Abstract
Background
Evidence from animal models and postmortem human studies points to the importance of the dopamine D3 receptor (D3R) in cocaine dependence (CD). The objective of this pilot study was to use the D3R-preferring radioligand [11C](+)PHNO to compare receptor availability in groups with and without CD.
Methods
Ten medically healthy, non-treatment seeking CD subjects (mean age 41±8) in early abstinence were compared to 10 healthy control (HC) subjects (mean age 41±6) with no history of cocaine or illicit substance abuse. Binding potential (BPND), a measure of available receptors, was determined with parametric images, computed using the simplified reference tissue model (SRTM2) with the cerebellum as the reference region.
Results
BPND in CD subjects was higher in D3R-rich areas including the substantia nigra ((SN) 29%; P=0.03), hypothalamus (28%; P=0.02) and amygdala (35%, P=0.03). No between-group differences were observed in the striatum or pallidum. BPND values in the SN (r = + 0.83; p =0.008) and pallidum (r = + 0.67; p = 0.03) correlated with years of cocaine use.
Conclusions
Between-group differences suggest an important role for dopaminergic transmission in the SN, hypothalamus and amygdala in CD. Such findings also highlight the potential relevance of D3R as a medication development target in CD.
Keywords: cocaine, D3, dopamine, PET, human, [11C](+)PHNO
1. INTRODUCTION
Research suggests that chronic cocaine use exerts long-lasting effects on dopaminergic systems, and these changes have been implicated in addictive processes (Koob and Volkow, 2010; Newman et al., 2012). While all dopamine receptor subtypes (Missale et al., 1998) are likely affected, the dopamine D3 receptor (D3R) may be particularly relevant to addictions like cocaine dependence (CD).
Animal and postmortem human studies support a role for the D3R in CD. Anatomically, D3R is densely present in the mesolimbic system where reward-related learning induced by cocaine occurs (Blaylock and Nader, 2012; Stanwood et al., 2000; Xi and Gardner, 2007). Specifically, D3R mRNA and protein in these areas show increased expression after exposure to stimulants and other drugs of abuse (Caine and Koob, 1993; Heidbreder and Newman, 2010; Neisewander et al., 2004; Staley and Mash, 1996; Xi and Gardner, 2007). Although some apparently inconsistent findings exist (Caine et al., 2012), D3R antagonists and partial agonists inhibit the actions of cocaine in preclinical models (Heidbreder et al., 2005; Le Foll et al., 2005; Newman et al., 2012; Xi and Gardner, 2007). Given these findings and the ineffectiveness of current pharmacologic treatments for CD, the D3R has become a target in medication development for CD.
The development of a D3R–preferring positron emission tomography (PET) ligand, [11C](+)PHNO, has allowed in vivo neuroimaging investigations in schizophrenia, Parkinson’s Disease and tobacco smoking (Boileau et al., 2009; Graff-Guerrero et al., 2009; Mizrahi et al., 2011; Mugnaini et al., 2012). Directly relevant to CD, two PET studies using this ligand were recently completed by the same group studying polysubstance methamphetamine drug users and CD subjects (Boileau et al., 2012; Payer et al., 2014).
The first of these studies (Boileau et al., 2013) focused on primary methamphetamine users, but hair analysis disclosed cocaine metabolites in the majority of users as well. Stimulant users versus non-users showed statistically significantly elevated receptor availability (+46%; p= 0.02) in the substantia nigra (SN), a region with a high level of expression of D3R, but not in areas with predominant D2R expression (e.g.. striatum). In a second study of primary CD subjects and healthy controls (HC; Payer et al., 2014), elevations in receptor availability in SN that approached statistical significance (+24%; p= 0.06) were also found in the cocaine group. In combination, these studies provide evidence of D3R upregulation in the SN in stimulant abuse. It is not known, however, whether this process extends to other D3R rich areas (e.g., such as the hypothalamus) that would suggest a more global D3R upregulation in subcortical areas. In addition, the authors noted that the CD subject sample included had abstinence durations varying from 7–240 days on scan day, which is divergent from previous studies involving PET that focused on early cocaine abstinence (e.g, Martinez et al, 2004, 2011). Given the importance in addiction on length of abstinence in brain circuitry (Koob and Volkow, 2010), it remains relevant to elucidate any possible D3R differences with an early cocaine abstinence cohort.
The objective of the current pilot study is to use the D3R-preferring PET radioligand [11C](+)PHNO to investigate whether individuals with primary CD have elevated binding potential values in D3R-expressing regions (e.g. the SN and hypothalamus) versus comparison subjects in early abstinence.
2. PATIENTS ANDMETHODS
2.1. Subjects
Ten medically healthy, non-treatment seeking CD subjects were compared to 10 age-matched healthy control (HC) subjects without significant alcohol or illicit substance use in the past 3 months (demographic and injection measures are shown in Table 1). Eligibility was confirmed through comprehensive psychiatric histories and clinical semi-structured interviews (e.g., the Mini-International Neuropsychiatric Interview or M.I.N.I.) or SCID-1 (Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Axis I disorders), a physical exam with medical history, routine laboratory studies, pregnancy tests, urine toxicology and electrocardiograms (ECGs).
Table 1.
Subject characteristics and radioligand information in cocaine-dependent (CD) and healthy control (HC) participants. Mean values (and standard deviation) are shown.
| CD | HC | P Value | |
|---|---|---|---|
| Number of Subjects | 10 | 10 | - |
| Age, in years (mean (S.D.)) | 41 (8) | 41 (6) | 0.98 |
| Gender | 8 males; 2 females | 8 males; 2 females | - |
| Race/Ethnicity | 7 AA; 2 EA; 1 Hispanic | 3 AA; 7 EA | - |
| Body Mass Index (kg/m2) | 30 (7) | 29 (6) | 0.84 |
| Injected Mass (μg/kg) | 0.028 (0.003) | 0.026 (0.007) | 0.30 |
| Radioactive Dose (MBq) | 307(121) | 376 (131) | 0.24 |
| Specific Activity (MBq/nmol) | 32 (15) | 46 (24) | 0.15 |
AA: African American, EA: European American
CD participants were required to meet DSM IV criteria for CD, be between the ages of 18 and 50, use cocaine via a high-potency, rapid-onset route of administration (i.e., smoked or intravenous), have a history of regular and recent cocaine use and provide objective evidence of recent use (i.e., benzoylecgonine positivity) on urine toxicology testing. Clinical characteristics of all participants are shown (Table 2).
Table 2.
Substance use characteristics of CD and HC participants. Mean values (and standard deviation) are shown.
| CD | HC | |
|---|---|---|
| Years of cocaine use | 19 (7) | 0 |
| Weekly cocaine use in USD and grams | $395 (262) 2.8 g (1.9) |
0 |
| Weekly ETOH use (drinks) | 14 (23) | 1 (2) |
| Daily nicotine use (cigarettes) | 11 (6) | 0 |
| Cannabis use in the last week (joints) | 1 (3) | 0 |
| Days from last cocaine use to PET scan | 7 (4) | N/A |
Individuals were excluded for evidence of a diagnosis of current or lifetime severe Axis I psychiatric disorders (e.g., schizophrenia or bipolar disorder), current or past serious medical or neurological illness (including a history of head injury with loss of consciousness), current pregnancy (as documented by pregnancy testing at screening and on the day of the PET imaging study), breast feeding or general MRI exclusion criteria. All subjects had not received any medications (pharmacotherapy) for a minimum of 6 weeks at the time of scanning.
The study was performed under protocols approved by the Yale Human Investigation, Yale University Radiation Safety, Yale-New Haven Hospital (YNHH) Radiation Safety, and Yale MRI Safety Committees. Subjects were recruited from New Haven and surrounding areas by advertisement, word of mouth and referrals. Written informed consent was obtained from all participants after a full explanation of study procedures.
2.2. Radiochemistry
Carbon 11–labeled (+)-4-propyl-9-hydroxynaphthoxazine [[11C](+)PHNO] is a D2/D3 receptor agonist radiotracer that has D3R preferring properties. [11C](+)PHNO was prepared as reported before by N-acylation of the despropyl precursor with [11C]propionyl chloride followed by reduction of the resulting amide with lithium aluminum hydride and purification by reverse-phase high performance liquid chromoatography (HPLC; Gallezot et al., 2012). The requisite radioisotope [11C]CO2 was produced with the PETtrace cyclotron (GE Medical Systems, Milwaukee, WI) and purified via the PETtrace Standard Chemistry System. The fraction containing the product was formulated into 9% ethanolic saline by solid-phase extraction, followed by filtration through 0.22-μm Millipore membrane, with a mean single intravenous injection of 341 ± 128MBq and a mean specific activity of 39± 20 MBq/nmol.
2.3. Scanning and imaging Procedures
All scans used a high-resolution research tomograph (HRRT) (Siemens/CTI, Knoxville, TN, USA), which acquired 207 slices (1.2mm slice separation) with a reconstructed image resolution of ~3mm. A transmission scan with a 137Cs point source was obtained before the emission scan. The PET scans were acquired for 120min at rest.
Structural Magnetic resonance images were performed on a Siemens 3-T Trio system (Siemens Medical Solutions, Malvern, Pennsylvania) with a circularly polarized head coil for each subject to exclude individuals with anatomical abnormalities and for coregistration. The dimension and voxel size of MR images were 256 × 256 × 176 and 0.98 × 0.98 × 1.0 mm3, respectively.
Dynamic PET scan data were reconstructed with all corrections (attenuation; normalization; scatter; randoms; deadtime and motion), using the MOLAR algorithm(Carson RE, 2003) with the following frame timing: 6 × 30 sec; 3 × 1 min; 2 × 2 min; 22 × 5 min. Motion correction was based on an optical detector (Vicra, NDI Systems, Waterloo, Ontario, Canada).
A summed image (0–10 min after injection) was created from the motion-corrected PET data and registered to the subject’s MR image, which in turn was nonlinearly registered to a MR template (Montreal Neurological Institute space). All transformations were performed with Bioimagesuite (version 2.5; http://www.bioimagesuite.com). PET data were used to produce a time–activity curve for the cerebellum, which has minimal D3R binding and was used as the reference as in previous studies (Boileau et al., 2012; Ginovart et al., 2007; Mizrahi et al., 2011; Searle et al., 2010). Parametric images of the binding potential (BPND), which is linearly proportional to the density of available D2/D3 receptors, were computed using a simplified reference tissue model (2-parameter version: SRTM2). This method has been previously validated (Wu and Carson, 2002) and was used to optimize the statistical quality of the SRTM used in prior studies by reducing noise of the functional images.
Regions of interest (ROI) included the amygdala, caudate, hypothalamus, pallidum, putamen, SN, thalamus and ventral striatum and were based on the Anatomical Automatic Labeling (AAL) template delineated on MR (Tzourio-Mazoyer et al., 2002) with the exception of hand-drawn ventral striatum and SN templates. The ventral striatum template was based on guidelines from Mawlawi et al. (2001) The SN was manually delineated on BPND images in template space as previously described (Lee DE, 2012).
2.4 Statistical Analysis
All outcomes were summarized descriptively and assessed for normality prior to analysis using normal probability plots and Kolmogorov test statistics. All outcomes were approximately normal. Linear mixed models were used to examine the independent and joint effects of group (between-subjects factor) and region of interest (within-subjects) on BPND values. Between-group contrasts within each region were estimated to explain significant interactions. Within-subject correlations were accounted for by fitting three variance-covariance structures to the data (unstructured, compound symmetry, and heterogeneous compound symmetry) with an unstructured form fitting the data best according to the Bayesian Information Criterion (BIC). Gender, age, BMI, and injection dose were considered as covariates in the above models but were not significant and dropped for parsimony. Among CD subjects, potential associations between background variables (e.g., years of use, age) and BPND levels within each region were evaluated using correlation analysis. Correlations were not adjusted for multiple tests given the exploratory nature of this analysis. All analyses were conducted using SAS, version 9.1 (Cary, NC).
3. RESULTS
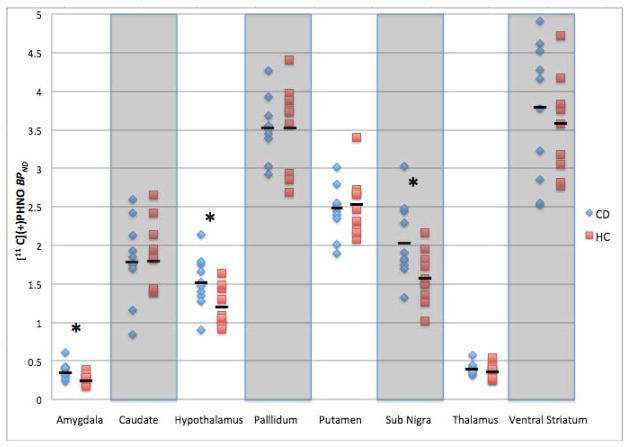
The main effect of diagnostic group on BPND levels was not significant (F1,18 =1.34, P = 0.26). However, a significant diagnostic-group-by-region interaction effect (F7,18 = 3.53, P=0.01) was observed. Table 3 shows mean BPND values for all ROIs. Figure 1 shows individual subject BPND values within each group and region. Higher BPND values in CD (versus HC) individuals were seen in the amygdala (F1,18 = 5.82, P = 0.03; +35%), hypothalamus (F1,18 = 6.22, P = 0.02; +28%) and SN (F1,18 = 5.96, P = 0.03; +29%). Findings persisted when covarying for age, gender, body mass index (BMI) and PET injection parameters.
Table 3.
Mean BPND values (with standard deviation) for each ROI. Percent difference between CD and HC subjects is tabulated.
| BPND Mean (S.D.) | CD | HC | ΔCD | P Value |
|---|---|---|---|---|
| Amygdala | 0.38(0.11) | 0.28(0.28) | +35% | 0.03 |
| Caudate | 1.81 (0.53) | 1.86 (0.45) | −2 % | 0.85 |
| Hypothalamus | 1.52(0.33) | 1.19(0.25) | +28% | 0.02 |
| Pallidum | 3.53 (0.39) | 3.56 (0.55) | −1 % | 0.88 |
| Putamen | 2.45 (0.33) | 2.54 (0.39) | −4 % | 0.58 |
| Substantia Nigra | 2.05(0.50) | 1.59(0.34) | +29% | 0.03 |
| Thalamus | 0.40 (0.07) | 0.38 (0.09) | +6 % | 0.55 |
| Ventral Striatum | 3.74 (0.89) | 3.57 (0.63) | +5 % | 0.63 |
Figure 1.
Individual subject BPND values are shown for each region (N=10 for each group; CD in blue and HC in red). Short bold lines denote group mean values (per Table 3). Asterisks denote statistical significance (p=0.03 for both the amygdala and SN and p=0.02 for the hypothalamus).
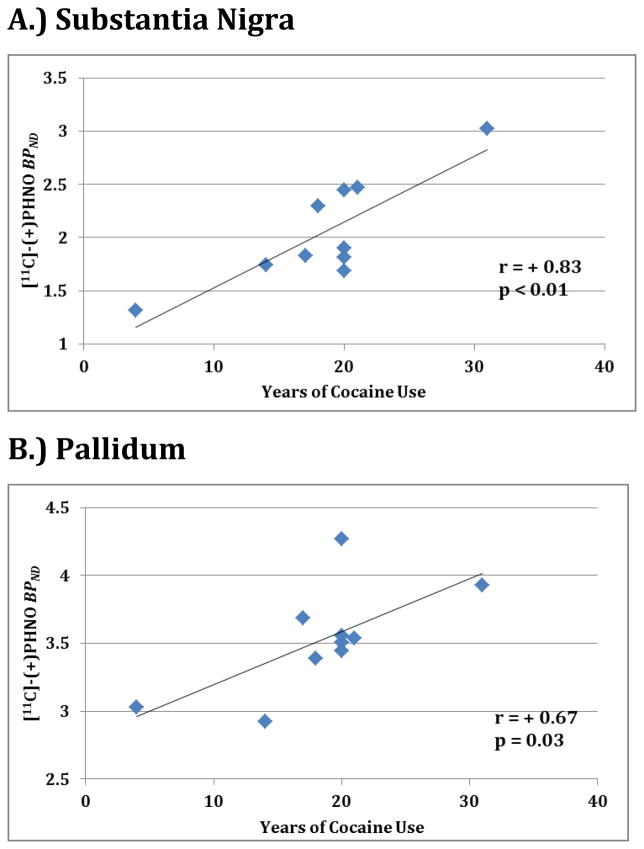
Within CD individuals, positive associations were observed between years of cocaine use and [11C](+)PHNO BPND in the SN (Figure 2A; r = + 0.83; p =0.008) and pallidum (Figure 2B; r = + 0.67; p = 0.03. Weekly cocaine use (in dollars spent) correlated with the amygdala (r = + 0.66; p = 0.04.) No other factors examined (age, gender, BMI, radioligand parameters, alcohol use, nicotine use, or days since last cocaine use) correlated with regional brain BPND availability within this cohort.
Figure 2.
BPND and years of cocaine use reported by participants are shown for the (A) substantia nigra and (B) pallidum. Correlation (r) and significance (p) values are displayed.
4. DISCUSSION
In this study, our major findings were higher [11C](+)PHNO BPND values in the SN, hypothalamus and amygdala in CD as compared with HC individuals. Among CD subjects, years of cocaine use were correlated with BPND values in the SN and pallidum. Despite differences in abstinence periods (on average 7 days for the current study versus 19 days from the polysubstance methamphetamine and 50 days for the previous CD study), overall the recent findings complement those from previous studies showing higher [11C](+)PHNO binding in the SN (Boileau et al., 2012; Payer et al., 2014). The results in the hypothalamus and amygdala have not been reported before, and as such represent novel findings. Implications are discussed in further detail below.
Previous work with [11C]raclopride and a D3R antagonist have indicated that the [11C](+)PHNO signal can be considered to be relatively specific for D2R or D3R depending upon brain region (Graff-Guerrero et al., 2008; Searle et al., 2010; Tziortzi et al., 2011), with some studies attributing 100% of the SN and hypothalamus to D3R (Gallezot et al., 2012; Searle et al., 2010; Tziortzi et al., 2011). Therefore, these two regions are arguably a reasonable representation of a “pure” D3R signal with [11C](+)PHNO and these elevated BPND values could represent a global D3R up-regulation in CD. Further, the correlation between BPND values and years of cocaine use suggests that chronic cocaine use may lead to D3R up-regulation, although longitudinal studies are needed to test this hypothesis directly. While up-regulation of the D3R seems the most plausible explanation, the increase in BPND values may alternatively result from decreased endogenous dopamine (leading to higher ligand binding) in CD participants. Any possible differences in endogenous dopamine levels between the groups could be especially sensitive to [11C](+)PHNO as the binding values of D3R have higher affinities for dopamine than do other dopamine receptors, making D3R-preferring ligands particularly sensitive to endogenous dopamine levels (Schotte et al., 1996; Sokoloff et al., 1992).
While the SN and hypothalamus have been previously studied with [11C](+)PHNO and found to be rich in D3R, the amygdala has not and our findings here should be viewed cautiously due to relatively low BPND values in this region (which adds more overall variability to these results). With that caveat stated, if confirmed, these preliminary results could be a potentially important finding as the amygdala contributes to learned associations between rewarding properties of drugs and cues (Koob, 2003; Koob and Volkow, 2010). In chronic cocaine users, decreased amygdalar volume and changes in functional connectivity involving the amygdala have been described (Gu et al., 2010; Makris et al., 2004) In rodents, D3R antagonists injected into the amygdala have decreased cocaine self-administration under second-order schedules of reinforcement (Di Ciano, 2008), anxiety-like behaviors (Diaz et al., 2011) and more recently cocaine seeking (Xi et al., 2012). In addition, the amygdala has been implicated in cocaine-induced behavioral inflexibility (Stalnaker et al., 2007), cocaine memory reconsolidation (Wells et al., 2012) and stress-induced relapse (Smith and Aston-Jones, 2008). Despite not knowing the attributable D3R percentage in the amygdala of humans, these findings suggest that the D3R receptors represent an attractive pharmacologic target in this region.
It is noteworthy to consider the absence of between-group differences in [11C](+)PHNO binding potential values in D2R-rich regions like the caudate and putamen. These findings contrast with those using the D2/D3 antagonist ligand [11C]raclopride to examine CD (Martinez et al., 2004, 2011; Volkow et al., 1997, 2006; Wong et al., 2006). Several explanations exist. First, low endogenous dopamine levels in CD could differentially increase available [11C](+)PHNO binding, muting more robust differences seen in D2R-rich areas with antagonist tracers. This possibility was given more credence recently as [11C](+)PHNO was found to be more sensitive than [11C]raclopride in detecting the fluctuations of extracellular dopamine in humans (Shotbolt et al., 2012), complementing earlier work on anesthetized animals in the striatum (Ginovart et al., 2006). Second, given the high affinity of dopamine for D3R (Schotte et al., 1996; Sokoloff et al., 1992), another plausible explanation could relate to striatal D3R. Although previous [11C](+)PHNO studies have suggested relatively negligible D3R binding in the caudate and putamen (Gallezot et al., 2012; Searle et al., 2010; Tziortzi et al., 2011), repeated exposure to L-dopa in rats can increase striatal D3R (Bordet et al., 1997). Thus, potential D2R losses could be concealed by up-regulation of the D3R (Boileau et al., 2012). Third, the agonist profile of [11C](+)PHNO has increased affinity to a proposed high-affinity state (D2high) that reflects receptors that are coupled to G-proteins (as opposed to a low-affinity state where receptors are uncoupled from G-proteins, D2low; Graff-Guerrero et al., 2009; Seeman, 2012; Skinbjerg et al., 2012). This differential binding could have also blunted differences, but this possibility has been called into question recently with evidence that agonists (including [11C](+)PHNO) are not selective for dopamine D2High receptors, but also bind to the D2Low state of the dopamine receptors (Seeman, 2012). Evidence for this phenomena explaining our results seems inconclusive, with a current review describing the controversy of whether D2high can be measured in-vivo (Skinbjerg et al., 2012).
The current study was limited by a small sample size that may have precluded the identification of some between-group differences in [11C](+)PHNO binding, particularly in other regions demonstrating moderate levels of D3R expression (e.g., the thalamus, ventral striatum and pallidum). In addition, despite not finding any correlations with the use of other substances (e.g. alcohol and nicotine) in the CD group, we cannot exclude the possibility that differences in non-cocaine substance use between the CD and HC groups and/or ethnicity may have partially confounded the results. That being said, we believe such effects are explanatory for the SN given previously positive findings in matched cohorts (Payer et al., 2014). Despite such limitations and some unresolved questions, this study lends support to the continued development of D3R-related treatments for CD. The first report of a D3R antagonist in the clinical treatment of substance abuse was recently reported to reduce self-reported craving in cigarette smoking (Mugnaini et al., 2012), and it possible that D3R antagonism might normalize a hypofunctional dopamine system in CD (Heidbreder et al., 2005; Xi and Gardner, 2007). Given these prospects, further studies can focus on reproducing these findings with a larger sample size to improve generalizability to CD clinical populations, investigating how long-lasting these differences exist in abstinence, and potentially linking clinically relevant constructs like craving, impulsivity, drug self-administration and deficits in executive functions (Ersche et al., 2012) with D3R availability.
Acknowledgments
We would like to thank the staff of the Clinical Neuroscience Research Unit (CNRU) at Connecticut Mental Health Center (CMHC), the Hospital Research Unit (HRU) in YNHH, the Yale PET Center, the Yale Magnetic Resonance Research Center (MRRC) and especially Julie Holub.
Financial Support
This work was supported by a NARSAD Young Investigator Award Grant (M132018; DM), the National Institute on Drug Abuse (NIDA) (K24 DA017899; 1R03DA027456-01; RTM; K12DA00167; JH; P20 DA027844; RTM, MNP), the National Institute of Mental Health (NIMH; T32 MH019961; DM/RTM), Yale PET Center, and YCCI Pilot Projects Utilizing Core Technologies (RTM)and the Department of Mental Health and Addiction Services (DMHAS) of the State of Connecticut. This publication was also made possible by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Science (NCATS), components of the National Institutes of Health (NIH), and NIH roadmap for Medical Research.
Footnotes
Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Conflicts of Interest and Disclosures: The authors report no conflicts of interest related to the current study. Dr. Potenza has served as a consultant or advisor to Boehringer Ingelheim, Somaxon, gambling businesses and organizations, law offices, the federal defender’s office in issues regarding impulse control disorders. He has received research support from the National Institutes of Health, Veteran’s Administration, Mohegan Sun Casino, the National Center for Responsible Gaming, Psyadon, Forest Laboratories, Ortho-McNeil, Oy-Control/Biotie, and GlaxoSmithKline.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blaylock BL, Nader MA. Dopamine D3 receptor function and cocaine exposure. Neuropsychopharmacology. 2012;37:297–298. doi: 10.1038/npp.2011.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Guttman M, Rusjan P, Adams JR, Houle S, Tong J, Hornykiewicz O, Furukawa Y, Wilson AA, Kapur S, Kish SJ. Decreased binding of the D3 dopamine receptor-preferring ligand [11C]-(+)-PHNO in drug-naive Parkinson’s disease. Brain. 2009;132:1366–1375. doi: 10.1093/brain/awn337. [DOI] [PubMed] [Google Scholar]
- Boileau I, Payer D, Chugani B, Lobo D, Behzadi A, Rusjan PM, Houle S, Wilson AA, Warsh J, Kish SJ, Zack M. The D2/3 dopamine receptor in pathological gambling: a positron emission tomography study with [11C]-(+)-propyl-hexahydro-naphtho-oxazin and [11C]raclopride. Addiction. 2013;108:953–963. doi: 10.1111/add.12066. [DOI] [PubMed] [Google Scholar]
- Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong J, Wilkins D, Selby P, George TP, Zack M, Furukawa Y, McCluskey T, Wilson AA, Kish SJ. Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. J Neurosci. 2012;32:1353–1359. doi: 10.1523/JNEUROSCI.4371-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordet R, Ridray S, Carboni S, Diaz J, Sokoloff P, Schwartz JC. Induction of dopamine D3 receptor expression as a mechanism of behavioral sensitization to levodopa. Proc Natl Acad Sci USA. 1997;94:3363–3367. doi: 10.1073/pnas.94.7.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science. 1993;260:1814–1816. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- Caine SB, Thomsen M, Barrett AC, Collins GT, Grundt P, Newman AH, Butler P, Xu M. Cocaine self-administration in dopamine D3 receptor knockout mice. Exp Clin Psychopharmacol. 2012;20:352–363. doi: 10.1037/a0029135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson RE, Barker WC, Liow J-S, Johnson CA. Design of a Motion-Compensation OSEM List-mode Algorithm for Resolution-Recovery Reconstruction of the HRRT Conf Record IEEE Nuclear Science Symposium and Medical Imaging Conference; Portland, OR. 2003. pp. 3281–3285. [Google Scholar]
- Di Ciano P. Drug seeking under a second-order schedule of reinforcement depends on dopamine D3 receptors in the basolateral amygdala. Behav Neurosci. 2008;122:129–139. doi: 10.1037/0735-7044.122.1.129. [DOI] [PubMed] [Google Scholar]
- Diaz MR, Chappell AM, Christian DT, Anderson NJ, McCool BA. Dopamine D3-like receptors modulate anxiety-like behavior and regulate GABAergic transmission in the rat lateral/basolateral amygdala. Neuropsychopharmacology. 2011;36:1090–1103. doi: 10.1038/npp.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Chamberlain SR, Muller U, Bullmore ET, Robbins TW. Cognitive dysfunction and anxious-impulsive personality traits are endophenotypes for drug dependence. Am J Psychiatry. 2012;169:926–936. doi: 10.1176/appi.ajp.2012.11091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallezot JD, Beaver JD, Gunn RN, Nabulsi N, Weinzimmer D, Singhal T, Slifstein M, Fowles K, Ding YS, Huang Y, Laruelle M, Carson RE, Rabiner EA. Affinity and selectivity of [(1)(1)C]-(+)-PHNO for the D3 and D2 receptors in the rhesus monkey brain in vivo. Synapse. 2012;66:489–500. doi: 10.1002/syn.21535. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Galineau L, Willeit M, Mizrahi R, Bloomfield PM, Seeman P, Houle S, Kapur S, Wilson AA. Binding characteristics and sensitivity to endogenous dopamine of [11C]-(+)-PHNO, a new agonist radiotracer for imaging the high-affinity state of D2 receptors in vivo using positron emission tomography. J Neurochem. 2006;97:1089–1103. doi: 10.1111/j.1471-4159.2006.03840.x. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Willeit M, Rusjan P, Graff A, Bloomfield PM, Houle S, Kapur S, Wilson AA. Positron emission tomography quantification of [11C]-(+)-PHNO binding in the human brain. J Cereb Blood Flow Metab. 2007;27:857–871. doi: 10.1038/sj.jcbfm.9600411. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Mizrahi R, Agid O, Marcon H, Barsoum P, Rusjan P, Wilson AA, Zipursky R, Kapur S. The dopamine D2 receptors in high-affinity state and D3 receptors in schizophrenia: a clinical [11C]-(+)-PHNO PET study. Neuropsychopharmacology. 2009;34:1078–1086. doi: 10.1038/npp.2008.199. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Willeit M, Ginovart N, Mamo D, Mizrahi R, Rusjan P, Vitcu I, Seeman P, Wilson AA, Kapur S. Brain region binding of the D2/3 agonist [11C]-(+)-PHNO and the D2/3 antagonist [11C]raclopride in healthy humans. Hum Brain Mapp. 2008;29:400–410. doi: 10.1002/hbm.20392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. NeuroImage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi ZX, Thanos PK, Mugnaini M, Hagan JJ, Ashby CR., Jr The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Brain Res Rev. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH. Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann NY Acad Sci. 2010;1187:4–34. doi: 10.1111/j.1749-6632.2009.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR, Sokoloff P. The dopamine D3 receptor and drug dependence: effects on reward or beyond? Neuropharmacology. 2005;49:525–541. doi: 10.1016/j.neuropharm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Lee DE, Wilson B, Li CSR, Carson RE. Evaluation of linear and nonlinear spatial transformation models for high resolution PET studies of small brain structures. J Cereb Blood Flow Metab. 2012;32:S128–S191. [Google Scholar]
- Makris N, Gasic GP, Seidman LJ, Goldstein JM, Gastfriend DR, Elman I, Albaugh MD, Hodge SM, Ziegler DA, Sheahan FS, Caviness VS, Jr, Tsuang MT, Kennedy DN, Hyman SE, Rosen BR, Breiter HC. Decreased absolute amygdala volume in cocaine addicts. Neuron. 2004;44:729–740. doi: 10.1016/j.neuron.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, Perez A, Frankle WG, Cooper T, Kleber HD, Fischman MW, Laruelle M. Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, Kumar D, Van Heertum R, Kleber HD, Nunes E. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am J Psychiatry. 2011;168:634–641. doi: 10.1176/appi.ajp.2010.10050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, Agid O, Borlido C, Suridjan I, Rusjan P, Houle S, Remington G, Wilson AA, Kapur S. Effects of antipsychotics on D3 receptors: a clinical PET study in first episode antipsychotic naive patients with schizophrenia using [11C]-(+)-PHNO. Schizophr Res. 2011;131:63–68. doi: 10.1016/j.schres.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Mugnaini M, Iavarone L, Cavallini P, Griffante C, Oliosi B, Savoia C, Beaver J, Rabiner EA, Micheli F, Heidbreder C, Andorn A, Merlo Pich E, Bani M. Occupancy of Brain Dopamine D(3) Receptors and Drug Craving: A Translational Approach. Neuropsychopharmacology. 2012;38:302–312. doi: 10.1038/npp.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Fuchs RA, Tran-Nguyen LT, Weber SM, Coffey GP, Joyce JN. Increases in dopamine D3 receptor binding in rats receiving a cocaine challenge at various time points after cocaine self-administration: implications for cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1479–1487. doi: 10.1038/sj.npp.1300456. [DOI] [PubMed] [Google Scholar]
- Newman AH, Blaylock BL, Nader MA, Bergman J, Sibley DR, Skolnick P. Medication discovery for addiction: translating the dopamine D3 receptor hypothesis. BiochemPharmacol. 2012;84:882–890. doi: 10.1016/j.bcp.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payer DE, Behzadi A, Kish SJ, Houle S, Wilson AA, Rusjan PM, Tong J, Selby P, George TP, McCluskey T, Boileau I. Heightened D3 dopamine receptor levels in cocaine dependence and contributions to the addiction behavioral phenotype: a positron emission tomography study with [(11)C]-(+)-PHNO. Neuropsychopharmacology. 2014;39:321–328. doi: 10.1038/npp.2013.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotte A, Janssen PF, Bonaventure P, Leysen JE. Endogenous dopamine limits the binding of antipsychotic drugs to D3 receptors in the rat brain: a quantitative autoradiographic study. Histochemical J. 1996;28:791–799. doi: 10.1007/BF02272152. [DOI] [PubMed] [Google Scholar]
- Searle G, Beaver JD, Comley RA, Bani M, Tziortzi A, Slifstein M, Mugnaini M, Griffante C, Wilson AA, Merlo-Pich E, Houle S, Gunn R, Rabiner EA, Laruelle M. Imaging dopamine D3 receptors in the human brain with positron emission tomography, [11C]PHNO, and a selective D3 receptor antagonist. Biol Psychiatry. 2010;68:392–399. doi: 10.1016/j.biopsych.2010.04.038. [DOI] [PubMed] [Google Scholar]
- Seeman P. Dopamine agonist radioligand binds to both D2High and D2Low receptors, explaining why alterations in D2High are not detected in human brain scans. Synapse. 2012;66:88–93. doi: 10.1002/syn.20987. [DOI] [PubMed] [Google Scholar]
- Shotbolt P, Tziortzi AC, Searle GE, Colasanti A, van der Aart J, Abanades S, Plisson C, Miller SR, Huiban M, Beaver JD, Gunn RN, Laruelle M, Rabiner EA. Within-subject comparison of [(11)C]-(+)-PHNO and [(11)C]raclopride sensitivity to acute amphetamine challenge in healthy humans. J Cereb Blood Flow Metab. 2012;32:127–136. doi: 10.1038/jcbfm.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinbjerg M, Sibley DR, Javitch JA, Abi-Dargham A. Imaging the high-affinity state of the dopamine D2 receptor in vivo: fact or fiction? Biochem. Pharmacol. 2012;83:193–198. doi: 10.1016/j.bcp.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Andrieux M, Besancon R, Pilon C, Bouthenet ML, Souil E, Schwartz JC. Localization and function of the D3 dopamine receptor. Arzneimittel-Forschung. 1992;42:224–230. [PubMed] [Google Scholar]
- Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker TA, Roesch MR, Calu DJ, Burke KA, Singh T, Schoenbaum G. Neural correlates of inflexible behavior in the orbitofrontal-amygdalar circuit after cocaine exposure. Ann NY Acad Sci. 2007;1121:598–609. doi: 10.1196/annals.1401.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanwood GD, Lucki I, McGonigle P. Differential regulation of dopamine D2 and D3 receptors by chronic drug treatments. J Pharmacol Exp Ther. 2000;295:1232–1240. [PubMed] [Google Scholar]
- Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, Laruelle M, Rabiner EA, Gunn RN. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. NeuroImage. 2011;54:264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AM, Arguello AA, Xie X, Blanton MA, Lasseter HC, Reittinger AM, Fuchs RA. Extracellular signal-regulated kinase in the basolateral amygdala, but not the nucleus accumbens core, is critical for context-response-cocaine memory reconsolidation in rats. Neuropsychopharmacology. 2012;38:753–762. doi: 10.1038/npp.2012.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, Brasic JR, Kimes AS, Maris MA, Kumar A, Contoreggi C, Links J, Ernst M, Rousset O, Zukin S, Grace AA, Lee JS, Rohde C, Jasinski DR, Gjedde A, London ED. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- Wu Y, Carson RE. Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab. 2002;22:1440–1452. doi: 10.1097/01.WCB.0000033967.83623.34. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gardner EL. Pharmacological actions of NGB 2904, a selective dopamine D3 receptor antagonist, in animal models of drug addiction. CNS Drug Rev. 2007;13:240–259. doi: 10.1111/j.1527-3458.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Li X, Li J, Peng XQ, Song R, Gaal J, Gardner EL. Blockade of dopamine D(3) receptors in the nucleus accumbens and central amygdala inhibits incubation of cocaine craving in rats. Addict Biol. 2013;18:665–677. doi: 10.1111/j.1369-1600.2012.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]