Abstract
Rationale
Social factors are important determinants of drug dependence and relapse.
Objectives
We reviewed preclinical literature examining the role of social experiences from early life through the development of drug dependence and relapse, emphasizing two aspects of these experiences: 1) whether the social interaction is appetitive or aversive and 2) whether the social interaction occurs within or outside of the drug-taking context.
Methods
The models reviewed include neonatal care, isolation, social defeat, chronic subordination, and prosocial interactions. We review results from these models in regard to effects on self-administration and conditioned place preference established with alcohol, psychostimulants, and opiates.
Results
We suggest that in general, when the interactions occur outside of the drug-taking context, prosocial interactions are protective against drug abuse-related behaviors whereas social stressors facilitate these behaviors. By contrast, positive or negative social interactions occurring within the drug-taking context may interact with other risk factors to enhance or inhibit these behaviors.
Conclusions
Despite differences in the nature and complexity of human social behavior compared to other species, the evolving animal literature provides useful models for understanding social influences on drug abuse-related behavior that will allow for research on the behavioral and biological mechanisms involved. The models have contributed to understanding social influences on initiation and maintenance of drug use, but more research is needed to understand social influences on drug relapse.
Keywords: Psychostimulants, opiates, alcohol, maternal separation, isolation, social defeat, social dominance, risk factors, stress, review, drug self-administration, conditioned place preference
Introduction
There is growing interest among neuroscience researchers in the role of social influences on the development of drug dependence and relapse. The development of drug dependence is thought to occur in stages and ultimately manifests as a chronic relapsing disorder (Kalivas and O'Brien 2008; Le Moal and Koob 2007). Initial drug use typically occurs during adolescence or early adulthood and is heavily influenced by peers (Bohnert et al. 2009; Ferguson and Meehan 2011; Fergusson et al. 2008; Schulden et al. 2009). In fact, human drug users often report that their initial desire to take drugs is to affiliate with other users (Baker et al. 2004; Sussman 2005). It is important to consider how social context, among other environmental factors, contributes to the initial rewarding effects of the drug-taking experience because individual differences in sensitivity to the drug and how rewarding one finds the initial drug experience are predictive of future dependence (Haertzen et al. 1983; Pomerleau 1995). Most people consume drugs without serious consequences, while others gradually lose control over drug taking and eventually meet the diagnostic criteria for substance abuse or dependence. Attempts to remain abstinent become increasingly difficult across the spectrum of abuse and dependence, and the risk of relapse is persistent (Gawin 1991; O'Brien et al. 1990). Drug craving and relapse are often precipitated by a lapse (i.e., sampling drug), exposure to drug-associated cues, and stress (O'Brien et al. 1998; Shaham et al. 2003; Wikler 1973) and the latter 2 factors are often social in nature (Bohnert et al. 2009; Childress et al. 1993; Sinha 2001).
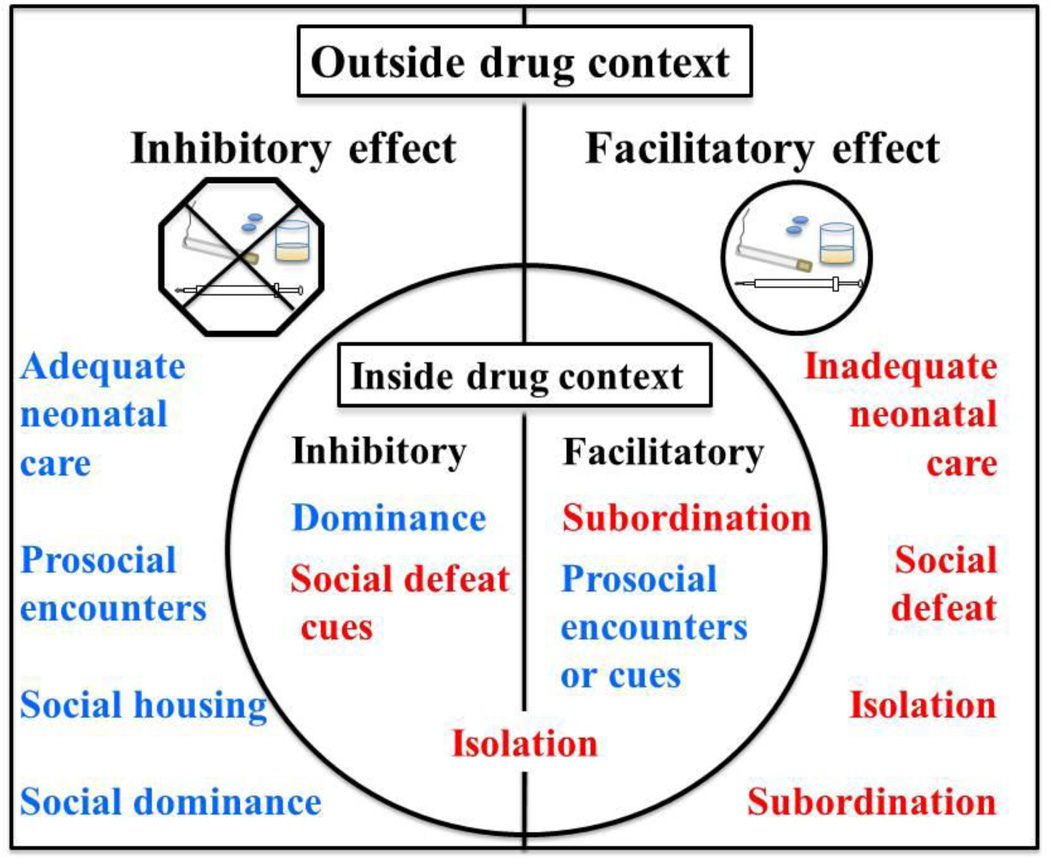
Social interactions are crucial for survival and reproduction, and accordingly, they are powerful determinants of behavior. This review emphasizes two aspects of social interactions that are important for understanding their impact on vulnerability to drug abuse: 1) the emotional valence (i.e., positive versus negative) of the social experience and 2) the context in which the social interaction occurs (i.e., within or outside of the drug-taking context; see Figure 1). First we review models related to early life social experience effects on drug abuse-related behaviors established later in life. These models include neonatal care and post-weaning rearing conditions, each of which has conditions that are positive (i.e., adequate care, social interactions with peers) or negative (i.e., inadequate care, isolation). These manipulations are far removed from the drug experience, and therefore distinctions regarding whether they occur within or outside of the drug-taking context are not considered. Next, we review social influences that occur outside of the drug context proximal to the time of drug experience, followed by social influences occurring within the drug context at the time of drug experience. Within both of these parts of the manuscript, we review positive social factors including social housing and acute appetitive social interactions and negative social factors including isolation housing, acute isolation, and repeated episodes of social defeat (SD) with or without chronic subordination. Based on this review, we suggest that in general social interactions occurring outside of the drug-taking context are protective against drug abuse when the interactions are positive but are risk factors when the interactions are negative. In contrast, positive or negative social interactions within the drug-taking context may interact with other situational events or individual differences to facilitate or inhibit vulnerability to initiate and maintain drug taking and seeking behaviors.
Figure 1.
Heuristic model summarizing inhibitory versus facilitatory social influences on drug abuse-related behaviors, depending to some degree on whether the experience occurs outside or within (circle) the context (i.e., place) where the drug is experienced. Positive social experiences appear in blue and negative social experiences appear in red. See text for some exceptions.
Early life social experiences prior to initiation of drug use
Clinical research has shown that negative childhood stressors such as household dysfunction, parental neglect, and parental emotional/physical abuse can increase risk of psychopathology in adulthood (Chapman et al. 2004; Dube et al. 2001; Wright et al. 2009). It is not surprising that these early life social stressors are risk factors for the development of drug and alcohol abuse (Anda et al. 2008; 1999; Dube et al. 2003; 2006; Strine et al. 2012; Young et al. 2006).
Neonatal care
Several models have been employed to examine effects of early life social experiences on subsequent vulnerability to substance abuse, including maternal deprivation models in which monkeys or rodents are either peer-reared or artificially-reared and models in which neonatal rodents are separated from their mother and placed on a heating pad either as a litter (maternal separation; MS) or individually (neonatal isolation; NI). When discussing the latter two models, we denote the separation time by a number representing minutes (e.g., NI15 = neonatal separation for 15 min; MS360 = maternal separation for 6 h). The type(s) of controls used in rodent studies varies but generally consists of at least one of the following: 1) animal facility reared (AFR) controls that are left undisturbed except for custodial care; 2) non-handled (NH) controls that are left undisturbed except for minimal cage changes by the experimenter; and 3) MS0 controls that are handled and returned to the dam each time experimental animals undergo separation (i.e., controls for handling).
The pioneering work of Harlow and colleagues demonstrated that maternal deprivation for the first 6 months of life in monkeys produced long-lasting behavioral disruption and difficulty coping with stress (Arling and Harlow 1967; Chamove et al. 1973; Harlow et al. 1965). In general, prolonged maternal deprivation or separation results in hyper-responsiveness of the hypothalamic-pituitary-adrenal (HPA) axis in adulthood (Anisman et al. 1998; Levine 2000; Walker et al. 1991). In contrast, the pioneering work of Levine (1957) gave rise to a literature demonstrating that brief separations, sometimes referred to as handling, increase resilience to stress in adulthood in rodents and monkeys (Campbell and Spear 1999; Denenberg et al. 1967; Levine and Mody 2003; Meaney et al. 1991). These findings suggest a quadratic function with respect to resilience across stress intensities, reminiscent of the Yerkes-Dodson Law which asserts an inverted U-shaped relationship between performance and stress or arousal intensity (Yerkes 1908).
The effects of maternal separation on drug abuse-related behaviors in rodents are generally consistent with an inverted U-shaped resilience function. Brief MS (≤15 min) typically protects animals against alcohol and stimulant self-administration compared to AFR and NH controls (see Table 1) whereas prolonged MS (≥60 min) can reverse the protective effects of brief separation and vulnerability to self-administer drugs compared to controls (see Table 2). In contrast to brief MS, NI15 male rats exhibit enhanced ethanol intake compared to NH controls (Lancaster 1998), suggesting that even brief NI enhances vulnerability perhaps because isolation is a more severe stressor than brief MS. Brief MS is actually most similar to natural rearing conditions in which brief parental separations may be necessary for food foraging (Moffett et al. 2006). Furthermore, in rats, maternal behavior toward the pups typically increases once they are returned (Der-Avakian and Markou 2010; Francis and Kuhar 2008; Marmendal et al. 2004) and is likely that the increase in maternal care offsets the stress of brief MS rendering the experience overall more positive compared to controls.
Table 1.
Effects of brief maternal separation (≤ 15 min) on drug abuse-related behaviors in rodents
| Model* | Drug | Dose | Effect** | References*** |
|---|---|---|---|---|
| CPP | Amphetamine | 2–5 mg/kg, i.p. | ↓ amphetamine-CPP | Campbell and Spear, 1999 |
| Self- administration | cocaine | 0.5–1.0 mg/kg, i.v. | ↓ cocaine intake | Flagel et al, 2003 (only males and only when handled during the 1st and not 2nd post-natal week); Moffett et al, 2006(Weinberg 1987) |
| 0.5–1.0 mg/kg, i.v. | ↑ cocaine intake | Flagel et al, 2003 (only in females and only when handled during the 2nd and not 1st post-natal week) | ||
| Ethanol | 2–10%, oral | ↓ ethanol preference and/or intake |
Hilakivi-Clarke et al, 1991; Jaworski et al, 2005 (NH but not AFR controls); Ploj et al, 2003a (effect in subgroup of high drinkers but not overall); Roman et al, 2003; 2005 (only in alcohol-preferring females) | |
| 2–20%, oral | Ø ethanol preference and/or intake |
Daoura and Nylander, 2011; Daura et al, 2011; Huot et al, 2001; Gustafsson and Nylander, 2006; Gustafsson et al, 2005; 2007; Oreland et al, 2011; Roman et al, 2004 | ||
| ↑ ethanol intake | Lancaster et al, 1998 (adolescent onset in males; adult onset in females) |
Controls were either AFR, NH, or MS0 unless otherwise specified
Ø represents no effect
Annotations refer to comparisons made within the cited reference
Table 2.
Effects of prolonged maternal separation (≥ 60 min) on drug abuse-related behaviors in rodents
| Model* | Drug | Dose | Effect** | References*** |
|---|---|---|---|---|
| Cocaine | 2 mg/kg, i.p. | ↓ cocaine-CPP | Hays et al, 2012 | |
| Methamphetamine | 1 mg/kg, i.p. | Ø methamphetamine-CPP | Faure et al, 2009 | |
| 1 mg/kg, i.p. | ↓ methamphetamine-CPP | Dimatelis et al, 2012 | ||
| Morphine | 3–10 mg/kg, s.c. 2 mg/kg, i.p. |
↑ morphine-CPP |
Michaels and Holtzman, 2008; Vazquez et al, 2005b, 2007 (evident as resistance to extinction) |
|
| Intracranial self-stimulation (ICSS) |
Amphetamine | 0.5 mg/kg, i.p. | ↑ amphetamine-induced lowering of ICSS threshold |
Der-Avakian and Markou, 2010 |
| 0.2–1.0 mg/kg, i.p. | Ø amphetamine-induced lowering of ICSS threshold |
Matthews and Robbins, 2003 | ||
| Morphine | 0.3–3.0 mg/kg, s.c. | Ø morphine-induced lowering of ICSS threshold |
Michaels et al, 2007 | |
| Heroin | 0.06–0.6 mg/kg, i.p. | ↓ heroin-induced lowering of ICSS threshold |
Matthews and Robbins, 2003 | |
| Self-administration | Amphetamine | 25 mg/l, oral | ↑ amphetamine intake | Vazquez et al, 2006 |
| Cocaine | 0.0625–0.5 mg/kg, i.v. |
↑ cocaine intake |
Kosten et al, 2000, 2004 (males only), 2006; Matthews et al, 1999 (females only and only at 0.08 mg/kg), Moffett et al, 2006; Zhang et al, 2005 |
|
| 100 mg/l, oral 1.5 mg/kg, i.v. |
Ø cocaine intake | Lynch et al, 2005; Vazquez et al, 2006 | ||
| 0.05–0.5 mg/kg, i.v. | ↓ cocaine intake | Kosten et al, 2004 (females only); Matthews et al, 1999 | ||
| Morphine | 25 mg/l, oral | ↑ morphine intake | Vazquez et al, 2005b, 2006 | |
| Ethanol | 6–20%, oral | ↑ ethanol intake | Cruz et al, 2008; Daora et al, 2011 (adults); Huot et al, 2001; Ploj et al, 2003a (effect in subgroup of high drinkers but not overall); Roman et al, 2005 (only in alcohol-preferring males) |
|
| 2–20%, oral | Ø ethanol preference or intake |
Daoura and Nylander, 2011; Daoura et al, 2011 (adolescents); Gustafsson et al., 2005; Gustafsson and Nylander, 2006; Marmendal et al, 2004, 2006; Oreland et al, 2011; Roman et al, 2004 (only females tested); Vazquez et al, 2006 | ||
| 8–10%, oral | ↓ ethanol preference and intake |
Gustafsson et al, 2007; Jaworski et al, 2005 (compared to NH but not AFR controls); Roman et al, 2005 (only in alcohol-preferring females) | ||
| Extinction | Cocaine history | ↑ cocaine seeking | Lynch et al, 2005 | |
| Cocaine | Cocaine history | 0.5–2.0 mg/kg,i.v. | Ø reinstatement of cocaine | Zhang et al, 2005 |
| reinstatement | priming dose | seeking | ||
| Cue reinstatement |
Cocaine history | ↑ cue reinstatement | Lynch et al, 2005 |
Controls were either AFR, NH, or MS0
Ø represents no effect
Annotations refer to comparisons made within the cited reference
Parental neglect is a risk factor for substance abuse in humans (Altman et al. 1996) that is reliably captured across species by animal models of inadequate neonatal care. For instance, offspring of rodent mothers displaying less attentive behavior toward their pups consume more ethanol and cocaine as adults than the offspring of high attentive mothers (Isengulova et al. 2009; van der Veen et al. 2008). Studies in rhesus monkeys have consistently found elevated ethanol consumption in both male and female peer-reared monkeys compared to mother-reared monkeys (Barr et al. 2009; Barr et al. 2004; Fahlke et al. 2000; Higley et al. 1991; 1996; Newman et al. 2009). Although findings with rodent MS/NI models have been less consistent, the bulk of evidence suggests that prolonged MS facilitates alcohol, stimulant and opiate self-administration later in life as summarized in Table 2. Table 2 also shows that a number of studies have failed to observe effects of MS on alcohol and cocaine self-administration or have found the opposite effect of a decrease in alcohol or cocaine intake (see table for references). The reason for these discrepancies is not clear; however, effects of MS/NI are complicated by several experimental parameters (e.g., litter composition, length and number of separations, age when separations and testing takes place) that may contribute to differences across studies. In some cases MS effects are uncovered after additional manipulations, such as naltrexone pretreatment or post-weaning isolation rearing (Advani et al. 2007; Daoura and Nylander 2011; Fahlke et al. 1997), suggesting that sensitivity to detecting MS/NI effects may be enhanced by additional stressors.
Sex differences likely contribute to the discrepancies across MS/NI studies. The studies that failed to detect effects of prolonged MS on ethanol consumption were conducted with female rats (Gustafsson et al. 2005; Roman et al. 2004) and some studies comparing the effects of brief NI or prolonged MS on ethanol intake across sexes only found effects in males (Lancaster 1998; Roman and Nylander 2005). These findings suggest that males are more sensitive to early rearing stress effects on propensity to drink ethanol later in life. Additionally, even though both male and female peer-reared monkeys exhibit enhanced drinking relative to mother-reared monkeys, sex differences in the genes involved have been found, with vulnerability linked to the serotonin transporter gene promoter in females (Barr et al. 2004) and to the dopamine D1 receptor in males (Newman et al. 2009). With stimulants, effects of MS/NI are more variable in females than males. Increases in stimulant intake have been detected in female NI60 (Kosten et al. 2006) and female MS180–360 rats (Matthews et al. 1999; Moffett et al. 2006) relative to female NH controls. However, decreases in intake have also been reported in female NI60 (Kosten et al. 2004) and in female MS360 rats on the first day of acquisition only (Matthews et al. 1999). The discrepancies across studies using females may be due to confounding effects of fluctuating gonadal steroid hormones that are known to alter cocaine abuse-related behavior in female rats (Fuchs et al. 2005; Jackson et al. 2006; Kippin et al. 2005; Roth et al. 2004). Furthermore, hormonal influences on cocaine effects in females are altered by NI60 (Kosten et al. 2005). Finally, sex may interact with timing of MS as Flagel et al. (2003) found sex differences in cocaine self-administration that were dependent upon the age at which the separations were initiated (see Table 1 for details).
Age at the time of testing for drug consumption is another important factor as MS/NI effects are more robust in adults than younger animals. For instance, MS360 adolescent rats fail to exhibit the enhanced ethanol intake that is observed in adults (Daoura et al. 2011). Although it is possible that the impact of MS is greater in adults, it seems more likely that MS effects are masked by higher alcohol consumption in adolescent rats relative to adult rats.
Effects of MS on stimulant abuse-related behavior may be due to changes in sensitivity to reward systems. For instance, male MS210 rats exhibit lower intracranial self-stimulation (ICSS) thresholds than NH controls in response to amphetamine (Der-Avakian and Markou 2010), consistent with sensitized reward as a result of prolonged MS. However, conditioned place preference (CPP) established with cocaine or methamphetamine is decreased in rodents undergoing NI15 or prolonged MS with or without other stressors (Campbell and Spear 1999; Dimatelis et al. 2012; Hays et al. 2012). These findings may reflect attenuation of stimulant reward or impaired associative processes needed to establish CPP. Nevertheless, other studies have failed to find differences in the rewarding or aversive effects of stimulants after prolonged MS (Faure et al. 2009; Matthews and Robbins 2003; Roma et al. 2008), and Matthews and Robbins (2003) found that MS360 females show greater ICSS elevating effects of the dopamine D2 antagonist raclopride relative to MS2 control females, consistent with enhanced anhedonia. Discrepancies regarding MS effects on stimulant reward enhancement may be due to differences in the MS protocols used (see Der-Avakian and Markou 2010 for discussion).
Effects of MS on opiate reward are also somewhat discrepant. A relatively harsh, prolonged MS procedure results in anhedonic-like increases in ICSS thresholds that are not altered by either morphine or naltrexone (Michaels et al. 2007). In contrast, morphine-CPP is enhanced in animals undergoing MS or NI for ≥180 min relative to NH or AFR controls (Michaels and Holtzman 2008; Vazquez et al. 2005; Vazquez et al. 2007), suggesting enhanced opiate reward. Consistent with this finding, morphine-CPP is also enhanced in artificially-reared male rats compared to mother-reared controls (Lomanowska et al. 2006). Interestingly, conditioned place aversion (CPA) produced by the kappa receptor agonist, spiradoline, is attenuated in males, but not females, undergoing prolonged MS relative to NH controls (Michaels and Holtzman 2008). Collectively, these findings suggest that early life stress may not only enhance the rewarding effects of morphine, but may also attenuate the aversive effects produced by kappa opiate receptors agonists.
Although few studies have examined the effects of maternal care on vulnerability to drug relapse, the evidence to date suggests that prolonged MS/NI may enhance vulnerability. Cocaine seeking-behavior during extinction or cue reinstatement, but not cocaine-primed reinstatement, is enhanced in NI60 rats (Lynch et al. 2005; Zhang et al. 2005). Furthermore, global withdrawal measures following cessation of a continuous morphine infusion are higher in MS180 male and female rats, as well as MS15 female rats, relative to respective NH controls (Kalinichev et al. 2001), factors that may motivate maintenance of drug use.
As shown in Figure 1, we conclude that models of neonatal care suggest that inadequate care increases vulnerability to drug abuse later in life whereas attentive care is protective. This relationship is observed across classes of abuse drugs.
Rearing conditions post-weaning
Housing conditions post-weaning provide a model for assessing early life social influences on drug abuse-related behaviors during adulthood. Several different housing conditions have been investigated that include varying degrees of enrichment (e.g., novel objects, opportunity to forage or exercise, etc.). Given that the present focus is on social influences, we limited our review to studies comparing isolation to social housing with two or more same-sex conspecifics during the post-weaning period.
The majority of the isolation rearing literature suggests that this condition increases alcohol, stimulant and opiate intake later in life compared to social rearing (see Table 3 for references). The increase in alcohol intake observed with isolation rearing manifests across a range of concentrations, as low as 5% (Advani et al. 2007; Wolffgramm 1990) and as high as 20% (Wolffgramm and Heyne 1991). Isolation rearing effects appear to enhance sensitivity to stimulants given that intake is increased relative to controls at low doses during both acquisition (Schenk et al. 1987) and maintenance of self-administration (Bardo et al. 2001; Boyle et al. 1991; Ding et al. 2005; Howes et al. 2000). Isolation-reared rats also acquire cocaine self-administration at a faster rate and escalate intake at a lower dose of cocaine reinforcement when switched from limited to extended daily access compared to social-housed rats (Gipson et al. 2011a). Similarly, isolation-reared rats self-administer more sufentanil aerosol, an opiate receptor agonist, during long access acquisition sessions compared to social controls, although this effect dissipates with limited access sessions during the maintenance phase (1993). An increase in oral morphine consumption also occurs in isolate-reared rats compared to social-reared rats (Alexander et al. 1978). Developmental studies of prolonged isolation suggest that animals are more sensitive to isolation effects during rearing than in adulthood (Lopez et al. 2011; Schenk et al. 1990). One caveat is that animals in these studies were tested for alcohol consumption at different ages and therefore it is unclear whether age at the time of isolation or testing accounts for these differences.
Table 3.
Effects of isolation rearing immediately post-weaning on drug abuse-related behaviors in rodents1
| Model | Drug | Dose | Effect | References |
|---|---|---|---|---|
| Self-administration | Amphetamine | 0.03 mg/kg, i.v. | ↑ amphetamine intake | Bardo et al, 2001 |
| 0.004–0.25 mg/kg, i.v.; 0.025 mg/ml, oral |
Ø amphetamine intake | Bardo et al, 2001; Schenk et al, 1988; Zimmerberg & Brett 1992 | ||
| 0.25 µg/side, intracranial (NAc) |
↓ amphetamine intake | Phillips et al, 1994a+ | ||
| Cocaine | 0.04–0.75 mg/kg, i.v. | ↑ cocaine intake | Boyle et al, 1991; Ding et al, 2005; Gipson et al, 201 1aΔ; Howes et al, 2000; Schenk et al, 1987; Phillips et al, 1994b+ | |
| 0.023–1.5 mg/kg, i.v. | Ø cocaine intake | Boyle et al, 1991; Howes et al, 2000; Phillips et al, 1994a+,b+; Schenk et al, 1987 | ||
| 0.047–1.5 mg/kg, i.v.; 40 mg %, oral |
↓ cocaine intake | Howes et al, 2000; Morse et al, 1993+; Phillips et al, 1994a+; Schenk et al, 1987+ | ||
| Morphine | 0.5 mg/ml, oral | ↑ morphine intake | Alexander et al, 1978+ | |
| Sufentanil aerosol | 50 µg/ml, inhalation | ↑ sufentanil intake | Weinhold et al, 1993+ | |
| Ethanol* | 5–20%, oral | ↑ ethanol intake |
Advani et al, 2007 (males, only in females with a history of MS); Deehan et al, 2007+; Hall et al, 1998; Lodge & Lawrence, 2003+; Lopez et al, 2011; McCool & Chappell 2009+; Schenk et al, 1990+; Wolffgramm 1990+; Wolffgramm & Heyne, 1991+ |
|
| 2–20%, oral | Ø ethanol intake | Advani et al, 2007; Doremus et al, 2005; McCool & Chappell, 2009†; Rockman et al, 1988+; Schenk et al, 1990+ | ||
| 2.5–10%, oral | ↓ ethanol intake | Pisu et al, 2011 | ||
| 10%, oral training dose | Ø extinction responding for ethanol |
McCool & Chappell, 2009 | ||
| CPP | Amphetamine | 0.031 – 2.0 mg/kg, s.c | Ø amphetamine CPP | Bowling & Bardo, 1994; Schenk et al, 1986 |
| 1.5, 5 mg/kg, i.p. | ↓ amphetamine CPP | Wongwitchdecha & Marsden 1995 | ||
| Cocaine | 5, 10 mg/kg, i.p. | ↑ cocaine CPP | Zakharova et al, 2009 | |
| 0.31 – 2.5 mg/kg, s.c. | ↓ cocaine CPP | Schenk et al, 1986 | ||
| Morphine | 5.0 mg/kg, s.c. | ↑ morphine CPP | Kennedy et al, 2011 (BALB: anti-social strain) | |
| 0.25 – 5.0 mg/kg, s.c. | Ø morphine CPP | Kennedy et al, 2011 (B6: social strain) | ||
| 0.25 mg/kg, s.c. | ↓ morphine CPP | Kennedy et al, 2011 (BALB: anti-social strain) | ||
| Heroin | 80 µg/kg, s.c. | Ø heroin CPP | Schenk et al, 1983 | |
| 20 – 40µg/kg, s.c. | ↓ heroin CPP | Schenk et al, 1985; Schenk et al, 1983 | ||
| Extinction CPP | Cocaine | 12.5 mg/kg, i.p. training | ↓ weeks to extinguish | Ribeiro Do Couto et al, 2009 |
| dose | cocaine CPP | |||
| Reinstatement CPP |
50 mg/kg, i.p. training dose | Ø reinstatement | Ribeiro Do Couto et al, 2009 (25 mg/kg, s.c. priming dose) | |
| 50 mg/kg, i.p. training dose | ↓ reinstatement | Ribeiro Do Couto et al, 2009 (12 mg/kg, s.c. priming dose) |
Isolation immediately post-weaning (i.e., beginning PND 20–25)
Isolation housing ≥ 90 days
Enriched environment control (Note - in all other studies controls were social-reared)
Forced consumption only
Choice ethanol consumption
Other behavioral measures in studies of isolation rearing effects on drug abuse-related behaviors provide some insight regarding potential mechanisms involved. For instance, Lodge and Lawrence (2003) observed increased anxiety-like behavior as well as preference for alcohol relative to water, suggesting that anxiety likely contribute to the increased vulnerability. Also, in a cocaine self-administration study, Ding and colleagues (2005) found higher response rates on both the active (i.e., activates reinforcer delivery) and inactive levers in isolated rats resulting in shorter inter-reinforcement intervals compared to social-housed rats. They suggest that the enhanced self-administration behavior in isolated rats reflects greater impulsivity, thereby rendering these rats more vulnerable to cocaine. More recent studies report that impulsive rats do not show indiscriminate responding (Belin et al. 2008; Dalley et al. 2007). Furthermore, the enhanced drug intake in isolated rats is not likely due to a nonspecific propensity to lever press because these rats initially show lower sucrose reinforcement rates than rats reared in an enriched environment, and this effect dissipates during maintenance (Bardo et al. 2001). One possibility is that isolation-reared rats become more aroused during self-administration than social-reared rats and direct this excess energy toward a drug-related behavior (i.e., indiscriminate lever pressing) while titrating drug intake by response rates on the active lever.
There is evidence suggesting that sensitivity to drug and natural rewards may be enhanced as a result of isolation rearing, which may contribute to enhanced drug self-administration. For instance, Hall et al. (1998) found that both alcohol and sucrose consumption are increased in isolation-reared rats, although in contrast, others have found no change or a transient decrease in sucrose consumption (Bardo et al. 2001; McCool and Chappell 2009). Early research examining effects of isolation rearing on drug-CPP suggest that sensitivity to opiate and stimulant reward is either reduced or unchanged relative to that observed in social-reared controls (Bowling and Bardo 1994; 1985; 1983; 1986; Wongwitdecha and Marsden 1996), in contrast to more recent studies suggesting enhanced sensitivity (Kennedy et al. 2011; Zakharova et al. 2009). The reason for these discrepancies is likely related to the apparatus. The latter studies used an unbiased apparatus to avoid preference shifts due soley to reduction of aversion (Carr 1989), whereas the former studies used either a biased apparatus or an apparatus with clear Plexiglas dividers to confine rats to a drug-paired quadrant of an open field. The ability to see other animals during conditioning introduces a social component (Gipson et al. 2011b) that may have interfered with conditioning in the isolation-reared rats relative to controls. Importantly, the enhanced sensitivity to opiate reward reported by Kennedy et al. (2011) is due to an interaction between isolation rearing and the genetic propensity for social behavior in mice to influence morphine-CPP. In the relatively social mouse strain (C57BL/6J), the dose-effect function for morphine-CPP was similar in isolate- and social-reared mice, but in a less social strain (BALB/cJ), the isolation-reared mice showed greater morphine-CPP at a high, but not a low, dose relative to social-reared mice. When collapsed across dose, there was a non-significant tendency in both strains for isolate-reared mice to exhibit higher magnitude morphine-CPP relative to social-reared mice. Collectively, these studies suggest that post-weaning isolate housing affects brain reward and stress systems such that under some genetic and experimental conditions these effects likely contribute to enhanced vulnerability to stimulants and opiates.
As shown on Table 3, a number of studies failed to find differences in drug self-administration between isolated and socially reared animals, and a few studies found a decrease in drug consumption in isolated animals relative to social-housed controls (see Table 3 for references). Although there are some exceptions, in general it appears that isolation rearing decreases stimulant self-administration when a relatively longer period of isolation is used (i.e., ≥ 90 days). Dose of cocaine may also contribute to discrepancies given that a high dose of 1.5 mg/kg, i.v. either produced a decrease or no effect (Phillips et al. 1994a; Phillips et al. 1994b). Higher doses of cocaine tend to be aversive (Nomikos and Spyraki 1988), and therefore, it is possible that isolated rats are more sensitive to the aversive effects of high cocaine doses than the social-reared controls, resulting in lower intake. These studies also used a different strain of rats, the Lister hooded strain, suggesting that strain differences may contribute to the discrepancies. Consistent with this idea, Morse et al. (1993) reported a strain-dependent decrease in oral cocaine intake in isolated mice DBA/2J compared to C57BL/6J mice.
Similar to inadequate neonatal care, studies investigating early life housing conditions reveal that animals reared in isolation display increased drug intake and motivation for drugs of abuse. Collectively, these pre-clinical findings are consistent with the human literature on adverse childhood experiences increasing risk of drug and alcohol abuse later in life.
Social experiences proximal in time to the drug experience but occurring outside of the drug context
Isolation versus social housing outside of drug context
Little is known about the effects of isolation versus social housing on intravenous drug intake because most studies employing intravenous drug self-administration utilize isolation housing for practical reasons, such as the ability to isolate drug intake for individual subjects and to avoid catheter damage. Furthermore, the majority of the literature on housing effects has compared isolation housing to environmental enrichment, but the latter model confounds social influences on drug intake with other components of enrichment. There is a literature examining housing effects on alcohol intake as reviewed previously (Becker et al. 2011). The present review will emphasize those studies that dissociated housing manipulations from other environmental stressors (e.g., novel context isolation, restraint). As summarized in Table 4, studies comparing isolation to social housing suggest that intake of ethanol and heroin is higher in isolated animals than social-housed animals, although some studies have failed to find housing effects on ethanol intake (see Table 4 for references). The only study to examine social housing effects outside of the cocaine self-administration context in adults found no effect during acquisition or maintenance (Bozarth et al. 1989). Also, no effects on morphine-, heroin-, or cocaine-CPP have been observed across isolated and social-housed adult rodents (Herzig and Schmidt 2005; Ribeiro Do Couto et al. 2009; Schenk et al. 1985).
Table 4.
Isolation housing effects outside of drug context on drug abuse-related behaviors in rodents1
| Model | Drug | Dose | Effect | References |
|---|---|---|---|---|
| Self-administration | Cocaine | 1.0 mg/kg, i.v. | Ø cocaine intake | Bozarth et al 1989 |
| Heroin | 0.1 mg/kg, i.v. | ↑ heroin intake | Bozarth et al, 1989 | |
| Ethanol * | 8 – 25%, oral | ↑ ethanol intake | Ehlers et al, 2007+(alcohol-preferring rats only); Juarez & Vazquez-Cortes, 2003†; Roske et al, 1994 | |
| 2 – 15%, oral | Ø ethanol intake | Lopez et al, 2011; Thorsell et al, 2005† | ||
| CPP | Cocaine | 3.125 – 50 mg/kg, i.p. | Ø cocaine CPP | Ribeiro Do Couto et al, 2009 |
| Heroin | 20 µg/kg, s.c. | Ø heroin CPP | Schenk et al, 1985 | |
| Morphine | 10 mg/kg, i.p. | Ø morphine CPP | Herzig & Schmidt 2005 | |
| Extinction | Cocaine history |
↑ resistance to extinction |
Ribeiro Do Couto et al, 2009; Thiel et al, 2009bΔ | |
| Cue reinstatement |
Cocaine history |
↑ reinstatement | Thiel et al, 2010 | |
| Cocaine reinstatement |
Cocaine history |
10 mg/kg, i.p. priming dose | Ø reinstatement | Thiel et al, 2009b |
| 12 mg/kg, i.p. priming dose | ↑ reinstatement | Ribeiro Do Couto et al, 2009 |
Isolation began in adolescence or adulthood; comparisons social-housed controls unless otherwise specified
Choice ethanol consumption
Isolation housing ≥ 90 days
Enriched environment control
Forced consumption only
Protective effects of social housing in adults have been found in the extinction/reinstatement model. Specifically, rats trained to self-administer cocaine while isolate-housed and then switched to social housing during a period of forced abstinence exhibit less cocaine-seeking behavior under extinction and less cue reinstatement of extinguished cocaine-seeking behavior relative to rats that remained isolated (Thiel et al. 2010; Thiel et al. 2009b) housing conditions failed to alter cocaine-primed reinstatement in this study. The protective effects of social housing are further enhanced by other environmental enrichments, which has been demonstrated using reinstatement of cocaine-CPP (Solinas et al. 2008; Solinas et al. 2009), and stress and cue reinstatement of cocaine-seeking behavior (Chauvet et al. 2009; Thiel et al. 2010). The detrimental effects of isolation housing have also been demonstrated in adult OF1 mice that are moved from group housing to isolation after establishing cocaine-CPP. These mice exhibit an increase in resistance to extinction of the CPP compared to those that remain group housed, as well as an increase in sensitivity to cocaine-primed reinstatement of extinguished cocaine-CPP (Ribeiro Do Couto et al. 2009).
In conclusion, isolation outside of the drug context appears to increase the propensity to self-administer ethanol and opiates, and to increase the incentive motivational effects of cocaine-associated cues.
Brief social defeat (SD) in rodents outside of drug context
Bullying or physical attack and the resulting psychological trauma from such social interactions are risk factors for substance abuse (Back et al. 2000; Brown et al. 1999; Niedhammer et al. 2011; Traweger et al. 2004). Although episodes of physical violence/trauma that are associated with increased vulnerability sometimes occur in the drug-taking context, this experience more often occurs outside of the drug-taking context, such as school or the workplace. One ethologically relevant rodent model of such experience is the resident-intruder model in which brief agonistic confrontations occur between a non-aggressive rodent (i.e., intruder) that is placed into the home cage of an aggressive rodent (i.e., resident). The resident then attacks the intruder and upon reaching a criterion of SD (i.e., usually the intruder lying motionless in a supine position for several seconds), the intruder is either removed from the cage or placed into a smaller protective, yet semi-open, enclosure within the resident’s cage in order for the defeated rat to experience continued threat (Tornatzky and Miczek 1993).
In general, SD increases vulnerability to stimulant self-administration, especially in animals with a history of stimulant self-administration. Miczek and colleagues reported that SD increases cocaine intake during maintenance of limited access self-administration sessions (Boyson et al. 2011), particularly when low doses of cocaine are available (Miczek and Mutschler 1996). This laboratory also consistently finds increases in cocaine intake as a result of episodic SD in animals given binge access (i.e., 24 h) to cocaine (Boyson et al. 2011; Covington et al. 2005; Covington and Miczek 2001; 2005; Quadros and Miczek 2009). Unlike other stressors, the activating effects of SD/threat stress persist for weeks to months without habituating (Covington et al. 2005; Miczek and Mutschler 1996). Under limited access maintenance sessions SD increases response rates during timeouts, suggesting a loss of control over cocaine seeking (Miczek and Mutschler 1996). Under binge conditions, SD shortens the inter-reinforcer interval at high doses of cocaine and increases the persistence of responding before the rat discontinues binging (Covington et al. 2005). Some studies find that SD increases cocaine intake on a progressive ratio (PR) schedule of reinforcement (Covington and Miczek 2005; Covington et al. 2008; Quadros and Miczek 2009), although other studies have failed to find this effect (Boyson et al. 2011; Covington and Miczek 2001; Yap and Miczek 2007). There are several issues regarding interpretation of changes in PR responding (Katz 1990), but the model provides insight into reinforcer effectiveness (Rowlett 2000) and motivation for drug (Markou et al. 1993). It seems unlikely that changes in motivation alone account for the less reliable effects of SD on PR versus binge responding because SD enhances cocaine-primed reinstatement of extinguished cocaine-CPP in OF1 mice (Ribeiro Do Couto et al. 2009), which is another model thought to assess incentive motivational effects of cocaine. It is possible that the differences in the sensitivity of PR versus binge responding to reliably detect SD effects may involve cocaine’s locomotor activating effects, which may be more pronounced during a binge due to higher drug intake relative to PR testing. In any case, together with findings from the other self-administration models, it seems that both cocaine reinforcement and incentive motivation for cocaine are enhanced as a result of SD.
Brief SD also has facilitative effects on initial acquisition of cocaine self-administration, although these effects are observed under more limited conditions than the effects of SD on maintenance. Male rats exposed to daily brief episodes of SD over 4 consecutive days acquire cocaine self-administration twice as fast as non-defeated controls when the rats are given continuous access (Tidey and Miczek 1997). With daily limited access, however, similar SD parameters fail to reliably produce effects during initial acquisition sessions (Covington and Miczek 2001; Cruz et al. 2011; Quadros and Miczek 2009), although one study found that an increase in cocaine intake emerged during the third session in stressed rats relative to controls (Haney et al. 1995). Male rats classified as either having a high response (HR) or a low response (LR) to amphetamine induced-locomotion are differentially affected by SD. Specifically, HR rats exhibit delayed acquisition following SD compared to non-defeated HR controls, but during later sessions intake is similar in defeated and non-defeated HR rats (Kabbaj et al. 2001). By contrast, LR rats exhibit similar intake during early acquisition sessions regardless of SD history, but during later trials defeated LR rats self-administer more than non-defeated LR controls. Overall, SD seems to have less impact on initial acquisition than it does on intake during maintenance sessions, perhaps due to greater sensitivity to detect SD effects during maintenance in individuals who would otherwise be at lower risk.
The effects of SD outside of the drug-taking context on alcohol intake are complex due to timing issues, variation in number of defeats and individual differences. Rats tested immediately before or after brief SD or threat of defeat during maintenance of alcohol self-administration exhibit either no change or a decrease in alcohol consumption (Funk et al. 2005; van Erp and Miczek 2001; van Erp et al. 2001). The decrease in alcohol consumption is consistent with the general suppressant effects that stressors have on ongoing behavior (e.g., Meerlo et al. 1996). In contrast, when access to alcohol is delayed 2 h post-defeat, rats exhibit an increase in alcohol intake (Caldwell and Riccio 2010). Croft et al. (2005) also found a delayed increase in alcohol preference in a selected subgroup of low alcohol-preferring C57/BL/10 mice with a history of repeated SD that emerged about 2 weeks after the last defeat episode. This effect is observed with daily SD episodes over 5 consecutive days, but not when the 5 episodes are experienced once/week nor when only a single episode is experienced. Sillaber et al. (2002) also reported time-dependent increases in alcohol intake in C57/BL/6J adult male CRH1 knockout mice in which exposure to brief episodes of SD stress for 3 consecutive days increased alcohol intake compared to pre-stress baselines, effects absent in control mice. The time delay in increased alcohol consumption in contrast to the immediate increases in stimulant consumption after brief SD outside of the drug context is likely related to differences in the effects of these drugs on performance. Under stress, it seems advantageous to consume stimulants to achieve increased energy and other performance-enhancing effects, whereas it seems disadvantageous to consume ethanol due to its performance-interfering effects.
The effects of brief intermittent SD stress on drug intake may also involve altered motivation for drug or changes in sensitivity of reward systems. For instance, Funk et al. (2004) found that after a period of protracted alcohol withdrawal when motivation is high, SD produced a transient increase in alcohol intake. In the CPP model, adult male rats experiencing a brief episode of SD/threat immediately before conditioning with an aversive dose of ethanol (1 g/kg) fail to acquire the CPA that is observed in controls, perhaps due to enhanced rewarding and/or attenuated aversive effects of ethanol (Funk et al. 2004). Alternatively, it is possible that the SD interfered with learning the drug-context association in the latter study. Brief SD enhances sensitivity to stimulant-CPP in both adolescents and adults (Burke et al. 2011; McLaughlin et al. 2006). The enhancement observed in adolescents is specific to SD stress, as no change in amphetamine-CPP is observed in adolescent rats pre-exposed to foot-shock stress (Burke et al. 2011). Collectively, these findings suggest that intermittent episodes of SD in rodents enhance sensitivity to the rewarding effects of drugs of abuse.
Only one study to our knowledge has examined the effects of brief SD on opiate intake, which failed to reveal any effect on heroin intake; however, defeated rats given access to cocaine in combination with heroin (i.e., speedball) continue to self-administer for a longer period of time during a binge than non-defeated controls (Cruz et al. 2011). Although the enhancement of speedball self-administration may be due to SD effects on cocaine reinforcement/motivation rather than heroin, it is possible that motivation to seek opiates may also contribute because SD reinstates extinguished morphine-CPP (Ribeiro Do Couto et al. 2006), a measure of incentive motivation for morphine.
In summary, the literature with stimulants suggests that brief SD outside of the drug context increases vulnerability. However, with alcohol there are important differences in the timing of SD effects with immediate suppressive effects and delayed facilitatory effects. More research is needed to examine effects of SD on opiate self-administration.
Social hierarchy outside of drug context
Social hierarchies develop over time among monkeys and rodents when they are housed in groups. Within these hierarchies chronic subordination is stressful in both species (Blanchard et al. 2001; Nader et al. 2012a). The physical and social environments vary from semi-natural habitats to standard home cages in which multiple animals are housed. Once established, dominance-subordinate hierarchies are maintained for prolonged periods (Blanchard et al. 1995; Blanchard et al. 1977). The strength of the agonistic interactions within these colonies varies considerably, with larger natural habitats, access to females, and competition for resources producing higher levels of aggression. In addition, specific colony features such as housing temperature, light/dark cycles, species, strain, colony group size, stability and age range all impact the quality of colony member interactions (Blanchard and Blanchard 1990; Honess and Marin 2006; Kitchen and Beehner 2007).
Chronic social subordination stress experienced outside of the drug-taking context generally increases vulnerability to drugs of abuse. Both moderately and severely fight-stressed subordinate NIH-Swiss mice increase ethanol intake; however, only severely fight-stressed subordinates prefer alcohol to water (Hilakivi-Clarke and Lister 1992). Importantly this difference is not seen prior to establishing social hierarchies, indicating that submissive mice escalate their alcohol intake only after severe subordination stress. Furthermore, subordinate male rats consume twice as much ethanol during weekly 24-h isolation sessions compared to dominant rats, and these effects persist for up to a year (Wolffgramm and Heyne 1991). A negative correlation between alcohol intake and dominance rank was observed under the stable social conditions of individual and contact caging (i.e., housing with separators that allow for limited social contact), but not under group housing; however, when housing conditions were rotated dominant rats increased their consumption. Following an 18-month washout period ethanol was reoffered and the influence of social dominance on ethanol choice was initially present but disappeared after four weeks; during the following ten weeks no significant differences between dominant and non-dominant rats were detected. Interestingly, this study also found that subordinate rats consume twice as much diazepam as dominant rats, suggesting that chronic social subordination stress increases the intake of anxiolytic drugs in general.
In contrast to the facilitatory effects of subordination on alcohol intake, chronic social stress involving daily 5-min SD episodes as well as 5 weeks of continuous threat while living in a protected enclosure within the resident’s cage reduces cocaine intake during 24-h binge access (Miczek et al. 2011). Conversely, chronic subordination stress enhances cocaine choice in group-housed male monkeys given concurrent FR and PR access to both food and cocaine (Czoty et al. 2005), and subordinate male monkeys self-administer more total cocaine relative to dominant monkeys (Morgan et al. 2002). Interestingly, after transitioning from individual to group housing, dominant male monkeys exhibit substantially reduced cocaine self-administration acquisition rates across a range of doses. However, after prolonged cocaine self-administration, the differences in drug intake between dominant and subordinate male monkeys disappears (Czoty et al. 2004), but re-emerges following 8 months of extended abstinence (Czoty et al. 2010). Dominant female monkeys also appear more sensitive to cocaine reinforcement because they acquire self-administration at lower doses than subordinate monkeys (Nader et al. 2012b). It is unclear why both increases and decreases in cocaine intake are observed with chronic social stress, but differences in species, sex, predictability of the stress or differences in the intensity of the stressors (i.e. daily physical defeat in rats vs. possible defeat in monkeys) are possibilities. In any case, these studies suggest that subordination stress generally increases vulnerability, not only for initiation of drug use, but also relapse.
The effects of chronic social subordination stress on opiate intake have not been investigated. However, Coventry et al. (1997) found time-dependent effects of chronic SD stress and its interaction with the social hierarchy on morphine-CPP. When rats were tested 3 days after SD, only subordinate rats exhibited morphine-CPP. In contrast, 7 days following defeat, morphine-CPP was evident in the defeated rats that maintained their dominant status but not in those that became subordinate. These data suggest that subordination stress causes dynamic regulatory changes in opiate systems that alter sensitivity to morphine-reward.
Prosocial experiences occurring outside of the drug context
The attitude of peers towards drugs can influence initiation of drug use, dependence and relapse (Bohnert et al. 2009; Buchanan and Latkin 2008; Valente et al. 2007). The key distinction is whether peers encourage or discourage drug-taking. Positive attitudes toward drug use may facilitate initiation and use (Bohnert et al. 2009; Buchanan and Latkin 2008), and we suggest that this risk factor is most salient among peers at the time of drug availability. In fact in some cases, social reinforcement in the drug-taking context may serve as the primary reinforcer, rather than the drug effect per se (Geckova 2005; Sussman 2005). In contrast, negative attitudes toward drug use among peers may protect against initiation of drug use. Furthermore, maintaining affiliations with such peers may be an alternative reinforcer that serves to discourage drug seeking and drug taking (Valente et al. 2007).
In animal models, positive social experiences outside of the drug context are protective against drug abuse-related behaviors. For instance, 60-min pairings with a conspecific immediately prior to oral access to morphine in a different environment attenuates isolation-induced increases in morphine consumption in isolate-housed rats (2010). Also, Fritz, Zernig, and colleagues (Fritz et al. 2011) found that cocaine and social rewards compete with each other in the CPP model. The CPP model utilizes two distinctly different conditioning compartments of an apparatus to create different contexts during conditioning. When rats receive either a conspecific or cocaine paired with one end compartment of the apparatus (i.e., reward context) and saline paired with the other end compartment (i.e., neutral context), young adult male rats exhibit a preference for the reward-paired context. However, if the conspecific is paired with one context and cocaine is paired with the other during conditioning, the rats fail to show CPP likely because each context is associated with a reward that is of equal value to the other, and therefore, the drug reward context and social reward context are equally preferred. In addition, after establishing a preference for a cocaine-paired context (i.e., cocaine-CPP), pairing a conspecific with the previously saline-paired side shifts the rats’ preferences away from the cocaine-paired side in favor of the social reward-paired side. Furthermore, after establishing cocaine-CPP to a given side and then extinguishing the CPP, social reward conditioning in the alternate side inhibits cocaine-primed reinstatement of cocaine-CPP (Fritz et al. 2011). Finally, Ribeiro Do Couto et al (2009) found that after establishing cocaine-CPP and then extinguishing the CPP, a brief encounter with a non-aggressive conspecific 30 min prior to testing prevents cocaine-primed reinstatement of the CPP in OF1 mice. Although there is little research to date on brief prosocial encounters outside of the drug context, the results thus far consistently support a protective effect in reducing drug intake and drug-primed incentive motivation.
Social experiences occurring within the drug context
Drug intake in the home cage of isolate versus social-housed animals
The influence of isolation versus social housing conditions on vulnerability to drugs of abuse has been investigated in animals that are housed and tested within the context in which they have access to drug. The findings from these studies are complicated by several factors including strain, age, length of isolation, and whether or not the housing conditions remain the same throughout the experiment or are changed during the course of the experiment. Rodents housed in isolation have been found to consume more ethanol (Deatherage 1972; Ehlers et al. 2007; Juarez and Vazquez-Cortes 2003; Parker and Radow 1974; Wolffgramm and Heyne 1991; Yanai and Ginsburg 1976) and morphine (Raz and Berger 2010) compared to social housed rodents; however, other studies find the opposite with isolated rodents consuming less ethanol (Anacker et al. 2011a; Doremus et al. 2005; Thorsell et al. 2005) and cocaine (Smith 2012) compared to social housed rodents, as well as other studies the found no difference in consumption between isolated and social-housed groups (Deatherage 1972; Kulkosky et al. 1980; Schenk et al. 1990).
In some cases, lower drug intake in isolated animals may be due to social facilitation of drug intake in the social condition rather than an inhibitory effect of isolation. Social facilitation was first observed in monkeys that self-administered more phencyclidine when in the presence of another monkey than when in isolation (Newman et al. 2007). Similarly, Smith (2012) housed rats in duplex operant conditioning chambers that were separated by a mesh barrier, allowing for some social interaction. He found that cocaine intake was increased when both rats in the dyad had access to cocaine compared to dyads in which only one of the two rats had access to cocaine and compared to isolated controls. Also, alcohol consumption increases in rats when they are transitioned from isolated to social housing (Weisinger et al. 1989). Using a highly social strain of voles, Anacker et al. (2011a) found that those living in duplex contact housing consumed more ethanol than those living in isolation housing. An experiment using the same design with access to a saccharin solution found no differences in consumption across groups, suggesting that the social facilitation observed is specific to ethanol.
In contrast to social facilitation, others have demonstrated social protection against drug intake. For instance, Raz and Berger (2010) have demonstrated that isolation increases oral consumption of morphine relative to social housing. In addition, when rats in this study were switched from social housing to isolation housing, they exhibited an increase in morphine consumption. Furthermore, a brief social interaction outside of the home cage attenuated the effects of isolation housing on morphine consumption. Similarly, Wolffgramm and Heyne (1991) demonstrated that when rats are switched from group housing to contact cages that allowed some social interaction with neighbors, alcohol consumption increased. Furthermore, rats that were housed in the latter condition and then switched to isolation housing also showed increased alcohol consumption. However, rats that were isolation housed and switched to group housing showed a decrease in alcohol consumption. In a recent study, Anacker et al. (2011b) found that isolated prairie voles classified as high drinkers decrease alcohol consumption when transferred to duplex contact housing with a low drinker, although in a few cases the low drinker increased alcohol consumption to match the high drinker (Anacker et al. 2011b). These findings suggest that voles adjust their drinking level to that of a peer, highlighting the importance of sociality on drug intake.
The conditions under which social housing facilitates versus protects animals against drug intake are presently unclear. Doremus et al. (2005) found a decrease in alcohol preference and intake in isolated adults, but not isolated adolescents, suggesting that age influences drug intake when drug is available in the home cage. The intensity of stress experienced may also play a role. There are individual differences in perceived stress that are due to genetic and environmental influences. Similar to the Yerkes-Dodson function, motivation for drug across intensities of stress may be an inverted U-shaped function for which it is reasonable to predict that initial isolation (relatively mild stressor) may produce arousal that motivates alcohol consumption whereas long-term isolation (severe stressor) may produce depression and anhedonia that may interfere with drug seeking. Along this line of reasoning, moving rats from social housing to a less social condition or isolation in the Wolffgramm study may have resulted in stress-induced increases in alcohol intake, whereas the highly social prairie voles in the Anacker study may find the isolation stress more severe resulting in less motivation to consume alcohol. Indeed the highly social prairie vole exhibits a decrease in alcohol preference when moved from social housing to isolation (Anacker et al. 2011a). Further research is needed to understand the circumstances and mechanisms of social housing conditions in the drug context on drug intake.
Transitioning animals from social housing to isolation results in a complex pattern of changes in ethanol intake that varies across sexes and with the procedures used. For instance, alternating periods of social versus isolated housing in rodents and monkeys increase ethanol intake during the isolation periods, and in monkeys, ethanol intake is elevated above that of controls continuously housed in isolation (Kraemer and Mckinney 1985; Mediratta et al. 2003). McKenzie-Quirk and Miczek (2008) found a similar effect in male monkeys transitioning from social housing to isolation, whereas ethanol intake in female monkeys was not affected by the transition. However, these authors also found that placing a monkey into a smaller clear Plexiglas enclosure within the home cage while also removing other cohabitating monkeys for 20 min suppressed all fluid intake regardless of sex. Thus, it is clear that procedural differences affect the outcome of transitions from social to isolation housing on ethanol intake, perhaps related to length of isolation and social history prior to isolation.
Social defeat-conditioned cues within the drug context
To our knowledge, the effects of brief SD within the drug context have not been examined. However, Funk et al (2005) paired an odor cue with SD outside of the drug-taking context and found that when the cue was presented within the drug context, rats decrease alcohol self-administration. This finding suggests that cues predictive of SD have an inhibitory effect on alcohol intake.
Social hierarchy within the drug context
Social hierarchy effects within the drug context have only been investigated for ethanol intake and the findings in both monkeys and rodents suggest that subordination increases vulnerability. Subordinate rats initiate ethanol intake faster and consume more than dominant rats regardless of whether there is high competition for resources (Blanchard 1992; Blanchard et al. 1987) or relatively little competition (Ellison 1987; Ellison et al. 1983; Pohorecky 2006; 2008; 2010). Social hierarchy effects on ethanol intake in male mice exposed to daily brief SD while also living in a cage that allowed for transmission of social threat cues differ across strains as increases in ethanol intake are observed in subordinate, but not dominant, male C57BL/6J mice (Kudryavtseva et al. 2006; Kudryavtseva et al. 1991), whereas dominant and subordinate CBA/lac did not differ (Kudryavtseva et al. 1991). These differences may simply reflect strain differences in stress susceptibility or an effect of stressor intensity as the C57BL/6J subordinates experienced attack three times more than the CBA/lac subordinates. More recently, McKenzie-Quirk and Miczek (2008) found dominance rank is inversely correlated with alcohol intake in male and female squirrel monkeys given daily 15-min sessions in a Plexiglas chamber located within a colony consisting of 4–10 adult monkeys in multi-generational, mixed-sex social groups. The increase in vulnerability to alcohol with subordination is an interesting contrast to the decrease in alcohol self-administration by a SD-associated cue, suggesting that animals in these models may have different coping strategies (e.g., stay with group versus flee) that interact with alcohol differently.
Prosocial-conditioned cues within the drug context
Cues (e.g., odor or drinking spout) associated with positive social experiences outside of the drug context can facilitate alcohol intake when animals are subsequently given access to alcohol. For instance, a drinking spout that is associated with access to a conspecific subsequently facilitates acquisition of ethanol intake and escalates consumption of higher concentrations of ethanol in rats (Tomie et al. 2004). The scent of alcohol on a peer can also be associated with the prosocial interaction with that peer and subsequently facilitate alcohol consumption. For example, pre-exposure to an intoxicated peer, but not a sober peer, facilitates drinking in pre-weanling and adolescent rats later given access to ethanol when alone (Hunt et al. 2001). Male rats appear more sensitive to the familiarity of the social conspecific because they only increase drinking when exposed to a familiar intoxicated peer, whereas females increase drinking regardless of partner familiarity (Hunt et al. 2000; Maldonado et al. 2008). Another example is that pre-exposure to an intoxicated peer later shifts approach behavior away from an initially preferred vanilla-scented hole to an alcohol-scented hole (Fernandez-Vidal and Molina 2004). These findings demonstrate that social conditioning readily occurs and influences drug intake even when an individual is alone with access to drug.
Brief prosocial encounters within the drug context
Most drug use is initiated among peers during adolescence and young adulthood in social contexts (Geckova 2005; Ramirez et al. 2012; Sussman 2005). There has been a recent surge of research investigating the effects of prosocial encounters within the drug context in animals that, for the most part, has shown social facilitation of drug self-administration and drug-CPP (see Table 5). Maintenance of amphetamine self-administration is facilitated in male rats that have a conspecific in an adjacent compartment sharing a clear Plexiglas wall with the self-administration cage (Gipson et al. 2011b). This effect is selective for amphetamine as rats reinforced with sucrose pellets showed a disruption of intake. Acquisition of nicotine self-administration paired with oral delivery of a sweet scented solution is also facilitated in male and female adolescent rats by the presence of a conspecific located in an adjacent cage who is drinking the scented solution (Chen et al. 2011); the divider between the cages was perforated to allow the transfer of olfactory cues. Without the conspecific, rats fail to increase licking on an active spout that resulted in the solution + nicotine compared to an inactive spout, and rats pretrained to lick for the solution without a conspecific present show a decrease in licking when nicotine delivery is also made contingent upon licking the active spout. The social facilitation is stronger in the presence of a familiar rat than a novel rat, suggesting that social, rather than novel, cues drive the facilitation. We have also found that male rats at the transition from adolescence to adulthood self-administer more nicotine on the first day of acquisition when they are in duplex self-administration chambers that have a wire mesh common wall than in chambers that have a solid common wall (Peartree et al. 2011). In contrast, nicotine self-administration in female rats was not affected by chamber type initially, however, only females in the solid wall condition showed escalation of nicotine intake across sessions, suggesting social suppression of nicotine intake in females.
Table 5.
Prosocial interactions occurring within the drug context on drug abuse-related behaviors in rodents
| Model | Drug | Dose | Effect | Type of social interaction | References |
|---|---|---|---|---|---|
| Self-administration | Amphetamine | 0.1 mg/kg, i.v. | ↑ amphetamine intake |
Conspecific behind Plexiglas wall | Gipson et al, 2011b |
| Nicotine | 0.015 mg/kg, i.v. | ↑ nicotine intake (males only) |
Conspecific self-administering in chamber with mesh separating wall |
Peartree et al, 2011 | |
| 0.015 mg/kg, i.v. | ↓ nicotine intake (females only) |
Conspecific self-administering in chamber with mesh separating wall |
Peartree et al, 2011 | ||
| 15–30 µg/kg, i.v. | ↑ nicotine intake | Conspecific self-administering olfactory- gustatory drug-CS in chamber with perforated Plexiglas separating wall |
Chen et al, 2011 | ||
| Ethanol* | 3–10%, oral | ↑ ethanol intake | Social-conditioned cue (scent of ethanol on intoxicated conspecific) |
Hunt et al, 2000, 2001; Maldonado et al, 2008 |
|
| CPP | Methamphetamine | 2 mg/kg, i.p. | ↑ amphetamine CPP | Drug +conspecific US during conditioning |
Watanabe, 2011 (only if conspecific is in similar drug state) |
| Cocaine | 2 mg/kg, i.p. | ↑ cocaine CPP | Drug +conspecific US during conditioning | Thiel et al, 2008 | |
| Nicotine | 0.1 mg/kg, s.c. | ↑ nicotine CPP | Drug +conspecific US during conditioning | Thiel et al, 2009a | |
| Morphine | 0.25–5 mg/kg, s.c. | ↑ morphine CPP | Drug +conspecific US during conditioning |
Kennedy et al, 2011 (anti-social strain of mice) |
|
| ↓ morphine CPP | Drug +conspecific US during conditioning |
Kennedy et al, 2011 (social strain of mice) |
|||
| Ethanol | 4 g/kg 20%, oral | ↓ ethanol CPA | Drug +conspecific US during conditioning | Gauvin et al, 1994 | |
| CPP-like procedure | Ethanol | ↑ approach to ethanol cue |
Social-conditioned cue (scent of ethanol on intoxicated conspecific) |
Fernandez-Vidal & Molina, 2004 |
Choice ethanol consumption
Increases in stimulant self-administration in a social context may be due to enhancement of drug reward by the social context (i.e., presence of another rat). Limited social contact through a mesh wall is rewarding in rats as both adolescents and adults exhibit CPP for a compartment in which another rat is present behind a wire mesh divider (Kummer et al. 2011; Peartree et al. 2012). We have shown that both nicotine and cocaine reward interact synergistically with social reward in adolescent rats using the CPP model (Thiel et al. 2008, 2009a). In these studies, neither the drug experience alone nor the social encounter alone was sufficient to produce CPP, but when experienced together, significant CPP was observed. In a follow up experiment, we paired nicotine or saline with both sides of the CPP apparatus during conditioning to remove the potential influence of drug conditioning while pairing another rat (US) with the CS side of the apparatus. We found that nicotine enhanced social reward-CPP, demonstrating that the nicotine-enhanced social reward-CPP observed previously was not due to additive effects of a weak drug-CPP and a weak social reward-CPP, but rather these two rewards interacted synergistically to produce a stronger reward than either alone (2009a). More recently, Watanabe (2011) showed that methamphetamine-CPP is enhanced in adult mice conditioned with another mouse given methamphetamine but not in adult mice conditioned with another mouse given saline, demonstrating social facilitation of stimulant reward only occurs when both partners share the drug experience.
In contrast to the above findings, Trezza, Vanderschuren and colleagues (2009) found an inhibitory interaction between methyphenidate and social rewards in rats, where both a conspecific and methyphenidate alone produce CPP, but when both are given together during conditioning no CPP is observed. Similarly, morphine-CPP is attenuated in mice conditioned with a conspecific, and this effect is more robust in singly housed mice than socially housed mice (Kennedy et al. 2011).
The reason for the differences in social and drug reward interactions across studies is unclear. Methylphenidate reduces play behavior (Vanderschuren et al. 2008), and therefore Trezza et al. (2009) suggest that the methylphenidate state reduces the incentive value of the social interaction, thereby inhibiting methylphenidate-CPP. However, nicotine and cocaine also reduce play behavior yet enhance social reward-CPP (Thiel et al. 2008; 2009a), suggesting that a reduction in play is not sufficient to predict an inhibitory interaction. Species and procedural differences may also contribute to the apparent discrepancies across studies.
It is also possible that a reduction of the aversive effects of initial drug exposure by the presence of a conspecific may contribute to the interaction between drug and social rewards. For instance, rats given a dose of ethanol that produces CPA fail to show CPA if ethanol is given in the presence of an intoxicated or sober conspecific (Gauvin et al. 1994). Furthermore, acute nicotine increases corticosterone in adolescent rats only when they experience the nicotine alone and not when they are with another nicotine-treated rat, suggesting that social context reduces the stressful effects of acute nicotine administration (Pentkowski et al. 2011).
In conclusion, these findings suggest that prosocial interactions occurring within the drug context likely facilitate stimulant reward and reinforcement, which may play an important role in the development of drug dependence. Further research is needed to determine whether brief prosocial interactions within the drug context facilitate the rewarding effects of other drugs of abuse and to understand the mechanisms underlying facilitatory drug and social reward interactions.
Potential mechanisms of social influences on drug abuse-related behavior and concluding remarks
We have emphasized that the emotional valence of social circumstances and whether they occur within or outside of the drug-taking context are important determinants of their influence on drug abuse-related behaviors. Social experiences occurring during early development impact an individual’s ability to cope with stress, increasing vulnerability to substance abuse later in life (Sinha 2008). In general, animal models of early life stress are consistent with the human literature and these models are useful for examining underlying genetic influences and neural mechanisms (e.g., Barr et al. 2004; Newman et al. 2009). Capturing the developmental underpinning of vulnerability to substance abuse is highly complex as reflected by the inconsistencies in the animal literature, and further research in this area is needed to refine the validity of these models.
Social experiences outside of the drug-taking context likely have an influence on responsivity to stress and cognitive processes, such as decision-making and impulsivity, that contribute to vulnerability to drug abuse (Lupien et al. 2009; Piazza and Le Moal 1998; Redish et al. 2008; Uhart and Wand 2009), with positive social experiences prior to initiation of drug use having an inhibitory effect and negative social experiences having a facilitatory effect. For instance, the reinforcing effects of positive social experiences outside of the drug-taking context may prevent or decrease the desire to initially seek and/or continue taking drugs, similar to the impact of other alternative reinforcers and environmental enrichment observed previously (Bardo et al. in press; Campbell and Carroll 2000; Carroll 1998; Podlesnik et al. 2006). In addition, positive social experiences outside of the drug-taking context may provide a social buffer that alleviates some of the negative effects of daily life stressors that might otherwise motivate drug seeking. In contrast, negative social experiences occurring outside of the drug-taking context may sensitize drug reward and/or stress pathways leading to increased risk for initiating drug use (Tidey and Miczek 1997) or to escalation of recreational drug use in an attempt to self-medicate (Boyson et al. 2011; Covington et al. 2005; Covington and Miczek 2001; 2005; Quadros and Miczek 2009). Indeed human adolescent and adult populations that have been exposed to bullying or physical attack outside of the drug-taking context exhibit increased vulnerability to initiate and continue using drugs of abuse (Back et al. 2000; Brown et al. 1999; Niedhammer et al. 2011; Traweger et al. 2004).
The impact of negative social encounters within the drug-taking context may vary depending on the nature and degree of the stressor. For instance, brief, predictable stressors that engage escape strategies, such as physical attack or cues predictive of threat, may initially suppress drug taking and seeking because the animals is focused on responding to the stress. This idea is consistent with the findings that a cue associated with SD inhibits alcohol self-administration (Funk et al. 2005) and with the suppressant effects that stressors have on ongoing behavior in general (e.g., Meerlo et al. 1996). However, once the physical threat is no longer present in the environment, animals may seek drug in an attempt to self-medicate. This idea is consistent with increased alcohol intake when access to alcohol is offered 2-h post-defeat (Caldwell and Riccio 2010). By contrast, chronic, unpredictable stressors that are unavoidable may heighten reward sensitivity or produce attempts to self-medicate resulting in facilitated drug taking and seeking behaviors. This idea is consistent with the findings that chronic social subordination experienced within the drug-taking context enhances drug intake and/or drug seeking (e.g., Blanchard et al. 1987; Ellison 1987; McKenzie-Quirk and Miczek 2008). Therefore, it is important to fully elucidate the impact that social stressors have on the underlying neural mechanisms regulating drug taking and seeking behaviors. Understanding these processes not only has implications for understanding initiation and escalation of drug use, but may also help inform the development of behavioral and pharmacological treatment strategies that alleviate or prevent the negative consequences of chronic social stress.
The first drug use almost always occurs in a social context and is sometimes motivated by social reinforcement rather than drug reinforcement (Geckova 2005; Ramirez et al. 2012; Sussman 2005). The impact of positive social encounters within the drug context likely depends in part on motivation for such encounters, with higher motivation related to higher vulnerability. This enhanced vulnerability may be related to a sense of safety or trust. For instance, the peptide neurotransmitter oxytocin is released in response to appetitive social stimuli (Baskerville and Douglas 2010; Insel 1992), and causes a decrease in anxiety (Bowen et al. 2011; McGregor et al. 2008; Windle et al. 1997) and an increase in trust in humans (Kosfeld et al. 2005). The increase in trust may then enhance the influence of peers to engage each other in drug use.
Social context may also decrease the salience of aversive drug effects (Gauvin et al. 1994). Such effects may involve differences in HPA axis function in social versus isolated contexts. For instance, rats given nicotine or amphetamine in a social context fail to exhibit the drug-induced elevation of plasma corticosterone that is observed in isolated rats (Pentkowski et al. 2011; Stairs et al. 2011). In addition, most drugs of abuse are known to enhance the effects of other rewarding stimuli (Wise 1996), and in the case of nicotine, can even render nonpharmacological stimuli reinforcing even though they intrinsically have little if any reinforcing effects (e.g., see Chaudhri et al. 2006 for recent review). Thus, social context likely provides a key constituent of the reward experience during initial drug use, with drug and social rewards interacting synergistically to produce a stronger, perhaps neurobiologically unique, reward experience than either of these stimuli alone (Thiel et al. 2008; Thiel et al. 2009a). Therefore, it is important to understand the neural mechanisms underlying interactions between social and drug reward that are experienced together in the drug context. Such interactions have implications for understanding initiation of drug use.
Another important reason to consider social reward and reinforcement occurring at the time of drug taking is the associative learning that occurs between these stimuli. There is a growing appreciation in neuroscience for the role of learning in the maintenance and relapse of drug abuse and dependence, with much attention given to the ability of environmental stimuli that have become associated with drug effects to elicit craving and/or habitual drug-seeking behavior (Everitt and Wolf 2002). Relatively few studies have investigated the reverse: the ability of drug stimulus effects to acquire conditioned reinforcing effects via pairing with social reinforcers. It is clear that drugs of abuse produce interoceptive stimulus effects that can serve as discriminative stimuli during operant conditioning, and as occasion setters in Pavlovian conditioning (Morrison and Stephenson 1969; Palmatier and Bevins 2007; 2005; Stolerman et al. 1984). Furthermore, several laboratories have demonstrated that drug effects can become conditioned via association with other reinforcers (e.g., Carey et al. 1999; Murray and Bevins 2007; Reilly and Revusky 1992). For instance, in humans preference for placebo over diazepam is reversed by pairing diazepam with earning a higher amount of money on an experimental task relative to that earned after placebo (Alessi et al. 2002). The authors suggest that the most parsimonious explanation for the switch in preference is that diazepam acquired conditioned reinforcing effects via pairing with the higher amount of money earned. We hypothesize that interoceptive effects of drugs that are experienced repeatedly with social reinforcement early during the course of drug use acquire conditioned reinforcing effects via association with social reinforcement. It is possible that the brain attributes high incentive value to the interoceptive effects of drugs as a result of their association with social reward, an effect that may be enhanced by drug-induced facilitation of learning. If this is the case, then in addition to the pharmacological effects of drugs, social reward-conditioned effects of drugs may also contribute to drug addiction. For instance, exposure to drug could increase subsequent drug intake due to social reward-conditioned effects, similar to the way exposure to drug-conditioned cues can motivate drug-seeking behavior in animals and humans when reinforced by the cues (e.g., cue reinstatement in rats, needle freak phenomenon in humans; O'Brien et al. 1993; Stewart 1983). It is possible that such learning engages neural pathways involved in social reward that may be unique from those engaged by the drug and that their discovery may lead to novel therapies for drug abuse.
In closing, we suggest that it is critical to consider social history and social context in animal models directed toward understanding initiation of drug use, the development of dependence, and relapse. Vulnerability to initiate drug use is influenced by sensitivity to both drug reward and aversive effects, which are determined in part by social history and social context. For instance, upon initial drug use, positive social contexts may interact synergistically with drug reward. Such an effect may be greater when social motivation is high due to an impoverished social history, whereas a rich social history may be protective against positive social context interactions with drug. Understanding the contribution of social factors to initial drug experience is important because the degree to which one finds initial drug experience pleasurable is related to risk of developing dependence (Haertzen et al. 1983). Escalation of drug intake and development of dependence are also influenced by social factors. For instance, negative social experiences may motivate attempts to self-medicate or to escalate recreational drug use, thereby contributing to the development of dependence. In contrast, positive social history or situations where positive social interaction is available as an alternative reinforcer likely are protective against the development of drug addiction. Finally, although little preclinical research has examined social influences on drug relapse, early findings suggest that positive social experiences outside of the drug taking context are protective against cue-elicited motivation for drug (Fritz et al. 2011; Ribeiro Do Couto et al. 2009; Thiel et al. 2009b). We suggest that associations between positive social interactions and drug within the drug taking context are likely powerful motivators of relapse. These ideas need further empirical support, but if upheld, the field may gain new insight to inform the development of behavioral and pharmacological treatment strategies for drug addiction.
Acknowledgements
The authors thank Suzanne Weber for her assistance with literature searches and formatting.
Funding: This work was supported by NIDA grants R01DA011064 and F32DA025413 and The More Graduate Education at Mountain State Alliance (MGE@MSA) Program
References
- Advani T, Hensler JG, Koek W. Effect of early rearing conditions on alcohol drinking and 5-HT1A receptor function in C57BL/6J mice. Int J Neuropsychopharmacol. 2007;10:595–607. doi: 10.1017/S1461145706007401. [DOI] [PubMed] [Google Scholar]
- Alessi SM, Roll JM, Reilly MP, Johanson CE. Establishment of a diazepam preference in human volunteers following a differential-conditioning history of placebo versus diazepam choice. Exp Clin Psychopharmacol. 2002;10:77–83. discussion 101-3. [PubMed] [Google Scholar]
- Alexander BK, Coambs RB, Hadaway PF. The effect of housing and gender on morphine self-administration in rats. Psychopharmacology (Berl) 1978;58:175–179. doi: 10.1007/BF00426903. [DOI] [PubMed] [Google Scholar]
- Altman J, Everitt BJ, Glautier S, Markou A, Nutt D, Oretti R, Phillips GD, Robbins TW. The biological, social and clinical bases of drug addiction: commentary and debate. Psychopharmacology (Berl) 1996;125:285–345. doi: 10.1007/BF02246016. [DOI] [PubMed] [Google Scholar]
- Anacker AM, Loftis JM, Kaur S, Ryabinin AE. Prairie voles as a novel model of socially facilitated excessive drinking. Addict Biol. 2011a;16:92–107. doi: 10.1111/j.1369-1600.2010.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AM, Loftis JM, Ryabinin AE. Alcohol intake in prairie voles is influenced by the drinking level of a peer. Alcohol Clin Exp Res. 2011b;35:1884–1890. doi: 10.1111/j.1530-0277.2011.01533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda RF, Brown DW, Felitti VJ, Dube SR, Giles WH. Adverse childhood experiences and prescription drug use in a cohort study of adult HMO patients. BMC Public Health. 2008:8. doi: 10.1186/1471-2458-8-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda RF, Croft JB, Felitti VJ, Nordenberg D, Giles WH, Williamson DF, Giovino GA. Adverse childhood experiences and smoking during adolescence and adulthood. JAMA. 1999;282:1652–1658. doi: 10.1001/jama.282.17.1652. [DOI] [PubMed] [Google Scholar]
- Anisman H, Zaharia MD, Meaney MJ, Merali Z. Do early-life events permanently alter behavioral and hormonal responses to stressors? Int J Dev Neurosci. 1998;16:149–164. doi: 10.1016/s0736-5748(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Arling GL, Harlow HF. Effects of social deprivation on maternal behavior of rhesus monkeys. J Comp Physiol Psychol. 1967;64:371–377. doi: 10.1037/h0025221. [DOI] [PubMed] [Google Scholar]
- Back S, Dansky BS, Coffey SF, Saladin ME, Sonne S, Brady KT. Cocaine dependence with and without posttraumatic stress disorder: A comparison of substance use, trauma history and psychiatric comorbidity. Am J Addict. 2000;9:51–62. doi: 10.1080/10550490050172227. [DOI] [PubMed] [Google Scholar]
- Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Annu Rev Psychol. 2004;55:463–491. doi: 10.1146/annurev.psych.55.090902.142054. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 2001;155:278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL, Kelley TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev. doi: 10.1124/pr.111.005124. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Dvoskin RL, Gupte M, Sommer W, Sun H, Schwandt ML, Lindell SG, Kasckow JW, Suomi SJ, Goldman D, Higley JD, Heilig M. Functional CRH variation increases stress-induced alcohol consumption in primates. Proc Natl Acad Sci U S A. 2009;106:14593–14598. doi: 10.1073/pnas.0902863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Lindell S, Becker ML, Shannon C, Champoux M, Suomi SJ, Higley JD. Early experience and sex interact to influence limbic-hypothalamic-pituitary-adrenal-axis function after acute alcohol administration in rhesus macaques (Macaca mulatta) Alcohol Clin Exp Res. 2004;28:1114–1119. doi: 10.1097/01.alc.0000130973.94350.8c. [DOI] [PubMed] [Google Scholar]
- Baskerville TA, Douglas AJ. Dopamine and oxytocin interactions underlying behaviors: potential contributions to behavioral disorders. Cns Neuroscience & Therapeutics. 2010;16:e92–e123. doi: 10.1111/j.1755-5949.2010.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology (Berl) 2011;218:131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Behavioral correlates of chronic dominance-subordination relationships of male rats in a seminatural situation. Neurosci Biobehav Rev. 1990;14:455–462. doi: 10.1016/s0149-7634(05)80068-5. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, Mcewen B, Sakai RR. Visible burrow system as a model of chronic social stress - behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20:117–134. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Flores T, Magee L, Weiss S, Blanchard DC. Pregrouping aggression and defense scores influence alcohol consumption for dominant and subordinate rats in visible burrow systems. Aggress Behav. 1992;18:459–467. [Google Scholar]
- Blanchard RJ, Hori K, Tom P, Blanchard DC. Social structure and ethanol consumption in the laboratory rat. Pharmacol Biochem Behav. 1987;28:437–442. doi: 10.1016/0091-3057(87)90502-8. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, McKittrick CR, Blanchard DC. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol Behav. 2001;73:261–271. doi: 10.1016/s0031-9384(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Takahashi LK, Blanchard DC. Development of intruder attack in colonies of laboratory rats. Anim Learn Behav. 1977;5:365–369. [Google Scholar]
- Bohnert AS, Bradshaw CP, Latkin CA. A social network perspective on heroin and cocaine use among adults: evidence of bidirectional influences. Addiction. 2009;104:1210–1218. doi: 10.1111/j.1360-0443.2009.02615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen MT, Carson DS, Spiro A, Arnold JC, McGregor IS. Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats. PLoS One. 2011;6:e27237. doi: 10.1371/journal.pone.0027237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling SL, Bardo MT. Locomotor and rewarding effects of amphetamine in enriched, social, and isolate reared rats. Pharmacol Biochem Behav. 1994;48:459–464. doi: 10.1016/0091-3057(94)90553-3. [DOI] [PubMed] [Google Scholar]
- Boyle AE, Gill K, Smith BR, Amit Z. Differential effects of an early housing manipulation on cocaine-induced activity and self-administration in laboratory rats. Pharmacol Biochem Behav. 1991;39:269–274. doi: 10.1016/0091-3057(91)90178-5. [DOI] [PubMed] [Google Scholar]
- Boyson CO, Miguel TT, Quadros IM, DeBold JF, Miczek KA. Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA. Psychopharmacology (Berl) 2011;218:257–269. doi: 10.1007/s00213-011-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth MA, Murray A, Wise RA. Influence of housing conditions on the acquisition of intravenous heroin and cocaine self-administration in rats. Pharmacol Biochem Behav. 1989;33:903–907. doi: 10.1016/0091-3057(89)90490-5. [DOI] [PubMed] [Google Scholar]
- Brown PJ, Stout RL, Mueller T. Substance use disorder and posttraumatic stress disorder comorbidity: Addiction and psychiatric treatment rates. Psychol Addict Behav. 1999;13:115–122. [Google Scholar]
- Buchanan AS, Latkin CA. Drug use in the social networks of heroin and cocaine users before and after drug cessation. Drug Alcohol Depend. 2008;96:286–289. doi: 10.1016/j.drugalcdep.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR, Watt MJ, Forster GL. Adolescent social defeat increases adult amphetamine conditioned place preference and alters D2 dopamine receptor expression. Neuroscience. 2011;197:269–279. doi: 10.1016/j.neuroscience.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell EE, Riccio DC. Alcohol self-administration in rats: Modulation by temporal parameters related to repeated mild social defeat stress. Alcohol. 2010;44:265–274. doi: 10.1016/j.alcohol.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Campbell J, Spear LP. Effects of early handling on amphetamine-induced locomotor activation and conditioned place preference in the adult rat. Psychopharmacology (Berl) 1999;143:183–189. doi: 10.1007/s002130050934. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Carroll ME. Acquisition of drug self-administration: environmental and pharmacological interventions. Exp Clin Psychopharmacol. 2000;8:312–325. doi: 10.1037//1064-1297.8.3.312. [DOI] [PubMed] [Google Scholar]
- Carey R, Damianopoulos E, DePalma G. Issues in the pharmacological modification of cocaine conditioning: evidence that the stimulus properties of drugs can interact with contextual cues to activate or inactivate cocaine conditioned stimuli. Behav Brain Res. 1999;101:189–206. doi: 10.1016/s0166-4328(98)00149-1. [DOI] [PubMed] [Google Scholar]
- Carr GD, Fibiger HC, Phillips AG. Conditioned place preference as a measure of drug reward. In: Liebman JM, Cooper SJ, editors. Neuropharmacological basis of reward. New York: Oxford University Press; 1989. pp. 264–319. [Google Scholar]
- Carroll ME. Acquisition and reacquisition (relapse) of drug abuse: modulation by alternative reinforcers. NIDA Res Monogr. 1998;169:6–25. [PubMed] [Google Scholar]
- Chamove AS, Rosenblu La, Harlow HF. Monkeys (Macaca-Mulatta) raised only with peers -Pilot-study. Anim Behav. 1973;21:316–325. doi: 10.1016/s0003-3472(73)80073-9. [DOI] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord. 2004;82:217–225. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Chauvet C, Lardeux V, Goldberg SR, Jaber M, Solinas M. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology. 2009;34:2767–2778. doi: 10.1038/npp.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Sharp BM, Matta SG, Wu QL. Social interaction promotes nicotine self-administration with olfactogustatory cues in adolescent rats. Neuropsychopharmacology. 2011;36:2629–2638. doi: 10.1038/npp.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O'Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Coventry TL, D’Aquila PS, Brain P, Willner P. Social influences on morphine conditioned place preference. Behav Pharmacol. 1997;8:575–584. doi: 10.1097/00008877-199711000-00015. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Jr., Miczek KA. Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–321. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology (Berl) 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology (Berl) 2005;183:331–340. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Tropea TF, Rajadhyaksha AM, Kosofsky BE, Miczek KA. NMDA receptors in the rat VTA: a critical site for social stress to intensify cocaine taking. Psychopharmacology (Berl) 2008;197:203–216. doi: 10.1007/s00213-007-1024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft AP, Brooks SP, Cole J, Little HJ. Social defeat increases alcohol preference of C57BL/10 strain mice; effect prevented by a CCKB antagonist. Psychopharmacology (Berl) 2005;183:163–170. doi: 10.1007/s00213-005-0165-6. [DOI] [PubMed] [Google Scholar]
- Cruz FC, Quadros IM, Hogenelst K, Planeta CS, Miczek KA. Social defeat stress in rats: escalation of cocaine and “speedball” binge self-administration, but not heroin. Psychopharmacology (Berl) 2011;215:165–175. doi: 10.1007/s00213-010-2139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Gage HD, Nader MA. Differences in D2 dopamine receptor availability and reaction to novelty in socially housed male monkeys during abstinence from cocaine. Psychopharmacology (Berl) 2010;208:585–592. doi: 10.1007/s00213-009-1756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, McCabe C, Nader MA. Assessment of the relative reinforcing strength of cocaine in socially housed monkeys using a choice procedure. J Pharmacol Exp Ther. 2005;312:96–102. doi: 10.1124/jpet.104.073411. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Morgan D, Shannon EE, Gage HD, Nader MA. Characterization of dopamine D1 and D2 receptor function in socially housed cynomolgus monkeys self-administering cocaine. Psychopharmacology (Berl) 2004;174:381–388. doi: 10.1007/s00213-003-1752-z. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoura L, Haaker J, Nylander I. Early environmental factors differentially affect voluntary ethanol consumption in adolescent and adult male rats. Alcohol Clin Exp Res. 2011;35:506–515. doi: 10.1111/j.1530-0277.2010.01367.x. [DOI] [PubMed] [Google Scholar]
- Daoura L, Nylander I. The response to naltrexone in ethanol-drinking rats depends on early environmental experiences. Pharmacol Biochem Behav. 2011;99:626–633. doi: 10.1016/j.pbb.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Deatherage G. Effects of housing density on alcohol intake in the rat. Physiol Behav. 1972;9:55–57. doi: 10.1016/0031-9384(72)90264-8. [DOI] [PubMed] [Google Scholar]
- Deehan GA, Cain ME, Kiefer SW. Differential rearing conditions alter operant responding for ethanol in outbred rats. Alcoholism-Clinical and Experimental Research. 2007;31:1692–1698. doi: 10.1111/j.1530-0277.2007.00466.x. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, Brumaghi JT, Haltmeye GC, Zarrow MX. Increased adrenocortical activity in neonatal rat following handling. Endocrinology. 1967;81:1047–1052. doi: 10.1210/endo-81-5-1047. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. Neonatal maternal separation exacerbates the reward-enhancing effect of acute amphetamine administration and the anhedonic effect of repeated social defeat in adult rats. Neuroscience. 2010;170:1189–1198. doi: 10.1016/j.neuroscience.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimatelis JJ, Russell VA, Stein DJ, Daniels WM. The effects of lobeline and naltrexone on methamphetamine-induced place preference and striatal dopamine and serotonin levels in adolescent rats with a history of maternal separation. Metab Brain Dis. 2012;3:351–361. doi: 10.1007/s11011-012-9288-8. [DOI] [PubMed] [Google Scholar]
- Ding Y, Kang L, Li B, Ma L. Enhanced cocaine self-administration in adult rats with adolescent isolation experience. Pharmacol Biochem Behav. 2005;82:673–677. doi: 10.1016/j.pbb.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Dube SR, Anda RF, Felitti VJ, Chapman DP, Williamson DF, Giles WH. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span - Findings from the adverse childhood experiences study. Jama-J Am Med Assoc. 2001;286:3089–3096. doi: 10.1001/jama.286.24.3089. [DOI] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics. 2003;111:564–572. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- Dube SR, Miller JW, Brown DW, Giles WH, Felitti VJ, Dong M, Anda RF. Adverse childhood experiences and the association with ever using alcohol and initiating alcohol use during adolescence. J Adolesc Health. 2006;38:e1–e10. doi: 10.1016/j.jadohealth.2005.06.006. 444. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Walker BM, Pian JP, Roth JL, Slawecki CJ. Increased alcohol drinking in isolate-housed alcohol-preferring rats. Behav Neurosci. 2007;121:111–119. doi: 10.1037/0735-7044.121.1.111. [DOI] [PubMed] [Google Scholar]
- Ellison G. Stress and alcohol intake: The socio-pharmacologic approach. Physiol Behav. 1987;40:387–392. doi: 10.1016/0031-9384(87)90066-7. [DOI] [PubMed] [Google Scholar]
- Ellison G, Levy A, Lorant N. Alcohol-preferring rats in colonies show withdrawal, inactivity, and lowered dominance. Pharmacol Biochem Behav. 1983;18:565–570. doi: 10.1016/0091-3057(83)90237-x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlke C, Hard E, Eriksson CJ. Effects of early weaning and social isolation on subsequent alcohol intake in rats. Alcohol. 1997;14:175–180. doi: 10.1016/s0741-8329(96)00141-3. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Lorenz JG, Long J, Champoux M, Suomi SJ, Higley JD. Rearing experiences and stress-induced plasma cortisol as early risk factors for excessive alcohol consumption in nonhuman primates. Alcohol Clin Exp Res. 2000;24:644–650. [PubMed] [Google Scholar]
- Faure J, Stein DJ, Daniels W. Maternal separation fails to render animals more susceptible to methamphetamine-induced conditioned place preference. Metab Brain Dis. 2009;24:541–559. doi: 10.1007/s11011-009-9158-1. [DOI] [PubMed] [Google Scholar]
- Ferguson CJ, Meehan DC. With friends like these…: Peer delinquency influences across age cohorts on smoking, alcohol and illegal substance use. Eur Psychiatry. 2011;26:6–12. doi: 10.1016/j.eurpsy.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ. The developmental antecedents of illicit drug use: Evidence from a 25-year longitudinal study. Drug Alcohol Depend. 2008;96:165–177. doi: 10.1016/j.drugalcdep.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Fernandez-Vidal JM, Molina JC. Socially mediated alcohol preferences in adolescent rats following interactions with an intoxicated peer. Pharmacol Biochem Behav. 2004;79:229–241. doi: 10.1016/j.pbb.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Vazquez DM, Robinson TE. Manipulations during the second, but not the first, week of life increase susceptibility to cocaine self-administration in female rats. Neuropsychopharmacology. 2003;28:1741–1751. doi: 10.1038/sj.npp.1300228. [DOI] [PubMed] [Google Scholar]
- Francis DD, Kuhar MJ. Frequency of maternal licking and grooming correlates negatively with vulnerability to cocaine and alcohol use in rats. Pharmacol Biochem Behav. 2008;90:497–500. doi: 10.1016/j.pbb.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz M, El Rawas R, Salti A, Klement S, Bardo MT, Kemmler G, Dechant G, Saria A, Zernig G. Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addict Biol. 2011;16:273–284. doi: 10.1111/j.1369-1600.2010.00285.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, See RE. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2005;179:662–672. doi: 10.1007/s00213-004-2080-7. [DOI] [PubMed] [Google Scholar]
- Funk D, Harding S, Juzytsch W, Le AD. Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2005;183:341–349. doi: 10.1007/s00213-005-0194-1. [DOI] [PubMed] [Google Scholar]
- Funk D, Vohra S, Le AD. Influence of stressors on the rewarding effects of alcohol in Wistar rats: studies with alcohol deprivation and place conditioning. Psychopharmacology (Berl) 2004;176:82–87. doi: 10.1007/s00213-004-1859-x. [DOI] [PubMed] [Google Scholar]
- Gauvin DV, Briscoe RJ, Goulden KL, Holloway FA. Aversive attributes of ethanol can be attenuated by dyadic social interaction in the rat. Alcohol. 1994;11:247–251. doi: 10.1016/0741-8329(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Geckova A, Stewart R, van Dijk JP, Orosova O, Groothoff VW, Post D. Influence of socio-economic status, parents and peers on smoking behaviour of adolescents. Eur Addict Res. 2005;11:204–209. doi: 10.1159/000086403. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT. Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology (Berl) 2011a;214:557–566. doi: 10.1007/s00213-010-2060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Yates JR, Beckmann JS, Marusich JA, Zentall TR, Bardo MT. Social facilitation of d-amphetamine self-administration in rats. Exp Clin Psychopharmacol. 2011b;19:409–419. doi: 10.1037/a0024682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson L, Nylander I. Time-dependent alterations in ethanol intake in male wistar rats exposed to short and prolonged daily maternal separation in a 4-bottle free-choice paradigm. Alcohol Clin Exp Res. 2006;30:2008–2016. doi: 10.1111/j.1530-0277.2006.00247.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson L, Ploj K, Nylander I. Effects of maternal separation on voluntary ethanol intake and brain peptide systems in female Wistar rats. Pharmacol Biochem Behav. 2005;81:506–516. doi: 10.1016/j.pbb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Gustafsson L, Zhou Q, Nylander I. Ethanol-induced effects on opioid peptides in adult male Wistar rats are dependent on early environmental factors. Neuroscience. 2007;146:1137–1149. doi: 10.1016/j.neuroscience.2007.02.037. [DOI] [PubMed] [Google Scholar]
- Haertzen CA, Kocher TR, Miyasato K. Reinforcements from the 1st drug experience can predict later drug habits and or addiction - Results with coffee, cigarettes, alcohol, barbiturates, minor and major tranquilizers, stimulants, marijuana, hallucinogens, heroin, opiates and cocaine. Drug Alcohol Depend. 1983;11:147–165. doi: 10.1016/0376-8716(83)90076-5. [DOI] [PubMed] [Google Scholar]
- Hall FS, Huang S, Fong GW, Pert A, Linnoila M. Effects of isolation-rearing on voluntary consumption of ethanol, sucrose and saccharin solutions in Fawn Hooded and Wistar rats. Psychopharmacology (Berl) 1998;139:210–216. doi: 10.1007/s002130050706. [DOI] [PubMed] [Google Scholar]
- Haney M, Maccari S, Le Moal M, Simon H, Piazza PV. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 1995;698:46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Dodswort Ro, Harlow MK. Total social isolation in monkeys. Proc Natl Acad Sci U S A. 1965;54:90–97. doi: 10.1073/pnas.54.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays SL, McPherson RJ, Juul SE, Wallace G, Schindler AG, Chavkin C, Gleason CA. Long-term effects of neonatal stress on adult conditioned place preference (CPP) and hippocampal neurogenesis. Behav Brain Res. 2012;227:7–11. doi: 10.1016/j.bbr.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig V, Schmidt WJ. Anti-craving drugs acamprosate and naloxone do not reduce expression of morphine conditioned place preference in isolated and group-housed rats. Neurosci Lett. 2005;374:119–123. doi: 10.1016/j.neulet.2004.10.036. [DOI] [PubMed] [Google Scholar]
- Higley JD, Hasert MF, Suomi SJ, Linnoila M. Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption. Proc Natl Acad Sci U S A. 1991;88:7261–7265. doi: 10.1073/pnas.88.16.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A nonhuman primate model of type II excessive alcohol consumption? Part 1. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations and diminished social competence correlate with excessive alcohol consumption. Alcohol Clin Exp Res. 1996;20:629–642. doi: 10.1111/j.1530-0277.1996.tb01665.x. [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke L, Lister RG. Social status and voluntary alcohol consumption in mice: interaction with stress. Psychopharmacology (Berl) 1992;108:276–282. doi: 10.1007/BF02245112. [DOI] [PubMed] [Google Scholar]
- Honess PE, Marin CM. Behavioural and physiological aspects of stress and aggression in nonhuman primates. Neurosci Biobehav Rev. 2006;30:390–412. doi: 10.1016/j.neubiorev.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Howes SR, Dalley JW, Morrison CH, Robbins TW, Everitt BJ. Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala- striatal FOS expression. Psychopharmacology (Berl) 2000;151:55–63. doi: 10.1007/s002130000451. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Holloway JL, Scordalakes EM. Social interaction with an intoxicated sibling can result in increased intake of ethanol by periadolescent rats. Dev Psychobiol. 2001;38:101–109. doi: 10.1002/1098-2302(200103)38:2<101::aid-dev1002>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Lant GM, Carroll CA. Enhanced intake of ethanol in preweanling rats following interactions with intoxicated siblings. Dev Psychobiol. 2000;37:90–99. [PubMed] [Google Scholar]
- Insel TR. Oxytocin--a neuropeptide for affiliation: evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology. 1992;17:3–35. doi: 10.1016/0306-4530(92)90073-g. [DOI] [PubMed] [Google Scholar]
- Isengulova AA, Kalmykova ZA, Miroshnichenko IV. The significance of maternal care for the formation of ethanol preference in rats periodically separated from mothers during the first half of the nest period. Bull Exp Biol Med. 2009;147:390–393. doi: 10.1007/s10517-009-0533-z. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Juarez J, Vazquez-Cortes C. Alcohol intake in social housing and in isolation before puberty and its effects on voluntary alcohol consumption in adulthood. Dev Psychobiol. 2003;43:200–207. doi: 10.1002/dev.10133. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Norton CS, Kollack-Walker S, Watson SJ, Robinson TE, Akil H. Social defeat alters the acquisition of cocaine self-administration in rats: role of individual differences in cocaine-taking behavior. Psychopharmacology (Berl) 2001;158:382–387. doi: 10.1007/s002130100918. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Easterling KW, Holtzman SG. Early neonatal experience of Long-Evans rats results in long-lasting changes in morphine tolerance and dependence. Psychopharmacology (Berl) 2001;157:305–312. doi: 10.1007/s002130100806. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Katz JL. Models of relative reinforcing efficacy of drugs and their predictive utility. Behav Pharmacol. 1990;1:283–301. doi: 10.1097/00008877-199000140-00003. [DOI] [PubMed] [Google Scholar]
- Kennedy BC, Panksepp JB, Runckel PA, Lahvis GP. Social influences on morphine-conditioned place preference in adolescent BALB/cJ and C57BL/6J mice. Psychopharmacology (Berl) 2011;219:923–932. doi: 10.1007/s00213-011-2421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE. Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology (Berl) 2005;182:245–252. doi: 10.1007/s00213-005-0071-y. [DOI] [PubMed] [Google Scholar]
- Kitchen DM, Beehner JC. Factors affecting individual participation in group-level aggression among non-human primates. Behaviour. 2007;144:1551–1581. [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Kehoe P. Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Res. 2000;875:44–50. doi: 10.1016/s0006-8993(00)02595-6. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Sanchez H, Jatlow PI, Kehoe P. Neonatal isolation alters the estrous cycle interactions on the acute behavioral effects of cocaine. Psychoneuroendocrinology. 2005;30:753–761. doi: 10.1016/j.psyneuen.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Sanchez H, Zhang XY, Kehoe P. Neonatal isolation enhances acquisition of cocaine self-administration and food responding in female rats. Behav Brain Res. 2004;151:137–149. doi: 10.1016/j.bbr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY, Kehoe P. Heightened cocaine and food self-administration in female rats with neonatal isolation experience. Neuropsychopharmacology. 2006;31:70–76. doi: 10.1038/sj.npp.1300779. [DOI] [PubMed] [Google Scholar]
- Kraemer GW, Mckinney WT. Social separation increases alcohol-consumption in rhesus-monkeys. Psychopharmacology (Berl) 1985;86:182–189. doi: 10.1007/BF00431706. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva N, Gerrits MAFM, Avgustinovich DF, Tenditnik MV, Van Ree JM. Anxiety and ethanol consumption in victorious and defeated mice; effect of kappa-opioid receptor activation. Eur Neuropsychopharmacol. 2006;16:504–511. doi: 10.1016/j.euroneuro.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva NN, Madorskaya IA, Bakshtanovskaya IV. Social success and voluntary ethanol consumption in mice of C57BL/6J and CBA/Lac strains. Physiol Behav. 1991;50:143–146. doi: 10.1016/0031-9384(91)90511-l. [DOI] [PubMed] [Google Scholar]
- Kulkosky PJ, Zellner DA, Hyson RL, Riley AL. Ethanol-consumption of rats in individual, group, and colonial housing conditions. Physiol Psychol. 1980;8:56–60. [Google Scholar]
- Kummer K, Klement S, Eggart V, Mayr MJ, Saria A, Zernig G. Conditioned place preference for social interaction in rats: contribution of sensory components. Front Behav Neurosci. 2011;5:80. doi: 10.3389/fnbeh.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster FE. Sex differences in voluntary drinking by Long Evans rats following early stress. Alcohol Clin Exp Res. 1998;22:830–836. [PubMed] [Google Scholar]
- Le Moal M, Koob GF. Drug addiction: Pathways to the disease and pathophysiological perspectives. Eur Neuropsychopharmacol. 2007;17:377–393. doi: 10.1016/j.euroneuro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405–405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Levine S. Influence of psychological variables on the activity of the hypothalamic-pituitary-adrenal axis. Eur J Pharmacol. 2000;405:149–160. doi: 10.1016/s0014-2999(00)00548-3. [DOI] [PubMed] [Google Scholar]
- Levine S, Mody T. The long-term psychobiological consequences of intermittent postnatal separation in the squirrel monkey. Neurosci Biobehav Rev. 2003;27:83–89. doi: 10.1016/s0149-7634(03)00011-3. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Lawrence AJ. The effect of isolation rearing on volitional ethanol consumption and central CCK/dopamine systems in Fawn-Hooded rats. Behav Brain Res. 2003;141:113–122. doi: 10.1016/s0166-4328(02)00328-5. [DOI] [PubMed] [Google Scholar]
- Lomanowska AM, Rana SA, McCutcheon D, Parker LA, Wainwright PE. Artificial rearing alters the response of rats to natural and drug-mediated rewards. Dev Psychobiol. 2006;48:301–314. doi: 10.1002/dev.20139. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Doremus-Fitzwater TL, Becker HC. Chronic social isolation and chronic variable stress during early development induce later elevated ethanol intake in adult C57BL/6J mice. Alcohol. 2011;45:355–364. doi: 10.1016/j.alcohol.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Mangini LD, Taylor JR. Neonatal isolation stress potentiates cocaine seeking behavior in adult male and female rats. Neuropsychopharmacology. 2005;30:322–329. doi: 10.1038/sj.npp.1300594. [DOI] [PubMed] [Google Scholar]
- Maldonado AM, Finkbeiner LM, Kirstein CL. Social interaction and partner familiarity differentially alter voluntary ethanol intake in adolescent male and female rats. Alcohol. 2008;42:641–648. doi: 10.1016/j.alcohol.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology (Berl) 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- Marmendal M, Eriksson CJ, Fahlke C. Early deprivation increases exploration and locomotion in adult male Wistar offspring. Pharmacol Biochem Behav. 2006;85:535–544. doi: 10.1016/j.pbb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Marmendal M, Roman E, Eriksson CJ, Nylander I, Fahlke C. Maternal separation alters maternal care, but has minor effects on behavior and brain opioid peptides in adult offspring. Dev Psychobiol. 2004;45:140–152. doi: 10.1002/dev.20027. [DOI] [PubMed] [Google Scholar]
- Matthews K, Robbins TW. Early experience as a determinant of adult behavioural responses to reward: the effects of repeated maternal separation in the rat. Neurosci Biobehav Rev. 2003;27:45–55. doi: 10.1016/s0149-7634(03)00008-3. [DOI] [PubMed] [Google Scholar]
- Matthews K, Robbins TW, Everitt BJ, Caine SB. Repeated neonatal maternal separation alters intravenous cocaine self-administration in adult rats. Psychopharmacology (Berl) 1999;141:123–134. doi: 10.1007/s002130050816. [DOI] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Early social isolation in male Long-Evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol self-administration. Alcohol Clin Exp Res. 2009;33:273–282. doi: 10.1111/j.1530-0277.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS, Callaghan PD, Hunt GE. From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use? Br J Pharmacol. 2008;154:358–368. doi: 10.1038/bjp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie-Quirk SD, Miczek KA. Social rank and social separation as determinants of alcohol drinking in squirrel monkeys. Psychopharmacology (Berl) 2008;201:137–145. doi: 10.1007/s00213-008-1256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Mitchell JB, Aitken DH, Bhatnagar S, Bodnoff SR, Iny LJ, Sarrieau A. The effects of neonatal handling on the development of the adrenocortical-response to stress -Implications for neuropathology and cognitive deficits in later life. Psychoneuroendocrinology. 1991;16:85–103. doi: 10.1016/0306-4530(91)90072-2. [DOI] [PubMed] [Google Scholar]
- Mediratta PK, Mahajan P, Sharma KK, Bhandari R, Dubey SP. Involvement of GABA-A receptor chloride channel complex in isolation stress-induced free choice ethanol consumption in rats. Indian J Exp Biol. 2003;41:47–52. [PubMed] [Google Scholar]
- Meerlo P, Overkamp GJF, Benning MA, Koolhaas JM, vandenHoofdakker RH. Long-term changes in open field behaviour following a single social defeat in rats can be reversed by sleep deprivation. Physiol Behav. 1996;60:115–119. doi: 10.1016/0031-9384(95)02271-6. [DOI] [PubMed] [Google Scholar]
- Michaels CC, Easterling KW, Holtzman SG. Maternal separation alters ICSS responding in adult male and female rats, but morphine and naltrexone have little affect on that behavior. Brain Res Bull. 2007;73:310–318. doi: 10.1016/j.brainresbull.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Michaels CC, Holtzman SG. Early postnatal stress alters place conditioning to both mu- and kappa-opioid agonists. J Pharmacol Exp Ther. 2008;325:313–318. doi: 10.1124/jpet.107.129908. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Mutschler NH. Activational effects of social stress on IV cocaine self-administration in rats. Psychopharmacology (Berl) 1996;128:256–264. doi: 10.1007/s002130050133. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Shimamoto A, Covington HE. Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J Neurosci. 2011;31:9848–9857. doi: 10.1523/JNEUROSCI.0637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett MC, Harley J, Francis D, Sanghani SP, Davis WI, Kuhar MJ. Maternal separation and handling affects cocaine self-administration in both the treated pups as adults and the dams. J Pharmacol Exp Ther. 2006;317:1210–1218. doi: 10.1124/jpet.106.101139. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Morrison CF, Stephenson JA. Nicotine injections as the conditioned stimulus in discrimination learning. Psychopharmacologia. 1969;15:351–360. doi: 10.1007/BF00403710. [DOI] [PubMed] [Google Scholar]
- Morse AC, Erwin VG, Jones BC. Strain and housing affect cocaine self-selection and open-field locomotor activity in mice. Pharmacol Biochem Behav. 1993;45:905–912. doi: 10.1016/0091-3057(93)90138-j. [DOI] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. Behavioral and neuropharmacological characterization of nicotine as a conditional stimulus. Eur J Pharmacol. 2007;561:91–104. doi: 10.1016/j.ejphar.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Czoty PW, Nader SH, Morgan D. Nonhuman primate models of social behavior and cocaine abuse. Psychopharmacology (Berl) 2012a doi: 10.1007/s00213-012-2843-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Nader SH, Czoty PW, Riddick NV, Gage HD, Gould RW, Blaylock BL, Kaplan JR, Garg PK, Davies HM, Morton D, Garg S, Reboussin BA. Social dominance in female monkeys: dopamine receptor function and cocaine reinforcement. Biol Psychiatry. 2012b;72:414–421. doi: 10.1016/j.biopsych.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JL, Perry JL, Carroll ME. Social stimuli enhance phencyclidine (PCP) self-administration in rhesus monkeys. Pharmacol Biochem Behav. 2007;87:280–288. doi: 10.1016/j.pbb.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman TK, Parker CC, Suomi SJ, Goldman D, Barr CS, Higley JD. DRD1 5’UTR variation, sex and early infant stress influence ethanol consumption in rhesus macaques. Genes Brain Behav. 2009;8:626–630. doi: 10.1111/j.1601-183X.2009.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedhammer I, David S, Degioanni S, Drummond A, Philip P, Phys O. Workplace bullying and psychotropic drug use: The mediating role of physical and mental health status. Ann Occup Hyg. 2011;55:152–163. doi: 10.1093/annhyg/meq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomikos GG, Spyraki C. Cocaine-Induced place conditioning - Importance of route of administration and other procedural variables. Psychopharmacology (Berl) 1988;94:119–125. doi: 10.1007/BF00735892. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, McLellan AT, Ehrman R. Developing treatments that address classical conditioning. Washington, DC: Cocaine Treatment: Research and Clinical Perspectives; 1993. pp. 71–91. [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, McLellan T, Ehrman R. Integrating systemic cue exposure with standard treatment in recovering drug dependent patients. Addict Behav. 1990;15:355–365. doi: 10.1016/0306-4603(90)90045-y. [DOI] [PubMed] [Google Scholar]
- Oreland S, Raudkivi K, Oreland L, Harro J, Arborelius L, Nylander I. Ethanol-induced effects on the dopamine and serotonin systems in adult Wistar rats are dependent on early-life experiences. Brain Res. 2011;1405:57–68. doi: 10.1016/j.brainres.2011.06.031. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Bevins RA. Facilitation by drug states does not depend on acquired excitatory strength. Behav Brain Res. 2007;176:292–301. doi: 10.1016/j.bbr.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Wilkinson JL, Metschke DM, Bevins RA. Stimulus properties of nicotine, amphetamine, and chlordiazepoxide as positive features in a pavlovian appetitive discrimination task in rats. Neuropsychopharmacology. 2005;30:731–41. doi: 10.1038/sj.npp.1300629. [DOI] [PubMed] [Google Scholar]
- Parker LF, Radow BL. Isolation stress and volitional ethanol consumption in rat. Physiol Behav. 1974;12:1–3. doi: 10.1016/0031-9384(74)90060-2. [DOI] [PubMed] [Google Scholar]
- Peartree NA, Hood LE, Adams MA, Chandler KN, Neisewander JL. Social context increases nicotine intake during initial opportunity for self-administration Society for Neuroscience. Washington, D.C: Society for Neuroscience; 2011. [Google Scholar]
- Peartree NA, Hood LE, Thiel KJ, Sanabria F, Pentkowski NS, Chandler KN, Neisewander JL. Limited physical contact through a mesh barrier is sufficient for social reward-conditioned place preference in adolescent male rats. Physiol Behav. 2012;105:749–756. doi: 10.1016/j.physbeh.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentkowski NS, Painter MR, Thiel KJ, Peartree NA, Cheung TH, Deviche P, Adams M, Alba J, Neisewander JL. Nicotine-induced plasma corticosterone is attenuated by social interactions in male and female adolescent rats. Pharmacol Biochem Behav. 2011;100:1–7. doi: 10.1016/j.pbb.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips GD, Howes SR, Whitelaw RB, Robbins TW, Everitt BJ. Isolation rearing impairs the reinforcing efficacy of intravenous cocaine or intra-accumbens d-amphetamine: impaired response to intra-accumbens D1 and D2/D3 dopamine receptor antagonists. Psychopharmacology (Berl) 1994a;115:419–429. doi: 10.1007/BF02245085. [DOI] [PubMed] [Google Scholar]
- Phillips GD, Howes SR, Whitelaw RB, Wilkinson LS, Robbins TW, Everitt BJ. Isolation rearing enhances the locomotor response to cocaine and a novel environment, but impairs the intravenous self-administration of cocaine. Psychopharmacology (Berl) 1994b;115:407–418. doi: 10.1007/BF02245084. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Pisu MG, Mostallino MC, Dore R, Maciocco E, Secci PP, Serra M. Effects of voluntary ethanol consumption on emotional state and stress responsiveness in socially isolated rats. Eur Neuropsychopharmacol. 2011;21:414–425. doi: 10.1016/j.euroneuro.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlesnik CA, Jimenez-Gomez C, Shahan TA. Resurgence of alcohol seeking produced by discontinuing non-drug reinforcement as an animal model of drug relapse. Behav Pharmacol. 2006;17:369–374. doi: 10.1097/01.fbp.0000224385.09486.ba. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Housing and rank status of male Long-Evans rats modify ethanol’s effect on open-field behaviors. Psychopharmacology (Berl) 2006;185:289–297. doi: 10.1007/s00213-005-0257-3. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Psychosocial stress and chronic ethanol ingestion in male rats: Effects on elevated plus maze behavior and ultrasonic vocalizations. Physiol Behav. 2008;94:432–447. doi: 10.1016/j.physbeh.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Acute novel stressors modify ethanol intake of psychosocially stressed rats. Pharmacol Biochem Behav. 2010;95:390–400. doi: 10.1016/j.pbb.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF. Individual differences in sensitivity to nicotine: implications for genetic research on nicotine dependence. Behav Genet. 1995;25:161–177. doi: 10.1007/BF02196925. [DOI] [PubMed] [Google Scholar]
- Quadros IMH, Miczek KA. Two modes of intense cocaine bingeing: increased persistence after social defeat stress and increased rate of intake due to extended access conditions in rats. Psychopharmacology (Berl) 2009;206:109–120. doi: 10.1007/s00213-009-1584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez R, Hinman A, Sterling S, Weisner C, Campbell C. Peer influences on adolescent alcohol and other drug use outcomes. J Nurs Scholarsh. 2012;44:36–44. doi: 10.1111/j.1547-5069.2011.01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz S, Berger BD. Social isolation increases morphine intake: behavioral and psychopharmacological aspects. Behav Pharmacol. 2010;21:39–46. doi: 10.1097/FBP.0b013e32833470bd. [DOI] [PubMed] [Google Scholar]
- Redish AD, Jensen S, Johnson A. A unified framework for addiction: Vulnerabilities in the decision process. Behav Brain Sci. 2008;31:415–437. doi: 10.1017/S0140525X0800472X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly S, Revusky S. Drug-drug heart rate conditioning in rats: effective USs when pentobarbital is the CS. Pharmacol Biochem Behav. 1992;42:633–643. doi: 10.1016/0091-3057(92)90009-5. [DOI] [PubMed] [Google Scholar]
- Ribeiro Do Couto B, Aguilar MA, Lluch J, Rodriguez-Arias M, Minarro J. Social experiences affect reinstatement of cocaine-induced place preference in mice. Psychopharmacology (Berl) 2009;207:485–498. doi: 10.1007/s00213-009-1678-1. [DOI] [PubMed] [Google Scholar]
- Ribeiro Do Couto B, Aguilar MA, Manzanedo C, Rodriguez-Arias M, Armario A, Minarro J. Social stress is as effective as physical stress in reinstating morphine-induced place preference in mice. Psychopharmacology (Berl) 2006;185:459–470. doi: 10.1007/s00213-006-0345-z. [DOI] [PubMed] [Google Scholar]
- Rockman GE, Hall AM, Markert LE, Glavin GB. Influence of rearing conditions on voluntary ethanol intake and response to stress in rats. Behav Neural Biol. 1988;49:184–191. doi: 10.1016/s0163-1047(88)90506-7. [DOI] [PubMed] [Google Scholar]
- Roma PG, Davis CM, Kohut SJ, Huntsberry ME, Riley AL. Early maternal separation and sex differences in the aversive effects of amphetamine in adult rats. Physiol Behav. 2008;93:897–904. doi: 10.1016/j.physbeh.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Roman E, Gustafsson L, Hyytia P, Nylander I. Short and prolonged periods of maternal separation and voluntary ethanol intake in male and female ethanol-preferring AA and ethanol-avoiding ANA rats. Alcohol Clin Exp Res. 2005;29:591–601. doi: 10.1097/01.alc.0000158933.70242.fc. [DOI] [PubMed] [Google Scholar]
- Roman E, Nylander I. The impact of emotional stress early in life on adult voluntary ethanol intake-results of maternal separation in rats. Stress. 2005;8:157–174. doi: 10.1080/10253890500188666. [DOI] [PubMed] [Google Scholar]
- Roman E, Ploj K, Nylander I. Maternal separation has no effect on voluntary ethanol intake in female Wistar rats. Alcohol. 2004;33:31–39. doi: 10.1016/j.alcohol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Roske I, Baeger I, Frenzel R, Oehme P. Does a relationship exist between the quality of stress and the motivation to ingest alcohol? Alcohol. 1994;11:113–124. doi: 10.1016/0741-8329(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Rowlett JK. A labor-supply analysis of cocaine self-administration under progressive-ratio schedules: antecedents, methodologies, and perspectives. Psychopharmacology (Berl) 2000;153:1–16. doi: 10.1007/s002130000610. [DOI] [PubMed] [Google Scholar]
- Sanna E, Talani G, Obili N, Mascia MP, Mostallino MC, Secci PP, Pisu MG, Biggio F, Utzeri C, Olla P, Biggio G, Follesa P. Voluntary ethanol consumption induced by social isolation reverses the increase of alpha(4)/delta GABA(A) receptor gene expression and function in the hippocampus of C57BL/6J mice. Front Neurosci. 2011;5:15. doi: 10.3389/fnins.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Ellison F, Hunt T, Amit Z. An examination of heroin conditioning in preferred and nonpreferred environments and in differentially housed mature and immature rats. Pharmacol Biochem Behav. 1985;22:215–220. doi: 10.1016/0091-3057(85)90380-6. [DOI] [PubMed] [Google Scholar]
- Schenk S, Gorman K, Amit Z. Age-dependent effects of isolation housing on the self-administration of ethanol in laboratory rats. Alcohol. 1990;7:321–326. doi: 10.1016/0741-8329(90)90090-y. [DOI] [PubMed] [Google Scholar]
- Schenk S, Hunt T, Colle L, Amit Z. Isolation versus grouped housing in rats: differential effects of low doses of heroin in the place preference paradigm. Life Sci. 1983;32:1129–1134. doi: 10.1016/0024-3205(83)90118-2. [DOI] [PubMed] [Google Scholar]
- Schenk S, Hunt T, Malovechko R, Robertson A, Klukowski G, Amit Z. Differential effects of isolation housing on the conditioned place preference produced by cocaine and amphetamine. Pharmacol Biochem Behav. 1986;24:1793–1796. doi: 10.1016/0091-3057(86)90523-x. [DOI] [PubMed] [Google Scholar]
- Schenk S, Lacelle G, Gorman K, Amit Z. Cocaine self-administration in rats influenced by environmental conditions: implications for the etiology of drug abuse. Neurosci Lett. 1987;81:227–231. doi: 10.1016/0304-3940(87)91003-2. [DOI] [PubMed] [Google Scholar]
- Schulden JD, Thomas YF, Compton WM. Substance abuse in the United States: findings from recent epidemiologic studies. Curr Psychiatry Rep. 2009;11:353–359. doi: 10.1007/s11920-009-0053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgansberger W, Wurst W, Holsboer F, Spanagel R. Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science. 2002;296:931–933. doi: 10.1126/science.1069836. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA. Peer influences on drug self-administration: Social facilitation and social inhibition of cocaine intake in male rats. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Chauvet C, Thiriet N, El Rawas R, Jaber M. Reversal of cocaine addiction by environmental enrichment. Proc Natl Acad Sci U S A. 2008;105:17145–17150. doi: 10.1073/pnas.0806889105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Thiriet N, El Rawas R, Lardeux V, Jaber M. Environmental enrichment during early stages of life reduces the behavioral, neurochemical, and molecular effects of cocaine. Neuropsychopharmacology. 2009;34:1102–1111. doi: 10.1038/npp.2008.51. [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Prendergast MA, Bardo MT. Environmental-induced differences in corticosterone and glucocorticoid receptor blockade of amphetamine self-administration in rats. Psychopharmacology (Berl) 2011;218:293–301. doi: 10.1007/s00213-011-2448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. Conditioned and unconditioned drug effects in relapse to opiate and stimulant drug self-adminstration. Prog Neuropsychopharmacol B ol Psychiatry. 1983;7:591–597. doi: 10.1016/0278-5846(83)90030-1. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Garcha HS, Pratt JA, Kumar R. Role of training dose in discrimination of nicotine and related compounds by rats. Psychopharmacology (Berl) 1984;84:413–419. doi: 10.1007/BF00555223. [DOI] [PubMed] [Google Scholar]
- Strine TW, Dube SR, Edwards VJ, Prehn AW, Rasmussen S, Wagenfeld M, Dhingra S, Croft JB. Associations between adverse childhood experiences, psychological distress, and adult alcohol problems. Am J Health Behav. 2012;36:408–423. doi: 10.5993/AJHB.36.3.11. [DOI] [PubMed] [Google Scholar]
- Sussman S. Risk factors for and prevention of tobacco use. Pediatr Blood Cancer. 2005;44:614–619. doi: 10.1002/pbc.20350. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: a model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008;96:202–212. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Pentkowski NS, Peartree NA, Painter MR, Neisewander JL. Environmental living conditions introduced during forced abstinence alter cocaine-seeking behavior and Fos protein expression. Neuroscience. 2010;171:1187–1196. doi: 10.1016/j.neuroscience.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Neisewander JL. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology (Berl) 2009a;204:391–402. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Pentkowski NS, Neisewander JL. Anti-craving effects of environmental enrichment. Int J Neuropsychopharmacol. 2009b:1–6. doi: 10.1017/S1461145709990472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, Khoury A, Mathe AA, Ehlers CL. Effect of social isolation on ethanol consumption and substance P/neurokinin expression in Wistar rats. Alcohol. 2005;36:91–97. doi: 10.1016/j.alcohol.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology (Berl) 1997;130:203–212. doi: 10.1007/s002130050230. [DOI] [PubMed] [Google Scholar]
- Tomie A, Burger KM, Di Poce J, Pohorecky LA. Social opportunity and ethanol drinking in rats. Prog Neuropsychopharmacol B ol Psychiatry. 2004;28:1089–1097. doi: 10.1016/j.pnpbp.2004.05.044. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol Behav. 1993;53:983–993. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- Traweger C, Kinzl JF, Traweger-Ravanelli B, Fiala M. Psychosocial factors at the workplace-do they affect substance use? Evidence from the Tyrolean workplace study. Pharmacoepidemiol Drug Saf. 2004;13:399–403. doi: 10.1002/pds.955. [DOI] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Vanderschuren LJ. Conditioned place preference induced by social play behavior: parametrics, extinction, reinstatement and disruption by methylphenidate. Eur Neuropsychopharmacol. 2009;19:659–669. doi: 10.1016/j.euroneuro.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhart M, Wand GS. Stress, alcohol and drug interaction: an update of human research. Addict Biol. 2009;14:43–64. doi: 10.1111/j.1369-1600.2008.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente TW, Ritt-Olson A, Stacy A, Unger JB, Okamoto J, Sussman S. Peer acceleration: effects of a social network tailored substance abuse prevention program among high-risk adolescents. Addiction. 2007;102:1804–1815. doi: 10.1111/j.1360-0443.2007.01992.x. [DOI] [PubMed] [Google Scholar]
- van der Veen R, Koehl M, Abrous DN, de Kloet ER, Piazza PV, Deroche-Gamonet V. Maternal environment influences cocaine intake in adulthood in a genotype-dependent manner. PLoS One. 2008;3:e2245. doi: 10.1371/journal.pone.0002245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp AM, Miczek KA. Persistent suppression of ethanol self-administration by brief social stress in rats and increased startle response as index of withdrawal. Physiol Behav. 2001;73:301–311. doi: 10.1016/s0031-9384(01)00458-9. [DOI] [PubMed] [Google Scholar]
- van Erp AM, Tachi N, Miczek KA. Short or continuous social stress: suppression of continuously available ethanol intake in subordinate rats. Behav Pharmacol. 2001;12:335–342. doi: 10.1097/00008877-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Vanderschuren L, Trezza V, Griffioen-Roose S, Schiepers OJG, Van Leeuwen N, De Vries TJ, Schoffelmeer ANM. Methylphenidate disrupts social play behavior in adolescent rats. Neuropsychopharmacology. 2008;33:2946–2956. doi: 10.1038/npp.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez V, Giros B, Dauge V. Maternal deprivation specifically enhances vulnerability to opiate dependence. Behav Pharmacol. 2006;17:715–724. doi: 10.1097/FBP.0b013e3280116e6f. [DOI] [PubMed] [Google Scholar]
- Vazquez V, Penit-Soria J, Durand C, Besson MJ, Giros B, Dauge V. Maternal deprivation increases vulnerability to morphine dependence and disturbs the enkephalinergic system in adulthood. J Neurosci. 2005;25:4453–4462. doi: 10.1523/JNEUROSCI.4807-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez V, Weiss S, Giros B, Martres MP, Dauge V. Maternal deprivation and handling modify the effect of the dopamine D3 receptor agonist, BP 897 on morphine-conditioned place preference in rats. Psychopharmacology (Berl) 2007;193:475–486. doi: 10.1007/s00213-007-0789-9. [DOI] [PubMed] [Google Scholar]
- Walker CD, Scribner KA, Cascio CS, Dallman MF. The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology. 1991;128:1385–1395. doi: 10.1210/endo-128-3-1385. [DOI] [PubMed] [Google Scholar]
- Watanabe S. Drug-social interactions in the reinforcing property of methamphetamine in mice. Behav Pharmacol. 2011;22:203–206. doi: 10.1097/FBP.0b013e328345c815. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Effects of early experience on responsiveness to ethanol - a preliminary-report. Physiol Behav. 1987;40:401–406. doi: 10.1016/0031-9384(87)90068-0. [DOI] [PubMed] [Google Scholar]
- Weinhold LL, Sharpe LG, Jaffe JH. Housing conditions influence acquisition of sufentanil aerosol self-administration in rats. Pharmacol Biochem Behav. 1993;44:141–144. doi: 10.1016/0091-3057(93)90291-z. [DOI] [PubMed] [Google Scholar]
- Weisinger RS, Denton DA, Osborne PG. Voluntary ethanol intake of individually- or pair-housed rats: effect of ACTH or dexamethasone treatment. Pharmacol Biochem Behav. 1989;33:335–341. doi: 10.1016/0091-3057(89)90510-8. [DOI] [PubMed] [Google Scholar]
- Wikler A. Dynamics of drug dependence - implications of a conditioning theory for research and treatment. Arch Gen Psychiatry. 1973;28:611–616. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J. Free choice ethanol intake of laboratory rats under different social conditions. Psychopharmacology (Berl) 1990;101:233–239. doi: 10.1007/BF02244132. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J, Heyne A. Social behavior, dominance, and social deprivation of rats determine drug choice. Pharmacol Biochem Behav. 1991;38:389–399. doi: 10.1016/0091-3057(91)90297-f. [DOI] [PubMed] [Google Scholar]
- Wongwitdecha N, Marsden CA. Effect of social isolation on the reinforcing properties of morphine in the conditioned place preference test. Pharmacol Biochem Behav. 1996;53:531–534. doi: 10.1016/0091-3057(95)02046-2. [DOI] [PubMed] [Google Scholar]
- Wright MO, Crawford E, Del Castillo D. Childhood emotional maltreatment and later psychological distress among college students: The mediating role of maladaptive schemas. Child Abuse Neglect. 2009;33:59–68. doi: 10.1016/j.chiabu.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Yanai J, Ginsburg BE. Increased sensitivity to chronic ethanol in isolated mice. Psychopharmacologia. 1976;46:185–189. doi: 10.1007/BF00421390. [DOI] [PubMed] [Google Scholar]
- Yap JJ, Miczek KA. Social defeat stress, sensitization, and intravenous cocaine self-administration in mice. Psychopharmacology (Berl) 2007;192:261–273. doi: 10.1007/s00213-007-0712-4. [DOI] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurol Psychol. 1908;18:459–482. [Google Scholar]
- Young SYN, Hansen CJ, Gibson RL, Ryan MAK. Risky alcohol use, age at onset of drinking, and adverse childhood experiences in young men entering the US marine corps. Arch Pediatr Adolesc Med. 2006;160:1207–1214. doi: 10.1001/archpedi.160.12.1207. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Miller J, Unterwald E, Wade D, Izenwasser S. Social and physical environment alter cocaine conditioned place preference and dopaminergic markers in adolescent male rats. Neuroscience. 2009;163:890–897. doi: 10.1016/j.neuroscience.2009.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Sanchez H, Kehoe P, Kosten TA. Neonatal isolation enhances maintenance but not reinstatement of cocaine self-administration in adult male rats. Psychopharmacology (Berl) 2005;177:391–399. doi: 10.1007/s00213-004-1963-y. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Brett MB. Effects of early environmental experience on self-administration of amphetamine and barbital. Psychopharmacology (Berl) 1992;106:474–478. doi: 10.1007/BF02244817. [DOI] [PubMed] [Google Scholar]