Abstract
Eusociality is taxonomically rare, yet associated with great ecological success. Surprisingly, studies of environmental conditions favouring eusociality are often contradictory. Harsh conditions associated with increasing altitude and latitude seem to favour increased sociality in bumblebees and ants, but the reverse pattern is found in halictid bees and polistine wasps. Here, we compare the life histories and distributions of populations of 176 species of Hymenoptera from the Swiss Alps. We show that differences in altitudinal distributions and development times among social forms can explain these contrasting patterns: highly social taxa develop more quickly than intermediate social taxa, and are thus able to complete the reproductive cycle in shorter seasons at higher elevations. This dual impact of altitude and development time on sociality illustrates that ecological constraints can elicit dynamic shifts in behaviour, and helps explain the complex distribution of sociality across ecological gradients.
Keywords: social behaviour, altitude, development time, species distributions, hymenoptera
1. Introduction
Altitude has a large effect on the ecology and composition of social insect communities [1,2], and variation in social behaviour has been documented for a number of social insect species along both altitudinal and latitudinal clines [3–6]. Surprisingly, the directionality of this relationship is not consistent across species and clades. Studies of halictid bees and polistine wasps suggest that social behaviour is not favoured at higher elevations and latitudes. For example, many species are solitary or forgo the production of workers at high altitudes and latitudes (Halictus rubicundus [7]; Augochlorella aurata [8]; Lasioglossum baleicum [9]; Lasioglossum calceatum [10]; Polistes biglimus [11]). Similarly, spider sociality is generally restricted to lowland tropical regions, and social species are replaced by non-social relatives at higher latitudes and altitudes [3,5,6] (cf. [12]). However, the opposite pattern has been documented in bumblebees [13,14], where queen–worker dimorphism and the number of first-brood workers tend to increase at higher latitudes as well as across entire bee communities where sociality increases with altitude [1]. Similarly, in ants, species in 17 of 19 genera and five of six subfamilies tend to have larger colony sizes at high latitudes [2] (cf. [4]).
In order to better understand the causes of these conflicting patterns in the distribution of social systems, we studied the ecological niche limits of solitary, intermediate and highly social bee species along altitudinal gradients. We hypothesize that the shorter amount of time available for growth and reproduction at higher elevations is a constraint for social species, which must produce two broods: first workers, then reproductives [15,16]. We test whether these environmental constraints impact the distribution of sociality across altitudinal gradients, and examine how bees might have adapted to them.
2. Results
(a). Environmental gradients and social complexity
We compared the altitudinal distribution of solitary, intermediate and highly social taxa in the hymenopteran superfamily Apoidea. We defined intermediate social species as those with largely indistinguishable queen and worker castes (e.g. many halictine bees), and highly social species as those with easily recognizable morphological differences between queens and workers (e.g. bumblebees). We used occurrence data obtained from the Swiss Centre for Faunal Cartography (CSCF), and combined this with a meta-analysis of life-history traits for hymenopteran species in order to examine the relationship between sociality and species altitudinal distributions. The Alps of Switzerland present the ideal geography for this analysis because they encompass a huge altitudinal range, from lakes at 195 m to mountains up to 4600 m. A description of the species included in the dataset is provided in figure 1, and the full dataset is included as electronic supplementary material, table S1.
Figure 1.
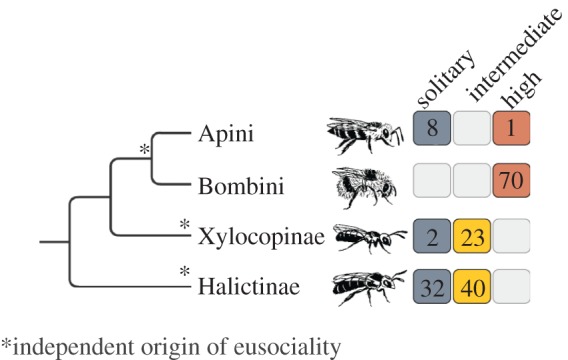
The phylogenetic relationships of the taxa examined indicate that the data encompass at least three independent contrasts of social complexity. Species were classified into three behavioral groups: solitary, intermediate social and highly social. Species with clearly distinguishable queen and worker castes were classified as highly social, whereas species with largely indistinguishable queen and worker castes were classified as intermediate social. The number of species is indicated inside each box. (Online version in colour.)
To examine the impact of climatic constraints on sociality, we estimated season length at the maximal elevation reached by each species. We compared the maximal number of foraging days at the cold niche limits for each social form using phylogenetic generalized least squares (pGLS) to account for phylogenetic relationships across species [17]. We calculated the number of foraging days at the cold niche limit as the number of days above 16°C obtained from meteorological stations in Switzerland and averaged over 40 years (16°C is generally the temperature above which bees will forage [18], and is therefore a good threshold indicator of the number of foraging days available throughout the season). The number of foraging days is strongly correlated with altitude (linear regression, F1,100 = 1155.2, R2 = 0.92, p < 0.0001; electronic supplementary material, figure S2).
We found that the cold niche limits of intermediate social species differ from those of solitary and highly social species (figure 2; R2 = 0.265, F3,59 = 10.62, p < 0.001). In line with previous studies documenting intraspecific variation in halictid bees and polistine wasps [7–11,19,20], intermediate social species are largely limited to regions at low elevation (roughly below 1000 m), with an average of 130 foraging days in the year. By contrast, both solitary and highly social species are able to cope with much colder conditions, with distributions ranging up to 2600 m (Bombus alpinus) and an average of 116 and 83 foraging days at the niche edge for solitary and highly social species, respectively.
Figure 2.
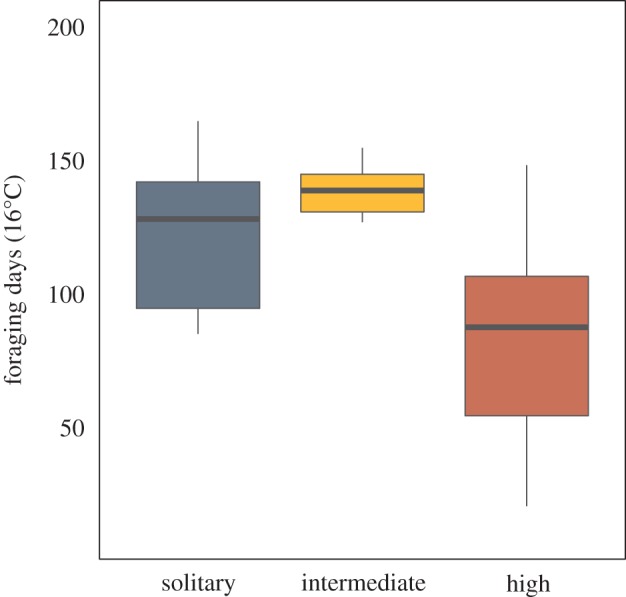
Cold niche limit (in number of foraging days throughout the season) in relation to the three categories of sociality (solitary, intermediate social and highly social). The number of foraging days at the cold niche limit differs significantly between social forms (pGLS; R2 = 0.265, F3,59 = 10.62, p < 0.001). (Online version in colour.)
It is important to note that the chosen temperature threshold (16°C) is a simplification, and that many species (particularly bumblebees) are capable of foraging at temperatures below this value. We note that foraging days and altitude are always strongly correlated, regardless of the temperature threshold chosen (electronic supplementary material, table S3), and that there are always more foraging days at lower elevations. Furthermore, the differences in distributions between solitary, intermediate and highly social species are the same when using altitude (instead of foraging days) as the response variable for the pGLS analyses (R2 = 0.1533, F3,100 = 10.23, p < 0.001).
(b). Phenology and social complexity
Compared with solitary species (which need only produce a single, reproductive brood), the time required to complete reproduction should be nearly doubled for social species because they must produce two broods: workers and then reproductives. Thus, season length might represent a major constraint for social species. One way they might overcome this constraint is by reducing the development time of the brood. We compared the egg-to-adult development times of solitary, intermediate and highly social species. We hypothesize that development time will correlate with degree of sociality in such a way that the combination of both factors will help to explain the ecological niche limits of solitary, intermediate and highly social species.
We searched existing literature for measures of egg-to-adult development times for bee species (data and references included in the electronic supplementary material, table S1). We selected this variable because, unlike colony development time (which is often very difficult to measure without controlled growth experiments), the time required from egg laying to emergence of the adult form is well studied in a number of species.
We found a significant association between egg-to-adult development time and degree of sociality (pGLS, R2 = 0.396, F3,20 = 6.56, p = 0.002; figure 3). Highly social species (primarily Bombus) tend to have consistently quick development times (23 ± 3 days), whereas intermediate social species have longer development times (32 ± 5 days). Solitary species vary greatly in development time (67 ± 27 days), but do not require two broods and are thus able to complete the reproductive cycle in shorter seasons. In this dataset, as in bees in general, species diversity is greatest in the solitary group. This could contribute to the high variance observed in development time for this group. However, most of the variance within these bees appears to segregate within subfamilies rather than between them, suggesting that the high variance is not an artefact of sampling diversity (see electronic supplementary material, figure S4 for breakdown by subfamily).
Figure 3.
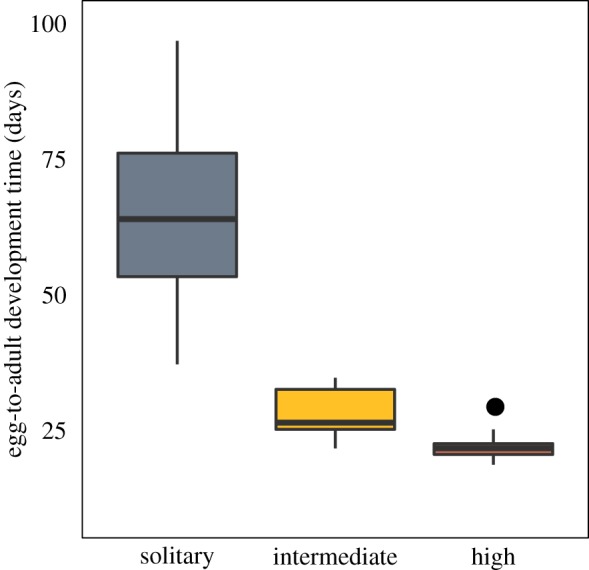
Development time is significantly associated with social complexity (pGLS; R2 = 0.396, F3,20 = 6.56, p = 0.002). (Online version in colour.)
(c). Ecological modelling
We developed an ecological model to study the interaction between development time and season length for each social form and determine whether these variables are sufficient to predict the differing distributions of solitary, intermediate and highly social species. Because each species must forage and provision their brood, we assumed that the minimum number of days required for a species to complete reproduction within a season was the sum of the number of days required for egg-to-adult development time plus 30% of the number of foraging days within a season. We assumed this value was normally distributed across taxa within each sociality level, with the parameters of each level's distribution determined from our dataset.
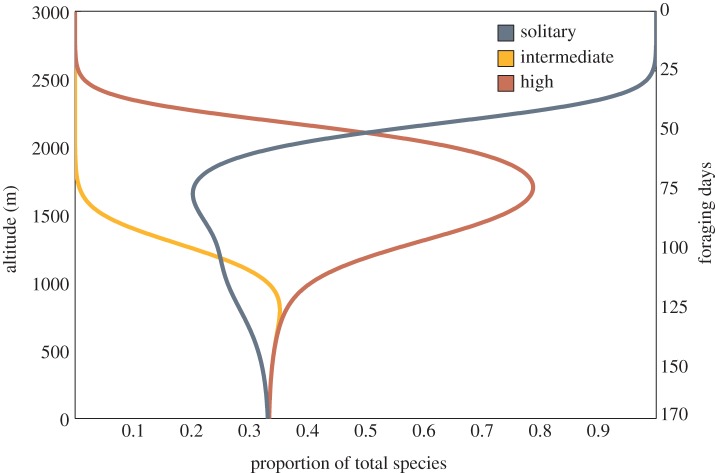
We found that the model was able to accurately predict the observed distributions of all three social forms across altitudes (figure 4). All species are present at low elevations, where season length is sufficiently long for each group to reproduce. Above approximately 1500 m, intermediate social species decline rapidly in the model; solitary and highly social species are predicted to account for the vast majority of species above this altitudinal threshold.
Figure 4.
Predicted distribution of solitary, intermediate and highly social bee species based on an ecological model considering only development time and season length across an altitudinal gradient. Notably, in this model intermediate social species do not occur in appreciable numbers above approximately 1500 m, which is in line with observations made in the field and with the altitudinal niche limit documented in this dataset. (Online version in colour.)
Two aspects of the distribution of development time within a social form explain its maintenance at high altitudes. First, shorter mean development times shift the entire distribution towards being able to survive in harsher climates with fewer foraging days. Second, a high variance in development time across taxa ensures that a significant proportion of species within a social form have sufficiently short development times that they are able to survive at high altitudes. Highly social species (taken as a group) are superior by the first criterion: they have a faster mean development time. Solitary species are able to survive at high altitudes by the second criterion: as a group, they exhibit a greater variance in development time. Intermediate social species are inferior on both counts because their development times are not sufficiently short, nor do they exhibit a high degree of variation.
Solitary species are observed in non-negligible numbers at all altitudes in the model, as in our dataset. Highly social species are also common across a wide range of altitudes—at high altitudes, their mean development time is much lower than that of solitary species and compensates for their lower variance in development time. Intermediate social species are essentially not observed above 1500 m in our occurrence dataset, and they comprise only 2.8% of the total number of species observed, whereas solitary and highly social species represent 27.8% and 69.4%, respectively. Thus, this model provides strong evidence that the ecological pressures of season length and development time are sufficient to produce differential distributions across altitude of solitary, intermediate and highly social species such as those observed in our data.
3. Discussion
It has long been accepted that the local environment has a large impact on species distributions and phenotypes. However, surprisingly few studies have explicitly examined these relationships with respect to social insects. The majority of these studies have focused on intraspecific variation in social structure [11,19–22]. Here, we document similar patterns of change in social complexity across a number of taxonomic groups. We show that altitude has a dual impact on the social structure of bee communities: intermediate social species appear to be filtered out at higher elevations, whereas solitary and highly social species persist at both high and low elevations.
Both the empirical and modelling results indicate that the combined constraints imposed by development time and season length may explain the different distributions of social forms along altitudinal gradients. Individuals of intermediate social species have longer development times than highly social ones, which develop approximately 30% more quickly than intermediates. As a result, as altitude increases, highly social species can persist in these short seasons, whereas intermediate social species are filtered out. Likewise, the high degree of variation in egg-to-adult development times in solitary species coupled with the need to produce only a single brood could enable some taxa in this group to survive in short seasons. We predict that these differences in distribution patterns are likely to be even more pronounced across latitudinal gradients, where differences in season length and temperature variability may be even more extreme [23,24], and future work could test this hypothesis.
Our data further suggest that a critical threshold in season length influences social evolution in bees. Above this threshold, species with intermediate levels of social organization can survive. However, below this threshold (when seasons are too short), intermediate social species may either forgo the production of the worker brood and revert to a solitary life history [21] or evolve towards higher degrees of sociality with shorter development times, larger colony sizes, increased division of labour and greater social cohesion. Indeed, through improved foraging efficiency and nest thermoregulation, highly social species can decrease brood development times [25–27], which may allow them to overcome the constraints of season length and colonize harsher environments. Highly social species are also capable of foraging at lower temperatures than many other species; this could also increase the number of foraging days available to them at any given altitude, and therefore increase the maximum altitudes at which these species could occur. More broadly, these findings also emphasize that bivoltinism (the production of two broods per season) constitutes a constraint for incipiently social species: mild climatic conditions are required to provide sufficient time for the production of both a worker and a reproductive brood [15].
The observed pattern in the altitudinal distributions of social species aligns with some but not all predictions of prevailing models to explain the evolution of worker behaviour and/or cooperative brood care (table 1). For example, the bet-hedging [28,29] hypothesis posits that in highly variable environments, individuals might remain to help in their natal colony, thereby sacrificing potential fitness to reduce the probability of complete reproductive failure. The fact that we find only highly social species in the harsher high-altitude environments suggest that this strategy may apply to these species, but not to intermediate social species where the constraint of a long development time could override the benefits of remaining in the natal nest as a worker in habitats with short breeding seasons. Instead, many intermediate social species might switch to a solitary life history, as has been observed in several halictid bees and polistine wasps [7–11,19,20]. Finally, reproductive skew within social groups is predicted to increase in situations with high variance in resources, while constant but low levels of food availability are predicted to favour groups with lower levels of reproductive skew [36–38]. Assuming that resource availability is less variable at low elevations, our results are consistent with these predictions: highly social species exhibit greater levels of reproductive skew than intermediate social species.
Table 1.
Evolutionary models attempting to explain the evolution of worker behaviour and cooperative breeding. Predictions are based on the application of these models in the context of the current, ecological dataset [15,16,28–34].
| hypothesis | citations | explanation | environmental inputs | relationship to phenology | prediction |
|---|---|---|---|---|---|
| the origin of worker behaviour | |||||
| assured fitness returns | Gadagkar 1990 [30]; Queller 1994 [31] | helping behaviour favoured when solitary foundresses are unlikely to live until their brood reach the age of independence. In this case, grouping is favoured, because all individuals in a group are likely to gain some fitness, whereas solitary nesters may frequently fail to rear brood | conditions unfavourable to the survival of a solitary foundress: high predation/parasitism rate, resource limitation, extreme environmental conditions | the lifespan of an adult relative to the development time of the brood is important; if offspring development is prolonged, a solitary foundress would be increasingly unlikely to survive until the age of independence | helping (workers) should be favoured in more extreme environments, where adults are unlikely to survive until brood mature |
| mating limitation + environmental control | Yanega 1997 [32] | female’s social role is dependent on whether she mates while young—this depends on male demography; production of one sex or the other depends on the temperature and/or photoperiod conditions experienced at the time the egg is laid | would occur in seasonal environments | importance of temperature effects on rate of development, in turn influencing male and female demography during the course of each warm season | workers should emerge at lower elevations, where the earlier onset of reproduction favours initial production of females, which become workers because there are no males available to mate |
| bivoltinism | Hunt & Amdam 2005 [15]; Hunt 2012 [16] | diapause, which evolved for overwintering, is co-opted to suppress ovarian development in the first brood of bivoltine wasps and bees | insects must live in a seasonal environment that allows for at least two broods to be produced each year | the development time of offspring relative to the length of the growing season dictates whether two broods can be produced in a given habitat | workers will only be produced at lower elevations, where two broods can be produced each year |
| the origin of cooperative breeding behaviour | |||||
| bet hedging | Seger & Brockmann 1987 [35]; Rubenstein 2011 [29] | in highly variable environments, individuals sacrifice some potential fitness to reduce probability of complete failure | group living should increase in more variable habitats | the lifespan of the helper relative to the brood would influence the potential fitness costs experienced by the helper | group living should increase at higher elevations, where conditions would be more variable |
| habitat saturation | Strassman & Queller 1989 [33]; Bull & Schwarz 1996 [34] | colony sizes (i.e. number of potentially reproductive females) will be greater in habitats where dispersal opportunities are limited, suggesting group living is a secondary option to independent reproduction | will occur in environments with shortages of nesting materials or other resources necessary to solitary nest founding, or with intraspecific competition | longer offspring development times would increase the strain on the resources of solitary foundresses | if resources are more limited at higher elevations, cooperation/colony sizes should increase at higher elevations |
| risk sensitivity | Caraco et al. 1995 [36]; Uetz 1996 [37]; Poethke & Liebig 2008 [38] | group formation and food sharing buffers against fluctuations in individual foraging success and may be a risk avoidance mechanism | cooperative breeding favoured in situations with constant, low levels of resources; high variance favours solitary individuals or groups with high reproductive skew | the main reproductive female must survive long enough to obtain the necessary resources to pass the threshold for reproduction | solitary individuals or groups with high reproductive skew should increase at high elevations if they have a higher variance in resources |
Over the past decade, climate change has been recognized as a major force driving changes in species distribution, ecology and behaviour throughout the world. Many species have shifted their range to increased altitudinal and latitudinal distributions, and have altered their seasonal timing towards those of equatorial populations [39]. Our results on the role of season length in social evolution can predict how individual bee species and community composition are likely to respond to increasing environmental extremes.
4. Methods
(a). Phenology and distribution of species in Switzerland
We collected meteorological observations from the Idaweb platform for 108 Swiss meteorological stations from 1970 to 2010. For each meteorological station, we computed the number of days above 16°C. To interpolate this variable, we averaged the number of days above 16°C for each meteorological station and related this to elevation using both linear and quadratic terms. We then projected the model using a digital elevation model at a resolution of 25 m. We investigated whether spatial autocorrelation remained in the residuals and used an inverse distance-weighted approach to interpolate residual errors (see [40] for further details). Finally, we summed modelled values and interpolated residual layers to obtain a climatic map of the number of days above 16°C in Switzerland. Electronic supplementary material, figure S5 shows the distributions of solitary, intermediate and highly social species projected onto this map.
Occurrences of the bee species present in Switzerland were obtained from the CSCF. As a measure of the colder niche edge, we calculated the niche extremes (10th percentile) as the number of days above 16°C for the sites where each species was found. We preferred this measure to a minimal value as it reduces the effect of outlier values (e.g. from georeference errors).
(b). Life-history traits
We collected information from the literature on social structure and egg-to-adult development times. For species with more than one recorded observation, all values were recorded, and the mean value for egg-to-adult development time was used for the aforementioned analyses. These data and supplemental references are presented in the electronic supplementary material, table S1. For the distributional analysis, we excluded Apis mellifera because it is an agricultural species bred and maintained across a wide range of habitats. We also excluded species that have evolved a parasitic life history because their presence is highly dependent on the presence of a suitable host species and they have evolved a unique behavioural repertoire that is not appropriately characterized as solitary or eusocial. The remaining focal species were categorized into three groups: solitary, intermediate social and highly social, where ‘intermediate social’ species are species with low differentiation between queen and worker castes, and ‘highly social’ species have easily distinguishable queen–worker dimorphism. We analysed the relationship between social behaviour and the number of foraging days at the niche's edge using a pGLS regression calculated with the ‘caper’ package in the R environment [17,41].
Phylogenetic relationships were inferred as in [42]. This tree was constructed using a super-matrix approach comprising 20 genes and over 17 000 aligned nucleotide sites, and included more than 1300 species.
(c). Ecological modelling
We have hypothesized that differences in development time (including required foraging time) drive the observed distribution of solitary, intermediate and highly social species across altitudes. The data demonstrate that highly social species have shorter development times than intermediate social and solitary species, and that the variance in development time is greater in solitary than in social species.
We assume required development time (with foraging) across species within each of solitary, intermediate and highly social levels to be normally distributed, with means and variances differing across each social form. Let TS, TI and TH be random variables of required development time (with foraging) for species of sociality levels solitary, intermediate and high, respectively. If we further assume that development time and required foraging time are independent for species within a sociality level, then the data suggest the following rough distributions (electronic supplementary material, figure S6):
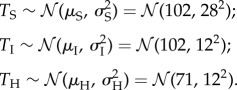 |
The results turn out to be robust to large changes in the parameters, as long as the qualitative conditions that 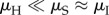 and
and 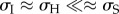 hold with sufficient strength (see electronic supplementary material, figure S7 for examples of robustness checks). It is important to note that the data on development time primarily come from studies of species reared in laboratory conditions where sufficient time was provided for the completion of a reproductive cycle, thus guarding against biases in our estimates.
hold with sufficient strength (see electronic supplementary material, figure S7 for examples of robustness checks). It is important to note that the data on development time primarily come from studies of species reared in laboratory conditions where sufficient time was provided for the completion of a reproductive cycle, thus guarding against biases in our estimates.
The number of foraging days across altitudes follows a roughly linear trend (electronic supplementary material, figure S2). If a is altitude in metres and F(a) the number of foraging days at a, then the data suggest
The proportion of species i that can survive at altitude a, denoted pi(a), is then
where Φ is the cumulative distribution function of the standard normal distribution. Ignoring the influence of brood size and other factors, we report in figure 4, for each altitude, the relative proportions of the three sociality levels; for sociality level S, for example, this is pS(a)/[pS(a) + pI(a) + pH(a)].
Supplementary Material
Acknowledgements
We thank Wenfei Tong for illustrations, L. Keller for hosting S.D.K. in his laboratory throughout the development of this project, and J. D. Crall, B. A. Souza de Medeiros and members of the Pierce and Chapuisat laboratories for helpful discussion. We also thank the Swiss Center for Fauna Cartography for providing bee distribution data.
Funding statement
S.D.K. was supported by two Putnam Expedition Grants from the Harvard Museum of Comparative Zoology, a John Templeton Foundation FQEB Prize Fellowship, and a USDA NIFA post-doctoral fellowship. M.C. and J.P. were supported by grants 31003A-125306 and 31003A-146641 from the Swiss National Science Foundation. L.P. was supported by The Danish Council for Independent Research grant 12-126430. N.E.P. and S.D.K. were supported by NSF SES-0750480 and NSF IOS-1257543. The authors declare no competing financial interests. S.D.K. and L.P. conceived and designed the project, analysed the data, and drafted the manuscript. C.V. and M.A.N. formulated the ecological model, analysed the results, and contributed to the drafting and revising of the manuscript. J.P. developed the table, and contributed to the drafting and revising of the manuscript. M.C. and N.E.P. participated in project design and data interpretation, and in the drafting and revising of the manuscript.
References
- 1.Hoiss B, Krauss J, Potts SG, Roberts S, Steffan-Dewenter I. 2012. Altitude acts as an environmental filter on phylogenetic composition, traits and diversity in bee communities. Proc. R. Soc. B 279, 4447–4456. ( 10.1098/rspb.2012.1581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaspari M, Vargo EL. 1995. Colony size as a buffer against seasonality: Bergmann's rule in social insects. Am. Nat. 145, 610–632. ( 10.1086/285758) [DOI] [Google Scholar]
- 3.Avilés L. 1997. Causes and consequences of cooperation and permanent-sociality in spiders. In The evolution of social behavior in insects and arachnids (eds Choe JC, Crespi BJ.), pp. 476–498. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 4.Purcell J. 2011. Geographic patterns in the distribution of social systems in terrestrial arthropods. Biol. Rev. Camb. Philos. Soc. 86, 475–491. ( 10.1111/j.1469-185X.2010.00156.x) [DOI] [PubMed] [Google Scholar]
- 5.Avilés L, Agnarsson I, Salazar PA, Purcell J, Iturralde G, Yip EC, Powers KS, Bukowski TC. 2007. Altitudinal patterns of spider sociality and the biology of a new midelevation social Anelosimus species in Ecuador. Am. Nat. 170, 783–792. ( 10.1086/521965) [DOI] [PubMed] [Google Scholar]
- 6.Purcell J, Avilés L. 2007. Smaller colonies and more solitary living mark higher elevation populations of a social spider. J. Anim. Ecol. 76, 590–597. ( 10.1111/j.1365-2656.2007.01228.x) [DOI] [PubMed] [Google Scholar]
- 7.Yanega D. 1988. Social plasticity and early-diapausing females in a primitively social bee. Proc. Natl Acad. Sci. USA 85, 4374–4377. ( 10.1073/pnas.85.12.4374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Packer L. 1990. Solitary and eusocial nests in a population of Augochlorella striata (Provancher) (Hymenoptera, Halictidae) at the northern edge of its range. Behav. Ecol. Sociobiol. 27, 339–344. ( 10.1007/BF00164004) [DOI] [Google Scholar]
- 9.Cronin AL, Hirata M. 2003. Social polymorphism in the sweat bee Lasioglossum (Evylaeus) baleicum (Cockerell) (Hymenoptera, Halictidae) in Hokkaido, northern Japan. Insect. Soc. 50, 379–386. ( 10.1007/s00040-003-0693-1) [DOI] [Google Scholar]
- 10.Sakagami SF, Munakata M. 1972. Distribution and bionomics of a transpalaeartic eusocial halictine bee, Lasioglossum (Evylaeus) calceatum, in northern Japan, with reference to its solitary life cycle at high altitude. J. Fac. Sci. Hokkaido Univ. Ser. 6 Zool. 18, 411. [Google Scholar]
- 11.Fucini S, Di Bona V, Mola F, Piccaluga C, Lorenzi MC. 2009. Social wasps without workers: geographic variation of caste expression in the paper wasp Polistes biglumis. Insect. Soc. 56, 347–358. ( 10.1007/s00040-009-0030-4) [DOI] [Google Scholar]
- 12.Jones T, Riechert S, Dalrymple S, Parker P. 2007. Fostering model explains variation in levels of sociality in a spider system. Anim. Behav. 73, 195–204. ( 10.1016/j.anbehav.2006.06.006) [DOI] [Google Scholar]
- 13.Laverty TM, Plowright RC. 1985. Comparative bionomics of temperate and tropical bumble bees with special reference to Bombus ephippiatus (Hymenoptera: Apidae). Can. Entomol. 117, 467–474. ( 10.4039/Ent117467-4) [DOI] [Google Scholar]
- 14.Dillon ME, Frazier MR, Dudley R. 2006. Into thin air: physiology and evolution of alpine insects. Integr. Comp. Biol. 46, 49–61. ( 10.1093/icb/icj007) [DOI] [PubMed] [Google Scholar]
- 15.Hunt JH, Amdam GV. 2005. Bivoltinism as an antecedent to eusociality in the paper wasp genus Polistes. Science 308, 264–267. ( 10.1126/science.1109724) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunt JH. 2012. A conceptual model for the origin of worker behaviour and adaptation of eusociality. J. Evol. Biol. 25, 1–19. ( 10.1111/j.1420-9101.2011.02421.x) [DOI] [PubMed] [Google Scholar]
- 17.Orme D. 2012. The caper package: comparative analysis of phylogenetics and evolution in R. See http://cran.r-project.org/web/packages/caper. [Google Scholar]
- 18.Couvillon MJ, Fitzpatrick G, Dornhaus A. 2010. Ambient air temperature does not predict whether small or large workers forage in bumble bees (Bombus impatiens). Psyche: J. Entomol. 2010, 1–8. ( 10.1155/2010/536430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plateaux-Quenu C, Plateaux L, Packer L. 2000. Population-typical behaviours are retained when eusocial and non-eusocial forms of Evylaeus albipes (F.) (Hymenoptera, Halictidae) are reared simultaneously in the laboratory. Insect. Soc. 47, 263–270. ( 10.1007/PL00001713) [DOI] [Google Scholar]
- 20.Lorenzi MC, Turillazzi S. 1986. Behavioural and ecological adaptations to the high mountain environment of Polistes biglumis bimaculatus. Ecol. Entomol. 11, 199–204. ( 10.1111/j.1365-2311.1986.tb00295.x) [DOI] [Google Scholar]
- 21.Field J, Paxton RJ, Soro A, Bridge C. 2010. Cryptic plasticity underlies a major evolutionary transition. Curr. Biol. 22, 2028–2031. ( 10.1016/j.cub.2010.10.020) [DOI] [PubMed] [Google Scholar]
- 22.Soucy SL, Danforth BN. 2002. Phylogeography of the socially polymorphic sweat bee Halictus rubicundus (Hymenoptera: Halictidae). Evolution 56, 330–341. ( 10.1111/j.0014-3820.2002.tb01343.x) [DOI] [PubMed] [Google Scholar]
- 23.Janzen DH. 1967. Why mountain passes are higher in the tropics. Am. Nat. 101, 233–249. ( 10.1086/282487) [DOI] [Google Scholar]
- 24.Ghalambor CK, Huey RB, Martin PR, Tewksbury JJ, Wang G. 2006. Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integr. Comp. Biol. 46, 5–17. ( 10.1093/icb/icj003) [DOI] [PubMed] [Google Scholar]
- 25.Heinrich B. 1979. Bumblebee economics. Cambridge, MA: Harvard University Press. [Google Scholar]
- 26.O'Donnell S, Foster RL. 2001. Thresholds of response in nest thermoregulation by worker bumble bees, Bombus bifarius nearcticus (Hymenoptera: Apidae). Ethology 107, 387–399. ( 10.1046/j.1439-0310.2001.00668.x) [DOI] [Google Scholar]
- 27.Jeanson R, Fewell JH, Gorelick R, Bertram SM. 2007. Emergence of increased division of labor as a function of group size. Behav. Ecol. Sociobiol. 62, 289–298. ( 10.1007/s00265-007-0464-5) [DOI] [Google Scholar]
- 28.Seger J. 1987. What is bet-hedging? Oxford Surv. Evol. Biol. 4, 182–211. [Google Scholar]
- 29.Rubenstein DR. 2011. Spatiotemporal environmental variation, risk aversion, and the evolution of cooperative breeding as a bet-hedging strategy. Proc. Natl Acad. Sci. USA 108, 10 816–10 822. ( 10.1073/pnas.1100303108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gadagkar R. 1990. Evolution of eusociality: the advantage of assured fitness returns. Phil. Trans. R. Soc. Lond. B 329, 17–25. ( 10.1098/rstb.1990.0146) [DOI] [Google Scholar]
- 31.Queller D. 1994. Extended parental care and the origin of eusociality. Proc. R. Soc. Lond. B 256, 105–111. ( 10.1098/rspb.1994.0056) [DOI] [Google Scholar]
- 32.Yanega D. 1997. Demography and sociality in halictine bees (Hymenoptera: Halictidae). In The evolution of social behavior in insects and arachnids (eds Choe JC, Crespi BJ.), pp. 293–315. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 33.Strassmann JE, Queller DC. 1989. Ecological determinants of social evolution. In The genetics of social evolution (eds Breed MD, Page RE.), pp. 81–101. CO: Westview Press. [Google Scholar]
- 34.Bull NJ, Schwarz MP. 1996. The habitat saturation hypothesis and sociality in an allodapine bee: cooperative nesting is not ‘making the best of a bad situation’. Behav. Ecol. Sociobiol. 39, 267–274. ( 10.1007/s002650050289) [DOI] [Google Scholar]
- 35.Seger J, Brockmann HJ. 1987. What is bet-hedging? In Oxford surveys in evolutionary biology, vol. 4 (eds Harvey PH, Partridge L.), pp. 182–211. Oxford, UK: Oxford University Press.
- 36.Caraco T, Uetz GW, Gillespie RG, Giraldeau L-A. 1995. Resource consumption variance within and among individuals: on coloniality in spiders. Ecology 76, 196–205. ( 10.2307/1940641) [DOI] [Google Scholar]
- 37.Uetz GW. 1996. Risk sensitivity and the paradox of colonial web-building in spiders. Integr. Comp. Biol. 36, 459–470. ( 10.1093/icb/36.4.459) [DOI] [Google Scholar]
- 38.Poethke H, Liebig J. 2008. Risk-sensitive foraging and the evolution of cooperative breeding and reproductive skew. BMC Ecol. 8, 2 ( 10.1186/1472-6785-8-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. 37, 637–669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 40.Zimmermann NE, Kienast F. 1999. Predictive mapping of alpine grasslands in Switzerland: species versus community approach. J. Vegetat. Sci. 10, 469–482. ( 10.2307/3237182) [DOI] [Google Scholar]
- 41.R Development Core Team. 2011. R: A Language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 42.Hedtke S, Patiny S, Danforth BN. 2013. The bee tree of life: a supermatrix approach to apoid phylogeny and biogeography. BMC Evol. Biol. 13, 138 ( 10.1186/1471-2148-13-138) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.