Abstract
High-throughput behavior-based screen in zebrafish is a powerful approach for the discovery of novel neuroactive small molecules for treatment of nervous system diseases such as epilepsy. To identify neuroactive small molecules, we first screened 36 compounds (1–36) derived from marine natural products xyloketals and marine isoprenyl phenyl ether obtained from the mangrove fungus. Compound 1 demonstrated the most potent inhibition on the locomotor activity in larval zebrafish. Compounds 37–42 were further synthesized and their potential anti-epilepsy action was then examined in a PTZ-induced epilepsy model in zebrafish. Compound 1 and compounds 39, 40 and 41 could significantly attenuate PTZ-induced locomotor hyperactivity and elevation of c-fos mRNA in larval zebrafish. Compound 40 showed the most potent inhibitory action against PTZ-induced hyperactivity. The structure-activity analysis showed that the OH group at 12-position played a critical role and the substituents at the 13-position were well tolerated in the inhibitory activity of xyloketal derivatives. Thus, these derivatives may provide some novel drug candidates for the treatment of epilepsy.
Keywords: behavior-based screen, zebrafish, PTZ, c-fos
1. Introduction
Disorders of the central nervous system (CNS) are very common and devastating. However, CNS diseases are usually poorly treated due to of the limited availability of selective neuroactive drugs. Thus, the development of novel neuroactive drugs is of high priority. It has been very difficult to discover novel neuroactive drugs in the past years [1]. Many current behavior-altering drugs were discovered by chance in the 1940s and 1950s. A major obstacle to the discovery of novel neuroactive drugs is the lack of available relevant model systems for screening large numbers of active compounds. Modeling the brain activity in vitro is problematic because of the complex networks of the brain. In addition, the screens in mice and rats are low-throughput due to the expense and ethical issues [2]. Recently, zebrafish has become a powerful model system for whole organism small molecule screening. Zebrafish are small, cheap to keep, fast to develop, and easy to breed. Similar to mammals, zebrafish larvae can display diverse behaviors including the optokinetic response [3], the optomotor response [4], prepulse inhibition [5] and sleep [6,7]. Combined with the video track system, several high-throughput behavior-based assays have been successfully applied to identify novel neuroactive small molecules in the zebrafish [8,9].
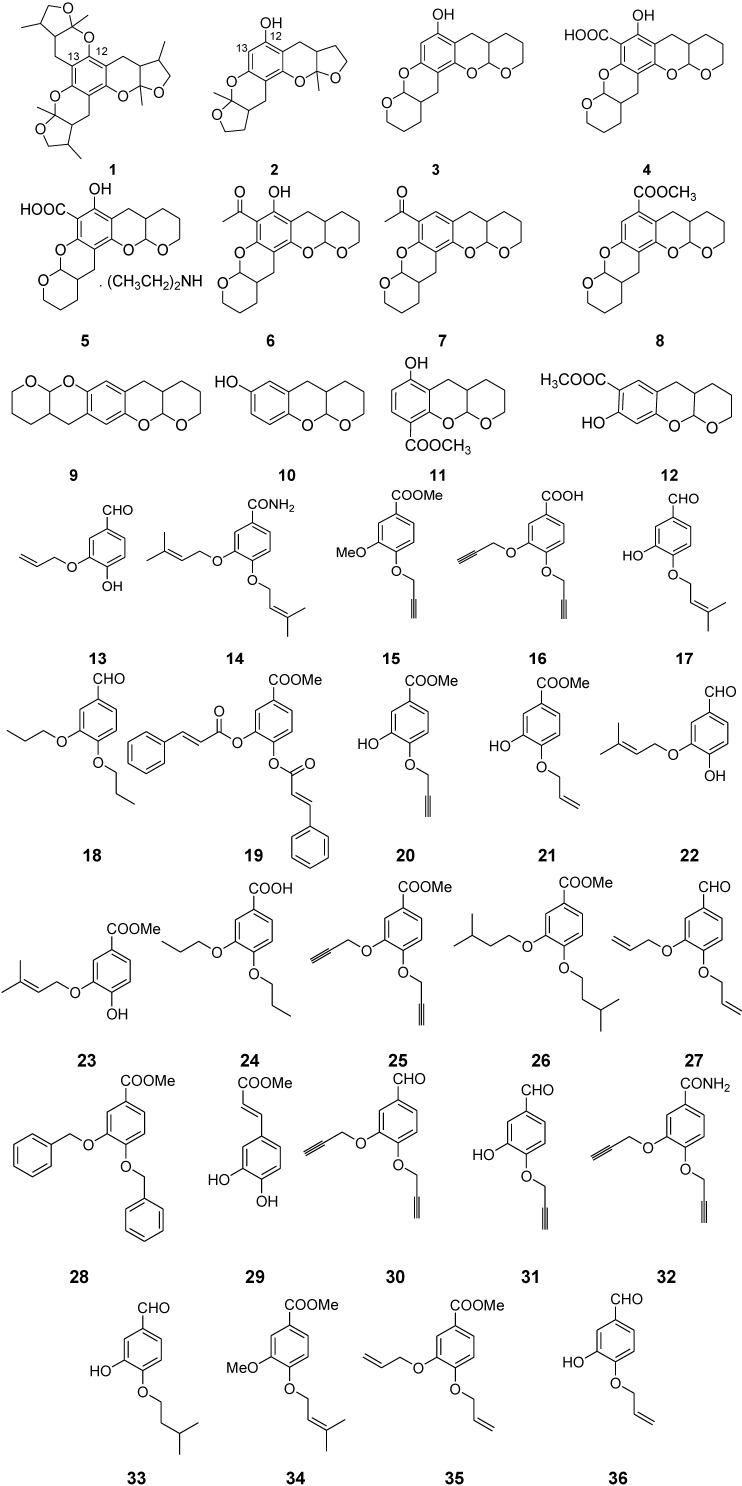
The marine habitat is a rich resource for the discovery of new drugs because of its vast chemical and biological diversity. However, most marine-derived lead compounds are stereochemically complex or have low activity. Thus, the appropriate structural modifications of lead compounds are important to develop chemically simple and active drug candidates [10]. In this paper, we conducted a behavior-based screen for neuroactive small molecules on 12 benzopynan compounds derived from natural xyloketals from marine mangrove fungus (NO. 2508) [11] and 24 isoprenyl phenyl ether derivatives modified from marine isoprenyl phenyl ether from Mangrove fungus (NO. B60) [12] (Chart 1). We further modified compound 1 (Chart 2) to study structure-activity relationships and optimize the biological activity of compound 1 derived compounds. Finally, we explored the potential of compound 40 as a new antiepileptic candidate in pentylenetetrazol (PTZ)-induced epilepsy model in zebrafish.
Chart 1.
Structures selected for neuroactive screening.
Chart 2.
Modification of compound 1.
2. Results and Discussion
2.1. Chemistry
Forty-two analogues of the natural xyloketals and isoprenyl phenyl ether were obtained, and the general synthetic routes of compounds 1–42 have been described previously [10,12,13,14]. Compounds 9–12 were new compounds synthesized by reduction and electrophilic aromatic substitution reactions of 3,4-dihydro-2H-pyran-5-carboxylate with different phenols with 29%–52% yield (Scheme 1). The reduction product of 3,4-dihydro-2H-pyran-5-carboxylate was unstable and was immediately used for the subsequent experiment. The title compounds were isolated as their bis- and mono- adducts in different phenol proportions. Different substituted compounds of methyl 2,4-dihydroxybenzoate 11 and 12 could be obtained in one pot reaction and detected using TLC, followed by purifying, easily done by flash chromatography. However, the final products were obtained in low yields and further optimizations were required. Compounds 9–12 were fully characterized by HRMS and NMR.
Scheme 1.

Synthesis of compounds 9–12.
2.2. Neuroactive Activity of 36 Compounds were Evaluated in Zebrafish Behavioral Assay
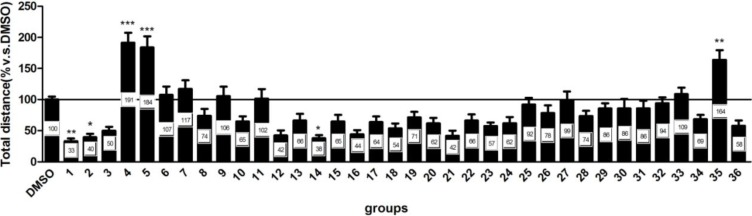
Zebrafish have neural networks similar to mammals and their locomotor activity is a measurable complex behavior. Similar to higher vertebrates, locomotor activity of zebrafish is also regulated by light. In zebrafish behavioral assays, the total swimming distance traveled is often measured to determine the changes in the locomotor activity. Therefore, we examine the effect of different compounds on the total distance during the initial screen. All marine-derived compounds (1–36) were dissolved in DMSO and diluted with E3 buffer (5 mM NaCl, 0.17 mM KCL, 0.33 mM CaCl2·2H2O, 0.33 mM MgSO4) to a final concentration of 20 μM. The results (Figure 1) showed that three compounds including compounds 1, 2 and 14 significantly inhibited locomotor activity (p < 0.01 vs. DMSO) to 33%, 40% and 38%, respectively. Meanwhile, several compounds exhibited a hyperactive effect on locomotor activity (p < 0.01 vs. DMSO). For example, compounds 4, 5 and 35 could significantly increase locomotor activity by 91%, 84% and 64%, respectively.
Figure 1.
The larval zebrafish behavioral assay was performed on 120-hpf zebrafish dosed with compounds at 20 µM concentrations in DMSO. Each group had 24 replicates and three independent experiments were performed. The data of total distance are normalized as the percentage of control and representative of three independent experiments. Data was analyzed using One-way ANOVA followed by Post Hoc test (Bonferroni’s Multiple Comparison Test). * p < 0.05 vs. DMSO, ** p < 0.01 vs. DMSO, *** p < 0.001 vs. DMSO.
2.3. Neuroactive Compounds Exhibited Different Behavioral Patterns
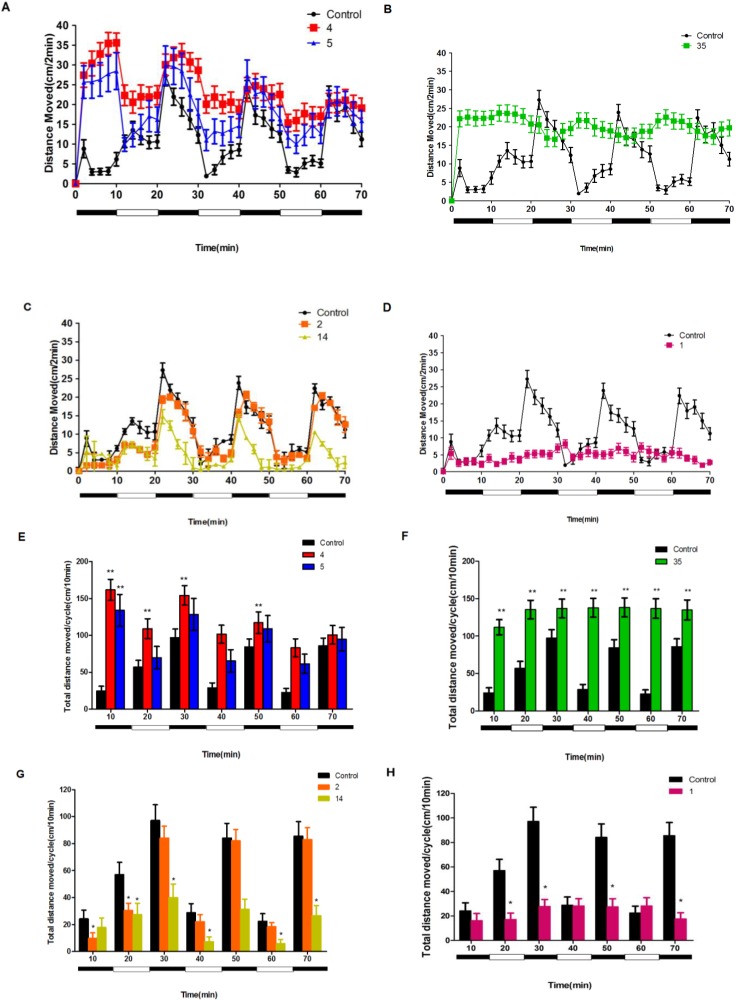
The behavioral assay used here has been well-characterized. During this assay, zebrafish typically exhibited robust but transient behavioral activity in response to sudden transitions from light to dark [15]. In the present study, we used a modified version of this test consisting of a single transition from light to dark. The basal swimming activity was recorded during 10 min with lights on. Immediately following the basal activity recording, the lights were suddenly turned off for 10 min. Consistent with previous reports, the control animals displayed a normal pattern of locomotor activity, i.e., the activity of zebrafish decreased when the visible light was on (light) whereas the activity of zebrafish rapidly and markedly increased when the light was off (dark). We further analyzed these six neuroactive (compounds 1, 2, 4, 5, 14, 35) compounds obtained from the initial screen. We found that neuroactive compounds exhibited distinct patterns and some neuroactive compounds altered the orderly normal activity pattern (Figure 2). Based on the behavior assay, marine-derived compounds tested could be simply categorized as two major types: hyperactive and hypoactive compounds. The behaviors also varied among different compounds within the same types. For example, among these hyperactive compounds, compounds 4 and 5 showed hyperactive activities which were more prevalent in darkness than in light (Figure 2A,E) whereas compound 35 induced a constant hyperactivity all the time regardless of dark or light conditions (Figure 2B,F). Among hypoactive compounds, compound 2 decreased activities during the first dark and light cycle and activities were then returned to normal in the subsequent cycles whereas compound 14 decreased activity during all the periods (Figure 2C,G). In addition, animals treated with compound 1 decreased activity in every dark and light period (Figure 2D,H).
Figure 2.
The behavioral assay of neuroactive compounds. The larvae were placed into the compound solutions (final concentration is 20 µM) and recording began 20 min later in alternating periods of darkness and light for a total duration of 70 min. (A–D) Total activity (distance moved, cm) in two-minute intervals for total duration of 70 min. (E–H) Total activity (distance moved, cm) in each 10-minute light and dark period. Values are reported as mean (n = 21–24 larvae/concentration/plate, for 2 plates) ±SEM. * p < 0.05 vs. DMSO, ** p < 0.01 vs. DMSO.
2.4. Compounds 1 and 37–41 Suppressed Locomotor Activity in Larval Zebrafish
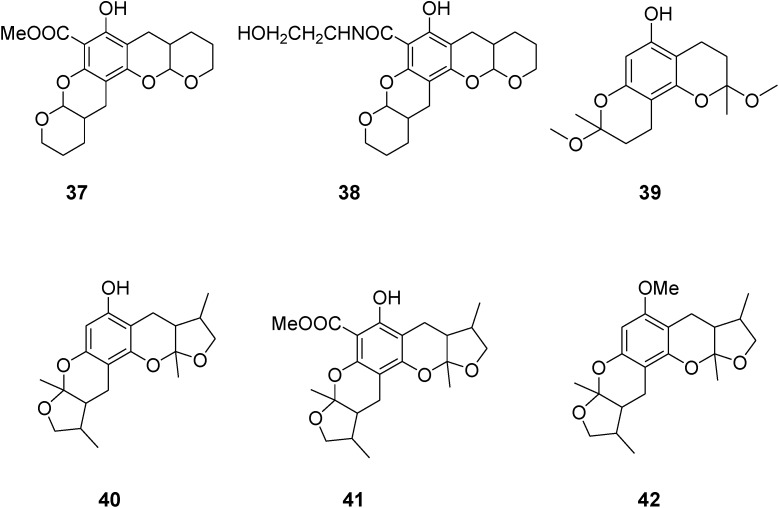
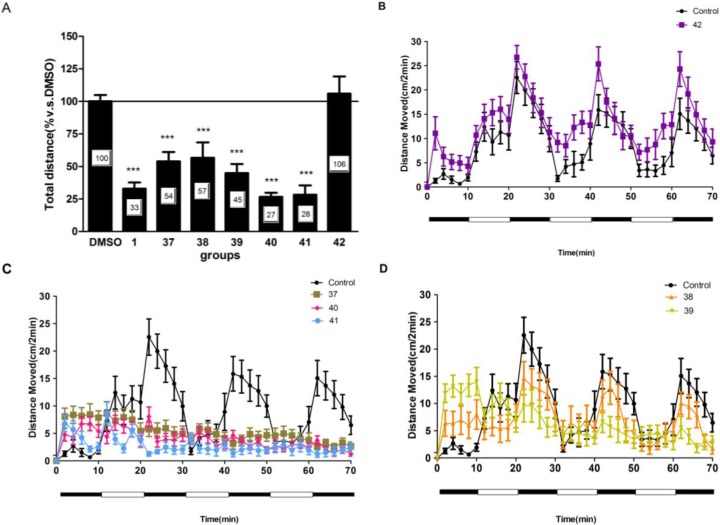
To further study the structure-activity relationships and optimize biological activity of compound 1, three compounds (40–42) derived from 1 and three more compounds(37–39) were synthesized (Scheme 1). The results (Figure 3A) showed that the majority of compounds inhibited the locomotor activity (total distance) at the concentration of 20 μM (p < 0.05 vs. DMSO). Among them, compounds 37–41 could significantly reduce total distance by up to 57%. Moreover, compounds 40 and 41 displayed more potent inhibition compared to the lead compound 1 (reduced activity level to 27% and 28%, respectively). Six derivatives displayed different patterns of locomotor activity (Figure 3B–D). Compound 42 displayed a reverse effect with a higher activity during all of the dark and light cycles. Similar to compound 1, compounds 37, 40 and 41 induced a constant inhibition on locomotor activity throughout every dark and light period. Furthermore, compounds 40 and 41 displayed much lower activity compare to compound 1. Although compounds 38 and 39 suppressed the locomotor activity, they did not disturb the normal orderly pattern of activity. Altogether, we found that compound 40 showed the most potent inhibitory action on locomotor activity.
Figure 3.
Compounds 1 and 37–41 suppressed larval zebrafish locomotor activity. (A) The behavior assay was performed with compounds 37–42. The relative distance of DMSO group was set as control and other groups were shown relative to DMSO group. (B–D) Different activity patterns of compound 1 and compounds 37–42. * p < 0.05 vs. DMSO, *** p < 0.001 vs. DMSO.
Structure–activity investigation was conducted to identify the active components within xyloketal derivatives. We found that xyloketal derivatives possessing the benzopyrano furan skeleton exhibited more potent inhibitory action compared to that possessing benzopyrano pyran. However, methyl ether derivative 42 did not show any suppression of activity compared to controls. Thus, an unsubstituted hydroxyl group or easily cleaved hydroxyl prodrug, such as a phenolic ester at 12-position of the benzopyran scaffold, may be important for the inhibitory action. Furthermore, the substituents at the 13-position may be tolerated but not necessary for the inhibitory activity. However, compound 1 does not possess an OH group at this position perhaps hinting at different binding modes between 1 and 40/41.
2.5. The Potential of Selected Compounds for New Antiepileptic Drug Development in PTZ-Induced Seizure Model
Epilepsy is one of the most common CNS disorders and affects about 50 million people worldwide [16]. A variety of pharmacological and genetic models of epilepsy have been developed to study seizure mechanisms and to identify new anti-epileptic drugs. PTZ is a noncompetitive antagonist of the GABA receptor complex and its epileptogenic properties have been widely used for anti-epileptic drug discovery. Similarly, PTZ can induce measurable seizure-related behaviors in zebrafish. These behaviors can be reversed by known anti-epileptic drugs. Thus, zebrafish are emerging as a useful model system for anti-epileptic drug discovery.
The potential of compounds 1 and 39–41 as anti-epileptic drugs was further explored in a PTZ-induced epilepsy model in zebrafish. Consistent with previous reports [17], PTZ at 10 mM induced a robust locomotor activity (hyperactivity) in zebrafish larvae (Figure 4A). In contrast, all three compounds (39–41) demonstrated significant inhibitory action (hypoactivity) in this PTZ model. Among compounds tested, compound 40 showed the most potent inhibitory activity (Figure 4B).
Figure 4.
The effect of compounds 1 and 39–41 on PTZ-induced hyperactivity in zebrafish. (A) The effect of PTZ on larval activity was initially tested across a broad concentration range (0–40 mM). *** p < 0.001 vs. DMSO. (B) The total distances in larval zebrafish exposure to 10 mM PTZ with or without compound 1 and compounds 39–41. *** p < 0.001 vs. PTZ. (C) The expression levels of c-fos mRNA in larval zebrafish exposure to PTZ (10 mM for 60 min) with or without tested compounds (** p < 0.01 vs. PTZ, ***, p < 0.0001).
PTZ induced changes not only in activity but also in gene expression. The expression levels of IEGs are well correlated with seizure activity. The expression of c-fos has been used as an indicator of neuronal activity in zebrafish which is often measured by using the electroencephalography (EEG) [18], To investigate whether compounds 1 and 39–41 affects PTZ-induced expression of the gene c-fos, quantitative real time PCR of c-fos was performed on larval zebrafish (Figure 4C). The results indicated that the PTZ treatment (10 mM for 60 min) induced about 43-fold expression of c-fos mRNA in five dpf larval zebrafish whereas compounds 1 and 39–41 significantly attenuated the PTZ-induced increase in c-fos expression. The inhibitory action on c-fos expression by compounds tested corresponded to their suppression of PTZ-induced movement in the larval zebrafish. Consistent with the behavior assay, compound 40 exhibited one of the most potent inhibitory actions. Given that PTZ induces c-fos expression exclusively in central nervous system in zebrafish, our data also suggests that the compounds are able to cross the blood brain barrier [19].
2.6. The Dose-Response Study of Compound 40 in PTZ Model
We then conducted a dose-response study on compound 40, the most potent inhibitory compound tested, to explore its efficacy and toxicity. We found that compound 40 at doses between 10 µm and 1 mM significantly reduced PTZ-induced hyperactivity in larval zebrafish (Figure 5F). To investigate whether compound 40 causes potential toxicity to zebrafish, we examined the morphology of compound 40-treated larval zebrafish. The results (Figure 5A–E) showed normal morphology in larval zebrafish receiving different doses of 40, indicating that compound 40 alone at doses between 1 µm and 1 mM did not cause toxic effects on larval zebrafish. Images of larval zebrafish were taken using an OLYMPUS IX71 inverted microscope at 4× magnification.
Figure 5.
The effect of compound 40 on PTZ-induced morphological changes. (A) The morphology of larval zebrafish at five dpf. (B–E) The morphology of five dpf larval zebrafish exposed to compound 40 at different concentrations (100 nM, 1, 10, 100 µm and 1 mM). Scale bar represents 100 µm. (F) The total distances in larval zebrafish exposure to PTZ with or without different concentrations of compound 40. * p < 0.05 vs. PTZ, ** p < 0.01 vs. PTZ.
Calcium has been implicated in the pathophysiology of seizure. It has been reported that calcium influx mediates seizure induction whereas blockage of calcium channels reduces epileptic activity [20,21,22]. Xyloketal derivatives have multiple-properties and their inhibitory action on L-calcium channel activity has been reported [23]. Therefore, xyloketal derivatives may exert their anti-convulsive effect by inhibiting the activity of calcium channels. In addition to the direct anti-convulsive action, calcium channel antagonists can potentiate the anticonvulsant action of other anti-epileptics such as diazepam. Xyloketal derivatives deserve further investigation as a potential anticonvulsant drug candidate.
3. Experimental Section
3.1. Chemistry
All commercial available reagents and solvents were used directly without further purification unless otherwise stated. 1H and 13C NMR data were recorded on a 300 MB NMR spectrometer operating at 400 MHz and 100 MHz for 1H and 13C, respectively. Tetramethylsilane (TMS) as internal standard and all chemical shifts are in ppm (δ). Mass spectra were detected on DSQ (Low Resolution Mass Spectrometer) and MAT95XP (High Resolution Mass Spectrometer).
3.2. General Procedure of Synthesizing Compounds 9–12; Take 9 for Example
3.2.1. Compound 9
To a suspension of lithium aluminum hydride (0.76 g, 20 mmol) in ether (30 mL) with an ice water bath was added a solution of 3,4-dihydro-2H-pyran-5-carboxylate (1.88 g, 13.2 mmol, 3.2 molar equivalent to the subsequent reagent hydroquinone) in ether (20 mL) dropwise. The resultant mixture was allowed to warm to room temperature and stirred for additional 1 hour. Then, aqueous solution of 2 M sodium hydroxide (1.2 g) was added at 0 °C. The resultant mixture was filtered, washed with ether (3 × 50 mL), and the combined filtrates were concentrated in vacuo below 10 °C to afford the reduction product as a colorless liquid. This material was immediately diluted with ether (20 mL) and used for the subsequent experiment without storage because of its instability.
To a suspension of hydroquinone (0.52 g, 4.1 mmol) and anhydrous magnesium sulfate (2 g) in 40 mL ether at 0 °C was added the prepared above ether solution as soon as possible. After stirring for 1 min, p-toluene sulfonic acid (0.1 g) was added. The resultant mixture was allowed to warm to room temperature and stirred for 30 min. The reaction mixture was then filtered and the filter-cake was washed with ether (3× 50 mL). The combined filtrates were washed with water (100 mL) and saturated brine (100 mL) followed by drying over anhydrous magnesium sulfate and concentrated in vacuo. Purification by flash chromatography using ether: dichloromethane (2:25) afforded 0.71 g title compound 9 as a colorless crystal in 50% yield. 1H NMR (400 MHz, CDCl3) δ 6.68 (s, 1H), 6.52 (s, 1H), 5.28–5.14 (m, 2H), 4.06–3.93 (m, 2H), 3.72–3.63 (m, 2H), 2.93–2.39 (m, 4H), 2.25–2.05 (m, 2H), 1.71–1.59 (m, 8H). EI-MS m/z 302 (M). EI-HR-MS m/z Found: 302.1510, Calcd. for C18H22O4: 302.1513.
3.2.2. Compound 10
The title compound 10 was obtained 0.48 g from hydroquinone as a colorless crystal in 52% yield. 1H NMR (400 MHz, CDCl3) δ 6.69 (d, J = 8.7 Hz, 1H), 6.58 (dd, J = 8.7, 3.0 Hz, 1H), 6.49 (d, J = 2.9 Hz, 1H), 6.13 (s, 1H), 5.25 (d, J = 2.6 Hz, 1H), 4.05–3.96 (m, 1H), 3.73–3.66 (m, 1H), 2.81 (dd, J = 16.7, 6.1 Hz, 1H), 2.57 (dd, J = 16.7, 5.3 Hz, 1H), 2.17–2.06 (m, 1H), 1.69–1.59 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 149.73, 146.07, 120.57, 116.82, 115.35, 114.46, 96.45, 62.76, 31.51, 28.50, 24.11, 23.12. EI-MS m/z 206 (M). EI-HR-MS m/z Found: 206.0940, Calcd. for C12H14O3: 206.0937.
3.2.3. Compound 11
The title compound was obtained 0.18 g from methyl 2,4-dihydroxybenzoate as a colorless crystal in 29% yield. 1H NMR (400 MHz, CDCl3) δ 11.18 (s, 1H), 7.62 (d, J = 8.9 Hz, 1H), 6.43 (d, J = 8.9 Hz, 1H), 5.34 (d, J = 2.5 Hz, 1H), 4.06–3.96 (m, 1H), 3.90 (s, 3H), 3.79–3.68 (m, 1H), 2.85–2.61 (m, 2H), 2.29–2.12 (m, 1H), 1.76–1.58 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 170.77, 161.21, 158.86, 128.67, 108.42, 107.88, 105.09, 96.96, 62.57, 51.87, 30.92, 24.09, 23.63, 23.02. EI-MS m/z 264 (M). EI-HR-MS m/z Found: 264.0998, Calcd. for C14H16O5: 264.0992.
3.2.4. Compound 12
The title compound 12 was obtained 0.21 g from methyl 2,4-dihydroxybenzoate as a colorless crystal in 34% yield. 1H NMR (400 MHz, CDCl3) δ 10.61 (s, 1H), 7.52 (s, 1H), 6.45 (s, 1H), 5.38 (s, 1H), 4.04–3.93 (m, 1H), 3.90 (s, 3H), 3.79–3.63 (m, 1H), 2.97–2.74 (m, 1H), 2.65–2.49 (m, 1H), 2.27–2.07 (m, 1H), 1.73–1.53 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 170.16, 161.81, 159.56, 130.88, 111.86, 106.15, 103.82, 97.13, 62.22, 51.84, 31.77, 28.41, 23.63, 23.57. EI-MS m/z 264 (M).
3.3. Zebrafish Behavior-Based Activity Screen
3.3.1. Zebrafish Maintenance
Zebrafish (Danio rerio) were maintained according to standard animal care protocols [24]. AB strain zebrafish were bred to yield embryos. Embryos and larvae were maintained on a re-circulating Tecniplast aquatic system at 28 ± 1 °C and between pH 7.0 and 7.5 on a 14/10 h light/dark (L/D) cycle. Embryos were collected from multiple AB/Tubingen breeding. Embryos were collected, rinsed in E3 buffer (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2·2H2O, 0.33 mM MgSO4), washed three times, transferred to Petri dishes and incubated at 28 °C until 4 dpf. They were washed twice every day to remove dead or unfertilized embryos.
3.3.2. Zebrafish Behavioral Assay
AB strain zebrafish were bred to yield embryos. At 4 dpf, larval zebrafish were transferred to 96-well microplates and acclimated at 28 °C overnight. Twenty-four h later, compounds were added and incubated with zebrafish for 30 min at 28 °C. The 96-well microplates were then put into the zebrafish tracking box (Viewpoint Life Sciences Inc., Montreal, Canada) and the activity of zebrafish was monitored using automated video-tracking (the Viewpoint video tracking system and software).
After zebrafish acclimated at the tracking box for 20 min, their behaviors were recorded in infrared light (which will hereafter be referred to as “dark”), and the infrared light remained on throughout the recording session. In the behavior assay, the visible light (or “light”) was switched on for 10 min and then switched off for 10 min (dark period). This light/dark transition was continued for a total duration of 70 min. Each drug challenge was conducted on at least two separate plates with n = 12 larvae/plate in the compounds challenges, and 24 larvae/plate in the PTZ challenge (for controls, n = 12 larvae/plate in the compounds challenge and 24 larvae/plate in the PTZ challenge). All experiments consisted of 20 min of acclimation in the dark followed by seven 10 min-cycles containing a darkness and light phase (90 min total). To capture the different types of activity, a threshold was set at 25 mm/s in order to separate the fast/darting activity and 4 mm/s to separate the slow activity.
3.3.3. Morphology Assay
Briefly, 5 dpf zebrafish with or without compounds were anaesthetized by treatment with 0.4% tricaine and mounted onto glass slide. The morphology was visualized at 4× magnification under an OLYMPUS IX71 inverted microscope.
3.3.4. Quantitative RT Real Time PCR
Total RNA from zebrafish was extracted using E.Z.N.A. total RNA extraction kit (OMEGA biotek, Inc., Norcross, GA, USA). Intact RNA was checked by running a 1.0% agarose/formaldehyde gel and quantified spectrometrically (Beckman Coulter DU 800) before proceeding to subsequent steps. Five hundred ng of total RNA was reverse-transcribed using PrimeScript™ RT Master Mix (Perfect Real Time) Kit (Takara Inc., Otsu, Japan) according to manufacturer’s instructions. Real-Time PCR was performed on an Opticon MONITORTM Software (MJ Research Inc., Quebec, Canada) using SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) (Takara Inc.). Expression levels for each target gene were calculated by the 2~DDCT method [25]. All analyses were performed in triplicates. Primers used for the RT real-time PCR are as follow:
c-fos Forward primer: 5′-GCTCCATCTCAGTCCCAGAG-3′
c-fos Reverse primer: 5′-AGAGTGGGCTCCAGATCAGA-3′
β-actin Forward primer: 5′-CCGTTGCCCCGAGGCTCTCT-3′
β-actin Reverse primer: 5′-CGCATCCTGAGTCAATGCGCCA-3′
3.3.5. Data Analysis
Dead larvae or larvae with physical abnormalities were not included in any data analyses or figures. Any observed death or abnormalities were not due to drug exposures, and the total amount of dead and abnormal animals on each plate represented less than 5% of the total population. Data were analyzed using Graph Pad Prism software. Replicate experiments were run on two separate days (n = 12/day) for both the therapeutic dilution series test and the PTZ dilution series test, with a carrier control group in each plate. The data for each drug were first assessed using a repeated measures analysis of variance (ANOVA) with time and dose as the independent variables and locomotor activity (distance moved/time) as the dependent variable. All data are presented as mean ± standard error of the mean (SEM).
4. Conclusions
In the present study, we have evaluated the neuroactive activity of 36 natural compounds and six designed novel derivatives in zebrafish model. Compound 1 and compounds 39, 40 and 41 could significantly attenuate PTZ-induced locomotor hyperactivity and elevation of c-fos mRNA in larval zebrafish. Compound 40 showed the most potent inhibitory action against PTZ-induced changes. The structure-activity analysis showed that the OH group at the 12-position had a critical role and the substituents at the 13-position had a favorable role in the inhibitory activity of xyloketal derivatives. Thus, these derivatives may provide some novel drug candidates for the treatment of epilepsy.
Acknowledgments
This study was supported by grants from the National Key Clinical Department, National Key Discipline, Guangdong Key Laboratory For Diagnosis and Treatment of Major Neurological Diseases, the National Natural Science Foundation of China (No. 81371255, 81100936, 21172271), Doctoral Program of Higher Education of China (No. 20110171110058), Guangdong Technological grant (No. 2010B050700024, 2011B050400031, 2012B031800107), and the Natural Science Foundation of Guangdong Province (No. S2011010004860). This study was also supported by the multi-year research grant, university of Macau, MYRG122 (Y1-L3)-ICMS12-SHX.
Abbreviations
- HT
high-throughput
- PTZ
pentylenetetrazol
- GABA
γ-Aminobutyric acid
- 5-HT
5-hydroxy-tryptamine
- NMDA
N-Methyl-d-aspartic acid
- CBZ
carbamazepine
- DMSO
dimethyl sulfoxide
- dpf
days post-fertilization
- hpf
hours post fertilization
- CNS
central nervous system
- IEGs
immediate early genes
- EEG
electroencephalography
Author Contributions
Conceived and designed the experiments: S.-M. Long, F.-Y. Liang, J.-Y. Pang, Z. Pei; Performed the experiments: S.-M. Long, F.-Y. Liang, Q. Wu, X.-L. Liu, X.-L. Yao, S.-C. Li, J. Li; Analyzed the data: S.-M. Long, H. Su, J.-Y. Pang; Wrote the paper: S.-M. Long, J.-Y. Pang, Z. Pei.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Pangalos M.N., Schechter L.E., Hurko O. Drug development for CNS disorders: Strategies for balancing risk and reducing attrition. Nat. Rev. Drug Discov. 2007;6:521–532. doi: 10.1038/nrd2094. [DOI] [PubMed] [Google Scholar]
- 2.Kokel D., Peterson R.T. Chemobehavioural phenomics and behaviour-based psychiatric drug discovery in the zebrafish. Brief. Funct. Genomics Proteomics. 2008;7:483–490. doi: 10.1093/bfgp/eln040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brockerhoff S.E., Hurley J.B., Janssen-Bienhold U., Neuhauss S.C., Driever W., Dowling J.E. A behavioral screen for isolating zebrafish mutants with visual system defects. Proc. Natl. Acad. Sci. USA. 1995;92:10545–10549. doi: 10.1073/pnas.92.23.10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neuhauss S.C., Biehlmaier O., Seeliger M.W., Das T., Kohler K., Harris W.A., Baier H. Genetic disorders of vision revealed by a behavioral screen of 400 essential loci in zebrafish. J. Neurosci. 1999;19:8603–8615. doi: 10.1523/JNEUROSCI.19-19-08603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgess H.A., Granato M. Sensorimotor gating in larval zebrafish. J. Neurosci. 2007;27:4984–4894. doi: 10.1523/JNEUROSCI.0615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prober D.A., Rihel J., Onah A.A., Sung R.J., Schier A.F. Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J. Neurosci. 2006;26:13400–13410. doi: 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhdanova I.V., Wang S.Y., Leclair O.U., Danilova N.P. Melatonin promotes sleep-like state in zebrafish. Brain Res. 2001;903:263–268. doi: 10.1016/S0006-8993(01)02444-1. [DOI] [PubMed] [Google Scholar]
- 8.Kokel D., Bryan J., Laggner C., White R., Cheung C.Y., Mateus R., Healey D., Kim S., Werdich A.A., Haggarty S.J., et al. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat. Chem. Biol. 2010;6:231–237. doi: 10.1038/nchembio.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rihel J., Prober D.A., Arvanites A., Lam K., Zimmerman S., Jang S., Haggarty S.J., Kokel D., Rubin L.L., Peterson R.T., et al. Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science. 2010;327:348–351. doi: 10.1126/science.1183090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li S., Shen C., Guo W., Zhang X., Liu S., Liang F., Xu Z., Pei Z., Song H., Qiu L., Lin Y., Pang J. Synthesis and neuroprotective action of xyloketal derivatives in Parkinson’s disease models. Mar. Drugs. 2013;11:5159–5189. doi: 10.3390/md11125159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu R., Zhou Z.Y., Jiang M.Y., Wang F., Liu J.K. A new isoprenyl phenyl ether riboside from the culture of basidiomycete Laccaria amethystea. J. Asian Nat. Prod. Res. 2010;12:723–726. doi: 10.1080/10286020.2010.499855. [DOI] [PubMed] [Google Scholar]
- 12.Li J., Zhang D., Zhu X., He Z., Liu S., Li M., Pang J., Lin Y. Studies on synthesis and structure-activity relationship (SAR) of derivatives of a new natural product from marine fungi as inhibitors of influenza virus neuraminidase. Mar. Drugs. 2011;9:1887–1901. doi: 10.3390/md9101887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pettigrew J.D., Wilson P.D. Synthesis of xyloketal A, B, C, D, and G analogues. J. Organ. Chem. 2006;71:1620–1625. doi: 10.1021/jo052371+. [DOI] [PubMed] [Google Scholar]
- 14.Xu Z., Li Y., Xiang Q., Pei Z., Liu X., Lu B., Chen L., Wang G., Pang J., Lin Y. Design and synthesis of novel xyloketal derivatives and their vasorelaxing activities in rat thoracic aorta and angiogenic activities in zebrafish angiogenesis screen. J. Med. Chem. 2010;53:4642–4653. doi: 10.1021/jm1001502. [DOI] [PubMed] [Google Scholar]
- 15.Guo S. Linking genes to brain, behavior and neurological diseases: What can we learn from zebrafish? Genes Brain Behav. 2004;3:63–74. doi: 10.1046/j.1601-183X.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- 16.Epilepsy Fact Sheet, World Health Organization. Oct, 2012. [(accessed on 10 May 2014)]. Available online: http://www.who.int/mediacentre/factsheets/fs999/en/
- 17.Mussulini B.H., Leite C.E., Zenki K.C., Moro L., Baggio S., Rico E.P., Rosemberg D.B., Dias R.D., Souza T.M., Calcagnotto M.E., et al. Seizures induced by pentylenetetrazole in the adult zebrafish: a detailed behavioral characterization. PLoS One. 2013;8:e54515. doi: 10.1371/journal.pone.0054515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scallet A.C., Kowalke P.K., Rountree R.L., Thorn B.T., Binienda Z.K. Electroencephalographic, behavioral, and c-fos responses to acute domoic acidexposure. Neurotoxicol. Teratol. 2004;26:331–342. doi: 10.1016/j.ntt.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Fleming A., Diekmann H., Goldsmith P. Functional characterisation of the maturation of the blood-brain barrier in larval zebrafish. PLoS One. 2013;8:e77548. doi: 10.1371/journal.pone.0077548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghasemi M., Shafaroodi H., Nazarbeiki S., Meskar H., Heydarpour P., Ghasemi A., Talab S.S., Ziai P., Bahremand A., Dehpour A.R. Voltage-dependent calcium channel and NMDA receptor antagonists augment anticonvulsant effects of lithium chloride on pentylenetetrazole-induced clonic seizures in mice. Epilepsy Behav. 2010;18:171–178. doi: 10.1016/j.yebeh.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Meyer F.B., Anderson R.E., Sundt T.M., Jr., Yaksh T.L., Sharbrough F.W. Suppression of pentylenetetrazole seizures by oral administration of a dihydropyridine Ca2+ antagonist. Epilepsia. 1987;28:409–414. doi: 10.1111/j.1528-1157.1987.tb03666.x. [DOI] [PubMed] [Google Scholar]
- 22.Moron M.A., Stevens C.W., Yaksh T.L. The antiseizure activity of dihydropyridine calcium channel antagonists in the conscious rat. J. Pharmacol. Exp. Ther. 1990;252:1150–1155. [PubMed] [Google Scholar]
- 23.Wu X.Y., Liu X.H., Lin Y.C., Luo J.H., She Z.G., Jin L.H., Chan W.L., Antus S., Kurtan T., Elsässer B., et al. Xyloketal F: A strong l-calcium channel blocker from the mangrove fungus xylaria sp. (#2508) from the South China Sea Coast. Eur. J. Org. Chem. 2005;2005:4061–4064. doi: 10.1002/ejoc.200500326. [DOI] [Google Scholar]
- 24.Westerfield M. The Zebrafish Book: A Guide for Laboratory Use of Zebrafish (Danio rerio) University of Oregon Press; Eugene, OR, USA: 1995. [Google Scholar]
- 25.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]