Abstract
Purpose
Conventional follow-up methods are not sufficient to identify adverse soft tissue reactions in patients with metal-on-metal hip replacements. The national guidelines regarding metal ion measurements are debatable. The aims of our study were to investigate (1) if there is a clinically significant change in whole blood (WB) cobalt (Co) or chrome (Cr) levels in repeated WB assessment in patients operated on with ASR hip replacements, and (2) what proportion of patients has WB Co or Cr level below the previously established safe upper limits (SUL) in the repeated WB metal ion assessment.
Methods
We identified all patients (n = 254) with unilateral ASR implants who had second blood sample taken eight to 16 months after the first.
Results
WB Co and Cr levels remained below SUL and within their initial values during a mean one-year measurement interval in the majority of patients with a high risk HR device. In contrast to this, 50 % of patients with THRs had metal ion levels exceeding the SUL in the first measurement. WB Co values significantly increased over the measurement interval in the THR group.
Conclusion
In patients with a high risk HR, repeated metal ion measurement did not provide useful information for clinical decision-making. In patients with a LD MoM THR repeated measurements revealed a large number of patients with metal ion levels exceeding SUL and might thus be clinically beneficial.
Keywords: Metal-on-metal, Whole blood, Cobalt, Chrome, Hip resurfacing, Total hip arthroplasty
Introduction
Adverse reactions to metal debris (ARMeD) associated with metal-on-metal (MoM) hip replacements have become a major issue in recent years [1–3]. It is clear that conventional follow-up methods (i.e., plain radiographs and clinical follow-up) are not sufficient to identify ARMeD in patients with MoM hips [4]. Hence metal ion assessment both from blood and synovial fluid, in addition to cross-sectional imaging, have been used to detect articulation related complications [4–8].
Numerous studies have been published reporting prospective whole blood (WB) cobalt (Co) and chrome (Cr) ion measurements in patients with metal-on-metal (MoM) hip resurfacing (HR) and with MoM total hip replacement (THR) with varying head diameters. WB metal ion levels after HR have been shown to peak nine to 12 months postoperatively, reaching plateau levels thereafter [9, 10]. Identical findings have been reported in patients with MoM THR, although some authors suggest peaking to occur later [11–13]. To the best of our knowledge, however, WB metal ion levels have not been reported in large diameter (LD) MoM THRs beyond two years.
The UK Medicines and Healthcare Products Regulation Agency (MHRA) has recommended that all stemmed MOM total hip replacements with femoral diameter greater than 36 mm should be followed-up on an annual basis [14]. The recommendation includes routine blood metal ion analyses to be performed for these patients. By contrast, the U.S. Food and Drug Administration (FDA) recently did not recommend using whole blood metal ion assessment in the routine screening of patients with MoM hip implants [15].
The primary aims of our study were therefore to investigate (1) if there is a clinically significant change in WB Co or Cr levels in repeated WB assessment performed more than eight to 16 months after the initial measurement in patients operated on with ASR hip replacements, and (2) what proportion of patients have WB Co or Cr levels below the previously established safe upper limits (SUL) in the repeated WB metal ion assessment [16].
Materials and methods
ASR (DePuy, Warsaw, In, USA) MOM hip replacements were used in 1,036 operations (887 patients) at our institution between March 2004 and December 2009. ASR hip resurfacing devices were implanted in 415 patients (497 hips) and 471 patients (537 hips) received an ASR XL THR. MHRA announced a medical device alert regarding the ASR hip replacements in September 2010 [17]. After the announcement we established a screening program to identify possible articulation related complications in patients with these implants. The screening was initiated at a mean of four years post-operatively. All patients received an Oxford hip score questionnaire, and were clinically examined (incl. Harris hip score) by a physiotherapist at our outpatient clinic. Anteroposterior and lateral radiographs of the hip and anteroposterior pelvic radiograph were taken prior to each visit. Each patient was also referred for WB Cr and Co ion concentration measurements. All patients were primarily referred for magnetic resonance imaging (MRI) using magnetic artifact reduction sequence (MARS). If MRI was contraindicated for medical reasons, or if the patient suffered from claustrophobia, an ultrasonography was used.
After the abovementioned procedures, all patients were evaluated by senior hip surgeons. Revision surgery was considered if (1) there was a clear pseudo-tumor in cross-sectional imaging regardless of symptoms or WB metal ion levels, or (2) despite a normal finding in cross-sectional imaging if the patient had both elevated (>5 ppb) WB metal ion levels and hip symptoms or (3) continuously symptomatic hip regardless of imaging findings or metal ion levels. All patients who did not meet the criteria for being considered for revision surgery were subsequently scheduled for annual or biennial repeat visits. Borderline cases were re-evaluated more frequently.
All patients participating in the screening protocol had their blood samples taken from the antecubital vein using a 21-gauge needle connected to a VacutainerTM system (Becton, Dickinson and Company Franklin Lakes, NJ, USA) and trace element blood tubes containing sodium EDTA. The first 10 ml of blood was used for other laboratory tests such as C-reactive protein and erythrocyte sedimentation rate measurement. The second 10 ml was used for cobalt and chromium analysis. At the Finnish Institute for Occupational Health, standard (operating) procedures were established for cobalt and chromium measurement using dynamic reaction cell inductively coupled plasma (quadruple) mass spectrometry (Agilent 7500 cx, Agilent Technologies, Santa Clara, CA, USA).
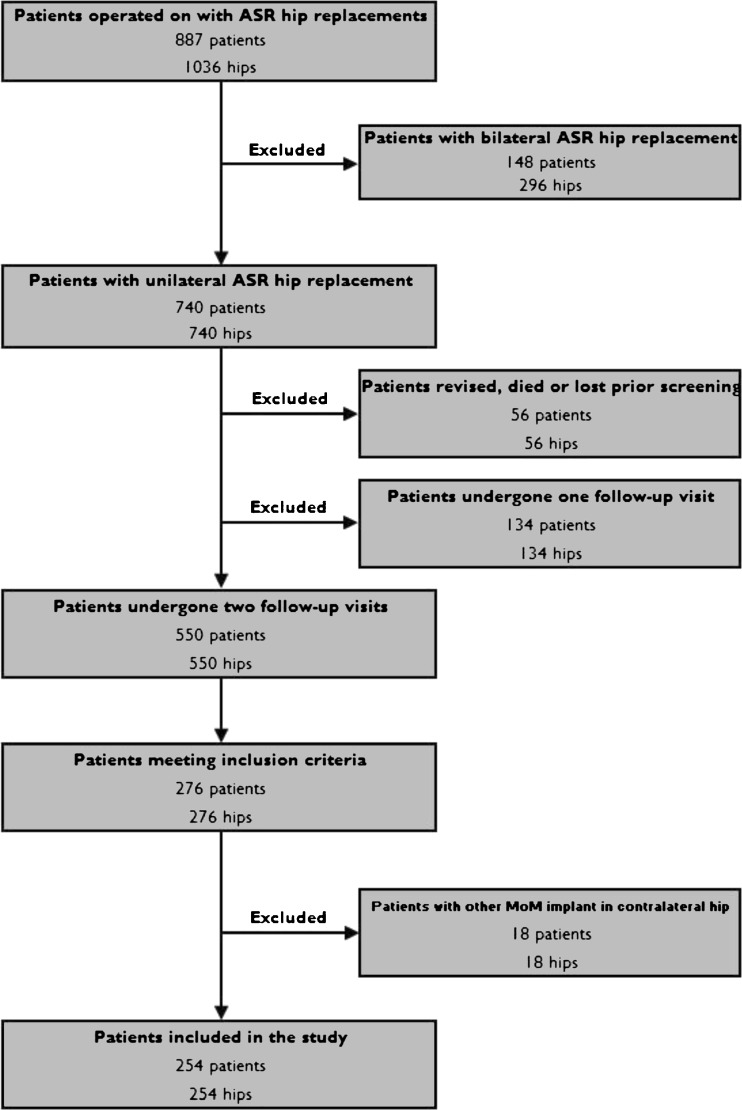
For the purposes of the present study, we identified all patients with unilateral ASR implants who had made two follow-up visits to our institution after initiation of the screening program (incl. OHS and WB metal ion measurements). Of the 740 unilateral patients, 56 were lost to follow-up, died or revised prior to the screening. A total of 134 patients (134 hips) made only one follow-up visit (Fig. 1). Seventy-six patients were revised, five died and 14 were lost to follow-up after the first visit. In 39 patients the second follow-up visit was postponed beyond three years and they have not yet attended, while 550 patients (550 hips) have undergone two follow-up visits. The mean time elapsing from the first metal ion assessment (initial measurement) to the second (control measurement) was 1.40 years (SD 0.60, range 0.1–2.74). Those patients from whom a second blood sample was taken eight to 16 months (mean value 1.05 years) after the first were included in the study. Eighteen patients who had another MoM implant in the contralateral hip were excluded from the study cohort (Fig. 1). This left 255 patients with 255 hips in the study group. All unilateral ASR patients operated on at our institution are hereafter referred to as the ASR group, whereas those patients included in the present study are referred to as the study group.
Fig. 1.
Flow chart of the study
Of the 254 patients included in the study, 156 had received an ASR XL THR and 98 patients an ASR HR. Mean follow-up duration of these patients was 5.2 years (SD 1.4). Mean follow-up duration at the time of the first metal ion measurement was 3.7 years (SD 1.3).
Age and inclination angle were normally distributed in the study group [p < 0.001 for both, (Kolmogoroff–Smirnoff goodness-of-fit test using the Lilliefors method of significance correction)]. Female patients and patients with ASR THR were overrepresented in the study group compared to the ASR group (Table 1). Median femoral diameter in the study group was significantly lower than in the ASR cohort (47 mm vs. 51 mm, p < 0.001). There were no other significant differences in demographics between the groups (Table 1).
Table 1.
Comparison of demographic variables between the ASR group and the study group
| Variable | ASR cohort | Study group | p-value | |
|---|---|---|---|---|
| Prevalence | THR | 54.7 % | 61.4 % | p = 0.003a |
| HR | 45.3 % | 38.6 % | ||
| Sex | Male | 58.0 % | 42.9 % | p < 0.001a |
| Female | 42.0 % | 57.1 % | ||
| Age (SD) | THR | 58.9 years (10.6) | 59.1 (10.0) | p = 0.9b |
| HR | 53.7 years (9.8) | 53.8 (9.3) | p = 0.9b | |
| Median femoral diameter (range) | THR | 49 mm (39-61) | 47 mm (41-59) | p = 0.01c |
| HR | 53 mm (43-63) | 49 mm (43-59) | p < 0.001a | |
| Mean acetabular inclination (SD) | THR | 46.2 (7.5) | 46.0 (7.4) | p = 0.71a |
| HR | 46.2 (6.3) | 46.4 (6.8) | p = 0.79a | |
THR total hip replacement, HR hip resurfacing
a One way chi-square test
b Kruskal-Wallis one-way analysis of variance
c One sample t-test
The time elapsing from the index operation to the first metal ion measurement (initial) is referred to as follow-up time. Patients were divided into follow-up time interval groups according to the time elapsing from the index operation to the first metal ion assessment. The time elapsing from the first metal ion measurement (initial) to the second measurement (control) in the same patient is referred to as the measurement interval. Thus total follow-up is defined as follow-up time plus measurement interval. The Wilcoxon signed-rank sum test was used to compare two consecutive metal ion measurements from the same patients. SUL values for WB Co were 4.0 ppb and for WB Cr 4.4 ppb as reported earlier [16]. Differences between groups were considered statistically significant if the p-values were less than 0.05 in a two-tailed test.
Results
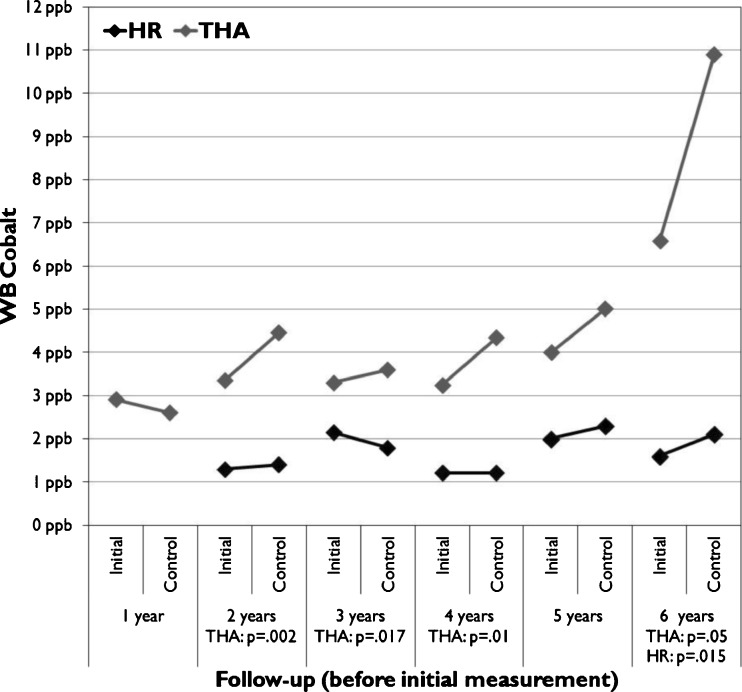
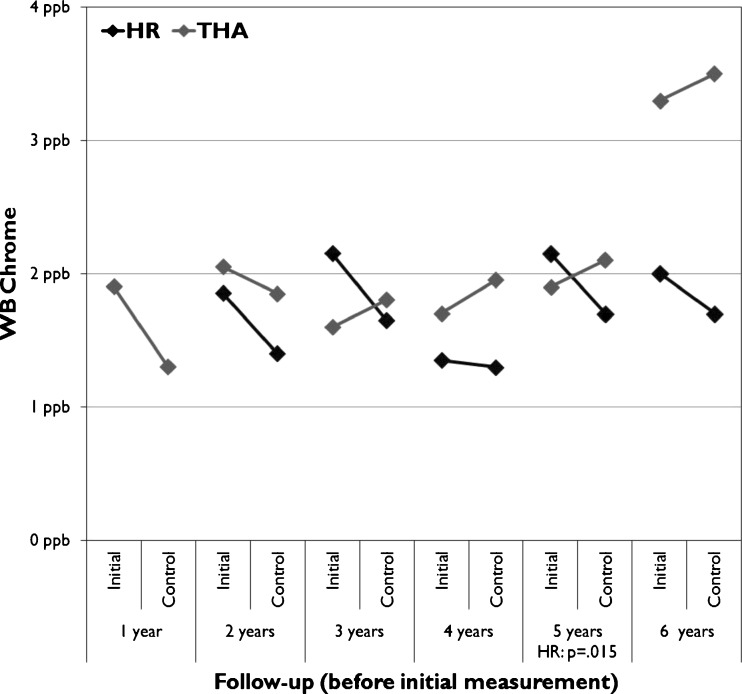
A significant change was seen in WB Cr values in the HR group and in WB Co values in the THR group (Table 2). In the HR group a significant increase in median WB cobalt level was seen in the six-year followup group (Fig. 2) and a significant decrease in WB chrome levels in the five year followup group (Fig. 3). In the THR group change in median WB cobalt values showed significant differences in several follow-up groups (Fig. 3).
Table 2.
Differences in WB Co and Cr levels
| Measure | Initial | Control | p value | |
|---|---|---|---|---|
| Median WB Cr (range) | HR | 1.85 ppb (0.60–18.0) |
1.60 ppb (0.50–17.4) |
p < 0.001 |
| THR | 1.90 ppb (0.60–13.3) |
2.00 ppb (0.40–18.5) |
p = 0.17 | |
| Median WB Co (range) | HR | 1.55 ppb (0.60–128.4) |
1.80 ppb (0.50–46.9) |
p = 0.9 |
| THR | 3.45 ppb (0.60–115.1) |
4.55 ppb (0.60–174.60) |
p < 0.001 | |
THR total hip replacement, HR hip resurfacing
Fig 2.
Median whole blood (WB) Co values divided across the follow-up time before initial measurement
Fig. 3.
Median whole blood (WB) Cr values divided across the follow-up time before initial measurement
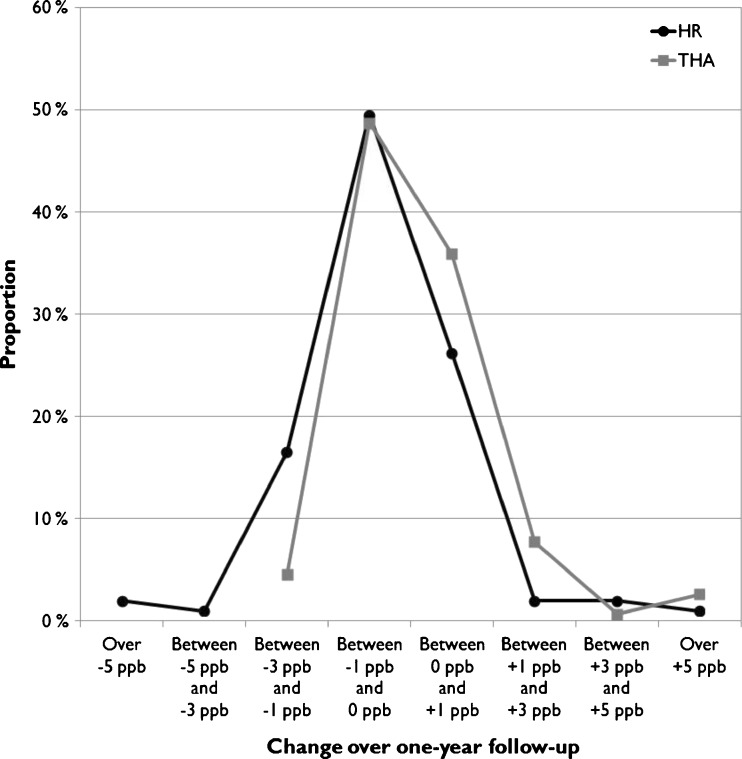
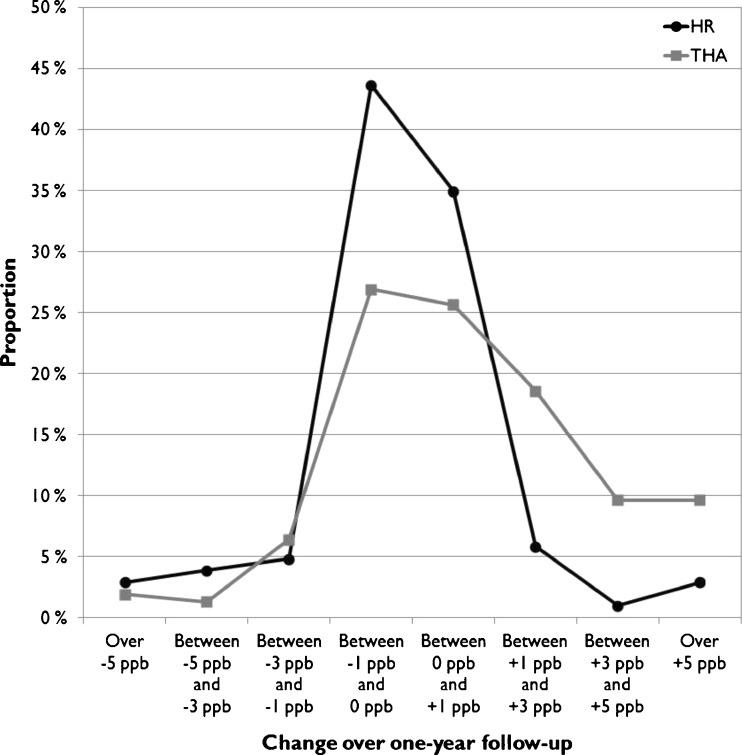
The change over a one-year measurement interval was calculated and plotted as frequency distributions for the HR and THR cohorts of the study group and for both metal ions separately (Figs. 4 and 5). Both Co and Cr concentrations remained within ±1 ppb of their initial value in most HR patients (76.5 % for Co, 75.5 % for Cr), with no trends towards increasing values (Figs. 4 and 5). In THR patients, however, there was a clear skewing towards increased Co values (40.3 % with more than +1 ppb change in WB Co, 83.3 % within ±1 ppb for Cr).
Fig. 4.
Frequency distribution of change in whole blood (WB) Cr levels over one-year follow-up
Fig. 5.
Frequency distribution of change in whole blood (WB) Co levels over one-year follow-up
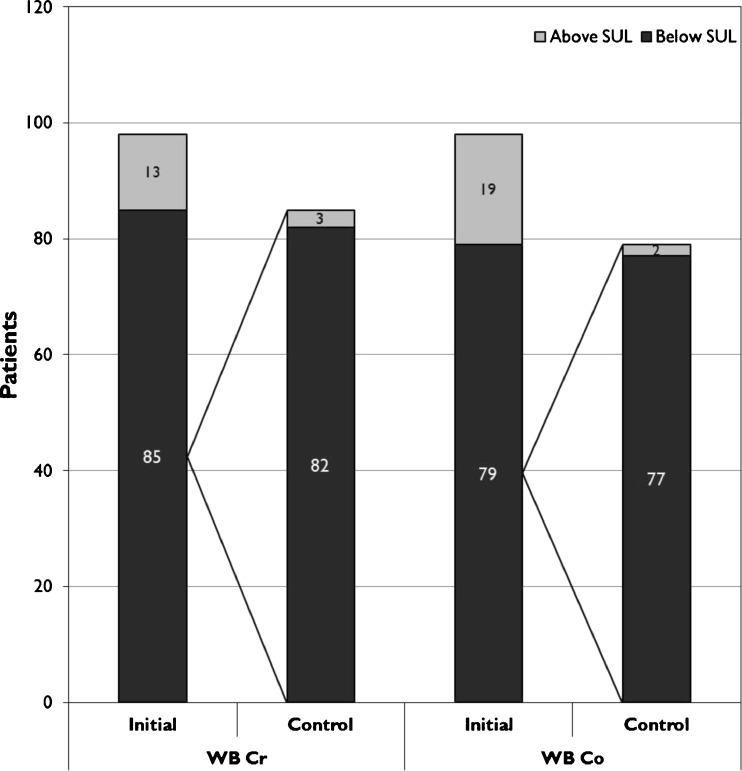
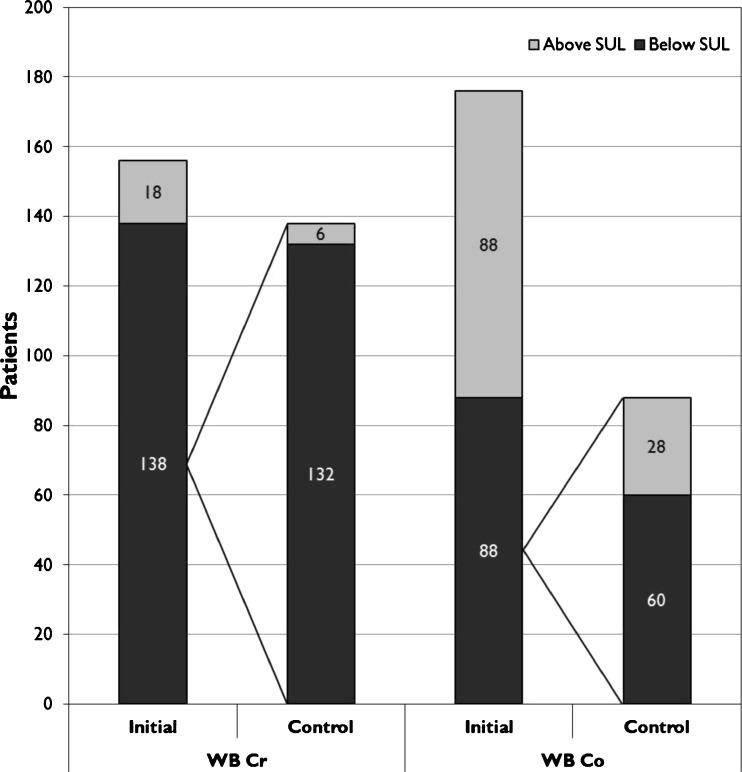
In the majority of HR patients both WB Cr and Co remained below SUL in the control measurement (Fig. 6). The same finding was also seen in WB Cr values in the THR group while 32 % of the patients with WB Co below SUL had WB Co above SUL in the control measurement (Fig. 7).
Fig. 6.
Proportion of HR patients having whole blood (WB) metal ion values below their safe upper limits (SUL)
Fig. 7.
Proportion of THR patients having whole blood (WB) metal ion values below their safe upper limits (SUL)
Discussion
The conventional follow-up methods for patients with hip replacements include clinical follow-up and plain radiographs. These are not, however, sufficient to detect possible articulation-related failures in patients with MoM hip replacements [4]. WB or serum metal ion measurements and cross-sectional imaging modalities such as MRI and US are therefore used to identify patients with suspected ARMeD. There is a relative paucity of reports in the literature on repeated WB metal ion measurements in patients with high risk MoM devices with follow-up exceeding two years. We therefore aimed to study (1) if there was a significant change in WB Co or Cr levels in repeated WB assessment performed after eight to 16 months after the initial measurement, (2) if there were differences in WB Co and Cr levels between two consecutive assessments, and (3) in what proportion of patients WB Co or Cr levels were below the previously established SULs in the repeated WB metal ion assessment.
We acknowledge a few limitations in our study. First, the inclusion criterion used in our study was arbitrary. We aimed to study changes in WB metal ion levels by repeated measurements and the practical measurement interval was one year as in current guidelines [14]. The time elapsing from the first measurement to the second was not, however, constant in our patients. Therefore we were compelled to select a time range and one year ± four months was deemed most suitable. Second, there is also a selection bias in our study. Female patients, THRs and small femoral size were overrepresented. All these variables are known to be associated with elevated WB Co and Cr levels [18, 19]. Hence our results may partly reflect the “worst case scenario”. Third, we only included one, subsequently recalled hip replacement design in our study. Therefore our results cannot be directly generalized to patients with all other types of MoM hip replacements.
There are numerous reports on the longitudinal follow-up of ion levels in patients with HRs and with both LD and 28-mm MoM THRs [11–13, 20, 21]. Behaviour of metal ions in HRs have been reported for up to ten years, whereas the follow-up time in studies reporting metal ion levels after LD MoM THRs has been less than two years. We observed a significant decrease in WB Cr levels, but not in the WB Co levels in the HR group. Decreases in WB metal ion levels are known to occur one to two years postoperatively but we observed a decrease in Cr levels also five years postoperatively. We cannot evince one single explanation for this observation. It must be noted, however, that the decrease in Cr levels was rather small (0.2 ppb) and its clinical significance may thus be minute. Significantly higher Co levels in WB and serum have been reported in LD MoM THRs compared to HRs with identical bearings, indicating the importance of the taper in the Co levels [18]. We observed a significant rise in WB Co levels in patients with THRs at several time points. We consider this indicative for annual WB metal ion measurement in patients with LD MoM THR as the MHRA guidelines recommend.
In 16 (10 %) THR patients control measurement revealed an increase of more than 5 ppb in WB Co levels. Moreover, the Co level increased more than 3 ppb in 29 THR patients over the measurement interval. In contrast, WB Cr level increased more than 3 ppb in only five patients with THRs. The WB Co levels increased significantly at several follow-up time points, which may indicate continuous corrosion or fretting process. This is a worrisome finding since WB Co levels do not correlate with the volumetric material loss in the taper and thus it has been proposed that cobalt ions originating from taper might evince higher cytotoxic or inflammatory properties [22].
The majority of HR patients with WB Co or Cr below SUL had a similar situation in the control measurement. This suggests that once conformity between head and cup is achieved it also remains and adverse wearing conditions are unlikely to develop later on even though ASR HR is a high risk device. A similar observation was also made in the THR group in relation to WB Cr levels indicating that Cr ion release from the taper is negligible. WB Cr remained below SUL in 132 of the 138 THR patients. However, only 50 % of the THR patients had WB Co below SUL in the initial measurement. This concurs with other studies reporting WB and serum Co values in patients with LD MoM THR. Moreover, in a relatively high proportion (32 %) of patients with WB Co below SUL in the initial measurement, the control measurement revealed Co value outside this limit. This is a novel finding and further supports the annual measurement of WB Co values in THR patients.
In conclusion, WB Co and Cr levels remained below SUL and within their initial values during a mean one-year measurement interval in the majority of patients with high risk HR device. By contrast, WB Co was above SUL in a high proportion of patients with LD MoM THR. In addition there was a clear trend towards increasing WB Co values in these patients. Longer follow-up and further research are needed to ascertain the clinical implications of repeated WB Co and Cr ion measurement in patients with high risk HR devices. In patients with a LD MoM THR, repeated WB metal ion measurement, especially Co, may be of clinical benefit.
Contributor Information
Aleksi Reito, Email: aleksi.reito@uta.fi.
Teemu Moilanen, Email: teemu.moilanen@coxa.fi.
Timo Puolakka, Email: timo.puolakka@coxa.fi.
Jorma Pajamäki, Email: j.pajamaki@coxa.fi.
Antti Eskelinen, Email: antti.eskelinen@coxa.fi.
References
- 1.Bosker BH, Ettema HB, Boomsma MF, Kollen BJ, Maas M, Verheyen CC. High incidence of pseudotumour formation after large-diameter metal-on-metal total hip replacement: a prospective cohort study. J Bone Joint Surg (Br) 2012;94(6):755–761. doi: 10.1302/0301-620X.94B6.28373. [DOI] [PubMed] [Google Scholar]
- 2.Reito A, Puolakka T, Elo P, Pajamaki J, Eskelinen A. High prevalence of adverse reactions to metal debris in small-headed ASR hips. Clin Orthop Relat. 2013;471(9):2954–2961. doi: 10.1007/s11999-013-3023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bisschop R, Boomsma MF, Van Raay JJ, Tiebosch AT, Maas M, Gerritsma CL. High prevalence of pseudotumors in patients with a Birmingham hip resurfacing prosthesis: a prospective cohort study of one hundred and twenty-nine patients. J Bone Joint Surg Am. 2013;95(17):1554–1560. doi: 10.2106/JBJS.L.00716. [DOI] [PubMed] [Google Scholar]
- 4.Donell ST, Darrah C, Nolan JF, Wimhurst J, Toms A, Barker TH. Early failure of the Ultima metal-on-metal total hip replacement in the presence of normal plain radiographs. J Bone Joint Surg (Br) 2010;92(11):1501–1508. doi: 10.1302/0301-620X.92B11.24504. [DOI] [PubMed] [Google Scholar]
- 5.Anderson H, Toms AP, Cahir JG, Goodwin RW, Wimhurst J, Nolan JF. Grading the severity of soft tissue changes associated with metal-on-metal hip replacements: reliability of an MR grading system. Skelet Radiol. 2011;40(3):303–307. doi: 10.1007/s00256-010-1000-7. [DOI] [PubMed] [Google Scholar]
- 6.Nishii T, Sakai T, Takao M, Yoshikawa H, Sugano N. Ultrasound screening of periarticular soft tissue abnormality around metal-on-metal bearings. J Arthroplasty. 2012;27(6):895–900. doi: 10.1016/j.arth.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Hart AJ, Sabah SA, Bandi AS, Maggiore P, Tarassoli P, Sampson B. Sensitivity and specificity of blood cobalt and chromium metal ions for predicting failure of metal-on-metal hip replacement. J Bone Joint Surg (Br) 2011;93(10):1308–1313. doi: 10.1302/0301-620X.93B10.26249. [DOI] [PubMed] [Google Scholar]
- 8.De Pasquale D, Stea S, Squarzoni S, Bordini B, Amabile M, Catalani S (2013) Metal-on-metal hip prostheses: Correlation between debris in the synovial fluid and levels of cobalt and chromium ions in the bloodstream. Int Orthop 2013 Oct 12 [DOI] [PMC free article] [PubMed]
- 9.Clarke IC, Donaldson T, Bowsher JG, Nasser S, Takahashi T. Current concepts of metal-on-metal hip resurfacing. Orthop Clin N Am. 2005;36(2):143–162. doi: 10.1016/j.ocl.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Daniel J, Ziaee H, Pradhan C, McMinn DJ. Six-year results of a prospective study of metal ion levels in young patients with metal-on-metal hip resurfacings. J Bone Joint Surg (Br) 2009;91(2):176–179. doi: 10.1302/0301-620X.91B2.21654. [DOI] [PubMed] [Google Scholar]
- 11.Maurer-Ertl W, Friesenbichler J, Sadoghi P, Pechmann M, Trennheuser M, Leithner A. Metal ion levels in large-diameter total hip and resurfacing hip arthroplasty - Preliminary results of a prospective five year study after two years of follow-up. BMC Musculoskelet Disord. 2012;13(1):56. doi: 10.1186/1471-2474-13-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garbuz DS, Tanzer M, Greidanus NV, Masri BA, Duncan CP. The John Charnley Award: metal-on-metal hip resurfacing versus large-diameter head metal-on-metal total hip arthroplasty: a randomized clinical trial. Clin Orthop Relat Res. 2010;468(2):318–325. doi: 10.1007/s11999-009-1029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernstein M, Desy NM, Petit A, Zukor DJ, Huk OL, Antoniou J. Long-term follow-up and metal ion trend of patients with metal-on-metal total hip arthroplasty. Int Orthop. 2012;36(9):1807–1812. doi: 10.1007/s00264-012-1570-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medicines and Healthcare Products Regulation Agency (2012) Medical device alert: all metal-on-metal (MoM) hip replacements (MDA/2012/036). http://www.mhra.gov.uk/home/groups/dts-bs/documents/medicaldevicealert/con155767.pdf. Accessed 24 February 2014
- 15.U.S. Food and Drug Administration (2013) Metal-on-metal hip implants. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/MetalonMetalHipImplants/. Accessed 24 February 2014
- 16.Van Der Straeten C, Grammatopoulos G, Gill HS, Calistri A, Campbell P, De Smet KA. The 2012 Otto Aufranc award: the interpretation of metal ion levels in unilateral and bilateral hip resurfacing. Clin Orthop Relat Res. 2012;471(2):377–385. doi: 10.1007/s11999-012-2526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medicines and Healthcare Products Regulation Agency (2010) Medical device alert: ASR™ hip replacement implants manufactured by DePuy International Ltd (MDA/2010/069). http://www.mhra.gov.uk/home/groups/dts-bs/documents/medicaldevicealert/con093791.pdf. Accessed 24 February 2014
- 18.Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg (Br) 2010;92(1):38–46. doi: 10.1302/0301-620X.92B1.22770. [DOI] [PubMed] [Google Scholar]
- 19.Langton DJ, Sprowson AP, Joyce TJ, Reed M, Carluke I, Partington P. Blood metal ion concentrations after hip resurfacing arthroplasty: a comparative study of articular surface replacement and Birmingham hip resurfacing arthroplasties. J Bone Joint Surg (Br) 2009;91(10):1287–1295. doi: 10.1302/0301-620X.91B10.22308. [DOI] [PubMed] [Google Scholar]
- 20.Beaule PE, Kim PR, Hamdi A, Fazekas A. A prospective metal ion study of large-head metal-on-metal bearing: a matched-pair analysis of hip resurfacing versus total hip replacement. Orthop Clin N Am. 2011;42(2):251–257. doi: 10.1016/j.ocl.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 21.deSouza RM, Parsons NR, Oni T, Dalton P, Costa M, Krikler S. Metal ion levels following resurfacing arthroplasty of the hip: serial results over a ten-year period. J Bone Joint Surg (Br) 2010;92(12):1642–1647. doi: 10.1302/0301-620X.92B12.24654. [DOI] [PubMed] [Google Scholar]
- 22.Matthies AK, Racasan R, Bills P, Blunt L, Cro S, Panagiotidou A. Material loss at the taper junction of retrieved large head metal-on-metal total hip replacements. J Orthop Res. 2013;31(11):1677–1685. doi: 10.1002/jor.22431. [DOI] [PubMed] [Google Scholar]