Abstract
Host plant resistance has been widely used for controlling the major rice pest brown planthopper (BPH, Nilaparvata lugens). However, adaptation of the wild BPH population to resistance limits the effective use of resistant rice varieties. Quantitative trait locus (QTL) analysis was conducted to identify resistance-breaking genes against the anti-feeding mechanism mediated by the rice resistance gene Bph1. QTL analysis in iso-female BPH lines with single-nucleotide polymorphism (SNP) markers detected a single region on the 10th linkage group responsible for the virulence. The QTL explained from 57 to 84% of the total phenotypic variation. Bulked segregant analysis with next-generation sequencing in F2 progenies identified five SNPs genetically linked to the virulence. These analyses showed that virulence to Bph1 was controlled by a single recessive gene. In contrast to previous studies, the gene-for-gene relationship between the major resistance gene Bph1 and virulence gene of BPH was confirmed. Identified markers are available for map-based cloning of the major gene controlling BPH virulence to rice resistance.
Keywords: host plant resistance, brown planthopper, rice, gene-for-gene interaction, quantitative trait locus
1. Introduction
Host plant resistance is an effective, economic and environmentally friendly approach to control crop pests. Among a large number of commercial rice varieties (Oryza sativa) resistant to rice insect pests, varieties that are resistant to the brown planthopper (BPH, Nilaparvata lugens (Stål)) are representative in their long history of breeding and successful application in the field [1–5]. More than 20 resistance genes to BPH have been identified from landrace cultivars and wild Oryza species [5], and the anti-feeding effects of these genes are highly valuable resources in breeding rice varieties resistant to BPH [4].
BPH is the most damaging insect pest of rice and causes severe yield losses by direct feeding and viral transmission of serious diseases [6]. While rice resistance to BPH has played a main role in the integrated control strategies for this pest throughout East and Southeast Asia [4], the development of new BPH ‘biotypes’ to overcome the resistance of these rice varieties in the field has threatened the deployment of resistant varieties and new rice breeding programmes [1,4,7,8]. For example, IR26, the first commercial resistant rice variety carrying Bph1 released in 1973 became susceptible after only a few years due to the emergence of local populations that conquered the anti-feeding mechanism of this variety [9,10]. The wild BPH population can adapt to resistant varieties of rice within seven to 10 generations when selected on those varieties [11,12]. The resistance genes Bph1 and bph2 have almost lost their resistance in the field owing to the development of virulent ‘biotypes’ of BPH. However, Bph3 and bph4 still possess practical resistance to some of the local BPH populations in Asia [5,13]. The longer lasting resistance of these genes reflects variation in the adaption ability of BPH populations to resistance genes. Therefore, the breeding and deployment of new resistant rice varieties should be considered in the context of genetic and physiological mechanisms of BPH adaptation to rice resistance [7,14].
Several genetic analyses of BPH ‘biotypes’ have been conducted, and most of the studies have suggested polygenic control of BPH virulence to rice resistance genes [15–17]. The recessive nature of the virulence to Bph1 was also reported [11,16]. Although the polygenic control of BPH virulence to resistant rice is partially explained by the high genetic diversity of BPH populations in the field [7,15,16], the mechanical uniqueness in BPH virulence to resistant plants has not been clarified.
Bph14 is a BPH resistance gene that encodes an immune receptor of the NBS–LRR (nucleotide-binding site–leucine-rich repeat) family of proteins and is a typical resistance gene for rice disease pathogens [18]. The discovery of the similarity in genes mediating BPH resistance and pathogen resistance, indicating typical gene-for-gene interaction, facilitated the opportunity to reconsider the BPH–rice resistance relationship [19]. Understanding the genetics and molecular mechanism between rice resistance and BPH virulence will lead to new agricultural solutions for controlling this serious rice pest [8,14].
In this paper, we re-examined the inheritance of the BPH virulence to the resistance gene Bph1 using iso-female lines of the BPH population and near-isogenic rice varieties. Next, quantitative trait locus (QTL) analysis was conducted to identify the responsible regions for the virulence on the BPH genetic map. Our purpose was to verify whether BPH virulence can be explained using a polygenic or monogenic theory—i.e. whether a gene-for-gene relationship is established between rice resistance and the corresponding BPH virulence [15]. Finally, to directly identify molecular markers linked to the putative virulence gene vBph1, bulked segregant analysis and next-generation sequencing (NGS) technologies were applied [20,21]. These systematic applications of genetics against BPH virulence to rice resistance contribute to understanding the nature of BPH ‘biotypes’ that has long been ambiguously discussed [7,12,15,16,17,22].
2. Material and methods
(a). Rice varieties
The japonica rice variety Saikai190, which carries the dominant Bph1 gene originating from the indica rice landrace Mudgo, was used as the resistant rice [3,23,24]. Koshihikari, which carries no resistance gene, was used as the control variety. Ten rice seeds were grown in a seedling case (155 × 60 × 100 mm) for 40 days at 25°C and used for the virulence tests of BPH.
(b). Production of inbred brown planthopper lines and crossing
The Izumo87 strain, collected in Izumo, Shimane Prefecture, Japan, in 1987, was full sib-mated for 10 generations (I87i) on Koshihikari to increase the homozygosity of the stock. I87i was tested for a virulence to Bph1-mediated BPH resistance. The Chikugo89 strain, collected in Chikugo, Kumamoto Prefecture, Japan, in 1989, was also full sib-crossed for four generations (C89i) on the resistant variety Saikai190 possessing Bph1 to increase the homozygosity and fix alleles related to the virulence. A virulent C89i virgin female was crossed with an I87i male in a test tube for F1 generations (F1-A-E). Seven populations of F2 generations were obtained by crossing one F1 female and one F1 male from the same parent pairs (F2-A, B and G are from F1-A; F2-C–F are from F1-B). BF1 progenies (F1 × I87i) were also generated to merge our new molecular markers to previously published genetic markers [25]. All the parents and hybrid progenies were reared on Koshihikari at 25°C under a 16 L : 8 D light regimen.
(c). Evaluation of virulence and DNA extraction
The Parafilm sachet test was applied to evaluate the individual virulence of the BPH after slight modification from the original test [17,26]. A sachet (50 × 25 mm) made from Parafilm (Bemis Flexible Packaging, Neenah, WI, USA) was attached to the leaf shelf of a Saikai190 rice plant. A female adult 2–3 days after eclosion was released in each sachet for 48 h. The quantity of honeydew excreted by each adult was measured using a microbalance (Shimadzu, Kyoto, Japan), and its pH was checked using pH indicator test paper (902 10; Macherey-Nagel, Düren, Germany). The pH determination was based on the BPH feeding behaviour on resistant rice on which the virulent individuals intake phloem sap and excrete a large volume of alkaline honeydew (pH > 8), whereas avirulent individuals excrete a small volume or no volume of neutral honeydew because they can feed only on xylem sap [6]. To analyse the segregation of the virulence in F2 generations, each adult female was classified as avirulent or virulent based on the quantity of honeydew with the threshold of 10 mg/48 h or 20 mg/48 h, or pH of the honeydew with the threshold of 7.2. Genomic DNA from all individuals who underwent the Parafilm sachet test was extracted using a modified method by Livak [27], followed by whole-genome amplification using GenomiPhi v. 2.0 (GE Healthcare, Little Chalfont, UK).
(d). Development of single-nucleotide polymorphism markers and genotyping
Total RNA from the I87i strain (adults, nymphs and eggs) or C89i strain (adults and nymphs) was extracted using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) and analysed by RNAseq (whole-transcriptome shotgun sequencing) on the Hiseq2000 platform (Illumina, San Diego, CA, USA). In total, 59.8 and 35.6 million paired-end, 101-base reads were obtained for I87i and C89i, respectively. To construct reference transcripts, raw read data from the I87i strain were subjected to de novo assembly using Trinity (release 17 March 2012) [28] and 79 824 assembled contigs were obtained larger than 200 bases, totalling 68 328 832 bases. Individual raw reads from each strain were separately mapped to the reference contigs in silico using BWA (v. 0.6.1) [29]. Single-nucleotide polymorphisms (SNPs) between two strains were identified using SAMtools/BCFtools (v. 0.10.12a) software [30]. SNPs and surrounding genomic sequences [25] were subjected to primer design for the MassArray genetic analysis system (Sequenom, San Diego, CA, USA). The MassArray system genotypes individual SNPs by measuring nucleic acid variations using mass spectrometry-based technology. Suitable primers harbouring SNPs were used for the individual genotyping of the F2-C (27 individuals), F2-E (54 individuals) and BF1 (92 individuals) populations.
(e). Single-nucleotide polymorphism data analysis, construction of the genetic linkage map and quantitative trait locus mapping
Genotypic data obtained by the MassArray system were filtered to exclude SNP markers with statistically irregular segregation in F2 populations (χ2 test; p < 0.01) and then were analysed using JoinMap v. 4.1 [31] to determine the linkage groups and order of the markers. To identify the linkage groups corresponding to the 17 linkage groups of BPH [25], 92 simple sequence repeat (SSR) markers were selected from the literature and genotyped with BF1 progenies. After generating a consensus genetic linkage map with framework SNP marker sets, QTL analyses were conducted using Cartographer v. 2.13 with the composite interval mapping method [32]. The total honeydew weight or the honeydew quality (alkaline or neutral) was used as phenotypic data. The significant logarithm of odds (LOD) threshold value for each treatment to determine QTLs was estimated using a simulator implemented in Cartographer with 1000 permutations.
(f). Bulk segregant analysis with next-generation sequencing
To directly survey SNP markers near the detected QTLs, association analysis was performed using bulk DNA from the F2 populations [20,21]. F2 individuals (F2-A–E, 176 individuals in total) were divided into two groups, one comprising 30 virulent individuals (more than 20 mg of alkaline honeydew in 48 h) and the other 139 avirulent individuals (less than 10 mg of alkaline honeydew). Seven intermediate individuals were excluded from grouping. The genomic DNA of the two groups was pooled separately and analysed by whole-genome shotgun sequencing on the Illumina HiSeq2000 platform. Paired-end, 101-base reads obtained from the ‘virulent DNA bulk’ (176 million reads, ≈15× genome size) and ‘avirulent DNA bulk’ (133 million reads, ≈11× genome size) were mapped to the reference-assembled contigs described above. If the virulence to Bph1 was controlled by a single recessive gene in the C89i strain, the virulent DNA bulk should contain only ‘C89i’ alleles of the gene. Conversely, the avirulent DNA bulk should contain ‘I87i’ alleles of the gene from ‘I87i/I87i’ and ‘C89i/I87i’ genotypes, and ‘C89i’ alleles from ‘C89i/I87i’ genotypes in the ratio of ‘I87i’ : ‘C89i’ = 0.67 : 0.33. SNPs that have these theoretical allele frequencies in DNA bulks were selected as the candidate markers linked to the hypothetical virulence gene vBph1 and designated as virulence-linked SNPs (VLSn). Individual genotypes of VLSn in F2-C and F2-E populations were detected by PCR and Sanger sequencing for graphical genotyping.
3. Results
(a). Inheritance of virulence
The honeydew quantity excreted by adult females of parental strain I87i, C89i, their F1 progenies and their F2 progenies were 0.8 ± 0.76, 25.3 ± 3.55, 2.1 ± 1.52 and 8.4 ± 1.37 (mg ± s.e.), respectively. Females from the parental strain (I87i) were avirulent to rice resistance mediated by Bph1, while females from the other parental strain (C89i) were virulent to the same resistance gene (table 1 and figure 1). The F1 generation of the parental cross were avirulent to Bph1 except for two virulent sister individuals (4%) from the F1-E population (table 1 and figure 1). In F2 populations, virulent females occupied 16.4, 21.5 or 23.4% of those tested depending on the methods for discriminating the virulence (table 1). The frequencies of virulent individuals as assessed by honeydew quantity (more than 10 mg) or pH (more than 7.2) yielded a good fit to the 1 (virulence) : 3 (avirulence) ratio (χ2 test; p > 0.05) expected for a single recessive gene (table 1).
Table 1.
Segregation of virulence or avirulence to ‘Saikai190’ rice (Bph1) among F2 females produced by F1 (C89i × I87i) parents. Asterisks indicate the significance of fitting the virulent/avirulent ratio for a χ2 distribution of 0.25.
| population | n | % virulence |
||
|---|---|---|---|---|
| more than 10 mga | more than 20 mgb | pHc | ||
| I87i C89i | 8 21 | 0 76.2 | 0 57.1 | 0 85.7 |
| F1-A F1-B F1-C F1-D F1-E | 10 14 9 10 11 | 0 0 0 0 18.2 | 0 0 0 0 18.2 | 0 0 0 0 18.2 |
| F1 total | 54 | 3.7 | 3.7 | 3.7 |
| F2-A F2-B F2-C F2-D F2-E F2-F F2-G | 34 30 28 30 54 21 17 | 17.6 33.3 25.0 10.0 25.9 9.5 23.5 | 17.6 20.0 21.4 6.7* 18.5 9.5 17.6 | 20.6 26.7 32.1 13.3 27.8 14.3 23.5 |
| F2 total | 214 | 21.5 | 16.4** | 23.4 |
* <0.05, ** <0.01
aIndividuals that produced more than 10 mg of honeydew in 48 h.
bIndividuals that produced more than 20 mg of honeydew in 48 h.
cIndividuals that produced alkaline honeydew (pH > 7.2) in 48 h.
Figure 1.
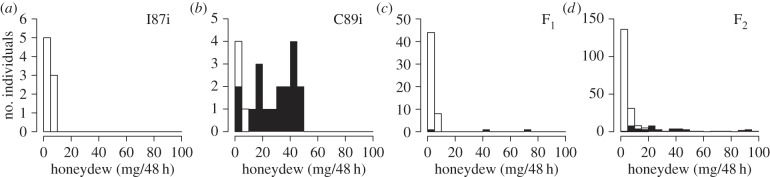
Distribution of the honeydew weight in two sib-lines of N. lugens and their progenies. Individuals with alkaline (pH > 7.2) honeydew are shown in black.
(b). Construction of a genetic linkage map with single-nucleotide polymorphism markers
In total, 109 159 SNPs across 26 946 (34%) assembled contigs were found in two parental strains (I87i versus C89i). Among them, 2344 SNPs and 200 bp of genomic sequences flanking these SNP sites were selected and subjected to MassArray primer design (electronic supplementary material, table S2). Using Joinmap, grouping produced 17 linkage groups from 767 SNPs in the F2-C population and 15 linkage groups from 937 SNPs in the F2-E population. The consensus genetic map for the two F2 populations was constructed using 123 framework SNP markers (electronic supplementary material, table S3 and figure S1). Genotyping of 92 published SSR markers and 191 SNP markers in the BF1 population connected our new linkage map to the published map successfully [25] (electronic supplementary material, table S4). Linkage groups 16 and 17 in the paper were merged to our linkage groups 9 and 15, respectively. Therefore, the total number of linkage groups in the new BPH genetic map was reduced to 15, the same number as the chromosomes of this species [33]. Because of the very low recombination frequency in the BF1 population, the location of SSR and SNP markers in each chromosome could not be analysed.
(c). Detection of quantitative trait loci
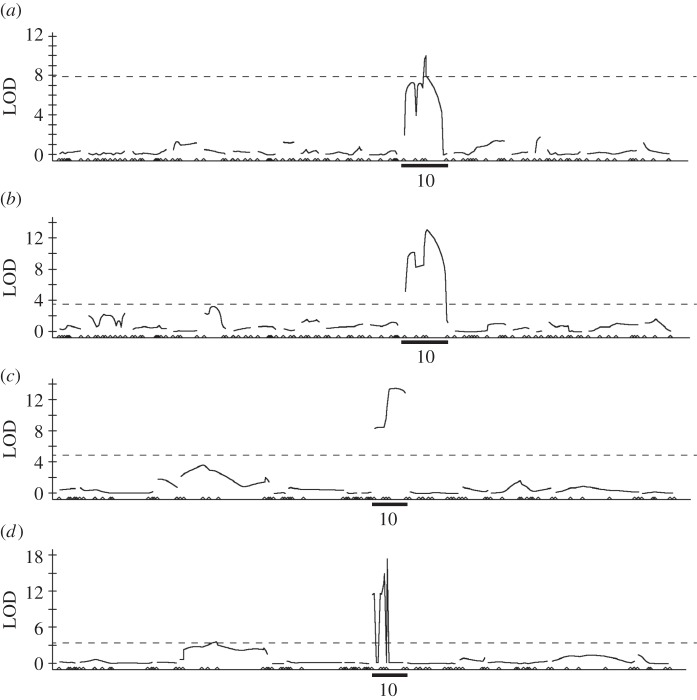
QTL analysis involving 123 SNP markers detected significant regions associated with the virulence of BPH to Bph1-mediated rice resistance (figure 2). In both phenotypes (weight or pH of honeydew), the marker S001204 was identified as the closest for the two populations (F2-C or F2-E; table 2).
Figure 2.
LOD plots from composite interval mapping of the QTL analyses of the virulence to resistant rice variety ‘Saikai190’ that carries Bph1. Horizontal lines indicate the significant LOD threshold. (a) Honeydew weight of the F2-C population. (b) Honeydew pH of the F2-C population. (c) Honeydew weight of the F2-E population. (d) Honeydew pH of the F2-E population.
Table 2.
Locations and biometrical characteristics of QTLs for BPH virulence to the resistant rice variety ‘Saikai190 (Bph1)’. LG, linkage group.
| trait | nearest marker | LG | estimated position (cM) | peak LOD | additive effecta | dominance effect | r2b |
|---|---|---|---|---|---|---|---|
| F2-C | |||||||
| honeydew weight | S001204 | 10 | 16.4 | 10.5 | –52.41 | –29.06 | 0.57 |
| honeydew pH | S001204 | 10 | 16.6 | 13.1 | –0.43 | –0.59 | 0.84 |
| F2-E | |||||||
| honeydew weight | S001204 | 10 | 10.6 | 13.5 | –13.27 | –10.81 | 0.65 |
| honeydew pH | S005185 | 4 | 18.5 | 3.6 | 0.24 | 0.04 | 0.12 |
| S001204 | 10 | 7.6 | 17.5 | –0.47 | –0.35 | 0.72 | |
aAdditive effect of I87i (avirulent) alleles.
bProportion of the phenotypic variance explained by the QTL.
(d). Bulk segregant analysis using next-generation sequencing and graphical genotypes of identified markers
Nineteen SNPs with allele frequencies well fitted to theoretical estimates (1.0 in the virulent DNA bulk and 0.33 in the avirulent DNA bulk) were selected as the candidate markers linked to the putative virulence gene vBph1 and designated as VLS00–VLS18. Five of these markers were mapped to linkage group 10 (electronic supplementary material, table S5). The markers VLS00 and S001204 used in QTL analysis were regarded as one marker, because both were found in the same assembled contig. QTLs in linkage group 10 were reanalysed with five VLS markers from the combined data of two populations, F2-C and F2-E (figure 3a). Graphical genotyping in two F2 populations revealed that vBph1 was located on the 1.8 cM genomic region flanked by SNP markers VLS01 and VLS05 of linkage group 10 (figure 3b,c).
Figure 3.
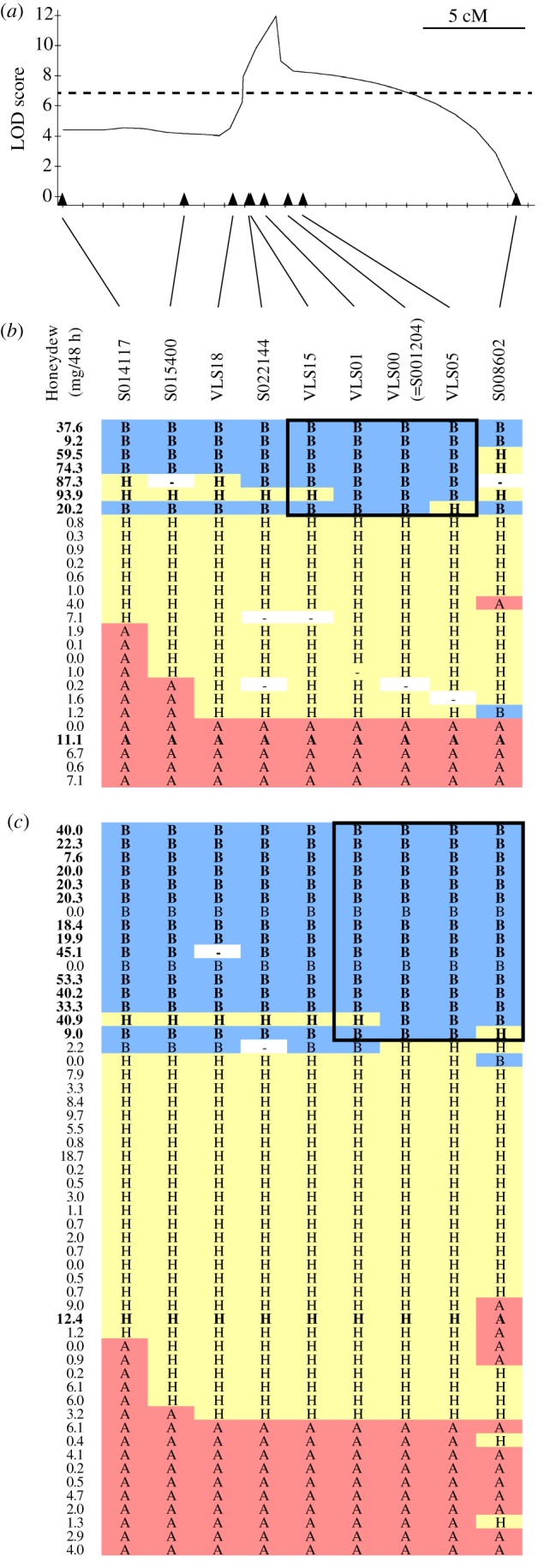
LOD plots of QTL analyses for the virulence to Bph1 and graphical genotypes of the SNP markers on linkage group 10. (a) The peaks of the LOD curves indicate the putative positions of virulence QTLs. Triangles indicate the genetic position of SNP markers. (b) Graphical genotypes of the F2-C population. (c) F2-E population. Individuals in bold represent those with a honeydew pH > 7.2. A, homozygous for the I87i strain; B, homozygous for the C89i strain; H, heterozygous; -, undetermined. (Online version in colour.)
4. Discussion
Re-examination of the genetic analysis of BPH virulence to the major rice resistance gene Bph1 suggests that virulence is controlled by one major gene, as supported by the following evidence. First, the manner of inheritance for the virulence to Bph1 followed the typical Mendelian inheritance of a single recessive gene. Second, QTL analyses identified a single unique region on linkage group 10 of the BPH genome responsible for the virulence. Third, SNPs identified by the bulk segregant analysis in F2 populations were strongly linked genetically to the individual phenotypes of virulence. These findings provide new insight into the genetics of the BPH virulence to resistant rice because previous studies have concluded that virulence is polygenically controlled [15–17]. In those studies, progenies in virulent/avirulent hybrids did not show typical segregation ratios for the virulence to resistant rice varieties. It has been suggested that the cause of indefinite segregations in the genetic analyses of BPH virulence is due to the polygenic nature of the physiological traits or the genetic heterogeneity of parental ‘biotype’ populations [15,16].
Our different conclusion regarding the genetics of BPH virulence from that of previous studies seems to largely depend on the purity of the biological materials and accurate evaluation of virulence. Our inbred strains originated from a single pair of parents from the stock population. The strong bottleneck at the initial isolation and following successive full-sibling matings are expected to result in decreased genetic diversity of the strains, much lower than that of wild populations. Loss of virulence to Bph1 in our F1 populations confirmed the results of previous studies that reported the recessive characteristics of the virulence [11,16]. The selection pressure on rice resistance during sib-crosses would rapidly exclude genetically avirulent individuals from the C89i strain and homogenize the recessive alleles of the virulence gene(s). In addition, all seven pairs of F2 populations in our experiments showed a 1 : 3 ratio of virulence to avirulence, indicating that virulence was mediated by a single recessive gene. Detection of the same single QTL for virulence in two F2 populations and the success of bulk segregant analysis with DNA pools from five F2 populations demonstrated that these populations shared the same single virulence gene, which was homozygous in the parents. These results support the major gene hypothesis in BPH virulence to Bph1 rice resistance. Further studies are needed to determine whether additional genetic factors in wild BPH populations affect the virulence to Bph1.
The virulence test in this study has an advantage over the traditional Parafilm sachet test by Pathak et al. [26], because a reasonable interpretation of the phenotype is adopted to assess the individual virulence. Tanaka [17] specified that a honeydew quantity threshold of 10–20 mg/48 h used in the test is critical for the development and oviposition of adult females, while a quantity greater than the threshold showed no biological effect on the individual. The qualitative nature of the BPH virulence was also confirmed by assessing the quality of honeydew. BPH excretes two types of honeydew after phloem and xylem ingestion [34,35], which can be distinguished by pH [36] because the phloem sap in higher plants is more alkaline (pH 7.8–8.4) than xylem sap (pH 5.4–6.5) [37]. When we classified our avirulent/virulent individuals, grouping by the threshold of 10 mg/48 h in total honeydew production was almost identical to grouping by pH (table 1). This result is in agreement with previous reports that female adults excreting less than 10 mg of honeydew cannot take in sufficient nutrition for ovary development [17]. Individual virulence to Bph1 should be interpreted as a qualitative characteristic of BPH virulence to resistant rice.
The major gene theory in BPH virulence can well explain the behavioural characteristics of the BPH responses to resistant rice varieties. According to the studies using electrical penetration graphs, BPH on resistant rice shows no difficulty in the insertion of stylets or in reaching the vascular bundles; however, phloem ingestion on resistant rice is not sustained [8,35,38–40]. In fact, the principal defence mechanism of resistance genes is to cause direct or indirect inhibition of feeding behaviour although inhibition of feeding behaviour has multiple effects on BPH, including lower survival, prolonged nymphal periods, lower weight and reduced oviposition [8,23,41,42]. Even if a simple genetic mechanism mediated by a major virulence gene controls the qualitative difference in BPH responses between ‘biotypes’, this genetic parsimony might have been masked in wild BPH populations by high levels of genetic diversity and/or heterogeneity [7,22,23,43].
Bph1 is dominant to susceptibility to BPH, and its resistance is specific to BPH [3]. The combination of a dominant resistance gene in rice and a recessive gene to virulence in BPH indicates the establishment of a gene-for-gene relationship between the two species. The gene-for-gene relationship first proposed in phytopathology has been applied to this day to explain the evolution of interactions between general hosts and parasites [44–47], including some pest insects [48]. In this theory, resistance occurs only when both a gene for resistance in the host and a gene for avirulence in the parasite are present. This interaction is the result of coevolution between host resistance and parasite virulence. Most of the pathogen-resistance genes isolated under this theory are known to be NBS–LRR-type proteins that are used as receptors for pathogen detection [47]. Notably, Bph14, the first BPH resistance gene isolated from rice, also encoded an NBS–LRR protein [18]. Possible molecular mechanisms were proposed based on the hypothetic receptor–effector interaction between rice and BPH [19]. Our result supports the presence of a BPH effector that interacts with the rice resistance gene Bph1 in the gene-for-gene relationship.
The traditional concept of a BPH ‘biotype’ to resistant rice has been under debate regarding gene-for-gene relationships [7,22,49,50]. A field BPH population and inbred ‘biotype’ stocks show considerable variations in virulence that overlap between each other [7], and the ‘biotypes’ of BPH are known to be polygenically determined [15–17]. However, genetic variations in a BPH ‘biotype’ or polygenic effects of their virulence do not prevent the formation of a gene-for-gene relationship between rice and BPH for two reasons. First, all interactions between species involve both gene-for-gene relationships and polygenic relationships, and different environmental conditions can magnify or mitigate the gene-for-gene effects in the population structure [45]. Second, the development of new ‘biotypes’ against host plants reflects a directional change in the virulent allele frequency in genetically variable populations, rather than the emergence of a new gene [50]. In the case of BPH resistance in rice, the uneven distribution of resistance genes in local rice cultivars indicates that the sources of the rice resistance genes were the specific regions around Sri Lanka and southern India [1]. Local BPH populations in Sri Lanka also show a wide difference in virulence, and they are closely adapted with their host variety [51]. Therefore, rice resistance to BPH and the corresponding BPH virulence to rice likely coevolved within small geographical areas of this region and would represent the origin of the interaction between a rice immune receptor (R gene) and a specific BPH factor (V gene) [19,52]. Gene flow, along with the spread of the rice cultivation and natural selection on dominant or resistant rice varieties, has led to the occurrence of variants adapted to specific rice varieties, called BPH ‘biotypes’ [43]. However, specific receptor–effector interactions that coevolved in the past may play a critical role between individual rice resistance and corresponding BPH virulence observed currently. Our experiments with iso-female BPH lines could shed light on one of the rice gene-for-BPH gene relationships hidden in the current field BPH populations due to a high level of genetic diversity and heterogeneity.
Isolation of the ‘virulence’ gene and functional analysis of the resistance-breaking mechanism at the molecular level will be crucial for understanding BPH adaptation to resistant rice [19]. Clarifying the resistance-breaking mechanism of BPH also contributes to deciding rice breeding objectives [8,14]. Major gene control of BPH virulence can explain why rice resistance by the single major gene Bph1 had a short-lasting effect on BPH control in the field [9,10]. Monoculture of the single resistance gene Bph1 in the rice field would rapidly change the gene frequency of the corresponding virulence gene vBph1 in the wild BPH population, leading to the emergence of a new ‘biotype’ in a short time. Pyramiding of known resistance genes in commercial rice varieties seems to be insufficient to prevent the development of new virulent populations in the field unless the resistance-breaking mechanism of BPH to each resistance gene was considered [8,14]. Identifying BPH virulence factors to resistant rice will contribute to the establishment of new strategies for efficient use of rice gene resources.
Supplementary Material
Supplementary Material
Acknowledgements
We thank U. Asaga, S. Nakane, M. Urio and T. Toyofuku for their technical support. The original Izumo strain was provided by Dr H. Noda. We also thank Dr M. Yano and Dr K. Hori for their valuable advice regarding QTL analysis, and Dr A. Kaga and T. Shimizu for their assistance with the MassArray analysis.
Data accessibility
The RNAseq raw data for I87i and C89i BPH strains have been deposited in the DDBJ BioProject Database under ID PRJDB1556. SNPs with flanking sequences and primer information are available in the Brown Planthopper Maps & Markers Database (http://sogo.dna.affrc.go.jp/cgi-bin/sogo.cgi?class=unka). The assembled contig sequences of I87i and raw read data for bulk segregant analysis are also available in the above database.
Funding statement
The research was funded by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (Genomics for Agricultural Innovation, LCT-12) and NIAS (National Institute of Agrobiological Sciences) strategic research fund.
References
- 1.IRRI (International Rice Research Institute). 1979. Brown planthopper: threat to rice production in Asia, pp. 171–320. Los Baños, Philippines: International Rice Research Institute. [Google Scholar]
- 2.Khush GS, Virk PS. 2005. IR varieties and their impact. Los Baños, Philippines: International Rice Research Institute. [Google Scholar]
- 3.Pathak MD, Cheng CH, Fortuno ME. 1969. Resistance to Nephotettix impicticeps and Nilaparvata lugens in varieties of rice. Nature 223, 502–504. ( 10.1038/223502a0) [DOI] [Google Scholar]
- 4.Brar DS, Virk PS, Jena KK, Khush GS. 2009. Breeding for resistance to planthoppers in rice. In Planthoppers: new threats to the sustainability of intensive rice production systems in Asia (eds Heong KL, Hardy B.), pp. 401–427. Los Baños, Philippines: International Rice Research Institute. [Google Scholar]
- 5.Jena KK, Kim S-M. 2010. Current status of brown planthopper (BPH) resistance and genetics. Rice 3, 161–171. ( 10.1007/s12284-010-9050-y) [DOI] [Google Scholar]
- 6.Sogawa K. 1982. The rice brown planthopper: feeding physiology and host plant interaction. Annu. Rev. Entomol. 27, 49–73. ( 10.1146/annurev.en.27.010182.000405) [DOI] [Google Scholar]
- 7.Claridge MF, Hollander JD. 1980. The ‘biotypes’ of the rice brown planthopper, Nilaparvata lugens. Entomol. Exp. Appl. 27, 23–30. ( 10.1111/j.1570-7458.1980.tb02942.x) [DOI] [Google Scholar]
- 8.Horgan F. 2009. Mechanisms of resistance: a major gap in understanding plantohopper–rice interactions. In Planthoppers: new threats to the sustainability of intensive rice production systems in Asia (eds Heong KL, Hardy B.), pp. 281–302. Los Baños, Philippines: International Rice Research Institute. [Google Scholar]
- 9.The International Rice Research Institute. 1976. Annual report for 1975, p. 418 Los Baños, Philippines: International Rice Research Institute. [Google Scholar]
- 10.Sogawa K. 1981. Biotypic variations in the brown planthopper, Nilaparvata lugens (Homoptera: Delphacidae) at the IRRI, the Philippines. Appl. Entomol. Zool. 16, 129–137. [Google Scholar]
- 11.Ito K, Kisimoto R. 1981. Selection of new biotypes of the brown planthopper, Nilaprvata lugens Stål, capable of surviving on resistant rice cultivars. J. Cent. Agric. Exp. Stn. 35, 139–154. [Google Scholar]
- 12.Pathak PK, Heinrichs EA. 1982. Selection of biotype populations 2 and 3 of Nilaparvata lugens by exposure to resistant rice varieties. Environ. Entomol. 11, 85–90. [Google Scholar]
- 13.Fujita D, Myint KKM, Matsumura M, Yasui H. 2009. The genetics of host-plant resistance to rice planthopper and leafhopper. In Planthoppers: new threats to the sustainability of intensive rice production systems in Asia (eds Heong KL, Hardy B.), pp. 389–399. Los Baños, Philippines: International Rice Research Institute. [Google Scholar]
- 14.Chen YH. 2009. Variation in planthopper–rice interactions: possible interactions among three species? In Planthoppers: new threats to the sustainability of intensive rice production systems in Asia (eds Heong KL, Hardy B.), pp. 315–326. Los Baños, Philippines: International Rice Research Institute. [Google Scholar]
- 15.den Hollander JD, Pathak PK. 1981. The genetics of the ‘biotypes’ of the rice brown planthopper, Nilaparvata lugens. Entomol. Exp. Appl. 29, 76–86. ( 10.1111/j.1570-7458.1981.tb03044.x) [DOI] [Google Scholar]
- 16.Sogawa K. 1981. Hybridization experiments on three biotypes of the brown planthopper, Nilaparvata lugens (Homoptera: Delphacidae) at the IRRI, the Philippines. Appl. Entomol. Zool. 16, 193–199. [Google Scholar]
- 17.Tanaka K. 1999. Quantitative genetic analysis of biotypes of the brown planthopper Nilaparvata lugens: heritability of virulence to resistant rice varieties. Entomol. Exp. Appl. 90, 279–287. ( 10.1046/j.1570-7458.1999.00448.x) [DOI] [Google Scholar]
- 18.Du B, et al. 2009. Identification and characterization of Bph14, a gene conferring resistance to brown planthopper in rice. Proc. Natl Acad. Sci. USA 106, 22 163–22 168. ( 10.1073/pnas.0912139106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng X, Zhu L, He G. 2013. Towards understanding of molecular interactions between rice and the brown planthopper. Mol. Plant 6, 621–634. ( 10.1093/mp/sst030) [DOI] [PubMed] [Google Scholar]
- 20.Schneeberger K, Weigel D. 2011. Fast-forward genetics enabled by new sequencing technologies. Trends Plant Sci. 16, 282–288. ( 10.1016/j.tplants.2011.02.006) [DOI] [PubMed] [Google Scholar]
- 21.Trick M, Adamski NM, Mugford SG, Jiang CC, Febrer M, Uauy C. 2012. Combining SNP discovery from next-generation sequencing data with bulked segregant analysis (BSA) to fine-map genes in polyploid wheat. BMC Plant Biol. 12, 14 ( 10.1186/1471-2229-12-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Claridge MF, Hollander JD. 1983. The biotype concept and its application to insect pests of agriculture. Crop Prot. 2, 85–95. ( 10.1016/0261-2194(83)90028-5) [DOI] [Google Scholar]
- 23.Sogawa K, Pathak MD. 1970. Mechanisms of brown planthopper resistance in Mudgo variety of rice (Hemiptera: Delphacidae). Appl. Entomol. Zool. 5, 145–158. [Google Scholar]
- 24.Kaneda C, Nemoto H, Ikeda R, Yokoo M, Kobayashi A. 1985. Breeding of rice Norin-PL 3, a new germplasm with brown planthopper resistance. Bull. Naric. Agric. Res. Control Jpn 5, 93–103. [Google Scholar]
- 25.Jairin J, et al. 2013. A simple sequence repeat- and single-nucleotide polymorphism-based genetic linkage map of the brown planthopper, Nilaparvata lugens. DNA Res. 20, 17–30. ( 10.1093/dnares/dss030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pathak PK, Saxena RC, Heinrichs EA. 1982. Parafilm sachet for measuring honeydew exceretion by Nilaparvata lugens on rice. J. Econ. Entomol. 75, 194–195. [Google Scholar]
- 27.Livak KJ. 1984. Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics 107, 611–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grabherr MG, et al. 2011. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat. Biotechnol. 15, 644–652. ( 10.1038/nbt.1883) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760. ( 10.1093/bioinformatics/btp324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, et al. 2004. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079. ( 10.1093/bioinformatics/btp352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Ooijwn JW. 2006. JoinMap 4, software for the calculation of genetic linkage maps in experimental populations. Wageningen, The Netherlands: Kyazma B. V. [Google Scholar]
- 32.Basten CJ, Weir BS, Zeng Z-B. 2002. QTL Cartographer, v. 1.16. Department of Statistics, North Carolina State University, Raleigh, NC, USA.
- 33.Noda H, Tatewaki R. 1990. Re-examination of chromosomes of three species of rice planthoppers (Homoptera: Delphacidae). Appl. Entomol. Zool. 25, 538–540. [Google Scholar]
- 34.Sogawa K. 1980. Chemical nature and excretory activity of honeydew in the brown planthopper, Nilaparvata lugens (Homoptera: Delphacidae). Proc. Assoc. Plant Prot. Hokuriku 28, 30–35. [In Japanese with English summary.] [Google Scholar]
- 35.Kimmins FM. 1989. Electrical penetration graphs from Nilaparvata lugens on resistant and susceptible rice varieties. Entomol. Exp. Appl. 50, 69–79. ( 10.1111/j.1570-7458.1989.tb02317.x) [DOI] [Google Scholar]
- 36.Pathak PK, Heinrichs EA. 1982. Bromocresol-green indicator for measuring feeding activity of Nilaparvata lugens on rice varieties. Philipp. Entomol. 5, 195–198. [Google Scholar]
- 37.Pate JS. 1980. Transport and partitioning of nitrogenous solutes. Annu. Rev. Plant Physiol. 31, 313–340. ( 10.1146/annurev.pp.31.060180.001525) [DOI] [Google Scholar]
- 38.Velusamy R, Heinrichs EA. 1986. Electronic monitoring of feeding behaviour of Nilaparvata lugens (Homoptera: Delphacidae) on resistant and susceptible rice cultivars. Environ. Entomol. 15, 678–682. [Google Scholar]
- 39.Hattori M. 2001. Probing behavior of the brown planthopper, Nilaparvata lugens Stål (Homoptera: Delphacidae) on a non-host barnyard grass, and resistant and susceptible varieties of rice. Appl. Entomol. Zool. 36, 83–89. ( 10.1303/aez.2001.83) [DOI] [Google Scholar]
- 40.Ghaffar MBAB, Pritchard J, Ford-Lloyd B. 2011. Brown planthopper (N. lugens Stål) feeding behaviour on rice germplasm as an indicator of resistance. PLoS ONE 6, e22137 ( 10.1371/journal.pone.0022137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Padgham DE. 1983. The influence of the host-plant on the development of the adult brown planthopper, Nilaparvata lugens (Stål) (Hemiptera: Delphacidae), and its significance in migration. Bull. Entomol. Res. 73, 117–128. ( 10.1017/s0007485300013857) [DOI] [Google Scholar]
- 42.Padgham DE, Woodhead S. 1988. Variety-related feeding patterns in the brown planthopper, Nilaparvata lugens (Stål) (Hemiptera: Delphacidae), on its host, the rice plant. Bull. Entomol. Res. 78, 339–349. ( 10.1017/s0007485300013109) [DOI] [Google Scholar]
- 43.Jing S, Liu B, Peng L, Peng X, Zhu L, Fu Q, He G. 2012. Development and use of EST-SSR markers for assessing genetic diversity in the brown planthopper (Nilaparvata lugens Stål). Bull. Entomol. Res. 102, 113–122. ( 10.1017/s0007485311000435) [DOI] [PubMed] [Google Scholar]
- 44.Flor HH. 1956. The complementary genetic systems in flax and flax rust. Adv. Genet. 8, 29–54. ( 10.1016/s0065-2660(08)60498-8) [DOI] [Google Scholar]
- 45.Thompson JN, Burdon JJ. 1992. Gene-for-gene coevolution between plants and parasites. Nature 360, 121–125. ( 10.1038/360121a0) [DOI] [Google Scholar]
- 46.Hogenhout SA, Van der Hoorn RA, Terauchi R, Kamoun S. 2009. Emerging concepts in effector biology of plant-associated organisms. Mol. Plant Microbe Interact. 22, 115–122. ( 10.1094/mpmi-22-2-0115) [DOI] [PubMed] [Google Scholar]
- 47.Spoel SH, Dong X. 2012. How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. 12, 89–100. ( 10.1038/nri3141) [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Anderson KM, Reber J, Stuart JJ, Cambron S, Harris MO. 2011. A reproductive fitness cost associated with hessian fly (Diptera: Cecidomyiidae) virulence to wheat's H gene-mediated resistance. J. Econ. Entomol. 104, 1055–1064. ( 10.1603/ec10116) [DOI] [PubMed] [Google Scholar]
- 49.Denno RF, Rderick GK. 1990. Population biology of planthoppers. Annu. Rev. Entomol. 35, 489–520. ( 10.1146/annurev.en.35.010190.002421) [DOI] [Google Scholar]
- 50.Downie DA. 2010. Baubles, bangles, and biotypes: a critical review of the use and abuse of the biotype concept. J. Insect Sci. 10, 1–18. ( 10.1673/031.010.14136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Claridge MF, Hollander JD, Furet I. 1982. Adaptations of brown planthopper (Nilaparvata lugens) populations to rice varieties in Sri Lanka. Entomol. Exp. Appl. 32, 222–226. ( 10.1111/j.1570-7458.1982.tb03209.x) [DOI] [Google Scholar]
- 52.Diehl SR, Bush GL. 1984. An evolutionaly and applied perspective of insect biotypes. Annu. Rev. Entomol. 29, 471–504. ( 10.1146/annurev.en.29.010184.002351) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNAseq raw data for I87i and C89i BPH strains have been deposited in the DDBJ BioProject Database under ID PRJDB1556. SNPs with flanking sequences and primer information are available in the Brown Planthopper Maps & Markers Database (http://sogo.dna.affrc.go.jp/cgi-bin/sogo.cgi?class=unka). The assembled contig sequences of I87i and raw read data for bulk segregant analysis are also available in the above database.