Abstract
Much of the plasticity that prey exhibit in response to predators is linked to the prey's immediate background level of risk. However, we know almost nothing of how background risk influences how prey learn to categorize predators and non-predators. Learning non-predators probably represents one of the most underappreciated aspects of anti-predator decision-making. Here, we provide larval damselfish (Pomacentrus chrysurus) with a high or low background risk and then try to teach them to recognize a cue as non-threatening through the process of latent inhibition. Prey from the low-risk background that were pre-exposed to the novel odour cues in the absence of negative reinforcement for 3 days, and then provided the opportunity to learn to recognize the odour as threatening, failed to subsequently respond to the odour as a threat. Fish from the high-risk background showed a much different response. These fish did not learn the odour as non-threatening, probably because the cost of falsely learning an odour as non-threatening is higher when the background level of risk is higher. Our work highlights that background level of risk appears to drive plasticity in cognition of prey animals learning to discriminate threats in their environment.
Keywords: learning, predator recognition, non-predator recognition, risk assessment, damselfish, coral reefs
1. Introduction
Young prey animals are exposed to a myriad of unknown species, any one of which can be a potential threat. It is therefore critical for prey to distinguish predators from non-predators or risky from safe situations. Not surprisingly, the field of behavioural ecology is rife with hundreds of examples of the way in which animals learn to respond to risk [1–6]. However, in stark contrast, there are relatively few studies that examine how prey animals learn to recognize non-predators and safety [7,8]. Latent inhibition is one such mechanism, in which prey repeatedly exposed to an unknown stimulus (e.g. the sight or smell of a novel species) in the absence of negative reinforcement gradually learn to label this stimulus as non-risky. In this case, a subsequent attempt to teach the prey to recognize the stimulus as risky would typically fail because the stimulus has already been classified as safe. For example, Acquistapace et al. [9] showed that exposure of crayfish (Orconectes virilis and Orconectes rusticus) to the cues of an unknown fish in the absence of risk prevented the crayfish from learning the fish as a threat when it was later associated with risk, via pairing with injured crayfish cues. Likewise, Ferrari & Chivers [10] showed that pre-exposure of fathead minnows (Pimephales promelas) to pike (Esox lucius) odour over several days prevented minnows from learning pike odour as a threat when it was associated with risk from injured minnow cues. In the most comprehensive study to date, Mitchell et al. [7] showed that latent inhibition in a coral reef fish, the lemon damselfish (Pomacentrus moluccensis), could be overridden with multiple subsequent risk-learning trials. In this case, the repeated pairing of the ‘safe’ novel stimulus with risk shifted the value of the stimulus from ‘safe’ to ‘risky’, illustrating the liability of information processing in prey species. This work indicates that latent inhibition is flexible and allows animals to use the most up-to-date information to adjust their classification of predators and non-predators.
Predation risk varies over both space and time, and this variation is a key factor determining how prey respond to risk [11,12]. Ecology has traditionally considered the importance of spatial variation in predation risk [13], but studies of temporal variation were quite limited until the publication of Lima & Bednekoff's [5] landmark paper on risk allocation. This model proposed that recent background level of risk is a key factor determining the intensity of responses of animals to cues of known predators and has received considerable empirical support [12,14,15]. Recently, Brown et al. [16] have also shown that recent background level of risk can promote plasticity in the way animals respond not only to predators, but also to unknown, novel cues in their environment. Both guppies (Poecilia reticulata) and woodfrog tadpoles (Lythobates sylvaticus) raised in a high-risk background environment respond to novel predators upon their first exposure. However, individuals from the same population that are raised in a low-risk background fail to respond to novel predators. This was the first demonstration that environmental risk could induce neophobic responses. In a follow-up study, Brown et al. [17] showed that there was gradation in neophobic responses that matched the level of background risk. These studies highlight that background level of risk is a key factor in prey responses to unknown predators. Here, we assess whether the behavioural plasticity that is associated with background risk in freshwater guppies and woodfrogs also occurs in our marine damselfish (Pomacentrus amboinensis) system, and whether this differential risk background has implications for how prey learn to respond to predators and non-predators. The biodiversity of tropical ecosystems is much greater than that of temperate ecosystems. With the exception of apex predators, the potential for finding low-risk habitats is much reduced for the vast majority of prey, given the number and variation in potential predators in those environments. Hence, it is possible that prey in these rich ecosystems may have evolved risk assessment and predator evasion strategies that may differ from those in much simpler temperate systems.
Lemon damselfish, our test species, exhibit a bipartite life history, starting with pelagic larvae that recruit back onto the reef after a couple of weeks to become benthic adults. Our test subjects were captured immediately prior to their settlement onto coral reefs (at the transition from pelagic to benthic), making our experiment a cognitive exercise that is naturally taking place at this point in their ontogeny. This transition represents a major life-history switch point for them; when the larvae (approx. 1 cm in length) arrive at the reef after their pelagic phase, they are confronted with hundreds of unknown species that may or may not be predators. Predation-related mortality is extreme at this time, with more than 60% of fish being captured in the first 2 days after settlement [18] by a diverse group of specialist and opportunistic predators. With so many predators, it may be wise to respond to all potential threats with an anti-predator response. However, an immense selection on size-mediated traits influencing the outcome of competitive interactions for food and habitat [19] is also taking place; hence, hiding (and thus by-passing feeding opportunities) is not a viable option. Damselfishes do not respond to predators without prior experience but very quickly start to categorize species as dangerous [7,20–23].
Here, we exposed larval damselfish to a high or low background level of risk right at the point of settlement to the reef. What makes an environment more or less risky is linked to a number of predation-related parameters, including predator encounter rate, predator density, proportion of predators to non-predators and success rate of predators. From a prey's point of view, these endpoints can be mediated via detection of risk-related cues in the water column; for instance, the frequency of detection of injured conspecific cues. We used repetitive exposures to injured conspecific cues (hereafter alarm cues) to mediate a high- versus low-risk environment without informing the prey on the exact nature of the predator. After providing fish with a high or low background for several days, we then tried to induce latent inhibition in both groups of fish by repeatedly exposing them to the odour of a common reef predator for several days. To test whether this pre-exposure was enough for the fish to learn the odour as non-threatening, we subsequently exposed them to the odour paired with a solution of injured conspecific cues. If the pre-exposure worked, the fish should fail to learn the odour as threatening. We predicted that changes in background levels of risk would alter the cost–benefit trade-offs between responding and non-responding to a potential threat. The likelihood of making a type II error (wrongly recognizing the odour as safe, when it was in fact risky) is probably higher in a high-risk environment than in a low-risk environment (see table 1 for an overview of our experimental design and associated predictions).
Table 1.
Predicted anti-predator responses for fish under various experimental regimes.
| background level of risk | pre-exposure | training to recognize the predator | test cue | predicted anti-predator response |
|---|---|---|---|---|
| low | water | yes yes | water predator cue | no response (−) anti-predator response present (+); training successful |
| predator cue | yes yes | water predator cue | no response (−) no anti-predator response (−); repeated exposure to predator without negative reinforcement leading to failed training (latent inhibition) | |
| high | water | yes yes | water predator cue | no response (−) anti-predator response present (+ or ++); high background of risk may lead to enhanced efficiency of training |
| predator cue | yes yes | water predator cue | no response (−) anti-predator response present; high background risk may impair latent inhibition. Impairment may be complete (++: pre-exposure is completely ignored) or partial (+: pre-exposure leads to a decrease in anti-predator response) |
2. Material and methods
(a). Fish collection and maintenance
This experiment took place in October and November 2013, at the Lizard Island Research Station, Great Barrier Reef, Australia (14°40′ S, 145°28′ E). Larval fish were captured approximately 100 m off the reef at night using light traps [24], in accordance with a Great Barrier Reef Marine Park Authority collection permit and a James Cook University Animal Ethics Committee Protocol (#A1593). Fish were immediately taken to the laboratory and placed in groups of 20 in a series of twenty-four 3 l plastic aquaria with a flow rate of approximately 3 l h−1. The fish were fed ad libitum with newly hatched brine shrimp three times per day.
(b). Experimental protocol
This experiment consisted of four phases. In the first phase, we manipulated the fish's background level of risk. Fish were exposed to high or low risk by introducing an alarm cue solution (high risk) or a seawater control (low risk) to the tanks three times per day for 4 days. Half the fish received high risk and the other half received low risk. The alarm cue solution was prepared by making six vertical cuts on each side of six freshly euthanized donor fish and then rinsing the fish in 60 ml of seawater. We injected 5 ml of this standard alarm cue solution into the conditioning tanks, giving us a concentration of 2 cuts l−1 when it was injected into the tanks. The timing of the three injections occurred at random times between 08.00 and 18.00, with a minimum of 1.5 h between injections.
The day following the end of the background risk exposure, six fish from each of the 24 tanks were transferred to 1 l plastic containers for the next phase of the experiment (a total of 144 fish). The remaining fish were used for a different experiment. The second phase was a pre-exposure phase, where we exposed half of the fish to predator odour three times per day for 3 days, while the other half received a saltwater injection on the same schedule. The background level of risk was also maintained throughout the predator pre-exposure phase such that each tank received a total of six injections per day during this phase. This gave us a design where high or low background risk was crossed with pre-exposure to predator odour or seawater. The six injections in each tank were separated by a minimum of 1.5 h. Five minutes after the injection of the predator odour or seawater, the water in the containers was changed to ensure that all tanks contained clean seawater for a minimum of 85 min prior to injection of the next stimulus. We used a 3-day pre-exposure with three injections per day, as Mitchell et al. [7] showed that this exposure schedule is more than enough to allow latent inhibition in lemon damselfish (P. moluccensis). Predator odour was obtained from a tank containing three adult dottybacks (Pseudochromis fuscus). The fish were held in 10 l of water for 2 h prior to stimulus collection. The predators were maintained on a diet of apogonids (Apongon sp.) to ensure that prey were responding to predator odour and not alarm cues in the predator's diet [25]. For the pre-exposure phase, we introduced 5 ml of predator odour and enough alarm cue solution to bring the concentration in the conditioning tank to 2 cuts l−1.
Upon completion of the pre-exposure phase, we initiated phase 3—predator training trials—where all of the fish were exposed to alarm cue solution (2 cuts l−1) paired with 5 ml of predator odour. The water in the containers was changed after 5 min. This method of predator training is a standard technique used to condition prey animals, including fishes, to recognize unknown threats [22,26,27].
The fourth and last phase was the testing phase. After the predator training phase, individual fish were transferred to plastic 13 l flow-through tanks (36 × 21 × 20 cm) and tested the next day for a response to either predator odour or seawater. Each tank had a sand substrate, a small piece of dead coral as a shelter, an airstone and an injection tube (length: 1.5 m) used to introduce predator odour or water into the tank. The tanks all had a 4 × 4 cm grid drawn on the side to aid recording of activity (number of lines crossed during the observation period). All tanks were covered on three sides with black plastic to avoid transfer of visual information between tanks. A black plastic curtain was hung in front of the tanks to minimize disturbance to the fish by the movement of the observer. One hour after adding fish to the conditioning tanks, and again 1 h prior to testing, the fish were fed ad libitum with Artemia larvae.
We followed a well-established protocol to quantify the anti-predator response of damselfish to predator odours [20,28]. At the beginning of each trial, fish were fed 2.5 ml of food (seawater containing approx. 250 Artemia larvae per millilitre) to remove the possibility of a ‘feeding frenzy’ effect at the start of the bioassay. Pre-stimulus observations began 4 min later, when another 5 ml of food was injected into the tank. At the end of the 4 min pre-stimulus observation period, 20 ml of dottyback odour or 20 ml of seawater were introduced into the tank followed by 5 ml of food, and the 4 min post-stimulus period started. For all injections, the cues (food or stimuli) were flushed in the tank by 60 ml of tank water previously drawn. During both the pre- and post-stimulus periods, we measured two behaviours: (i) the total number of lines the fish crossed during the observation period, using the grid drawn on the side of the tank (a line was counted as crossed when the entire body of the fish crossed it); and (ii) the total number of feeding strikes displayed by the fish, regardless of whether they were successful at capturing a food item. Prey fishes exposed to risk typically reduce feeding and activity, consequently if fish recognize the predator odour they should exhibit a reduction in feeding and activity from the pre-stimulus baseline.
We tested a total of 84 fish (n = 9–13 per treatment), which included between one and six fish from each of our 24 conditioning tanks, randomly chosen throughout the experiment. Fish size (total length: 3 ± 1 mm) did not differ among treatment groups (four-way nested ANOVA: p > 0.1 for all factors). All trials were conducted blind with respect to the treatments.
(c). Statistical analysis
The two variables used (feeding strikes and line crosses) were highly correlated (Pearson correlation coefficient: 0.84, p < 0.001), indicating that fish decreasing their activity also decreased their foraging. Thus, we used a MANOVA approach to take into account the interdependency of the two variables. After establishing that background level of risk or pre-exposure treatment did not affect basal behaviour of the fish (pre-stimulus values) prior to testing (MANOVA: risk: F2,79 = 0.58, p = 0.56), pre-exposure (F2,79 = 0.76, p = 0.47) and risk × pre-exposure (F2,79 = 0.02, p = 0.98), we computed a proportion change in behaviour from the pre-stimulus baseline ((post–pre)/pre) so as to standardize the response variables. We used those values as raw data in subsequent analyses. We did not perform the standard arcsine-square-root transformation because most values were negative. We first performed a four-way nested MANOVA, testing the effect of background level of risk (high versus low), pre-exposure (water versus predator odour) and testing cue (water versus predator odour) on our response variables (proportion change in foraging and line crosses). We added conditioning tank as a nested factor (‘testing cue’ was nested within ‘conditioning tanks’, using Type I SS) to account for the dependence of fish conditioned together in the tanks, making tank, not fish, our unit for replication. Significant interactions led to subsequent simpler MANOVAs, to investigate the nature of the interaction. All MANOVA results were reported using Pillai's trace computations. Results for individual ANOVAs were not provided, as both response variables showed the same pattern for all analyses (see figures 1 and 2). We also tested for any size difference among treatment groups using a similar analysis (reported here above).
Figure 1.
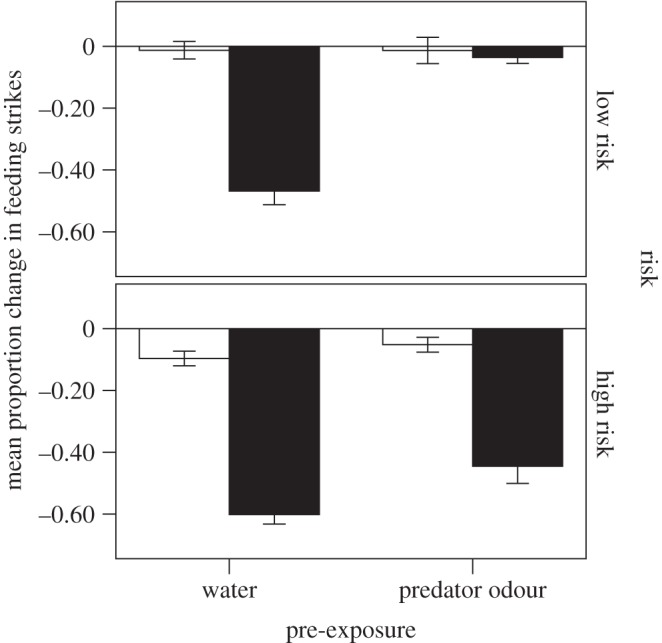
Mean (±s.e.) proportion change in feeding strikes for fish provided with a high or low background level of risk, and then pre-exposed to water or predator odour and tested for a response to seawater (open bars) or predator odour (solid bars).
Figure 2.
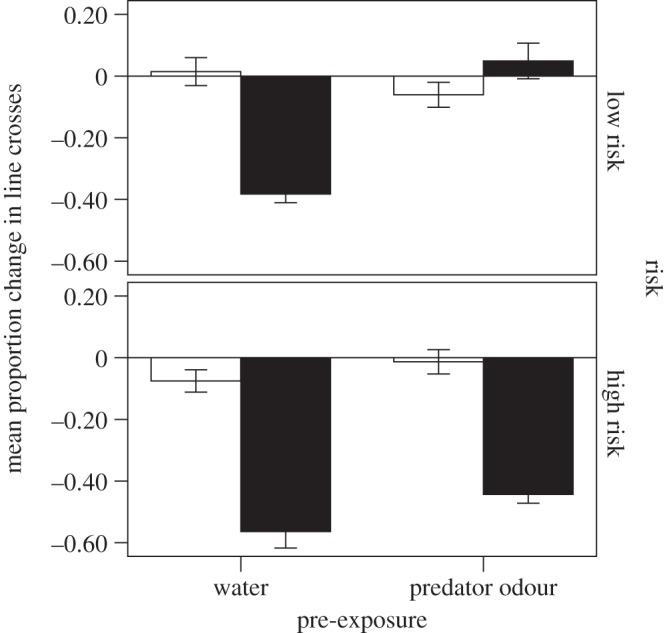
Mean (±s.e.) proportion change in line crosses for fish provided with a high or low background level of risk, and then pre-exposed to water or predator odour and tested for a response to seawater (open bars) or predator odour (solid bars).
3. Results
The four-way nested MANOVA revealed a significant three-way interaction among risk, pre-exposure and testing cue (F2,36 = 7.6, p = 0.002; figures 1 and 2) on the behaviour of the fish. Conditioning tank did not explain any variation in behaviour (F42,74 = 0.8, p = 0.77), and nor did conditioning tank × testing cue (F36,74 = 1.20, p = 0.28).
When we looked at the responses of fish to water only, we found that neither risk (F2,12 = 1.5, p = 0.26) nor pre-exposure (F2,12 = 0.2, p = 0.80) affected the behaviour of the fish, and we failed to find an interaction between the two factors (F2,12 = 1.0, p = 0.40). Again, conditioning tank did not have any effect of the fish behaviour (F38,26 = 0.5, p = 0.96). In other words, all fish responded similarly to water, regardless of risk or pre-exposure regimes.
The behaviours of fish exposed to the predator odour were affected both by the risk and pre-exposure regime (risk × pre-exposure: F2,23 = 9.1, p = 0.001). However, conditioning tank did not have an effect (F40,48 = 1.4, p = 0.11). The interaction was investigated by a series of post hoc comparisons. First, the responses of fish pre-exposed to water only (no latent inhibition) were affected by risk level (F2,11 = 6.9, p = 0.012), with fish from a high-risk background displaying stronger anti-predator responses to the learned predator odour than fish from the low-risk background. Conditioning tank did not have any effect (F20,24 = 1.2, p = 0.33).
Fish pre-exposed to the predator odour (latent inhibition group) were also affected by risk (interaction: F2,11 = 48.8, p < 0.001). In this case, the low-risk fish did not respond to the predator odour (not different from water: p = 0.33), while high-risk fish still displayed a significant anti-predator response to the predator odour (different from water: p < 0.001), a pattern indicating a failure of latent inhibition in the high-risk fish. To establish whether the failure of latent inhibition was complete or partial, we tested the effect of pre-exposure on the response intensity of the high-risk fish and found a significant effect of pre-exposure (F2,10 = 4.7, p = 0.036) on fish behaviour. Fish pre-exposed to water (no latent inhibition) responded to the predator odour with a stronger intensity than those fish pre-exposed to the predator odour, indicating a partial failure of latent inhibition. In all the above tests, conditioning tank did not have an effect (p > 0.2).
4. Discussion
The results of our study highlight that background level of risk is a key factor influencing the ability of prey animals to learn non-threatening stimuli. Consistent with previous studies, we showed that prey fish that were pre-exposed to novel odours for several days fail to learn the identity of those cues when the prey were subsequently taught that the cues represented risk. This means that learning the cue as threatening did not occur because the cues were already categorized as safe. This latent inhibition phenomenon was restricted to fish that were held under a low background level of risk, as is the case in all of the previous studies on latent inhibition of predator cues [7,9,10,29]. In sharp contrast, when we held fish under a high-risk background, which consisted of three exposures to alarm cues in each of 7 days (4 days in phase 1 and 3 days in phase 2), the fish failed to exhibit full latent inhibition. They did not learn the odour as non-threatening. Perhaps most interesting was that for fish with a high background level of risk, pre-exposure did influence their intensity of response to the odour cue. Fish responded more strongly when they had no pre-exposure to predator cues than when they had pre-exposure, indicating that the predator pre-exposure had an effect on reducing the intensity of the response to the odour. In this way, we conclude that under high risk, the pre-exposure regime led to only partial elimination of the latent inhibition effect.
Why should the background level of risk influence the ability of prey to learn to recognize non-threatening stimuli? Perhaps the best way to explain such a phenomenon is to think about the probability of making a mistake. If an animal is in a low-risk environment, then chances are that most unknown animals that the prey encounters are non-threatening. However, in a higher-risk environment, there is a greater chance that any given animal is a predator, hence a mistake (classifying a predator as a non-predator because no risk-related cues were associated with it during the previous exposure) is more likely to occur in a high-risk environment. This increasing ambiguity clearly reduced learning in the high-risk environment. Future studies should examine whether prey from a high-risk environment are able to exhibit full latent inhibition with a greater number of pre-exposures to the cues. Here, we gave the prey three exposures per day for 3 days. Increasing the number of pre-exposures could increase the likelihood of the prey exhibiting latent inhibition. It would also be interesting to determine how many times it would take to override any latent inhibition effects. It should take prey from a low-risk background much longer to override latent inhibition than fish from a high-risk background.
Our manipulation of background level of risk not only influenced learning of non-predators, but also learning of predators. Fish from the high-risk background environment that were conditioned to recognize the dottyback odour as a threat showed a significantly stronger-intensity anti-predator response to the dottyback odour than fish that were conditioned in the low-risk environment. Fish from the high-risk background may be more likely to respond strongly after the initial conditioning. However, we know that repeated conditioning allows fish to adjust the intensity of their response to predator cues [10]. Having a higher initial response in a high-risk environment probably represents a cautious strategy when estimating the risk posed by a predator. The only other study to test whether background risk influences learning was completed on woodfrog tadpoles. Similar to what we found, Ferrari also documented that the intensity of the learned response was higher with an increase in background level of risk [30]. Moreover, she showed that tadpoles raised under high risk prior to learning maintained responses to the predator cues for much longer that those that were raised under low risk prior to learning [30].
In our experiment, we manipulated different background levels of risk and found striking effects on predator and non-predator learning. A next logical step would be to tease apart how prey deal with intermediate levels of risk. Such experiments should be easy to conduct but before researchers attempt to titrate risk and learning effects, they need to carefully consider the risk level to which prey are normally exposed. Here, we gave three pulses of alarm cue every day for a week in our high-risk treatment. Is this actually a high-risk level? How often would damselfish normally experience a pulse of risk? Could it be that our high-risk treatment is actually relatively low in an absolute sense? Research aimed at quantifying in situ risk and variability in risk for different prey would be invaluable to both theoreticians and empiricists.
With the publication of Lima & Bednekoff's [5] risk allocation model, ecologists began to look for evidence that behavioural decisions were modified in large part due to background level of risk. Recently, Brown et al. [16] extended the examination of temporal variability to consider how prey respond to unknown predators. We further extend this paradigm to show that the intensity of learning of predators is intricately linked to background risk, and most importantly that background level of risk influences the ability of prey to learn non-predators. These results highlight the complex nature of anti-predator decision-making in prey animals.
Acknowledgements
We thank the staff at the Lizard Island Research Station for assistance with this project.
Funding statement
Funding was provided to M.C.O.F. and D.P.C. from the Natural Sciences and Engineering Council of Canada, to M.C.O.F., D.C. and M.I.M. from the Australian Research Council, and to M.I.M. from the ARC Centre of Excellence for Coral Reef Studies.
References
- 1.Stankowich T, Blujmstein DT. 2005. Fear in animals: a meta-analysis and review of risk assessment. Proc. R. Soc. B 272, 2627–2634. ( 10.1098/rspb.2005.3251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumstein DT. 2010. Flush early and avoid the rush: a general rule of antipredator behavior? Behav. Ecol. 21, 440–442. ( 10.1093/beheco/arq030) [DOI] [Google Scholar]
- 3.Zanette LY, White AF, Allen MC, Clinchy M. 2011. Perceived predation risk reduces the number of offspring songbirds produce per year. Science 334, 1398–1401. ( 10.1126/science.1210908) [DOI] [PubMed] [Google Scholar]
- 4.Lima SL. 1998. Stress and decision making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. Adv. Study behav. 27, 215–290. [Google Scholar]
- 5.Lima SL, Bednekoff PA. 1999. Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am. Nat. 153, 649–659. ( 10.1086/303202) [DOI] [PubMed] [Google Scholar]
- 6.Wisenden BD. 2000. Olfactory assessment of predation risk in the aquatic environment. Phil. Trans. R. Soc. Lond. B 355, 1205–1208. ( 10.1098/rstb.2000.0668) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell MD, McCormick MI, Ferrari MCO, Chivers DP. 2011. Friend or foe? The role of latent inhibition in predator and non-predator labelling by coral reef fishes. Anim. Cogn. 14, 707–714. ( 10.1007/s10071-011-0405-6) [DOI] [PubMed] [Google Scholar]
- 8.Ferrari MCO, Chivers DP. 2011. Learning about non-predators and safe places: the forgotten elements of risk assessment. Anim. Cogn. 14, 309–316. ( 10.1007/s10071-010-0363-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acquistapace P, Hazlett BA, Gherardi F. 2003. Unsuccessful predation and learning of predator cues by crayfish. J. Crustac. Biol. 23, 364–370. ( 10.1163/20021975-99990346) [DOI] [Google Scholar]
- 10.Ferrari MCO, Chivers DP. 2006. The role of latent inhibition in acquired predator recognition by fathead minnows. Can. J. Zool. 84, 505–509. ( 10.1139/z06-027) [DOI] [Google Scholar]
- 11.Sih A, Ziemba R, Harding KC. 2000. New insights on how temporal variation in predation risk shapes prey behavior. Trends Ecol. Evol. 15, 3–4. ( 10.1016/S0169-5347(99)01766-8) [DOI] [PubMed] [Google Scholar]
- 12.Sih A, McCarthy TM. 2002. Prey responses to pulses of risk and safety: testing the risk allocation hypothesis. Anim. Behav. 63, 437–443. ( 10.1006/anbe.2001.1921) [DOI] [Google Scholar]
- 13.Werner EE, Gilliam JF, Hall DJ, Mittelbach GG. 1983. An experimental test of the effects of predation risk on habitat use in fish. Ecology 64, 1540–1548. ( 10.2307/1937508) [DOI] [Google Scholar]
- 14.Ferrari MCO, Elvidge CK, Jackson CD, Chivers DP, Brown GE. 2010. The responses of prey fish to temporal variation in predation risk: sensory habituation or risk assessment? Behav. Ecol. 21, 532–536. ( 10.1093/beheco/arq023) [DOI] [Google Scholar]
- 15.Foam PE, Mirza RS, Chivers DP, Brown GE. 2005. Juvenile convict cichlids (Archocentrus nigrofasciatus) allocate foraging and antipredator behaviour in response to temporal variation in predation risk. Behaviour 142, 129–144. ( 10.1163/1568539053627631) [DOI] [Google Scholar]
- 16.Brown GE, Ferrari MCO, Elvidge CK, Ramnarine I, Chivers DP. 2013. Phenotypically plastic neophobia: a response to variable predation risk. Proc. R. Soc. B 280, 20122712 ( 10.1098/rspb.2012.2712) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown GE, Chivers DP, Elvidge CK, Jackson CD, Ferrari MCO. 2013. Background level of risk determines the intensity of predator neophobia in juvenile convict cichlids. Behav. Ecol. Sociobiol. 68, 127–133. ( 10.1007/s00265-013-1629-z) [DOI] [Google Scholar]
- 18.Almany GR, Webster MS. 2006. The predation gauntlet: early post-settlement mortality in reef fishes. Coral Reefs 25, 19–22. ( 10.1007/s00338-005-0044-y) [DOI] [Google Scholar]
- 19.McCormick MI, Weaver CJ. 2012. It pays to be pushy: intracohort interference competition between two reef fishes. PLoS ONE 7, e42590 ( 10.1371/journal.pone.0042590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrari MCO, Manassa RP, Dixson DL, Munday PL, McCormick MI, Meekan MG, Sih A, Chivers DP. 2012. Effects of ocean acidification on learning in coral reef fishes. PLoS ONE 7, e31478 ( 10.1371/journal.pone.0031478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell MD, McCormick MI, Chivers DP, Ferrari MCO. 2013. Generalization of learned predator recognition in coral reef ecosystems: how cautious are damselfish? Funct. Ecol. 27, 299–304. ( 10.1111/1365-2435.12043) [DOI] [Google Scholar]
- 22.Lonnstedt OM, McCormick MI, Meekan MG, Ferrari MCO, Chivers DP. 2012. Learn and live: predator experience and feeding history determines prey behaviour and survival. Proc. R. Soc. B 279, 2091–2098. ( 10.1098/rspb.2011.2516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell MD, McCormick MI, Ferrari MCO, Chivers DP. 2011. Coral reef fishes rapidly learn to identify multiple unknown predators upon recruitment to the reefs. PLoS ONE 6, e15764 ( 10.1371/journal.pone.0015764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meekan MG, Wilson SG, Halford A, Retzel A. 2001. A comparison of catches of fishes and invertebrates by two light trap designs, in tropical NW Australia. Mar. Biol. 139, 373–381. ( 10.1007/s002270100577) [DOI] [Google Scholar]
- 25.Mathis A, Smith RJF. 1993. Chemical labeling of northern pike (Esox lucius) by the alarm pheromone of fathead minnows (Pimephales promelas). J. Chem. Ecol. 19, 1967–1979. ( 10.1007/BF00983800) [DOI] [PubMed] [Google Scholar]
- 26.Mathis A, Smith RJF. 1993. Fathead minnows, Pimephales promelas, learn to recognize northern pike, Esox lucius, as predators on the basis of chemical stimuli from minnows in the pike's diet. Anim. Behav. 46, 645–656. ( 10.1006/anbe.1993.1241) [DOI] [Google Scholar]
- 27.Wisenden BD, Chivers DP, Smith RJF. 1997. Learned recognition of predation risk by Enallagma damselfly larvae (Odonata, Zygoptera) on the basis of chemical cues. J. Chem. Ecol. 23, 137–151. ( 10.1023/B:JOEC.0000006350.66424.3d) [DOI] [Google Scholar]
- 28.Chivers DP, McCormick MI, Nilsson GE, Munday PL, Watson S-A, Meekan MG, Mitchell MD, Corkill KC, Ferrari MCO. 2014. Impaired learning of predators and lower prey survival under elevated CO2: a consequence of neurotransmitter interference. Glob. Change Biol. 20, 515–522. ( 10.1111/gcb.12291) [DOI] [PubMed] [Google Scholar]
- 29.Ferrari MCO, Chivers DP. 2009. Latent inhibition of predator recognition by embryonic amphibians. Biol. Lett. 5, 160–162. ( 10.1098/rsbl.2008.0641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrari MCO. In press. Short-term environmental variation in predation risk leads to differential performance in predation-related cognitive function. Anim. Behav. [Google Scholar]


