Abstract
Purpose
Legg-Calve-Perthes disease is a paediatric condition encompassing idiopathic osteonecrosis of the femoral head (ONFH). Preventing collapse and the need for subsequent joint replacement remains the major goal of clinical management. This exploratory study utilises a porcine model of surgically induced ONFH.
Methods
rhBMP-2 with and without zoledronic acid (ZA) was delivered by intra-osseous injection in the phase-transitioning sucrose acetate isobutyrate (SAIB) in an attempt to prevent femoral head collapse. Epiphyseal quotient (EQ) at eight weeks post-surgery was the primary outcome measure. Heterotopic ossification in the joint capsule and bisphosphonate retention in the femoral head were key secondary outcomes.
Results
Femoral heads with ONFH and no treatment all collapsed (3/3, EQ < 0.4, P < 0.05 compared to no ONFH). Local delivery of rhBMP-2/SAIB into the femoral head prevented collapse by EQ measurement one of four samples; however, this specimen still showed evidence of significant collapse. In contrast, the combination of local rhBMP-2 and local ZA prevented collapse in two of four samples. Confocal fluorescence microscopy showed locally dosed bisphosphonate entered and was retained in the femoral head. This group also showed strong Calcein signal, indicating new bone formation. Treatment with rhBMP-2 was associated with a limited amount of heterotrophic ossification in the joint capsules in some specimens.
Conclusions
Operators reported SAIB to be an efficient way to deliver rhBMP-2 to the femoral head. These data suggest that rhBMP-2 is ineffective for preventing femoral head collapse without the addition of bisphosphonate. Further research will be required to validate the clinical efficacy of a combined local rhBMP-2/bisphosphonate approach.
Keywords: Perthes disease, ONFH, Bisphosphonate, rhBMP-2, SAIB
Introduction
Legg-Calve-Perthes disease is a condition that describes an idiopathic osteonecrosis of the femoral head (ONFH) in children, typically between the ages of four and ten [1]. Non-operative interventions such as bracing are the frontline of treatment, but osteoarthritic degeneration and/or femoral head collapse can still result. In these cases total hip arthroscopy or resurfacing is advised [2]. While a single total hip arthroplasty may suffice for treatment of ONFH in older patients, it is not an adequate treatment option in the paediatric field. These interventions typically require multiple revisions over a lifetime, which leads to depletion of bone stock and the need for further hip replacements.
It has been proposed the use of bisphosphonate therapy may ameliorate the risk of collapse by preventing bone loss while the vasculature becomes re-established and bone anabolism recovers [3, 4]. Previous preclinical studies have shown improvements with systemic bisphosphonates in a spontaneously hypertensive rat model [5] and a surgical model of induced avascular necrosis [6]. In a piglet model that involves using ligature to disrupt blood supply to the femoral head, resulting in tissue necrosis [7, 8], systemic dosing with ibandronate led to significant improvements [9].
However, there is limited clinical evidence that systemic intravenous bisphosphonate treatment can reduce the deformity of the femoral head in this condition [10–12]. The presence of a vascular insult at the vascular head draws question as to whether systemic bisphosphonate treatments can adequately penetrate the femoral head suggesting a local dosing system may yield superior results. In addition, it was speculated that therapeutic augmentation of bone formation using recombinant human bone morphogenetic proteins (rhBMPs) may also reduce the risk of collapse.
In 2011, Vandermeer et al. published a seminal report describing a combination of rhBMP-2 and the bisphosphonate ibandronate being co-delivered locally to the femoral head by percutaneous intraosseous injection [13]. This led to a significant increase in epiphyseal quotient with this intervention compared to saline controls.
We hypothesized that superior infiltration and drug delivery could be achieved in the femoral head using an alternative injectable carrier to saline. Sucrose acetate isobutyrate (SAIB) is a highly viscous, mixed sucrose ester that can be lowered to a working level with the addition of a solvent, such as ethanol [14]. Following implantation, the solvent rapidly disperses leaving a semi-solid depot for slow release of agents, a property that has been utilized for the delivery of anti-psychotics (clinicaltrials.gov NCT015921100) and for local anaesthetics (clinicaltrials.gov NCT01052012, NCT00974350).
In this paper we describe the results of a pilot study for treating avascular necrosis in the pig with rhBMP-2 and bisphosphonate via intra-osseus injection of SAIB. A mixture of the potent third-generation bisphosphonate Zoledronic acid and fluorescently labelled Pamidronate for biodistribution were utilized. The model was examined at an endpoint of eight weeks by a range of measures including X-ray, dual-energy X-ray absorptiometry (DEXA), tissue histology, and fluorescent bisphosphonate tracking.
Materials and methods
Pharmaceuticals
SAIB was purchased from SAFC, a branch of Sigma Aldrich (Missouri, USA). rhBMP-2 was purchased as part as of the INFUSE bone graft kit from Medtronic (Minnesota, USA). Purified ZA was sourced from AXXORA, LLC (San Diego, USA). Pamidronate (Cipla, Bombay, India) was fluorescently labelled with a AlexaFluor 555 labelling kit (Life Technologies, Victoria, Australia) using the manufacturer’s instructions to generate AlexaPAM. While the covalent attachment of dye moiety could compromise the anti-resorptive activity of Pamidronate, the ‘bone hook’ region responsible for mineral avidity is unable to be labelled.
SAIB preparation
SAIB was prepared as an 80:15 stock solution and allowed to spin on a rotary spinner overnight. Prior to surgery, rhBMP-2 was mixed into the SAIB solution in ethanol to yield an 80:20 SAIB/ethanol ratio. This solution was then loaded into a 1 mL syringe prior to surgery.
Surgical procedure
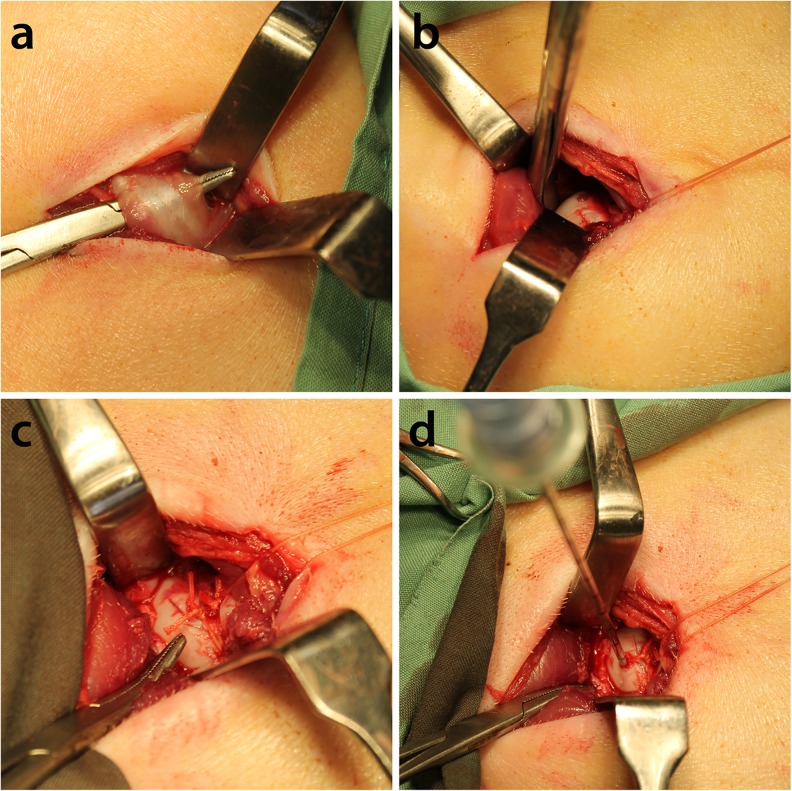
Twelve large white/Landrace cross piglets with weights ranging from 10 kg to 15 kg (six to eight weeks old) were allowed to acclimatize for one week in the vivarium. Animals were fed twice daily, and allowed free access to water but were fasted overnight prior to surgery. Animals were pre-anaesthetized using Zoletil 4.4 mg/kg, Atropine 0.05 mg/kg, and Xylazine 2.2 mg/kg delivered intramuscularly and maintained under general anaesthesia via Isoflurane following intubation. Pigs underwent the operative procedure described by Kim et al. [7]. Briefly, the joint capsule was opened, the ligamentum teres was cut and a suture was tied around the base of the femoral head. An EZ-IO cannula (Vidacare, Texas, USA) was used to deliver the treatments to the femoral head (Fig. 1). Three groups (n = 4/group) received treatment as outlined in Table 1. The first group received saline, the second group SAIB/rhBMP-2 and the third group SAIB/rhBMP-2/bisphosphonate. All animals were randomised into treatment groups.
Fig. 1.
Surgical procedure to induce osteonecrosis of the femoral head. a Division of the short abductor muscle. b The joint capsule is opened, the ligamentum teres cut, and a suture placed around the base of the femoral head c Sutures tied. d EZ-IO cannula is used to deliver bisphosphonates and SAIB containing rhBMP-2 into the femoral head
Table 1.
Experimental design
| Group | N | rhBMP-2 (200 μL) | Local BP (200 μL) |
|---|---|---|---|
| 1. Saline | 4 | SAIB only | Saline only |
| 2. rhBMP-2 | 4 | 200 μg | Saline only |
| 3. rhBMP-2 + local BP | 4 | 200 μg | 250 μg |
At the completion of surgery, animals were given saline and the antibiotic Enrofloxacin 50 mg/kg intravenously. Piglets were allowed to recover on a heat mat. Post-operatively, animals received the analgesic Buprenorphine 0.01 mg/kg two to three times daily. Calcein was delivered via subcutaneous injection at weeks six and seven post surgery. Calcein labelling is a commonly used technique that becomes incorporated into new bone, and is used to identify newly mineralised bone. Pigs were culled at eight weeks. All experiments were carried out with approval from the South Western Area Health Service (SWAHS) Animal Ethics Committee (Protocol number 5086, issued April 2011).
Epiphyseal quotient (EQ) and area analyses
Area calculations were taken using Image J from flatbed scanned images of bisected femoral heads. The value for each sample was an average of three separate measurements of the femoral head, bounded by the growth plate. Epiphyseal quotient was calculated as described by Gong et al. as a ratio of the height of the femoral head from the centre of the growth plate to the length of the growth plate [15]. In a healthy femoral head this ratio is ~0.5, with a value of <0.4 representing significant collapse.
Radiography
Samples were digitally X-rayed (Faxitron X-ray Corp, Illinois, USA) at 25 kV, 1.5× magnification. X-rays were used to image the hard tissues and identify collapse of the femoral head. Soft tissues of the joint were imaged using dual-energy absorptiometry (DXA, Piximus, Wisconsin, USA) to identify any instances of heterotrophic ossification (HO).
Confocal imaging
Imaging was conducted on a Leica SP5 confocal microscope (Leica Microsystems, Wetzlar, Germany) using the 488 nm, and 633 nm laser lines and with 500–560 nm, and 640–720 nm emission bands, respectively. Bone surfaces were scanned with a 5× objective using the Tile Scan (300–400 images)and Z stack (four to five slices at 150 μm intervals) functions, and images were processed with Leica Application Suite Advanced Fluorescence software (Version 2.5.1-6757) and Adobe Photoshop.
Results
Ease of percutaneous injection of SAIB into the femoral head
Following cannulation, manual infusion of SAIB-containing agents was a rapid and simple procedure (Fig. 1). Injection of the SAIB:solvent solution did not require excessive pressure to infuse the head. After removal of the cannula, no leakage of SAIB into the operative space was observed. In contrast, saline was poorly contained after removal of the cannula.
One pig from the control group was culled early due to excessive limping. All of the SAIB infused animals survived without complications during monitoring for the eight-week duration of the study.
Prevention of femoral head collapse with SAIB/rhBMP-2/bisphosphonate
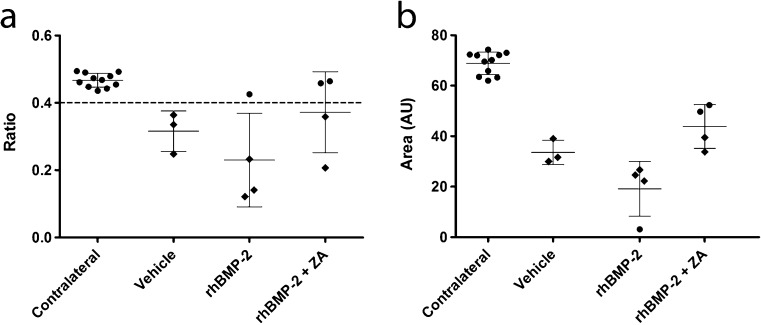
Based on calculations of EQ, the three surviving saline treated animals all showed an EQ < 0.4 in the operative side indicating collapse at eight weeks after surgical intervention. In contrast, the contralateral hips of all groups retained their sphericity (Fig. 2).
Fig. 2.
Quantification of femoral head collapse in the porcine model of Perthes disease, with and without interventions. Epiphyseal quotient (a) and femoral head area (b) as determined from bisected samples of either non-operated or operated femoral heads. Circles represent un-collapsed samples; diamonds represent collapsed samples
In the rhBMP-2 intervention group, one hip showed an apparent prevention of collapse with an EQ value of 0.42. However, subsequent analysis showed that this corresponded to the hip with the lowest 2D cross-sectional area and that this hip showed histological evidence of significant collapse. In comparison, two pigs from the SAIB/rhBMP-2/bisphosphonate treatment group showed a lack of collapse both histologically and by EQ > 0.4. This group demonstrated a higher mean EQ and femoral head area than the saline and rhBMP-2 treated groups.
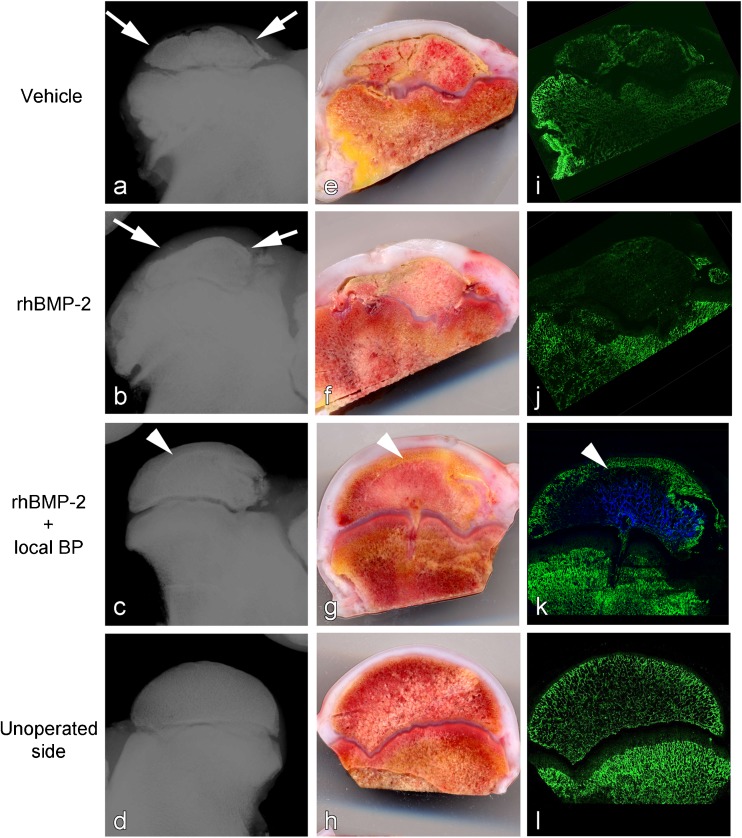
X-rays of the operated femoral heads showed clear radiological evidence of collapse and deformity, and this was reduced with SAIB/rhBMP-2/bisphosphonate treatment (Fig. 3a–d). An acute effect of bisphosphonate treatment is a horizontal line of radiodense sclerotic bone at the metaphysis, termed bisphosphonate lines, and these were seen in ZA/AlexaPAM-treated specimens. Flatbed scans of the bisected femoral heads revealed thickened articular cartilage, particularly in the saline and SAIB/rhBMP-2 groups (Fig. 3e–h). These groups were also the most irregular with respect to shape of the femoral head and growth plate. The SAIB/rhBMP-2/bisphosphonate treated hips were the most similar to the non-operated side.
Fig. 3.
Visualisation of femoral head collapse in a surgical model of Perthes disease in the pig. Samples from each group are shown as X-ray (a–d), flat bed scan (e–h), and confocal images (i–l). In the confocal images: Green, calcein; blue, local BP. Arrows indicate collapsed head, arrowhead indicates bisphosphonate line
Fluorescent tags for new bone growth and local bisphosphonate delivery
Fluorescent labelling of new bone formation using Calcein and the distribution of bisphosphonate in the femoral head were examined by confocal microscopy (Fig. 3i–l).
Bisphosphonate was only observed in the SAIB/rhBMP-2/bisphosphonate group, and a robust signal was observed in the hip at eight weeks after injection indicating long-term retention in the hip. Systemic Calcein dosing demarking bone formed between six and eight weeks after operative insult showed a limited signal the saline and rhBMP-2 treated groups; this indicated minimal new bone formation. In contrast, the SAIB/rhBMP-2/bisphosphonate group revealed a strong Calcein signal peripheral to the bisphosphonate line indicating robust new bone formation.
SAIB/rhBMP-2 induces limited heterotrophic ossification in the joint capsule
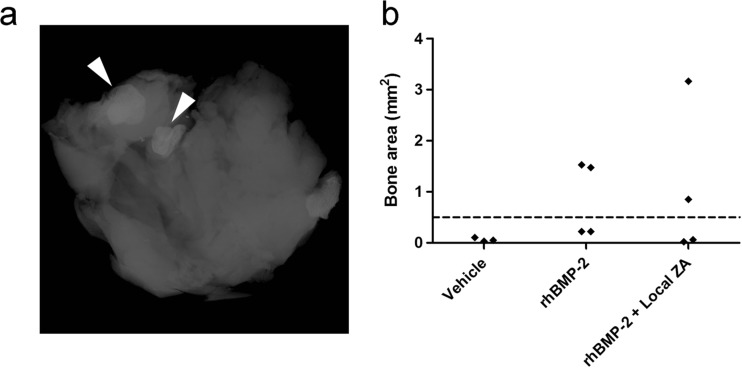
HO was observed in the soft tissues of the joint in a number of specimens treated with rhBMP-2. The bone area of the soft tissues was quantified by DEXA and a bimodal distribution was noted corresponding with the presence of heterotopic bone by X-ray (Fig. 4). HO was absent in 50 % of animals receiving SAIB/rhBMP-2, suggesting that further optimization of this procedure may be able to minimize this potential complication.
Fig. 4.
Quantification of heterotrophic ossification in the joint capsule in treated hips. a X-ray example of bone formation in the soft tissue of the joint. Arrowheads highlight bone nodules. b Quantification of bone area in the soft tissue of the joint
Discussion
In this study, we were able to replicate a surgical model of avascular necrosis of the femoral head in the pig [16] and demonstrate that the local delivery of rhBMP-2 and bisphosphonates in the sugar-based carrier SAIB was able to prevent collapse in half of the cases. Clinically, the severity and prognosis of Perthes disease correlates with the sphericity of the femoral head [17]. A femoral head with an EQ ≤ 0.4 commonly progresses to osteoarthritis [18], and treatments therefore, aim to maintain the sphericity.
Injectable solutions have been previously investigated for the treatment of ONFH, where an injectable calcium-based ceramic combined with core decompression showed promising results clinically [19]. The injectable carrier SAIB has been previously employed to delay rhBMP-2 release from a collagen-chondroitin scaffold by infusing it into the scaffold similar to our injection into the femoral head [20]. More recently, we have proposed direct injection of SAIB containing rhBMP-2 and rhBMP-2/bisphosphonate as a novel system for bone tissue engineering [21]. This system was selected based on its capacity to phase transition allowing it to both be injected and infuse the femoral head, but minimize its leakage into the joint capsule. While some heterotopic bone was observed with the SAIB/rhBMP-2 groups, we predict that this would be a more substantive issue if rhBMP-2 was delivered via saline and was poorly contained within the femoral head as previously reported by Vandermeer et al. who injected rhBMP-2 and ibandronate in saline [13]. HO is a major concern with the use of rhBMP-2, especially in joint spaces, as they can decrease the flexibility and structural properties of the soft tissues surrounding the joint. It is possible that further optimization of the SAIB system and/or injection technique may reduce or eliminate this potential adverse event.
We demonstrated that labelled Pamidronate injected in SAIB was retained within the femoral head out to eight weeks at the experimental end point. In a comparable pig model, bisphosphonate distribution was examined at one week after surgery [22] and showed that 14C tagged ibandronate delivered locally to the femoral head was retained. We hypothesize the release of rhBMP-2 may be more advantageous from a slow release carrier such as SAIB; however, as bisphosphonates can bind the bone of the femoral head it is unclear whether there is a specific advantage to delayed release. Nevertheless, rapid loss of bisphosphonate into the join capsule would likely be less favourable than remaining in the femoral head, thus we speculate that use of a phase-transitioning carrier such as SAIB may be advantageous in this regard.
Treatment of femoral head osteonecrosis typically targets preventing resorption of the dead bone that can result in collapse. In this study, we noted that anabolic intervention with SAIB/rhBMP-2 was unable to restore femoral head sphericity. Treatment with rhBMP-2 has been associated with increased osteoclast activity and bone catabolism [23]. Clinically, rhBMP-2 has been associated with inflammation, which has been associated with adverse events [24]. In a segmental defect model in a rat, high doses of rhBMP-2 were seen to cause a significant inflammatory response with an associated increase in osteoclast-like cells [25]. These data suggest that combined anabolic and anti-resorptive treatments may yield improved outcomes. Our group has previously described a synergistic relationship between rhBMPs and bisphosphonates in other animal models [26–28].
The selected growing pig model reflects the intention of developing a superior intervention for Perthes disease, and the results of this study could also be applicable to traumatically induced or adult onset ONFH. Adult onset ONFH has many causes [29, 30]; however, much like Perthes disease, the femoral head presents with necrosis of the bone that may lead to collapse. In such a setting our system could be used on its own, or perhaps in combination with a surgical procedure such as core decompression.
In conclusion, this study uses an established porcine model of avascular necrosis to examine the novel use of SAIB to deliver rhBMP-2 and/or bisphosphonate. We demonstrate the feasibility of this carrier system and also note that two out of four of the SAIB/rhBMP-2/bisphosphonate treated hips were able to maintain sphericity with an EQ > 0.4. This study demonstrates that with this dosing regimen bisphosphonate is retained in the femoral head eight weeks after a single dose, and that this treatment results in new bone formation.
Acknowledgments
This work received funding support from the Australian Orthopaedic Association (AOA) in the form of a grant. Tegan Cheng was supported by funding from an Australian Postgraduate Award (APA) from the Australian Research Council.
The Leica SP5 in the CLEM Suite at KRI was supported by the following grants: Cancer Institute New South Wales Research Equipment [10/REG/1-23], Australian National Health and Medical Research Council [2009-02759], the Ian Potter Foundation [20100508], the Perpetual Foundation [730], the Ramaciotti Foundation [3037/2010], and the Sydney Medical School Research Infrastructure Major Equipment Scheme.
Conflict of interest
The authors (TLC, AS, DGL) have an IP position relating to the approach used in this study. No commercial funding support was received.
References
- 1.Fabry G. Clinical practice—The hip from birth to adolescence. Eur J Pediatr. 2010;169(2):143–148. doi: 10.1007/s00431-009-1025-x. [DOI] [PubMed] [Google Scholar]
- 2.Costa CR, Johnson AJ, Naziri Q, Mont MA. Review of total hip resurfacing and total hip arthroplasty in young patients who had Legg-Calve-Perthes disease. Orthop Clin N Am. 2011;42(3):419–422. doi: 10.1016/j.ocl.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Little DG, Kim HK. Potential for bisphosphonate treatment in Legg-Calve-Perthes disease. J Pediatr Orthop. 2011;31(2 Suppl):S182–S188. doi: 10.1097/BPO.0b013e318223b541. [DOI] [PubMed] [Google Scholar]
- 4.Young ML, Little DG, Kim HKW. Evidence for using bisphosphonate to treat Legg-Calve-Perthes disease. Clin Orthop Relat Res. 2012;470(9):2462–2475. doi: 10.1007/s11999-011-2240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Little DG, McDonald M, Sharpe IT, Peat R, Williams P, McEvoy T. Zoledronic acid improves femoral head sphericity in a rat model of perthes disease. J Orthop Res. 2005;23(4):862–868. doi: 10.1016/j.orthres.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Little DG, Peat RA, McEvoy A, Williams PR, Smith EJ, Baldock PA. Zoledronic acid treatment results in retention of femoral head structure after traumatic osteonecrosis in young Wistar rats. J Bone Miner Res. 2003;18(11):2016–2022. doi: 10.1359/jbmr.2003.18.11.2016. [DOI] [PubMed] [Google Scholar]
- 7.Kim HKW, Su PH, Qiu YS. Histopathologic changes in growth-plate cartilage following ischemic necrosis of the capital femoral epiphysis—An experimental investigation in immature pigs. J Bone Joint Surg Am. 2001;83A(5):688–697. doi: 10.2106/00004623-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Kim HKW, Su PH. Development of flattening and apparent fragmentation following ischemic necrosis of the capital femoral epiphysis in a piglet model. J Bone Joint Surg Am. 2002;84A(8):1329–1334. doi: 10.2106/00004623-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Kim HK, Randall TS, Bian H, Jenkins J, Garces A, Bauss F. Ibandronate for prevention of femoral head deformity after ischemic necrosis of the capital femoral epiphysis in immature pigs. J Bone Joint Surg Am. 2005;87A(3):550–557. doi: 10.2106/JBJS.D.02192. [DOI] [PubMed] [Google Scholar]
- 10.Ramachandran M, Ward K, Brown RR, Munns CF, Cowell CT, Little DG. Intravenous bisphosphonate therapy for traumatic osteonecrosis of the femoral head in adolescents. J Bone Joint Surg Am. 2007;89A(8):1727–1734. doi: 10.2106/JBJS.F.00964. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen T, Zacharin MR. Pamidronate treatment of steroid associated osteonecrosis in young patients treated for acute lymphoblastic leukaemia—two-year outcomes. J Pediatr Endocrinol Metab. 2006;19(2):161–167. doi: 10.1515/JPEM.2006.19.2.161. [DOI] [PubMed] [Google Scholar]
- 12.Kotecha RS, Powers N, Lee S-J, Murray KJ, Carter T, Cole C. Use of bisphosphonates for the treatment of osteonecrosis as a complication of therapy for childhood acute lymphoblastic leukaemia (ALL) Pediatr Blood Cancer. 2010;54(7):934–940. doi: 10.1002/pbc.22428. [DOI] [PubMed] [Google Scholar]
- 13.Vandermeer JS, Kamiya N, Aya-ay J, Garces A, Browne R, Kim HKW. Local administration of ibandronate and bone morphogenetic protein-2 after ischemic osteonecrosis of the immature femoral head: a combined therapy that stimulates bone formation and decreases femoral head deformity. J Bone Joint Surg Am. 2011;93A(10):905–913. doi: 10.2106/JBJS.J.00716. [DOI] [PubMed] [Google Scholar]
- 14.Lu YX, Yu YL, Tang X. Sucrose acetate isobutyrate as an in situ forming system for sustained risperidone release. J Pharm Sci. 2007;96(12):3252–3262. doi: 10.1002/jps.21091. [DOI] [PubMed] [Google Scholar]
- 15.Gong SY, Kim HW, Park HW, Lee SY, Lee KS. Effects of multiple drilling on the ischemic capital femoral epiphysis of immature piglets. Yonsei Med J. 2011;52(5):809–817. doi: 10.3349/ymj.2011.52.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HKW. Pathophysiology and new strategies for the treatment of Legg-Calvé-Perthes disease. J Bone Joint Surg Am. 2012;94A(7):659–669. doi: 10.2106/JBJS.J.01834. [DOI] [PubMed] [Google Scholar]
- 17.Tsuchida Y, Kim WC, Takahashi KA, Horii M, Mikami Y, Fujioka M, Kusakabe T, Chang K, Hosokawa M, Kubo T. Usefulness of epiphyseal quotient measurement on magnetic resonance images for outcome prediction in patients with early-stage Legg-Calve-Perthes disease. J Pediatr Orthop B. 2005;14(1):16–23. doi: 10.1097/01202412-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Stulberg SD, Cooperman DR, Wallensten R. The natural history of Legg-Calvé-Perthes disease. J Bone Joint Surg Am. 1981;63(7):1095–1108. [PubMed] [Google Scholar]
- 19.Civinini R, De Biase P, Carulli C, Matassi F, Nistri L, Capanna R, Innocenti M. The use of an injectable calcium sulphate/calcium phosphate bioceramic in the treatment of osteonecrosis of the femoral head. Int Orthop. 2012;36(8):1583–1588. doi: 10.1007/s00264-012-1525-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keskin DS, Tezcaner A, Korkusuz P, Korkusuz F, Hasirci V. Collagen-chondroitin sulfate-based PLLA-SAIB-coated rhBMP-2 delivery system for bone repair. Biomaterials. 2005;26(18):4023–4034. doi: 10.1016/j.biomaterials.2004.09.063. [DOI] [PubMed] [Google Scholar]
- 21.Cheng TL, Valtchev P, Murphy CM, Cantrill LC, Dehghani F, Little DG, Schindeler A. A sugar-based phase-transitioning delivery system for bone tissue engineering. Eur Cell Mater. 2013;26:208–221. doi: 10.22203/ecm.v026a15. [DOI] [PubMed] [Google Scholar]
- 22.Aya-ay J, Athavale S, Morgan-Bagley S, Bian H, Bauss F, Kim HKW. Retention, distribution, and effects of intraosseously administered ibandronate in the infarcted femoral head. J Bone Miner Res. 2007;22(4):642–642. doi: 10.1359/jbmr.060817e. [DOI] [PubMed] [Google Scholar]
- 23.Kaneko H, Arakawa T, Mano H, Kaneda T, Ogasawara A, Nakagawa M, Toyama Y, Yabe Y, Kumegawa M, Hakeda Y. Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone. 2000;27(4):479–486. doi: 10.1016/S8756-3282(00)00358-6. [DOI] [PubMed] [Google Scholar]
- 24.Smucker JD, Rhee JM, Singh K, Yoon ST, Heller JG. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine. 2006;31(24):2813–2819. doi: 10.1097/01.brs.0000245863.52371.c2. [DOI] [PubMed] [Google Scholar]
- 25.Zara JN, Siu RK, Zhang X, Shen J, Ngo R, Lee M, Li W, Chiang M, Chung J, Kwak J, Wu BM, Ting K, Soo C. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng A. 2011;17(9–10):1389–1399. doi: 10.1089/ten.tea.2010.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Little DG, McDonald M, Bransford R, Godfrey CB, Amanat N. Manipulation of the anabolic and catabolic responses with OP-1 and zoledronic acid in a rat critical defect model. J Bone Miner Res. 2005;20(11):2044–2052. doi: 10.1359/JBMR.050712. [DOI] [PubMed] [Google Scholar]
- 27.Yu NYC, Schindeler A, Peacock L, Mikulec K, Fitzpatrick J, Ruys AJ, Cooper-White JJ, Little DG. Modulation of anabolic and catabolic responses via a porous polymer scaffold manufactured using thermally induced phase separation. Eur Cells Mater. 2013;25:190–203. doi: 10.22203/ecm.v025a14. [DOI] [PubMed] [Google Scholar]
- 28.Schindeler A, Birke O, Yu NYC, Morse A, Ruys A, Baldock PA, Little DG. Distal tibial fracture repair in a neurofibromatosis type 1-deficient mouse treated with recombinant bone morphogenetic protein and a bisphosphonate. J Bone Joint Surg Br. 2011;93B(8):1134–1139. doi: 10.1302/0301-620X.93B8.25940. [DOI] [PubMed] [Google Scholar]
- 29.Kang JS, Moon KH, Kwon DG, Shin BK, Woo MS. The natural history of asymptomatic osteonecrosis of the femoral head. Int Orthop. 2013;37(3):379–384. doi: 10.1007/s00264-013-1775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim HS, Bae SC, Kim TH, Kim SY. Endothelial nitric oxide synthase gene polymorphisms and the risk of osteonecrosis of the femoral head in systemic lupus erythematosus. Int Orthop. 2013;37(11):2289–2296. doi: 10.1007/s00264-013-1966-6. [DOI] [PMC free article] [PubMed] [Google Scholar]