Abstract
Background
The role of serotonin-1B receptors (5-HT1BRs) in modulating cocaine abuse-related behaviors has been controversial due to discrepancies between pharmacological and gene knockout approaches, and opposite influences on cocaine selfadministration versus cocaine-seeking behavior. We hypothesized that modulation of these behaviors via 5-HT1BRs in the mesolimbic pathway may vary depending on the stage of the addiction cycle.
Methods
To test this hypothesis, we examined the effects of increasing 5-HT1BR production by microinfusing a viral vector expressing either green fluorescent protein (GFP) and 5-HT1BR or GFP alone into the medial nucleus accumbens shell of rats either during maintenance of cocaine self-administration (i.e. active drug use) or during protracted withdrawal.
Results
5-HT1BR-gene transfer during maintenance shifted the dose–response curve for cocaine self-administration upward and to the left and increased break points and cocaine intake on a progressive ratio (PR) schedule, consistent with enhanced reinforcing effects of cocaine. In contrast, following 21 days of forced abstinence 5-HT1BR-gene transfer attenuated break points and cocaine intake on a PR schedule of reinforcement, as well as cue- and cocaine-primed reinstatement of cocaineseeking behavior.
Conclusions
This unique pattern of effects suggests that mesolimbic 5-HT1BRs differentially modulate cocaine abuse-related behaviors, with a facilitative influence during periods of active drug use in striking contrast to an inhibitory influence during protracted withdrawal. These findings suggest that targeting 5-HT1BRs may lead to a novel treatment for cocaine dependence and that the therapeutic efficacy of these treatments may vary depending on the stage of the addiction cycle.
Keywords: reward, addiction, reinforcement, relapse, craving, reinstatement
Introduction
Polymorphisms of serotonin-1B receptors (5-HT1BRs) have been linked to substance abuse (1–5), yet the role of these receptors in cocaine abuse-related behaviors is unclear due to inconsistencies across studies examining their involvement in the rewarding, reinforcing and incentive motivational effects of psychostimulants. For instance, studies examining cocaine self-administration in 5-HT1BR knockout mice (6, 7) or amphetamine self-administration in rats (8, 9) suggest that 5-HT1BRs inhibit psychostimulant reinforcement. However, self-administration studies in rats using fixed (FR) and progressive (PR) ratio schedules suggest that 5-HT1BRs enhance cocaine reinforcement (10–12). Furthermore, 5-HT1BR knockout mice fail to exhibit cocaine-conditioned place preference (CPP; 13), whereas 5-HT1BR agonists alone produce conditioned place aversion in rats yet enhance cocaine-CPP (14), effects that depend on the timing of cocaine-context pairings (15). 5-HT1BR agonists also elevate intracranial self-stimulation (ICSS) thresholds and prevent cocaine-induced decreases in ICSS thresholds, suggesting blunted reward mechanisms (16). Furthermore, these agonists attenuate cue- and cocaine-primed reinstatement of extinguished cocaine-seeking behavior, suggesting decreased incentive motivation for cocaine (12, 17). These discrepancies may be due to differences in 5-HT1BR manipulations or the timing of their use.
5-HT1BRs are synthesized in medium spiny neurons throughout the striatum and are transported to axon terminals in the ventral tegmental area (VTA), ventral pallidum and substantia nigra (18–20) where they exert inhibitory control over neuronal activity (21, 22). Stimulation of VTA 5-HT1BRs potentiates cocaine effects via inhibiting local GABA release from neurons that inhibit dopaminergic neurons projecting to the nucleus accumbens shell (NAcsh; 23, 24). Increased expression of 5-HT1BRs on terminals of GABAergic neurons projecting from the NAcsh to the VTA shifts the cocaine-CPP dose-response curve to the left (15, 25), suggesting that this 5-HT1BR population modulates cocaine reward.
The present study aimed to further elucidate the role of 5-HT1BRs in cocaine abuserelated behaviors utilizing viral-mediated gene transfer (VMGT) to transiently increase 5-HT1BRs in terminals of medial NAcsh neurons. Following VMGT, rats were tested for cocaine intake on a FR5 schedule of reinforcement across a range of cocaine doses during maintenance (i.e. active drug use), and for break points and cocaine intake on a PR schedule during both maintenance and protracted withdrawal. Additional experiments examined the effects of VMGT on cue- and cocaine-primed reinstatement of cocaine-seeking behavior during protracted withdrawal. Anxiety-like behavior in the elevated plus-maze (EPM) was also measured as anxiogenic effects of 5-HT1BR agonists may contribute to their effects on drug-seeking behavior (12, 26).
Methods and Materials
For detailed methodology see the Supplement.
Subjects
Male Sprague–Dawley rats weighing 268–308 g at the time of surgery were individually housed in a climate-controlled colony room with a 12-h reversed light/dark cycle (lights off at 6 AM).
Surgery
Surgical procedures were performed as described previously (27). Briefly, rats (n=74) were anesthetized using isoflurane and catheters were implanted into their jugular veins. Next, the rats were placed into a stereotaxic alignment system (Kopf Instruments, Tujunga, California) and guide cannulae (26G, Plastics One, Roanoke, VA) were implanted bilaterally into the NAcsh using coordinates obtained from the Rat Brain Atlas (28): +1.60 mm anterior to bregma, +/−1.1 mm from the midline and −6.6 mm ventral from the surface of the skull; coordinates were based on previous research (15). Following surgery, rats were returned to their home cages for 7–10 days recovery prior to starting self-administration training.
Drugs/viral vectors
Cocaine hydrochloride (RTI International, Research Triangle Park, NC) was dissolved in sterile saline. The herpes simplex virus (HSV) based vector system utilized in the present study (see Figure 1B) has been reviewed previously (25, 29, 30). Briefly, 5-HT1BR-green fluorescent protein (GFP) expresses both hemagglutinin-tagged 5-HT1BR and GFP from separate transcriptional cassettes, whereas GFP-only expresses only the GFP transcript. The GFP-only control vector does not alter drug reward or behavior compared with sham or vehicle microinfusions (25, 29, 31). This 5-HT1BR-GFP vector produces a 3-fold increase in 5-HT1BR mRNA in neuronal (30), but not glial (15) cells, and hemagglutinin epitope tagging does not alter the function of 5-HT1BRs (30).
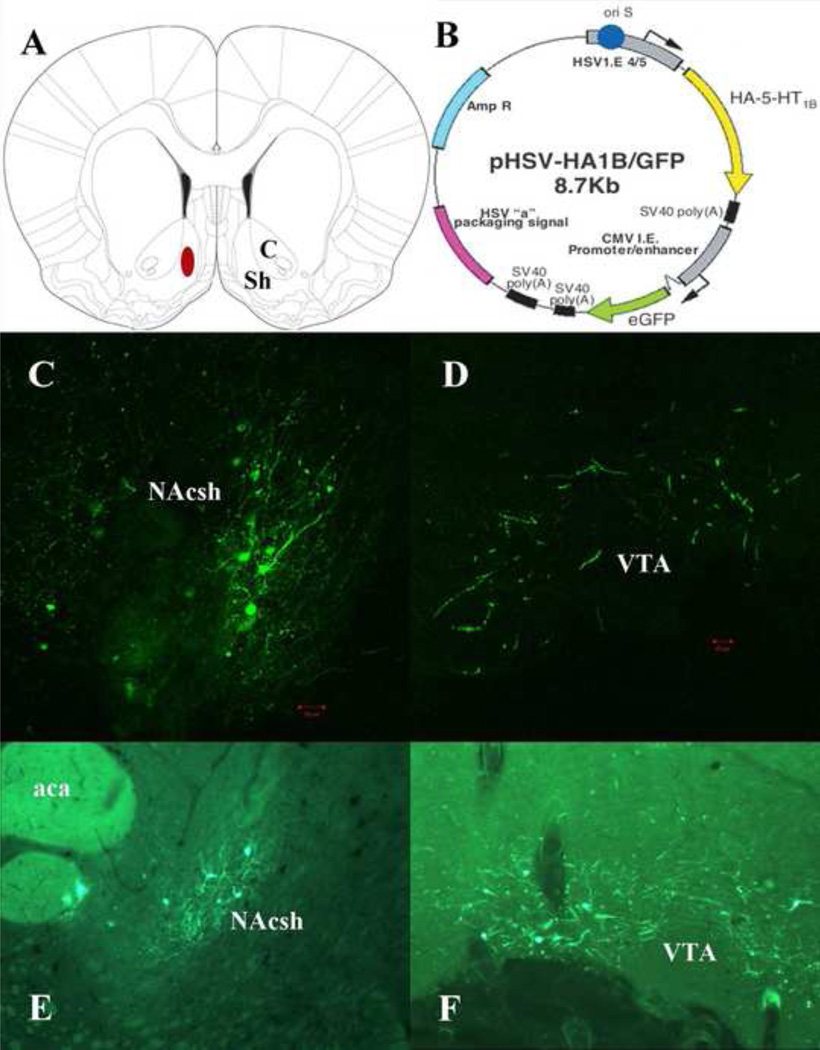
Figure 1.
Illustration of the anatomical target within the medial NAcsh for viral microinfusions (A) (reproduced with permission from (28)), schematic drawing of the HSV-hemagglutinin-tagged 5-HT1BR-GFP transgene amplicon (B), and representative photomicrographs depicting viral-mediated gene transfer evident as GFP expression in sections from the NAcsh (C and E) and VTA (D and F) 5 days after viral microinfusions of the 5-HT1BR-GFP transgene. Viral-mediated gene transfer into the medial NAcsh induced local GFP expression in cell bodies and transport of transgenes from the NAcsh to axon terminals of median spiny neurons in the VTA. Scale bar, 50 µm. Abbreviations: C (nucleus accumbens core); Sh (nucleus accumbens shell); aca (anterior commissure).
Self-administration training
Rats were trained to self-administer cocaine (0.75 mg/kg/0.1 ml, i.v.) progressing from a FR1 to a FR5 schedule of reinforcement 6 days/week during 2-h sessions. Schedule completions on the active lever resulted in the simultaneous activation of a cue light, house light, and tone generator followed 1 s later by a 6-s cocaine infusion; following each infusion the house light remained activated signaling a 20-s timeout period. Inactive lever presses were recorded but produced no consequences. Once selfadministration infusion rates stabilized, defined as less than 10% variability/session across 3 consecutive days with no upward or downward trends (16–22 sessions), rats included in the cocaine dose-response experiments were given 30 min access to varying cocaine doses presented in ascending order (0.0, 0.032, 0.1, 0.32 and 1.0 mg/kg/0.1 ml, i.v.) on a FR5 schedule with a 10-min timeout period between doses (32). Within session dose-response training occurred every 2 days alternating with access to the training dose of cocaine for 2-h sessions. For the reinstatement and PR experiments during protracted withdrawal, once infusion rates stabilized on the training dose of cocaine (0.75 mg/kg/0.1 ml, i.v.; 18 sessions), rats began extinction training (17 sessions across 21 days of abstinence) or were placed into forced abstinence (21 days), respectively.
Effects of VMGT on cocaine intake during maintenance
Testing procedures began once within-session cocaine dose-effect functions stabilized to less than 10% variability across 3 consecutive sessions. Rats were assigned to groups counterbalanced for previous total cocaine intake and received bilateral microinfusions (2.0 µl/side over 10 min) containing approximately 200,000 infective units of either 5-HT1BR-GFP (n=10) or GFP-only (n=9) into the medial NAcsh under light isoflurane (2%) anesthesia. To allow for maximal viral expression, rats were given access to their training dose of cocaine for the next 3 days and were then tested on the within-session cocaine dose-effect function on day 4 post-infection; a subset of rats were tested on a PR schedule on day 5. Testing occurred on day 4 and 5 post infection as this time course corresponds with peak viral-mediated 5-HT1BR expression for the vector utilized in this study (15, 25). Break points were defined as the highest ratio attained once rats failed to receive a cocaine infusion during a 1-h period on a schedule that progressed exponentially from an FR1 according to the formula 5*e^(0.2n)−5, with n reflecting the number of reinforcers the rat received during the session (33).
Extinction training
For the reinstatement experiments, extinction training began the day after selfadministration ended, which consisted of daily 1-h (6 days/wk) exposures to the selfadministration environment. During extinction active and inactive lever responses were recorded but produced no consequences. Responding on the active lever in the absence of cocaine reinforcement is the operational definition of cocaine-seeking behavior. Extinction training continued until response rates on the active lever declined to less than 20% of the highest rate observed during extinction (17 sessions).
Effects of VMGT on cue- and cocaine-primed reinstatement of extinguished cocaine-seeking behavior
Following 14 days of extinction, rats received microinfusions of either 5-HT1BR-GFP (n=15) or GFP-only (n=14) as detailed above. To allow for maximal 5-HT1BR expression prior to testing, rats continued extinction training for the next 3 days and were then tested for cue- and cocaine-primed reinstatement on day 4 post-infection. For cueelicited reinstatement, rats received response-contingent cue presentations on a FR 1 schedule during a 1-h test session. Immediately following the cue test phase, rats received an i.p. saline injection and were returned to the operant chambers for a 1-h extinction session. Lever responses during this test phase produced no consequences and served as the baseline for the cocaine-primed reinstatement test. Immediately after the saline-primed extinction phase, rats received a cocaine-priming injection (10 mg/kg, i.p.) and were returned to the operant chambers for a 1-h test during which responses produced no consequences.
Effects of VMGT on cue-elicited cocaine-seeking behavior and cocaine intake during protracted withdrawal
For these experiments, a period of forced abstinence (21 days) began the day after the last self-administration session. On the 17th day of abstinence, rats received microinfusions of either 5-HT1BR-GFP (n=11) or GFP-only (n=9) as detailed above. Tests for cue-elicited cocaine seeking and responding under a PR schedule of reinforcement were conducted on day 4 and 5 post-infection, respectively. Parameters for both tests were identical to those described above, except that testing commenced following 21 days of forced abstinence.
Effects of VMGT on anxiety-like behavior in the EPM
Testing for anxiety-like behavior occurred on day 5 post-infection as described previously (34). Rats were individually placed in the center of the EPM and their exploratory behavior was measured for 5 min.
Histology
After testing, rats were anesthetized and perfused transcardially with 0.9% saline followed by 4% paraformaldehyde. Brains were then harvested, post-fixed and cryoprotected. A series of coronal sections (40µm) were taken throughout the NAc and VTA and were analyzed using dual laser confocal microscopy (Zeiss) in order to verify cannulae tip placement and GFP expression. Rats with misplaced cannulae or that lacked GFP expression in both the NAcsh and VTA were excluded from the analyses.
Statistical analyses
Infusion and response rates during self-administration and extinction training were analyzed using independent sample t-tests. For the within session cocaine dose-response experiment, baseline responding was defined as the average response rates during the three within sessions prior to VMGT. The total number of infusions at each dose of cocaine were analyzed using a mixed-factor ANOVA with viral condition as the between subjects factor and cocaine dose as the repeated-measure. For PR testing, and cue testing following protracted withdrawal, the total number of infusions, lever responses and the highest ratios achieved were analyzed using independent sample t-tests. Lever response rates during cue- and cocaine-primed reinstatement tests were analyzed using mixedfactor ANOVAs with viral condition as the between subjects factor and test session (extinction baseline and cue- or cocaine-primed test session) as the repeated-measure. Percent open-arm duration, the number of open-arm entries and total locomotor activity in the EPM were analyzed using independent sample t-tests. Significant ANOVAs were followed by post-hoc Newman-Keuls tests; α was set at 0.05.
Results
Histology and attrition
The histological boundaries of injector tip placements for rats included in the analyses are shown in Figure 1A and representative photomicrographs within the NAcsh and VTA are shown in Figure 1C, E and 1D, F, respectively. GFP expression was visible in neurons located within the NAcsh (Figure 1C and E) and VTA (Figure 1D and F), consistent with previous reports utilizing this HSV packaging system (15, 25). Virtually all cell bodies expressing transgenic GFP were located within the NAcsh, although we occasionally observed limited GFP expression in VTA cell bodies, suggesting that a few cells were infected retrogradely (0–2 cells per VTA section; see Figure 1F). GFP was extensively localized in beaded axon terminals within the VTA (Figure 1D, F), suggesting viral uptake into medial NAcsh medium spiny projection neurons and translocation of the receptors from the somata of these neurons to their terminals, including the VTA. See Supplement for detailed attrition rates.
Effects of VMGT on cocaine intake during maintenance
Prior to VMGT, rats readily acquired cocaine self-administration. There were no group differences in rates of acquisition (data not shown), the total number of cocaine infusions throughout training or across the last five days of training (Table 1), or the number of active lever presses on the last day of self-administration training (Table 1). Depending on individual performance, rats received 36–60 sessions, including those that occurred during the training and testing phases.
Table 1.
Cocaine reinforcers and response rates (mean +SEM) during self-administration (SA) training and extinction.
| Viral condition/experiment | Infusions/session last 5 training days |
Total infusions | Active lever presses |
|
|---|---|---|---|---|
| Last SA day | First extinction day | |||
| Reinforcement: | ||||
| 5-HT1B–GFP (n= 9) | 26.36 ± 2.58 | 678.67 ± 58.31 | 83.17 ± 14.50 | N/A |
| GFP-only (n=8) | 28.05 ± 4.22 | 719.25 ± 63.63 | 73.31 ± 14.91 | N/A |
| Reinstatement: | ||||
| 5-HT1B–GFP (n=15) | 25.56 ± 0.95 | 424.43 ± 18.63 | 72.10 ± 3.98 | 100.33 ± 12.29 |
| GFP-only (n=14) | 27.49 ± 1.51 | 453.85 ± 26.46 | 88.39 ± 17.28 | 122.54 ± 18.43 |
| Relapse | ||||
| 5-HT1B–GFP (n=9) | 22.40 ± 1.05 | 306.63 ± 23.31 | 58.19 ± 1.61 | N/A |
| GFP-only (n=7) | 22.67 ± 0.92 | 315.83 ± 24.55 | 63.14 ± 3.70 | N/A |
Each i.v. cocaine infusion contained 0.75 mg/kg/0.1 ml.
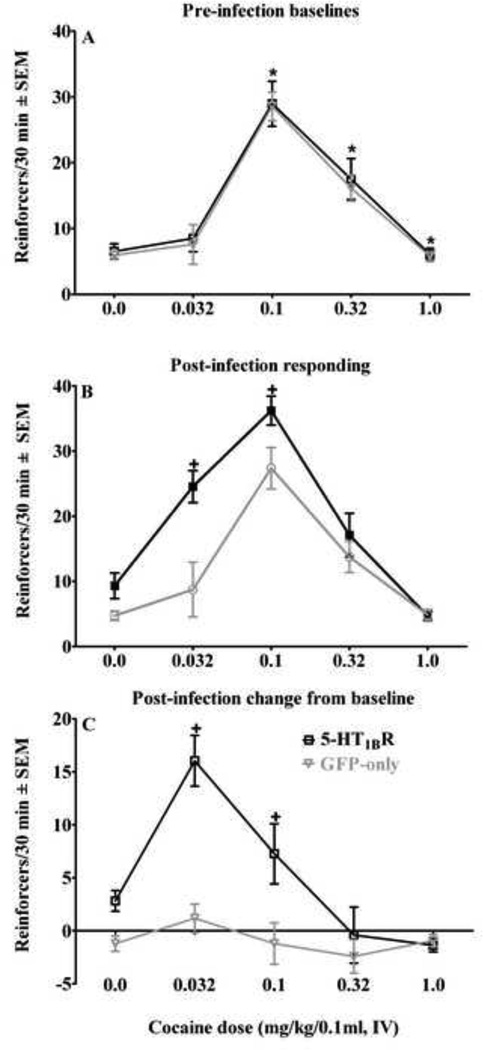
Figure 2 illustrates the effects of VMGT into the medial NAcsh on the cocaine selfadministration dose-effect function. Prior to VMGT, varying the unit doses of cocaine produced a characteristic inverted U-shaped dose-effect function (Figure 2A), with the ANOVA indicating a significant quadratic trend and a main effect of cocaine dose [F(4, 64)=59.49, p<0.0001] but no viral group by cocaine dose interaction; there were no differences between 5-HT1BR-GFP and GFP-only groups at any cocaine dose prior to VMGT. Post-hoc Newman-Keuls analysis revealed that on the ascending limb of the cocaine dose-response curve, increasing the unit dose of cocaine reliably increased intake at each dose relative to the previous dose of cocaine, while on the descending limb, intake decreased at each subsequent cocaine dose (p<0.05 in each case); selfadministration was not reliably maintained when saline was substituted for cocaine.
Figure 2.
Effects of viral-mediated 5-HT1BR-gene transfer into the medial NAcsh on the cocaine self-administration dose-effect functions expressed as the mean number of reinforcers obtained/30 min ± SEM on a FR5 schedule of reinforcement during baseline training (A), post-infection testing (B) and as a difference from baseline (C). Prior to viral-mediated gene transfer, rats in both the 5-HT1BR-GFP (black squares) and GFP-only (gray triangles) viral treated groups (n=8–9/group) exhibited characteristic inverted U-shaped cocaine dose-response functions, whereas post-infection the dose-effect function was shifted upward and to the left in 5-HT1BR-GFP treated rats. Asterisk (*) represents a within-group change in cocaine intake compared to the previous cocaine dose (Newman-Keuls, p<0.05); there were no differences between 5-HT1BR-GFP and GFP-only groups at any cocaine dose during baseline sessions. Plus sign (+) represents an elevation compared to GFP-only controls (Newman-Keuls, p<0.05 in each case).
Following VMGT (Figure 2B), there was a main effect of viral treatment [F(4, 60)=46.94, p<0.0001] and a cocaine dose by viral treatment interaction [F(4, 60)=3.90, p<0.001]. 5-HT1BR-GFP microinfusions shifted the cocaine dose-effect function upward and to the left, with 5-HT1BR-GFP treated rats taking more infusions of the 0.032 and 0.1 mg/kg cocaine doses compared to both their pre-infection baselines and GFP-only controls (p<0.05 in each case); there were no changes in the number of reinforcers at any cocaine dose in GFP-only treated controls compared to baseline following VMGT. In 5-HT1BR-GFP treated rats, the 0.032 mg/kg dose of cocaine supported self-administration; 0.1 mg/kg remained the peak cocaine dose, but intake was elevated compared to GFP-only controls (p<0.05 in each case). Lever presses on the inactive lever were negligible and did not vary across groups (data not shown).
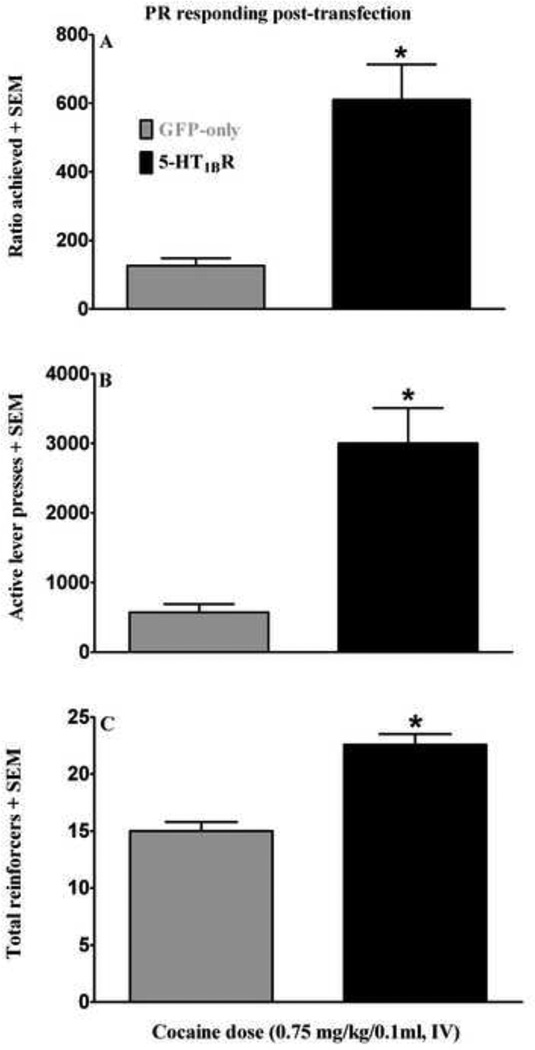
Figure 3 illustrates the effects of VMGT into the medial NAcsh on PR responding. 5-HT1BR-GFP microinfused rats achieved higher ratios [t(11)= 4.28, p<0.005], self-administered more cocaine infusions [t(11)=5.94, p<0.0001] and emitted more total active lever responses [t(11)=4.33, p<0.005] compared to GFP-only controls. Lever presses on the inactive lever were negligible and did not vary across groups (data not shown).
Figure 3.
Effects of viral-mediated 5-HT1BR-gene transfer into the medial NAcsh on responding during cocaine self-administration (0.75 mg/kg/0.1 ml, i.v.) break point testing on an exponential PR schedule of reinforcement expressed as the highest ratio achieved (A), the number of active lever responses emitted (B) and the number of cocaine reinforcers obtained (C). Five days following viral-mediated gene transfer, 5-HT1BR-GFP (black bars) treated rats achieved higher final ratios, emitted more active lever responses and received more cocaine infusions compared to GFP-only (gray bars) controls; data expressed as mean + SEM. Asterisk (*) represents an increase compared to GFP-only treated rats (t-tests, p<0.05 in each case).
Effects of VMGT on cue- and cocaine-primed reinstatement of extinguished cocaine-seeking behavior
Prior to VMGT, rats readily acquired cocaine self-administration. There were no group differences in rates of acquisition (data not shown), the total number of cocaine infusions throughout training or across the last five days of training (Table 1), or the number of active lever presses on the last day of self-administration training (Table 1). Active lever presses readily decreased during extinction training (data not shown), with a significant decrease across each of the first three days of extinction (p<0.05 in each case). There were no group differences in the number of active lever presses on the first day of extinction (Table 1) or across the 17 days of extinction prior to reinstatement testing (data not shown).
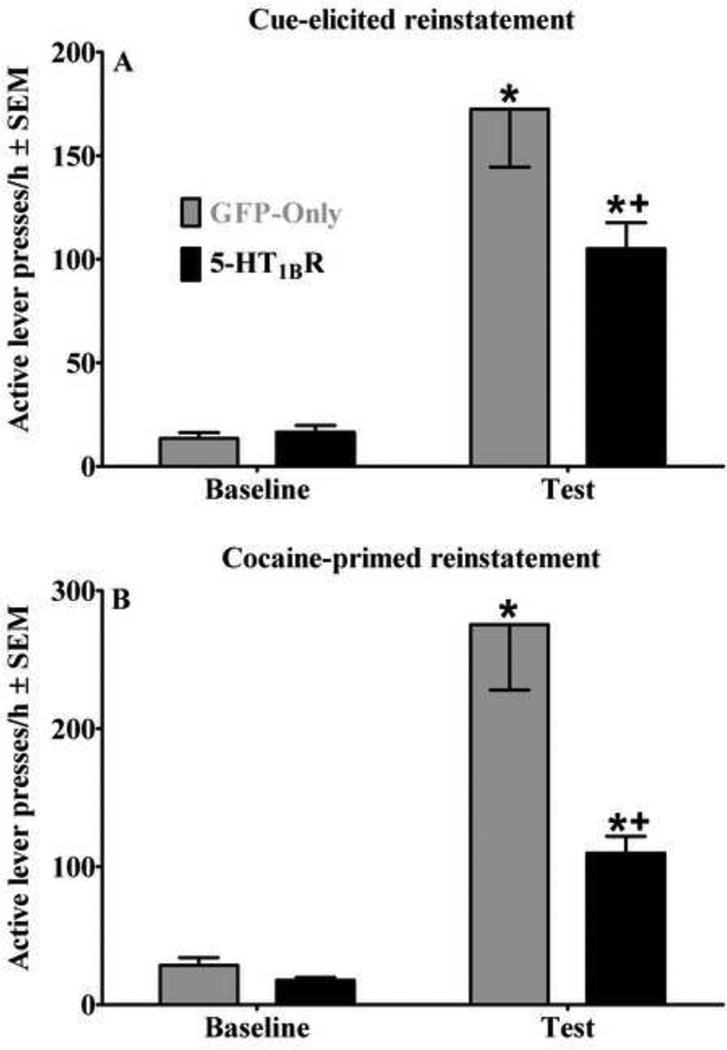
Figure 4A illustrates the effects of VMGT into the medial NAcsh on active lever responding during cue-elicited reinstatement testing. There was a main effect of viral treatment [F(1, 27)=4.64, p<0.05] and a viral treatment by test session interaction [F(1, 27)=5.34, p<0.05]. Post-hoc analysis indicated that both viral groups increased responding on the active lever when response-contingent cues were available, relative to baseline when responses produced no consequences (Newman-Keuls, p<0.05 in each case), indicating cue-elicited reinstatement of extinguished cocaine-seeking behavior regardless of viral condition. However, 5-HT1BR-GFP microinfusions attenuated response rates compared to GFP-only microinfusions, indicating that elevated 5-HT1BR expression attenuated cue-elicited reinstatement of extinguished cocaine-seeking behavior. Lever presses on the inactive lever were negligible and did not vary across groups (data not shown).
Figure 4.
Effects of viral-mediated 5-HT1BR-gene transfer into the medial NAcsh on cue-(A) and cocaine-primed (B) reinstatement of extinguished cocaine-seeking behavior expressed as the mean number of active lever responses during a 1-hr test session ± SEM. Baselines represent mean responses during the extinction session preceding the cueelicited test session (A), and mean responses during the saline-primed extinction session preceding the cocaine-primed test session (B). Rats (n=14–15/group) received microinfusions of a viral vector containing either 5-HT1BR-GFP (black bars) or GFP-only (gray bars) into the medial NAcsh four days prior to testing. For cue-elicited reinstatement, cues were available response-contingently during the test session on an FR1 schedule of reinforcement. For cocaine-primed reinstatement, the cocaine prime (10 mg/kg, i.p.) was administered immediately before testing and no cues were presented during the test sessions. Asterisk (*) represents a difference from baseline and plus sign (+) represents a difference from GFP-only controls (Newman-Keuls, p<0.05 in each case).
Figure 4B illustrates the effects of VMGT into the medial NAcsh on active lever responding during cocaine-primed reinstatement testing. There was a main effect of viral treatment [F(1, 27)=12.137, p=0.005] and a viral treatment by test session interaction [F(1, 27)=11.86, p=0.005]. Post-hoc analysis indicated that both viral groups increased responding when cocaine-priming injections were administered, relative to baseline when saline-priming injections were administered (Newman-Keuls, p<0.05 in each case), indicating cocaine-primed reinstatement of extinguished cocaine-seeking behavior regardless of viral condition. However, 5-HT1BR-GFP microinfusions attenuated response rates compared to GFP-only controls, indicating that elevated expression of 5-HT1BRs attenuated cocaine-primed reinstatement of extinguished cocaine-seeking behavior. Lever presses on the inactive lever were negligible and did not vary across groups (data not shown).
Effects of VMGT on cue-elicited cocaine-seeking behavior and cocaine intake during protracted withdrawal
Prior to VMGT, rats readily acquired cocaine self-administration. There were no group differences in rates of acquisition (data not shown), the total number of cocaine infusions throughout training or across the last five days of training (Table 1), or the number of active lever presses on the last day of self-administration training (Table 1).
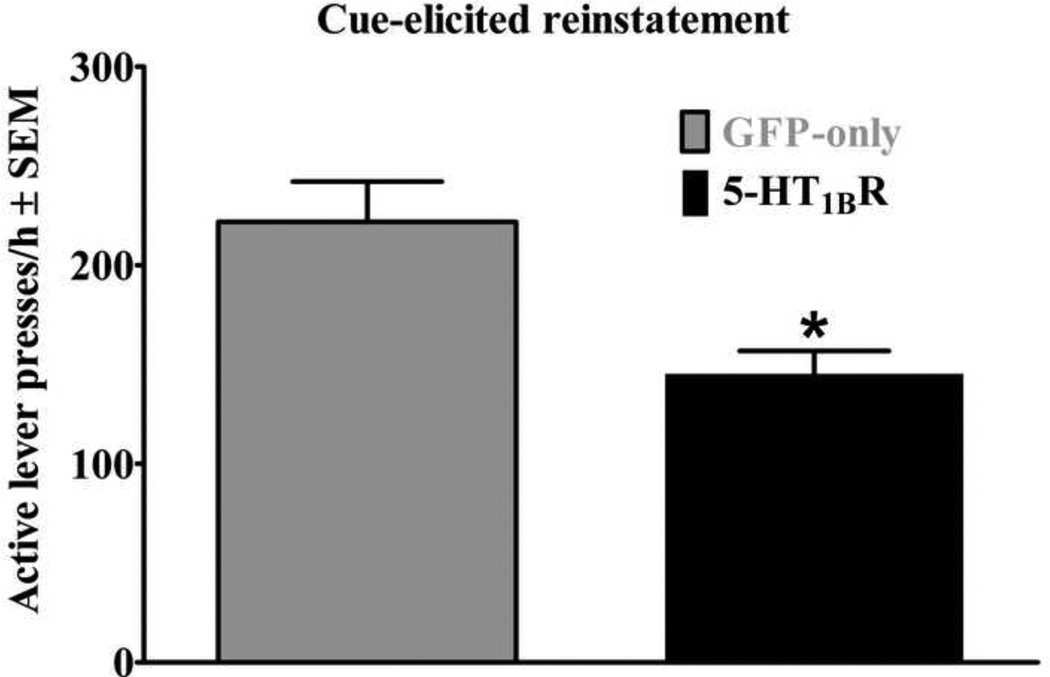
Figure 5 illustrates the effects of VMGT into the medial NAcsh on active lever responding during the cue-elicited cocaine-seeking test. Following VMGT, 5-HT1BR-GFP treated rats exhibited decreased responding on the active lever compared to GFP-only controls [ t(14)=3.46, p<0.005], indicating that elevated expression of 5-HT1BRs attenuated cue-elicited cocaine-seeking behavior. Lever presses on the inactive lever were negligible and did not vary across groups (data not shown).
Figure 5.
Effects of viral-mediated 5-HT1BR-gene transfer into the medial NAcsh on cueelicited cocaine-seeking behavior following 21 days of forced abstinence from chronic cocaine self-administration expressed as the mean number of active lever responses during a 1-hr test session + SEM. Rats (n=7–9/group) received microinfusions of a viral vector containing either 5-HT1BR-GFP (black bars) or GFP-only (gray bars) into the medial NAcsh four days prior to testing. Cues were available response-contingently during the test session on an FR1 schedule of reinforcement. Asterisk (*) represents a difference compared to GFP-only controls (t-test, p<0.05).
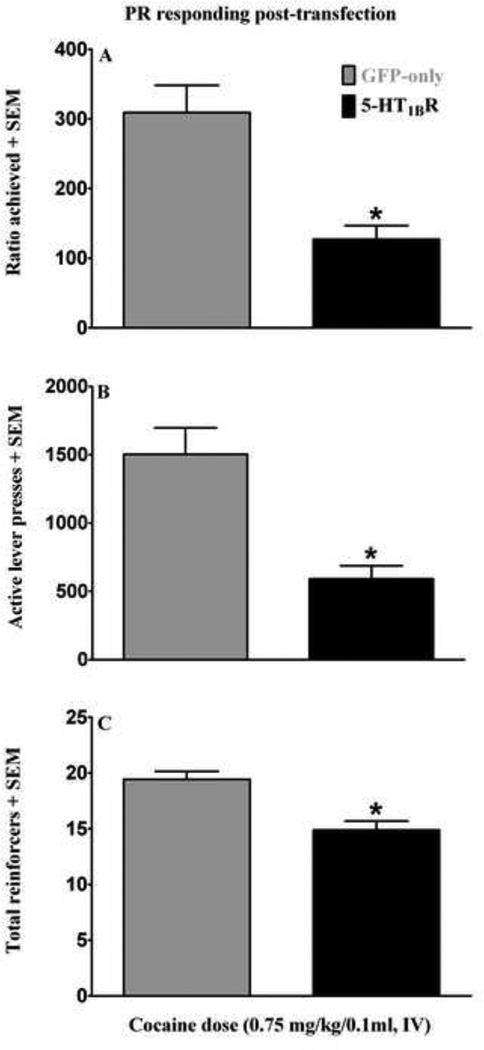
Figure 6 illustrates the effects of VMGT into the medial NAcsh on PR response rates. Following VMGT, 5-HT1BR-GFP microinfused rats achieved lower ratios [t(14)= 4.52, p<0.0001], self-administered fewer cocaine infusions [ t(14)=4.07, p<0.005] and emitted less total active lever responses [ t(14)=4.57, p<0.0001] compared to GFP-only controls. Lever presses on the inactive lever were negligible and did not vary across groups (data not shown).
Figure 6.
Effects of viral-mediated 5-HT1BR-gene transfer into the medial NAcsh following 21 days of protracted withdrawal (i.e. forced abstinence) from chronic cocaine self-administration on responding during cocaine self-administration (0.75 mg/kg/0.1 ml, i.v.) break point testing on an exponential PR schedule of reinforcement. Data are expressed as the highest ratio achieved (A), the number of active lever responses emitted (B) and the number of cocaine reinforcers obtained (C). Five days following viralmediated gene transfer (n=7–9/group), 5-HT1BR-GFP (black bars) treated rats achieved lower final ratios, emitted fewer active lever responses and received fewer cocaine infusions compared to GFP-only (gray bars) controls; data expressed as mean + SEM. Asterisk (*) represents an increase compared to GFP-only treated rats (t-tests, p<0.05 in each case).
Effects of VMGT on anxiety-like behavior in the EPM
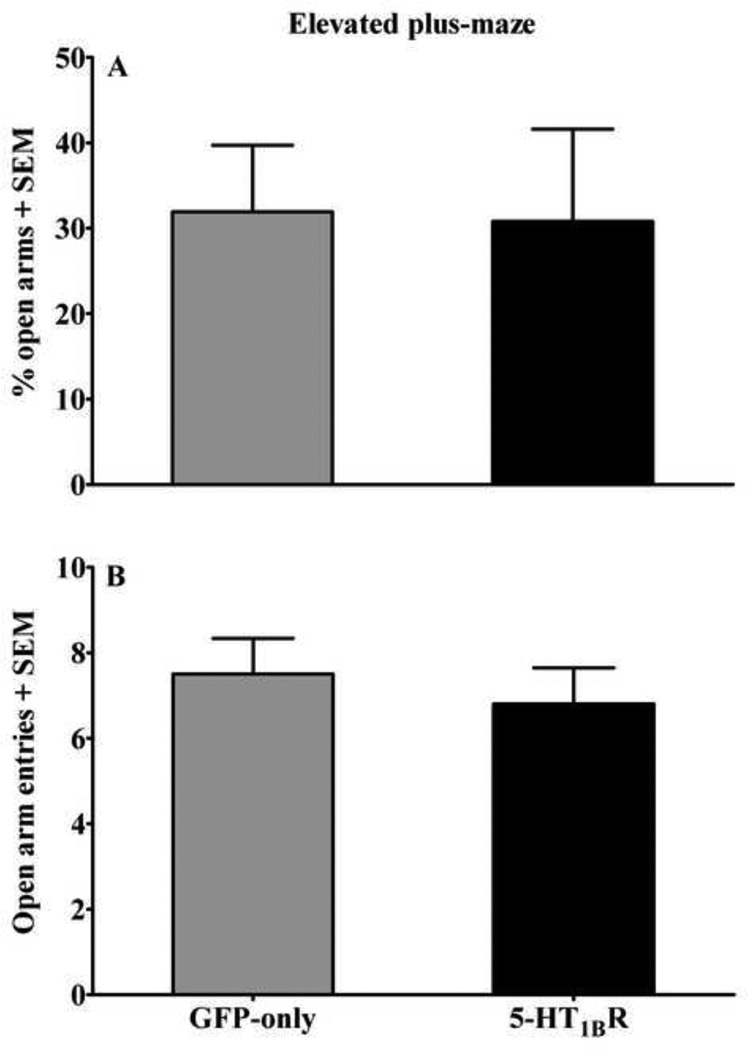
5-HT1BR-GFP microinfusions into the medial NAcsh failed to alter anxiety-like behavior in the EPM. There were no group differences in the percentage of time spent in the open arms or the number of open-arm entries (Figure 7). Furthermore, following VMGT, there were no differences between viral groups in total locomotor activity (data not shown).
Figure 7.
Effects of viral-mediated 5-HT1BR-gene transfer into the medial NAcsh on anxiety-like behavior during testing in the EPM expressed as the percentage of time spent in (A) the open arms and number of entries into (B) the open arms + SEM during a 5 min test session. Rats (n=14–15/group) received microinfusions of a viral vector containing either 5-HT1BR-GFP (black bars) or GFP-only (gray bars) into the medial NAcsh five days prior to testing.
Discussion
The results provide convincing support for the hypothesis that 5-HT1BRs modulate cocaine-abuse related behaviors in opposing directions depending on the stage of addiction. During maintenance (i.e. active drug use), 5-HT1BR-VMGT shifted the dose–response curve for cocaine self-administration upward and to the left on a FR5 schedule of reinforcement (Figure 2), and increased cocaine intake, active lever responses and the highest ratio achieved (i.e., break points) on a PR schedule (Figure 3). This pattern of changes is similar to the effects produced by increasing the unit dose of self-administered cocaine (35, 36), suggesting increased reinforcing effects of cocaine. In contrast, during protracted withdrawal 5-HT1BR-VMGT decreased cue- and cocaine-primed reinstatement of extinguished cocaine-seeking behavior (Figures 4, 5), and decreased cocaine intake, active lever responses and the highest ratio achieved on a PR schedule (Figure 6). This pattern suggests a decrease in the incentive motivational effects elicited by cocaine priming injections and exposure to cocaine-associated cues, and a decrease in motivation and/or reinforcement on a PR schedule.
These findings extend upon previous research demonstrating that elevated 5-HT1BR expression in these same neuronal populations increases cocaine-CPP (15, 25), and that 5-HT1BR agonists administered during maintenance produce a leftward shift in the cocaine self-administration dose-effect function and increase responding on PR schedules of reinforcement (10, 11). In contrast, 5-HT1BR agonists administered during protracted withdrawal decrease cue- and cocaine-primed reinstatement of extinguished cocaineseeking behavior (12, 17), consistent with the inhibitory influence of increased 5-HT1BR expression in the present study. The bi-directional effects of VMGT on PR responding may indicate that 5-HT1BRs are differentially engaged in reinforcement processes during maintenance of cocaine self-administration versus motivation processes after a period of abstinence. Alternatively, the opposing influence on PR responding may indicate a withdrawal-induced shift in 5-HT1BR regulation over cocaine reinforcement and/or motivation. Indeed, a functional switch in multiple neurotransmitter systems has been reported following withdrawal from several drugs of abuse (37–39); however, this is the first data to suggest a withdrawal-induced switch in 5-HT systems. Although future research is needed to determine the precise mechanism by which 5-HT1BRs modulate PR responding before and during withdrawal, the present pattern of effects indicates that 5-HT1BR modulation over drug seeking/taking is addiction stage-dependent with a facilitative influence during periods of active drug use in contrast to an inhibitory influence during protracted withdrawal.
The opposing effects observed at different time points mitigate impaired performance explanations, which predict consistent behavioral changes. Furthermore, there were no differences in locomotion between 5-HT1BR-GFP and GFP-only groups during EPM testing. The finding that anxiety-like behavior was not altered during EPM testing (Figure 7) suggests that anxiety did not interfere with cocaine-seeking behavior as suggested previously for systemic 5-HT1BR agonist effects (12, 26). 5-HT1BR-mediated anxiety-like effects likely involve neurons originating in the dorsal raphe (30), but the present findings suggest that the target regions involved in the behavior are outside of the mesolimbic pathway. Although all groups in the present study received virus infusions, previous studies utilizing this identical vector system detected no behavioral differences between no-surgery, sham surgery, and GFP-only groups (25, 31). Furthermore, this vector system produces a 3-fold increase in 5-HT1BR mRNA (30) exclusively in neurons (15) and hemagglutinin epitope tagging does not alter 5-HT1BR function (30). Collectively, these findings preclude nonspecific motor, anxiety or viral-vector effects.
The medial NAcsh projects predominantly to VTA (40). Although 5-HT1BR expression was not quantified in the present study, transgene expression in the VTA was evident in the form of GFP located within beaded axons (Figure 1D and F), consistent with previously confirmed transgene expression within the VTA in the form of immunoblotted hemagglutinin-tagged 5-HT1BR protein (15). The majority (>90%) of NAcsh neurons are GABAergic medium spiny projection neurons (41), thus it is likely that the majority of transgenic 5-HT1BRs were expressed in these neurons. GABA release in the VTA is inhibited via 5-HT1BRs localized on axon terminals of GABAergic neurons that originate in the NAcsh (20, 21). These neurons presumably synapse onto dopamine neurons directly (42) or glutamatergic neurons that provide excitatory input onto dopamine neurons (43). Therefore, the present behavioral effects likely resulted from 5-HT1BR-induced inhibition of GABA release in the VTA and subsequent disinhibition of dopaminergic neurons (23, 24, 44–46) that influence drug abuse-related behaviors associated with cocaine and other drugs of abuse (47–49). However, HSV vectors are not selective for any neuronal subtype (29), and therefore, it is likely that some cells projecting to the ventral pallidum, hypothalamus or collaterals within the NAcsh were also infected and may have contributed to the observed behavioral effects. Indeed, 5-HT1BR-VMGT within a single or small ensemble of medium spiny neurons would be expected to increase 5-HT1BRs in all of the related axon terminal fields (40). Such changes closely model the consequences of increased 5-HT1BR expression in NAc medium spiny neurons that occurs during exposure to cocaine (50, 51).
The present results suggest that 5-HT1BRs should be pursued as a target for medication development for treating cocaine dependence and relapse. Indeed, it may be possible to exploit the ability of 5-HT1BR agonists to inhibit cocaine intake during a relapse (i.e. reduced intake on PR schedules during protracted withdrawal), while simultaneously decreasing incentive motivational effects of stimuli that elicit craving (12, 17), thereby decreasing the incidence of relapse. Importantly, “pure” cocaine addiction is a rare clinical disorder and poly-drug use in cocaine addicts presents a major challenge in developing treatments for drug dependence (52, 53). In particular, cocaine-dependent patients often exhibit co-morbidity for alcohol and opiate abuse (54–56). Interestingly, polymorphisms of 5-HT1BRs have been linked to substance abuse not only for cocaine, but also opiates and alcohol (1–5). Neuroimaging studies in humans have also revealed that alcohol dependence is associated with increased ventral striatal 5-HT1BRs (57), effects similar to those detected following cocaine administration in rats (50, 51). Furthermore, systemic 5-HT1BR agonist administration decreases ethanol (58, 59), amphetamine (60) and cocaine (10–12) intake, particularly on the descending limb of the self-administration dose-effect function. Collectively these data indicate that 5-HT1BRs are involved in modulating drug intake across a range of abused substances, including alcohol, amphetamine and cocaine; however, further research investigating the effects of 5-HT1BRs on poly-drug intake and the influence of these receptors on the incentive motivational effects of stimuli that elicit craving for alcohol and opiates is needed.
Conclusions
The present report offers the strongest evidence to date that during the different stages of the addiction cycle 5-HT1BRs produce opposite modulatory effects on cocaine abuse-related behaviors, enhancing cocaine intake during periods of active drug use in contrast to inhibiting cocaine intake and incentive motivation for cocaine elicited by exposure to cocaine-associated cues or cocaine-priming injections following protracted withdrawal. The pattern of behavioral effects observed in the present study, as well as with 5-HT1BR agonists, is unique and strongly suggests that 5-HT1BRs should be pursued as a target for medication development for treating cocaine dependence and relapse.
Supplementary Material
Acknowledgements
This research was supported by National Institute on Drug Abuse (NIDA) grants to JLN (DA11064) and JFN (DA16432); NSP was supported by a NIDA Individual National Research Service Award (DA025413). The authors thank Kenny Thiel, Natalie Peartree, Lara Pockros, Ryan Bastle, Alyssa Jensen, Claudia Valles, Jose Alba, Shinban Lui, Lindsey Robertson, and Mike Painter for their technical assistance, and Cheryl Conrad and Ronald P. Hammer Jr. for use of equipment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Proudnikov D, LaForge KS, Hofflich H, Levenstien M, Gordon D, Barral S, et al. Association analysis of polymorphisms in serotonin 1B receptor (HTR1B) gene with heroin addiction: a comparison of molecular and statistically estimated haplotypes. Pharmacogenet Genomics. 2006;16:25–36. doi: 10.1097/01.fpc.0000182782.87932.d6. [DOI] [PubMed] [Google Scholar]
- 2.Sun HF, Chang YT, Fann CS, Chang CJ, Chen YH, Hsu YP, et al. Association study of novel human serotonin 5-HT(1B) polymorphisms with alcohol dependence in Taiwanese Han. Biol Psychiatry. 2002;51:896–901. doi: 10.1016/s0006-3223(01)01366-x. [DOI] [PubMed] [Google Scholar]
- 3.Huang YY, Oquendo MA, Friedman JM, Greenhill LL, Brodsky B, Malone KM, et al. Substance abuse disorder and major depression are associated with the human 5-HT1B receptor gene (HTR1B) G861C polymorphism. Neuropsychopharmacology. 2003;28:163–169. doi: 10.1038/sj.npp.1300000. [DOI] [PubMed] [Google Scholar]
- 4.Lee SY, Lin WW, Huang SY, Kuo PH, Wang CL, Wu PL, et al. The relationship between serotonin receptor 1B polymorphisms A-161T and alcohol dependence. Alcohol Clin Exp Res. 2009;33:1589–1595. doi: 10.1111/j.1530-0277.2009.00990.x. [DOI] [PubMed] [Google Scholar]
- 5.Cao JX, Hu J, Ye XM, Xia Y, Haile CA, Kosten TR, et al. Association between the 5-HTR1B gene polymorphisms and alcohol dependence in a Han Chinese population. Brain Res. 2011;1376:1–9. doi: 10.1016/j.brainres.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 6.Castanon N, Scearce-Levie K, Lucas JJ, Rocha B, Hen R. Modulation of the effects of cocaine by 5-HT1B receptors: a comparison of knockouts and antagonists. Pharmacol Biochem Behav. 2000;67:559–566. doi: 10.1016/s0091-3057(00)00389-0. [DOI] [PubMed] [Google Scholar]
- 7.Rocha BA, Scearce-Levie K, Lucas JJ, Hiroi N, Castanon N, Crabbe JC, et al. Increased vulnerability to cocaine in mice lacking the serotonin-1B receptor. Nature. 1998;393:175–178. doi: 10.1038/30259. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher PJ, Azampanah A, Korth KM. Activation of 5-HT(1B) receptors in the nucleus accumbens reduces self-administration of amphetamine on a progressive ratio schedule. Pharmacol Biochem Behav. 2002;71:717–725. doi: 10.1016/s0091-3057(01)00717-1. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher PJ, Korth KM. Activation of 5-HT1B receptors in the nucleus accumbens reduces amphetamine-induced enhancement of responding for conditioned reward. Psychopharmacology (Berl) 1999;142:165–174. doi: 10.1007/s002130050876. [DOI] [PubMed] [Google Scholar]
- 10.Parsons LH, Weiss F, Koob GF. Serotonin1B receptor stimulation enhances cocaine reinforcement. J Neurosci. 1998;18:10078–10089. doi: 10.1523/JNEUROSCI.18-23-10078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Przegalinski E, Golda A, Frankowska M, Zaniewska M, Filip M. Effects of serotonin 5-HT1B receptor ligands on the cocaine- and food-maintained self-administration in rats. Eur J Pharmacol. 2007;559:165–172. doi: 10.1016/j.ejphar.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Pentkowski NS, Acosta JI, Browning JR, Hamilton EC, Neisewander JL. Stimulation of 5-HT(1B) receptors enhances cocaine reinforcement yet reduces cocaine-seeking behavior. Addict Biol. 2009;14:419–430. doi: 10.1111/j.1369-1600.2009.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belzung C, Scearce-Levie K, Barreau S, Hen R. Absence of cocaine-induced place conditioning in serotonin 1B receptor knock-out mice. Pharmacol Biochem Behav. 2000;66:221–225. doi: 10.1016/s0091-3057(00)00238-0. [DOI] [PubMed] [Google Scholar]
- 14.Cervo L, Rozio M, Ekalle-Soppo CB, Carnovali F, Santangelo E, Samanin R. Stimulation of serotonin1B receptors induces conditioned place aversion and facilitates cocaine place conditioning in rats. Psychopharmacology (Berl) 2002;163:142–150. doi: 10.1007/s00213-002-1145-8. [DOI] [PubMed] [Google Scholar]
- 15.Barot SK, Ferguson SM, Neumaier JF. 5-HT(1B) receptors in nucleus accumbens efferents enhance both rewarding and aversive effects of cocaine. Eur J Neurosci. 2007;25:3125–3131. doi: 10.1111/j.1460-9568.2007.05568.x. [DOI] [PubMed] [Google Scholar]
- 16.Harrison AA, Parsons LH, Koob GF, Markou A. RU 24969, a 5-HT1A/1B agonist, elevates brain stimulation reward thresholds: an effect reversed by GR 127935, a 5-HT1B/1D antagonist. Psychopharmacology (Berl) 1999;141:242–250. doi: 10.1007/s002130050831. [DOI] [PubMed] [Google Scholar]
- 17.Acosta JI, Boynton FA, Kirschner KF, Neisewander JL. Stimulation of 5-HT1B receptors decreases cocaine- and sucrose-seeking behavior. Pharmacol Biochem Behav. 2005;80:297–307. doi: 10.1016/j.pbb.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Ghavami A, Stark KL, Jareb M, Ramboz S, Segu L, Hen R. Differential addressing of 5-HT1A and 5-HT1B receptors in epithelial cells and neurons. J Cell Sci. 1999;112(Pt 6):967–976. doi: 10.1242/jcs.112.6.967. [DOI] [PubMed] [Google Scholar]
- 19.Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, et al. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417:181–194. [PubMed] [Google Scholar]
- 20.Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA, et al. Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994;33:367–386. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 21.Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev. 2004;28:565–582. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, et al. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Dell LE, Parsons LH. Serotonin1B receptors in the ventral tegmental area modulate cocaine-induced increases in nucleus accumbens dopamine levels. J Pharmacol Exp Ther. 2004;311:711–719. doi: 10.1124/jpet.104.069278. [DOI] [PubMed] [Google Scholar]
- 24.Yan QS, Zheng SZ, Yan SE. Involvement of 5-HT1B receptors within the ventral tegmental area in regulation of mesolimbic dopaminergic neuronal activity via GABA mechanisms: a study with dual-probe microdialysis. Brain Res. 2004;1021:82–91. doi: 10.1016/j.brainres.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 25.Neumaier JF, Vincow ES, Arvanitogiannis A, Wise RA, Carlezon WA., Jr Elevated expression of 5-HT1B receptors in nucleus accumbens efferents sensitizes animals to cocaine. J Neurosci. 2002;22:10856–10863. doi: 10.1523/JNEUROSCI.22-24-10856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin D, Parsons LH. Anxiogenic-like effect of serotonin(1B) receptor stimulation in the rat elevated plus-maze. Pharmacol Biochem Behav. 2002;71:581–587. doi: 10.1016/s0091-3057(01)00712-2. [DOI] [PubMed] [Google Scholar]
- 27.Pentkowski NS, Duke FD, Weber SM, Pockros LA, Teer AP, Hamilton EC, et al. Stimulation of medial prefrontal cortex serotonin 2C (5-HT(2C)) receptors attenuates cocaine-seeking behavior. Neuropsychopharmacology. 2010;35:2037–2048. doi: 10.1038/npp.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. Amsterdam ; Boston: Academic Press/Elsevier; 2007. [Google Scholar]
- 29.Carlezon WA, Nestler EJ, Neve RL., Jr Herpes simplex virus-mediated gene transfer as a tool for neuropsychiatric research. Crit Rev Neurobiol. 2000;14:47–67. doi: 10.1080/08913810008443546. [DOI] [PubMed] [Google Scholar]
- 30.Clark MS, Sexton TJ, McClain M, Root D, Kohen R, Neumaier JF. Overexpression of 5-HT1B receptor in dorsal raphe nucleus using Herpes Simplex Virus gene transfer increases anxiety behavior after inescapable stress. J Neurosci. 2002;22:4550–4562. doi: 10.1523/JNEUROSCI.22-11-04550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell ES, Sexton T, Neumaier JF. Increased expression of 5-HT6 receptors in the rat dorsomedial striatum impairs instrumental learning. Neuropsychopharmacology. 2007;32:1520–1530. doi: 10.1038/sj.npp.1301284. [DOI] [PubMed] [Google Scholar]
- 32.Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology. 2004;1(47 Suppl):256–273. doi: 10.1016/j.neuropharm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 34.Pentkowski NS, Litvin Y, Blanchard DC, Vasconcellos A, King LB, Blanchard RJ. Effects of acidic-astressin and ovine-CRF microinfusions into the ventral hippocampus on defensive behaviors in rats. Horm Behav. 2009;56:35–43. doi: 10.1016/j.yhbeh.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caine SB, Koob GF. Pretreatment with the dopamine agonist 7-OH-DPAT shifts the cocaine self-administration dose-effect function to the left under different schedules in the rat. Behav Pharmacol. 1995;6:333–347. [PubMed] [Google Scholar]
- 36.Roberts DC, Loh EA, Vickers G. Self-administration of cocaine on a progressive ratio schedule in rats: dose-response relationship and effect of haloperidol pretreatment. Psychopharmacology (Berl) 1989;97:535–538. doi: 10.1007/BF00439560. [DOI] [PubMed] [Google Scholar]
- 37.Laviolette SR, Gallegos RA, Henriksen SJ, van der Kooy D. Opiate state controls bi-directional reward signaling via GABAA receptors in the ventral tegmental area. Nat Neurosci. 2004;7:160–169. doi: 10.1038/nn1182. [DOI] [PubMed] [Google Scholar]
- 38.Bickerdike MJ, Abercrombie ED. Striatal acetylcholine release correlates with behavioral sensitization in rats withdrawn from chronic amphetamine. J Pharmacol Exp Ther. 1997;282:818–826. [PubMed] [Google Scholar]
- 39.Krawczyk M, Sharma R, Mason X, Debacker J, Jones AA, Dumont EC. A switch in the neuromodulatory effects of dopamine in the oval bed nucleus of the stria terminalis associated with cocaine self-administration in rats. J Neurosci. 2011;31:8928–8935. doi: 10.1523/JNEUROSCI.0377-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- 41.Gerfen CR. Synaptic organization of the striatum. J Electron Microsc Tech. 1988;10:265–281. doi: 10.1002/jemt.1060100305. [DOI] [PubMed] [Google Scholar]
- 42.Steffensen SC, Taylor SR, Horton ML, Barber EN, Lyle LT, Stobbs SH, et al. Cocaine disinhibits dopamine neurons in the ventral tegmental area via use-dependent blockade of GABA neuron voltage-sensitive sodium channels. Eur J Neurosci. 2008;28:2028–2040. doi: 10.1111/j.1460-9568.2008.06479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dobi A, Margolis EB, Wang HL, Harvey BK, Morales M. Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J Neurosci. 2010;30:218–229. doi: 10.1523/JNEUROSCI.3884-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parsons LH, Koob GF, Weiss F. RU 24969, a 5-HT1B/1A receptor agonist, potentiates cocaine-induced increases in nucleus accumbens dopamine. Synapse. 1999;32:132–135. doi: 10.1002/(SICI)1098-2396(199905)32:2<132::AID-SYN6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 45.Yan QS, Yan SE. Activation of 5-HT(1B/1D) receptors in the mesolimbic dopamine system increases dopamine release from the nucleus accumbens: a microdialysis study. Eur J Pharmacol. 2001;418:55–64. doi: 10.1016/s0014-2999(01)00913-x. [DOI] [PubMed] [Google Scholar]
- 46.Yan QS, Yan SE. Serotonin-1B receptor-mediated inhibition of [(3)H]GABA release from rat ventral tegmental area slices. J Neurochem. 2001;79:914–922. doi: 10.1046/j.1471-4159.2001.00643.x. [DOI] [PubMed] [Google Scholar]
- 47.Balfour DJ. The neuronal pathways mediating the behavioral and addictive properties of nicotine. Handb Exp Pharmacol. 2009:209–233. doi: 10.1007/978-3-540-69248-5_8. [DOI] [PubMed] [Google Scholar]
- 48.Morales M, Pickel VM. Insights to drug addiction derived from ultrastructural views of the mesocorticolimbic system. Ann N Y Acad Sci. 2011 doi: 10.1111/j.1749-6632.2011.06299.x. [DOI] [PubMed] [Google Scholar]
- 49.Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoplight BJ, Vincow ES, Neumaier JF. Cocaine increases 5-HT1B mRNA in rat nucleus accumbens shell neurons. Neuropharmacology. 2007;52:444–449. doi: 10.1016/j.neuropharm.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 51.Neumaier JF, McDevitt RA, Polis IY, Parsons LH. Acquisition of and withdrawal from cocaine self-administration regulates 5-HT mRNA expression in rat striatum. J Neurochem. 2009;111:217–227. doi: 10.1111/j.1471-4159.2009.06313.x. [DOI] [PubMed] [Google Scholar]
- 52.Preti A. New developments in the pharmacotherapy of cocaine abuse. Addict Biol. 2007;12:133–151. doi: 10.1111/j.1369-1600.2007.00061.x. [DOI] [PubMed] [Google Scholar]
- 53.Rowan-Szal GA, Chatham LR, Simpson DD. Importance of identifying cocaine and alcohol dependent methadone clients. Am J Addict. 2000;9:38–50. doi: 10.1080/10550490050172218. [DOI] [PubMed] [Google Scholar]
- 54.Leri F, Bruneau J, Stewart J. Understanding polydrug use: review of heroin and cocaine co-use. Addiction. 2003;98:7–22. doi: 10.1046/j.1360-0443.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- 55.Prinzleve M, Haasen C, Zurhold H, Matali JL, Bruguera E, Gerevich J, et al. Cocaine use in Europe - a multi-centre study: patterns of use in different groups. Eur Addict Res. 2004;10:147–155. doi: 10.1159/000079835. [DOI] [PubMed] [Google Scholar]
- 56.Miller NS, Summers GL, Gold MS. Cocaine dependence: alcohol and other drug dependence and withdrawal characteristics. J Addict Dis. 1993;12:25–35. doi: 10.1300/J069v12n01_03. [DOI] [PubMed] [Google Scholar]
- 57.Hu J, Henry S, Gallezot JD, Ropchan J, Neumaier JF, Potenza MN, et al. Serotonin 1B receptor imaging in alcohol dependence. Biol Psychiatry. 2010;67:800–803. doi: 10.1016/j.biopsych.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tomkins DM, O'Neill MF. Effect of 5-HT(1B) receptor ligands on self-administration of ethanol in a MF (operant procedure in rats. Pharmacol Biochem Behav. 2000;66:129–136. doi: 10.1016/s0091-3057(00)00232-x. [DOI] [PubMed] [Google Scholar]
- 59.Wilson AW, Costall B, Neill JC. Manipulation of operant responding for an ethanol-paired conditioned stimulus in the rat by pharmacological alteration of the serotonergic system. J Psychopharmacol. 2000;14:340–346. doi: 10.1177/026988110001400402. [DOI] [PubMed] [Google Scholar]
- 60.Miszkiel J, Adamczyk P, Filip M, Przegalinski E. The effect of serotonin 5HT(1B) receptor ligands on amphetamine self-administration in rats. Eur J Pharmacol. 2012;677:111–115. doi: 10.1016/j.ejphar.2011.12.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.