Abstract
Purpose
Low-intensity pulsed ultrasound (LIPUS) has been used successfully to accelerate healing of fresh fractures and non-unions. It also improved callus maturation with distraction osteogenesis in animal trials. However, only few clinical studies are available to support its widespread use for the latter indication in humans.
Methods
Twenty-one patients undergoing callus distraction for posttraumatic tibial defects were randomized into two groups: the trial group (12 men; mean age 32 years) which received 20 minutes LIPUS daily during treatment and the control group (six men and three women; mean age 29 years) without LIPUS treatment. The Ilizarov ring fixator was used in all cases. Results were examined clinically and radiologically, analysing callus maturation with a computer-assisted measurement.
Results
Patients in the LIPUS group needed a mean of 33 days to consolidate every 1 cm of new bone in comparison to 45 days in the control group. The healing index was therefore shortened by 12 days/cm in the LIPUS group. This means that callus maturation was 27 % faster in the LIPUS group. The fixator time was shortened by 95 days in the LIPUS group. The overall daily increase in radiographic callus density was 33 % more in the LIPUS group than in the control group.
Conclusions
LIPUS treatment is an effective non-invasive adjuvant method to enhance callus maturation in distraction osteogenesis. With the help of this treatment, the healing time and the duration of external fixation can be reliably shortened.
Keywords: Low-intensity pulsed ultrasound, Ilizarov, Callus distraction, Bone defects
Introduction
Callus distraction was introduced by Codivilla in 1904 through traction on a calcaneal pin [1]. His idea was grasped and evolved in Italy 14 years later by Putti [2]. Ilizarov [3] developed and used his extremely versatile circular fixator since the early 1950s. Over his 40 years of work in Kurgan’s Russian Scientific Center, he expanded the indications for his technique and the method of transosseous osteosynthesis it harnesses from acute fracture care to obscure cases such as Ollier’s disease and thromboangitis obliterans with bone transport being the most frequent application [4].
One of the main problems associated with bone distraction is the long duration of treatment in a cumbersome external fixation frame with subsequent socioeconomic and psychological drawbacks as well as higher risk of complications like pin tract infections and soft tissue contractures [5, 6]. Shortening the treatment time, particularly the maturation period, would therefore reduce the costs, complications and burden on the patient. However, neither direct electric stimulation [7] nor the application of electromagnetic fields [8] have proved themselves as adjuvant treatment in the clinical setting.
The use of low-intensity pulsed ultrasound (LIPUS) offers an alternative to assist callus maturation. It has been theorized that the micromechanical strains produced by its pressure waves in biological tissues may result in biochemical events that regulate fracture healing [9]. Several studies showed the benefits of LIPUS stimulation in enhancing bone healing after fresh fractures [10–16] as well as in cases with delayed or non-union [17–22] with accelerated fracture healing by 24 %–42 % and a good success rate in treating delayed and non-union of humeral (67 %), femoral (82 %), tibial (87 %) and forearm fractures (90 %) [23]. Furthermore, a number of animal studies demonstrated significant benefits for its use as an adjuvant during distraction osteogenesis [24–29]. However, its clinical application in humans to enhance callus maturation has been not sufficiently studied. The aim of this work was to study the effect of LIPUS on bone maturation after tibial callus distraction regarding the healing index, fixation time and radiographic bone density in the clinical setting.
Materials and methods
In a randomized controlled trial (RCT) performed between January 2000 and January 2006, 21 skeletally mature patients (older than 18 years) undergoing callus distraction (average distraction distance of 79 mm) after comminuted tibial fractures were assigned to either of two groups: the trial (LIPUS) group included patients whose callus distraction was supported with LIPUS stimulation and the control group where patients were not subjected to LIPUS during distraction. The trial group composed of 12 male patients with a mean age of 32 years, a mean body weight of 77 kg and a mean height of 179 cm. Seven patients were alcoholics and six were smokers. The control group consisted of nine patients (six men and three women) with a mean age of 29 years, mean body weight was 82 kg and the mean height was 177 cm. There were four patients with alcohol problems and four were smokers. Exclusion criteria were pregnancy, lack of compliance, discontinuation or change of therapy, re-operation and amputation.
In all 21 cases, Ilizarov ring fixators were used. The technique of bone cutting was a low-energy corticotomy using osteotome cuts in between drill bit holes (rarely using a Gigli saw) with the preassembled fixator mounted on the leg while considering the important anatomical structures and the location of both safe and hazardous corridors for wire/pin placement. The dorsal cortex is broken in external torsion of the distal rings in relation to the proximal ring as to avoid traction injury of the peroneal nerve. Ten days after corticotomy, callus distraction was started with a rate of 1 mm per day (¼ turn every six hours). All patients in the trial group received LIPUS treatment using a Sonic Accelerated Fracture Healing System—SAFHS device (Exogen Inc., Piscataway, NJ, USA and Exogen Europe GmbH, Dießen a.A.) according to the values of Duarte [30]:
Frequence: 1.5 MHz
Form: pulsed
Impulse length: 200 μsec
Signal repetition frequency: 1 kHz
Intensity: 30 mW/cm2
The device was used once a day for 20 minutes without interruption. The exact site of ultrasound (US) stimulation was determined and marked under fluoroscopy. To check for patient’s compliance in using LIPUS, the US device was supplied by a memory chip that makes the regular application of LIPUS at the end of treatment controllable.
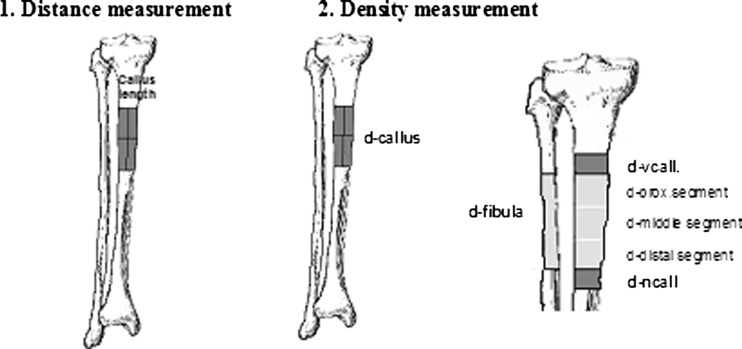
Clinical follow-up was done every two weeks and control radiographs were ordered every four weeks, the first of which was done immediately postoperatively to check the completeness of the corticotomy and the pin/wire locations. All radiographs were done using a Siemens 150/30/50 C-100 tube and then scanned using Lumiscan 75 (Lumisys. Inc., Sunnyvale CA) with the digital science software from Kodak. The images were then transferred to a standard Pentium III PC 256 MB RAM supplemented with the evaluation program Applicare Medical Imaging B.V. (RADWorks Review 2.1). Using this program, it is possible to measure distances and radiologic density. To avoid misinterpretations from different X-ray penetrations, the fibula was taken as a reference to neutralize the penetration factor. A calibration scale (ruler) was added to eliminate the magnification factor from differences in the distance between the extremity and the X-ray tube. In every film, the corticotomy site and the new callus were defined and referred to as the d-Callus. The whole callus surface was divided into three equal parts “d-prox., d-middle and d-distal” (Fig. 1). The bone density in the neighbouring fibula was then measured followed by the density of the tibia immediately proximal and distal to the callus (d-vcall und d-ncall). The distance of callus distraction was called “callus length”. Its measurement took in consideration the correction factor from the added calibration. This was then correlated to the duration of distraction as mm/day. All distance values were given in the software program in units, which were then transferred to real values using a specific formula.
Fig. 1.
Length and density measurement in the follow-up radiographs
The healing time was defined as the time between frame application and removal. For a differentiated analysis, the whole process of callus distraction was divided into three phases: the latent phase, the distraction phase and the consolidation (maturation) phase. The end of treatment was defined in both groups as fixator removal.
Results
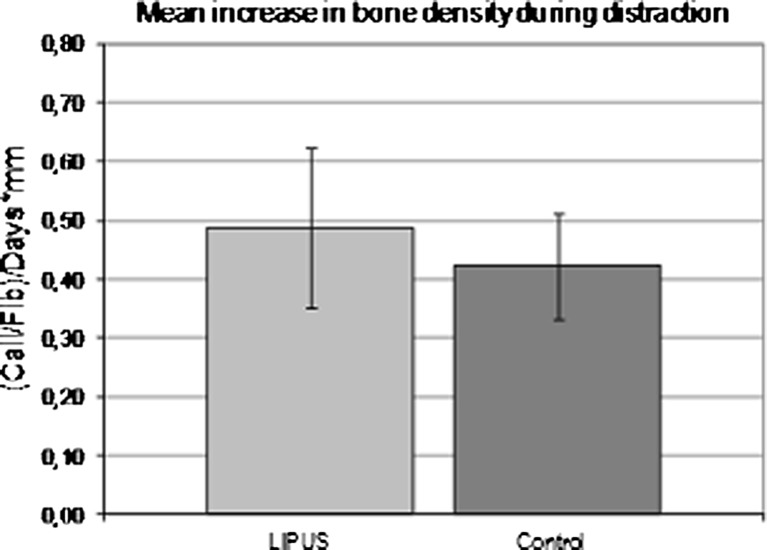
The distraction phase began after a ten-day-latent period. Callus distraction of 1-mm per day was done in both groups with additional 20 minutes of US treatment per day in the LIPUS group. The daily increase in bone density was measured by means of the above-mentioned program and corresponded to the average value, a measure of the average density in a radiograph. Only the factor callus/fibula was used to describe the increase in density. In the distraction phase, the mean daily increase in radiologic bone density was 0.49 ± 0.14 in the LIPUS group in comparison to 0.42 ± 0.09 in the control group, which meant an improvement by 14 % in the LIPUS group (Fig. 2).
Fig. 2.
Differences in bone density in the trial and control groups during distraction
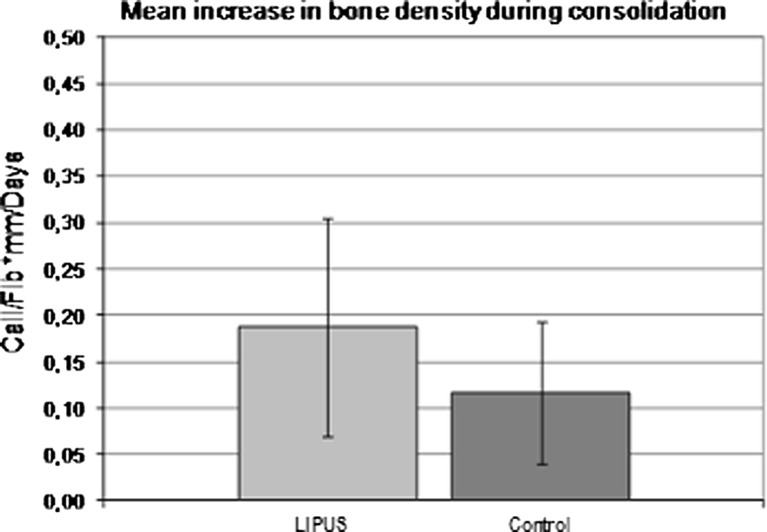
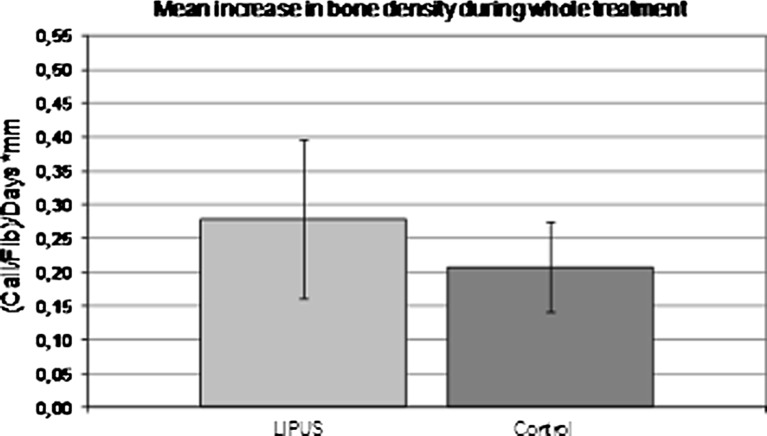
The consolidation or healing phase started after the end of distraction till removal of the fixator. During this phase, the daily 20-minute-LIPUS treatment was continued in the trial group. The increase in bone density in the consolidation phase was measured similar to the distraction phase. The difference in its daily increase between both groups was minimal: 0.27 % ± 0.002 in the LIPUS group and 0.25 % ± 0.001 in the control group. A length corrected increase in the bone density per day (because of the different distraction lengths) was therefore considered. In the LIPUS group, there was a daily increase of bone density of 0.19 ± 0.12 in comparison to a 0.12 ± 0.08 in the control group (Fig. 3). This amounts to an increase of 58 %. The collective increase in radiographic bone density during both phases showed a mean daily increase of 0.28 ±0.12 in the LIPUS group in comparison to 0.21 ± 0.07 in the control group. Accordingly, the daily increase of bone density was 33 % more in the LIPUS group (Fig. 4).
Fig. 3.
Differences in bone density in the trial and control groups during consolidation
Fig. 4.
Differences in bone density in the trial and control groups during the whole treatment
As regards the healing time, patients in the LIPUS group needed a mean of 33 days to consolidate every 1 cm of new bone formation. On the other hand, patients in the control group needed a mean of 45 days to achieve the same goal. The healing index was therefore shortened by 12 days/cm (27 %) in the LIPUS group (Fig. 5). With an average distraction distance of 79 mm, the healing time for patients in the LIPUS group was reduced by approximately 95 days.
Fig. 5.
The healing time
Discussion
LIPUS is a special type of acoustic pulsed energy that is increasingly used as an adjuvant therapy to enhance bone and wound healing [31]. Its energy is absorbed at a rate proportional to the density of the tissues in which it passes through [9]. Although US at high intensities causes thermal damage, lower doses were shown to stimulate bone healing with minimal heating effects [9, 32, 33]. This was first reported by Maintz in 1950 after radius osteotomy in rabbits [34]. Corradi and Cozzolino were the first to document that US treatment led to periosteal callus formation [35]. Duarte first used LIPUS to stimulate bone healing in osteotomized rabbit fibulae [30]. In 1983, Xavier and Duarte successfully applied LIPUS to trigger healing of human fractures [36]. Subsequently, the effect of LIPUS on bone healing was clinically established in the 1990s for fresh fractures as well as delayed and non-unions [9, 37], so that it gained Food and Drug Administration (FDA) approval for fresh fractures in 1994 and for established non-unions in 2000 [38].
A number of prospective randomised controlled studies proved an accelerated fracture healing using LIPUS treatment. Heckman et al. demonstrated a 38 % reduction in the healing time of tibial fractures using LIPUS [10]. Similarly, Kristiansen et al. found a 30 % acceleration in fractures of the distal radius [11]. Comparable results were reported by Mayr et al. for scaphoid fractures [12]. In their meta-analysis of these three studies with a total of 158 fractures, Busse et al. found that LIPUS shortens the healing time by 64 days in conservatively treated fractures [13]. Similar positive effects were observed by Cook et al. [14] in tibial and distal radius fractures and by Leung et al. [15] in complex tibial fractures. Warden et al. concluded that from animal and human studies, the use of LIPUS could accelerate the rate of fracture repair by a factor of 1.4–1.6 in animals and reduce the healing time by 38 % in humans [16]. Mayr et al. showed an overall success rate of 91 % for delayed unions and 86 % in cases with non-union using LIPUS treatment [17]. Further studies supported LIPUS treatment of delayed and non-unions [18–23]. Most recently, Urita et al. showed that LIPUS shortens the time to cortical union by 27 % and to endosteal union by 18 % after forearm (ulnar or radial) bone shortening osteotomies [39].
The promising results of LIPUS treatment in fracture healing consequently led many investigators to study its effect on callus maturation during distraction osteogenesis using different animal models [24–29]. Three research groups investigated the effect of LIPUS application in rabbit tibiae [24–26]. Shimazaki et al. [24] and Sakurakichi et al. [25] found larger callus formation, higher bone mineral density (BMD) and a correspondingly higher biomechanical stiffness and strength in the LIPUS group. Tis et al. found LIPUS to increase the size of the distraction callus and improve its composition with less fibrous tissue but without a positive effect on its density or mechanical parameters [26]. Mayr et al. [27] and Claes et al. [28] used LIPUS in the maturation phase after callus distraction in sheep metatarsals. The LIPUS group showed more BMD [27], a significantly higher axial compression and indentation stiffness and significantly more callus formation [28] than the control group. Eberson et al. [29] showed better mechanical callus properties (20 % stiffer and 30 % stronger) with LIPUS application in femoral lengthening in rats.
Only few human trials studied LIPUS effects on distraction osteogenesis. El-Mowafi and Mohsen [40] noted a significant reduction (p = 0.001) of the healing index in tibial distraction (5–8 cm) using Ilizarov fixators by 18 days/cm (30 %) in LIPUS-treated patients (30 days) in comparison to controls (48 days). Tsumaki et al. [41] applied LIPUS in bilateral one-stage opening wedge high tibial osteotomy to treat varus osteoarthritis in elderly patients (mean age 68 years) with significant increase in the mean BMD of the distraction callus (p = 0.02) but no significant change in the consolidation period or the duration of external fixation. Chan et al. reported accelerated bone remodelling and harder callus volume with significantly increased BMD, bone mineral content (BMC), and bone strength index in the LIPUS group [42]. In a recent prospective randomized controlled trial, Dudda et al. reported shortening the external fixation time to 43.6 days with a mean distraction/consolidation index of 32.8 days/cm in the US group compared to 44.6 days/cm for the control group [43]. Furthermore, Gebauer and Correll [44] reported solid healing through LIPUS treatment of all 17 cases of delayed unions (three to seven months) and non-unions (more than eight months) after limb lengthening in children without additional operations. This was also reported by Esenwein et al. for delayed callus maturation in adults [45].
The exact mechanism of action of LIPUS is complex. In vitro cellular evaluation and in vivo animal trials revealed that US induces a variety of non-thermal effects [9, 33, 46] that promote proliferation of human and animal cells with increased protein and DNA synthesis, membrane permeability and calcium influx [47]. Zhou et al. attributed US-induced cell proliferation to the activation of integrin receptors [31]. Kumagai et al. described the homing of circulating osteogenic progenitors to fracture sites as a possible mechanism of US contribution to new bone formation [48]. The enhanced angiogenesis, increased synthesis of fibroblast, vascular endothelial and platelet-derived growth factors and prostaglandin E2 and the activation of nitric oxide pathways and aggrecan messenger RNA expression have also been proposed as potential mechanisms of stimulating bone healing [37, 46–50].
The timing of LIPUS application in distraction osteogenesis is still controversial. Most authors [24, 26–28, 40–42] studied the influence of LIPUS treatment on the maturation (consolidation) phase because this makes up the longest part of the treatment time and thus the benefit of stimulation would be at greatest. Chan et al. [42] found that the effective period of LIPUS treatment was at the initial stage of consolidation (without comparing this to the distraction phase). These findings were deferred by Sakurakichi et al. [25] in Japanese white rabbits. They found greater BMD and mechanical strength when LIPUS was performed during the lengthening phase as compared to the latent and the maturation phase. In our study, we applied LIPUS in both the distraction and the maturation phase in order to achieve a maximal benefit, that is why we are unable to make a recommendation as regards the best timing of LIPUS treatment. However, the increase in bone density was greater in the consolidation than in the distraction phase.
Our study is limited by its small number of patients. However, the results we obtained and the extensive literature we reviewed support the clinical use of LIPUS to enhance callus maturation, reduce the healing time and shorten the period of external fixation in distraction osteogenesis. This may prevent delayed union or the development of pseudarthrosis as well as other fixator-related complications like pin tract infection and joint contractures in such lengthy and complex procedures.
Footnotes
Investigation performed at the Department of Trauma, Hand, Plastic and Reconstructive Surgery, Ulm University, Germany.
References
- 1.Codivilla A. On the means of lengthening, in the lower limbs, the muscles and tissues which are shortened through deformity. 1904 [classical article] Clin Orthop Relat Res. 1994;301:4–9. [PubMed] [Google Scholar]
- 2.Putti V. The operative lengthening of the femur. 1921 [classical article] Clin Orthop Relat Res. 1990;250:4–7. [PubMed] [Google Scholar]
- 3.Ilizarov GA. Transosseous osteosynthesis: theoretical and clinical aspects of the regeneration and growth of tissue. New York: Springer-Verlag; 1992. [Google Scholar]
- 4.Maiocchi AB, Aronson J (1991) Operative principles of Ilizarov. Medi Surgical Video. Milan, Italy
- 5.Paley D. Problems, obstacles, and complications of limb lengthening by the Ilizarov technique. Clin Orthop Relat Res. 1990;250:81–104. [PubMed] [Google Scholar]
- 6.Faber FW, Keessen W, van Roermund PM. Complications of leg lengthening. 46 procedures in 28 patients. Acta Orthop Scand. 1991;62(4):327–332. doi: 10.3109/17453679108994463. [DOI] [PubMed] [Google Scholar]
- 7.Hamanashi C, Kawabata T, Yoshii T, Tanaka S. Bone mineral density changes in distracted callus stimulated by pulsed direct electrical current. Clin Orthop Relat Res. 1995;312:247–252. [PubMed] [Google Scholar]
- 8.Taylor KF, Inoue N, Rafiee B, Tis JE, McHale KA, Chao EY. Effect of pulsed electromagnetic fields on maturation of regenerate bone in a rabbit limb lengthening model. J Orthop Res. 2006;24(1):2–10. doi: 10.1002/jor.20014. [DOI] [PubMed] [Google Scholar]
- 9.Claes L, Willie B. The enhancement of bone regeneration by ultrasound. Prog Biophys Mol Biol. 2007;93(1–3):384–398. doi: 10.1016/j.pbiomolbio.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Heckman JD, Ryaby JP, McCabe J, Frey JJ, Kilcoyne RF. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg Am. 1994;76-A:26–34. doi: 10.2106/00004623-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Kristiansen TK, Ryaby JP, McCabe J, Frey JJ, Roe LR. Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound. J Bone Joint Surg Am. 1997;79-A:961–973. doi: 10.2106/00004623-199707000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Mayr E, Rudzki MM, Rudzki M, Borchardt B, Häusser H, Rüter A. Does low intensity, pulsed ultrasound speed healing of scaphoid fractures? Handchir Mikrochir Plast Chir. 2000;32(2):115–122. doi: 10.1055/s-2000-19253. [DOI] [PubMed] [Google Scholar]
- 13.Busse JW, Bhandari M, Kulkarni AV, Tunks E. The effect of low-intensity pulsed ultrasound therapy on time to fracture healing: a meta-analysis. CMAJ. 2002;166:437–441. [PMC free article] [PubMed] [Google Scholar]
- 14.Cook SD, Ryaby JP, McCabe J, Frey JJ, Heckman JD, Kristiansen TK. Acceleration of tibia and distal radius fracture healing in patients who smoke. Clin Orthop Relat Res. 1997;337:198–207. doi: 10.1097/00003086-199704000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Leung KS, Lee WS, Tsui HF, Liu PP, Cheung WH. Complex tibial fracture outcomes following treatment with low-intensity pulsed ultrasound. Ultrasound Med Biol. 2004;30:389–395. doi: 10.1016/j.ultrasmedbio.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Warden SJ, Bennell KL, McMeeken JM, Wark JD. Acceleration of fresh fracture repair using the Sonic Accelerated Fracture Healing System (SAFHS): a review. Calcif Tissue Int. 2000;66:157–163. doi: 10.1007/s002230010031. [DOI] [PubMed] [Google Scholar]
- 17.Mayr E, Frankle V, Rüter A. Ultrasound–an alternative healing method for nonunions? Arch Ortop Trauma Surg. 2000;120:1–8. doi: 10.1007/pl00021234. [DOI] [PubMed] [Google Scholar]
- 18.Nolte PA, Van der Krans A, Patka P, Janssen IM, Ryaby JP, Albers GH. Low-intensity pulsed ultrasound in the treatment of nonunions. J Trauma. 2001;51:693–703. doi: 10.1097/00005373-200110000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Lerner A, Stein H, Soudry M. Compound high energy limb fractures with delayed union: our experience with adjuvant ultrasound stimulation (Exogen) Ultrasonics. 2004;42:915–917. doi: 10.1016/j.ultras.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Rutten S, Nolte PA, Guit GL, Bouman DE, Albers GH. Use of low intensity pulsed ultrasound for posttraumatic nonunions of the tibia: a review of patients treated in the Netherlands. J Trauma. 2007;62:902–908. doi: 10.1097/01.ta.0000238663.33796.fb. [DOI] [PubMed] [Google Scholar]
- 21.Jingushi S, Mizuno K, Matsushita T, Itoman M. Low intensity pulsed ultrasound treatment for postoperative delayed union or nonunion of long bone fractures. J Orthop Sci. 2007;12:35–41. doi: 10.1007/s00776-006-1080-3. [DOI] [PubMed] [Google Scholar]
- 22.Schofer MD, Block JE, Aigner J, Schmelz A. Improved healing response in delayed unions of the tibia with low-intensity pulsed ultrasound: results of a randomized sham-controlled trial. BMC Musculoskelet Disord. 2010;11:229. doi: 10.1186/1471-2474-11-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe Y, Matsushita T, Bhandari M, Zdero R, Schemitsch EH. Ultrasound for fracture healing: current evidence. J Orthop Trauma. 2010;24(Suppl 1):S56–S61. doi: 10.1097/BOT.0b013e3181d2efaf. [DOI] [PubMed] [Google Scholar]
- 24.Shimazaki A, Inui K, Azuma Y, Nishimura N, Yamano Y. Low-intensity pulsed ultrasound accelerates bone maturation in distraction osteogenesis in rabbits. J Bone Joint Surg Br. 2000;82-B:1077–1082. doi: 10.1302/0301-620X.82B7.9948. [DOI] [PubMed] [Google Scholar]
- 25.Sakurakichi K, Tsuchiya H, Uehara K, Yamashiro T, Tomita K, Azuma Y. Effects of timing of low-intensity pulsed ultrasound on distraction osteogenesis. J Orthop Res. 2004;22:395–403. doi: 10.1016/S0736-0266(03)00181-5. [DOI] [PubMed] [Google Scholar]
- 26.Tis JE, Meffert CR, Inoue N, McCarthy EF, Machen MS, McHale KA, Chao EY. The effect of low intensity pulsed ultrasound applied to rabbit tibiae during the consolidation phase of distraction osteogenesis. J Orthop Res. 2002;20:793–800. doi: 10.1016/S0736-0266(02)00003-7. [DOI] [PubMed] [Google Scholar]
- 27.Mayr E, Laule A, Suger G, Rüter A, Claes L. Radiographic results of callus distraction aided by pulsed low-intensity ultrasound. J Orthop Trauma. 2001;15:407–414. doi: 10.1097/00005131-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Claes L, Rüter A, Mayr E. Low-intensity ultrasound enhances maturation of callus after segmental transport. Clin Orthop Relat Res. 2005;430:189–194. doi: 10.1097/01.blo.0000150456.39608.bc. [DOI] [PubMed] [Google Scholar]
- 29.Eberson CP, Hogan KA, Moore DC, Ehrlich MG. Effect of low-intensity ultrasound stimulation on consolidation of the regenerate zone in a rat model of distraction osteogenesis. J Pediatr Orthop. 2003;23(1):46–51. [PubMed] [Google Scholar]
- 30.Duarte LR. The stimulation of bone growth by ultrasound. Arch Orthop Trauma Surg. 1983;101:153–159. doi: 10.1007/BF00436764. [DOI] [PubMed] [Google Scholar]
- 31.Zhou S, Schmelz A, Seufferlein T, Li Y, Zhao J, Bachem MG. Molecular mechanisms of low intensity pulsed ultrasound in human skin fibroblasts. J Biol Chem. 2004;279(52):54463–54469. doi: 10.1074/jbc.M404786200. [DOI] [PubMed] [Google Scholar]
- 32.Reher P, Elbeshir el-NI, Harvey W, Meghji S, Harris M. The stimulation of bone formation in vitro by therapeutic ultrasound. Ultrasound Med Biol. 1997;23(8):1251–1258. doi: 10.1016/S0301-5629(97)00031-8. [DOI] [PubMed] [Google Scholar]
- 33.Chang WH, Sun JS, Chang SP, Lin JC. Study of thermal effects of ultrasound stimulation on fracture healing. Bioelectromagnetics. 2002;23(4):256–263. doi: 10.1002/bem.10009. [DOI] [PubMed] [Google Scholar]
- 34.Maintz G. Tierexperimentelle Untersuchungen über die Wirkung der Ultraschallwellen auf die Knochenregeneration. Strahlentherapie. 1950;82:631–638. [PubMed] [Google Scholar]
- 35.Corradi C, Cozzolino A. Effect of ultrasonics on the development of osseous callus in fractures. Arch Ortop. 1953;66(1):77–98. [PubMed] [Google Scholar]
- 36.Xavier CAM, Duarte LR. Estimulaca ultra-sonica de calo osseo: Applicaca clinica. Rev Bras Ortop. 1983;18:73–80. [Google Scholar]
- 37.Bashardoust Tajali S, Houghton P, MacDermid JC, Grewal R. Effects of low-intensity pulsed ultrasound therapy on fracture healing: a systematic review and meta-analysis. Am J Phys Med Rehabil. 2012;91(4):349–367. doi: 10.1097/PHM.0b013e31822419ba. [DOI] [PubMed] [Google Scholar]
- 38.Busse JW, Kaur J, Mollon B, Bhandari M, Tornetta P, 3rd, Schünemann HJ, Guyatt GH. Low intensity pulsed ultrasonography for fractures: systematic review of randomised controlled trials. BMJ. 2009;338:b351. doi: 10.1136/bmj.b351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Urita A, Iwasaki N, Kondo M, Nishio Y, Kamishima T, Minami A. Effect of low-intensity pulsed ultrasound on bone healing at osteotomy sites after forearm bone shortening. J Hand Surg [Am] 2013;38(3):498–503. doi: 10.1016/j.jhsa.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 40.El-Mowafi H, Mohsen M. The effect of low intensity pulsed ultrasound on callus maturation in tibial distraction osteogenesis. Int Orthop. 2005;29:121–124. doi: 10.1007/s00264-004-0625-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsumaki N, Kakiuchi M, Sasaki J, Ochi T, Yoshikawa H. Low-Intensity pulsed ultrasound accelerates maturation of callus in patients treated with opening-wedge high tibial osteotomy by hemicallotasis. J Bone Joint Surg Am. 2004;86-A:2399–2405. doi: 10.2106/00004623-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Chan CW, Qin L, Lee KM, Zhang M, Cheng JC, Leung KS. Low intensity pulsed ultrasound accelerated bone remodeling during consolidation stage of distraction osteogenesis. J Orthop Res. 2006;24(2):263–270. doi: 10.1002/jor.20015. [DOI] [PubMed] [Google Scholar]
- 43.Dudda M, Hauser J, Muhr G, Esenwein SA. Low-intensity pulsed ultrasound as a useful adjuvant during distraction osteogenesis: a prospective, randomized controlled trial. J Trauma. 2011;71(5):1376–1380. doi: 10.1097/TA.0b013e31821912b2. [DOI] [PubMed] [Google Scholar]
- 44.Gebauer D, Correll J. Pulsed low-intensity ultrasound: a new salvage procedure for delayed unions and nonunions after leg lengthening in children. J Pediatr Orthop. 2005;25(6):750–754. doi: 10.1097/01.bpo.0000173245.12184.7e. [DOI] [PubMed] [Google Scholar]
- 45.Esenwein SA, Dudda M, Pommer A, Hopf KF, Kutscha-Lissberg F, Muhr G. Efficiency of low-intensity pulsed ultrasound on distraction osteogenesis in case of delayed callotasis-clinical results. Zentralbl Chir. 2004;129(5):413–420. doi: 10.1055/s-2004-820398. [DOI] [PubMed] [Google Scholar]
- 46.Malizos KN, Hantes ME, Protopappas V, Papachristos A. Low-intensity pulsed ultrasound for bone healing: an overview. Injury. 2006;37(Suppl 1):S56–S62. doi: 10.1016/j.injury.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 47.Khan Y, Laurencin CT. Fracture repair with ultrasound: clinical and cell-based evaluation. J Bone Joint Surg Am. 2008;90-A(Suppl 1):138–144. doi: 10.2106/JBJS.G.01218. [DOI] [PubMed] [Google Scholar]
- 48.Kumagai K, Takeuchi R, Ishikawa H, Yamaguchi Y, Fujisawa T, Kuniya T, Takagawa S, Muschler GF, Saito T. Low-intensity pulsed ultrasound accelerates fracture healing by stimulation of recruitment of both local and circulating osteogenic progenitors. J Orthop Res. 2012;30(9):1516–1521. doi: 10.1002/jor.22103. [DOI] [PubMed] [Google Scholar]
- 49.Reher P, Harris M, Whiteman M, Hai HK, Meghji S. Ultrasound stimulates nitric oxide and prostaglandin E2 production by human osteoblasts. Bone. 2002;31:236–241. doi: 10.1016/S8756-3282(02)00789-5. [DOI] [PubMed] [Google Scholar]
- 50.Yang KH, Parvizi J, Wang SJ, Lewallen DG, Kinnick RR, Greenleaf JF, Bolander ME. Exposure to low-intensity ultrasound increases aggrecan gene expression in a rat femur fracture model. J Orthop Res. 1996;14:802–809. doi: 10.1002/jor.1100140518. [DOI] [PubMed] [Google Scholar]