Abstract
Deregulation of signaling pathways involving phosphorylation is a hallmark of malignant transformation. Degradation of phosphoproteins generates cancer-specific phosphopeptides that are associated with MHC-I and II molecules and recognized by T-cells. We identified 95 phosphopeptides presented on the surface of primary hematological tumors and normal tissues, including 61 that were tumor-specific. Phosphopeptides were more prevalent on more aggressive and malignant samples. CD8 T-cell lines specific for these phosphopeptides recognized and killed both leukemia cell lines and HLA-matched primary leukemia cells ex vivo. Healthy individuals showed surprisingly high levels of CD8 T-cell responses against many of these phosphopeptides within the circulating memory compartment. This immunity was significantly reduced or absent in some leukemia patients, which correlated with clinical outcome, and was restored following allogeneic stem cell transplantation. These results suggest that phosphopeptides may be targets of cancer immune surveillance in humans, and point to their importance for development of vaccine-based and T-cell adoptive transfer immunotherapies..
Introduction
Hematologic malignancies are susceptible to curative immunological therapies, such as stem cell transplantation, donor lymphocyte infusion and manipulation of endogenous immunity through immunostimulatory cytokines (1-4). The clinical utility of cellular immunotherapies provides powerful direct evidence that the adaptive immune response can control and eradicate tumors(5-9). However, immunotherapies targeting identified tumor antigens have met with limited success, suggesting the optimal antigens remain undiscovered (10-12). Moreover, the vast majority of identified tumor antigens are not derived from oncoproteins orchestrating the transformation process. Efforts targeting these antigens may be compromised by tolerance mechanisms and immune escape.
The importance of signal-transduction pathway deregulation in cancer pathogenesis is well-established (13-17). Small molecule therapies targeting these pathways have met with considerable clinical success, providing a powerful argument for immunotherapies that target similar deregulated pathways (18-20). Protein phosphorylation is the dominant mechanism involved in oncogenic signaling processes (21). We previously showed that phosphorylation is preserved on peptides during antigen processing for presentation by both MHC-I and -II molecules (22-24). This suggests that phosphopeptide antigens derived from cancer-related phosphoproteins could serve as immunological signatures of ‘transformed self’ (22-26). Moreover, phosphorylation can enhance the binding of peptides to MHC-I molecules, creating “neo-antigens”. Collectively these results suggest that phosphopeptides are attractive targets for cancer immunotherapy (27).
To date, no studies have examined MHC-I-bound phosphopeptides displayed on primary human tumor samples, nor has the human immune response against MHC-I restricted phosphopeptides been characterized. Here, we identify novel phosphopeptides from primary leukemia samples and show that healthy individuals display immune responses with memory characteristics against many of them. Immunity is lost in some patients with leukemia, but can be restored. This unexpected pre-existing immunity to phosphopeptides suggests that they could be targets of immune surveillance, and play a role in enabling immunological control of malignancies in humans.
Results
Characterization of Leukemia-Associated MHC Class-I Restricted Phosphopeptides
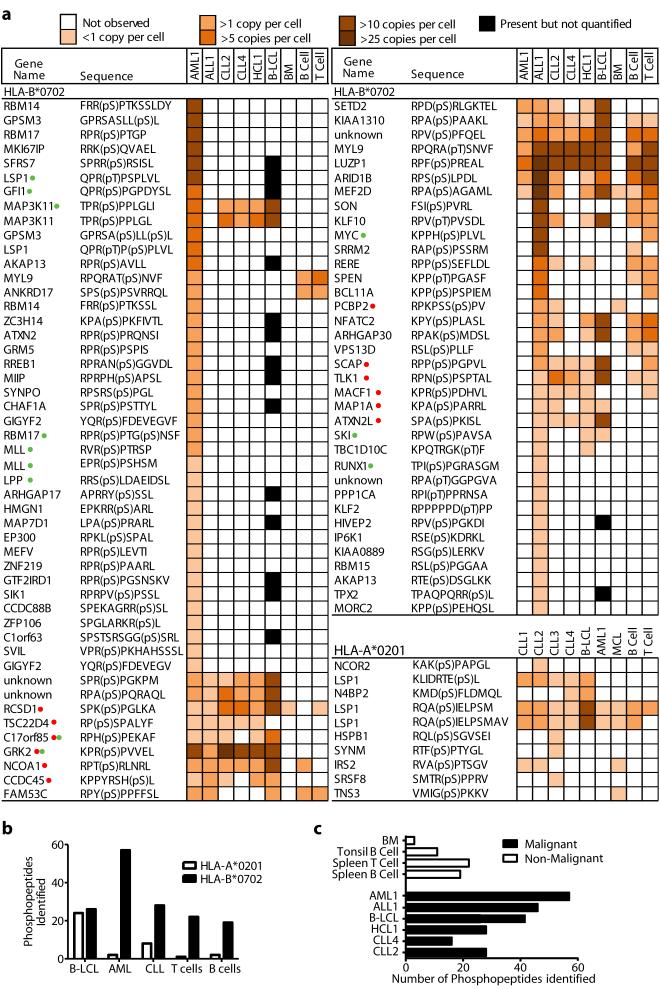
To identify tumor-associated phosphopeptides, we affinity-isolated HLA-A*0201 (HLA-A2) and HLA-B*0702 (HLA-B7) peptide complexes from 4 primary chronic lymphocytic leukemia (CLL) tumors, a primary hairy-cell leukemia (HCL), a primary mantle cell lymphoma (MCL), a primary acute lymphoblastic leukemia (ALL), and a primary acute myeloid leukemia (AML), in addition to normal splenic T and B-cells, bone marrow cells (BM), and cultured B-lymphoblastoid cell lines (B-LCL). Collectively, 10 HLA-A2-restricted and 85 HLA-B7-restricted phosphopeptides were identified (Fig. 1a). All tumor types and normal tissues expressed a greater number of HLA-B7 than HLA-A2 phosphopeptides (Fig. 1b).
Figure 1.
Characterizing HLA-bound phosphopeptides displayed on tumor and matched healthy tissue. (a) Phosphopeptide display isolated from HLA-A2 and HLA-B7 molecules from 11 primary tumor samples (ALL1, AML1, CLL1-4, HCL1, MCL), EBV-transformed B-cells (B-LCL) and HLA-matched healthy tissue (T-cells, B-cells and bone marrow). Red dots and green dots indicate phosphopeptide antigens selected for further study in patients with CLL and AML respectively. (b) Comparison of the number of individual phosphopeptides identified between HLA-A2 and HLA-B7 in both normal and malignant tissue. (c) Comparison of the number of unique phosphopeptides identified between normal, indolent malignant and aggressive malignant tissue.
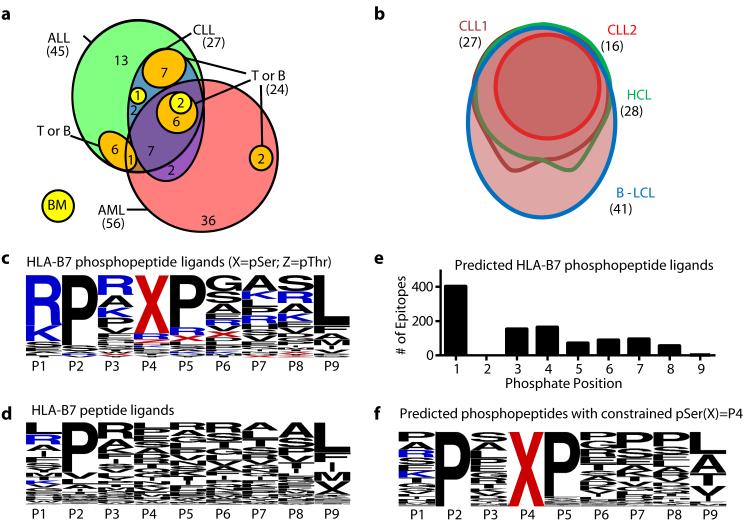
The large number of HLA-B7-restricted phosphopeptides enabled us to compare their representation on different tumor types and healthy tissue. On average more than twice as many were found on aggressive (AML and ALL) as on indolent (CLL and HCL) tumors or normal tissue (Fig. 1c). Of 56 HLA-B7-restricted phosphopeptides identified on AML, 36 were not found on any other leukemia and only two were found on bone marrow (Figs. 1a, 2a). Of 45 phosphopeptides identified on ALL, 19 were not found on other leukemias and 13/19 were also not observed on T- or B-cells (Fig. 2a). Seven were derived from oncogenes implicated in leukemogenesis: MYC, EP300, SKI, GFI-1, Bcl-11A, MEF2D, and MLL (Fig. 1a). Twenty-seven HLA-B7 phosphopeptides were identified on two CLL tumors, all of which were shared with either AML or ALL. Sixteen were observed on normal tissue but seven were common to AML, ALL and CLL and not normal tissue (CCDC45, GRK2, SETD2, C17orf85, TSC22D4 and SPR(pS)PGKPM, derived from an unknown protein). These proteins have not been previously associated with leukemic malignancies and the functions of most remain undefined. Twenty-six of 27 CLL peptides were also found on HCL, which itself expressed one additional unique phosphopeptide. Twenty-four of these were also shared by B-LCL (Fig. 2b). These results identify a cohort of phosphopeptides expressed on multiple leukemic malignancies, but not normal tissue, which represent potential immunotherapeutic targets.
Figure 2.
The distribution and characteristics of leukemia-associated phosphopeptides. (a, b) Euler diagrams depicting the distribution of HLA-B7-restricted phosphopeptides among different leukemias normal tissues (a) and within different B-cell malignancies (b). (c,d,f) Logoplots of residue frequency at each position of all 9mer HLA-B7 phosphopeptides (c); 9mer non-phosphorylated HLA-B7 peptides from the ImmuneEpitope database (d); and predicted HLA-B7 phosphopeptide binders with a pSer at position 4 (f). The position of phosphoserine for all B7-predicted binders (e).
Characteristics of the HLA-A2 bound phosphopeptides were similar to those previously reported (22, 23). Of 85 HLA-B7-restricted phosphopeptides, two were dually phosphorylated and the remainder monophosphorylated, with 77/85 containing phosphoserine and 9/85 containing phosphothreonine. The phosphate was found at position four in 72% of HLA-B7 phosphopeptides, similar to the distribution in HLA-A2 phosphopeptides (Fig. S1a,b) (27). When compared with 1038 non-phosphorylated HLA-B7-restricted 9mer peptides in the ImmuneEpitope database (28), HLA-B7-restricted phosphopeptides showed a similar strong preference for proline at P2 and common hydrophobic C-terminal anchor residues (Fig. 2c, d). However, they showed an unusual bias for basic residues at P1 and proline at P5 (Fig 2c, Fig. S1c). To test whether these biases were imposed by an underlying kinase recognition motif, potential HLA-B7 binding 9mer peptides were identified in the Phosphosite dataset (29) of known serine phosphorylation sites. The position of the phosphoserine within 1031 phosphopeptides predicted to bind to HLA-B7 was not skewed towards P4 (Fig. 2e). Thus, these biases likely reflect roles in binding to HLA-B7 analogous to those demonstrated for HLA-A2-associated phosphopeptides (27). Of 164 predicted HLA-B7 binding peptides that had phosphoserine at P4 there was no apparent bias for basic residues at P1 (Fig. 2f). However 87% contained a proline at P5 suggesting this bias reflects an underlying kinase motif, rather than being imposed by HLA binding.
Phosphopeptide-specific T-cell responses in healthy donors
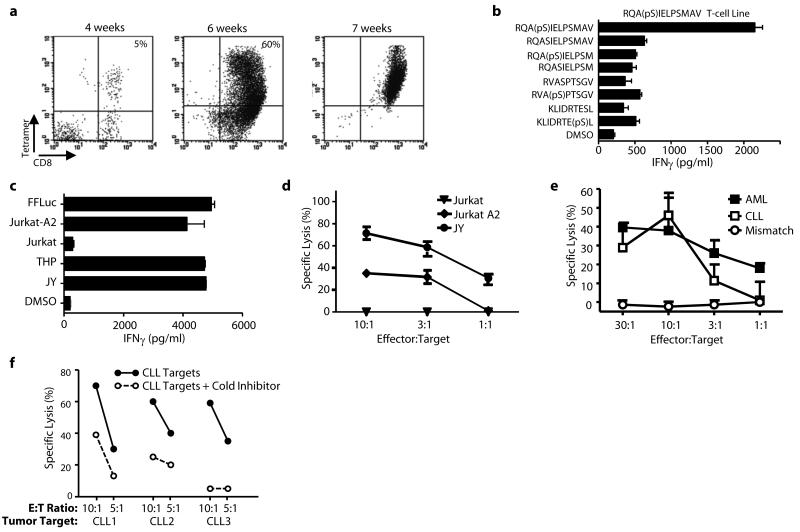
Three HLA-A2 and two HLA-B7 phosphopeptides were derived from LSP-1, a lymphoma marker (30). One, RQA(pS)IELPSMAV, is present on all HLA-A2+ tumor samples at high copy number. RQA(pS)IELPSMAV-pulsed dendritic cells were used to prime autologous T-cells. Responses to this phosphopeptide could be elicited in 3/3 healthy individuals. Specific T-cells were enriched using HLA-A2-RQA(pS)IELPSMAV tetramers to produce T-cell lines (Fig. 3a). These lines secreted IFNγ in response to stimulators pulsed with RQA(pS)IELPSMAV, but not with unphosphorylated RQASIELPSMAV, nor to other phosphopeptides, including the closely related RQA(pS)IELPSM (Fig. 3b). Recognition was therefore both phosphate-dependent and peptide sequence-specific. These lines also killed the HLA-A2+ AML cell line THP-1 and the HLA-A2 transfected ALL cell line, Jurkat-A2, but not untransfected Jurkat (Fig. 3c-d). Most importantly, RQA(pS)IELPSMAV-specific T-cells killed HLA-A2+ primary AML and CLL tumors, but not an HLA-A2neg CLL tumor (Fig. 3e). T-cell lines were also elicited against an HLA-B7 restricted phosphopeptide (RPT(pS)RLNRL) derived from NCOA-1 and showed remarkable killing towards three primary CLL tumors (Fig. 3f).
Figure 3.
Generation of phosphopeptide-specific T-cells, from healthy donors, which recognize and kill leukemic targets. (a) Expansion of LSP-1 (RQA(pS)IELPSMAV) specific T-cells from healthy donor PBMCs using DCs and tetramer selection. (b,c) Peptide antigen specificity (b) and recognition (c) of leukemia cell lines by LSP-1-specific T-cells. (d,e) T-cell mediated killing of leukemia cell lines (d) or primary leukemia samples (e) by phosphopeptide-specific T-cell lines but not an HLA-mismatched primary tumor. (f) NCOA1 (RPT(pS)RLNRL)-specific T-cell mediated killing of three HLA-matched CLL tumor samples was inhibited by adding autologous NCOA1 loaded cold targets.
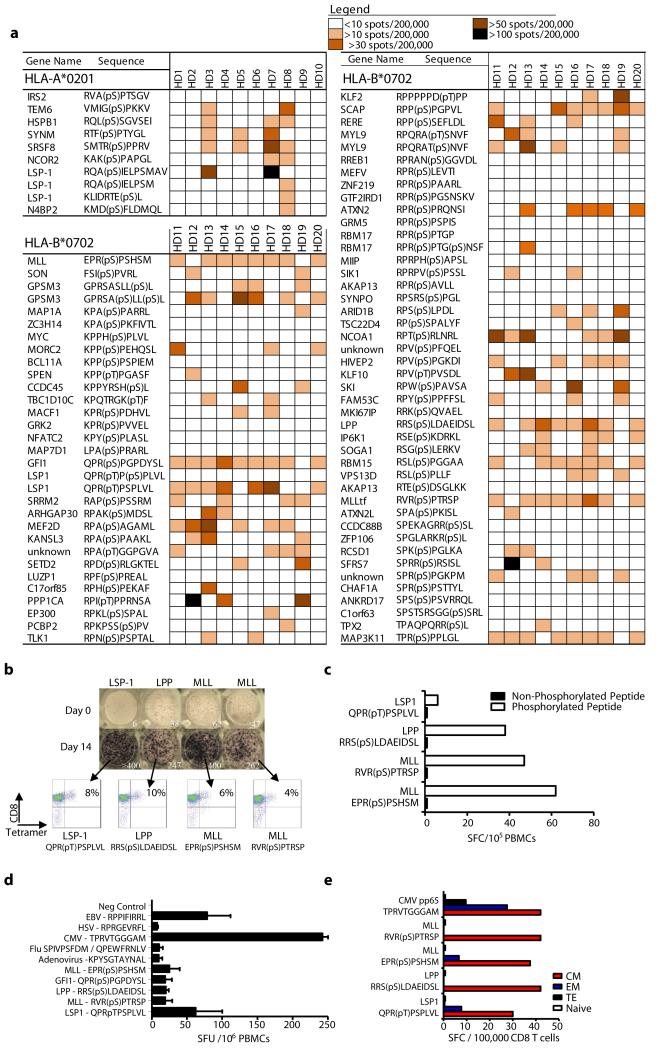
We next evaluated immunity in 10 healthy donors against 10 HLA-A2 and 76 HLA-B7 leukemia-associated phosphopeptides (Fig. 4a). Immune responses to 50/76 HLA-B7 and 9/10 HLA-A2 phosphopeptides were observed. Each individual responded to an average of 16/76 (range 11-22) HLA-B7 and 2/10 (range 0-4) HLA-A2 phosphopeptides. Importantly, these responses were observed in 7-day in vitro cultures without addition of exogenous cytokines. No responses of a similar magnitude were observed for several phosphopeptides encoded by well-established leukemia oncogenes, most notably MYC, BCL-11A and EP300. However, the MYC and BCL-11A phosphopeptides were also present on normal T-cells (Fig. 1a). Phosphopeptides from other leukemia oncogenes (MLL, LPP, SKI, GFI-1 and MEF2D) elicited strong immune responses in several individuals. There was substantial donor to donor variation as to which phosphopeptides stimulated these unusually strong responses: the GFI1 phosphopeptide stimulated responses in 9/10 individuals; the MLL, LPP, and MAP3K11 phosphopeptides in 8/10; and those from LSP1, SCAP and RBM15 in 7/10. For four phosphopeptides that were recognized by most healthy donors, T-cell specificity was confirmed in 14-day cultures using HLA-phosphopeptide multimers (Fig. 4b). Furthermore, these T-cells did not recognize the unphosphorylated counterpart peptides (Fig. 4c, Fig S3b). Collectively, these data demonstrate that the majority of leukemia-associated phosphopeptides elicited surprisingly strong and specific responses in a significant fraction of healthy individuals.
Figure 4.
Phosphopeptide-specific immunity is present in healthy individuals within the circulating memory T-cell compartment. (a) Marked heterogeneity of phosphopeptide-specific immunity in 10 healthy donors against 86 antigens revealed by 7-day ELISpot assays. (b) ELISpot results for immunodominant phosphopeptides directly ex vivo and following 14-days in vitro expansion when tetramer binding is evident. (c) Expanded T-cells do not recognize the unphosphorylated counterpart peptides. (d) The relative magnitude of phosphopeptide-specific T-cells compared against common viral epitopes following overnight culture (n=3). (e) Analysis of memory-flow-sorted CD8+ enriched T-cells by 7-day ELISpot reveal phosphopeptide-specific responses reside in the memory compartment.
Leukemia-associated phosphopeptide-specific immunity in healthy donors
Because responses to phosphopeptides were observed after only 7 days of in vitro culture in the absence of exogenous cytokines, we compared them with responses to immunodominant epitopes from three persistent viruses (CMV, EBV and HSV) and two non-persistent viruses (influenza and adenovirus). Responses against four phosphopeptides were even evident in ex vivo ELISpot analysis of peripheral blood mononuclear cells (PBMC) from some donors (Fig. 4b,d). These responses were similar to, or higher than, those to both non-persistent viral epitopes, but lower than responses to CMV and EBV epitopes. This high level of phosphopeptide-specific T-cells led us to investigate whether the responding cells resided within memory or naïve T-cell compartments. Enriched CD8 T-cells from peripheral blood of two HLA-B7+ healthy donors were flow-sorted into naïve (TN), central memory (TCM), effector memory (TEM) and terminal effector memory cells (TEMRA) based on expression of CD45RA and CD27 (31). After 7-day in vitro culture, ELISpot analysis demonstrated that T-cells responding to individual phosphopeptides were exclusively in the memory compartment, and predominantly had a TCM phenotype (Fig. 4e, Fig. S2). This suggests that the majority of healthy individuals have been previously exposed to a stimulus that establishes immunological memory to tumor-associated phosphopeptides.
Absent phosphopeptide-specific immunity in leukemia patients
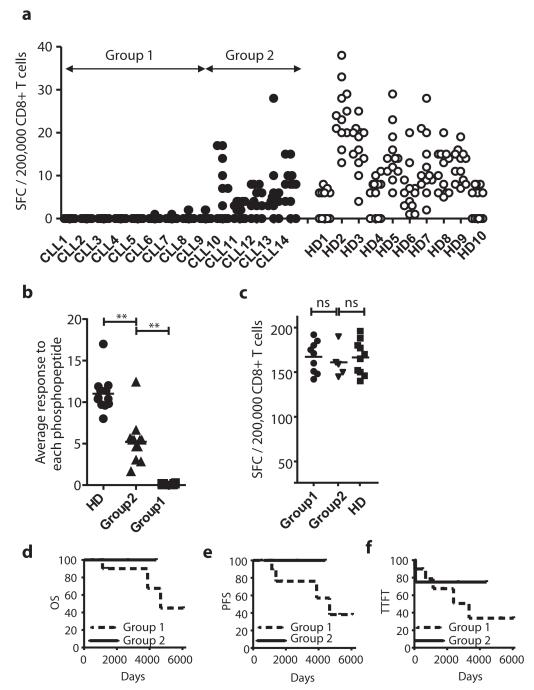
We next evaluated the level of phosphopeptide-specific CD8 T-cell immunity in immunocompetent patients with early-stage CLL (Table S1). As tumor cells are present at high levels in PBMC from CLL patients, comparison with responses in PBMC from healthy individuals is problematic. Therefore, responses against a group of 12 CLL-associated phosphopeptides (identified by red dots in Fig. 1) were assessed by direct ex vivo ELISpot of purified CD8 T-cells (Fig. S3). As expected, we detected immune responses to most of these phosphopeptides in all 10 healthy HLA-B7+ individuals (Fig. 5a). However, 9/14 CLL patients (Group 1) had low or absent immunity to all 12 phosphopeptides (less than a combined total of 10 spots for all 12 phosphopeptides/200,000 CD8 T-cells). Five CLL patients (Group 2) showed breadth of recognition of different phosphopeptides similar to that of the healthy donors. However, average responses to all phosphopeptides were significantly lower in Group 2 patients than in healthy individuals (Fig 5b).
Figure 5.
Leukemia-associated phosphopeptide-specific immunity is lacking in CLL patients. (a) Comparative ELISpot analysis of enriched CD8+ T-cells from patients with CLL and healthy donors against 12 CLL-associated antigens reveals two distinct groupings. (b,c) Analysis of the average patient response against each phosphopeptide between the groups and healthy donors (b) reveals both Group1 (n=12) and Group2 (n=12) have suppressed phosphopeptide-immunity yet responses to mitogens are intact (c). (d,e,f) Kaplan-Meier analysis of overall survival (OS, d), progression free survival (PFS, e) and time to first treatment (TTFT, f) is reduced in Group1 patients, but not statistically significant. **P < 0.01 by Student’s t test comparing average responses for each phosphopeptide between HD and either Group 1 or Group 2.
This lack of phosphopeptide-specific immunity in CLL patients might have been due to an overall depression of T-cell immunity. However, bulk CD8 T-cell responses to low level anti-CD3 were comparable in Group 1 and 2 patients and healthy donors (Fig. 5c). To assess whether the lack of phosphopeptide-specific immunity in Group 1 patients reflected T-cell anergy, we analyzed patient responses to the pNCOA-1 phosphopeptide, which is immunogenic in 9/10 healthy donors, in the presence of IL-2 as this cytokine has been shown to reverse the anergic state(32). Responses to anti-CD3 increased in the presence of IL-2, but no anti-phosphopeptide immunity was detected (Fig. S4). This suggests that pre-existing phosphopeptide immune T-cells in Group 1 patients had been deleted, or had never developed, rather than being anergized.
Although CLL is a less aggressive tumor than either AML or ALL, the disease is more aggressive in a subgroup of patients. Thus, we assessed whether the level of phosphopeptide-specific immunity was associated with patient outcome. Despite selecting patients with early stage disease, there were large differences in progression-free survival, overall survival and time to first treatment between Group 1 and Group 2. Group 2 patients survived longer and required less treatment than Group 1 patients (Figs. 5d-f). However, due to the small study size these differences did not reach statistical significance.
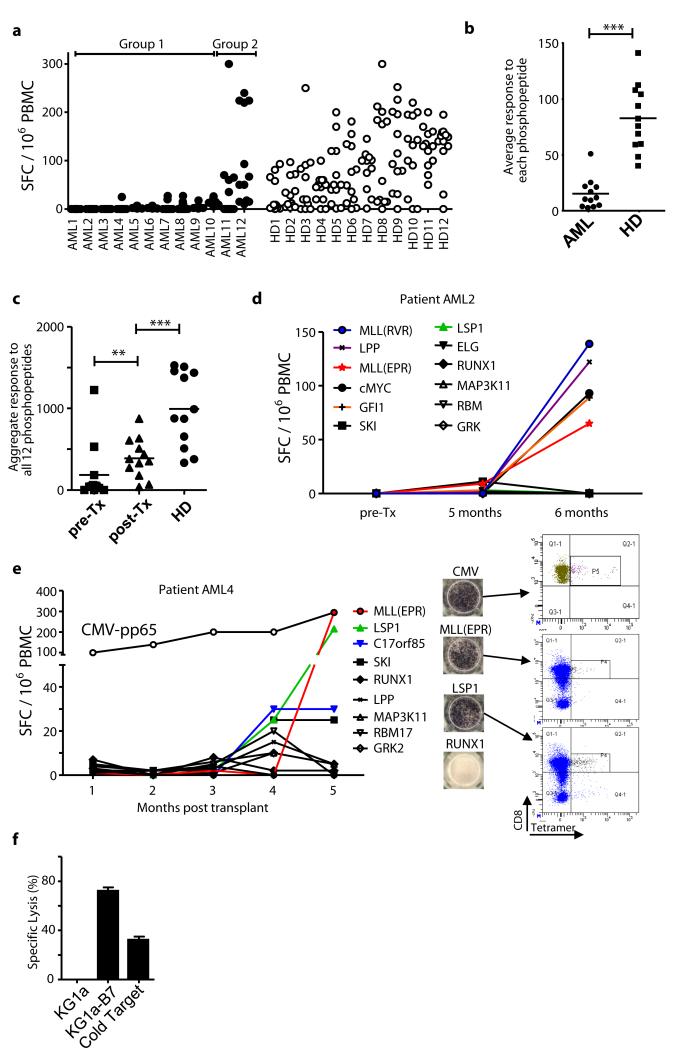
We extended these studies to a cohort of 12 HLA-B7+ AML patients in complete remission (Table S2) using a panel of 12 AML-specific phosphopeptides (green dots in Fig. 1). Responses to most of these phosphopeptides were detected in 12 healthy donors after 7-day in vitro culture (Fig. 6a, Fig. S5). However, as with CLL patients, the average responses of AML patients to individual phosphopeptides were significantly lower (Fig 6b, P<0.0001). AML patients could also be stratified: Group 1 patients (10/12) showed a profound lack of phosphopeptide-specific immunity, while Group 2 (2/12) showed responses that were similar to those of normal donors. Again, responses to low dose anti-CD3 were similar between the groups, indicating that the lack pre-existing anti-phosphopeptide responses in patients is not due to immune incompetence (Fig. S6). In keeping with this, absolute lymphocyte counts for all 12 AML patients were within the normal range pre-transplant (Table S2). Collectively, these findings demonstrate that the majority of patients with either CLL or AML have reduced pre-existing immunity specific for leukemia-associated phosphopeptides.
Figure 6.
Phosphopeptide-specific immunity is lacking in patients with AML and restored following stem cell transplantation. (a) AML-associated phosphopeptide-specific immunity is lacking in AML patients in remission compared against healthy individuals using a panel of 12 phosphopeptide antigens. (b) Analysis of the average patient response against each phosphopeptide (n=12) between the groups and healthy donors reveals patients have suppressed immunity. (c) Analysis of total immunity against all 12 antigens between healthy donors (n=12) and each patient pre- and post-transplant (n=12) reveal the recovery of AML-associated phosphopeptide immunity following SCT. (d,e) Immune reconstitution of donor anti-phosphopeptide immunity in two AML patients demonstrating large expansions of phosphopeptide-specific T-cells. (f) T-cell line specific to MLL(EPR) generated from patient AML4 is able to kill the HLA-B7 transfected KG-1a leukemia cell line. ***P < 0.001, **P<0.01 by Student’s t test.
Restoration of phosphopeptide-specific immunity after stem cell transplantation (SCT)
A graft versus leukemia (GvL) response following allogeneic SCT correlates with a positive clinical outcome in AML patients. While it is believed that GvL is directed to minor histocompatibility antigens (mHAgs), the targets have been only partly identified (32). We hypothesised that SCT might also reconstitute potentially protective immunity against leukemia-associated phosphopeptides in AML patients. All 12 AML patients went on to have allogeneic SCT. Immunity against the 12 AML-specific phosphopeptides was at least partly restored in the majority of patients studied (Fig. 6c, Fig. S5). Marked expansion of immune responses to six different phosphopeptides was observed in patient AML2 (Fig. 6d), while patient AML4 showed dramatic expansion of responses to MLL(EPR) and LSP1(QPR) phosphopeptides, and modestly increased responses to C17orf85 and SKI (Fig 6e). Some of these responses were as large as those against an immunodominant CMV epitope (pp65) and the resulting T-cells bound HLA-phosphopeptide tetramers. Furthermore, in vitro expanded MLL(EPR)-specific T-cells from patient AML4 killed AML cells (Fig. 6f) confirming their functional relevance. These data reveal a linkage between immunity to leukemia-associated phosphopeptides and GvL following allogeneic SCT
Discussion
Posttranslationally modified antigens are increasingly being shown to play important roles in human disease (33-37). Here we have tested the hypothesis that phosphopeptide neoantigens could be identified on primary human malignant tissue and would be immunogenic in healthy donors or patients with cancer. We now report 95 phosphopeptides displayed on the surface of primary hematological malignant tissue in association with two dominant human MHC-I molecules. We observed substantial differences in the number of phosphopeptides presented by different HLA alleles, although the underlying mechanism for these differences is unclear. Our results establish that MHC-I displayed phosphopeptides are over-represented on multiple leukemic malignancies, and that more aggressive malignancies display a greater diversity of them. Many of these tumor-associated phosphopeptides are derived from oncogenes linked to leukemogenesis, making these of particular interest as immunotherapeutic targets.
Unexpectedly, many of these phosphopeptides were the targets of pre-existing immunity, based on high levels of responding CD8 T-cells with a predominantly central memory phenotype. The levels of responding cells were similar to those directed against immunodominant epitopes from some non-persistent viruses. These T-cells, when expanded in vitro, bound to HLA-B7-phosphopeptide tetramers, and recognized and killed primary tumor cells. These observations contrast with those reported for other tumor-associated antigens. Immunity to cancer-testis antigens is generally not present in healthy individuals, and becomes detectable in patients with cancer, but is associated with a poor prognosis (38, 39). Immunity to tissue-associated differentiation antigens, particularly those defined as targets for melanoma-specific T-cells, is also elevated in melanoma patients (40, 41), but is believed to be compromised by self-tolerance (42, 43). Importantly, none of the healthy donors in which these memory-compartment associated immune responses were evident showed any signs of autoimmune disease. This suggests that these phosphopeptides are displayed on normal tissue at levels that are substantially lower than those on tumors. In keeping with this, T-cells specific for LSP1 (RQAV) and NCOA-1 recognize tumors, but not resting B-cells, despite the presence of these epitopes on the latter cells.
Although a small study, these findings suggest that the majority of healthy individuals have been previously exposed to a stimulus that establishes immunological memory to substantial numbers of phosphopeptides. For a small number of these phosphopeptides, immunological memory is prevalent among most individuals examined. The mechanism that underlies the development of phosphopeptide-specific memory CD8 T-cells is of great interest. While it is possible that a distinct, cross-reactive stimulus could be responsible for the development of a generic phosphate-directed immunity, all of the T-cells studied to date are specific for the phosphate, the specific peptide sequence, and the MHC molecule that presents it. On the other hand, hematolymphoid transformation by Epstein-Barr virus (EBV) is ubiquitous in humans, and T cell immunosuppressed individuals commonly develop EBV-related malignancies (44). This points to ongoing immune surveillance directed against virally-transformed, and not just virally infected, cells. While much of this is directed at latent EBV gene products, T-cells directed against EBV transformed cells also recognize EBV negative tumors (45). We hypothesize that phosphopeptides that are shared by different kinds of malignancies are among the targets of these T-cells. In addition, molecular techniques have shown that 69% of healthy individuals have detectable myeloid-transforming transcripts in peripheral blood (46), and 5-12% of healthy individuals show a monoclonal B-cell lymphocytosis, a recognized precursor of CLL (47, 48). Finally, since some of these phosphopeptides are also found on cell lines from solid tumor malignancies (22), immune surveillance against a broad range of early-stage non-hematopoietic cancers may also lead to the development of phosphopeptide-specific immune memory. The extensive variation among individuals in which phosphopeptides are the targets of this pre-existing immunity is consistent with immune surveillance operating on the unique tumors that arise in each person. While it is difficult to prove definitively in humans, we hypothesize that phosphopeptide-specific memory cells are evidence of previous encounters with nascent tumors that have deregulated some of their phosphorylation-based signaling cascades.
Interestingly, the majority of patients with CLL and AML lacked evidence of pre-existing immunity to substantial subsets of phosphopeptides, and the clinical outcome was more favorable in CLL patients where phosphopeptide immunity was present. It is possible that the lack of phosphopeptide-specific immunity reflects a lack of exposure during the immune surveillance phase preceding tumor development. Alternatively, it may reflect the operation of immunosuppressive mechanisms, or the occurrence of clonal exhaustion, in conjunction with tumor outgrowth. Regardless of the exact mechanism, the results point to a role for phosphopeptide-specific immunity as a component of tumor recognition and control.
Although the antigenic targets for the GvL response include mHAgs (49-51), multiple studies have revealed that SCT between identical twins, where mHAg differences are not present, can lead to curative outcomes (52-54). In AML, deficient phosphopeptide-specific immunity could be restored following adoptive transfer of T-cells from a stem cell donor. This indicates that immunity against phosphopeptide tumor antigens may define a component of the GvL response (1, 5). It is known that survival of CMV+ patients can be enhanced by pre-selecting donors for SCT who have pre-existing CMV-specific immunity (55). It is therefore possible SCT outcome may also be improved by matching donors with pre-existing immunity to particular phosphopeptides based on the display of phosphopeptides on leukemia cells.
In this present study we have been unable to demonstrate a direct causal relationship between the lack of cellular immunity against phosphorylated antigens in patients, with disease development and/or progression. Neither have we been able to connect, at the molecular level, deregulated oncogenic signaling present in each primary tumor, to the phosphorylation events present within the defined antigens. Larger, more complex and extensive studies, will be needed to define these relationships.
Collectively, however, our results suggest that the display of MHC-associated phosphopeptides may play a role in preventing either the development or progression of malignant disease. Thus, enhancing immunity to these tumor-associated antigens should become a focus of future cancer immunotherapeutic strategies.
Materials and Methods
Tumor samples and cell lines
Blood or leukapheresis samples were taken from patients with high-burden leukemia in heparin, and tumor cells were isolated on Ficoll density gradients. The purity of the tumor was >98% pure in all cases as assessed by flow cytometry. Healthy T and B-cell populations were isolated from normal spleen and tonsil samples processed by mechanical disruption, followed by density gradient separation and enrichment using anti-CD19 or anti-CD3 microbeads (Miltenyi Biotec) to >98% purity. Bone marrow sample was obtained from an elective orthopedic procedure and red cell depleted using hypotonic lysis. All cell lines were grown at 37°C with 5% CO2 in medium consisting of RPMI-1640 supplemented with 10% Fetal Bovine Serum (FBS) and 2 mM L-glutamine (all from Sigma-Aldrich).
Isolation of HLA-associated peptides
Class-I MHC molecules were immunoaffinity purified from samples, and their associated peptides were extracted, as described(22). Briefly 1.2 to 14 ×109 cells were lysed in 20 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% CHAPS, 1 mM PMSF, 5 μg/mL aprotinin, 10 μg/mL leupeptin, 10 μg/mL pepstatin A, 1 μg/ml calyculin A (Sigma-Aldrich) and phosphatase inhibitor cocktails I and II (Sigma-Aldrich). For primary tumors or the HLA-A2, B7 homozygote B-LCL JY the mixture was centrifuged at 100, 000 × g for 1 hour and the resulting supernatant was passed over protein A Sepharose pre-loaded with the HLA-A2-specific antibody BB7.2 or HLA-B7-specific antibody ME1. For the HLA-A3, B7 homozygous B-LCL GM03107 columns were loaded with the HLA-B, C specific antibody B123.2 to recover HLA-B7 molecules. Peptides were eluted from the purified class-I MHC molecules with 10% acetic acid and separated by ultrafiltration (ULTRAFREE-MC, Millipore).
Sequence analysis of HLA-Associated Phosphopeptides
Immunoaffinity-purified class I peptides were converted to d0- or d3-methyl esters and subjected to Fe+3-immobilized metal-affinity chromatography to isolate phosphopeptides, as described(22). Phosphopeptide methyl esters were then analyzed by a combination of nanoflow HPLC, microelectrospray ionization, and collision activated dissociation on LTQ/FT or Orbitrap tandem mass spectrometers (Thermo Scientific)(22). Electron transfer dissociation (ETD) spectra were acquired on an in-house modified LTQ mass spectrometer(56). Peptide sequences were determined by manual interpretation of CAD and ETD spectra recorded on the above peptide esters. If necessary, phosphopeptide sequences were confirmed by recording tandem mass spectra on the corresponding synthetics.
Epitope Prediction
This was performed using SYFPEITHI and a threshold score of 20.
Peptides
Peptides used in this study were synthesized with Fmoc chemistry, isolated by HPLC to >90% purity, and validated with mass spectrometry (EZ-Biolabs,and Genscript,).
HLA-phosphopeptide Tetramers
HLA tetramers were produced as described (57)
Generation of Human Phosphopeptide-Specific CD8 Cytotoxic T-cell lines
HLA-A2 restricted RQA(pS)IELPSMAV phosphopeptide-specific cytotoxic CD8 T-cells were generated from healthy donors as described previously (58). All cytokines were from PeproTech except where stated. Briefly, PBMCs were cultured in flat-bottom 6-well plates at 107 cells per well in RPMI-1640 plus 10% heat-inactivated human AB serum (Biosera) (10% media). GM-CSF (800 IU/ml) and IL-4 (1000U/mL) were added on day 0 to generate dendritic cells (DC). On day 1, a maturation cocktail containing 100ng/ml TNFα, 100ng/ml IL-1β, 10,000IU/ml IL-6, 8000 IU/ml GM-CSF and 10 μg/ml PGE2 (Sigma-Aldrich) was added. On day 2, DCs were harvested and loaded with phosphopeptide (20μg/ml) for 4 hours in the absence of FBS or human serum. Following three washes, T-cells were added at a ratio of five T-cells per DC in 10% media. Recombinant IL-7 (10ng/ml) and IL-15 (10ng/ml) were added on day 5. On day 9, T-cells were harvested and re-stimulated by adding 107 irradiated PBMCs and 106 irradiated autologous LCLs pulsed with phosphopeptide with IL-7 (5ng/ml), IL-15 (5ng/ml) and IL-2 (20IU/ml). Cultures were re-stimulated every 7 days thereafter in the same manner. At each re-stimulation T-cells were enriched using either anti-CD8 microbeads (Miltenyi Biotec) or by labeling with HLA-phosphopeptide tetramers and using anti-PE microbeads (Miltenyi Biotec).
HLA-B7 restricted anti-phosphopeptide T-cells were grown in the absence of dendritic cells by plating 5x106 PBMCs in 48 well plates in 10% media with individual phosphopeptides at 10μg/ml for 7 days without cytokines. Re-stimulations with irradiated phosphopeptide-pulsed autologous PBMCs took place every 7 days with cytokines added 3 days after each re-stimulation (final concentration 20IU/ml IL-2, 5ng/ml IL-7 and 5ng/ml IL-15). Functional and cytotoxicity assays were then performed from day 13.
T-cell recognition assays
For ELISpot analysis, PBMCs or CD8 T-cells were isolated fresh from in heparinized blood taken from healthy donors and patients. 1×106 PBMCs were isolated from both AML patients and healthy donors and re-suspended in AIM-V media (Invitrogen) with 10% human AB serum (Biosera) in a 96 well plate. For 7-day assays peptide or phosphopeptides were added individually at 10μg/ml and placed at 37°C in CO2 incubator for 7 days. For some experiments (figure 4b) the 7-day culture was restimulated by adding irradiated phosphopeptide-pulsed autologous dendritic cells for a further 7-days. For the ELISpot, cells were then harvested, washed 4 times in AIM-V and incubated for 16 hours with either phosphopeptides (10ug/ml), peptides (10ug/ml), or anti-CD3 (OKT3, 100ng/ml, Mabtech). Cytokine-producing cells were identified as dark spots after a 15 min reaction with 5-bromo-4-chloro-3-indolyl phosphate and NBT by means of an alkaline phosphatase conjugate substrate (Mabtech). Spots were counted using an automated reader (AID-Diagnostika), and results displayed as number of spot-forming cells (SFC) per 105 CD8 T-cells or 106 PBMCs.
For CLL patients a different approach had to be taken as PBMCs contain largely tumor cells. Therefore CD8 T-cells were magnetically enriched using anti-CD8 microbeads (Miltenyi Biotec) to a purity of >99% for both healthy donors and patients with CLL. 200,000 CD8 T-cells from both patients and healthy donors were used for ELISpot analysis as described above.
Cytotoxicity assays
Cytotoxic activity was evaluated in a standard 4 hour 51Cr release assay, as previously described(57).
Statistical Analysis
We analyzed the data using the Kaplan-Meier for the survival rate, the unpaired t-test for two-group comparisons. We performed the statistical analyses using Prism version 5 (GraphPad). P values <0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Dr Peter Nightingale for his valuable input on statistical analysis.
Funding: Supported by NIH grants AI33993 to D.F.H., AI20963 and CA134060 to V.H.E, Kay Kendall Leukaemia Research grant KKL3227 and Leukaemia Lymphoma Research Fund grant 08038 to M.C. and by NIH Cancer Center Support Grant P30 CA45579 to the University of Virginia.
Footnotes
Author contributions: M.C., V.H.E and D.F.H. formulated the hypothesis and designed the experiments; M.C., H.d.l.P., A.N., J.P., J.Q., A.M.E, J.E.T., J.C., J.G.A., S.A.M., H-W.H., S.A.P. and O.C.G. performed all experiments; M.E.W., C.C., G.P. and S.F. provided patient samples and clinical input into the study; A.N., J.P., J.Q., A.M.E., J.C., J.G.A. and J.S. performed and analysed mass spectrometry studies; M.C., H.d.l.P., S.A.P. J.E.T., H-W. H. and O.C.G performed and analysed human in vitro experiments; M.C. and A.L.Z. conducted MHC-peptide extraction.
Ethical approval. The University of Virginia Institutional Review Board approved all protocols. Patients with CLL were recruited from specialist clinics at the University Hospital NHS Trust and Heart of England NHS Trust (Birmingham, United Kingdom). The study received approval from the local ethics committees at South Birmingham, Birmingham East, North, and Solihull, and informed written consent was obtained in accordance with the Declaration of Helsinki in all cases.
Reference list
- 1.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 2.Preudhomme C, Guilhot J, Nicolini FE, Guerci-Bresler A, Rigal-Huguet F, Maloisel F, Coiteux V, Gardembas M, Berthou C, Vekhoff A, Rea D, Jourdan E, Allard C, Delmer A, Rousselot P, Legros L, Berger M, Corm S, Etienne G, Roche-Lestienne C, Eclache V, Mahon FX, Guilhot F. Imatinib plus peginterferon alfa-2a in chronic myeloid leukemia. N.Engl.J.Med. 2010;363:2511–2521. doi: 10.1056/NEJMoa1004095. [DOI] [PubMed] [Google Scholar]
- 3.Talpaz M, Kantarjian HM, McCredie K, Trujillo JM, Keating MJ, Gutterman JU. Hematologic remission and cytogenetic improvement induced by recombinant human interferon alpha A in chronic myelogenous leukemia. N.Engl.J.Med. 1986;314:1065–1069. doi: 10.1056/NEJM198604243141701. [DOI] [PubMed] [Google Scholar]
- 4.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, Rimm AA, Ringden O, Rozman C, Speck B. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 5.Kolb HJ, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, Heim M, Wilmanns W. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–2465. [PubMed] [Google Scholar]
- 6.Baron F, Maris MB, Sandmaier BM, Storer BE, Sorror M, Diaconescu R, Woolfrey AE, Chauncey TR, Flowers ME, Mielcarek M, Maloney DG, Storb R. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J.Clin.Oncol. 2005;23:1993–2003. doi: 10.1200/JCO.2005.08.136. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 8.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N.Engl.J.Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr.Opin.Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat.Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong Waun Ki, Bast Robert C., Jr, Hait William, Kufe Donald W., Pollock Raphael E., Weichselbaum Ralph R., Holland James F., Frei Emil., Iii . Holland-Frei Cancer Medicine. (10 A.D.) McGraw-Hill Medical; [Google Scholar]
- 12.Chi KR. Cancer research: Promise of protection. Nature. 2011;471:537–538. doi: 10.1038/nj7339-537a. [DOI] [PubMed] [Google Scholar]
- 13.Ley TJ, Mardis ER, Ding L, Fulton B, McLellan MD, Chen K, Dooling D, Dunford-Shore BH, McGrath S, Hickenbotham M, Cook L, Abbott R, Larson DE, Koboldt DC, Pohl C, Smith S, Hawkins A, Abbott S, Locke D, Hillier LW, Miner T, Fulton L, Magrini V, Wylie T, Glasscock J, Conyers J, Sander N, Shi X, Osborne JR, Minx P, Gordon D, Chinwalla A, Zhao Y, Ries RE, Payton JE, Westervelt P, Tomasson MH, Watson M, Baty J, Ivanovich J, Heath S, Shannon WD, Nagarajan R, Walter MJ, Link DC, Graubert TA, DiPersio JF, Wilson RK. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature. 2008;456:66–72. doi: 10.1038/nature07485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsons DW, Li M, Zhang X, Jones S, Leary RJ, Lin JC, Boca SM, Carter H, Samayoa J, Bettegowda C, Gallia GL, Jallo GI, Binder ZA, Nikolsky Y, Hartigan J, Smith DR, Gerhard DS, Fults DW, Vandenberg S, Berger MS, Marie SK, Shinjo SM, Clara C, Phillips PC, Minturn JE, Biegel JA, Judkins AR, Resnick AC, Storm PB, Curran T, He Y, Rasheed BA, Friedman HS, Keir ST, McLendon R, Northcott PA, Taylor MD, Burger PC, Riggins GJ, Karchin R, Parmigiani G, Bigner DD, Yan H, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE. The Genetic Landscape of the Childhood Cancer Medulloblastoma. Science. 2011;331:435–439. doi: 10.1126/science.1198056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van DR, Zoutman WH, Dijkman R, de Menezes RX, Commandeur S, Mulder AA, van der Velden PA, Vermeer MH, Willemze R, Yan PS, Huang TH, Tensen CP. Epigenetic profiling of cutaneous T-cell lymphoma: promoter hypermethylation of multiple tumor suppressor genes including BCL7a, PTPRG, and p73. J.Clin.Oncol. 2005;23:3886–3896. doi: 10.1200/JCO.2005.11.353. [DOI] [PubMed] [Google Scholar]
- 17.Tiacci E, Trifonov V, Schiavoni G, Holmes A, Kern W, Martelli MP, Pucciarini A, Bigerna B, Pacini R, Wells VA, Sportoletti P, Pettirossi V, Mannucci R, Elliott O, Liso A, Ambrosetti A, Pulsoni A, Forconi F, Trentin L, Semenzato G, Inghirami G, Capponi M, Di RF, Patti C, Arcaini L, Musto P, Pileri S, Haferlach C, Schnittger S, Pizzolo G, Foa R, Farinelli L, Haferlach T, Pasqualucci L, Rabadan R, Falini B. BRAF mutations in hairy-cell leukemia. N.Engl.J.Med. 2011;364:2305–2315. doi: 10.1056/NEJMoa1014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Druker BJ, Guilhot F, O’brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM, Cervantes F, Hochhaus A, Powell BL, Gabrilove JL, Rousselot P, Reiffers J, Cornelissen JJ, Hughes T, Agis H, Fischer T, Verhoef G, Shepherd J, Saglio G, Gratwohl A, Nielsen JL, Radich JP, Simonsson B, Taylor K, Baccarani M, So C, Letvak L, Larson RA. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N.Engl.J.Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 19.Harrison C. Trial watch: BTK inhibitor shows positive results in B cell malignancies. Nat.Rev.Drug Discov. 2012;11:96. doi: 10.1038/nrd3656. [DOI] [PubMed] [Google Scholar]
- 20.Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM, Silverman MH, Gilliland DG, Shorr J, Tefferi A. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J.Clin.Oncol. 2011;29:789–796. doi: 10.1200/JCO.2010.32.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Zarling AL, Polefrone JM, Evans AM, Mikesh LM, Shabanowitz J, Lewis ST, Engelhard VH, Hunt DF. Identification of class I MHC-associated phosphopeptides as targets for cancer immunotherapy. Proc.Natl.Acad.Sci.U.S.A. 2006;103:14889–14894. doi: 10.1073/pnas.0604045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zarling AL, Ficarro SB, White FM, Shabanowitz J, Hunt DF, Engelhard VH. Phosphorylated peptides are naturally processed and presented by major histocompatibility complex class I molecules in vivo. J.Exp.Med. 2000;192:1755–1762. doi: 10.1084/jem.192.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Depontieu FR, Qian J, Zarling AL, McMiller TL, Salay TM, Norris A, English AM, Shabanowitz J, Engelhard VH, Hunt DF, Topalian SL. Identification of tumor-associated, MHC class II-restricted phosphopeptides as targets for immunotherapy. Proc.Natl.Acad.Sci.U.S.A. 2009;106:12073–12078. doi: 10.1073/pnas.0903852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hogan KT, Eisinger DP, Cupp SB, III, Lekstrom KJ, Deacon DD, Shabanowitz J, Hunt DF, Engelhard VH, Slingluff CL, Jr., Ross MM. The peptide recognized by HLA-A68.2-restricted, squamous cell carcinoma of the lung-specific cytotoxic T lymphocytes is derived from a mutated elongation factor 2 gene. Cancer Res. 1998;58:5144–5150. [PubMed] [Google Scholar]
- 26.Meyer VS, Drews O, Gunder M, Hennenlotter J, Rammensee HG, Stevanovic S. Identification of natural MHC class II presented phosphopeptides and tumor-derived MHC class I phospholigands. J.Proteome.Res. 2009;8:3666–3674. doi: 10.1021/pr800937k. [DOI] [PubMed] [Google Scholar]
- 27.Mohammed F, Cobbold M, Zarling AL, Salim M, Barrett-Wilt GA, Shabanowitz J, Hunt DF, Engelhard VH, Willcox BE. Phosphorylation-dependent interaction between antigenic peptides and MHC class I: a molecular basis for the presentation of transformed self. Nat.Immunol. 2008;9:1236–1243. doi: 10.1038/ni.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beaver JE, Bourne PE, Ponomarenko JV. EpitopeViewer: a Java application for the visualization and analysis of immune epitopes in the Immune Epitope Database and Analysis Resource (IEDB) Immunome.Res. 2007;3:3. doi: 10.1186/1745-7580-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hornbeck PV, Chabra I, Kornhauser JM, Skrzypek E, Zhang B. PhosphoSite: A bioinformatics resource dedicated to physiological protein phosphorylation. Proteomics. 2004;4:1551–1561. doi: 10.1002/pmic.200300772. [DOI] [PubMed] [Google Scholar]
- 30.Marafioti T, Mancini C, Ascani S, Sabattini E, Zinzani PL, Pozzobon M, Pulford K, Falini B, Jaffe ES, Muller-Hermelink HK, Mason DY, Pileri SA. Leukocyte-specific phosphoprotein-1 and PU.1: two useful markers for distinguishing T-cell-rich B-cell lymphoma from lymphocyte-predominant Hodgkin’s disease. Haematologica. 2004;89:957–964. [PubMed] [Google Scholar]
- 31.Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA. Phenotypic and functional separation of memory and effector human CD8+ T cells. J.Exp.Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz RH. T cell anergy. Annu.Rev.Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 33.Girbal-Neuhauser E, Durieux JJ, Arnaud M, Dalbon P, Sebbag M, Vincent C, Simon M, Senshu T, Masson-Bessiere C, Jolivet-Reynaud C, Jolivet M, Serre G. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J.Immunol. 1999;162:585–594. [PubMed] [Google Scholar]
- 34.Arentz-Hansen H, Korner R, Molberg O, Quarsten H, Vader W, Kooy YM, Lundin KE, Koning F, Roepstorff P, Sollid LM, McAdam SN. The intestinal T cell response to alpha-gliadin in adult celiac disease is focused on a single deamidated glutamine targeted by tissue transglutaminase. J.Exp.Med. 2000;191:603–612. doi: 10.1084/jem.191.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wuttge DM, Bruzelius M, Stemme S. T-cell recognition of lipid peroxidation products breaks tolerance to self proteins. Immunology. 1999;98:273–279. doi: 10.1046/j.1365-2567.1999.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Utz PJ, Hottelet M, Schur PH, Anderson P. Proteins phosphorylated during stress-induced apoptosis are common targets for autoantibody production in patients with systemic lupus erythematosus. J.Exp.Med. 1997;185:843–854. doi: 10.1084/jem.185.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mamula MJ, Gee RJ, Elliott JI, Sette A, Southwood S, Jones PJ, Blier PR. Isoaspartyl post-translational modification triggers autoimmune responses to self-proteins. J.Biol.Chem. 1999;274:22321–22327. doi: 10.1074/jbc.274.32.22321. [DOI] [PubMed] [Google Scholar]
- 38.Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol.Rev. 2002;188:22–32. doi: 10.1034/j.1600-065x.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 39.Okada T, Akada M, Fujita T, Iwata T, Goto Y, Kido K, Okada T, Matsuzaki Y, Kobayashi K, Matsuno S, Sunamura M, Kawakami Y. A novel cancer testis antigen that is frequently expressed in pancreatic, lung, and endometrial cancers. Clin.Cancer Res. 2006;12:191–197. doi: 10.1158/1078-0432.CCR-05-1206. [DOI] [PubMed] [Google Scholar]
- 40.Rosenberg SA, White DE. Vitiligo in patients with melanoma: normal tissue antigens can be targets for cancer immunotherapy. J.Immunother.Emphasis.Tumor Immunol. 1996;19:81–84. [PubMed] [Google Scholar]
- 41.Dunbar PR, Chen JL, Chao D, Rust N, Teisserenc H, Ogg GS, Romero P, Weynants P, Cerundolo V. Cutting edge: rapid cloning of tumor-specific CTL suitable for adoptive immunotherapy of melanoma. J.Immunol. 1999;162:6959–6962. [PubMed] [Google Scholar]
- 42.Touloukian CE, Leitner WW, Schnur RE, Robbins PF, Li Y, Southwood S, Sette A, Rosenberg SA, Restifo NP. Normal tissue depresses while tumor tissue enhances human T cell responses in vivo to a novel self/tumor melanoma antigen, OA1. J.Immunol. 2003;170:1579–1585. doi: 10.4049/jimmunol.170.3.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colella TA, Bullock TN, Russell LB, Mullins DW, Overwijk WW, Luckey CJ, Pierce RA, Restifo NP, Engelhard VH. Self-tolerance to the murine homologue of a tyrosinase-derived melanoma antigen: implications for tumor immunotherapy. J.Exp.Med. 2000;191:1221–1232. doi: 10.1084/jem.191.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gottschalk S, Rooney CM, Heslop HE. Post-transplant lymphoproliferative disorders. Annu.Rev.Med. 2005;56:29–44. doi: 10.1146/annurev.med.56.082103.104727. [DOI] [PubMed] [Google Scholar]
- 45.Long HM, Zuo J, Leese AM, Gudgeon NH, Jia H, Taylor GS, Rickinson AB. CD4+ T-cell clones recognizing human lymphoma-associated antigens: generation by in vitro stimulation with autologous Epstein-Barr virus-transformed B cells. Blood. 2009;114:807–815. doi: 10.1182/blood-2008-12-194043. [DOI] [PubMed] [Google Scholar]
- 46.Bose S, Deininger M, Gora-Tybor J, Goldman JM, Melo JV. The presence of typical and atypical BCR-ABL fusion genes in leukocytes of normal individuals: biologic significance and implications for the assessment of minimal residual disease. Blood. 1998;92:3362–3367. [PubMed] [Google Scholar]
- 47.Nieto WG, Almeida J, Romero A, Teodosio C, Lopez A, Henriques AF, Sanchez ML, Jara-Acevedo M, Rasillo A, Gonzalez M, Fernandez-Navarro P, Vega T, Orfao A. Increased frequency (12%) of circulating chronic lymphocytic leukemia-like B-cell clones in healthy subjects using a highly sensitive multicolor flow cytometry approach. Blood. 2009;114:33–37. doi: 10.1182/blood-2009-01-197368. [DOI] [PubMed] [Google Scholar]
- 48.Rawstron AC, Bennett FL, O’Connor SJ, Kwok M, Fenton JA, Plummer M, de TR, Owen RG, Richards SJ, Jack AS, Monoclonal P. Hillmen. B-cell lymphocytosis and chronic lymphocytic leukemia. N.Engl.J.Med. 2008;359:575–583. doi: 10.1056/NEJMoa075290. [DOI] [PubMed] [Google Scholar]
- 49.den Haan JM, Meadows LM, Wang W, Pool J, Blokland E, Bishop TL, Reinhardus C, Shabanowitz J, Offringa R, Hunt DF, Engelhard VH, Goulmy E. The minor histocompatibility antigen HA-1: a diallelic gene with a single amino acid polymorphism. Science. 1998;279:1054–1057. doi: 10.1126/science.279.5353.1054. [DOI] [PubMed] [Google Scholar]
- 50.Bonnet D, Warren EH, Greenberg PD, Dick JE, Riddell SR. CD8(+) minor histocompatibility antigen-specific cytotoxic T lymphocyte clones eliminate human acute myeloid leukemia stem cells. Proc.Natl.Acad.Sci.U.S.A. 1999;96:8639–8644. doi: 10.1073/pnas.96.15.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brickner AG, Warren EH, Caldwell JA, Akatsuka Y, Golovina TN, L A, Shabanowitz Zarling, J., C L, D Eisenlohr, V F. Hunt, Engelhard H, Riddell SR. The immunogenicity of a new human minor histocompatibility antigen results from differential antigen processing. J.Exp.Med. 2001;193:195–206. doi: 10.1084/jem.193.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pavletic SZ, Zhou G, Sobocinski K, Marti G, Doney K, DiPersio J, Feremans W, Foroni L, Goodman S, Prentice G, LeMaistre C, Bandini G, Ferrant A, Jacobsen N, Khouri I, Gale RP, Wiestner A, Giralt S, Montserrat E, Chan WC, Bredeson C. Genetically identical twin transplantation for chronic lymphocytic leukemia. Leukemia. 2007;21:2452–2455. doi: 10.1038/sj.leu.2404928. [DOI] [PubMed] [Google Scholar]
- 53.Gale RP, Horowitz MM, Ash RC, Champlin RE, Goldman JM, Rimm AA, Ringden O, Stone JA, Bortin MM. Identical-twin bone marrow transplants for leukemia. Ann.Intern.Med. 1994;120:646–652. doi: 10.7326/0003-4819-120-8-199404150-00004. [DOI] [PubMed] [Google Scholar]
- 54.Kroger N, Brand R, van BA, Bron D, Blaise D, Hellstrom-Lindberg E, Gahrton G, Powles R, Littlewood T, Chapuis B, Zander A, Koza V, Niederwieser D, de WT. Stem cell transplantation from identical twins in patients with myelodysplastic syndromes. Bone Marrow Transplant. 2005;35:37–43. doi: 10.1038/sj.bmt.1704701. [DOI] [PubMed] [Google Scholar]
- 55.Ozdemir E, Saliba RM, Champlin RE, Couriel DR, Giralt SA, de LM, Khouri IF, Hosing C, Kornblau SM, Anderlini P, Shpall EJ, Qazilbash MH, Molldrem JJ, Chemaly RF, Komanduri KV. Risk factors associated with late cytomegalovirus reactivation after allogeneic stem cell transplantation for hematological malignancies. Bone Marrow Transplant. 2007;40:125–136. doi: 10.1038/sj.bmt.1705699. [DOI] [PubMed] [Google Scholar]
- 56.Syka JE, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc.Natl.Acad.Sci.U.S.A. 2004;101:9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cobbold M, Khan N, Pourgheysari B, Tauro S, McDonald D, Osman H, Assenmacher M, Billingham L, Steward C, Crawley C, Olavarria E, Goldman J, Chakraverty R, Mahendra P, Craddock C, Moss PA. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J.Exp.Med. 2005;202:379–386. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ho WY, Nguyen HN, Wolfl M, Kuball J, Greenberg PD. In vitro methods for generating CD8+ T-cell clones for immunotherapy from the naive repertoire. J.Immunol.Methods. 2006;310:40–52. doi: 10.1016/j.jim.2005.11.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.