 10.1042/bj1730739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Santella RM. Immunological methods for detection of carcinogen-DNA damage in humans. Cancer Epidemiology Biomarkers & Prevention. 1999;8(9):733–9. [PubMed] [Google Scholar]
- 76.Pero RW, Ostlund C. Direct comparison, in human resting lymphocytes, of the inter-individual variations in unscheduled DNA synthesis induced by N-acetoxy-2-acetylaminofluorene and ultraviolet radiation. Mutation Research. 1980;73(2):349–61. doi: 10.1016/0027-5107(80)90200-6. [DOI] [PubMed] [Google Scholar]
- 77.Kennedy DO, Agrawal M, Shen J, Terry MB, Zhang FF, Senie RT, Motykiewicz G, Santella RM. DNA repair capacity of lymphoblastoid cell lines from sisters discordant for breast cancer. J Natl Cancer Inst. 2005;97(2):127–32. doi: 10.1093/jnci/dji013. [DOI] [PubMed] [Google Scholar]
- 78.Paz-Elizur T, Elinger D, Leitner-Dagan Y, Blumenstein S, Krupsky M, Berrebi A, Schechtman E, Livneh Z. Development of an enzymatic DNA repair assay for molecular epidemiology studies: Distribution of OGG activity in healthy individuals. DNA Repair. 2007;6(1):45–60. doi: 10.1016/j.dnarep.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 79.Redaelli A, Magrassi R, Bonassi S, Abbondandolo A, Frosina G. AP endonuclease activity in humans: Development of a simple assay and analysis of ten normal individuals. Teratogenesis Carcinogenesis and Mutagenesis. 1998;18(1):17–26. [PubMed] [Google Scholar]
- 80.Shen GP, Galick H, Inoue M, Wallace SS. Decline of nuclear and mitochondrial oxidative base excision repair activity in late passage human diploid fibroblasts. DNA Repair. 2003;2(6):673–93. doi: 10.1016/s1568-7864(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 81.Parsons JL, Dianova, Dianov GL. APE1-dependent repair of DNA single-strand breaks containing 3 '-end 8-oxoguanine. Nucleic Acids Res. 2005;33(7):2204–9. doi: 10.1093/nar/gki518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilson DM, Kim D, Berquist BR, Sigurdson AJ. Variation in base excision repair capacity. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2011;711(1):100–12. doi: 10.1016/j.mrfmmm.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Georgiadis P, Polychronaki N, Kyrtopoulos SA. Progress in high-throughput assays of MGMT and APE1 activities in cell extracts. Mutat Res-Fundam Mol Mech Mutagen. 2012;736(1-2):25–32. doi: 10.1016/j.mrfmmm.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 84.Lu AL, Clark S, Modrich P. Methyl-directed repair of DNA base-pair mismatches in vitro. Proceedings of the National Academy of Sciences of the United States of America-Biological Sciences. 1983;80(15):4639–43. doi: 10.1073/pnas.80.15.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu RS, Hurstcalderone S, Kohn KW. Measurement of O-6-alkylguanine DNA alkyltransferase activity in human cells and tumor tissues by restriction endonuclease inhibition. Cancer Research. 1987;47(23):6229–35. [PubMed] [Google Scholar]
- 86.Mu D, Hsu DS, Sancar A. Reaction mechanism of human DNA repair excision nuclease. J Biol Chem. 1996;271(14):8285–94. doi: 10.1074/jbc.271.14.8285. [DOI] [PubMed] [Google Scholar]
- 87.Zhong Q, Boyer TG, Chen PL, Lee WH. Deficient nonhomologous end-joining activity in cell-free extracts from Brca1-null fibroblasts. Cancer Research. 2002;62(14):3966–70. [PubMed] [Google Scholar]
- 88.Guainazzi A, Scharer OD. Using synthetic DNA interstrand crosslinks to elucidate repair pathways and identify new therapeutic targets for cancer chemotherapy. Cell Mol Life Sci. 2010;67(21):3683–97. doi: 10.1007/s00018-010-0492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kucherlapati RS, Spencer J, Moore PD. Homologous recombination catalyzed by human cell extracts. Molecular and Cellular Biology. 1985;5(4):714–20. doi: 10.1128/mcb.5.4.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O'Donnell PNS, Barber PV, Margison GP, Povey AC. Association between O-6-alkylguanine-DNA- alkyltransferase activity in peripheral blood lymphocytes and bronchial epithelial cells. Cancer Epidemiology Biomarkers & Prevention. 1999;8(7):641–5. [PubMed] [Google Scholar]
- 91.Crosbie PAJ, Watson AJ, Agius R, Barber PV, Margison GP, Povey AC. Elevated N3-methylpurine-DNA glycosylase DNA repair activity is associated with lung cancer. Mutat Res-Fundam Mol Mech Mutagen. 2012;732(1-2):43–6. doi: 10.1016/j.mrfmmm.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 92.Calvo JA, Moroski-Erkul CA, Lake A, Eichinger LW, Shah D, Jhun I, Limsirichai P, Bronson RT, Christiani DC, Meira LB, Samson LD. Aag DNA Glycosylase Promotes Alkylation-Induced Tissue Damage Mediated by Parp1. Plos Genetics. 2013;9(4) doi: 10.1371/journal.pgen.1003413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Azqueta A, Collins AR. The essential comet assay: a comprehensive guide to measuring DNA damage and repair. Archives of Toxicology. 2013;87(6):949–68. doi: 10.1007/s00204-013-1070-0. [DOI] [PubMed] [Google Scholar]
- 94.McKenna DJ, McKeown SR, McKelvey-Martin VJ. Potential use of the comet assay in the clinical management of cancer. Mutagenesis. 2008;23(3):183–90. doi: 10.1093/mutage/gem054. [DOI] [PubMed] [Google Scholar]
- 95.Decordier I, Loock KV, Kirsch-Volders M. Phenotyping for DNA repair capacity. Mutation Research-Reviews in Mutation Research. 2010;705(2):107–29. doi: 10.1016/j.mrrev.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 96.Gaivão I, Piasek A, Brevik A, Shaposhnikov S, Collins AR. Comet assay-based methods for measuring DNA repair in vitro; estimates of inter-and intra-individual variation. Cell biology and toxicology. 2009;25(1):45–52. doi: 10.1007/s10565-007-9047-5. [DOI] [PubMed] [Google Scholar]
- 97.Kalthur G, Kumar P, Devi U, Ali S, Upadhya R, Pillai S, Rao A. Susceptibility of peripheral lymphocytes of brain tumour patients to in vitro radiation-induced DNA damage, a preliminary study. Clinical and Experimental Medicine. 2008;8(3):147–50. doi: 10.1007/s10238-008-0171-1. [DOI] [PubMed] [Google Scholar]
- 98.Jaloszynski P, Kujawski M, Czub-Swierczek M, Markowska J, Szyfter K. Bleomycin-induced DNA damage and its removal in lymphocytes of breast cancer patients studied by comet assay. Mutation Research-DNA Repair. 1997;385(3):223–33. doi: 10.1016/s0921-8777(97)00046-3. [DOI] [PubMed] [Google Scholar]
- 99.Rajaee-Behbahani N, Schmezer P, Risch A, Rittgen W, Kayser KW, Dienemann H, Schulz V, Drings P, Thiel S, Bartsch H. Altered DNA repair capacity and bleomycin sensitivity as risk markers for non-small cell lung cancer. Int J Cancer. 2001;95(2):86–91. doi: 10.1002/1097-0215(20010320)95:2<86::aid-ijc1015>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 100.El-Zein RA, Monroy CM, Cortes A, Spitz MR, Greisinger A, Etzel CJ. Rapid method for determination of DNA repair capacity in human peripheral blood lymphocytes amongst smokers. BMC Cancer. 2010;10 doi: 10.1186/1471-2407-10-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Palyvoda O, Polanska J, Wygoda A, Rzeszowska-Wolny J. DNA damage and repair in lymphocytes of normal individuals and cancer patients: Studies by the comet assay and micronucleus tests. Acta Biochim Pol. 2003;50(1):181–90. [PubMed] [Google Scholar]
- 102.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EROTC) International Journal of Radiation Oncology Biology Physics. 1995;31(5):1341–6. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 103.Alapetite C, Thirion P, de la Rochefordiere A, Cosset JM, Moustacchi E. Analysis by alkaline comet assay of cancer patients with severe reactions to radiotherapy: Defective rejoining of radioinduced dna strand breaks in lymphocytes of breast cancer patients. Int J Cancer. 1999;83(1):83–90. doi: 10.1002/(sici)1097-0215(19990924)83:1<83::aid-ijc16>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 104.Wood DK, Weingeist DM, Bhatia SN, Engelward BP. Single cell trapping and DNA damage analysis using microwell arrays. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(22):10008–13. doi: 10.1073/pnas.1004056107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Johnson JM, Latimer JJ. Analysis of DNA Repair Using Transfection-Based Host Cell Reactivation. In: Keohavong P, Grant GG, editors. Molecular Toxicology Protocols. Humana Press; Totowa, NJ: 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Athas WF, Hedayati MA, Matanoski GM, Farmer ER, Grossman L. Development and field-test validation of an assay for DNA-repair in circulating human lymphocytes. Cancer Research. 1991;51(21):5786–93. [PubMed] [Google Scholar]
- 107.Wei QY, Lee JE, Gershenwald JE, Ross MI, Mansfield PF, Strom SS, Wang LE, Guo ZZ, Qiao YW, Amos CI, Spitz MR, Duvic M. Repair of UV light-induced DNA damage and risk of cutaneous malignant melanoma. J Natl Cancer Inst. 2003;95(4):308–15. doi: 10.1093/jnci/95.4.308. [DOI] [PubMed] [Google Scholar]
- 108.Wang LE, Li CY, Strom SS, Goldberg LH, Brewster A, Guo ZZ, Qiao YW, Clayman GL, Lee JJ, El-Naggar AK, Prieto VG, Duvic M, Lippman SM, Weber RS, Kripke ML, Wei QY. Repair capacity for UV light-induced DNA damage associated with risk of nonmelanoma skin cancer and tumor progression. Clinical Cancer Research. 2007;13(21):6532–9. doi: 10.1158/1078-0432.CCR-07-0969. [DOI] [PubMed] [Google Scholar]
- 109.Wei QY, Gu J, Cheng L, Bondy ML, Jiang H, Hong WK, Spitz MR. Benzo(a)pyrene diol epoxide-induced chromosomal aberrations and risk of lung cancer. Cancer Research. 1996;56(17):3975–9. [PubMed] [Google Scholar]
- 110.Wei Q, Cheng L, Amos CI, Wang LE, Guo Z, Hong WK, Spitz MR. Repair of tobacco carcinogen-induced DNA adducts and lung cancer risk: a molecular epidemiologic study. J Natl Cancer Inst. 2000;92(21):1764–72. doi: 10.1093/jnci/92.21.1764. [DOI] [PubMed] [Google Scholar]
- 111.Spitz MR, Wei QY, Dong Q, Amos CI, Wu XF. Genetic susceptibility to lung cancer: The role of DNA damage and repair. Cancer Epidemiology Biomarkers & Prevention. 2003;12(8):689–98. [PubMed] [Google Scholar]
- 112.Wang L, Wei QY, Shi QL, Guo ZS, Qiao YW, Spitz MR. A modified host-cell reactivation assay to measure repair of alkylating DNA damage for assessing risk of lung adenocarcinoma. Carcinogenesis. 2007;28(7):1430–6. doi: 10.1093/carcin/bgm029. [DOI] [PubMed] [Google Scholar]
- 113.Shen HB, Spitz MR, Qiao YW, Guo ZZ, Wang LE, Bosken CH, Amos CI, Wei QY. Smoking, DNA repair capacity and risk of nonsmall cell lung cancer. Int J Cancer. 2003;107(1):84–8. doi: 10.1002/ijc.11346. [DOI] [PubMed] [Google Scholar]
- 114.Ramos JM, Ruiz A, Colen R, Lopez ID, Grossman L, Matta JL. DNA repair and breast carcinoma susceptibility in women. Cancer. 2004;100(7):1352–7. doi: 10.1002/cncr.20135. [DOI] [PubMed] [Google Scholar]
- 115.Shi QL, Wang LE, Bondy ML, Brewster A, Singletary SE, Wei QY. Reduced DNA repair of benzo a pyrene diol epoxide-induced adducts and common XPD polymorphisms in breast cancer patients. Carcinogenesis. 2004;25(9):1695–700. doi: 10.1093/carcin/bgh167. [DOI] [PubMed] [Google Scholar]
- 116.Matta J, Echenique M, Negron E, Morales L, Vargas W, Gaetan FS, Lizardi ER, Torres A, Rosado JO, Bolanos G, Cruz JG, Laboy J, Barnes R, Medina SS, Romero A, Martinez R, Dutil J, Suarez E, Alvarez-Garriga C, Bayona M. The association of DNA Repair with breast cancer risk in women. A comparative observational study. BMC Cancer. 2012;12 doi: 10.1186/1471-2407-12-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cheng L, Eicher SA, Guo ZZ, Hong WK, Spitz MR, Wei QY. Reduced DNA repair capacity in head and neck cancer patients. Cancer Epidemiology Biomarkers & Prevention. 1998;7(6):465–8. [PubMed] [Google Scholar]
- 118.Wang LE, Hu ZB, Sturgis EM, Spitz MR, Strom SS, Amos CI, Guo ZZ, Qiao YW, Gillenwater AM, Myers JN, Clayman GL, Weber RS, El-Naggar AK, Mao L, Lippman SM, Hong WK, Wei QY. Reduced DNA Repair Capacity for Removing Tobacco Carcinogen-Induced DNA Adducts Contributes to Risk of Head and Neck Cancer but not Tumor Characteristics. Clinical Cancer Research. 2010;16(2):764–74. doi: 10.1158/1078-0432.CCR-09-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lin J, Kadlubar FF, Spitz MR, Zhao H, Wu XF. A modified host cell reactivation assay to measure DNA repair capacity for removing 4-aminobiphenyl adducts: A pilot study of bladder cancer. Cancer Epidemiology Biomarkers & Prevention. 2005;14(7):1832–6. doi: 10.1158/1055-9965.EPI-04-0902. [DOI] [PubMed] [Google Scholar]
- 120.Tyson J, Caple F, Spiers A, Burtle B, Daly AK, Williams EA, Hesketh JE, Mathers JC. Inter-individual variation in nucleotide excision repair in young adults: effects of age, adiposity, micronutrient supplementation and genotype. British Journal of Nutrition. 2009;101(9):1316. doi: 10.1017/S0007114508076265. [DOI] [PubMed] [Google Scholar]
- 121.Mendez P, Taron M, Moran T, Fernandez MA, Requena G, Rosell R. A modified host-cell reactivation assay to quantify DNA repair capacity in cryopreserved peripheral lymphocytes. DNA Repair. 2011;10(6):603–10. doi: 10.1016/j.dnarep.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 122.Machella N, Terry MB, Zipprich J, Gurvich I, Liao YY, Senie RT, Kennedy DO, Santella RM. Double-strand breaks repair in lymphoblastoid cell lines from sisters discordant for breast cancer from the New York site of the BCFR. Carcinogenesis. 2008;29(7):1367–72. doi: 10.1093/carcin/bgn140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Slebos RJC, Taylor JA. A novel host cell reactivation assay to assess homologous recombination capacity in human cancer cell lines. Biochemical and Biophysical Research Communications. 2001;281(1):212–9. doi: 10.1006/bbrc.2001.4335. [DOI] [PubMed] [Google Scholar]
- 124.Kiziltepe T, Yan A, Dong M, Jonnalagadda VS, Dedon PC, Engelward BP. Delineation of the chemical pathways underlying nitric oxide-induced homologous recombination in mammalian cells. Chemistry & Biology. 2005;12(3):357–69. doi: 10.1016/j.chembiol.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 125.Lei XF, Zhu Y, Tomkinson A, Sun LZ. Measurement of DNA mismatch repair activity in live cells. Nucleic Acids Res. 2004;32(12) doi: 10.1093/nar/gnh098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Raetz AG, Xie Y, Kundu S, Brinkmeyer MK, Chang C, David SS. Cancer-associated variants and a common polymorphism of MUTYH exhibit reduced repair of oxidative DNA damage using a GFP-based assay in mammalian cells. Carcinogenesis. 2012 doi: 10.1093/carcin/bgs270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rahal EA, Henricksen LA, Li YL, Williams RS, Tainer JA, Dixon K. ATM regulates Mre11-dependent DNA end-degradation and microhomology-mediated end joining. Cell Cycle. 2010;9(14):2866–77. doi: 10.4161/cc.9.14.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sun Y, Moses R. Reactivation of psoralen-reacted plasmid DNA in Fanconi anemia, xeroderma pigmentosum, and normal human fibroblast cells. Somatic cell and molecular genetics. 1991;17(3):229–38. doi: 10.1007/BF01232819. [DOI] [PubMed] [Google Scholar]
- 129.Hlavin EM, Smeaton MB, Noronha AM, Wilds CJ, Miller PS. Cross-link structure affects replication-independent DNA interstrand cross-link repair in mammalian cells. Biochemistry. 2010;49(18):3977–88. doi: 10.1021/bi902169q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brooks PJ, Wise DS, Berry DA, Kosmoski JV, Smerdon MJ, Somers RL, Mackie H, Spoonde AY, Ackerman EJ, Coleman K, Tarone RE, Robbins JH. The oxidative DNA lesion 8,5 ′-(S)-cyclo-2 ′-deoxyadenosine is repaired by the nucleotide excision repair pathway and blocks gene expression in mammalian cells. J Biol Chem. 2000;275(29):22355–62. doi: 10.1074/jbc.M002259200. [DOI] [PubMed] [Google Scholar]
- 131.Spivak G, Hanawalt PC. Host cell reactivation of plasmids containing oxidative DNA lesions is defective in Cockayne syndrome but normal in UV-sensitive syndrome fibroblasts. DNA Repair. 2006;5(1):13–22. doi: 10.1016/j.dnarep.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 132.Nagel ZD, Thompson CM, Chaim IA, McRee SK, Mazzucato P, Ahmad A, Abo RP, Butty VL, Forget AL, Samson LD. Multiplexed DNA repair assays for multiple lesions and multiple doses via transcription inhibition and transcriptional mutagenesis. Proceedings of the National Academy of Sciences. 2014 doi: 10.1073/pnas.1401182111. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reeves R, Gorman CM, Howard B. Minichromosome assembly of non-integrated plasmid DNA transfected into mammalian cells. Nucleic Acids Res. 1985;13(10):3599–615. doi: 10.1093/nar/13.10.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jeong S, Stein A. DNA sequence affects nucleosome ordering on replicating plasmids in transfected COS-1 cells and in vitro. J Biol Chem. 1994;269(3):2197–205. [PubMed] [Google Scholar]
- 135.Jeong SW, Stein A. Micrococcal nuclease digestion of nuclei reveals extended nucleosome ladders having anomalous lengths for chromatin assembled on nonreplicating plasmids in transfected cells. Nucleic Acids Res. 1994;22(3):370–5. doi: 10.1093/nar/22.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mladenova V, Mladenov E, Russev G. Organization of plasmid DNA into nucleosome-like structures after transfection in eukaryotic cells. Biotechnology & Biotechnological Equipment. 2009;23(1):1044–7. [Google Scholar]
- 137.Khobta A, Anderhub S, Kitsera N, Epe B. Gene silencing induced by oxidative DNA base damage: association with local decrease of histone H4 acetylation in the promoter region. Nucleic Acids Res. 2010;38(13):4285–95. doi: 10.1093/nar/gkq170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fedier A, Fink D. Mutations in DNA mismatch repair genes: Implications for DNA damage signaling and drug sensitivity (review). Int J Oncol. 2004;24(4):1039–47. [PubMed] [Google Scholar]
- 139.Stojic L, Brun R, Jiricny J. Mismatch repair and DNA damage signalling. DNA Repair. 2004;3(8-9):1091–101. doi: 10.1016/j.dnarep.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 140.Kat A, Thilly WG, Fang WH, Longley MJ, Li GM, Modrich P. An alkylation-tolerant, mutator human cell line is deficient in strand-specific mismatch repair. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(14):6424–8. doi: 10.1073/pnas.90.14.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10(4):293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Leitner-Dagan Y, Sevilya Z, Pinchev M, Kramer R, Elinger D, Roisman LC, Rennert HS, Schechtman E, Freedman L, Rennert G, Livneh Z, Paz-Elizur T. N-Methylpurine DNA Glycosylase and OGG1 DNA Repair Activities: Opposite Associations With Lung Cancer Risk. J Natl Cancer Inst. 2012;104(22):1765–9. doi: 10.1093/jnci/djs445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sevilya Z, Leitner-Dagan Y, Pinchev M, Kremer R, Elinger D, Rennert HS, Schechtman E, Freedman LS, Rennert G, Paz-Elizur T. Low integrated DNA repair score and lung cancer risk. Cancer Prevention Research. 2013 doi: 10.1158/1940-6207.CAPR-13-0318. canprevres. 0318.2013. [DOI] [PubMed] [Google Scholar]
- 144.Posnick LM, Samson LD. Imbalanced base excision repair increases spontaneous mutation and alkylation sensitivity in Escherichia coli. Journal of Bacteriology. 1999;181(21):6763–71. doi: 10.1128/jb.181.21.6763-6771.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jungmichel S, Rosenthal F, Altmeyer M, Lukas J, Hottiger MO, Nielsen ML. Proteome-wide Identification of Poly(ADP-Ribosyl)ation Targets in Different Genotoxic Stress Responses. Molecular cell. 2013;52(2):272–85. doi: 10.1016/j.molcel.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 146.Sobol RW, Kartalou M, Almeida KH, Joyce DF, Engelward BP, Horton JK, Prasad R, Samson LD, Wilson SH. Base excision repair intermediates induce p53-independent cytotoxic and genotoxic responses. J Biol Chem. 2003;278(41):39951–9. doi: 10.1074/jbc.M306592200. [DOI] [PubMed] [Google Scholar]
- 147.Berger NA. Poly(ADP-ribose) polymerase in the cellular response to DNA damage. Radiat Res. 1985;101(1):4–15. [PubMed] [Google Scholar]
- 148.Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nature Reviews Molecular Cell Biology. 2006;7(7):517–28. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 149.Horton JK, Wilson SH. Predicting Enhanced Cell Killing through PARP Inhibition. Molecular Cancer Research. 2013;11(1):13–8. doi: 10.1158/1541-7786.MCR-12-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Strom CE, Johansson F, Uhlen M, Szigyarto CA, Erixon K, Helleday T. Poly (ADP-ribose) polymerase (PARP) is not involved in base excision repair but PARP inhibition traps a single-strand intermediate. Nucleic Acids Res. 2011;39(8):3166–75. doi: 10.1093/nar/gkq1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Murai J, Huang SN, Renaud A, Zhang YZ, Ji J, Takeda S, Morris J, Teicher B, Doroshow JH, Pommier Y. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Molecular Cancer Therapeutics. 2014;13(2):12. doi: 10.1158/1535-7163.MCT-13-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Heacock ML, Stefanick DF, Horton JK, Wilson SH. Alkylation DNA damage in combination with PARP inhibition results in formation of S-phase-dependent double-strand breaks. DNA Repair. 2010;9(8):929–36. doi: 10.1016/j.dnarep.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kedar PS, Stefanick DF, Horton JK, Wilson SH. Increased PARP-1 Association with DNA in Alkylation Damaged, PARP-Inhibited Mouse Fibroblasts. Molecular Cancer Research. 2012;10(3):360–8. doi: 10.1158/1541-7786.MCR-11-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tang JB, Svilar D, Trivedi RN, Wang XH, Goellner EM, Moore B, Hamilton RL, Banze LA, Brown AR, Sobol RW. N-methylpurine DNA glycosylase and DNA polymerase beta modulate BER inhibitor potentiation of glioma cells to temozolomide. Neuro-oncology. 2011;13(5):471–86. doi: 10.1093/neuonc/nor011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Horton JK, Watson M, Stefanick DF, Shaughnessy DT, Taylor JA, Wilson SH. XRCC1 and DNA polymerase beta in cellular protection against cytotoxic DNA single-strand breaks. Cell Res. 2008;18(1):48–63. doi: 10.1038/cr.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tobin LA, Robert C, Rapoport AP, Gojo I, Baer MR, Tomkinson AE, Rassool FV. Targeting abnormal DNA double-strand break repair in tyrosine kinase inhibitor-resistant chronic myeloid leukemias. Oncogene. 2013;32(14):1784–93. doi: 10.1038/onc.2012.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Caldecott KW. Single-strand break repair and genetic disease. Nature Reviews Genetics. 2008;9(8):619–31. doi: 10.1038/nrg2380. [DOI] [PubMed] [Google Scholar]
- 158.Gomez-Herreros F, Romero-Granados R, Zeng ZH, Alvarez-Quilon A, Quintero C, Ju LM, Umans L, Vermeire L, Huylebroeck D, Caldecott KW, Cortes-Ledesma F. TDP2-Dependent Non-Homologous End-Joining Protects against Topoisomerase II-Induced DNA Breaks and Genome Instability in Cells and In Vivo. Plos Genetics. 2013;9(3) doi: 10.1371/journal.pgen.1003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zeng ZH, Sharma A, Ju LM, Murai J, Umans L, Vermeire L, Pommier Y, Takeda S, Huylebroeck D, Caldecott KW, El-Khamisy SF. TDP2 promotes repair of topoisomerase I-mediated DNA damage in the absence of TDP1. Nucleic Acids Res. 2012;40(17):8371–80. doi: 10.1093/nar/gks622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.van Loon B, Hubscher U. An 8-oxo-guanine repair pathway coordinated by MUTYH glycosylase and DNA polymerase lambda. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(43):18201–6. doi: 10.1073/pnas.0907280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yang N, Galick H, Wallace SS. Attempted base excision repair of ionizing radiation damage in human lymphoblastoid cells produces lethal and mutagenic double strand breaks. DNA Repair. 2004;3(10):1323–34. doi: 10.1016/j.dnarep.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 162.Berger SH, Pittman DL, Wyatt MD. Uracil in DNA: consequences for carcinogenesis and chemotherapy. Biochemical Pharmacology. 2008;76(6):10. doi: 10.1016/j.bcp.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dou H, Mitra S, Hazra TK. Repair of oxidized bases in DNA bubble structures by human DNA glycosylases NEIL1 and NEIL2. J Biol Chem. 2003;278(50):49679–84. doi: 10.1074/jbc.M308658200. [DOI] [PubMed] [Google Scholar]
- 164.Hang B, Medina M, Fraenkel-Conrat H, Singer B. A 55-kDa protein isolated from human cells shows DNA glycosylase activity toward 3,N-4-ethenocytosine and the G/T mismatch. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(23):13561–6. doi: 10.1073/pnas.95.23.13561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ringvoll J, Moen MN, Nordstrand LM, Meira LB, Pang B, Bekkelund A, Dedon PC, Bjelland S, Samson LD, Falnes PO, Klungland A. AlkB homologue 2-mediated repair of ethenoadenine lesions in mammalian DNA. Cancer Research. 2008;68(11):4142–9. doi: 10.1158/0008-5472.CAN-08-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fu D, Samson LD. Direct repair of 3,N4-ethenocytosine by the human ALKBH2 dioxygenase is blocked by the AAG/MPG glycosylase. DNA repair. 2011 doi: 10.1016/j.dnarep.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Deans AJ, West SC. DNA interstrand crosslink repair and cancer. Nat Rev Cancer. 2011;11(7):467–80. doi: 10.1038/nrc3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rivera-Munoz P, Malivert L, Derdouch S, Azerrad C, Abramowski V, Revy P, Villartay J-Pd. DNA repair and the immune system: From V (D) J recombination to aging lymphocytes. European journal of immunology. 2007;37(S1):S71–S82. doi: 10.1002/eji.200737396. [DOI] [PubMed] [Google Scholar]
- 169.van der Burg M, Ijspeert H, Verkaik NS, Turul T, Wiegant WW, Morotomi-Yano K, Mari P-O, Tezcan I, Chen DJ, Zdzienicka MZ, van Dongen JJ, van Gent DC. A DNA-PKcs mutation in a radiosensitive T-B- SCID patient inhibits Artemis activation and nonhomologous end-joining. The Journal of clinical investigation. 2009;119(1):91. doi: 10.1172/JCI37141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Moshous D, Callebaut I, de Chasseval R, Corneo B, Cavazzana-Calvo M, Le Deist F, Tezcan I, Sanal O, Bertrand Y, Philippe N, Fischer A, de Villartay J-P. Artemis, a novel DNA double-strand break repair/V (D) J recombination protein, is mutated in human severe combined immune deficiency. Cell. 2001;105(2):177–86. doi: 10.1016/s0092-8674(01)00309-9. [DOI] [PubMed] [Google Scholar]
- 171.Moshous D, Pannetier C, de Chasseval Rg, le Deist Fo, Cavazzana-Calvo M, Romana S, Macintyre E, Canioni D, Brousse N, Fischer A, Casanova J-L, de Villartay J-P, Partial T. B lymphocyte immunodeficiency and predisposition to lymphoma in patients with hypomorphic mutations in Artemis. Journal of Clinical Investigation. 2003;111(3):381–7. doi: 10.1172/JCI16774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.O'Driscoll M, Cerosaletti KM, Girard P-M, Dai Y, Stumm M, Kysela B, Hirsch B, Gennery A, Palmer SE, Seidel J. DNA ligase IV mutations identified in patients exhibiting developmental delay and immunodeficiency. Molecular cell. 2001;8(6):1175–85. doi: 10.1016/s1097-2765(01)00408-7. [DOI] [PubMed] [Google Scholar]
- 173.Buck D, Malivert L, de Chasseval R, Barraud A, Fondaneche M-C, Sanal O, Plebani A, Stephan J-L, Hufnagel M, le Deist F. Cernunnos, a novel nonhomologous end-joining factor, is mutated in human immunodeficiency with microcephaly. Cell. 2006;124(2):287–99. doi: 10.1016/j.cell.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 174.Petersen S, Casellas R, Reina-San-Martin B, Chen HT, Difilippantonio MJ, Wilson PC, Hanitsch L, Celeste A, Muramatsu M, Pilch DR. AID is required to initiate Nbs1/ γ-H2AX focus formation and mutations at sites of class switching. Nature. 2001;414(6864):660–5. doi: 10.1038/414660a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Petersen-Mahrt SK, Harris RS, Neuberger MS. AID mutates E. coli suggesting a DNA deamination mechanism for antibody diversification. Nature. 2002;418(6893):99–104. doi: 10.1038/nature00862. [DOI] [PubMed] [Google Scholar]
- 176.Rada C, Di Noia JM, Neuberger MS. Mismatch recognition and uracil excision provide complementary paths to both Ig switching and the A/T-focused phase of somatic mutation. Molecular cell. 2004;16(2):163–71. doi: 10.1016/j.molcel.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 177.Imai K, Slupphaug G, Lee W-I, Revy P, Nonoyama S, Catalan N, Yel L, Forveille M, Kavli B, Krokan HE, Ochs HD, Fischer A, Durandy A. Human uracil-DNA glycosylase deficiency associated with profoundly impaired immunoglobulin class-switch recombination. Nature immunology. 2003;4(10):1023–8. doi: 10.1038/ni974. [DOI] [PubMed] [Google Scholar]
- 178.Péron S, Metin A, Gardes P, Alyanakian M-A, Sheridan E, Kratz CP, Fischer A, Durandy A. Human PMS2 deficiency is associated with impaired immunoglobulin class switch recombination. The Journal of experimental medicine. 2008;205(11):2465–72. doi: 10.1084/jem.20080789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.de Miranda N, Bjorkman A, Pan-Hammarstrom Q, Annals NYAS. DNA repair: the link between primary immunodeficiency and cancer. AnnNY AcadSci. 2011;1246:50–63. doi: 10.1111/j.1749-6632.2011.06322.x. [DOI] [PubMed] [Google Scholar]
- 180.de Miranda NF, Peng R, Georgiou K, Wu C, Sörqvist EF, Berglund M, Chen L, Gao Z, Lagerstedt K, Lisboa S. DNA repair genes are selectively mutated in diffuse large B cell lymphomas. The Journal of experimental medicine. 2013;210(9):1729–42. doi: 10.1084/jem.20122842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Grigera F, Bellacosa A, Kenter AL. Complex Relationship between Mismatch Repair Proteins and MBD4 during Immunoglobulin Class Switch Recombination. PloS one. 2013;8(10):e78370. doi: 10.1371/journal.pone.0078370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Alter BP. Diagnostic Evaluation of FA. In: Eiler ME, Frohnmayer D, Frohnmayer L, Larsen K, Owen J, editors. Fanconi Anemia: Guidelines for Diagnosis and Management. 3 ed Fanconi Anemia Research Fund, Inc.; 2008. [Google Scholar]
- 183.Bernstam VA. CRC Handbook of Gene Level Diagnostics in Clinical Practice. CRC Press; 1992. [Google Scholar]
- 184.Aebi S, KurdiHaidar B, Gordon R, Cenni B, Zheng H, Fink D, Christen RD, Boland CR, Koi M, Fishel R, Howell SB. Loss of DNA mismatch repair in acquired resistance to cisplatin. Cancer Research. 1996;56(13):3087–90. [PubMed] [Google Scholar]
- 185.Carethers JM, Hawn MT, Chauhan DP, Luce MC, Marra G, Koi M, Boland CR. Competency in mismatch repair prohibits clonal expansion of cancer cells treated with N-methyl-N′-nitro-N-nitrosoguanidine. Journal of Clinical Investigation. 1996;98(1):199–206. doi: 10.1172/JCI118767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wyatt MD, Wilson DM. Participation of DNA repair in the response to 5-fluorouracil. Cell Mol Life Sci. 2009;66(5):788–99. doi: 10.1007/s00018-008-8557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Olaussen KA, Dunant A, Fouret P, Brambilla E, Andre F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH, Stahel R, Sabatier L, Pignon J, Tursz T, Le Chevalier T, Soria JC, Investigators IB. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. New England Journal of Medicine. 2006;355(10):983–91. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 188.Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB, Herman JG. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. New England Journal of Medicine. 2000;343(19):1350–4. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 189.Sarkaria JN, Kitange GJ, James CD, Plummer R, Calvert H, Weller M, Wick W. Mechanisms of chemoresistance to alkylating agents in malignant glioma. Clinical Cancer Research. 2008;14(10):2900–8. doi: 10.1158/1078-0432.CCR-07-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Boland CR. Clinical uses of microsatellite instability testing in colorectal cancer: an ongoing challenge. Journal of clinical oncology. 2007;25(7):754–6. doi: 10.1200/JCO.2006.09.4607. [DOI] [PubMed] [Google Scholar]
- 191.Öhrling K, Edler D, Hallström M, Ragnhammar P. Mismatch repair protein expression is an independent prognostic factor in sporadic colorectal cancer. Acta Oncologica. 2010;49(6):797–804. doi: 10.3109/02841861003705786. [DOI] [PubMed] [Google Scholar]
- 192.Stupp R, Tonn JC, Brada M, Pentheroudakis G, Grp EGW. High-grade malignant glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2010;21:v190–v3. doi: 10.1093/annonc/mdq187. [DOI] [PubMed] [Google Scholar]
- 193.Fruh M, De Ruysscher D, Popat S, Crino L, Peters S, Felip E, Grp EGW. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2013;24:99–105. doi: 10.1093/annonc/mdt178. [DOI] [PubMed] [Google Scholar]
- 194.Horwich A, Parker C, de Reijke T, Kataja V, Grp EGW. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2013;24:106–14. doi: 10.1093/annonc/mdt208. [DOI] [PubMed] [Google Scholar]
- 195.Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandala M, Cervantes A, Arnold D, Grp EGW. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2013;24:64–72. doi: 10.1093/annonc/mdt354. [DOI] [PubMed] [Google Scholar]
- 196.Senkus E, Kyriakides S, Penault-Llorca F, Poortmans P, Thompson A, Zackrisson S, Cardoso F, Grp EGW. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2013;24:7–23. doi: 10.1093/annonc/mdt284. [DOI] [PubMed] [Google Scholar]
- 197.Vansteenkiste J, De Ruysscher D, Eberhardt WEE, Lim E, Senan S, Felip E, Peters S, Grp EGW. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2013;24:89–98. doi: 10.1093/annonc/mdt241. [DOI] [PubMed] [Google Scholar]
- 198.Bhatti P, Sigurdson AJ, Thomas CB, Iwan A, Alexander BH, Kampa D, Bowen L, Doody MM, Jones IM. No evidence for differences in DNA damage assessed before and after a cancer diagnosis. Cancer Epidemiology Biomarkers & Prevention. 2008;17(4):990–4. doi: 10.1158/1055-9965.EPI-07-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Research. 2000;60(1):184–90. [PubMed] [Google Scholar]
- 200.Tsoncheva VL, Todorova KA, Ivanov IG, Maximova VA. Influence of interferons on the repair of UV-damaged DNA. ZNaturforsch(C) 2008;63(3-4):303–7. doi: 10.1515/znc-2008-3-423. [DOI] [PubMed] [Google Scholar]
- 201.Chechlinska M, Kowalewska M, Nowak R. Systemic inflammation as a confounding factor in cancer biomarker discovery and validation. Nat Rev Cancer. 2010;10(1):2–U13. doi: 10.1038/nrc2782. [DOI] [PubMed] [Google Scholar]
- 202.Machida K, Tsukamoto H, Liu JC, Han YP, Govindarajan S, Lai MMC, Akira S, Ou JHJ. c-Jun Mediates Hepatitis C Virus Hepatocarcinogenesis Through Signal Transducer and Activator of Transcription 3 and Nitric Oxide-Dependent Impairment of Oxidative DNA Repair. Hepatology. 2010;52(2):480–92. doi: 10.1002/hep.23697. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 203.Yakovlev VA. Nitric Oxide-Dependent Downregulation of BRCA1 Expression Promotes Genetic Instability. Cancer Research. 2013;73(2):706–15. doi: 10.1158/0008-5472.CAN-12-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Soreide K. Molecular testing for microsatellite instability and DNA mismatch repair defects in hereditary and sporadic colorectal cancers - Ready for prime time? Tumor Biol. 2007;28(5):290–300. doi: 10.1159/000110427. [DOI] [PubMed] [Google Scholar]
- 205.Paz-Elizur T, Krupsky M, Blumenstein S, Elinger D, Schechtman E, Livneh Z. DNA repair activity for oxidative damage and risk of lung cancer. J Natl Cancer Inst. 2003;95(17):1312–9. doi: 10.1093/jnci/djg033. [DOI] [PubMed] [Google Scholar]
- 206.Myrnes B, Giercksky KE, Krokan H. Interindividual variation in the activity of O6-methyl guanine DNA methyltransferase and uracil DNA glycosylase in human organs. Carcinogenesis. 1983;4(12):1565–8. doi: 10.1093/carcin/4.12.1565. [DOI] [PubMed] [Google Scholar]
- 207.Gerson SL. Clinical relevance of MGMT in the treatment of cancer. Journal of Clinical Oncology. 2002;20(9):2388–99. doi: 10.1200/JCO.2002.06.110. [DOI] [PubMed] [Google Scholar]
- 208.Park IH, Zhao R, West JA, Yabuuchi A, Huo HG, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451(7175):141–U1. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 209.Chan SSL, Longley MJ, Copeland WC. The common A467T mutation in the human mitochondrial DNA polymerase (POLG) compromises catalytic efficiency and interaction with the accessory subunit. J Biol Chem. 2005;280(36):31341–6. doi: 10.1074/jbc.M506762200. [DOI] [PubMed] [Google Scholar]
- 210.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–95. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 211.Lauritzen KH, Moldestad O, Eide L, Carlsen H, Nesse G, Storm JF, Mansuy IM, Bergersen LH, Klungland A. Mitochondrial DNA Toxicity in Forebrain Neurons Causes Apoptosis, Neurodegeneration, and Impaired Behavior. Molecular and Cellular Biology. 2010;30(6):1357–67. doi: 10.1128/MCB.01149-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Liu PF, Demple B. DNA Repair in Mammalian Mitochondria: Much More Than We Thought? Environ Mol Mutagen. 2010;51(5):417–26. doi: 10.1002/em.20576. [DOI] [PubMed] [Google Scholar]
- 213.Mao PZ, Reddy PH. Aging and amyloid beta-induced oxidative DNA damage and mitochondrial dysfunction in Alzheimer's disease: Implications for early intervention and therapeutics. Biochimica Et Biophysica Acta-Molecular Basis of Disease. 2011;1812(11):1359–70. doi: 10.1016/j.bbadis.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Meyer JN, Bess AS. Involvement of autophagy and mitochondrial dynamics in determining the fate and effects of irreparable mitochondrial DNA damage. Autophagy. 2012;8(12):1822–3. doi: 10.4161/auto.21741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Furda AM, Marrangoni AM, Lokshin A, Van Houten B. Oxidants and not alkylating agents induce rapid mtDNA loss and mitochondrial dysfunction. DNA Repair. 2012;11(8):684–92. doi: 10.1016/j.dnarep.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Du L, Wang H, Xiong T, Ma Y, Yang J, Huang J, Zeng D, Wang X, Huang H, Huang J. The polymorphisms in the MGMT gene and the risk of cancer: a meta-analysis. Tumor Biol. 2013:1–11. doi: 10.1007/s13277-013-0893-x. [DOI] [PubMed] [Google Scholar]
- 217.Fishel R, Lescoe MK, Rao M, Copeland NG, Jenkins NA, Garber J, Kane M, Kolodner R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. cell. 1993;75(5):1027–38. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- 218.Leach FS, Nicolaides NC, Papadopoulos N, Liu B, Jen J, Parsons R, Peltomäki P, Sistonen P, Aaltonen LA, Nyström-Lahti M. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell. 1993;75(6):1215–25. doi: 10.1016/0092-8674(93)90330-s. [DOI] [PubMed] [Google Scholar]
- 219.Bronner C, Baker S, Morrison P, Warren G, Smith L, Lescoe M, Kane M, Earabino C, Lipford J, Lindblom A. Mutation in the DNA mismatch repair gene homologue hMLH1 is. Nature. 1994;368(6468):258–61. doi: 10.1038/368258a0. [DOI] [PubMed] [Google Scholar]
- 220.Papadopoulos N, Nicolaides NC, Wei Y-F, Ruben SM, Carter KC, Rosen CA, Haseltine WA, Fleischmann RD, Fraser CM, Adams MD. Mutation of a mutL homolog in hereditary colon cancer. Science. 1994;263(5153):1625–9. doi: 10.1126/science.8128251. [DOI] [PubMed] [Google Scholar]
- 221.Wu Y, Berends MJ, Mensink RG, Kempinga C, Sijmons RH, van der Zee AG, Hollema H, Kleibeuker JH, Buys CH, Hofstra RM. Association of Hereditary Nonpolyposis Colorectal Cancer-Related Tumors Displaying Low Microsatellite Instability with MSH6 Germline Mutations. The American Journal of Human Genetics. 1999;65(5):1291–8. doi: 10.1086/302612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ripperger T, Beger C, Rahner N, Sykora KW, Bockmeyer CL, Lehmann U, Kreipe HH, Schlegelberger B. Constitutional mismatch repair deficiency and childhood leukemia/lymphoma-report on a novel biallelic MSH6 mutation. Haematologica. 2010;95(5):841–4. doi: 10.3324/haematol.2009.015503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Couronné L, Ruminy P, Waultier-Rascalou A, Rainville V, Cornic M, Picquenot J-M, Figeac M, Bastard C, Tilly H, Jardin F. Mutation mismatch repair gene deletions in diffuse large B-cell lymphoma. Leukemia & lymphoma. 2013;54(5):1079–86. doi: 10.3109/10428194.2012.739687. [DOI] [PubMed] [Google Scholar]
- 224.Cleaver JE, Lam ET, Revet I. Disorders of nucleotide excision repair: the genetic and molecular basis of heterogeneity. Nature Reviews Genetics. 2009;10(11):756–68. doi: 10.1038/nrg2663. [DOI] [PubMed] [Google Scholar]
- 225.Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer. 2011;12(1):68–78. doi: 10.1038/nrc3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chrzanowska KH, Gregorek H, Dembowska-Bagińska B, Kalina MA, Digweed M. Nijmegen breakage syndrome (NBS). Orphanet J Rare Dis. 2012;7(1):13. doi: 10.1186/1750-1172-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Jones S, Emmerson P, Maynard J, Best JM, Jordan S, Williams GT, Sampson JR, Cheadle JP. Biallelic germline mutations in MYH predispose to multiple colorectal adenoma and somatic G: C→ T: A mutations. Human Molecular Genetics. 2002;11(23):2961–7. doi: 10.1093/hmg/11.23.2961. [DOI] [PubMed] [Google Scholar]
- 228.Baglioni S, Melean G, Gensini F, Santucci M, Scatizzi M, Papi L, Genuardi M. A kindred with MYH - associated polyposis and pilomatricomas. American Journal of Medical Genetics Part A. 2005;134(2):212–4. doi: 10.1002/ajmg.a.30585. [DOI] [PubMed] [Google Scholar]
- 229.Povey AC, Margison GP, Santibanez-Koref MF. Lung cancer risk and variation in MGMT activity and sequence. DNA Repair. 2007;6(8):1134–44. doi: 10.1016/j.dnarep.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 230.Saviozzi S, Ceppi P, Novello S, Ghio P, Iacono ML, Borasio P, Cambieri A, Volante M, Papotti M, Calogero RA. Non–Small Cell Lung Cancer Exhibits Transcript Overexpression of Genes Associated with Homologous Recombination and DNA Replication Pathways. Cancer research. 2009;69(8):3390–6. doi: 10.1158/0008-5472.CAN-08-2981. [DOI] [PubMed] [Google Scholar]
- 231.Johannessen T-CA, Prestegarden L, Grudic A, Hegi ME, Tysnes BB, Bjerkvig R. The DNA repair protein ALKBH2 mediates temozolomide resistance in human glioblastoma cells. Neuro-oncology. 2013;15(3):269–78. doi: 10.1093/neuonc/nos301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kane MF, Loda M, Gaida GM, Lipman J, Mishra R, Goldman H, Jessup JM, Kolodner R. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Research. 1997;57(5):808–11. [PubMed] [Google Scholar]
- 233.Mueller J, Gazzoli I, Bandipalliam P, Garber JE, Syngal S, Kolodner RD. Comprehensive molecular analysis of mismatch repair gene defects in suspected Lynch syndrome (hereditary nonpolyposis colorectal cancer) cases. Cancer Research. 2009;69(17):7053–61. doi: 10.1158/0008-5472.CAN-09-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Drost M, van Dijk L, Morreau H, Tops CM, Vasen HF, Wijnen JT, de Wind N. A cell-free assay for the functional analysis of variants of the mismatch repair protein MLH1. Human mutation. 2010;31(3):247–53. doi: 10.1002/humu.21180. [DOI] [PubMed] [Google Scholar]
- 235.Drost M, Zonneveld J, van Hees S, Rasmussen LJ, Hofstra RM, de Wind N. A rapid and cell-free assay to test the activity of lynch syndrome-associated MSH2 and MSH6 missense variants. Human mutation. 2012;33(3):488–94. doi: 10.1002/humu.22000. [DOI] [PubMed] [Google Scholar]
- 236.Cheng L, Sturgis EM, Eicher SA, Spitz MR, Wei Q. Expression of nucleotide excision repair genes and the risk for squamous cell carcinoma of the head and neck. Cancer. 2002;94(2):393–7. doi: 10.1002/cncr.10231. [DOI] [PubMed] [Google Scholar]
- 237.Vodicka P, Stetina R, Polakova V, Tulupova E, Naccarati A, Vodickova L, Kumar R, Hanova M, Pardini B, Slyskova J. Association of DNA repair polymorphisms with DNA repair functional outcomes in healthy human subjects. Carcinogenesis. 2006;28(3):657–64. doi: 10.1093/carcin/bgl187. [DOI] [PubMed] [Google Scholar]
- 238.Vogel U, Dybdahl M, Frentz G, Nexo BA. DNA repair capacity: inconsistency between effect of over-expression of five NER genes and the correlation to mRNA levels in primary lymphocytes. Mutation Research/DNA Repair. 2000;461(3):197–210. doi: 10.1016/s0921-8777(00)00051-3. [DOI] [PubMed] [Google Scholar]
- 239.Cobo M, Isla D, Massuti B, Montes A, Sanchez JM, Provencio M, Viñolas N, Paz-Ares L, Lopez-Vivanco G, Muñoz MA, Felip E, Alberola V, Camps C, Domine M, Sanchez JJ, Sanchez-Ronco M, Danenberg K, Taron M, Gandara D, Rosell R. Customizing cisplatin based on quantitative excision repair cross-complementing 1 mRNA expression: a phase III trial in non-small-cell lung cancer. Journal of Clinical Oncology. 2007;25(19):2747–54. doi: 10.1200/JCO.2006.09.7915. [DOI] [PubMed] [Google Scholar]
- 240.Wei Q, Wang L-E, Sturgis EM, Mao L. Expression of nucleotide excision repair proteins in lymphocytes as a marker of susceptibility to squamous cell carcinomas of the head and neck. Cancer Epidemiology Biomarkers & Prevention. 2005;14(8):1961–6. doi: 10.1158/1055-9965.EPI-05-0101. [DOI] [PubMed] [Google Scholar]
- 241.Allione A, Russo A, Ricceri F, Loock KV, Guarrera S, Voglino F, Kirsch-Volders M, Matullo G. Validation of the nucleotide excision repair comet assay on cryopreserved PBMCs to measure inter-individual variation in DNA repair capacity. Mutagenesis. 2013;28(1):65–70. doi: 10.1093/mutage/ges054. [DOI] [PubMed] [Google Scholar]
- 242.Qiao YW, Spitz MR, Guo ZZ, Hadeyati M, Grossman L, Kraemer KH, Wei QY. Rapid assessment of repair of ultraviolet DNA damage with a modified host-cell reactivation assay using a luciferase reporter gene and correlation with polymorphisms of DNA repair genes in normal human lymphocytes. Mutat Res-Fundam Mol Mech Mutagen. 2002;509(1-2):165–74. doi: 10.1016/s0027-5107(02)00219-1. [DOI] [PubMed] [Google Scholar]
- 243.Thoms KM, Baesecke J, Emmert B, Hermann J, Roedling T, Laspe P, Leibeling D, Truemper L, Emmert S. Functional DNA repair system analysis in haematopoietic progenitor cells using host cell reactivation. Scand J Clin Lab Invest. 2007;67(6):580–8. doi: 10.1080/00365510701230481. [DOI] [PubMed] [Google Scholar]
- 244.Marsit CJ, Liu M, Nelson HH, Posner M, Suzuki M, Kelsey KT. Inactivation of the Fanconi anemia/BRCA pathway in lung and oral cancers: implications for treatment and survival. Oncogene. 2003;23(4):1000–4. doi: 10.1038/sj.onc.1207256. [DOI] [PubMed] [Google Scholar]
- 245.Szaumkessel M, Richter J, Giefing M, Jarmuz M, Kiwerska K, Tönnies H, Grenman R, Heidemann S, Szyfter K, Siebert R. Pyrosequencing-based DNA methylation profiling of Fanconi anemia/BRCA pathway genes in laryngeal squamous cell carcinoma. Int J Oncol. 2011;39(2):505–14. doi: 10.3892/ijo.2011.1039. [DOI] [PubMed] [Google Scholar]
- 246.Starcevic D, Dalal S, Sweasy JB. Is there a link between DNA polymerase beta and cancer? Cell Cycle. 2004;3(8):996–9. [PubMed] [Google Scholar]
- 247.Pons B, Belmont A-S, Masson-Genteuil G, Chapuis V, Oddos T, Sauvaigo S. Age-associated modifications of Base Excision Repair activities in human skin fibroblast extracts. Mechanisms of ageing and development. 2010;131(11):661–5. doi: 10.1016/j.mad.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 248.Hsu C-F, Tseng H-C, Chiu C-F, Liang S-Y, Tsai C-W, Tsai M-H, Bau D-T. Association between DNA double strand break gene Ku80 polymorphisms and oral cancer susceptibility. Oral oncology. 2009;45(9):789–93. doi: 10.1016/j.oraloncology.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 249.Berwick M, Satagopan JM, Ben-Porat L, Carlson A, Mah K, Henry R, Diotti R, Milton K, Pujara K, Landers T. Genetic heterogeneity among Fanconi anemia heterozygotes and risk of cancer. Cancer research. 2007;67(19):9591–6. doi: 10.1158/0008-5472.CAN-07-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]