Abstract
Purpose
The aim of this study was to quantify the amount of bone morphogenic protein 7 (BMP-7) in bone samples in different storage and treatment conditions used in bone banks and thereby evaluate the benefit of this test as a routine measure before bone grafting.
Methods
Fresh as well as frozen bone chips, each with and without antibiotic impregnation, were screened for their BMP-7 content. Human bone chips were produced from femoral heads of two female donors who had undergone total hip replacement surgery. The amount of BMP-7 was detected using a commercially available enzyme-linked immunosorbent assay (ELISA) test.
Results
There were no significant differences between groups in samples obtained from the first femoral head. Bone-chip samples derived from the second femoral head showed significant differences between groups. The actual amount of these differences was small and most likely biologically irrelevant. It is important to note that there was a significant difference between groups when comparing both femoral heads, reflecting donor-to-donor variability.
Conclusion
ELISA testing for BMP-7 as a qualitative measurement of bone grafts should be considered a routine quality-control test for bone banks.
Keywords: Bone grafts, Antibiotics, BMP-7, Bone banking, Storage, Quality control
Introduction
Reconstruction of skeletal defects after trauma or disease [1–3], as well as repairing damage caused during surgery, has been a challenging problem in orthopaedics. The use of bone grafts quickly became a standard solution, since bone serves as a biologically active filling material and grows perfectly into spaces where a lack of material provides such a niche. Bone grafts have an osteoinductive (ability to induce new bone formation through factors favouring cell migration and differentiation) and osteoconductive (providing space and structure for bone formation) potential. Further, grafts can be used as carriers for antibiotics directly to the site of surgical intervention to prevent bacterial infections [4, 5]. Gentamicin sulphate has widely been used in bone cements and bone chips. We published a paper on the release rate and effectiveness of gentamicin palmitate as treatment for bone chips, adding a second phase of gentamicin release [6]. Both autografts (tissue from the same patient) and allografts (tissue from the same species) can be used for bone reconstruction. Although autografts bear the disadvantage of donor-site morbidity and low availability, allografts can lead to transmission of infectious diseases [1].
Several tests are routinely performed by bone banks to determine donor compatibility and reduce the risks of transmitting diseases. For example, the following tests are performed by the bone bank of Innsbruck, according to the Austrian Tissue Safety Law (GSG, German acronym), the Tissue Bank Act (GBVO, German acronym) and the Tissue Removal Department Act (GEEVO, German acronym): smear tests for fungi, aerobic and anaerobic germs, long-term incubation and histological examination. Serological tests include but are not limited to HIV [HIV 1/2 antibodies and polymerase chain reaction (PCR)], hepatitis A (PCR), hepatitis B (HBs antigen, HBc antibodies and HBV PCR], hepatitis C (HCV antibodies and HCV PCR), parvovirus B 19 (PCR), Lues-serology [enzyme-linked immunosorbent assay (ELISA)] and glutamate pyruvate transaminase (GPT, enzymatically). In addition, donors complete a specific anamnesis questionnaire [7–9]. If all requirements are fulfilled and there is no risk of infection, bone grafts obtained during surgery are stored at −80 °C for up to five years. Importantly, though, until now, no tests have been carried out by the bone bank to determine the osteogenic capacity of the graft material. One way to do so is to use bone morphogenetic protein 7 (BMP-7)—also known as osteogenic protein-1 (OP-1)—as a surrogate marker.
BMP-7, as with all BMPs, belongs to the transforming growth factor beta (TGF-β) superfamily, with >30 members containing three TGF-β isoforms, activins, inhibins and Müllerian-inhibiting substance [10, 11]. TGF-β signaling pathway is part of the formation and differentiation cycle of osteoblasts and osteoclasts [10], cells that play an important role in the bone remodeling process. BMPs are released during fracture-healing processes, during which they support ossification, and during remodeling of morselised and impacted bone grafts [12–14]. BMP-7 is able to initiate cartilage resorption and promotes primary bone formation in vivo [14, 15]. Bone grafts substituted with BMP-7 have a far superior ingrowth and remodeling rate than grafts without additional BMP-7 [12].
Whereas an experimental study showed the amount and activity of BMP-7 from human femoral bone grafts after treatment at various temperatures [15], to date there are no studies evaluating the amount of BMP-7 in bone grafts after they are coated with antibiotics. In this study, we quantified the amount of BMP-7 in human bone grafts in fresh and frozen samples each coated with or free of antibiotics in order to gain information on the potential osteogeneration of grafts treated with these standard means. We believe it is highly important to conduct a quality rating test to measure BMP content in bone grafts and controls regarding pathogenic contamination before bone grafting. This test could become a new standard by which to determine the bioactivity of bone grafts and could ultimately lead to improved quality of bone-reconstruction surgery.
Materials and methods
Preparation of bone chips
Femoral heads were obtained from two female donors who had undergone total hip replacement surgery at the Department of Orthopedics at the Innsbruck Medical University. Both donors approved the use of the samples for research purposes. During osteotomy, the bone was flushed with 0.9 % saline and cooled to prevent damage. Cartilage and cortical tissues were removed from the femoral heads using a bone saw. Bone chips (3- to 5-mm diameter) were obtained from the remaining spongious tissue using a bone mill (Noviumagus Bone Mill, Spierings Meische Techniek BV, Nijmegen, The Netherlands).
Sample arrangement and preparation
Bone chips were divided into six groups containing 1 g each (three samples per group) (Table 1):
Fresh bone chips (F)
Fresh bone chips frozen at −80 °C for four weeks (F80)
Fresh bone chips mixed with 50 mg gentamicin sulphate powder, equivalent to 30 mg gentamicin basis (F-GS)
Fresh bone chips mixed with 50 mg gentamicin sulphate powder, equivalent to 30 mg gentamicin basis + 140 mg gentamicin palmitate powder; equivalent to 30 mg gentamicin basis (F-GS + GP);
Fresh bone chips mixed with 50 mg gentamicin sulphate powder and stored at −80 °C for four weeks (F80-GS)
Fresh bone chips mixed with 50 mg gentamicin powder + 140 mg gentamicin palmitate powder and stored at −80 °C for four weeks (F80-GS + GP).
Table 1.
Treatment of bone chips in the experimental groups
| Group | Aquired fresh | Frozen at −80 °C | Added gentamicin sulfate | Added gentamicin palmitate |
|---|---|---|---|---|
| F | + | |||
| F-GS | + | + | ||
| F-GS + GP | + | + | + | |
| F80 | + | + | ||
| F80-GS | + | + | + | |
| F80-GS + GP | + | + | + | + |
See text for abbreviations
Antibiotics (Heraeus Medical GmbH, Wehrheim, Germany) were mechanically added to bone chips by mixing and vortexing for one minute; 2.5 ml phosphate-buffered saline (PBS) was added to all fresh and not previously frozen samples. After vortexing for one minute and centrifugation at 3,000 rpm for five minutes, three layers formed: a yellow fat layer on top, a light-red layer in the middle and a bone layer at the bottom. We then removed 1.2 ml from the mid layer, transferred it to Eppendorf tubes and stored it at −20 °C until ELISA was performed. All frozen samples were kept at −80 °C for four weeks, then removed from the freezer and thawed at room temperature. Then, 2.5 ml of PBS was added to each sample, and the same procedure was performed for fresh samples.
ELISA measurements
Human BMP-7 ELISA Kit (Sigma-Aldrich, Schnelldorf, Germany) was used to quantify the amount of BMP-7 from human femoral bone grafts according to the manufacturer’s instructions. All samples were thawed at room temperature and vortexed until uniform. We transferred 100 μl of each sample into the wells of the human BMP-7 antibody-coated ELISA plate. Each sample was run in duplicate. As suggested by the manufacturer, absorbance was measured at 450 nm using a microplate reader.
Statistical analysis
According to the standard curve, the concentration was calculated after the regression model using Excel 2010 (Microsoft Corporation, Redmont, WA, USA). Statistical analyses were executed using SPSS 20 (IBM, Armonk, NY, USA), followed by Kolmogorow–Smirnov test, Kruskall–Wallis test and Mann–Whitney U test. GraphPad Prism (Version 5) was used to generate the illustration graphs.
Results
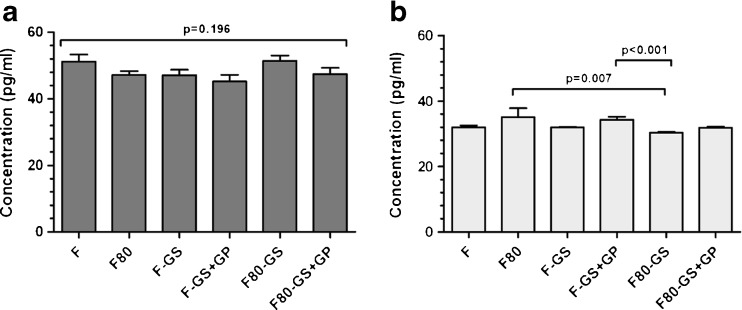
BMP-7 could be detected in all six groups. The average concentration in each group was calculated as the mean of both measured samples for both femoral heads. For femoral head one, groups showed the following concentrations: 51.20 pg/ml (F); 47.20 pg/ml (F80); 47.20 pg/ml (F-GS); 45.20 pg/ml (F-GS + GP); 51.50 pg/ml (F80-GS); 47.50 pg/ml (F80-GS + GP). There was no significant difference between groups (Fig. 1a).Femoral head two showed the following concentrations: 32.00 pg/ml (F); 35.10 pg/ml (F80); 32.00 pg/ml (F-GS); 34.30 pg/ml (F-GS + GP); 30.40 pg/ml (F80-GS); and 31.80 pg/ml (F80-GS + GP). Significant differences were detected between group sF80 and F80-GS and between groups F-GS + GP and F80-GS of the second femoral head (Fig. 1b). Significant differences were detected between the two heads in the amount of BMP-7 for each group (p < 0.001).
Fig. 1.
Concentration of bone morphogenetic protein 7 (BMP-7) from the first (a) and second (b) femoral head after different storage conditions: fresh bones (F; served as control group), fresh bones frozen at −80 °C for 4 weeks (F80), fresh bones mixed with gentamicin sulfate (F-GS), fresh bones mixed with gentamicin sulfate and gentamicin palmitate (F-GS + GP), fresh bones mixed with gentamicin sulfate stored at −80 °C for 4 weeks (F80-GS), fresh bones mixed with gentamicin sulfate and gentamicin palmitate stored at −80 °C for 4 weeks (F80-GS + GP)
Discussion
Several ex vivo tests are performed by bone banks to minimise the risk of transferring a contaminated allogenic bone graft and thus transmitting disease. Besides these preclinical tests, mixing bone tissue with antibiotic substances is an additional way to avoid recipient infection. So far, however, no studies describe the effects of antibiotic treatment on inducing capability of the transferred graft. Our study shows that the quantity of released BMP-7, an important marker of osteogenic potential in human bone grafts, does not generally change in the bone graft material during freezing or coating with antibiotic powder.
Previous studies show a slight BMP increase in in frozen and decrease in heat-treated bone grafts [15–17]. In the study reported here, an insignificant increase in BMP-7 was observed only between groups F (control) and F80-GS (frozen at −80 °C and treated with gentamicin) for the first femoral head and between groups F and F80 (frozen at −80 °C) of the second femoral head. The observed increase in BMP-7 could be explained by the formation of ice crystals during the freezing process, which can lead to concentrated buffer salts and proteins. Furthermore, an increased concentration of soluble protein can result in protein aggregation and subsequent self-stabilisation [18]. We found a slight (<4 pg/ml) but statistically significant decrease in the amount of BMP-7 between groups F-GS + GP and F80-GS but could not determine the effect of freezing and adding the palmitate form of gentamicin.
To prevent and treat bacterial infections and related infectious diseases, antibiotics can be administered systemically and locally on the surface of bone grafts [1, 3, 5]. It is highly important that the ingrowth and ossification process is not inhibited by the presence of such antibiotics. We found statistically significant differences in bone grafts from the second femoral head between groups F80 and F80-GS; however, actual difference in the amount of BMP-7 was minute (<5 pg/ml) and is most likely biologically irrelevant. The concentration of BMP-7 necessary to induce new bone formation in vivo is not yet known. In vitro tests on the differentiation of progenitor cells use human bone isolates on mouse cells (NIH3T3 [14] or C2C12 [4]). Co-culture of bone grafts with these cells directly did not lead to the up-regulation of alkaline phosphatase, a standard measure of differentiation of osteogenic lineage into cells [4].
The amount of BMP-7 seemed to be stable within the two femoral heads studied here, but there were significant differences between the two donors. In our opinion, this may be related to a number of factors, including but not limited to gender, age, osteoporosis and previous diseases. Previous studies show different BMP-7 levels from our results, which hint at great donor variability [14].
To date, bone banks do not carry out routine tests to classify the quality of human bone grafts. Measurements of BMP-7 by ELISA could help to arrange human bone grafts into different grades of quality. In this way, patients with severe health conditions could benefit from bone grafts with higher amounts of BMP-7, offering them better chances of healing [4]. ELISAs are usually fast and inexpensive and thus could be easily incorporated into the bone bank routine for quality control.
Conclusion
Our study results show that antibiotic coating as well as freezing (−80 °C) did not significantly reduce the amount of BMP-7 in bone graft material. In fact, in some samples, BMP-7 levels were slightly increased, which could be beneficial to the graft recipient. We here provide evidence that ELISA testing for BMP-7 as a measure to characterise the osteoinductive ability of bone grafts should be considered by bone banks as a routine quality-control test.
Acknowlegments
We thank Birgit Ladner from the Bone Bank and Department of Blood Transfusion and Immunology, Innsbruck Medical University, for their comments and improvement of this manuscript. We thank Prof. Klaus-Dieter Kühn from Heraeus Medical GmbH for sponsoring the antibiotics.
Conflicts of interest
This study was carried out with internal funds from Experimental Orthopedics, Innsbruck Medical University.
References
- 1.Coraca-Huber DC, Hausdorfer J, Fille M, Nogler M. Effect of storage temperature on gentamicin release from antibiotic-coated bone chips. Cell Tissue Bank. 2012 doi: 10.1007/s10561-012-9339-8. [DOI] [PubMed] [Google Scholar]
- 2.Hinsenkamp M, Muylle L, Eastlund T, Fehily D, Noel L, Strong DM. Adverse reactions and events related to musculoskeletal allografts: reviewed by the World Health Organisation Project NOTIFY. Int Orthop. 2012;36:633–641. doi: 10.1007/s00264-011-1391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis CS, Katz J, Baker MI, Supronowicz PR, Gill E, Cobb RR. Local antibiotic delivery with bovine cancellous chips. J Biomater Appl. 2011;26:491–506. doi: 10.1177/0885328210375729. [DOI] [PubMed] [Google Scholar]
- 4.Bormann N, Pruss A, Schmidmaier G, Wildemann B. In vitro testing of the osteoinductive potential of different bony allograft preparations. Arch Orthop Trauma Surg. 2010;130:143–149. doi: 10.1007/s00402-009-0908-7. [DOI] [PubMed] [Google Scholar]
- 5.Mathijssen NM, Hannink G, Pilot P, Schreurs BW, Bloem RM, Buma P. Impregnation of bone chips with alendronate and cefazolin, combined with demineralized bone matrix: a bone chamber study in goats. BMC Musculoskelet Disord. 2012;13:44. doi: 10.1186/1471-2474-13-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coraca-Huber DC, Putzer D, Fille M, Hausdorfer J, Nogler M, Kuhn KD. Gentamicin palmitate as a new antibiotic formulation for mixing with bone tissue and local release. Cell Tissue Banking. 2013 doi: 10.1007/s10561-013-9384-y. [DOI] [PubMed] [Google Scholar]
- 7.Bundesministerium fuer Gesundheit FuJ (2013) Verordnung der Bundesministerin fuer Gesundheit, Familie und Jugend, mit der naehere Regelungen fuer den Betrieb von Gewebebanken getroffen werden (Gewebebankenverordenung-GBVO). In: Austria B (ed) 32006L0017. Bundeskanzleramt Austria, Vienna, Austria. p. 8
- 8.Bundesministerium fuer Gesundheit FuJ (2013) Verordnung der Bundesministerin fuer Gesundheit, Familie und Jugend zur Festlegung von Standards fuer die Gewinnung von zur Verwendung beim Menschen bestimmter menschlicher Zellen und Geweben (Gewebeentnahmeeinrichtungsverordnung-GEEVO). In: Austria B (ed) 32006L0017. Bundeskanzleramt Austria, Vienna, Austria. p. 8
- 9.Bundesministerium fuer Gesundheit FuJ (2013) Bundesgesetz ueber die Festlegung von Qualitaets- und Sicherheitsstandards fuer die Gewinnung, Verarbeitung, Lagerung und Verteilung von menschlichen Zellen und Geweben zur Verwendung beim Menschen (Gewebesicherheitsgesetz-GSG). In: Austria B (ed) 32006L0086. Bundeskanzleramt Austria, Vienna, Austria. p. 18
- 10.Chen G, Deng C, Li YP. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8:272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar V, Abbas AK, Fausto N, Aster JC. Tissue Renewal, Regeneration, and Repair. In: Kumar V, Abbas AK, Fausto N, Aster JC, editors. Robbins and Cotran Pathologic Basis of Disease Saunders. Philadelphia, PA: Elsevier; 2010. p. 1450. [Google Scholar]
- 12.Belfrage O, Flivik G, Sundberg M, Kesteris U, Tagil M. Local treatment of cancellous bone grafts with BMP-7 and zoledronate increases both the bone formation rate and bone density: a bone chamber study in rats. Acta Orthop. 2011;82:228–233. doi: 10.3109/17453674.2011.566138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maurer T, Zimmermann G, Maurer S, Stegmaier S, Wagner C, Hansch GM. Inhibition of osteoclast generation: a novel function of the bone morphogenetic protein 7/osteogenic protein 1. Mediat Inflamm. 2012;2012:171209. doi: 10.1155/2012/171209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ai-Aql ZS, Alagl AS, Graves DT, Gerstenfeld LC, Einhorn TA. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J Dent Res. 2008;87:107–118. doi: 10.1177/154405910808700215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takata M, Sugimoto N, Yamamoto N, Shirai T, Hayashi K, Nishida H, Tanzawa Y, Kimura H, Miwa S, Takeuchi A, Tsuchiya H. Activity of bone morphogenetic protein-7 after treatment at various temperatures: freezing vs. pasteurization vs. allograft. Cryobiology. 2011;63:235–239. doi: 10.1016/j.cryobiol.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Ohta H, Wakitani S, Tensho K, Horiuchi H, Wakabayashi S, Saito N, Nakamura Y, Nozaki K, Imai Y, Takaoka K. The effects of heat on the biological activity of recombinant human bone morphogenetic protein-2. J Bone Miner Metab. 2005;23:420–425. doi: 10.1007/s00774-005-0623-6. [DOI] [PubMed] [Google Scholar]
- 17.Nakanishi K, Sato K, Sato T, Takahashi M, Fukaya N, Miura T. Preservation of bone morphogenetic protein in heat-treated bone. Nihon Seikeigeka Gakkai zasshi. 1992;66:949–955. [PubMed] [Google Scholar]
- 18.Pikal-Cleland KA, Rodrıguez-Hornedo N, Amidon GL, Carpenter JF. Protein Denaturation during Freezing and Thawing in Phosphate Buffer Systems: Monomeric and Tetrameric b-Galactosidase. Arch Biochem Biophys. 2000;384:398–406. doi: 10.1006/abbi.2000.2088. [DOI] [PubMed] [Google Scholar]