Abstract
Altitudinal gradients are characterized by steep changes of the physical and biotic environment that present challenges to plant adaptation throughout large parts of the world. Hybrid zones may form where related species inhabit different neighbouring altitudes and can facilitate interspecific gene flow and potentially the breakdown of species barriers. Studies of such hybrid zones can reveal much about the genetic basis of adaptation to environmental differences stemming from changes in altitude and the maintenance of species divergence in the face of gene flow. Furthermore, owing to recombination and transgressive effects, such hybrid zones can be sources of evolutionary novelty. We document plant hybrid zones associated with altitudinal gradients and emphasize similarities and differences in their structure. We then focus on recent studies of a hybrid zone between two Senecio species that occur at high and low altitude on Mount Etna, Sicily, showing how adaptation to local environments and intrinsic selection against hybrids act to maintain it. Finally, we consider the potential of altitudinal hybrid zones for generating evolutionary novelty through adaptive introgression and hybrid speciation. Examples of homoploid hybrid species of Senecio and Pinus that originated from altitudinal hybrid zones are discussed.
Keywords: adaptation, altitudinal hybrid zones, hybridization, introgression, selection, speciation
1. Introduction
How plants respond to environmental change is fundamental to our understanding of processes of plant adaptation and speciation. Since the early landmark studies of Turesson [1] and Clausen et al. [2] on ecotypic divergence within species, many plant evolutionary biologists have focused on the type and extent of genetic alterations that occur within and between species in response to environmental change. Whereas Turesson and Clausen et al. examined what were considered to be local races to demonstrate habitat-correlated patterns of genetic variation within species, later workers focused on genetic divergence across environmental gradients, where changes in environment occurred either gradually, for example with increases of latitude or altitude [3], or suddenly such as at the boundary between soils containing high or trace levels of toxic heavy metals [4].
Altitudinal gradients are particularly interesting in that they are characterized by steep changes in numerous features of the physical environment, such as temperature, atmospheric pressure, moisture, hours of sunshine, ultraviolet (UV) radiation, wind, season length and geology [5], and also aspects of the biotic environment, for example, number and type of pollinator, herbivore and competitor. Approximately a quarter of the land surface of the Earth is covered by mountains, which host at least one-third of terrestrial plant species’ diversity [5]. Altitudinal gradients therefore present major and recurrent challenges to successful plant adaptation throughout large parts of the world. Although some plant species can grow over a wide range of altitudes by adapting to changed conditions through phenotypic plasticity and genetic modification [2,6–9], most species are restricted in their distribution to narrower ranges of altitude. Consequently, as you ascend a mountain it is notable that particular plant species are replaced by other species, giving rise to different types of vegetation at different altitudes, for example, forest, sub-alpine and alpine vegetation zones at low, intermediate and high altitudes, respectively. In situations where related species occur at different altitudes over relatively short distances, it is possible for hybrid zones to form between them, which may serve as bridges for interspecific gene flow. Under such circumstances, the analysis of hybrid zones can reveal much about the genetic basis of adaptation to conditions at different altitudes and also the maintenance of species divergence in the face of gene flow. Furthermore, it is expected that owing to recombination and transgressive effects, hybrid zones will often contain greater levels of genetic and phenotypic variation than either parent and consequently provide the potential for generating evolutionary novelty and possibly new hybrid taxa [10].
In this review, we first document a number of plant hybrid zones that occur across altitudinal gradients between species of equivalent ploidy to emphasize similarities and differences in structure. We then focus on a hybrid zone between two Senecio species that occur on Mount Etna, Sicily, and finally discuss the potential of such altitudinal hybrid zones to generate evolutionary novelty, particularly new homoploid hybrid species.
2. Plant hybrid zones across altitudinal gradients
Broadly speaking, three different types of hybrid zone structure are recognized by theoretical and empirical evolutionary biologists. These are tension zones [11], bounded hybrid superiority zones [12] and mosaic hybrid zones [13–15]. These three models of hybrid zone (reviewed in [16]) vary according to how selection acts on hybrids and parent species within the context of hybridization and gene dispersal. The tension zone model assumes that hybrids are of low fitness (reduced viability and/or sterility) relative to parent species owing to inherent defects caused for example by negative interactions (epistasis) between genes inherited from each parent. Under these circumstances, selection against hybrids is environment independent (intrinsic selection) and hybrids tend to be restricted to a narrow clinal transition between parent species. The bounded hybrid superiority model may also be characterized by a smooth transition between one parent species and the other via the hybrid zone; however, in this model hybrids have higher fitness than either parent species in intermediate habitats, but lower fitness in parental habitats. Thus, in contrast to the tension zone model, selection on hybrids is environment dependent (extrinsic selection). Bounded hybrid superiority hybrid zones tend to occupy ecotones, i.e. distinctive intermediate environmental zones located between those occupied by the parent species. In the mosaic hybrid zone model, different environments are patchily distributed within an area of species overlap. Under these conditions, it is envisaged that hybrid zones comprise a mosaic of different parental genotype frequencies with parent species adapted to different environments. In this model, selection may both act against hybrids and be environment independent (as in the tension zone model) or favour hybrids and be environmental dependent (as in the bounded hybrid superiority model).
We conducted a trawl of the literature for examples of plant hybrid zones that occur across altitudinal gradients and which had been subject to genetic analysis using molecular markers. For this we used ISI Web of Knowledge to conduct a search using the terms ‘hybridization × altitude’ and ‘hybridization × elevation’. We also used the same key words to search a range of journals that might be expected to publish articles on the topic. Our search revealed 12 examples of plant hybrid zones occurring across altitudinal gradients in North and Central America, Europe and Asia (table 1). The species pairs that hybridize encompass trees (Picea [17], Pinus [18,19], Populus [20–22] and Quercus [23]), woody shrubs (Artemisia [24–26] and Rhododendron [27]) and herbaceous perennials (Aquilegia [28–30], Ipomopsis [31–34], Penstemon [35–37], Senecio [38–40] and Silene [41]), all of which are known or presumed to reproduce by outcrossing apart from Artemisia and Rhododendron, which are reported to exhibit a mixed mating system of selfing and outcrossing. Additional examples of hybridization occurring between species across altitudinal gradients have been proposed in Armeria [42,43], Impatiens [44], Pinus [45] and other Rhododendron [46], but detailed analyses of hybrid zone structure are lacking in these examples.
Table 1.
Locations, characteristics and structure of hybrid zones across altitudinal gradients reported in trees, shrubs and herbaceous perennials.
| species | altitude (m) | location | mating system | pollinators | pollinator preference | ecological differences | location of hybrids | hybrid zone structure model | hybrid fitness analysis | reciprocal transplant analysis | type of selection on hybrids | references |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| trees | ||||||||||||
| Picea | ||||||||||||
| P. glauca × P. engelmannii | <600 >1800 |
British Columbia, Canada | monoecy—outcrossing | wind | none | moisture, length of growing season, snowfall amount | intermediate altitude | bounded hybrid superiority—clinal variation evident | various fitness components measured on hybrids and parents. Hybrids fitter in intermediate environments | yes—in common gardens at different altitudes | extrinsic | [17] |
| Pinus | ||||||||||||
| P. parviflora var. pentaphyla × P. pumila | <1200 >1200 |
Mt. Asahidake, Japan | monoecy—outcrossing assumed | wind | no | not specified—associated with altitude differences | intermediate altitude | unknown—smooth cline present, all individuals of P. pumila are introgressed | no | no | unknown | [18,19] |
| Populus | ||||||||||||
| P. fremontii× P. angustifolia | <1500 >2300 |
Weber River, UT, USA | dioecy—outcrossing | wind | no | temperature and moisture | intermediate and high altitudes | tension zone—some cytoplasms and nuclear genes introgressed into high-altitude species indicating certain hybrids have high fitness | hybrids more susceptible to aphid attack, but as fit as one parent in sexual reproduction and two to four times greater than parents in asexual reproduction | no | intrinsic for parts of genome, extrinsic for other parts of genome | [20–22] |
| Quercus | ||||||||||||
| Q. magnoliifolia× Q. resinosa nnii | <1500 >1900 |
Tequila volcano, Jalisco, Mexico | monoecy —outcrossing assumed | wind | no | not specified—associated with altitude differences | intermediate altitude | unknown—cline in morphology, but hybrids present across entire altitude gradient based on molecular markers | hybrids exhibited a higher level of developmental instability | no | possibly intrinsic and extrinsic | [23] |
| shrubs | ||||||||||||
| Artemisia | ||||||||||||
| A. tridentata ssp. tridentata × A. t. ssp. vaseyana | <1740 >1840 |
Wasatch Mts, UT, USA | self-compatible, mixed mating system | wind | no | edaphic and associated with altitude | sites of intermediate or more variable ecology | bounded hybrid superiority | hybrids exhibit greater fitness in intermediate zone | yes—seed germination, survival and reproduction recorded | extrinsic | [24–26] |
| Rhododendron | ||||||||||||
| R. ponticum × R. caucasicum | <2000 >2000 |
Tiryal Dag, Turkey | self-compatible, mixed mating system | generalists—large no. of pollinator species recorded | no | not specified—associated with altitude differences | intermediate altitude | bounded hybrid superiority—Only fertile F1s occur in hybrid zone. Later generation hybrids and backcrosses are absent | no—however, hybrid intercrossing and backcrossing produce good germinable seed. Assumed all post-F1 hybrids eliminated by postgermination selection | no | extrinsic/intrinsic | [27] |
| herbaceous perennials | ||||||||||||
| Aquilegia | ||||||||||||
| A. formosa × A. pubescens | <3200 >2750 |
Sierra Nevada, CA, USA | self-compatible, protogynous | hummingbirds, bees, flies | no [25] yes [26] |
soil type, moisture, exposure | sites of intermediate ecology | mosaic | no | no | unknown | [28–30] |
| Ipomopsis | ||||||||||||
| I. aggregata × I. tenuituba | <2900 >3100 |
Rocky Mts, CO, USA | self-incompatible, protandrous | hummingbirds, hawkmoths | yes | not specified—associated with altitude differences | intermediate altitudes | none yet suitable—smooth cline present | yes—but hybrid fitness varies according to pollinator visitation, seed herbivory, reciprocal transplant analysis | yes | extrinsic | [31–34] |
| Penstemon | ||||||||||||
| P. newberryi × P. davidsonii | <3025 >3450 |
Sierra Nevada, CA, USA | unknown—outcrossing presumed | general—62 different pollinator species observed | no, but some pollinators absent at high altitude | temperature and moisture | intermediate altitudes | bounded hybrid superiority indicated—smooth cline present | yes—hybrids physiologically different and performed as well or better than parents in greenhouse and field | yes—compared survival of F1s and parents in common gardens at different altitudes | extrinsic | [35–37] |
| Senecio | ||||||||||||
| S. chrysanthemifolius × S. aethnensis | <600 >2500 |
Mount Etna, Sicily, Italy | self-incompatible, outcrossing | generalists—many different pollinator species | no | temperature and moisture | intermediate altitude | tension zone—post-F1 hybrid breakdown | F2 progeny exhibit decreased germination and survivorship | no | intrinsic | [38,39] |
| S. ovatus × S. hercynicus | <800 >1040 |
Mt Brocken, Harz Mountains, Germany | outcrossing assumed | assumed to be generalists | no | not specified—associated with altitude differences | intermediate altitude | mosaic | F1 and backcrosses are fertile | no | extrinsic suggested—but no hard evidence | [40] |
| Silene | ||||||||||||
| S. latifolia × S. dioica | <1120 >1440 |
Swiss Alps, Switzerland | dioecy—outcrossing | bumblebees, butterflies, moths | partial | temperature, moisture, disturbance | intermediate altitude | narrow hybrid zones are bimodal comprising mainly backcross individuals | F1 and backcrosses are fertile | no | extrinsic suggested—but no hard evidence | [41] |
In some cases, the species pairs that hybridize have different pollinators (Aquilegia, Ipomopsis and Silene), though pollinator preference is not sufficient to prevent at least some hybridization from occurring. However, in the majority of cases species are pollinated by a range of generalist pollinator species (Penstemon, Rhododendron and Senecio) or are wind pollinated (Artemisia, Picea, Pinus, Populus and Quercus). The most notable differences in the abiotic environment occupied by low- and high-altitude species are related to temperature and moisture, although differences in soil type and snowfall quantity were recorded in some instances. For four of the 12 hybrid zones, the bounded hybrid superiority model was thought to provide the best description of structure (Artemisia, Penstemon, Picea and Rhododendron), for two the tension zone was considered a better fit (Populus and Senecio: S. aethnensis × S. chrysanthemifolius), and for another two a mosaic hybrid zone description was indicated (Aquilegia and Senecio: S. ovatus × S. hercynicus). In the case of Ipomopsis, smooth clinal change was evident for both morphological and genetic changes, but the hybrid zone is complex and neither the bounded hybrid superiority nor tension zone model currently appear to describe the system exactly. Smooth clinal change is also evident in the Pinus hybrid zone, but there is insufficient known about this example to indicate whether it represents a tension zone or bounded hybrid superiority zone. In Quercus, there was smooth clinal change in morphology associated with altitude, but both hybrid and parental molecular genotypes were distributed across the altitudinal gradient making classification of the hybrid zone difficult. In Silene, a bimodal structure was evident in the hybrid zones studied with many backcross individuals but few F1s present. These zones were not classified according to existing models.
Although attempts were made by authors to classify most of the hybrid zones into one or other of the three well-known categories, it seems impossible to do this completely satisfactorily, owing to absence of critical information. Even in the cases of Artemisia, Penstemon and Picea where reciprocal transplant analyses were conducted and indicated that hybrids have highest fitness at intermediate altitudes and reduced fitness in parental habitats, estimates of lifetime fitness are lacking over the entire life cycle and are based on records made in one or a few years only. Furthermore, in one of the two examples of a tension zone (in Senecio), reduced hybrid fitness was based mainly on indirect assessment [36], whereas in Populus there was evidence for and against hybrids having low fitness in the hybrid zone. In neither of these two cases were reciprocal transplant analyses conducted. The Rhododendron hybrid zone is possibly an example of a bounded hybrid superiority zone, but is unusual in being entirely composed of fertile F1s capable of producing germinable seed when intercrossed or backcrossed to either parent. These F1 hybrids occupy a zone at intermediate altitudes where post-F1 hybrids are not recruited, possibly owing to both extrinsic and intrinsic selection acting against them. A transplant analysis is required to establish the relative fitness of parents, F1 hybrids and post-F1 hybrids across this hybrid zone to provide a more precise understanding of how it is maintained.
From this review of existing examples of plant hybrid zones across altitudinal gradients, it is evident that a range of different structures is exhibited, but none of them at the present time can be classified with complete confidence according to the three well-known hybrid zone models. More detailed investigation is required on each of these hybrid zones before all the factors and interactions affecting their maintenance might be fully understood. This is not to undervalue the studies conducted so far, which, despite their incompleteness, have yielded valuable information on the genetic basis of adaptive divergence and the nature and genetic basis of reproductive barriers between species. As emphasized by Hewitt [47, p. 158], hybrid zones are ‘natural laboratories for evolutionary studies’.
3. Determining the selective forces acting across hybrid zones: the Senecio hybrid zone on Mount Etna, Sicily
(a). Clinal variation, gene dispersal and selection
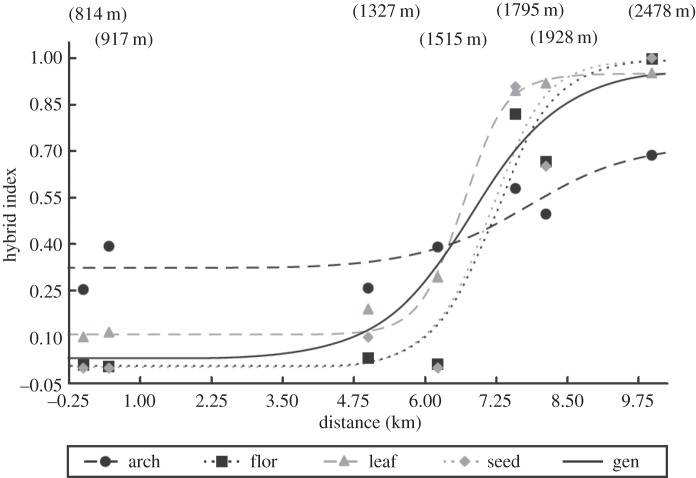
Two closely related ragwort species, Senecio aethnensis and Senecio chrysanthemifolius (Asteraceae), occur at high (more than 2500 m) and low altitudes (less than 600 m), respectively, on Mount Etna and form a hybrid zone at intermediate altitudes [38,39]. Both species are diploid (2n = 20), self-incompatible, short-lived herbaceous perennials that are easily distinguished from each other by differences in leaf shape, flower head (capitulum) size and fruit (achene) size. Senecio aethnensis has entire glaucous leaves and large flower heads and fruits, in contrast to the highly dissected, non-glaucous leaves and relatively small flower heads and fruits produced by S. chrysanthemifolius. Clinal change across the altitudinal gradient on Mount Etna is evident for these morphological characters (figure 1, [39]) and also for molecular marker genetic assignments to the two parental species (figure 1) [38,39]. A detailed analysis of this variation for a wide range of quantitative traits and several molecular markers indicated that although extrinsic selection was important in the adaptation of the two species to different altitudes, intrinsic selection against hybrids restricted gene flow between them [39]. Thus, narrower cline widths or shifts in the centre of a cline were recorded for three groups of quantitative traits (leaf shape, inflorescence structure and fruit structure) relative to molecular clines, indicating that such traits were under extrinsic environmental selection, and that differences between species reflected adaptations to different altitudes maintained by extrinsic selection. Furthermore, because cline widths and their centres varied slightly between quantitative trait groups, it was likely that the locations of selective optima varied for different traits, with selection for opposing leaf shapes occurring at a lower altitude on Mount Etna than selection for opposing floral structure and seed type. However, the molecular and quantitative genetic clines also indicated that strong intrinsic selection acted against hybrids. This was evident from tests of different models of hybrid zone structure which showed that dispersal-dependent tests, based on a balance of gene flow and intrinsic selection against hybrids, were more effective at explaining molecular variation and quantitative trait variation than were dispersal-independent tests where extrinsic selection due to local altitude would cause reduced hybrid fitness. Intrinsic selection against hybrids explained elevated linkage disequilibrium (LD) for molecular markers observed at intermediate hybrid indices in this study, and from the LD values, indirect estimates of both strong selection against hybrids and high rates of gene dispersal per generation were calculated. Thus, it was concluded that the Senecio hybrid zone on Mount Etna was characterized by strong intrinsic selection against hybrids and high dispersal rates [39].
Figure 1.
Changes in mean hybrid index for molecular and quantitative trait variation across the altitudinal gradient on Mount Etna, Sicily. Each quantitative trait group consists of two to three uncorrelated quantitative traits that are involved in similar ecological functions: arch, plant architecture; flor, inflorescence structure; leaf, leaf structure; seed, fruit and seed structure; gen, indicates the cline for molecular variation. Altitudes of sampled sites are indicated in parentheses at top of figure. (Adapted from [39]; reproduced with permission of John Wiley & Sons Inc.)
(b). Experimental measurement of fitness
Although analyses of clinal variation of the type described above are instructive of how selection structures and maintains a hybrid zone, experimental measures of the relative fitness of parent species and hybrids are crucial for an understanding of how selection operates. Ideally, reciprocal transplant analyses should be conducted over several years to estimate the relative fitness of hybrids and parent taxa, and this has yet to be done for the Senecio hybrid zone on Mount Etna. The only direct experimental comparison of fitness conducted so far on parents and hybrids in this hybrid zone comes from a study of germination and early seedling growth of progeny raised from crosses within and between populations [48]. This study revealed that seeds from higher altitudinal populations germinated better over low to intermediate temperatures, while at warmer temperatures there was a slight advantage in survival of seedlings derived from seed from lower altitude. These differences might reflect adaptations to high and low altitudinal conditions, respectively. Interestingly, the study produced almost no evidence of intrinsic selection against hybrids for the characters measured. Thus, seed of natural hybrids examined and also F1 hybrids between low- and high-altitudinal populations generally exhibited intermediate germination, and seedling growth and survival. The only exceptions to this occurred under cold conditions where seedling growth and survival of natural hybrids were inferior to both parents (significantly so for height), while seedling growth (but not survival) of F1s was significantly greater than either parent. This suggests that F1s show heterosis for seedling growth, which is lost in more advanced generation hybrids. It was concluded that evidence for intrinsic selection against hybrids during early stages of the life cycle was very limited. Rather, it was likely that at intermediate altitudes, hybrids exhibited more adapted phenotypes than parental species and that extrinsic selection favoured hybrids at these altitudes [48]. These results therefore tend to support a bounded hybrid superiority model, rather than a tension zone model, for the Senecio hybrid zone on Mount Etna.
In contrast to the above, direct evidence of hybrids exhibiting low intrinsic fitness has come from other studies involving crossing the parent species. Fertile F1 hybrids are easily produced from artificial crosses between the two Senecio species on Mount Etna [49], which initially suggested that hybrids might not be adversely affected by intrinsic selection [38]. However, the hypothesis that intrinsic selection acts against hybrids within the hybrid zone on Mount Etna [39] has since received support from two studies in which post-F1 hybrids were examined. The first of these studies examined germination and seedling survivorship of F1–F5 progeny produced from a reciprocal cross between S. aethnensis and S. chrysanthemifolius [50]. Although this study was aimed primarily at comparing differences in gene expression between the two species and hybrids, germination and seedling survivorship was also monitored during the course of the experiment. Interestingly, a sharp decrease in percentage seed germination was recorded, falling from approximately 94% to approximately 73% between the F1 and F2 generations and to approximately 18% in the F3 (figure 2), before a return to higher percentages was observed in the F4 and F5 generations. Also, between the F1 and F2 generations, seedling survivorship fell from 100% to approximately 87%. These results indicate that intrinsic selection against hybrids becomes apparent after the F1 generation as a result of hybrid breakdown. The return to higher levels of seedling survival after the F2, and also seed germination after the F3 generation, is likely to be a consequence of indirect selection in the experiment for hybrid genotypes exhibiting high seed germination and seedling survivorship [50].
Figure 2.
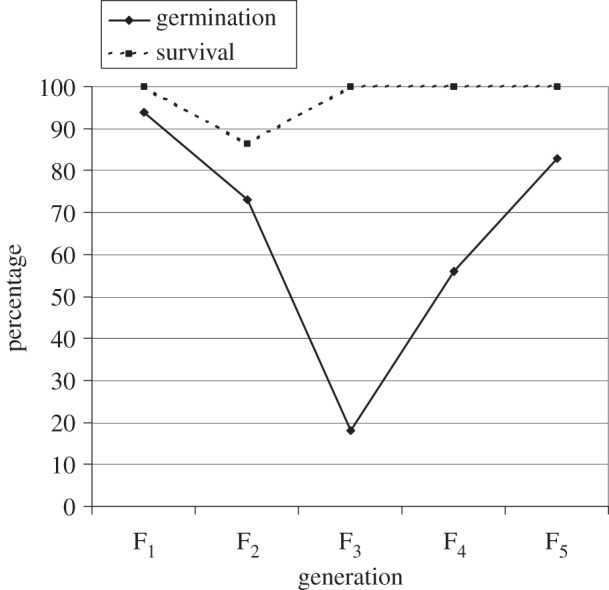
Percentage seed germination (after three weeks) and seedling survival (after six weeks) of F1–F5 progeny of a reciprocal cross between S. aethnensis and S. chrysanthemifolius. (Adapted from [50]; reproduced with permission of John Wiley & Sons Inc.)
Further evidence of reductions in fitness of later generation hybrids between S. aethnensis and S. chrysanthemifolius emerged from an analysis of genetic segregation among F2 progeny of a reciprocal cross between the two species [51]. Of 120 F2 progeny examined, 20 (16.7%) failed to either germinate or flower and were not genotyped. Among the remaining offspring, it was evident that genetic segregation at many loci was significantly distorted from expected Mendelian ratios, i.e. there was significant transmission ratio distortion (TRD). In fact, significant TRD was evident for approximately a quarter of 127 marker loci examined in the F2. These TRD loci were mapped to nine distinct clusters across seven of the 10 linkage groups detected, indicating widespread genomic effects of incompatibility between the species. Interestingly, levels of TRD recorded could not be entirely explained by loss of F2 individuals that failed to germinate or flower, indicating that incompatibility could affect seed production in addition to seed viability/germination and ability to flower. An examination of possible causes of TRD indicated that prezygotic events such as meiotic drive in F1 parents or gametophytic selection contributed to the TRD at four loci, cytonuclear incompatibility contributed to TRD at five loci, Bateson–Dobzhansky–Muller incompatibilities involving epistatic interactions between loci contributed to TRD at four loci, and underdominance was a possible cause of TRD at one locus. It was concluded that intrinsic selection against hybrids as evidenced by the occurrence of TRD across multiple genomic regions in the F2 would be an important factor in structuring the hybrid zone between S. aethnensis and S. chrysanthemifolius on Mount Etna according to the tension zone model.
In summary, from the relevant studies that have been conducted on the Mount Etna Senecio hybrid zone, it is clear that determining how selection acts on hybrids—in favour (extrinsically) or against (intrinsically)—is not an easy task. However, we consider that there is now very good evidence for intrinsic selection acting against hybrids between the two Senecio species based on the findings of the hybrid cline analysis conducted by Brennan et al. [39] and the crossing experiments of Hegarty et al. [50] and Brennan et al. [51]. Nevertheless, it should be emphasized that the lifetime fitnesses of different hybrid classes and parent species have yet to be measured across the altitudinal gradient on Mount Etna. Until this is done, our analysis of how extrinsic and intrinsic selection structures the hybrid zone will remain incomplete.
(c). Genetic basis of species differences and time since species divergence
Studies of molecular divergence between species that form hybrid zones can provide information on the genetic basis of adaptive divergence and how long ago such species diverged from their common ancestor. A recent examination of molecular genetic divergence within and among populations sampled from across part of the Mount Etna hybrid zone (from 230 to 2285 m altitude) has shown that most variation for expression-invariant genes and microsatellites was distributed within populations with less genetic differentiation occurring between low- and high-altitudinal populations [52]. By contrast, genetic divergence between low- and high-altitudinal populations was significantly higher for expressed genes that were possibly important in local adaptation to low and high altitudes, given their involvement in sulfur metabolism, response to cold temperature and cuticular wax biosynthesis. Similarly, two recent comparisons of the transcriptomes of plants from low (763–870 m) and high (2036–2471 m) altitudes have revealed that despite the marked phenotypic divergence between these plants divergence for synonymous nucleotide variation is very low, as is the proportion of fixed single nucleotide polymorphisms in approximately 10 000 genes analysed [53,54]. Moreover, only a few genes exhibit significantly elevated differentiation (i.e. are outliers) and even fewer show divergence in gene expression. From this, it was suggested that with regard to the two Senecio species on Mount Etna ‘Diversifying selection at only a handful of loci may be enough for the formation and maintenance of taxonomically well-defined species, despite ongoing gene flow.’ [54, p. 2553].
The molecular marker [52] and transcriptome [53,54] datasets from these studies were also used to estimate when the two Mount Etna species originated and the occurrence of gene flow between them. A coalescent analysis conducted on the set of expression-invariant genes and microsatellites indicated that divergence occurred approximately 32 000 years ago [52], while earlier times of divergence (approx. 108 000 to approx. 150 000 years ago) were estimated from the analyses of transcriptome data [53,54]. The analyses of both forms of data showed that significant gene flow had occurred between the two species during their history. Interestingly, time of divergence estimated from one of the transcriptome analyses [53] was correlated with the growth of Mount Etna to an altitude more than 1000 m where S. chrysanthemifolius is not found, and it was suggested that S. aethnensis possibly originated on Mount Etna ‘as a response to the emergence of a new, high-altitude niche as the volcano grew’ [53, p. 1704]. It was concluded from all of these studies that species divergence most likely took place along the altitudinal cline in the face of gene flow and was driven by extrinsic selection, and that the origin of the two species would ‘appear to be a classic example of ecological speciation in response to rapid geological upheaval’ [53, p. 1712]. That said, it was pointed out that the results of these analyses did not rule out the possibility of an initial period of allopatric divergence followed by secondary contact.
Caution is required in accepting the dates of divergence and levels of gene flow estimated from these studies, owing to the material chosen to represent each species. On Mount Etna, the most genetically divergent populations representing S. chrysanthemifolius and S. aethnensis occur below 900 m and above 2500 m, respectively, and populations between these altitudes contain plants that are admixed to varying degrees [38,55]. In the analysis of expression-invariant genes and microsatellites [52], material representing S. chrysanthemifolius was sampled from populations between 230 and 1085 m, while that representing S. aethnensis was collected from populations occurring between 1810 and 2285 m. For both species, therefore, samples were collected from some populations comprising admixed individuals rather than from the most divergent populations representing the two species. For the transcriptome analyses, material sampled from the lower altitudes (750–870 m) is likely to represent the most genetically divergent form of S. chrysanthemifolius, whereas plants derived from seed sampled from between 2036 and 2471 m are expected to be admixed and, therefore, unlikely to represent the most divergent form of S. aethnensis. Thus, in each of these studies divergence time between species is likely to have been underestimated and gene flow overestimated.
4. Generation of evolutionary novelty
(a). Adaptive introgression
It is now well known that introgression can lead to improved adaptation of a species to changed conditions [56–59], and so it is worth considering how this process might influence changes to plant hybrid zone structure across altitudinal gradients in response to climate change. Adaptive introgression can result in the formation of a bounded hybrid superiority hybrid zone across an altitudinal gradient and a recent attempt has been made to predict how such a hybrid zone in Picea (spruce), might respond to future climate change [17]. In this hybrid zone, which occurs in British Columbia, Canada, P. glauca, P. engelmannii and their hybrids grow at low, high and intermediate altitudes, respectively. Picea glauca outcompetes hybrids and P. engelmannii at low altitudes owing to an ability to grow faster, P. engelmannii performs best at high altitudes owing to greater tolerance to heavy snow loads or long, cold winters, and hybrids are best suited to intermediate altitudes owing to their ability to grow taller than P. engelmanii and set buds earlier than P. glauca. By 2050, mean annual temperature in the interior of British Columbia is predicted to rise by 2–3°C and the climate will become drier [17]. Models of the response of the hybrid zone to these climate changes predict that P. glauca will be favoured at both low and intermediate altitudes, while at high altitudes P. engelmannii will retain its advantage and hybrid index will not change much. These predictions, therefore, suggest that the current hybrid zone will become reduced in width in response to climate change, although caution is advised in accepting these predictions, given the uncertainty of future climates [17]. A more general belief is that climate warming will result in species adapted to high-altitude migrating to yet higher altitudes [60] and consequently hybrid zones will also be displaced to higher elevations. Should a high-altitudinal species eventually run out of habitat to which it is adapted, it is feasible that it will become extinct. Higher altitudes would then be occupied by hybrids if the hybrids represent a bounded hybrid superiority zone and do not require recurrent formation from crosses between parent species or by the lower altitudinal species in the case of hybrid tension zones with competitively inferior hybrids.
(b). Hybrid speciation
As a consequence of being adapted to novel conditions, it is also feasible for a hybrid to evolve into a new species without change in ploidy level (homoploid hybrid speciation [10,16,61–63]). This is favoured by both ecological and spatial isolation from the parent species [64] and consequently is more likely to occur if the hybrid or a group of hybrids is dispersed to a novel environment away from the hybrid zone. There are currently two examples of homoploid hybrid species thought to have originated from a hybrid zone across an altitudinal gradient, Pinus densata and Senecio squalidus. Pinus densata is a homoploid hybrid species that originated following hybridization between Pinus tabuliformis and Pinus yunnanensis [65,66]. The three species currently have allopatric geographical distributions in China with P. tabuliformis widely distributed in northern and central China, P. yunnanensis occurring in southwest China, while P. densata is extensively distributed in the southeastern part of the Tibetan Plateau. Whereas P. tabuliformis mainly grows at low to medium altitudes (400–1770 m, although occasionally reaches 3000 m), P. yunnanensis usually grows at higher altitudes (2000 to more than 3000 m), while P. densata is normally found at even higher altitudes (up to 4200 m) [67] showing enhanced drought-tolerance that is interpreted as an adaptation to high-altitude habitats [68]. From a survey of chloroplast and mitochondrial DNA throughout the ranges of each of these species, a putative ancestral hybrid zone between P. tabuliformis and P. yunnanensis was identified in the northeastern periphery of P. densata's distribution [67]. It is proposed that this was formed during a time when the distributions of the two parent species overlapped and that P. densata became established by migrating westwards from this hybrid zone to colonize a novel high-altitude habitat on the Tibetan Plateau, from which both parent species are excluded [67,69]. A subsequent survey of nucleotide variation over eight nuclear loci in P. densata indicated an origin of this species in the Late Miocene associated with a recent uplift of the Tibetan Plateau [70].
Because of the relatively ancient origin of P. densata and putative status of the hybrid zone from which it is thought to have originated, details of hybrid zone structure at the time of its origin are lacking. This is not the case for S. squalidus, which originated in Britain following the introduction of plants from the hybrid zone between S. aethnensis and S. chrysanthemifolius on Mount Etna approximately 300 years ago [38,63,71,72]. Thus, in this case it is possible to compare the new homoploid hybrid species with parental and hybrid plants from the hybrid zone from which it was recently derived to determine what changes may have occurred during its origin. Initial comparisons have shown that S. squalidus is clearly distinguished genetically from both parent species and hybrids present on Mount Etna (figure 3a) owing to changes in allele frequencies including loss of some alleles and gain of a few others [73]. It is also different, although less so, in morphology (figure 3b). Whereas for some morphological traits it has an intermediate mean, for others it resembles one or other of the parents, and for a few traits it is transgressive, i.e. exhibits a more extreme mean than either parent species. Transgressive effects were also evident in gene expression of 203 expressed sequence tags (ESTs) when S. squalidus was compared with its parent species using microarrays [50]. Two of the ESTs showing transgressively lower expression in S. squalidus (confirmed by qPCR) are involved in response to sulfur deficiency, which may reflect an adaptation to reduced sulfur levels in UK soils relative to soils on Mount Etna [50]. Transgressive segregation has been proposed as an important generator of evolutionary novelty [74] and a cause of homoploid hybrid species being adapted to novel environments [61,62]. It is feasible that it has played a role in the adaptation of S. squalidus to UK soil conditions, although this requires rigorous testing.
Figure 3.
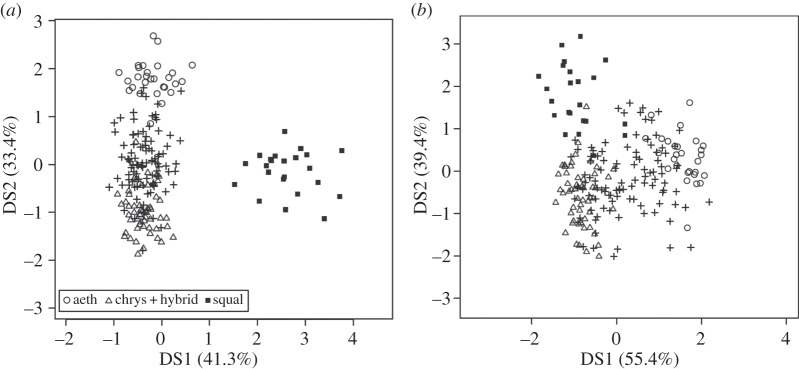
Plots of first and second discriminant coordinate scores resulting from analyses of (a) between-individual genetic distances based on 22 molecular genetic markers and (b) 20 quantitative traits. The analyses were conducted on samples of S. aethnensis, S. chrysanthemifolius and their hybrids from Mount Etna, Sicily, and also S. squalidus from the UK. Axes are labelled with the percentage variance explained by the first and second discriminant functions, respectively. (Adapted from [73]; reproduced with permission of Nature Publishing Group.)
The fact that there is now a body of evidence that strong intrinsic selection acts against hybrids on Mount Etna (see above) raises the question as to how such hybrids were able to give rise to a highly successful homoploid hybrid species. It has been repeatedly emphasized, however, that hybrid fitness varies between hybrid genotypic classes and across environments [10,16,75] and consequently it is entirely feasible for a given set of hybrids showing low fitness under one set of conditions to exhibit high fitness under another set of conditions or give rise to a further set of hybrids with particular parental combinations showing high fitness under the same set of conditions. This makes it feasible that a new homoploid hybrid species, S. squalidus, emerged in the UK following cultivation in the Oxford Botanic Garden for almost a century, even though the original hybrids from within the hybrid zone on Mount Etna were relatively unfit compared with parents there.
5. Conclusion
Altitudinal gradients reflecting steep changes in a wide range of abiotic and biotic variables are excellent systems for examining adaptive divergence and its maintenance between plant species. Such gradients are very common and available for investigation throughout large parts of the world. Rather surprisingly, relatively few plant hybrid zones across altitudinal gradients have been investigated in detail, although those that have encompass trees, shrubs and herbaceous perennials, and also the three main types of hybrid zone model: tension, bounded hybrid superiority and mosaic. It is feasible that the relatively low number of hybrid zones investigated simply reflects the small number of plant evolutionary biologists engaged in work on them. Alternatively, it is possible that such hybrid zones are in fact rare in nature. Support for this second possibility comes from a recent study of range overlap and niche divergence among sister species pairs in the California Floristic Province [76]. This study showed that shifts in altitude were rare between sister species, which suggested ‘ … a surprisingly limited role of altitudinal zonation in promoting speciation … despite the fact that we can often find ecotypes adapted to elevation … ’ [76, p. 6]. If this finding is generally true for other regions as well, it could partly account for why relatively few hybrid zones across altitudinal gradients have been studied to date, in that such hybrid zones might be rare because they are more likely to form between closely related sister species than between more distantly related taxa.
Classifying hybrid zones according to the three well-known models and, therefore, establishing how they are maintained in the wild is difficult owing to limitations of different approaches taken and a lack of critical information with regard to measures of relative lifetime fitness across the environmental gradient and in different years. A particular issue is to determine accurately whether hybrids are subject to extrinsic selection and favoured in intermediate environments (bounded hybrid superiority model), or are selected against owing to intrinsic incompatibilities between genes inherited from progenitor species (tension zone model). Studies on Senecio emphasize the point that it is at least necessary to examine fitness beyond the F1 generation before ruling out the possibility that intrinsic selection acts against hybrids. Hybrid zones across altitudinal gradients have the potential to generate evolutionary novelty through introgression and the establishment of new hybrid taxa. This has been demonstrated through the recent origin of the homoploid hybrid species S. squalidus in the UK from hybrid material introduced from Mount Etna, Sicily, and also the origin of the high-altitudinal homoploid hybrid species P. densata from a hybrid zone in China. The study of such hybrid zones across altitudinal gradients and the hybrid species they generate provide excellent opportunities for investigating how plants adapt to conditions that vary across these gradients and also to novel habitats away from the hybrid zones where the hybrid species are favoured.
Acknowledgements
We thank our research collaborators Simon Hiscock, Matthew Hegarty and Daniel Barker for their contributions to the Senecio research reviewed in this paper. R.J.A. acknowledges with fondness the inspirational effect that Leslie Gottlieb had on his research following their first meeting at Duke University in 1982 when both were on sabbatical leave there.
Funding statement
This work was supported by a grant from the Natural Environment Research Council (NE/D014166/1).
References
- 1.Turesson G. 1925. The plant species in relation to habitat and climate. Hereditas 6, 147–236. ( 10.1111/j.1601-5223.1925.tb03139.x) [DOI] [Google Scholar]
- 2.Clausen J, Keck DD, Hiesey WM. 1940. Experimental studies on the nature of species. I. The effect of varied environments on Western North American plants, pp. 1–452. Washington, DC: Carnegie Institution of Washington Publications. [Google Scholar]
- 3.Briggs D, Walters SM. 1997. Plant variation and evolution, 3rd edn Cambridge, UK: Cambridge University Press. [Google Scholar]
- 4.Antonovics J, Bradshaw AD. 1970. Evolution in closely adjacent plant populations. VIII. Clinal patterns at a mine boundary. Heredity 25, 349–362. ( 10.1038/hdy.1970.36) [DOI] [PubMed] [Google Scholar]
- 5.Korner C. 2007. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 22, 569–574. ( 10.1016/j.tree.2007.09.006) [DOI] [PubMed] [Google Scholar]
- 6.Byars SG, Papst W, Hoffmann AA. 2007. Local adaptation and cogradient selection in the alpine plant, Poa hiemata, along a narrow altitudinal gradient. Evolution 61, 2925–2941. ( 10.1111/j.1558-5646.2007.00248.x) [DOI] [PubMed] [Google Scholar]
- 7.Cordell S, Goldstein G, Mueller-Dombois D, Webb D, Vitousek PM. 1998. Physiological and morphological variation in Metrosideros polymorpha, a dominant Hawaiian tree species, along an altitudinal gradient: the role of phenotypic plasticity. Oecologia 113, 188–196. ( 10.1007/s004420050367) [DOI] [PubMed] [Google Scholar]
- 8.Hassel K, Pedersen B, Soderstrom L. 2005. Changes in life-history traits in an expanding moss species: phenotypic plasticity or genetic differentiation? A reciprocal transplantation experiment with Pogonatum dentatum. Ecography 28, 71–80. ( 10.1111/j.0906-7590.2005.03910.x) [DOI] [Google Scholar]
- 9.Hovenden MJ, Vander Schoor JK. 2004. Nature vs nurture in the leaf morphology of Southern beech, Nothofagus cunninghamii [Nothofagaceae]. New Phytol. 161, 585–594. ( 10.1046/j.1469-8137.2003.00931.x) [DOI] [PubMed] [Google Scholar]
- 10.Arnold ML. 2006. Evolution through genetic exchange. Oxford, UK: Oxford University Press. [Google Scholar]
- 11.Barton NH, Hewitt GM. 1985. Analysis of hybrid zones. Annu. Rev. Ecol. Syst. 16, 113–148. ( 10.1146/annurev.es.16.110185.000553) [DOI] [Google Scholar]
- 12.Moore WS. 1977. An evaluation of narrow hybrid zones in vertebrates. Q. Rev. Biol. 52, 263–78. ( 10.1086/409995) [DOI] [Google Scholar]
- 13.Harrison RG. 1986. Pattern and process in a narrow hybrid zone. Heredity 56, 337–349. ( 10.1038/hdy.1986.55) [DOI] [Google Scholar]
- 14.Harrison RG. 1990. Hybrid zones: windows on evolutionary process. Oxford Surveys Evol. Biol. 7, 69–128. [Google Scholar]
- 15.Howard DJ. 1986. A zone of overlap and hybridization between two ground cricket species. Evolution 40, 34–43. ( 10.2307/2408601) [DOI] [PubMed] [Google Scholar]
- 16.Arnold ML. 1999. Natural hybridization and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 17.De La Torre A, Wang T, Jaquish B, Aitken SN. 2014. Adaptation and exogenous selection in a Picea glauca × P. engelmannii hybrid zone: implications for forest management under climate change. New Phytol. 201, 687–699. ( 10.1111/nph.12540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watano Y, Imazu M, Shmizu T. 1995. Chloroplast DNA typing by PCR-SSCP in the Pinus pumila–P. parviflora var. pentaphylla complex (Pinaceae). J. Plant Res. 108, 493–499. ( 10.1007/BF02344239) [DOI] [Google Scholar]
- 19.Watano Y, Kania A, Tani N. 2004. Genetic structure of hybrid zones between Pinus pumila–P. parviflora var. pentaphylla [Pinaceae] revealed by molecular hybrid index analysis. Am. J. Bot. 91, 65–72. ( 10.3732/ajb.91.1.65) [DOI] [PubMed] [Google Scholar]
- 20.Whitham TG. 1989. Plant hybrid zones as sinks for pests. Science 244, 1490–1493. ( 10.1126/science.244.4911.1490) [DOI] [Google Scholar]
- 21.Martinsen GD, Whitham TG, Turek RJ, Keim P. 2001. Hybrid populations selectively filter gene introgression between species. Evolution 55, 1325–1335. ( 10.1111/j.0014-3820.2001.tb00655.x) [DOI] [PubMed] [Google Scholar]
- 22.Schweitzer JA, Martinsen GD, Whitham TG. 2002. Cottonwood hybrids gain fitness of both parents: a mechanism for their long-term persistence? Am. J. Bot. 89, 981–990. ( 10.3732/ajb.89.6.981) [DOI] [PubMed] [Google Scholar]
- 23.Albarrán-Lara AL, Mendoza-Cuenca L, Valencia-Avalos S, González-Rodríguez A, Oyama K. 2010. Leaf fluctuating asymmetry increases with hybridization and introgression between Quercus magnoliifolia and Quercus resinosa (Fagaceae) through an altitudinal gradient in Mexico. Int. J. Plant Sci. 171, 310–322. ( 10.1086/650317) [DOI] [Google Scholar]
- 24.Freeman DC, Turner WA, McArthur ED, Graham JH. 1991. Characterization of a narrow hybrid zone between two subspecies of Big sagebrush (Artemisia tridentata: Asteraceae). Am. J. Bot. 78, 805–815. ( 10.2307/2445072) [DOI] [Google Scholar]
- 25.Wang H, McArthur ED, Sanderson SC, Graham JH, Freeman DC. 1997. Narrow hybrid zone between two subspecies of Big sagebrush (Artemisia tridentata: Asteraceae). IV. Reciprocal transplant experiments. Evolution 51, 95–102. ( 10.2307/2410963) [DOI] [PubMed] [Google Scholar]
- 26.Miglia KJ, McArthur ED, Redman RS, Rodriguez RJ, Zak JC, Freeman DC. 2007. Genotype, soil type, and locale effects on reciprocal transplant vigor, endophyte growth, and microbial functional diversity of a narrow sagebrush hybrid zone in Salt Creek Canyon, Utah. Am. J. Bot. 94, 425–436. ( 10.3732/ajb.94.3.425) [DOI] [PubMed] [Google Scholar]
- 27.Milne RI, Terzioglu S, Abbott RJ. 2003. A hybrid zone dominated by fertile F1s: maintenance of species barriers in Rhododendron. Mol. Ecol. 12, 2719–2729. ( 10.1046/j.1365-294X.2003.01942.x) [DOI] [PubMed] [Google Scholar]
- 28.Chase VC, Raven PH. 1975. Evolutionary and ecological relationships between Aquilegia formosa and A. pubescens [Ranunculaceae], two perennial plants. Evolution 29, 474–486. ( 10.2307/2407260) [DOI] [PubMed] [Google Scholar]
- 29.Fulton M, Hodges SA. 1999. Floral isolation between Aquilegia formosa and A. pubescens. Proc. R. Soc. Lond. B 266, 2247–2252. ( 10.1098/rspb.1999.0915) [DOI] [Google Scholar]
- 30.Hodges SA, Arnold ML. 1994. Floral and ecological isolation between Aquilegia formosa and A. pubescens. Proc. Natl Acad. Sci. USA 91, 2493–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell DR, Waser NM, Meledez-Ackerman EJ. 1997. Analyzing pollinator-mediated selection in a plant hybrid zone: hummingbird visitation patterns on three spatial scales. Am. Nat. 149, 295–315. ( 10.1086/285991) [DOI] [Google Scholar]
- 32.Campbell DR, Waser NM. 2001. Genotype-by-environment interaction and the fitness of plant hybrids in the wild. Evolution 55, 669–676. ( 10.1554/0014-3820(2001)055[0669:GBEIAT]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 33.Campbell DR, Waser NM, Pederson GT. 2002. Predicting patterns of mating and potential hybridization from pollinator behavior. Am. Nat. 159, 438–450. ( 10.1086/339457) [DOI] [PubMed] [Google Scholar]
- 34.Campbell DR, Waser NM, Aldridge G, Wu CA. 2008. Lifetime fitness in two generations of Ipomopsis hybrids. Evolution 62, 2616–2627. ( 10.1111/j.1558-5646.2008.00460.x) [DOI] [PubMed] [Google Scholar]
- 35.Kimball S. 2008. Links between floral morphology and floral visitors along an elevational gradient in a Penstemon hybrid zone. Oikos 117, 1064–1074. ( 10.1111/j.2008.0030-1299.16573.x) [DOI] [Google Scholar]
- 36.Kimball S, Campbell DR, Lessin C. 2008. Differential performance of reciprocal hybrids in multiple environments. J. Ecol. 96, 1306–1318. ( 10.1111/j.1365-2745.2008.01432.x) [DOI] [Google Scholar]
- 37.Kimball S, Campbell D. 2009. Physiological differences among two Penstemon species and their hybrids in field and common garden environments. New Phytol. 181, 478–488. ( 10.1111/j.1469-8137.2008.02654.x) [DOI] [PubMed] [Google Scholar]
- 38.James JK, Abbott RJ. 2005. Recent, allopatric, homoploid hybrid speciation: the origin of Oxford ragwort, Senecio squalidus [Asteraceae], in the British Isles from a hybrid zone on Mount Etna, Sicily. Evolution 59, 2533–2547. ( 10.1111/j.0014-3820.2005.tb00967.x) [DOI] [PubMed] [Google Scholar]
- 39.Brennan AC, Bridle JR, Wang A-L, Hiscock SJ, Abbott RJ. 2009. Adaptation and selection in the Senecio [Asteraceae] hybrid zone on Mount Etna, Sicily. New Phytol. 183, 702–717. ( 10.1111/j.1469-8137.2009.02944.x) [DOI] [PubMed] [Google Scholar]
- 40.Raudnitschka D, Hensen C, Oberprieler C. 2007. Introgressive hybridization of Senecio hercynicus and S. ovatus [Compositae, Senecioneae] along an altitudinal gradient in Harz National Park [Germany]. Syst. Biodivers. 5, 333–344. ( 10.1017/S1477200007002435) [DOI] [Google Scholar]
- 41.Minder AM, Rothenbuehler C, Widmer A. 2007. Genetic structure of hybrid zones between Silene latifoia and Silene dioica [Caryophyllaceae]: evidence for introgressive hybridization. Mol. Ecol. 16, 2504–2516. ( 10.1111/j.1365-294X.2007.03292.x) [DOI] [PubMed] [Google Scholar]
- 42.Gutierrez Larena B, Fuertes Aguilar J, Nieto Feliner G. 2002. Glacial-induced altitudinal migrations in Armeria (Plumbaginaceae) inferred from patterns of chloroplast DNA haplotype sharing. Mol. Ecol. 11, 1965–1974. ( 10.1046/j.1365-294X.2002.01594.x) [DOI] [PubMed] [Google Scholar]
- 43.Fuertes Aguilar J, Gutierrez Larena B, Nieto Feliner G. 2011. Genetic and morphological diversity in Armeria (Plumbaginacae) is shaped by glacial cycles in Mediterranean refugia. Ann. Jard. Bot. Madrid 68, 175–197. ( 10.3989/ajbm.2260) [DOI] [Google Scholar]
- 44.Tsukaya H. 2004. Gene flow between Impatiens radicans and I. javensis (Balsaimaceae) in Gunung Pangrango, central Java, Indonesia. Am. J. Bot. 91, 2119–2123. ( 10.3732/ajb.91.12.2119) [DOI] [PubMed] [Google Scholar]
- 45.Matos JA, Schaal BA. 2000. Chloroplast evolution in the Pinus montezumae complex: a coalescent approach to hybridization. Evolution 54, 1218–1233. ( 10.1111/j.0014-3820.2000.tb00556.x) [DOI] [PubMed] [Google Scholar]
- 46.Kobayashi N, Handa T, Yoshimura K, Tsumura Y, Arisumi K, Takayanagi K. 2000. Evidence for introgressive hybridization based on chloroplast DNA polymorphisms and morphological variation in wild evergreen azalea populations of the Kirishima mountains, Japan. Edinb. J. Bot. 57, 209–219. ( 10.1017/S0960428600000147) [DOI] [Google Scholar]
- 47.Hewitt GM. 1988. Hybrid zones: natural laboratories for evolutionary studies. Trends Ecol. Evol. 3, 158–167. ( 10.1016/0169-5347(88)90033-X) [DOI] [PubMed] [Google Scholar]
- 48.Ross RIC, Ågren JA, Pannell JR. 2012. Exogenous selection shapes germination behaviour and seedling traits of populations at different altitudes in a Senecio hybrid zone. Ann. Bot. 110, 1439–1447. ( 10.1093/aob/mcs211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chapman MA, Forbes DG, Abbott RJ. 2005. Pollen competition among two species of Senecio (Asteraceae) that form a hybrid zone on Mt Etna, Sicily. Am. J. Bot. 92, 730–735. ( 10.3732/ajb.92.4.730) [DOI] [PubMed] [Google Scholar]
- 50.Hegarty MJ, Barker GL, Brennan AC, Edwards KJ, Abbott RJ, Hiscock SJ. 2009. Extreme changes to gene expression associated with homoploid hybrid speciation. Mol. Ecol. 18, 877–889. ( 10.1111/j.1365-294X.2008.04054.x) [DOI] [PubMed] [Google Scholar]
- 51.Brennan AC, Hiscock SJ, Abbott RJ. In press. Interspecific crossing and genetic mapping reveal intrinsic genomic incompatibility between two Senecio species that form a hybrid zone on Mount Etna, Sicily. Heredity. ( 10.1038/hdy.2014.14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muir G, Osborne OG, Sarasa J, Hiscock SJ, Filatov DA. 2013. Recent ecological selection on regulatory divergence is shaping clinal variation in Senecio on Mount Etna. Evolution 67, 3032–3042. ( 10.1111/evo.12157) [DOI] [PubMed] [Google Scholar]
- 53.Osborne OG, Batstone TE, Hiscock SJ, Filatov DA. 2013. Rapid speciation with gene flow following the formation of Mt. Etna. Genome Biol. Evol. 5, 1704–1715. ( 10.1093/gbe/evt127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chapman MA, Hiscock SJ, Filatov DA. 2013. Genomic divergence during speciation driven by adaptation to altitude. Mol. Biol. Evol. 30, 2553–2567. ( 10.1093/molbev/mst168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.James JK. 1999. Molecular analysis of the hybrid origin of Oxford ragwort, Senecio squalidus L., and related studies. PhD thesis, University of St Andrews, St Andrews, UK. [Google Scholar]
- 56.Martin NH, Bouck AC, Arnold ML. 2006. Detecting adaptive trait introgression between Iris fulva and I. brevicaulis in highly selective field conditions. Genetics 172, 2481–2489. ( 10.1534/genetics.105.053538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whitney KD, Randell RA, Rieseberg LH. 2010. Adaptive introgression of abiotic tolerance traits in the sunflower Helianthus annuus. New Phytol. 187, 230–239. ( 10.1111/j.1469-8137.2010.03234.x) [DOI] [PubMed] [Google Scholar]
- 58.Abbott R, et al. 2013. Hybridization and speciation. J. Evol. Biol. 26, 229–246. ( 10.1111/j.1420-9101.2012.02599.x) [DOI] [PubMed] [Google Scholar]
- 59.Hedrick PW. 2013. Adaptive introgression in animals: examples and comparisons to new mutation and standing variation as sources of adaptive variation. Mol. Ecol. 22, 4606–4618. ( 10.1111/mec.12415) [DOI] [PubMed] [Google Scholar]
- 60.Crawford RMM. 2008. Plants at the margin: ecological limits and climate change. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 61.Rieseberg LH, et al. 2003. Major ecological transitions in wild sunflowers facilitated by hybridization. Science 301, 1211–1216. ( 10.1126/science.1086949) [DOI] [PubMed] [Google Scholar]
- 62.Gross BL, Rieseberg LH. 2005. The ecological genetics of homoploid hybrid speciation. J. Hered. 96, 241–252. ( 10.1093/jhered/esi026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abbott RJ, Hegarty MJ, Hiscock SJ, Brennan AC. 2010. Homoploid hybrid speciation in action. Taxon 59, 1375–1386. [Google Scholar]
- 64.Buerkle CA, Morris RJ, Asmussen MA, Rieseberg LH. 2000. The likelihood of homoploid hybrid speciation. Heredity 84, 441–451. ( 10.1046/j.1365-2540.2000.00680.x) [DOI] [PubMed] [Google Scholar]
- 65.Wang XR, Szmidt AE. 1994. Hybridization and chloroplast DNA variation in a Pinus species complex from Asia. Evolution 48, 1020–1031. ( 10.2307/2410363) [DOI] [PubMed] [Google Scholar]
- 66.Song BH, Wang XQ, Wang XR, Ding KY, Hong DY. 2003. Cytoplasmic composition in Pinus densata and population establishment of the diploid hybrid pine. Mol. Ecol. 12, 2995–3001. ( 10.1046/j.1365-294X.2003.01962.x) [DOI] [PubMed] [Google Scholar]
- 67.Wang B, Mao J-F, Gao J, Zhao W, Wang X-R. 2011. Colonization of the Tibetan Plateau by the homoploid hybrid pine Pinus densata. Mol. Ecol. 20, 3796–3811. ( 10.1111/j.1365-294X.2011.05157.x) [DOI] [PubMed] [Google Scholar]
- 68.Ma F, Zhao C-M, Milne RI, Ji M-F, Chen L-T, Liu J-Q. 2010. Enhanced drought-tolerance in the homoploid hybrid species Pinus densata: implications for its habitat divergence from two progenitors. New Phytol. 185, 204–216. ( 10.1111/j.1469-8137.2009.03037.x) [DOI] [PubMed] [Google Scholar]
- 69.Mao J-F, Wang X-R. 2011. Distinct niche divergence characterizes the homoploid hybrid speciation of Pinus densata on the Tibetan Plateau. Am. Nat. 177, 424–439. ( 10.1086/658905) [DOI] [PubMed] [Google Scholar]
- 70.Gao J, Wang B-S, Mao J-F, Ingvarsson P, Zeng Q-Y, Wang X-R. 2012. Demography and speciation history of the hybrid pine Pinus densata on the Tibetan Plateau. Mol. Ecol. 21, 4811–4827. ( 10.1111/j.1365-294X.2012.05712.x) [DOI] [PubMed] [Google Scholar]
- 71.Harris SA. 2002. Introduction of Oxford ragwort, Senecio squalidus L. [Asteraceae], to the United Kingdom. Watsonia 24, 31–43. [Google Scholar]
- 72.Abbott RJ, Brennan AC, James JK, Forbes DF, Hegarty MJ, Hiscock SJ. 2009. Recent hybrid origin and invasion of the British Isles by a self-incompatible species, Oxford ragwort [Senecio squalidus L., Asteraceae]. Biol. Invasions 11, 1145–1158. ( 10.1007/s10530-008-9382-3) [DOI] [Google Scholar]
- 73.Brennan AC, Barker D, Hiscock SJ, Abbott RJ. 2012. Molecular genetic and quantitative trait divergence associated with recent homoploid hybrid speciation: a study of Senecio squalidus [Asteraceae]. Heredity 108, 87–95. ( 10.1038/hdy.2011.46) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dittrich-Reed DR, Fitzpatrick BM. 2013. Transgressive hybrids as hopeful monsters. Evol. Biol. 40, 310–315. ( 10.1007/s11692-012-9209-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arnold ML, Ballerini ES, Brothers AN. 2012. Hybrid fitness, adaptation and evolutionary diversification: lessons learned from Louisiana irises. Heredity 108, 159–166. ( 10.1038/hdy.2011.65) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anacker BL, Strauss SY. 2014. The geography and ecology of plant speciation: range overlap and niche divergence in sister species. Proc. R. Soc. B 281, 20132980 ( 10.1098/rspb.2013.2980) [DOI] [PMC free article] [PubMed] [Google Scholar]