Abstract
Purpose
Cordycepin, a nucleoside derivative isolated from Cordyceps, has been reported to exert anti-inflammatory, antitumor, antidiabetic and renoprotective effects. Osteoarthritis (OA) is a degenerative joint disease with an inflammatory component that drives the degradation of cartilage extracellular matrix. This study aimed to assess the effects of cordycepin on human OA chondrocytes.
Methods
In this study, human OA chondrocytes were pretreated with cordycepin at 10, 50 or 100 μM and subsequently stimulated with interleukin-1β (IL-1β) (5 ng/ml) for 24 h. Production of prostaglandin E2 (PGE2) and nitric oxide (NO) were evaluated by the Griess reaction and an enzyme-linked immunosorbent assay (ELISA). Gene expression of matrix metalloproteinase (MMP)-13, IL-6, inducible nitric oxide synthase (iNOS) and cyclo-oxygenase (COX-2) was measured by real-time polymerase chain reaction (PCR). MMP-13 and IL-6 proteins in culture medium were determined using cytokine-specific ELISA. Western immunoblotting was used to analyse the iNOS and COX-2 protein production in culture medium. Nuclear factor kappa-B (NF-κB) activity regulation was explored using Western immunoblotting.
Results
Pretreatment with cordycepin significantly inhibited the production of PGE2 and NO induced by IL-1β. Cordycepin also significantly decreased the IL-1β-stimulated gene expression and production of MMP-13, IL-6, iNOS and COX-2 in OA chondrocytes. Pretreatment with cordycepin attenuated IL-1β-induced activation of NF-κB by suppressing degradation of its inhibitory protein nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκB-α) in the cytoplasm.
Conclusions
We show for the first time the anti-inflammatory activity of cordycepin in human OA chondrocytes. Thus, with this unique profile of actions, cordycepin may prove to be a potentially attractive and new therapeutic/preventive agent for OA.
Keywords: Cordycepin, Interleukin-1β (IL-1β), Nitric oxide, Osteoarthritis
Introduction
Osteoarthritis (OA) is a degenerative disease of the joints leading to progressive cartilage damage, osteophyte formation and subchondral bone sclerosis, producing pain and loss of function. Although its aetiology is unknown, hereditary, developmental and abnormal metabolic and mechanical processes are all considered to contribute to joint degeneration. In OA, cell matrix signaling is disrupted by depletion of collagen and proteoglycan due to the production of matrix metalloproteinases (MMPs), aggrecanases (ADAMTSs) and other proteases, resulting in matrix degradation and mechanical loss of tissue resilience [1]. Production of these substances is induced by inflammatory cytokines and mediators, such as interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-a), IL-6, nitric oxide (NO), and prostaglandins, which are found at elevated levels in OA joint tissues [2–4].
IL-1β appears to play a central role in OA pathogenesis. It is implicated in the degeneration of articular cartilage due to its induction of proteoglycan loss and matrix degradation [5]. Elevated levels of IL-1 occur in the synovial fluid and cartilage tissue of patients with OA [6]. IL-1 receptor antagonist, a natural competitor of IL-1, suppresses cartilage degradation, further supporting the role of IL-1 in cartilage breakdown [7]. Therefore, there is an interest in developing disease-modifying OA drugs (DMOADs) for treating OA, which may inhibit both IL-1β-mediated inflammation and extracellular matrix (ECM) degradation.
Cordyceps militaris is the rare Chinese caterpillar fungus [8] and has benefits to the human body, including the circulatory, immune, respiratory and glandular systems. Extract of Cordyceps exhibits antitumour effects on cancers and inhibitory effects on the production of inflammatory mediators [9–11]. Cordycepin, a nucleoside derivative isolated from Cordyceps, is reported to exert inhibitory effects on macrophages based on its anti-inflammatory property [12]. Cordycepin has also been studied for antitumor [13], antidiabetic [14], and renoprotective effects [15]. Noh et al [16] demonstrated that cordycepin has anti-rheumatic and anti-inflammatory effects on IL1β-stimulated rheumatoid arthritis synovial fibroblasts (RASFs). Shin et al [17] discovered that cordycepin could suppress expression of diabetes-regulating genes by inhibiting lipopolysaccharide-induced inflammation in macrophages.
The excellent therapeutic properties of cordycepin have been demonstrated in various cell types [15, 18–21]. However, the role of cordycepin in inflammatory responses in articular chondrocytes is still unclear. In this study, we investigated whether cordycepin exerted anti-inflammatory activities in human OA chondrocytes.
Materials and methods
Chondrocyte isolation and cultures
Tissue collection was in accordance with the terms of the Medical Ethical Committee of the Second Affiliated Hospital, Wenzhou Medical College, and followed the guidelines of the Declaration of Helsinki and Tokyo. OA cartilage tissues were obtained from three OA patients (aged 53–68 years, two men and one woman) who underwent total knee replacement at the Second Affiliated Hospital of Wenzhou Medical College. The patients met the American College of Rheumatology (ACR) classification criteria for OA diagnosis [22]. Full ethical consent was obtained from all patients. Cartilage was separated from underlying bone and connective tissues, and joint cartilage slices were isolated with hyaluronidase (Sigma) for five minutes, similar to previously published methods [23, 24]. Chondrocytes were plated at 1 × 105 cells/well in six well plate, serum starved overnight, pretreated with various concentrations of cordycepin (10, 50 or 100 μM) for two hours prior to IL-1β (5 ng/ml) for 24 hours. Conditioned media was collected for NO, prostaglandin E2 (PGE2), MMP-13, IL-6, cyclo-oxygenase (COX-2) and inducible nitric oxide synthase (iNOS) measurement. In another study, cells were pretreated with cordycepin (10, 50 or 100 μM) for two hours and then stimulated with IL-1β for one hour. Then, cells were harvested for analysis of nuclear factor kappa B (NF-κB) activation.
Cell viability assay
Effects of cordycepin on cell viability in OA chondrocytes was assessed using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H tetrazolium bromide (MTT). After incubation with various concentrations of cordycepin in a 96-well plate at a density of 6 × 103/well for 24 hours, cells were incubated with 20 μl of 0.5 % MTT for four hours. The supernatant was removed and 150 μl of dimethylsulfoxide (DMSO) was added. Absorbance at 570 nm was measured with a microplate reader (Bio-Rad, Hercules, CA, USA). The culture medium was used as a blank.
NO and PGE2 measurement
Nitrite levels in culture medium were assessed by Griess reaction, as previously described [25] . PGE2 levels were investigated using a commercially available enzyme-linked immunosorbent assay (ELISA) kit according to the manufacture’s protocol (R&D Systems, Minneapolis, MN, USA). All assays were performed in duplicate.
RNA isolation and real-time polymerase chain reaction
Real-time polymerase chain reaction (PCR) was used to detect the expression of MMP-13, IL-6, COX-2 and iNOS. Total RNA (tRNA) was extracted using TriZol (Invitrogen), according to the manufacturer’s instructions, and quantified. Its concentration was determined spectrophotometrically at 260 nm (HP 8452A Diode Array Spectrophotometer). First-strand complementary DNAs (cDNAs) were synthesized from 0.3 mg of the isolated RNA by oligo(deoxythymidine) [oligo(dT)] using DyNamoTM cDNA Synthesis Kit (Fermentas) and used as templates for real-time PCR. The expression of messenger RNAs (mRNAs) was determined quantitatively using DyNamo SYBR1 Green qPCR kit (Takara, Japan). The PCR was performed on a final volume of 25 μl containing 2 μl cDNA, 7.5 pmol of each primer, 1 μl 6-carboxyl-x-rhodamine (ROX) reference dye and 12.5 μl of SYBR Green Master Mix (Tiangen), with ABI Prism 7300 (Applied Biosystems, Foster City, CA, USA). The samples underwent 40 cycles consisting of the following steps: initial denaturation at 95 °C for five minutes, followed by a set cycle of denaturation at 94 °C for ten seconds different annealing temperatures for each pair of primers (ranging between 53 and 62 °C) for ten seconds, extension at 72 °C for 28 seconds and a final elongation at 72 °C for five minutes. Fold increment of any assayed gene was calculated by normalizing its expression level to that of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene, which was used as an internal control. Each gene analysis was performed in triplicate. Primer sequences of the targeted genes are listed in Table 1.
Table 1.
Primer sequences used in real-time polymerase chain reaction (PCR)
| Gene | Primer sequences (5’-3’) | |
|---|---|---|
| MMP-13 | Forward | CGC CAG AAG AAT CTG TCT TTA AA |
| Reverse | CCA AAT TAT GGA GGA GAT GC | |
| IL-6 | Forward | GAC AGC CAC TCA CC TCTT CA |
| Reverse | TTC ACC AGG CAA GTC TCC TC | |
| iNOS | Forward | CCT TAC GAG GCG AAG AAG GAC AG |
| Reverse | CAG TTT GAG AGA GGA GGC TCC G | |
| COX-2 | Forward | GAG AGA TGTATC CTC CCA CAG TCA |
| Reverse | GAC CAG GCA CCA GAC CAA AG | |
| GAPDH | Forward | TCT CCTCTG ACT TCA ACA GCG AC |
| Reverse | CCC TGT TGC TGT AGC CAA ATT C |
Measuring MMP-13 and IL-6 by ELISA
Human OA chondrocytes were pretreated with cordycepin (10, 50 or 100 μM) for two hours and then stimulated or not with IL-1β (5 ng/ml) for 24 hours. The effect of IL-1β and/or cordycepin on the level of MMP-13 and IL-6 secreted by chondrocytes in the culture medium was further confirmed by sandwich ELISA (R&D Systems), and all assays were performed according to the manufacturer’s instructions. Results were rounded to one decimal place.
Western blot analysis
Stimulated cells were washed twice with ice-cold phosphate-buffered saline (PBS), harvested by a scraper and cytoplasmic proteins were isolated using an extraction kit (Beyotime Institute of Biotechnology, Jiangsu, China). The protein was resolved using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes. Membranes were incubated with blocking buffer (5 % w/v skimmed milk powder in 1 × Tris-buffered saline (TBS) containing 0.1 % Tween-20 for one hour then incubated with rabbit polyclonal antibodies for iNOS, COX-2, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκB-α), mouse monoclonal antibody (mAb) phospho-IκB-α, mouse mAb phospho-p65, mouse mAb p65 and β-actin overnight at 4 °C (Santa Cruz Biotechnology, CA, USA). Membranes were washed and incubated for one hour at room temperature with horseradish-peroxidase (HRP)-linked secondary antibodies. After washing, membranes were detected by enhanced chemiluminescence (ECL) and exposed to X-ray films (Kodak, China).
Statistical analysis
All experiments were performed independently at least three times, and data, expressed as mean ± standard deviation (SD), from a representative experiment are shown. Results are expressed as mean ± SD. Statistical significance was assessed by one-way analysis of variance (ANOVA) followed by Dunnett’s test; values of P < 0.05 were considered significant.
Results
Potential cytotoxic effects of cordycepin
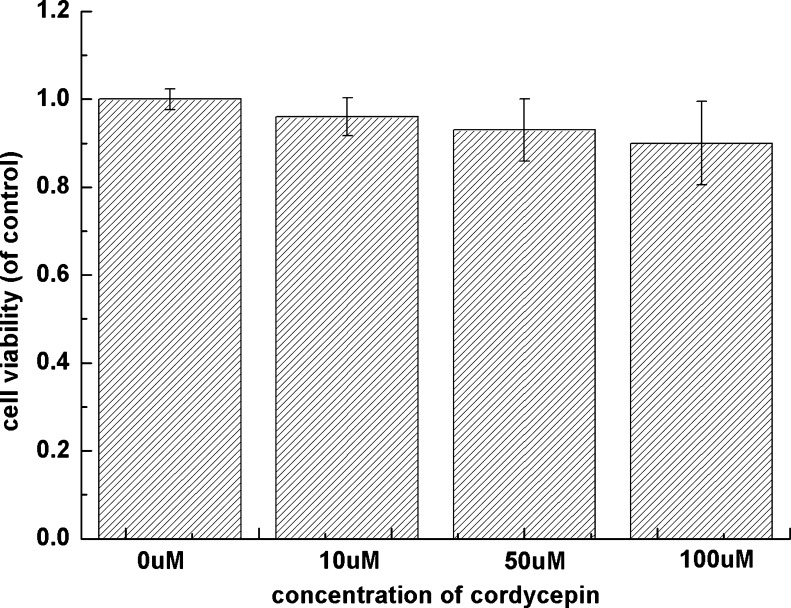
Cytotoxicity of cordycepin on human OA chondrocytes has not yet been reported. Therefore, we used MTT to assess the effect of cordycepin on cellular toxicity of human OA chondrocyte. As shown in Fig. 1, cell viability of human OA chondrocytes was not significantly impaired under the concentration of cordycepin (10, 50 or 100 μM) after cultured for 24 hours.
Fig. 1.
Effects of cordycepin on cell viability in osteoarthritis (OA) chondrocytes was assessed by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H tetrazolium bromide (MTT). Chondrocyte viability was not significantly impaired under 10-, 50- or 100-μM concentrations of cordycepin cultured for 24 h. Values are mean ± standard deviation (SD)
Effect of cordycepin on IL-1β-induced NO and PGE2 production
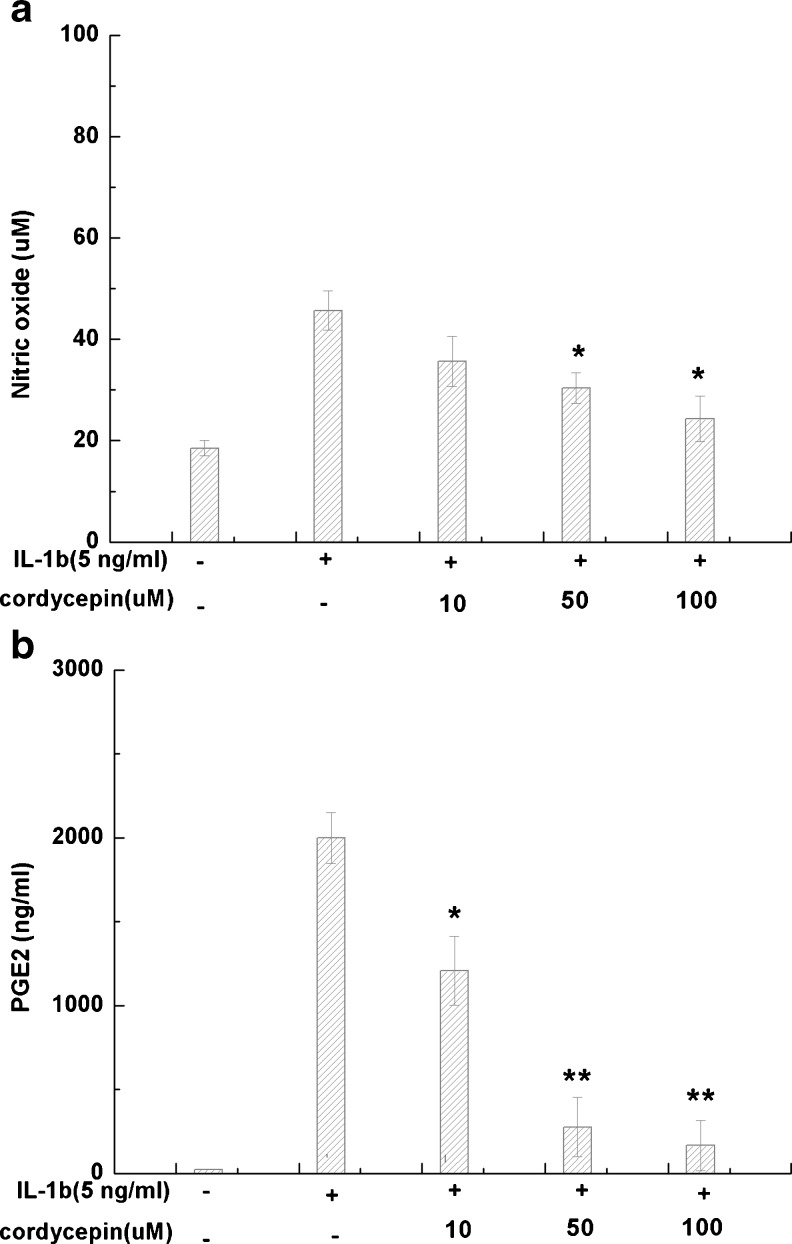
The effect of cordycepin on IL-1β-induced NO and PGE2 production is shown in Fig. 2a, b. Human OA chondrocytes were pretreated with cordycepin (0, 10, 50 or 100 μM) for two hours and stimulated or not with IL-1β (5 ng/ml) for 24 hours. Stimulation with IL-1β significantly increased the production of NO (p < 0.05) and PGE2 (p < 0.05) compared to unstimulated control. Pretreatment with cordycepin 50 or 100 μM partly blocked upregulation of NO and PGE2 induced by IL-1β (P < 0.05).
Fig. 2.
Effects of cordycepin on the production of nitric oxide (NO) and prostaglandin E2 (PGE2). Human osteoarthritis (OA) chondrocytes were pretreated with cordycepin at 10, 50 or 100 μM for 2 h, then stimulated or not with interleukin-1β (IL-1β) (5 ng/ml) for 24 h. a Nitrite levels in culture medium were assessed by Griess reaction. b PGE2 levels were assessed using the enzyme-linked immunosorbent assay (ELISA) kit. Values are mean ± standard deviation. *P < 0.05, **P < 0.01 compared with IL-1β group
Effect of cordycepin on IL-1β-induced iNOS and COX-2 mRNA and protein expression in OA chondrocytes
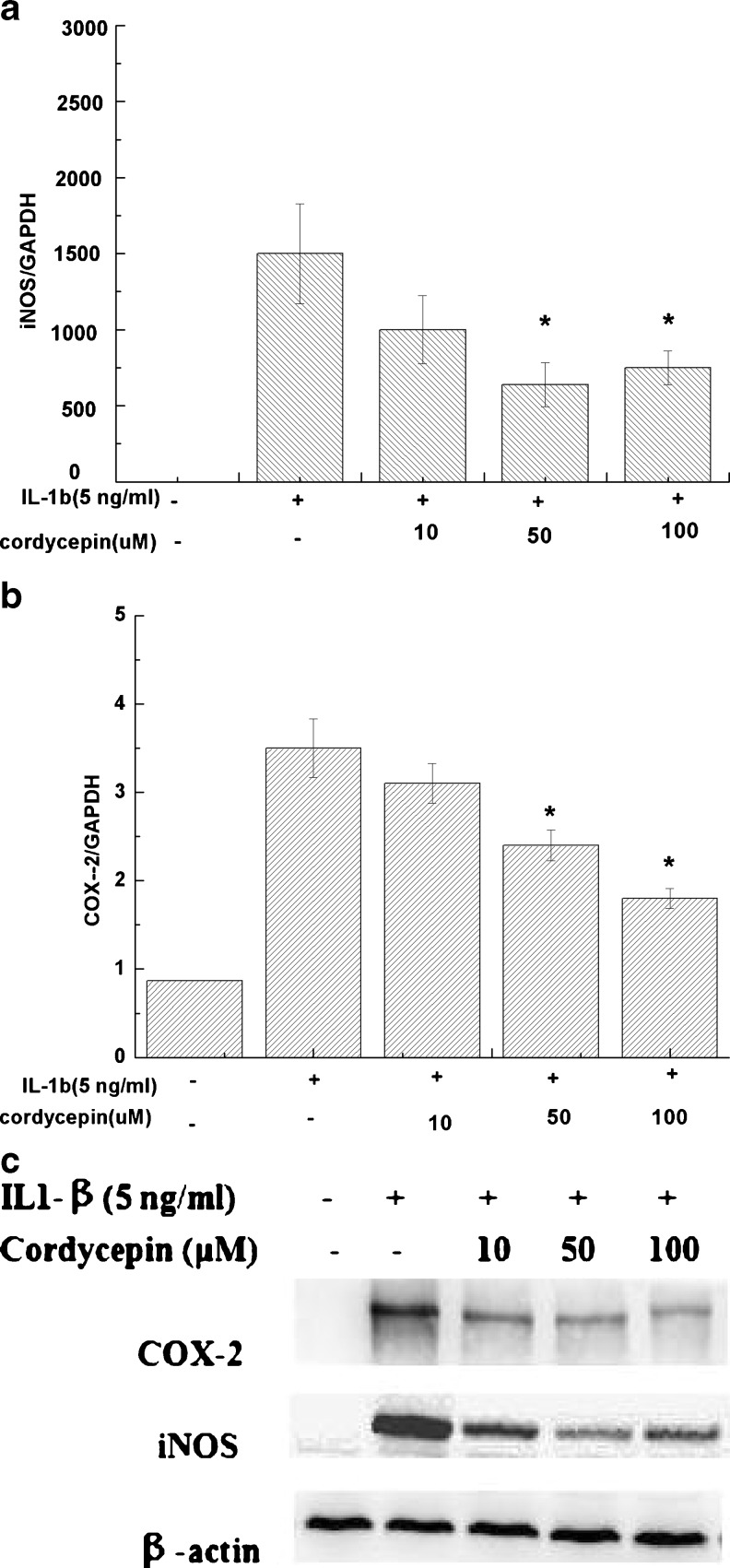
The mRNA expression of iNOS (Fig. 3a) and COX-2 (Fig. 3b) were quantitatively determined by real-time PCR. We found cordycepin exhibited a broad spectrum of inhibitory effects on the expression of proinflammatory mediators. Cordycepin (50 or 100 μM) significantly reduced gene expression of iNOS and COX-2 induced by IL-1β (P < 0.05). Data concerning iNOS and COX-2 expression were confirmed at the protein level. As shown in Fig. 3c, cordycepin inhibited upregulation of iNOS and COX-2 protein induced by IL-1β in OA chondrocytes by Western blot analysis.
Fig. 3.
Effect of cordycepin on interleukin-1β (IL-1β)-induced expression of inducible nitric oxide synthase (iNOS) and cyclo-oxygenase (COX-2) in osteoarthritis (OA) chondrocytes pretreated with cordycepin at 10, 50 or 100 μM for 2 h and stimulated or not with IL-1β (5 ng/ml, 24 h) for a iNOS and b COX-2 mRNA. The expression of mRNA was analysed with real-time polymerase chain reaction (PCR). c Protein levels of iNOS and COX-2 were assayed using Western blot analysis. Values are mean ± standard deviation. *P < 0.05 compared with IL1-β group
Effect of cordycepin on IL-1β induced MMP-13 and IL-6 mRNA and protein expression in OA chondrocytes
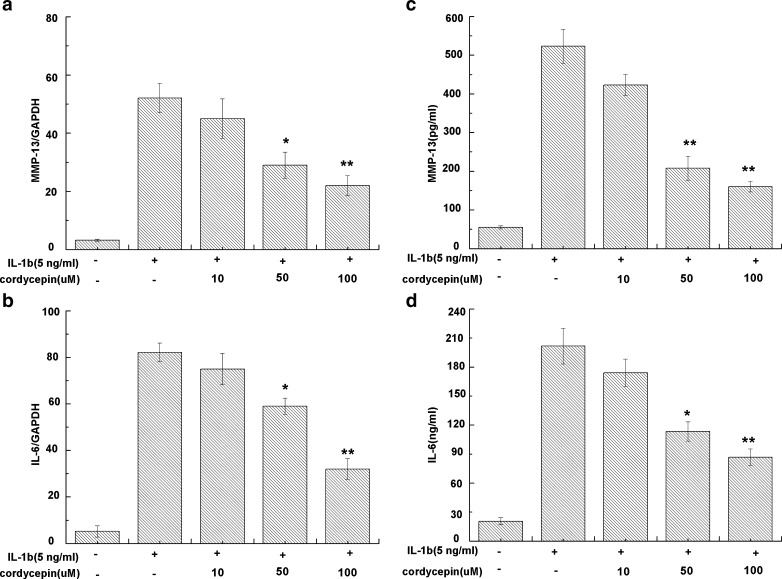
OA chondrocytes, stimulated with IL-1β, showed a significant expression of MMP-13 and IL-6 mRNA in comparison with unstimulated cells. Pretreatment with cordycepin (10, 50 or 100 μM) downregulated MMP-13 (Fig. 4a) and IL-6 (Fig. 4b) mRNA expression in IL-1β-stimulated OA chondrocytes during 24 h (P < 0.05). We then used ELISA to assay the effect of cordycepin on IL-1β-induced protein production of MMP-13 (Fig. 4c) and IL-6 (Fig. 4d). Protein expression of MMP-13 and IL-6 was up-regulated significantly in IL-1β-stimulated OA chondrocytes (P < 0.05). However, MMP-13 and IL-6 levels were significantly reduced after pretreatment with cordycepin.
Fig. 4.
Effect of cordycepin on interleukin-1β (IL-1β)-induced expression of matrix mellaproteinase-13 (MMP-13) and IL-6 in human osteoarthritis (OA) chondrocytes pretreated with cordycepin at 10, 50 or 100 μM for 2 h and then stimulated or not with IL-1β (5 ng/ml, 24 h) for a MMP-13 messenger RNA (mRNA) and b IL-6 mRNA. The mRNA expression was analyzed by real-time polymerase chain reaction (PCR). Levels of c MMP-13 and d IL-6 in culture supernatants were quantified by sandwich enzyme-linked immunosorbent assay (ELISA) for at 24 h. Values are mean ± standard deviation. *P < 0.05, **P <0.01 compared with the IL-1β group
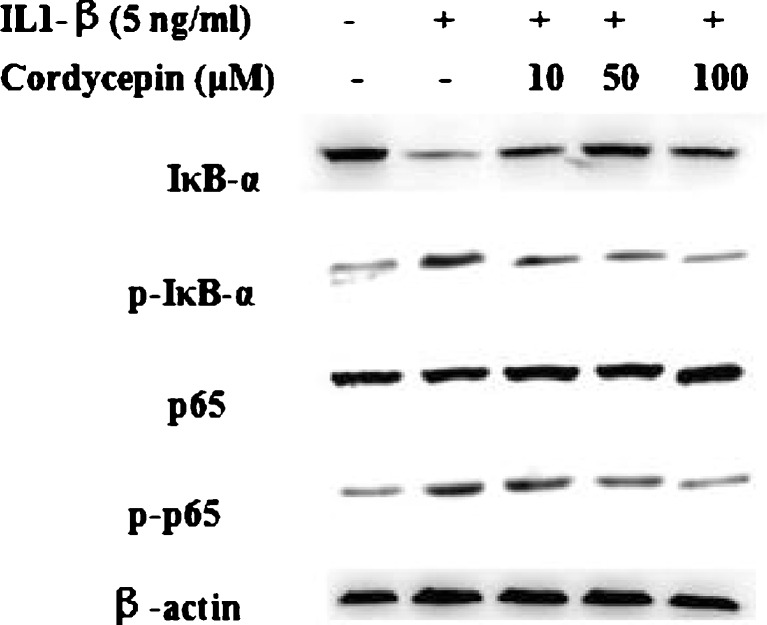
Effect of cordycepin on NF-κB activation
Activation of NF-κB was investigated by Western blot analysis due to its importance in the upregulation of COX-2 and iNOS expression in the pathogenesis of inflammation. IL-1β treatment activated the NF-κB signaling pathway and increased IκB-α phosphorylation. Also, we found the levels of the p65 subunit of NF-κB were increased in the nucleus and decreased in the cytoplasm. In contrast, pretreatment with cordycepin (10, 50 or 100 μM) inhibited IκB-α phosphorylation and translocation of the p65 subunit (Fig. 5).
Fig. 5.
Effect of cordycepin on interleukin-1β (IL-1β)-induced activation of nuclear factor kappa B (NF-κB) in IL-1β-stimulated human osteoarthritis (OA) chondrocytes pretreated with cordycepin at 10, 50 or 100 μM for 2 h and then stimulated or not with IL-1β (5 ng/ml) for 1 h. Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκB-α) degradation and phosphorylation and nuclear factor kappa B (NF-κB) p65 activation were analysed by Western blotting
Discussion
Long-term use of nonsteroidal anti-inflammatory drugs (NSAIDs) is recommended for treating some chronic inflammatory diseases, such as OA. As individual response to drugs can vary, and as potential side effects remain one of the problems for long-term use of NSAIDs, phytochemicals are potential candidates as a novel agent to prevent inflammation for the treatment of many diseases, including OA [26]. Cordycepin, a nucleoside derivative isolated from Cordyceps, is reported to exert inhibitory effects on macrophages and RA synovial fibroblasts due to its anti-inflammatory effect [12, 16]. In this study, we evaluated the role of cordycepin in inflammatory responses in human articular chondrocytes. The most important finding of this study was that cordycepin could prevent the expression of key molecules, such as PGE2, NO, MMP-13, IL-6 and COX-2 in IL-1β-stimulated human OA chondrocytes. We found the anti-inflammatory effects of cordycepin are at least in part due to its inhibitory effect on the NF-κB pathway.
We found that cordycepin had no significant effect on chondrocyte viability at the concentrations used in 24 hour cultures. Our results were consistent with the research from Noh et al. [16], who found no significant change RA synovial fibroblast cell viability following treatment with cordycepin (50 uM or 100 uM). Also, Seulmee et al. [17] showed that the exposure of mouse leukaemic monocyte macrophage cell line (RAW 264.7) to cordycepin at 5∼40 μg/ml for 24 hours showed no significant adverse effect on cell viability versus the untreated control.
Overproductions of NO and PGE2 are correlated with the pathophysiology of OA. NO is an inflammatory mediator produced by the NOS family of enzymes [27]. There are three types of NOS: iNOS, neuronal NOS (nNOS) and endothelial NOS (eNOS). The latter two are constitutive NOS, whereas iNOS is induced by cytokines to produce NO. In this study, we demonstrated that cordycepin prevented IL-1β-induced NO production as well as iNOS expression at the mRNA and protein levels in human OA chondrocytes. Results were consistent with Seulmee et al.’s results [17]. They found that cordycepin could inhibit NO production as well as iNOS expression on lipopolysaccharide-induced inflammation in RAW 264.7 cells. PGE2 is also an inflammatory factor elevated by COX-2. There are two isoforms of COX enzymes: COX-1 and COX-2. COX-1 contributes to gastric defense and COX-2 is the target in OA treatment. In this study, we also found that the induction of PGE2 production and COX-2 expression by IL-1β were blocked by cordycepin. Our results are consistent with previous study showing that cordycepin prevents PGE2 production and COX-2 expression on lipopolysaccharide-induced inflammation in RAW 264.7 cells [17].
MMPs are a family of enzymes normally required for degradation of ECM components, including type II collagen, during cartilage remodeling [28]. Recent studies demonstrate elevated MMP-13 in arthritic joints [29], and this protease is considered to be the major MMP responsible for cartilage breakdown [30]. Thus, blocking MMP-13 activity is a therapeutic approach to OA. IL-1β is a proinflammatory cytokine that plays a synergistic effect with IL-1β in OA [31]. The study reported here shows that addition of cordycepin effectively suppresses overexpression of MMP-13 and IL-6 in IL-1β-stimulated OA chondrocytes, indicating that cordycepin may act as a potent anti-inflammatory agent in chondrocytes.
We also investigated molecular mechanisms by which cordycepin inhibited inflammatory mediators in response to IL-1β in chondrocytes. We focused on NF-κB due to its importance in inflammatory process [32]. NF-κB is retained in the cytoplasm with IκB-α inactivity. IL-1β activates NF-κB by triggering IκB-α degradation. NF-κB activation resulted in the up-regulation of a group of responsive genes that contributed to inflammation, including iNOS and COX-2 [33, 34]. In our study, IL-1β induced IκB-α degradation in chondrocytes; this degradation was blocked by cordycepin. Our results are partly supported by Seulmee et al. [17], who found that cordycepin inhibits lipopolysaccharide-induced COX-2 and NO gene expression through down-regulating NF-κB in RAW 264.7 cells. However, Noh et al. [16] found that cordycepin inhibited MMP-1 and -3 production by suppressing the activator protein 1 (AP-1) but not NF-κB in RASFs. We speculated that the differences maybe attribute to the different types of cells. In RASFs, AP-1 is an essential component for MMP-1 synthesis, and cooperation between mitogen-activated protein kinase (MAPK) and NF-κB signalling pathways is necessary for IL-1β-induced synthesis of MMP-1 [35]. Ren et al. [36] found that cordycepin inhibited NF-κB signaling by suppressing NF-κB and IκB-α activity in human embryonic kidney (HEK)-293 T cells. However, the exact mechanism implicated in the regulation of inflammation in chondrocytes by cordycepin is still unclear. Further studies are needed to elucidate the precise mechanism of cordycepin regulation on inflammatory processes in OA chondrocytes.
This study is limited in terms of its in vitro experimental design and that extrapolations to in vivo conditions should be carried out with caution. Future studies are necessary to define in vivo actions of cordycepin using animal models of OA and further delineate the molecular mechanisms evoked by cordycepin.
Conclusions
In conclusion, we demonstrated that cordycepin prevented PGE2, NO, MMP-13, IL-6 and COX-2 production on IL-1β-stimulated human OA chondrocytes. With this unique profile of actions, cordycepin may prove to be a potentially attractive and new therapeutic/preventive agent for OA.
Acknowledgments
The authors thank all the staff in the Laboratory of Orthopaedic Research Institute and Scientific Research Center of Second Affiliated Hospital of Wenzhou Medical College. This work was supported by grants from Hainan Provincial Health Department (2011-34).
References
- 1.Flugge LA, Miller-Deist LA, Petillo PA. Towards a molecular understanding of arthritis. Chem Biol. 1999;6(6):R157–166. doi: 10.1016/S1074-5521(99)80043-X. [DOI] [PubMed] [Google Scholar]
- 2.Goldring MB, Otero M, Tsuchimochi K, Ijiri K, Li Y. Defining the roles of inflammatory and anabolic cytokines in cartilage metabolism. Ann Rheum Dis. 2008;67(Suppl 3):iii75–82. doi: 10.1136/ard.2008.098764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loeser RF. Molecular mechanisms of cartilage destruction: mechanics, inflammatory mediators, and aging collide. Arthritis Rheum. 2006;54(5):1357–1360. doi: 10.1002/art.21813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44(6):1237–1247. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, Feige U, Poole AR. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005;52(1):128–135. doi: 10.1002/art.20776. [DOI] [PubMed] [Google Scholar]
- 6.Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004;427 Suppl:S27–36. doi: 10.1097/01.blo.0000144854.66565.8f. [DOI] [PubMed] [Google Scholar]
- 7.Pelletier JP, Caron JP, Evans C, Robbins PD, Georgescu HI, Jovanovic D, Fernandes JC, Martel-Pelletier J. In vivo suppression of early experimental osteoarthritis by interleukin-1 receptor antagonist using gene therapy. Arthritis Rheum. 1997;40(6):1012–1019. doi: 10.1002/art.1780400604. [DOI] [PubMed] [Google Scholar]
- 8.Han ES, Oh JY, Park HJ. Cordyceps militaris extract suppresses dextran sodium sulfate-induced acute colitis in mice and production of inflammatory mediators from macrophages and mast cells. J Ethnopharmacol. 2011;134(3):703–710. doi: 10.1016/j.jep.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Hubbell HR, Pequignot EC, Willis DH, Lee C, Suhadolnik RJ. Differential antiproliferative actions of 2′,5′ oligo A trimer core and its cordycepin analogue on human tumor cells. Int J Cancer. 1985;36(3):389–394. [PubMed] [Google Scholar]
- 10.Won SY, Park EH. Anti-inflammatory and related pharmacological activities of cultured mycelia and fruiting bodies of Cordyceps militaris. J Ethnopharmacol. 2005;96(3):555–561. doi: 10.1016/j.jep.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Lee HH, Jeong JW, Lee JH, Kim GY, Cheong J, Jeong YK, Yoo YH, Choi YH. Cordycepin increases sensitivity of Hep3B human hepatocellular carcinoma cells to TRAIL-mediated apoptosis by inactivating the JNK signaling pathway. Oncol Rep. 2013 doi: 10.3892/or.2013.2589. [DOI] [PubMed] [Google Scholar]
- 12.Sjoholm A, Nystrom T. Endothelial inflammation in insulin resistance. Lancet. 2005;365(9459):610–612. doi: 10.1016/S0140-6736(05)17912-4. [DOI] [PubMed] [Google Scholar]
- 13.Yoo HS, Shin JW, Cho JH, Son CG, Lee YW, Park SY, Cho CK. Effects of Cordyceps militaris extract on angiogenesis and tumor growth. Acta Pharmacol Sin. 2004;25(5):657–665. [PubMed] [Google Scholar]
- 14.Kan WC, Wang HY, Chien CC, Li SL, Chen YC, Chang LH, Cheng CH, Tsai WC, Hwang JC, Su SB, Huang LH, Chuu JJ. Effects of Extract from Solid-State Fermented Cordyceps sinensis on Type 2 Diabetes Mellitus. Evid Based Complement Alternat Med. 2012;2012:743107. doi: 10.1155/2012/743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, He D, Yang J, Wang X. Cordycepin inhibits renal interstitial myofibroblast activation probably by inducing hepatocyte growth factor expression. J Pharmacol Sci. 2011;117(4):286–294. doi: 10.1254/jphs.11127FP. [DOI] [PubMed] [Google Scholar]
- 16.Noh EM, Kim JS, Hur H, Park BH, Song EK, Han MK, Kwon KB, Yoo WH, Shim IK, Lee SJ, Youn HJ, Lee YR. Cordycepin inhibits IL-1beta-induced MMP-1 and MMP-3 expression in rheumatoid arthritis synovial fibroblasts. Rheumatology (Oxford) 2009;48(1):45–48. doi: 10.1093/rheumatology/ken417. [DOI] [PubMed] [Google Scholar]
- 17.Shin S, Lee S, Kwon J, Moon S, Lee CK, Cho K, Ha NJ, Kim K. Cordycepin Suppresses Expression of Diabetes Regulating Genes by Inhibition of Lipopolysaccharide-induced Inflammation in Macrophages. Immune Netw. 2009;9(3):98–105. doi: 10.4110/in.2009.9.3.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baik JS, Kwon HY, Kim KS, Jeong YK, Cho YS, Lee YC. Cordycepin induces apoptosis in human neuroblastoma SK-N-BE(2)-C and melanoma SK-MEL-2 cells. Indian J Biochem Biophys. 2012;49(2):86–91. [PubMed] [Google Scholar]
- 19.Jeong MH, Seo MJ, Park JU, Kang BW, Kim KS, Lee JY, Kim GY, Kim JI, Choi YH, Kim KH, Jeong YK. Effect of cordycepin purified from Cordyceps militaris on Th1 and Th2 cytokines in mouse splenocytes. J Microbiol Biotechnol. 2012;22(8):1161–1164. doi: 10.4014/jmb.1203.03039. [DOI] [PubMed] [Google Scholar]
- 20.Lee HJ, Burger P, Vogel M, Friese K, Bruning A. The nucleoside antagonist cordycepin causes DNA double strand breaks in breast cancer cells. Investig New Drugs. 2012;30(5):1917–1925. doi: 10.1007/s10637-012-9859-x. [DOI] [PubMed] [Google Scholar]
- 21.Liu Q, Hong IP, Ahn MJ, Yoo HS, Han SB, Hwang BY, Lee MK. Anti-adipogenic activity of Cordyceps militaris in 3 T3-L1 cells. Nat Prod Commun. 2011;6(12):1839–1841. [PubMed] [Google Scholar]
- 22.Wu CW, Morrell MR, Heinze E, Concoff AL, Wollaston SJ, Arnold EL, Singh R, Charles C, Skovrun ML, FitzGerald JD, Moreland LW, Kalunian KC. Validation of American College of Rheumatology classification criteria for knee osteoarthritis using arthroscopically defined cartilage damage scores. Semin Arthritis Rheum. 2005;35(3):197–201. doi: 10.1016/j.semarthrit.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Ying X, Cheng S, Shen Y, Cheng X, An Rompis F, Wang W, Lin Z, Chen Q, Zhang W, Kou D, Peng L, Tian XQ, Lu CZ. Nicotine promotes proliferation and collagen synthesis of chondrocytes isolated from normal human and osteoarthritis patients. Mol Cell Biochem. 2012;359(1–2):263–269. doi: 10.1007/s11010-011-1020-1. [DOI] [PubMed] [Google Scholar]
- 24.Ying X, Chen X, Cheng S, Shen Y, Peng L, Xu HZ. Piperine inhibits IL-beta induced expression of inflammatory mediators in human osteoarthritis chondrocyte. Int Immunopharmacol. 2013 doi: 10.1016/j.intimp.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 25.Akhtar N, Miller MJ, Haqqi TM. Effect of a Herbal-Leucine mix on the IL-1beta-induced cartilage degradation and inflammatory gene expression in human chondrocytes. BMC Complement Altern Med. 2011;11:66. doi: 10.1186/1472-6882-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim KS, da Oh H, Choi HM, Bang JS, Ryu CJ, Kim JH, Yoo MC, Yang HI. Pyrrolidine dithiocarbamate, a NF-kappaB inhibitor, upregulates MMP-1 and MMP-13 in IL-1beta-stimulated rheumatoid arthritis fibroblast-like synoviocytes. Eur J Pharmacol. 2009;613(1–3):167–175. doi: 10.1016/j.ejphar.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 27.Wei XQ, Charles IG, Smith A, Ure J, Feng GJ, Huang FP, Xu D, Muller W, Moncada S, Liew FY. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375(6530):408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 28.Heard BJ, Martin L, Rattner JB, Frank CB, Hart DA, Krawetz R. Matrix metalloproteinase protein expression profiles cannot distinguish between normal and early osteoarthritic synovial fluid. BMC Musculoskelet Disord. 2012;13:126. doi: 10.1186/1471-2474-13-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF, Hambor JE. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97(3):761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, Shah M, Thompson EW. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60(12):3723–3733. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flannery CR, Little CB, Hughes CE, Curtis CL, Caterson B, Jones SA. IL-6 and its soluble receptor augment aggrecanase-mediated proteoglycan catabolism in articular cartilage. Matrix Biol. 2000;19(6):549–553. doi: 10.1016/S0945-053X(00)00111-6. [DOI] [PubMed] [Google Scholar]
- 32.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107(1):7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilston V, Jones HW, Soo CC, Coumbe A, Blades S, Kaltschmidt C, Baeuerle PA, Morris CJ, Blake DR, Winyard PG. NF-kappa B activation in human knee-joint synovial tissue during the early stage of joint inflammation. Biochem Soc Trans. 1997;25(3):518S. doi: 10.1042/bst025518s. [DOI] [PubMed] [Google Scholar]
- 34.Miagkov AV, Kovalenko DV, Brown CE, Didsbury JR, Cogswell JP, Stimpson SA, Baldwin AS, Makarov SS. NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci U S A. 1998;95(23):13859–13864. doi: 10.1073/pnas.95.23.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu HT, Liang YC, Sheu MT, Ho HO, Lin YT, Hsieh MS, Chen CH. Disease-modifying effects of glucosamine HCl involving regulation of metalloproteinases and chemokines activated by interleukin-1beta in human primary synovial fibroblasts. J Cell Biochem. 2008;104(1):38–50. doi: 10.1002/jcb.21597. [DOI] [PubMed] [Google Scholar]
- 36.Ren Z, Cui J, Huo Z, Xue J, Cui H, Luo B, Jiang L, Yang R. Cordycepin suppresses TNF-alpha-induced NF-kappaB activation by reducing p65 transcriptional activity, inhibiting IkappaBalpha phosphorylation, and blocking IKKgamma ubiquitination. Int Immunopharmacol. 2012;14(4):698–703. doi: 10.1016/j.intimp.2012.10.008. [DOI] [PubMed] [Google Scholar]