Abstract
Purpose
This study evaluates two basic hypotheses: (1) the risk of an isolated dorsal approach to ventral lumbar spondylodiscitis based on clinical and radiographic results and (2) the risk of anterior radical debridement due to using a titanium implant in the site of bone infection.
Methods
Group A consisting of 23 patients was treated only by a dorsal transmuscular approach and group B consisting of eight patients was treated by two-stage posteroanterior surgery. Both evaluated groups were assessed before surgery, six weeks and one year after surgery with the Japanese Orthopaedic Association (JOA) score, visual analogue scale (VAS) and Kirkaldy-Willis functional criteria. To evaluate the sagittal balance restoration, measurement by the Cobb modified angle of the affected segment was performed.
Results
Differences (p < 0.001) in group A were found between JOA values before surgery (average 9.30) and at six weeks after surgery (average 11.82) and 12 months after surgery (13.27) and VAS differences before surgery (average 7.39), six weeks after surgery (average 3.82) and 12 months after surgery (average 2.36) in group A. According to the Kirkaldy-Willis functional criteria, 11 patients were evaluated as excellent, nine patients as good and two patients as poor. The values of the JOA score in group B showed an improvement compared with the JOA values before surgery (average 9.38) at six weeks after surgery (average 11.75) and 12 months after surgery (average 13.63), and the VAS score before surgery (average 7.38) was found to have improved six weeks after surgery (average 4.63) and 12 months after surgery (average 2.25). The functional evaluation according to the Kirkaldy-Willis functional criteria assessed three patients as excellent, four patients as good and one patient as fair. Radiographic examinations of group A revealed the following findings before surgery (average 1.75), six months after surgery (average −3.73) and 12 months after surgery (average −0.79) and in group B before surgery (average 3.71), six weeks after surgery (average −8.21) and 12 months after surgery (average −6.45).
Conclusions
The results demonstrate the minimum serious surgical complications and greater loss of sagittal balance without clinical correlation in group A. We did not find any relapse or persistence of the infection in the post-operative period in group B.
Keywords: Spondylodiscitis, Dorsal approach, Anterior debridement, Titanium implants
Introduction
Due to the anatomic characteristics, spinal infections represent a heterogeneous group of skeletal disorders that form 0.15–7 % of all bone infections [1, 2]. Haematogenous ventral spondylodiscitis is the most common of them; the nature of vascularisation predisposes it to the formation of an infectious focus in the vertebral end plate and the adjacent intervertebral disc space with potential to influence the anterior spinal column stability and the formation of a deformity. The definitive diagnosis of spinal infection in patients suffering from co-morbidities and immunosuppression is complicated by the nature and course of their disease. The subsequent selection of inadequate therapy can lead to other complications [3–5]. Ventral spondylodiscitis is treated conservatively in most cases [6]. Surgery is indicated if conservative therapy of the infectious focus fails, if there is a clinically significant instability, if spine deformities are formed or in the presence of a neurologic deficit [7, 8]. The effect of early mobilisation after surgery, especially in elderly patients with co-morbidities, is disputable [9].
The aim of this retrospective study is the analysis of a group of patients treated surgically due to ventral lumbar spine specific/non-specific spondylodiscitis from the point of view of the methods of treatment and instruments used, the spondylodiscitis site and complications. The study evaluates two basic hypotheses. Hypothesis one is defined as: the risk of greater loss of sagittal balance without clinical significance and serious complications using the isolated dorsal approach. Hypothesis two is defined as: the risk of recurrence of deep infection in anterior debridement and defect reconstruction in relation to using a titanium implant.
Materials and methods
In the period from January 2006 to December 2012, an overall number of 124 patients were treated for spinal infections in our department. The lumbar spine was affected in 71 (57 %) patients, 43 of whom (61 %) underwent surgery. Patients operated for dorsal spondylitis (three patients) and patients treated only by neural decompression, epidural or other spine locality abscess drainage (nine patients) were excluded from our study. The study included 31 patients (20 men and 11 women) with an average age of 60.5 years surgically treated for ventral lumbar spondylodiscitis. Table 1 shows the patients’ significant co-morbidities, affected spinal level and microbial culture of the study group. Spinal instability was assessed according to clinical (local pain, radiculopathy at axial loading) and radiographic signs (pathologic fracture, segmental kyphosis, end plate destruction) and was an indication for surgery in 17 patients. Six patients suffered from nerve root deficit, three patients from paraparesis and five patients from persistence of infection.
Table 1.
Demographic data of the study groups (A and B)
| Gender | Age | Lumbar spine level | Risk factors | Microbial culture | CRP | Treatment before definitive surgery | Definitive surgery | Surgery complication | VAS (pre-op/6 weeks post-op/1 year post-op | JOA score (pre-op/6 weeks post-op/1 year post-op | Kirkaldy-Willis functional score | X-ray | X-ray Cobb modified sagittal balance (pre-op/6 weeks post-op/1 year post-op | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 28 | L4–5 | Developing country (TB endemic) | TB | 36 | – | Dorsal instrumentation (1 + 1) + limited TLIF | – | 7–3–1 | 10–13–14 | Excellent | −10.3/−14.6/−10.5 | |
| 2 | Male | 63 | L2–3 | – | Staphylococcus aureus, Escherichia coli | 162 | Decompression, epidural abscess drainage | Dorsal instrumentation (1 + 1) + limited TLIF | – | 9–4–2 | 8–11–12 | Good | Clear zones, distal transpedicular screw | −3.6/−5.4/−4.2 |
| 3 | Male | 56 | T12–L1 | Cerebral palsy, pulmonary emphysema | TB | 81 | Decompression | Dorsal instrumentation (2 + 2) + limited TLIF | Acute respiratory failure | 7–4–2 | 10–13–13 | Good | 28.8/22.4/26.5 | |
| 4 | Male | 40 | L4–5 | – | Staphylococcus epidermidis | 56 | CT-guided biopsy, drainage | Dorsal instrumentation (1 + 1) + limited TLIF | – | 7–3–1 | 12–14–15 | Excellent | −4.8/−4.9/−2.3 | |
| 5 | Male | 61 | L4–5 | Hepatopathy | Acinetobacter baumannii | 98 | Blood cultures | Dorsal instrumentation (1 + 1) + limited TLIF | – | 6–2–1 | 11–13–14 | Excellent | −12.4/−13.7/−13.1 | |
| 6 | Male | 66 | L2–3 | DM | Staphylococcus epidermidis | 111 | Blood cultures + CT-guided biopsy, drainage | Dorsal instrumentation (2 + 2) + limited TLIF | Revision instrumentation failure | 8–4–3 | 7–9–13 | Good | Instrumentation, windshield wiping effect | −9.4/−9.1/−8.7 |
| 7 | Female | 72 | T12–L1 | Ulcerative colitis | Staphylococcus aureus | 78 | Decompression | Dorsal instrumentation (2 + 2) + limited TLIF | Chronic fistulation | 7–4–4 | 9–11–11 | Poor | 20.4/17.5/18.8 | |
| 8 | Male | 35 | L5–S1 | Chronic OM of the ulna | Staphylococcus aureus | 143 | Decompression, epidural abscess drainage | Dorsal instrumentation (1 + 1) + limited TLIF | – | 8–5–2 | 10–13–15 | Excellent | −11.6/−18.7/−14.3 | |
| 9 | Male | 60 | L4–5 | Wegener’s granulomatosis, chronic immunosuppression, chronic renal failure | Staphylococcus epidermidis | 108 | CT-guided biopsy, drainage | Dorsal instrumentation (1 + 1) + limited TLIF | – | 7–3–2 | 10–12–14 | Excellent | −18.6/−19.2/−18.2 | |
| 10 | Female | 73 | L1–2 | DM, chronic renal failure | Staphylococcus species | 56 | CT-guided biopsy, drainage | Dorsal instrumentation (2 + 2) + limited TLIF | – | 8–4–3 | 7–10–13 | Good | Clear zones, proximal transpedicular screw | 3.2/−5.4/−2.3 |
| 11 | Male | 70 | T12–L1 | RA | Pseudomonas aeruginosa | 81 | Decompression, epidural abscess drainage | Dorsal instrumentation (2 + 2) + limited TLIF | Exitus letalis | 7– | 6 - | 26.4/ | ||
| 12 | Male | 63 | L5–S1 | DM | Staphylococcus aureus | 62 | CT-guided biopsy, drainage | Dorsal instrumentation (1 + 1) + limited TLIF | – | 8–4–2 | 6–11–15 | Excellent | −8.2/−12.3/−9.6 | |
| 13 | Male | 68 | T12–L1 | Lyme disease-seropositive | Staphylococcus hominis | 86 | CT-guided biopsy, drainage | Dorsal instrumentation (2 + 2) + limited TLIF | – | 7–3–2 | 13–13–15 | Excellent | 6.1/4.3/5.5 | |
| 14 | Female | 62 | L5–S1 | DM, bronchial asthma | Staphylococcus aureus | 101 | Blood cultures | Dorsal instrumentation (1 + 1) + limited TLIF | – | 6–4–4 | 10–12–12 | Good | −18.3/−12.1/−10.9 | |
| 15 | Male | 58 | L4–5 | – | TB | 48 | – | Dorsal instrumentation (1 + 1) + limited TLIF | – | 7–4–2 | 11–13–14 | Excellent | −5.9/12.8/−5.5 | |
| 16 | Female | 72 | L1–2 | DM, chronic renal failure | Staphylococcus species | 73 | Blood cultures | Dorsal instrumentation (2 + 2) + limited tlif | Superficial infection | 8–4–3 | 8–11–14 | Good | Instrumentation failure rod/distal transpedicular screw | 21.3/5.1/8.6 |
| 17 | Female | 76 | L3–4 | Chronic renal failure | Escherichia coli | 82 | CT-guided biopsy, drainage | Dorsal instrumentation (2 + 2) + limited TLIF | Pulmonary embolism | 8–5–3 | 9–11–12 | Good | −11.0/−8.3/−0.6 | |
| 18 | Female | 43 | L5–S1 | – | Staphylococcus aureus | 102 | CT-guided biopsy, drainage | Dorsal instrumentation (1 + 1) + limited TLIF | – | 7–3–2 | 12–14–15 | Excellent | 7.0/5.2/5.8 | |
| 19 | Male | 81 | T12–L1 | DM, decubitus | Staphylococcus aureus, Corynebacterium species | 116 | Decompression | Dorsal instrumentation (2 + 2) + limited TLIF | Deep infection-revision | 8–6–4 | 3–6–8 | poor | Clear zones, proximal transpedicular screw | 9.7/6.4/10.4 |
| 20 | Female | 75 | L1–2 | Renal transplantation, chronic immunosuppression | Klebsiella pneumoniae | 40 | CT-guided biopsy, drainage | Dorsal instrumentation (2 + 2) + limited TLIF | Superficial infection | 7–5–3 | 11–12–13 | Good | 12.1/0/7.6 | |
| 21 | Female | 47 | L5–S1 | – | Staphylococcus aureus | 93 | Blood cultures | Dorsal instrumentation (1 + 1) + limited TLIF | – | 7–3–1 | 12–14–15 | Excellent | −5.6/−19.3/−19.3 | |
| 22 | Female | 79 | L3–4 | Chronic renal failure | Streptococcus pyogenes group G | 81 | CT-guided biopsy, drainage | Dorsal instrumentation (1 + 1) + limited TLIF | – | 8–3–2 | 10–12–14 | Excellent | 23.1/4.7/19.8 | |
| 23 | Male | 63 | L2–3 | Ischaemic cardiac disease | Staphylococcus aureus | 103 | CT-guided biopsy, drainage | Dorsal instrumentation (2 + 2) + limited TLIF | – | 8–4–3 | 9–12–13 | Good | ||
| 1 | Female | 21 | T12–L1 | Drug abuse, HBV+ | Staphylococcus aureus | 116 | CT-guided biopsy, drainage | Dorsal instrumentation + anterior debridement + fusion | – | 7–2–1 | 11–13–14 | Excellent | 28.5/15.4/18.5 | |
| 2 | Male | 60 | L2–3 | Ischaemic cardiac failure, stp. pyelonephritis, hallux phlegmon | Streptococcus pyogenes group G | 138 | CT-guided biopsy, drainage | Dorsal instrumentation + anterior debridement + fusion | – | 7–4–2 | 9–12–14 | Good | 0.4/−8.1/−5.9 | |
| 3 | Male | 62 | L2–3 | DM | Staphylococcus epidermidis | 126 | CT-guided biopsy, drainage | Dorsal instrumentation + anterior debridement + fusion | Superficial infection | 8–6–2 | 9–11–13 | Good | 10.2/−7.2/−6.2 | |
| 4 | Male | 64 | L4–5 | Ulcerative colitis, chronic immunosuppression | Staphylococcus aureus | 101 | Blood cultures | Dorsal instrumentation + anterior debridement + fusion | Paralytic ileus | 7–5–2 | 10–13–14 | Excellent | −8.6/−17.4/−17.0 | |
| 5 | Female | 51 | L4–5 | – | Staphylococcus aureus | 126 | Blood cultures | Dorsal instrumentation + anterior debridement + fusion | – | 7–5–2 | 10–13–15 | Excellent | −10.5/−18.7/−18.0 | |
| 6 | Male | 68 | L3–4 | HCV+, cannula sepsis | Staphylococcus aureus | 126 | Decompression, epidural abscess drainage | Dorsal instrumentation + anterior debridement + fusion | – | 8–5–3 | 11–13–14 | Good | −2.3/−15.4/−14.6 | |
| 7 | Male | 75 | L3–4 | DM, chronic renal failure | Escherichia coli | 112 | Decompression, epidural abscess drainage – |
Dorsal instrumentation + anterior debridement + fusion | Acute revision for haemorrhage (segmental a.) | 7–6–3 | 6–8–12 | Fair | −4.4/−26.1/−26.0 | |
| 8 | Male | 63 | L1–2 | – | TB | 71 | – | Dorsal instrumentation + anterior debridement + fusion | Acute respiratory failure | 8–4–3 | 9–11–13 | Good | 16.4/11.8/12.6 |
CRP C-reactive protein, VAS visual analogue scale, JOA Japanese Orthopaedic Association, TB tuberculosis, TLIF transforaminal lumbar interbody fusion, CT computed tomography, DM diabetes mellitus, OM osteomyelitis, RA rheumatoid arthritis, HBV hepatitis B virus, HCV hepatitis C virus, stp status post
The patients were divided into two groups according to the surgical therapy. The first group (group A, 23 patients) was treated only by the dorsal approach. We used the transmuscular technique with a limited transarticular resection of the infected disc with subsequent necrotic end plate debridement. The anterior column defect was subsequently reconstructed using an autogenous bone graft combined with tobramycin-impregnated calcium sulphate pellets (OSTEOSET T, Wright Medical Technology Inc., Arlington, TN, USA). Limited transarticular interbody fusion [mini-transforaminal lumbar interbody fusion (TLIF)] was completed using transpedicular instrumentation and lateral fusion.
The second group (group B) consisted of eight patients (26 %) who were treated by two-stage, posteroanterior surgery: the primarily dorsal transpedicular instrumentation (first stage) was followed by anterior retroperitoneal radical debridement (second stage) and the defect was reconstructed by a titanium body replacement. Indications for the surgery were: large vertebral body osteolysis affecting more than a third of the vertebral body, pathologic fracture, formation of segmental kyphosis and radiographic signs of persisting instability after a previous dorsal stabilisation.
The definitive surgical intervention was preceded as follows: by computed tomography (CT)-guided biopsy (14 patients), by epidural abscess drainage (five patients) and by neural decompression (three patients). A microbial agent was found six times in a haemoculture.
The patients of both evaluated groups (A and B) were assessed retrospectively in the following periods of time: before surgery, six weeks after surgery and one year after surgery. For clinical evaluation, the Japanese Orthopaedic Association (JOA) score (with 0–15 points), visual analogue scale (VAS) for pain (graded 1–10) and Kirkaldy-Willis functional criteria (graded poor, fair, good and excellent) were used. Treatment complications in the individual patient groups were recorded at the same time.
Plain radiographs in anterodorsal and lateral spine projections in the supine position were performed before surgery, six weeks after surgery and one year after surgery. Measurement by the Cobb modified angle of the upper end plate of the proximal and the lower end plate of the distal vertebra of the affected segment was used in order to evaluate the sagittal balance restoration. At the same time, instrumentation failure and signs of non-fusion were recorded.
Within the statistical processing of the given set in time, standard descriptive and graphic representations by means of box plots were used for the set description. The statistical significance of differences among three different visits (before surgery, six weeks after surgery and one year after surgery) was assessed by means of repeated measures analysis of variance (ANOVA) (statistically significant difference between measurements p < 0.05). Scheffe’s post hoc test was used for subsequent comparison determining the decisive mutual difference between the specific pairs of measurement.
Results
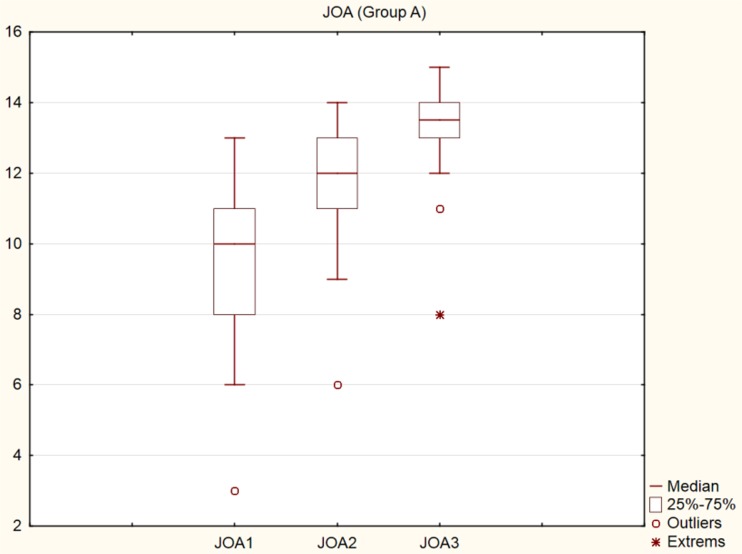
From the overall number of 23 patients in group A, one patient with rheumatoid arthritis and chronic immunosuppression died five weeks after the surgery due to cardiac failure. A pulmonary embolism developed in one patient on the fourth day after the surgery, and full recovery was achieved by conservative therapy. Acute post-operative respiratory failure was found immediately after the surgery in one patient operated for tuberculous spondylodiscitis at T12–L1 and the patient’s condition fully improved. We found a superficial infection in two patients, and a deep infection in two patients. The superficial infection healed spontaneously. The patients with deep infection were revised while leaving the instruments in place. One patient healed and the second patient was, due to his overall condition, treated by conservative therapy and suffered from chronic fistulation. There were no instances of neurologic impairment after the surgery. Statistically significant differences in the ANOVA test (p < 0.001) were found between JOA values before surgery (average 9.30) and at six weeks after surgery (average 11.82), and 12 months (average 13.27) after surgery (Fig. 1). The VAS values in group A showed statistically significant differences (p < 0.001) before surgery (average 7.39), six weeks after surgery (average 3.82) and 12 months (average 2.36) after surgery. According to the Kirkaldy-Willis functional criteria, 11 patients were finally evaluated as “excellent”, nine patients as “good” and two patients as “poor”.
Fig. 1.
Graph (box plots), JOA score, group A, preoperative, 6 weeks post-operative, 1 year post-operative
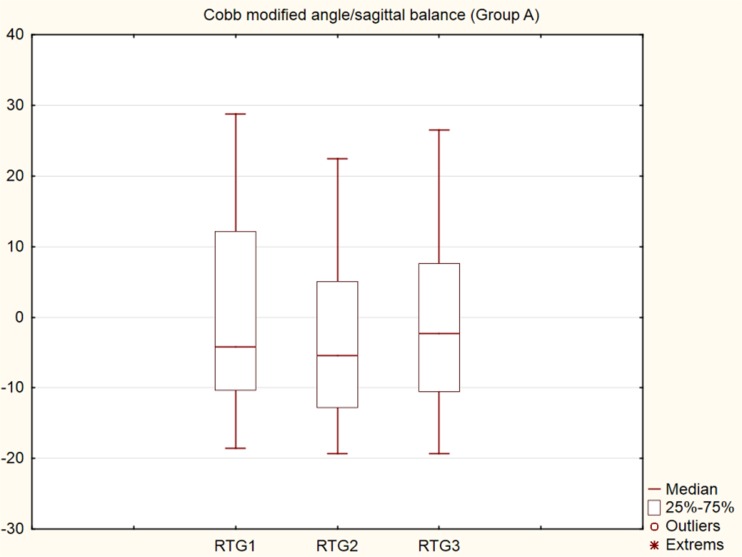
From the overall number of eight patients in group B, one patient operated in the L3–4 segment was revised acutely after the surgery due to bleeding in the retroperitoneum from a segmental artery. Paralytic ileus developed in one patient and was successfully treated conservatively. A superficial infection in one patient was treated by revision surgery and subsequently healed. The final values of the JOA showed significant improvement (p < 0.001) when comparing the JOA values before surgery (average 9.38) to six weeks after surgery (average 11.75) and 12 months after surgery (average 13.63) (Fig. 2). The resulting p value of the ANOVA test (p < 0.001) is for VAS before surgery (average 7.38), six weeks after surgery (average 4.63) and 12 months after surgery (average 2.25). The functional evaluation according to the Kirkaldy-Willis functional criteria assessed three patients as “excellent”, four patients as “good” and one patient as “fair”. In the modified Cobb angle measurement, the lordosis angle is a negative number (−0.0) while the kyphosis angle is a positive number (+0.0). The resulting p value of the ANOVA test (p < 0.001) of radiographic examinations of the dorsal approach (Group A) was found as follows: average 1.75 before surgery, average −3.73 6 weeks after surgery and average −0.79 12 months after surgery, respectively. In the subsequent Scheffe’s post hoc tests, the values in the first and second periods and in the second and third periods differed significantly, while the values in the first and third periods do not differ (Fig. 3). Plain radiographs showed dorsal instrumentation failure in one patient due to the so-called windshield wiping effect, followed by ventrodorsal revision surgery (Fig. 4a–d). Failure of the rod-screw fixation (distal transpedicular screws) was found in one patient (Fig. 5a–e) and clear zones were found on the plain radiographs of another three patients. The therapy of all four patients was finished in a brace without the necessity of revision surgery.
Fig. 2.
Graph (box plots), JOA score, group B, preoperative, 6 weeks post-operative, 1 year post-operative
Fig. 3.
Graph (box plots), Cobb modified angle/sagittal balance, group A, preoperative, 6 weeks post-operative, 1 year post-operative
Fig. 4.
Male, 66, ventral spondylodiscitis L2–3. a Pre-operative plain radiograph lateral view. b Post-operative plain radiograph lateral view, dorsal transpedicular fixation, mini-TLIF, anterior debridement, fusion, autogenous graft. c Post-operative plain radiograph lateral view, instrumentation failure, windshield wiping. d Post-operative plain radiograph lateral view, revision surgery, anterior debridement, titanium body replacement reconstruction, transpedicular instrumentation
Fig. 5.
Female, 72, ventral spondylodiscitis L1–2. a Pre-operative plain radiograph lateral view. b Post-operative plain radiograph AP view, dorsal transpedicular fixation, mini-TLIF, anterior debridement, fusion, autogenous graft. c Post-operative plain radiograph lateral view. d Post-operative plain radiograph AP view, distal transpedicular screw/rod failure. e Post-operative plain radiograph lateral view, segment L1–2 fusion, conservative
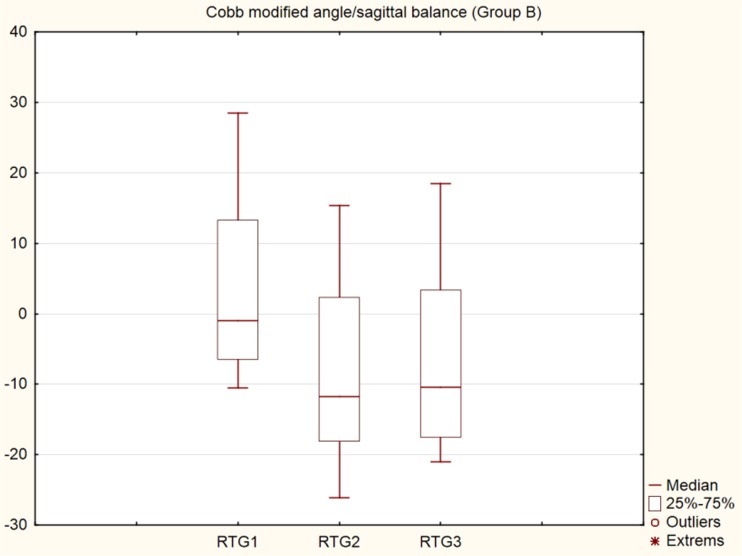
Significant differences (p < 0.001) were found in patients in group B between the radiologic values before surgery (average 3.71) and at six weeks after surgery (average −8.21) and at 12 months after surgery (average −6.45). In the subsequent Scheffe’s post hoc tests, the values in the first and second periods and in the first and third periods differed significantly (Fig. 6). No instrumentation failure was found in group B.
Fig. 6.
Graph (box plots), Cobb modified angle/sagittal balance, group B, preoperative, 6 weeks post-operative, 1 year post-operative
Discussion
Surgical therapy of ventral spondylodiscitis should be aimed at infection healing, prevention of neurologic deficit and at the restoration of spinal column stability and balance [10, 11]. A neurologic deficit always develops in an unstable segment in the presence of a pathologic fracture of the infected vertebra or in the presence of significant osteolysis with subsequent vertebral segmental kyphosis [12].
Hypothesis one was confirmed, because the results demonstrate the minimum serious surgical complications and greater loss of sagittal balance without clinical correlation after the isolated dorsal approach and mini-TLIF (group A). We did not find a neurologic deficit or complication involving a dural sac tear in the group. A deep infection appeared in two patients after a previous surgical decompression due to a neurologic deficit. One patient underwent revision surgery (debridement, lavage and drainage) while leaving the stable instrumentation intact. In general, we prefer a limited transmuscular approach that allows resection of the infectious focus and spine balance and stability restoration under a minimum risk of injury to neurogenic structures via the central dorsal approach. Within the access, decompression at the transverse process can be extended by total facetectomy with detachment of the lateral wall of the spinal canal and good visibility of the intervertebral disc space. Due to the risk of transpedicular instrumentation infection and biofilm formation, the surgery should be performed during the subacute phase of infection after a previous abscess drainage and specific antibiotic (ATB) therapy.
The extent of the anterior column affection represents another limitation. We have been using transpedicular instrumentation in the thoracolumbar and upper lumbar spine covering two segments above and below the affected segment; instrumentation in the lower lumbar spine has been limited to one segment above and below the affected segment. The extended instrumentation is justified on account of the higher risk of anterior spinal column kyphosis at the thoracolumbar junction and upper lumbar spine. In group A we found five patients with radiographic signs of instrumentation failure. One patient was revised and anterior debridement and column restoration using titanium body replacement was performed. The other four patients were treated conservatively in a brace. Radiographic evaluation of group A using the modified Cobb angle showed greater loss of sagittal balance correction compared to group B, but without the clinical correlate. Hempelmann et al. [13] prefer the dorsal access in seriously ill elderly patients. Dorsal approaches to the lumbar spine in ventral spondylodiscitis should be extended by treatment of the anterior spinal column pathology in order to preserve the spinal column stability. In general, posterior lumbar interbody fusion (PLIF) or TLIF is preferred. Zaveri and Mehta [14] presented a group of 55 patients treated for spondylodiscitis, 15 of whom were operated by TLIF. No complications, infection recurrence or the necessity of a revision surgery were found in any of the patients. They indicate surgery in patients with segmental kyphosis and pain lasting for more than three months under conservative therapy in the case of patient mobilisation and a neurologic deficit. The indication criterion is defined by a limited destruction of the vertebral bodies up to the half of the body height. Lee et al. [9] use PLIF in the indication to prevent loss of sagittal balance correction. Madert et al. [15] use TLIF in patients with thoracolumbar non-specific spondylodiscitis. In the group of 114 patients, they achieved successful fusion in 90 %, and of 39 patients with pre-operative neurologic deficit, regression was observed in 26 patients.
Hypothesis two was not confirmed, because there was no risk of the recurrence of infection in anterior debridement and defect reconstruction in relation to using a titanium implant (group B). This group of patients was treated by anterior retroperitoneal resection of the infectious focus and anterior column reconstruction using the titanium body replacement system. According to our results, there is no significant risk of using titanium implants in the site of infection in the subacute phase of ventral spondylodiscitis. The implants were used in large anterior defects and primarily provided very good stability and restoration of the sagittal balance of the spine. We did not find any relapse or persistence of the infection in the post-operative period. The metal implant use, however, is conditioned by the possibility of a radical removal of the whole focus and achievement of full implant stability in the spine. It is thus important to operate a previously treated site without any active abscess focus in an acute infection phase.
Restoration of the anterior column integrity after radical anterior debridement could be the problem of the anterior treatment [16, 17]. Most often, a structural autograft with good osteoinductive/conductive properties and a relatively low risk of inflammation relapse is recommended for the reconstruction [18]. Use of the titanium body replacement system, especially if large anterior defects must be reconstructed, represents another possibility of the ventral spondylodiscitis treatment. The current systems enable very good correction of kyphosis and restoration of the anterior spinal column stability, but the high risk of a biofilm formation on the implant surface in an infected site and a deep infection relapse remains a controversial issue [1, 3]. Korovessis et al. [19] presented a group of 14 patients operated on for pyogenic spondylodiscitis, primarily by anterior resection and use of the Harms cage and dorsal instrumentation in the second stage without any deep infection recurrence. They recommend an intervention even in the acute phase of infection when they place stress on the radical removal of the infectious focus. Lim et al. [20] do not consider the use of the titanium implant to be a contraindication if an infection is present, while the restoration of the spinal sagittal balance is considered to be an advantage. Some authors report lower adhesion of bacteria on the titanium implant’s surface as compared to other metals [14, 18]. Due to the infectious focus localisation, radical anterior debridement with anterior neural decompression of the neurogenic structure and subsequent restoration of the spine stability and shape have been preferred at present [21]. Disadvantages of the surgical anterior approach to the lumbar spine, especially in forced revisions, have often been discussed [22, 23]. The anatomic retroperitoneum visibility is often complicated due to the presence of granulation tissue and neighbouring adhesions. Our study included one patient in group B in whom bleeding in an early post-operative stage appeared; acute revision and participation of a vascular surgeon were required.
This study has the following strengths. It provides additional access options in the treatment of ventral lumbar spondylodiscitis. The results suggest the possibility of isolated dorsal and combined surgical approach with their limitations.
The study is limited by several factors. First, it is a retrospective evaluation of the group of patients, when during the assessment period use of the isolated dorsal transmuscular approach combined with the mini-TLIF method in the treatment of ventral spondylodiscitis started. The patient heterogeneity with respect to age, co-morbidities, previous therapy, anatomic localisation of the infection, the time of definitive diagnosis and no clear therapeutic guidelines are potential limitations for a future prospective study. The statistical evaluation is limited by a small number of patients due to the lower disease incidence. The higher percentage of operated patients as compared to the conservatively treated ones can be explained by the primary patient selection from the referenced centres. Patients treated conservatively are primarily reported with proposed subsequent therapy in local hospitals, so the study includes only “difficult” patients treated and controlled in our department.
Conclusion
In the treatment of ventral lumbar spondylodiscitis we prefer the dorsal transmuscular approach with transpedicular instrumentation, lateral fusion, limited facet joint resection with removal of the infectious focus of the intervertebral disc space and part of the end plate with subsequent autogenous bone grafting and bone substitute combined with local ATB delivery. In cases of large vertebral body osteolysis affecting more than a third of the vertebral body, the presence of pathologic fracture, formation of segmental kyphosis and radiographic signs of persisting instability, we add an anterior radical retroperitoneal debridement and defect reconstruction with titanium implant in the second stage.
References
- 1.Akbar M, Lehner B, Doustdar S, Fürstenberg CH, Hemmer S, Bruckner T, et al. Pyogenic spondylodiscitis of the thoracic and lumbar spine: a new classification and guide for surgical decision-making. Orthopade. 2011;40:614–623. doi: 10.1007/s00132-011-1742-5. [DOI] [PubMed] [Google Scholar]
- 2.Lange T, Schulte TL, Ullmann V. Two recurrences of adjacent spondylodiscitis after initial surgical intervention with posterior stabilization, debridement, and reconstruction of the anterior column in a patient with spondylodiscitis: a case report. Spine. 2010;35:E804–E810. doi: 10.1097/BRS.0b013e3181d56955. [DOI] [PubMed] [Google Scholar]
- 3.Asamoto A, Doi H, Kobayashi N, Endoh T, Sakagawa H, et al. Spondylodiscitis: diagnosis and treatment. Surg Neurol. 2005;64:103–108. doi: 10.1016/j.surneu.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 4.Cervan A, Colmenero Jde D, Del Arco A, Villanueva F, Guerado E. Spondylodiscitis in patients under haemodialysis. Int Orthop. 2012;36(2):421–426. doi: 10.1007/s00264-011-1433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osenbach RK, Hitchon PW, Menezes AH. Diagnosis and management of pyogenic vertebral osteomyelitis in adults. Surg Neurol. 1990;33:266–275. doi: 10.1016/0090-3019(90)90047-S. [DOI] [PubMed] [Google Scholar]
- 6.Carrega G, Arena S, Bartolacci V, Gavino D, Mecca D, Sandrone C, Santoriello L, Tabasso G, Riccio G. Non-tubercular vertebral osteomyelitis: diagnosis and therapy of 45 patients from a single Italian centre. Infez Med. 2003;11:183–188. [PubMed] [Google Scholar]
- 7.Quiñones-Hinojosa A, Jun P, Jacobs R, Rosenberg WS, Weinstein PR. General principles in the medical and surgical management of spinal infections: a multidisciplinary approach. Neurosurg Focus. 2004;17:E1–E15. doi: 10.3171/foc.2004.17.6.1. [DOI] [PubMed] [Google Scholar]
- 8.Rath SA, Neff U, Schneider O, Richter HP. Neurosurgical management of thoracic and lumbar vertebral osteomyelitis and discitis in adults: a review in 43 consecutive surgically treated patients. Neurosurgery. 1996;38:926–933. doi: 10.1097/00006123-199605000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Lee JS, Moon KP, Kim SJ, et al. Posterior lumbar interbody vision and posterior instrumentation in the surgical management of lumbar tuberculous spondylitis. J Bone Joint Surg Br. 2007;89:210–214. doi: 10.1302/0301-620X.89B2.17849. [DOI] [PubMed] [Google Scholar]
- 10.Zarghooni K, Röllinghoff M, Sobottke R, Eysel P. Treatment of spondylodiscitis. Int Orthop. 2012;36(2):405–411. doi: 10.1007/s00264-011-1425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Martino A, Papapietro N, Lanotte A, Russo F, Vadalà G, Denaro V. Spondylodiscitis: standards of current treatment. Curr Med Res Opin. 2012;28:689–699. doi: 10.1185/03007995.2012.678939. [DOI] [PubMed] [Google Scholar]
- 12.Karadimas EJ, Bunger C, Lindblad BE, Hansen ES, Høy K, Helmig P, Kannerup AS, Niedermann B. Spondylodiscitis. A retrospective study of 163 patients. Acta Orthop. 2008;79:650–659. doi: 10.1080/17453670810016678. [DOI] [PubMed] [Google Scholar]
- 13.Hempelmann RG, Mater E, Schön R. Septic hematogenous lumbar spondylodiscitis in elderly patients with multiple risk factors: efficacy of posterior stabilization and interbody vision with iliac crest bone graft. Eur Spine J. 2010;19:1720–1727. doi: 10.1007/s00586-010-1448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaveri GR, Mehta SS. Surgical treatment of lumbar tuberculous spondylodiscitis by transforaminal lumbar interbody vision (TLIF) and posterior instrumentation. J Spinal Disord Tech. 2009;22:257–262. doi: 10.1097/BSD.0b013e31818859d0. [DOI] [PubMed] [Google Scholar]
- 15.Madert J, Liem M, Frosch KH, Niemeyer T. Dorsolateral access and interbody spinal vision in spondylodiscitis of the thoracolumbar spine (TLIF technique) Oper Orthop Traumatol. 2013;25:262–272. doi: 10.1007/s00064-012-0214-3. [DOI] [PubMed] [Google Scholar]
- 16.Guaredo E, Cerván A. Surgical treatment of spondylodiscitis. An update. Int Orthop. 2012;36(2):413–420. doi: 10.1007/s00264-011-1441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oxland TR, Grant JP, Dvorak MF, et al. Effects of endplate removal on the structural properties of the lower lumbar vertebral bodies. Spine. 2003;28:771–777. [PubMed] [Google Scholar]
- 18.Pee YH, Park JD, Choi Y, Lee S. Anterior debridement and fusion followed by posterior pedicle screw fixation in pyogenic spondylodiscitis: autologous iliac bone strut versus cage. J Neurosurg Spine. 2008;8:405–412. doi: 10.3171/SPI/2008/8/5/405. [DOI] [PubMed] [Google Scholar]
- 19.Korovessis P, Petsinis G, Koureas G, Iliopoulos P, Zacharatos S. Anterior surgery with insertion of titanium mesh cage and posterior instrumented fusion performed sequentially on the same day under one anesthesia for septic spondylitis of thoracolumbar spine: is the use of titanium mesh cages safe? Spine (Phila Pa 1976) 2006;31:1014–1019. doi: 10.1097/01.brs.0000215049.08622.9d. [DOI] [PubMed] [Google Scholar]
- 20.Lim JK, Kim SM, Jo DJ, Lee TO. Anterior interbody grafting and instrumentation for advanced spondylodiscitis. J Korean Neurosurg Soc. 2008;43:5–10. doi: 10.3340/jkns.2008.43.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klöckner C, Valencia R. Sagittal alignment after anterior debridement and fusion with or without additional posterior instrumentation in the treatment of pyogenic and tuberculous spondylodiscitis. Spine. 2003;28:1036–1042. doi: 10.1097/01.BRS.0000061991.11489.7F. [DOI] [PubMed] [Google Scholar]
- 22.Rajasekaran S, Soundarapandian S. Progression of kyphosis in tuberculosis of the spine treated by anterior arthrodesis. J Bone Joint Surg Am. 1989;71:1314–1323. [PubMed] [Google Scholar]
- 23.Stulik J, Vyskocil T, Bodlák P, et al. Injury to major blood vessels in anterior thoracic and lumbar spinal surgery. Acta Chir Orthop Traumatol Cech. 2006;73:92–98. [PubMed] [Google Scholar]