Abstract
Background
Appropriate pain management after total shoulder arthroplasty (TSA) facilitates rehabilitation and may improve clinical outcomes.
Questions/purposes
This prospective, observational study evaluated a multimodal analgesia clinical pathway for TSA.
Methods
Ten TSA patients received an interscalene nerve block (25 cm3 0.375% ropivacaine) with intraoperative general anesthesia. Postoperative analgesia included regularly scheduled non-opioid analgesics (meloxicam, acetaminophen, and pregabalin) and opioids on demand (oral oxycodone and intravenous patient-controlled hydromorphone). Patients were evaluated twice daily to assess pain, anterior deltoid strength, handgrip strength, and sensory function.
Results
The nerve block lasted an average of 18 h. Patients had minimal pain after surgery; 0 (median score on a 0–10 scale) in the Post-Anesthesia Care Unit (PACU) but increased on postoperative day (POD) 1 to 2.3 (0.0, 3.8; median (25%, 75%)) at rest and 3.8 (2.1, 6.1) with movement. Half of the patients activated the patient-controlled analgesia four or fewer times in the first 24 h after surgery. Operative anterior deltoid strength was 0 in the PACU but returned to 68% by POD 1. Operative hand strength was 0 (median) in the PACU, but the third quartile (75%) had normalized strength 49% of preoperative value.
Conclusions
Patients did well with this multimodal analgesic protocol. Pain scores were low, half of the patients used little or no intravenous opiate, and some patients had good handgrip strength. Future research can focus on increasing duration of analgesia from the nerve block, minimizing motor block, lowering pain scores, and avoiding intravenous opioids.
Electronic supplementary material
The online version of this article (doi:10.1007/s11420-014-9381-0) contains supplementary material, which is available to authorized users.
Keywords: postoperative pain, total shoulder arthroplasty, interscalene nerve block, multimodal analgesia, non-opioid analgesics, clinical pathway
Introduction
Total shoulder arthroplasty (TSA) can cause moderate to severe postoperative pain [22]. Appropriate pain management improves patient satisfaction, facilitates postoperative rehabilitation, and may improve clinically relevant outcomes. Interscalene nerve blocks provide excellent postoperative analgesia and improve recovery for outpatient shoulder surgery [7, 12]. Following TSA, patients often receive intravenous opioids and transition to oral opioid analgesics. Concomitant utilization of adjunctive analgesics can minimize side effects from opioid administration and improve the quality of analgesia [26]. Pre-emptive multimodal analgesia combined with peripheral nerve blockade for total hip and knee arthroplasty was associated with reductions in pain scores, opioid use, and length of hospital stay [8]. Such a pathway for TSA patients may provide similar benefits. A pathway based on continuous interscalene nerve block for TSA resulted in decreased postoperative pain, opioid requirements, opioid-related side effects [9], decreased time until readiness for discharge [10], and enhanced range of motion (ROM) [11]. While a multimodal analgesic pathway with emphasis on peripheral nerve blockade for TSA shows promise, the optimal technique remains to be defined.
A clinical pathway for TSA was generated based on intraoperative general anesthetic with a peripheral nerve block for postoperative analgesia [2, 4]. Ultrasound-guided nerve blockade for shoulder surgery has a high success rate (99.83%) and low complication rate (0.4% rate of temporary numbness and tingling, no serious complications were observed in 1,169 blocks (95% CI, 0–0.3%)) [14]. Ropivacaine was used because it is a long-lasting local anesthetic, has less toxicity than bupivacaine, and displays preferential sensory blockade at lower concentrations [4, 9, 10, 13, 18]. TSA is often performed in the sitting “beach-chair” position. Although general anesthesia and positive-pressure ventilation in the sitting position are associated with a high rate of cerebral oxygen desaturation events, compared with the lateral decubitus position (80.3 vs 0%, respectively) [17], cerebral oxygen desaturation is rare when general anesthesia and positive-pressure ventilation are avoided [27]. Requests for increased muscle relaxation can often be safely met by increasing the isoflurane concentration, which provides significant muscle relaxation [24] but allows for spontaneous ventilation via laryngeal mask airway (LMA). The addition of low-dose ketamine during surgery may reduce postoperative opioid use [15, 16], improve surgical outcomes [15], and decrease pain [16].
In addition to intraoperative administration of IV ketamine and ketorolac, oral analgesic adjuncts (meloxicam, pregabalin, and acetaminophen) were included in the multimodal postoperative analgesic regimen. Prior studies showed that nonsteroidal anti-inflammatory drugs reduce postoperative pain [6, 20], pregabalin reduces opioid use and nausea [28] and improves patient-reported analgesia [1, 21, 26], and addition of acetaminophen to morphine patient-controlled analgesia (PCA) reduces opioid intake [19]. Despite the analgesia provided by an interscalene nerve block, opioid supplementation is generally required after TSA [4]. Thus, along with the preoperative interscalene nerve block and adjunctive oral medications, postoperative opioids (IV hydromorphone PCA + oral oxycodone) were provided as needed.
A successful clinical pathway would facilitate rehabilitation, heighten overall patient satisfaction, and promote an expedited recovery process. This prospective cohort study describes an analgesic and anesthetic protocol for TSA, combining single-injection ultrasound-guided interscalene nerve blockade and multimodal analgesia. Time until discharge, extent of motor and sensory blockade, pain scores, and analgesic use are reported.
Patients and Methods
After Institutional Review Board approval, informed written consent was obtained in the holding (pre-surgical) area from ten patients scheduled for primary TSA, aged 18 to 80 years old, with enrollment from December 2011 to February 2012. Inclusion criteria included American Society of Anesthesiologists (ASA) status of I, II, or III, planned use of general anesthesia via LMA, planned peripheral nerve block, and judged patient ability to follow study protocol. Exclusion criteria included allergy or intolerance to one of the study medications, hepatic or renal insufficiency, and chronic opioid use (regular administration for longer than 3 months). There were 17 patients considered for the study, but 4 were not eligible (two were taking opioids chronically and two were ineligible as per the surgeon (one had a tendon tear, one had rotator cuff repair)) and 3 declined to enroll. Patients had an average age of 72 and an average Body Mass Index (BMI) of 31 (Table 1); 50% of patients were female.
Table 1.
Demographics
| n = 10 | |
|---|---|
| Age (years) | 72 ± 4 |
| Gender (male/female; %) | 50/50 |
| Body Mass Index (BMI) | 31 ± 4 |
| BMI (n (%)) | |
| Normal (<25) | 1 (10%) |
| Overweight (25≥ and <30) | 3 (30%) |
| Obese (30≥ and <40) | 6 (60%) |
| Race | |
| Caucasian | 10 |
| ASA 1/2/3 | 0/6/4 |
| Length of stay (hours) | 63 ± 18 |
| Duration of analgesia from nerve block (hours) | 18 ± 7 |
| Duration of PCA (hours) | 33 ± 10 |
Data reported in mean (±standard deviation), unless otherwise noted
Abbreviation: PCA patient-controlled analgesia
The following pathway was employed (Table 2). Prior to induction of general anesthesia, patients received intravenous midazolam sedation and an ultrasound-guided interscalene block using a single injection of 25cm3 0.375% ropivacaine. The nerve block needle (22 G Chiba (Hakko Co Ltd., Chikuma-shi, Nagano-ken, Japan) or Stimuplex (B. Braun, Meisungen AG, Meisungen, Germany)) was placed between the C5 and C6 nerve roots (Fig. 1). Propofol was used to induce general anesthesia. A LMA (The Laryngeal Mask Company, Limited., Le Rocher, Victoria, Mahe, Seycelles) was placed, and anesthesia was maintained with a propofol infusion and inhaled nitrous oxide and isoflurane. Patients received ketamine (50 mg), ketorolac (15–30 mg IV; 30 mg unless age > 70 or weight < 60 kg), famotidine (20 mg), and anti-emetic prophylaxis with IV dexamethasone (4 mg) and IV ondansetron (4 mg). No intraoperative opioids were given. Intra-arterial catheters (20 G catheter for A-Line, Smiths Medical International, LTD, Lancashire, UK) were used to measure blood pressure with the transducer situated at the external auditory meatus, to approximate the level of the Circle of Willis. Hypotension was managed with intravenous ephedrine boluses and/or epinephrine infusions to maintain a mean arterial pressure goal of 60 mmHg.
Table 2.
Clinical pathway protocol
| Operating room anesthesia procedure |
|---|
| 1. Single-injection ultrasound-guided interscalene block (25 cm3 0.375% ropivacaine between C5 and C6) |
| 2. General anesthesia via LMA |
| Propofol/Midazolam induction |
| Propofol/nitrous oxide/Isoflurane maintenance |
| Spontaneous ventilation |
| 3. Ketamine (50 mg) |
| 4. IV Dexamethasone (4 mg), Ondansetron 4 mg, and Famotidine (20 mg) |
| 5. Ketorolac, if no contraindication (15–30 mg IV; 30 mg unless age is >70 or weight is <60 kg) |
| Postoperative |
| 1. Pregabalin (75–100 mg PO q 8 h × 3 days; 100 mg unless age is >70 or weight is <60 kg) |
| 2. Meloxicam, if no contraindication (7.5–15 mg daily × 5 days; 15 mg unless age is >70 or weight is <60 kg) |
| 3. Acetaminophen (650 mg q 6 h × 3 days) |
| 4. Oxycodone (5–10 mg q 4 h PRN) |
| 5. IV Hydromorphone PCA |
Abbreviation: LMA laryngeal mask airway, IV Intravenous, PCA patient-controlled analgesia, PRN pro re nata (as necessary), PO per os
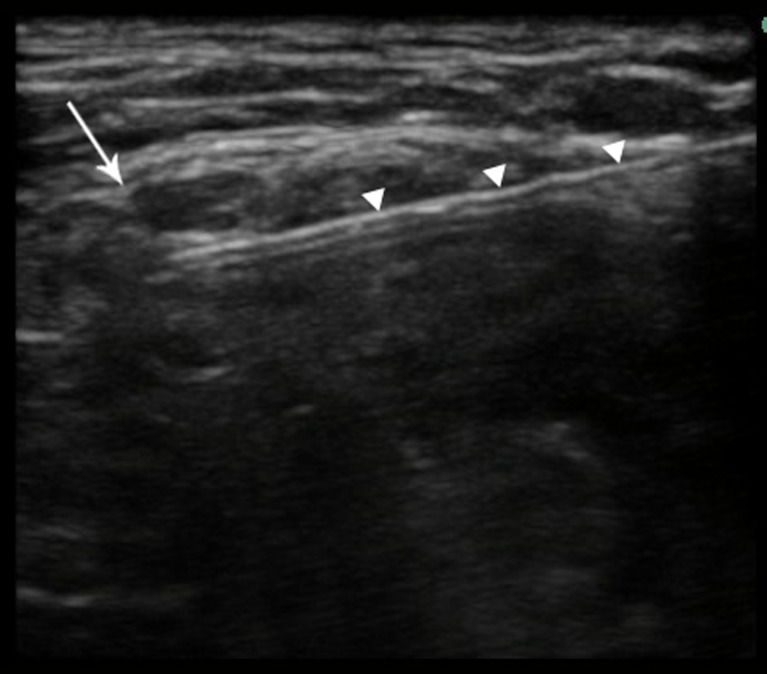
Fig. 1.
Ultrasonographic image of interscalene nerve block. The C5 nerve root (arrow) and block needle (arrowheads) are indicated.
Multimodal analgesia included the interscalene nerve block and intravenous PCA (IV hydromorphone; 0 basal rate / 0.2 mg / activation / 10 min lockout / maximum of 8 activations / h), first available to patients in the Post-Anesthesia Care Unit (PACU). Oral oxycodone (5–10 mg q 4 h pro re nata) was available on demand. In addition, three oral analgesic adjuncts were provided: meloxicam (7.5 to 15 mg daily for 5 days; 15 mg unless age is >70 or weight is <60 kg), pregabalin (75–100 mg po q 8 h × 3 days; 100 mg unless age is >70 or weight is <60 kg), and acetaminophen (650 mg q 6 h × 3 days). Meloxicam was not given to patients with renal insufficiency, peptic ulcer disease, or intolerance to NSAIDs. Patients were instructed that hydromorphone and oxycodone were available for moderate to severe pain but could cause nausea, sedation, or itching. They were also told that pain relief from oral oxycodone typically lasts longer than pain relief from intravenous hydromorphone.
Patients were seen preoperatively, postoperatively on the day of surgery, and twice daily after surgery on postoperative days (POD) 1 and 2. The following information was collected: age, sex, race, BMI, ASA status, and preoperative pain medication use. Patients were asked to rate their pain at rest and with activity (numeric rating scale, NRS, with 0 representing no pain and 10 representing the worst imaginable pain). Sensory function was assessed by pinprick in both the operative and nonoperative arms. Pinprick was measured using a paperclip, asking patients to report normal sensation (=2), loss of sensation to pinprick (=1), or loss of sensation to light touch (=0). Four dermatomes (C5, C6, C7, and C8) were evaluated. Handgrip strength and anterior deltoid strength were measured in both arms. Handgrip strength was assessed using the Baseline Smedley Digital Hand Dynamometer Model 12-0286 (Fabrication Enterprises, Inc., White Plains, NY, USA), whereas anterior deltoid strength was assessed using the Lafayette Manual Muscle Test System Model 01163 (Lafayette Instrument Company, Lafayette, IN, USA) in kilogram-force units. To assess anterior deltoid strength, patients were asked to make a vertical fist with arms bent at a 90° angle and parallel to the floor. With the patients’ fist in contact with the handheld device, patients were asked to press into the device using only their arm/shoulder without leaning forward with their bodies. All of the assessments described above were performed at each follow-up visit. Patient-reported duration of analgesia from interscalene block was also obtained.
Surgical Technique Relevant to Anesthetic and Analgesic Outcomes
Procedures were carried out with the patient in the beach-chair position at 35° to 40°. Proper soft tissue exposure, critically important for anatomic total shoulder replacement, is dependent upon proper surgical technique and appropriate anesthesia. Muscle relaxation is especially important in larger males and in patients with severe bony deformity. Relaxation of the pectoralis major and latissimus dorsi are critical for gaining proper exposure, particularly for the glenoid, which is a small surface, deep in the wound.
The deltopectoral approach was used. Once the deltopectoral interval was developed, the subdeltoid scar and bursa were excised. If external rotation was less than 25°, a subscapularis tendon lengthening was carried out by skeletonizing the tendon off the lesser tuberosity with or without an osteotomy of the lesser tuberosity. If the rotation was more than 25°, a simple tenotomy and release of the subscapularis muscle tendon unit was carried out to expose the humerus and dislocate the joint after the biceps tendon had been tenotomized. Occasionally if the pectoralis major was very tight, the superior portion was released.
Proper muscle relaxation is also important during placement of the humeral components, as motion and stability testing are hindered by muscle tension. After placement, the subscapularis tendon is closed either through a drill hole in the humeral neck, tendon to tendon or by transosseous tendon to tendon repair. The long head of the biceps was either let free or tenodesed.
In-hospital Postoperative Rehabilitation Protocol
Patients were maintained in a sling and swathe for comfort. Therapy began on POD 1 under the care of our physical therapists, directed by the findings at surgery. Depending upon the native pathology and strength of repair, patients started on a passive then active assistive ROM in elevation and abduction in line with the scapula soon after the procedure. External rotation was initially limited to 0° to 30°, to allow subscapularis healing.
Statistical Methods
Descriptive statistics were used (SPSS 14.0 for Windows (Chicago, IL)). Data are presented as mean (standard deviation) unless one or more of the results were skewed, in which case data are presented as median (lower and upper quartiles). Skew (non-normal distribution) often occurs if some of the data points are 0 and all others are positive. Normality of distribution was determined by using the Shapiro–Wilk test. A significance of >0.05 indicated the data were normally distributed; a significance of ≤0.05 indicated a non-normal distribution.
Results
Data were analyzed for all ten enrolled patients. Patients reported an average duration of analgesia from the nerve block of 18 h and had an average hospital length of stay of 63 h.
Strength could not be assessed for one patient in the PACU due to excessive sedation. One patient declined the strength assessments on the morning of POD 2 due to soreness in the shoulder muscles. The interscalene nerve block reduced operative side anterior deltoid strength to 0 in the PACU, but strength returned to 68% by POD 1, presumably as the effects of the nerve block dissipated (Table 3). On the nonoperative side, the anterior deltoid strength in the PACU was 86% of baseline, presumably reflecting sedating effects from the general anesthetic. Hand strength (operative side) was also reduced to a median of 0 in the PACU, but the third quartile (75%) had a normalized strength of 49% of preoperative value. For comparison, the median nonoperative handgrip strength in the PACU was 76% of baseline.
Table 3.
Strength measurement
| Number | Median (25%, 75%) | |
|---|---|---|
| Anterior deltoid strength operative | ||
| Preop (kgf) | 10 | 5.0 (3.6, 6.3) |
| PACU (normalized %) | 9 | 0% (0, 0) |
| POD1 (am; normalized %) | 10 | 68% (58, 85) |
| POD1 (pm; normalized %) | 10 | 63% (56, 76) |
| POD2 (am; normalized %) | 9 | 77% (52,119) |
| Anterior deltoid strength (nonoperative) | ||
| Preop (kgf) | 10 | 5.7 (4.8, 8.4) |
| PACU (normalized %) | 9 | 86% (77, 90) |
| POD1 (am; normalized %) | 10 | 102% (89, 117) |
| POD1 (pm; normalized %) | 10 | 108% (80, 155) |
| POD2 (am; normalized %) | 9 | 122% (76, 152) |
| Handgrip strength (operative) | ||
| Preop (kg) | 10 | 17.9 (13.1, 25.6) |
| PACU (normalized %) | 9 | 0% (0, 49) |
| POD1 (am; normalized %) | 10 | 87% (74, 95) |
| POD1 (pm; normalized %) | 10 | 81% (73, 99) |
| POD2 (am; normalized %) | 9 | 90% (76, 99) |
| Handgrip strength (nonoperative) | ||
| Preop (kg) | 10 | 20.9 (13.6, 23.2) |
| PACU (normalized %) | 9 | 76% (67, 87) |
| POD1 (am; normalized %) | 10 | 105% (91, 117) |
| POD1 (pm; nnrmalized %) | 10 | 108% (99, 123) |
| POD2 (am; normalized %) | 9 | 107% (94, 118) |
Abbreviation: PACU post-anesthesia care unit, POD postoperative day, kgf kilogram-force, kg kilogram
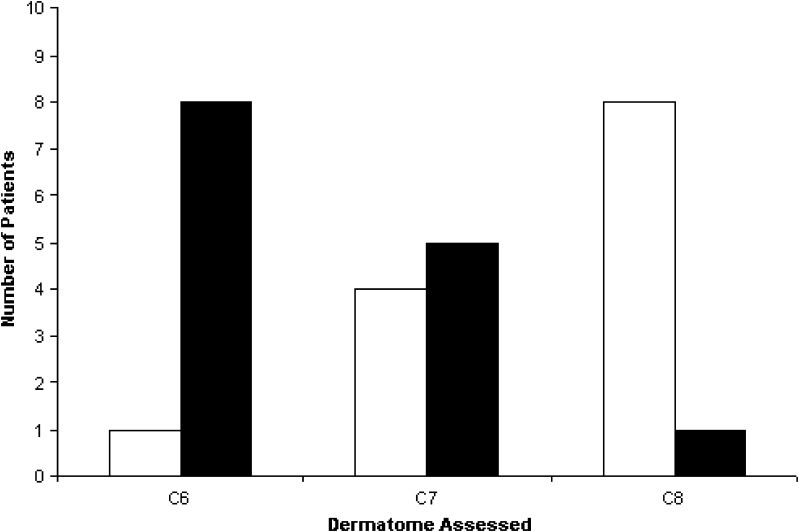
Dermatomes could not be assessed in the PACU for one patient due to sedation. A majority of patients had reduced sensation in their C6 and C7 dermatomes (Fig. 2). Dressings allowed assessment of the C5 dermatome in three patients, all of whom had decreased sensation. Only one patient had some numbness/tingling in her fingers in the morning on POD 1. All interscalene blocks were resolved by the evening visit on POD 1.
Fig. 2.
Pinprick assessment of sensory function on day of surgery in the PACU. The black bars indicate the number of patients with reduced sensation in a dermatome. The white bars indicate the number of patients with normal sensation in a dermatome.
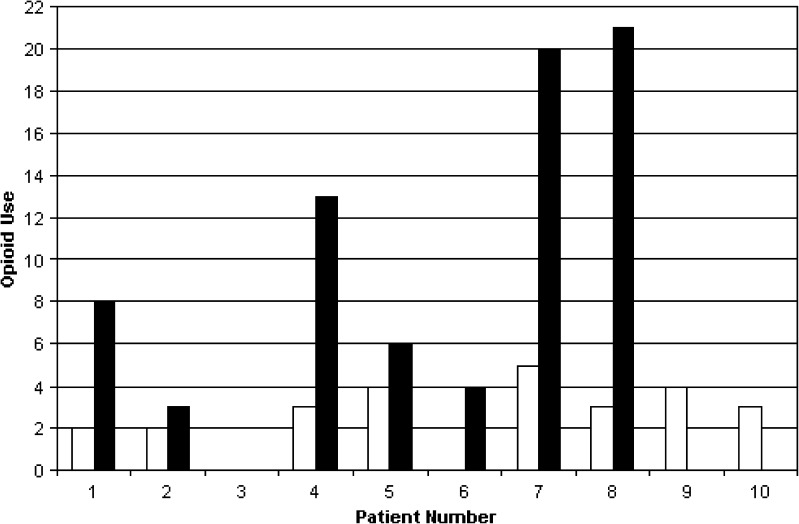
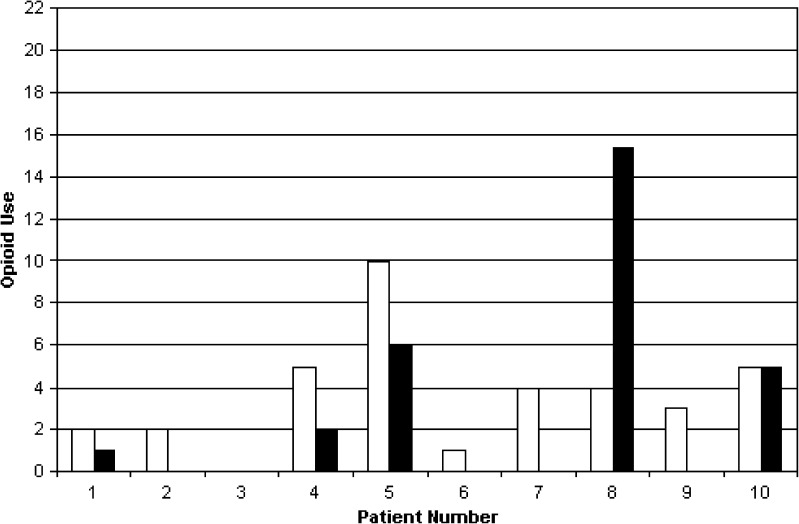
Patients had less pain after the operation than before, in the context of the multimodal analgesic protocol (Table 4). Pain scores (at rest and with movement) were a median of 0 in the PACU but increased on POD 1. Patients used highly variable amounts of oral and intravenous opioids (Figs. 3 and 4). In the first 24 h after the operation, three patients did not activate the PCA at all, but two activated the PCA 20 or more times.
Table 4.
Pain scores and opioid use
| Number | Median (25%, 75%) | Mean ± standard deviation | |
|---|---|---|---|
| Median NRS at rest (0–10) | |||
| Preop | 10 | 2.5 (1.1, 6.4) | |
| PACU | 9 | 0.0 (0.0, 0.0) | |
| POD1 (am) | 10 | 2.3 (0.0, 3.8) | |
| POD1 (pm) | 10 | 2.5 (1.3, 4.1) | |
| POD2 (am) | 10 | 0.8 (0, 1.5) | |
| Median NRS pain with movement (0–10) | |||
| Preop | 10 | 8.5 (8.0, 9.9) | |
| PACU | 4 | 0.0 (0.0, 0.6) | |
| POD1 (am) | 10 | 3.8 (2.1, 6.1) | |
| POD1 (pm) | 10 | 3.3 (2.1, 5.6) | |
| POD2 (am) | 10 | 2.5 (1.1, 4.6) | |
| Oral medication | |||
| Opioid intake (0–24 h; equivalent mg Morphine PO) | 10 | 22.8 ± 15.1 | |
| Opioid intake (24–48 h) | 10 | 29.5 ± 20.9 | |
| Opioid intake (total; 0–48 h) | 10 | 52.3 ± 32.7 | |
| PCA | |||
| Opioid intake (0–24 h; equivalent mg Morphine PO) | 10 | 39.0 ± 41.5 | |
| Opioid intake (24–48 h) | 10 | 15.4 ± 25.7 | |
| Opioid intake (total; 0–48 h) | 10 | 54.4 ± 58.6 | |
| PCA + PO combined | |||
| Opioid intake (0–24 h; equivalent mg Morphine PO) | 10 | 61.8 ± 49.3 | |
| Opioid intake (24–48 h) | 10 | 44.9 ± 38.7 | |
| Opioid intake (total; 0–48 h) | 10 | 106.7 ± 76.5 | |
Abbreviation: NRS numeric rating scale, PACU post-anesthesia care unit, POD postoperative day, PO per os, PCA patient-controlled analgesia
Fig. 3.
Opioid use, 0–24 h. The black bars indicate the number of successful activations of hydromorphone PCA. The white bars indicate the number of oral analgesic pills taken.
Fig. 4.
Opioid use, 24–48 h. The black bars indicate the number of successful activations of hydromorphone PCA. The white bars indicate the number of oral analgesic pills taken.
In the context of clinical trials, it is often instructive to divide patients into responders and nonresponders [23]. We propose a cut off of five activations in the first 24 h—patients who activate the PCA 4 or fewer times in 24 h (i.e., at most once every 6 h, on average) can be viewed as successfully responding to the multimodal analgesic regimen. Using this definition, half (5) of the patients were responders and would likely not have needed the IV hydromorphone PCA. Responders and nonresponders were similar on characteristics such as age (72 ± 5 and 71 ± 3, respectively), gender (60% and 40% female), BMI (31 ± 5 and 32 ± 4), and preoperative pain at rest (4.1 ± 3.6 and 3.0 ± 3.3), but the study is underpowered for detailed analysis.
Discussion
This prospective, observational study evaluated a clinical pathway for TSA patients that made extensive use of non-opioid analgesics (interscalene nerve blockade, intraoperative ketamine; postoperative meloxicam, pregabalin, and acetaminophen). Most patients still used oral oxycodone and intravenous hydromorphone. Information about time until discharge, extent of motor and sensory blockade, pain scores, and analgesic use was used to consider what an optimal perioperative regimen would be for TSA patients.
All nerve blocks were successful, and on average patients had less pain after the operation than they had prior to the operation. Extent of use of intravenous hydromorphone (via PCA) was highly variable—some patients used none in the first 24 h while others activated the PCA at least 20 times.
Limited information is available about other proposed pathways for TSA. Ambulatory perineural brachial plexus catheters have been found to promote readiness for discharge after TSA [10] and have been advocated for use as the primary analgesic for ambulatory TSA [5].
Prolonged postoperative motor blockade is not desirable after TSA, particularly in the hand, as some patients find this disconcerting. The interscalene nerve block (0.375% ropivacaine) caused motor blockade of the anterior deltoid which dissipated by the morning of POD 1, but it did not universally cause distal motor blockade. Although the median handgrip strength on the day of surgery was 0%, the 75th% was 49% of baseline. This indicates that some of the patients had partial preservation of distal (handgrip) strength. Other studies have not examined the effect of the interscalene block on anterior deltoid strength and handgrip strength. These additional data provide a foundation for the development of new studies directed at minimizing motor blockade.
There are a number of choices available for anesthesia and analgesia after TSA. A recent review of analgesia after shoulder surgery found the single-injection interscalene block and continuous interscalene block to be the best options but noted that the continuous interscalene block is a technically challenging procedure [3]. Alternative regional anesthesia techniques include the suprascapular nerve block and the supraclavicular block.
This was constructed as a pilot study. Future work could include efforts to prolong the duration of analgesia from the nerve blockade. This could reduce or eliminate intravenous opioids and thereby facilitate early hospital discharge. Two methods can be considered: continuous perineural infusion of local anesthetic via a nerve block catheter [11] or use of additives to the local anesthetic for the nerve block to prolong the duration of analgesia [25]. Alternatively, one could investigate use of additional non-opioid analgesic adjuncts. Additional research could use control groups and focus on issues such as: Does use of this clinical pathway reduce pain scores or increase patient satisfaction? Does the clinical pathway lead to improved rehabilitation or shorter length of stay? Does increased distal strength improve patient satisfaction? Which components of the pathway are needed? Is brachial plexus blockade needed in the context of multimodal analgesia?
This multimodal analgesic protocol provided excellent results for patients undergoing TSA. Pain scores were low, half of the patients used little or no intravenous opiate, and some of the patients had good handgrip strength. This work provides a basis for continued research aiming at increased duration of analgesia from the nerve block, lower pain scores, avoidance of intravenous opioids, and minimal impairment of handgrip strength.
Electronic Supplementary Material
(PDF 1225 kb)
(PDF 1224 kb)
(PDF 1225 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1225 kb)
(PDF 1224 kb)
Acknowledgments
Acknowledgment
This study was supported by the Department of Anesthesiology Research and Education Fund and approved by the Hospital for Special Surgery.
Disclosures
ᅟ
Conflict of Interest:
Amanda K. Goon, BA, Michael A. Gordon, MD, Enrique A. Goytizolo, MD, Yi Lin, MD, PhD, Emily Lin, MD, and Jacques T. YaDeau, MD have declared that they have no conflict of interest. David M. Dines, MD reports royalties from Biomet Inc., outside the work. Edward V. Craig, MD, MPH reports royalties from Biomet Inc., and patent with Biomet Inc., outside the work.
Human/Animal Rights:
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed Consent:
Informed consent was obtained from all patients for being included in the study.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Work was performed at the Hospital for Special Surgery, New York, NY.
Level of Evidence: Therapeutic study, level IV
References
- 1.Baidya DK, Agarwal A, Khanna P, Arora MK. Pregabalin in acute and chronic pain. J Anaesthesiol Clin Pharmacol. 2011;27:307–314. doi: 10.4103/0970-9185.83672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borgeat A, Ekatodramis G. Anaesthesia for shoulder surgery. Best Pract Res Clin Anaesthesiol. 2002;16:211–225. doi: 10.1053/bean.2002.0234. [DOI] [PubMed] [Google Scholar]
- 3.Fredrickson MJ, Krishnan S, Chen CY. Postoperative analgesia for shoulder surgery: a critical appraisal and review of current techniques. Anaesthesia. 2010;65:608–624. doi: 10.1111/j.1365-2044.2009.06231.x. [DOI] [PubMed] [Google Scholar]
- 4.Fredrickson MJ, Smith KR, Wong AC. Importance of volume and concentration for ropivacaine interscalene block in preventing recovery room pain and minimizing motor block after shoulder surgery. Anesthesiology. 2010;112:1374–1381. doi: 10.1097/ALN.0b013e3181d6929d. [DOI] [PubMed] [Google Scholar]
- 5.Gallay SH, Lobo JJ, Baker J, Smith K, Patel K. Development of a regional model of care for ambulatory total shoulder arthroplasty: a pilot study. Clin Orthop Relat Res. 2008;466:563–572. doi: 10.1007/s11999-007-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilron I, Orr E, Tu D, Mercer CD, Bond D. A randomized, double-blind, controlled trial of perioperative administration of gabapentin, meloxicam and their combination for spontaneous and movement-evoked pain after ambulatory laparoscopic cholecystectomy. Anesth Analg. 2009;108:623–630. doi: 10.1213/ane.0b013e318193cd1b. [DOI] [PubMed] [Google Scholar]
- 7.Hadzic A, Williams BA, Karaca PE, et al. For outpatient rotator cuff surgery, nerve block anesthesia provides superior same-day recovery over general anesthesia. Anesthesiology. 2005;102:1001–1007. doi: 10.1097/00000542-200505000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Hebl JR, Dilger JA, Byer DE, et al. A pre-emptive multimodal pathway featuring peripheral nerve block improves perioperative outcomes after major orthopedic surgery. Reg Anesth Pain Med. 2008;33:510–517. doi: 10.1097/00115550-200811000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Ilfeld BM, Morey TE, Wright TW, Chidgey LK, Enneking FK. Continuous interscalene brachial plexus block for postoperative pain control at home: a randomized, double-blinded, placebo-controlled study. Anesth Analg. 2003;96:1089–1095. doi: 10.1213/01.ANE.0000049824.51036.EF. [DOI] [PubMed] [Google Scholar]
- 10.Ilfeld BM, Vandenborne K, Duncan PW, et al. Ambulatory continuous interscalene nerve blocks decrease the time to discharge readiness after total shoulder arthroplasty: a randomized, triple-masked, placebo-controlled study. Anesthesiology. 2006;105:999–1007. doi: 10.1097/00000542-200611000-00022. [DOI] [PubMed] [Google Scholar]
- 11.Ilfeld BM, Wright TW, Enneking FK, Morey TE. Joint range of motion after total shoulder arthroplasty with and without a continuous interscalene nerve block: a retrospective, case–control study. Reg Anesth Pain Med. 2005;30:429–433. doi: 10.1016/j.rapm.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Kinnard P, Truchon R, St-Pierre A, Montreuil J. Interscalene block for pain relief after shoulder surgery. A prospective randomized study. Clin Orthop Relat Res. 1994;304:22–24. [PubMed] [Google Scholar]
- 13.Klein SM, Nielsen KC. Brachial plexus blocks: infusions and other mechanisms to provide prolonged analgesia. Curr Opin Anaesthesiol. 2003;16:393–399. doi: 10.1097/01.aco.0000084477.59960.92. [DOI] [PubMed] [Google Scholar]
- 14.Liu SS, Gordon MA, Shaw PM, Wilfred S, Shetty T, YaDeau JT. A prospective clinical registry of ultrasound-guided regional anesthesia for ambulatory shoulder surgery. Anesth Analg. 2010;111:617–623. doi: 10.1213/ANE.0b013e3181ea5f5d. [DOI] [PubMed] [Google Scholar]
- 15.Menigaux C, Fletcher D, Dupont X, Guignard B, Guirimand F, Chauvin M. The benefits of intraoperative small-dose ketamine on postoperative pain after anterior cruciate ligament repair. Anesth Analg. 2000;90:129–135. doi: 10.1097/00000539-200001000-00029. [DOI] [PubMed] [Google Scholar]
- 16.Mortero RF, Clark LD, Tolan MM, Metz RJ, Tsueda K, Sheppard RA. The effects of small-dose ketamine on propofol sedation: respiration, postoperative mood, perception, cognition, and pain. Anesth Analg. 2001;92:1465–1469. doi: 10.1097/00000539-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Murphy GS, Szokol JW, Marymont JH, et al. Cerebral oxygen desaturation events assessed by near-infrared spectroscopy during shoulder arthroscopy in the beach chair and lateral decubitus positions. Anesth Analg. 2010;111:496–505. doi: 10.1213/ANE.0b013e3181e33bd9. [DOI] [PubMed] [Google Scholar]
- 18.Owen MD, Dean LS. Ropivacaine. Expert Opin Pharmacother. 2000;1:325–336. doi: 10.1517/14656566.1.2.325. [DOI] [PubMed] [Google Scholar]
- 19.Remy C, Marret E, Bonnet F. Effects of acetaminophen on morphine side-effects and consumption after major surgery: meta-analysis of randomized controlled trials. Br J Anaesth. 2005;94:505–513. doi: 10.1093/bja/aei085. [DOI] [PubMed] [Google Scholar]
- 20.Thompson JP, Sharpe P, Kiani S, Owen-Smith O. Effect of meloxicam on postoperative pain after abdominal hysterectomy. Br J Anaesth. 2000;84:151–154. doi: 10.1093/oxfordjournals.bja.a013395. [DOI] [PubMed] [Google Scholar]
- 21.Tiippana EM, Hamunen K, Kontinen VK, Kalso E. Do surgical patients benefit from perioperative gabapentin/pregabalin? A systemic review of efficacy and safety. Anesth Analg. 2007;104:1545–1556. doi: 10.1213/01.ane.0000261517.27532.80. [DOI] [PubMed] [Google Scholar]
- 22.Tuominen M, Pitkanen M, Rosenberg PH. Postoperative pain relief and bupivacaine plasma levels during continuous interscalene brachial plexus block. Acta Anaesthesiol Scand. 1987;31:276–278. doi: 10.1111/j.1399-6576.1987.tb02565.x. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Department of Health and Human Services. Accessed 21 May 2012. Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims. December 2009. <http://www.ispor.org/workpaper/FDA%20PRO%20Guidance.pdf> [DOI] [PMC free article] [PubMed]
- 24.Vanini G, Watson CJ, Lydic R, Baghdoyan HA. Gamma-aminobutyric acid–mediated neurotransmission in the pontine reticular formation modulates hypnosis, immobility, and breathing during isoflurane anesthesia. Anesthesiology. 2008;109:978–988. doi: 10.1097/ALN.0b013e31818e3b1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vieira PA, Pulai I, Tsao GC, Manikantan P, Keller B, Connelly NR. Dexamethasone with bupivacaine increases duration of analgesia in ultrasound-guided interscalene brachial plexus blockade. Eur J Anaesthesiol. 2010;27:285–288. doi: 10.1097/EJA.0b013e3283350c38. [DOI] [PubMed] [Google Scholar]
- 26.White PF. The changing role of non-opioid analgesic techniques in the management of postoperative pain. Anesth Analg. 2005;101:S5–S22. doi: 10.1213/01.ANE.0000177099.28914.A7. [DOI] [PubMed] [Google Scholar]
- 27.YaDeau JT, Liu SS, Bang H, et al. Cerebral oximetry desaturation during shoulder surgery performed in a sitting position under regional anesthesia. Can J Anaesth. 2011;58:986–992. doi: 10.1007/s12630-011-9574-7. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Ho KY, Wang Y. Efficacy of pregabalin in acute postoperative pain: a meta-analysis. Br J Anaesth. 2011;106:454–462. doi: 10.1093/bja/aer027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1225 kb)
(PDF 1224 kb)
(PDF 1225 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1225 kb)
(PDF 1224 kb)