Abstract
This study examined cocaine self-administration after pretreatments with three structurally related compounds that bind selectively to dopamine D3 receptors (D3Rs) relative to the D2 receptor subtype (D2Rs) and exhibit varying intrinsic activities in the forskolin-stimulated adenylyl cyclase assay. The compounds are: a) WC10, a D3R weak partial agonist/ antagonist with 42-fold D3R:D2R selectivity, b) WC26, a 51-fold selective D3R partial agonist, c) WC44, a 23-fold selective D3R agonist. Rats were stabilized on a multiple variable-interval 60-sec (VI60) schedule with alternating components of sucrose (45 mg pellets) or cocaine reinforcement (0.375 mg/kg, IV) and then tested for effects of the WC compounds (0.0, 1.0, 3.0, 5.6, or 10.0 mg/kg, IP). Another cohort was trained to self-administer cocaine (0.75 mg/kg, IV) on a VI60 schedule then tested with various doses of cocaine available (0.0–1.5 mg/kg, IV) following pretreatment with WC10 (5.6 or 10.0 mg/kg) or WC44 (10.0 mg/kg). WC10 and WC26 decreased both cocaine and sucrose reinforcement rates at the 10.0 mg/kg dose, whereas WC44 decreased only cocaine reinforcement rate at this dose. Furthermore, WC26 and WC44 increased response latency for cocaine but not sucrose. In the cocaine dose-response experiment, WC10 and WC44 flattened the dose-effect function of cocaine reinforcement rate. All compounds decreased spontaneous locomotion. WC10 and WC26 also reduced cocaine-induced locomotion. These results support the targeting of D3Rs for treatments for cocaine dependence. WC26 and WC44, in particular, show promise as they increased the latency to respond for cocaine but not sucrose, suggesting selective reduction of the motivation for cocaine.
1. Introduction
The dopamine D3 receptor (D3R) subtype is a viable target for development of novel therapeutic agents for the treatment of psychostimulant addiction, as well as L-DOPA-induced dyskinesia and schizophrenia (Blaylock and Nader 2012; Heidbreder and Newman 2010; Joyce and Millan 2005; Kumar et al. 2009; Le Foll et al. 2005; Levant 1997; Luedtke and Mach 2003; Newman et al. 2005). Interest in D3 receptors as a therapeutic target for the treatment of psychostimulant abuse initially stemmed from the unique anatomical distribution of this receptor, including expression in the nucleus accumbens, which is known to play a key role in addiction-related behaviors (Kalivas and Volkow 2005; Wise 2004). Up-regulation of D3R expression has been reported to occur in response to administration of several drugs of abuse (Le Foll et al. 2003; Spangler et al. 2003; Vengeliene et al. 2006). For instance, Mash and colleagues found elevated D3R binding and mRNA expression in the ventral striatum of cocaine overdose fatalities (Segal et al. 1997; Staley and Mash 1996). In rodents, D3Rs are up-regulated following cocaine self-administration and the magnitude of elevation is related to measures of motivation for cocaine-seeking behaviors (Conrad et al. 2010; Neisewander et al. 2004).
There is 78% homology in the transmembrane spanning regions of D3Rs and D2Rs, (Sokoloff et al. 1990), which has made it difficult to develop highly selective D3R compounds. Those that have been developed are antagonists or low efficacy partial agonists with approximately 100-fold selectivity for D3Rs over D2Rs (Heidbreder and Newman 2010; Wang et al. 2010). These compounds alter several psychostimulant-conditioned and unconditioned behaviors including: 1) decreasing self-administration under fixed and progressive ratio schedules of reinforcement (Higley et al. 2011; Peng et al. 2009; Song et al. 2011; Xi and Gardner 2007; Xi et al. 2005; Xi et al. 2006), 2) decreasing drug-primed or cue-elicited reinstatement of extinguished psychostimulant–seeking behavior (Gal and Gyertyan 2006; Gilbert et al. 2005; Higley et al. 2011; Vorel et al. 2002; Xi et al. 2006), 3) attenuating psychostimulant reward as measured by electrical brain self-stimulation and conditioned place preference (Cervo et al. 2005; Duarte et al. 2003; Spiller et al. 2008; Vorel et al. 2002; Xi et al. 2006), and 4) attenuating cocaine-conditioned hyperactivity (Le Foll et al. 2002). However, a property of the dopamine D3R-selective antagonists and partial agonists that has been studied so far is that they have relatively poor bioavailability (Heidbreder and Newman 2010). Thus, there is a need to develop new D3R-selective compounds with improved bioavailability that can successfully target D3 receptors in the brain when administered systemically (Chu et al. 2005; Grundt et al. 2005; Luedtke and Mach 2003; Mach et al. 2003).
We have developed a series of 2-methoxy substituted phenylpiperazine compounds with varying degrees of selectivity and intrinsic activity (Chu et al. 2005; Kumar et al. 2009; Xu et al. 2009). We have previously demonstrated that these WC-series compounds have therapeutic potential for the treatment of L-DOPA-induced dyskinesia (Kumar et al. 2009) and that they are effective in diminishing dopamine receptor agonist-induced deficits in pre-pulse inhibition of startle, an animal model of sensory integration impairment in schizophrenia (Weber et al. 2009). The present study examined the ability of these compounds to suppress cocaine self-administration and their effects on responding for sucrose reinforcement. The effects of these compounds on spontaneous and cocaine-induced locomotion were also evaluated.
2. Methods and materials
2.1 Animals
Male Sprague-Dawley rats (Charles River, USA) weighing 250–300 g at the time of surgery were maintained on a 12:12 reverse light:dark cycle (lights on at 7:00 pm). Rats were given ad libitum food and water, unless otherwise specified. Subjects were acclimated to handling for approximately 2 min/day for at least 5 days prior to surgery. All procedures and housing conditions were in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 1996) and were approved by the Institutional Animal Care and Use Committee at Arizona State University.
2.2 Drugs
Cocaine hydrochloride (RTI International, Research Triangle Park, NC, USA) was dissolved in saline and filtered through 0.2 µm filters. The compounds WC10 and WC44 were mixed in 5% dimethyl sulfoxide in 0.9% saline, then 25 µl/ml 1.0 N hydrochloric acid was added to aid solubility. For the compound WC26, the concentration of dimethyl sulfoxide was increased to 25%. All WC compounds were administered intraperitoneally at a volume of 1 ml/kg.
2.3 Surgery
Rats were anesthetized using 2–3% isoflurane gas (Phoenix Pharmaceuticals, St. Joseph, MO, USA). Meloxicam (1 mg/kg SC, Boehringer Ingelheim Vetmedica, St. Joseph, MO, USA) was also administered to decrease inflammation. A Silastic (Dow Corning, Midland, MI, USA) catheter was implanted into the right jugular vein with the tip of the catheter positioned just rostral to the right atrium of the heart (3.0 cm from catheter insertion point). The catheter was then secured in position with sutures. The other end of the catheter was attached to a screw connector (Plastics One, Roanoke, VA, USA), fed subcutaneously to exit through an incision on the top of the head, and then fixed to the skull with dental acrylic cement as described in detail previously (Neisewander et al. 2000). Immediately following the surgery, rats were given 0.05 mg/kg, SC buprenorphine hydrochloride (PharmaForce, Hilliard, OH, USA). This analgesic treatment was repeated 24 hours later. Rats were given 1 week to recover, during which time they were handled 2 min per day. Catheters were flushed daily with a mixture of Timentin (66.7 mg/ml, GlaxoSmithKline, Research Triangle Park, NC, USA) and heparin saline (70 U/ml, APP Pharmaceuticals, Schaumburg, IL, USA) to maintain patency for the duration of the study. Proper catheter function was tested periodically by infusing 0.05 ml methohexital sodium (16.7 mg/ml, JHP Pharmaceuticals, Rochester, MI, USA) via the catheter, which produces brief anesthetic effects only when administered IV.
2.4 Dose-effects of WC compounds on sucrose and cocaine reinforcement
Separate cohorts of rats were used for each WC compound (WC10, n = 8; WC26, n = 8; WC44, n = 9). Training progressed in three phases: a sucrose-only phase, a cocaine-only phase, and a multiple-schedule phase. Rats were restricted to 16 g of food/day beginning 2 days before the sucrose-only phase to facilitate exploration. In the sucrose-only phase, daily sessions lasted 2 hrs or until the rat had earned 50 reinforcers, whichever occurred first. Sucrose pellets (45 mg, Bio-Serv, Frenchtown, NJ, USA) were used as the reinforcer. During the sucrose-only phase, the right lever was the active lever, a cue light was above the active lever to signal reinforcer availability, and the left lever was inactive. Pressing the active lever when a reinforcer had been scheduled for delivery produced the following consequences: a sucrose pellet was delivered, both levers retracted, the cue light turned off, a tone (500 Hz, 10 db above background) played for 6 sec, the house light turned on after 6 sec and remained on for the next 24 sec to signal a time-out. After the time-out period, the stimuli were reset so that the levers were reinserted, the cue light above the active lever was turned on and the house light was turned off. The schedule of reinforcement progressed within daily sessions from fixed ratio 1 (FR1) to variable interval 10 sec (VI10), VI30, and then finally to VI60. The schedule was advanced if the rat received 5 reinforcers within 40 minutes. Once the rat had ended the session on VI60 for 3 consecutive sessions, the VI60 schedule was in effect exclusively thereafter. All rats remained food-restricted to 16 g/day until a criterion of ≥ 14 reinforcers was achieved during VI60-only sessions, after which the food in the home cage was progressively increased to 18 g/day, then to 22 g/day, then to ad libitum across several sessions. Ad libitum food was then available in the home cage for the remainder of the experiment. Training in the sucrose-only phase continued for each rat until the VI60-only schedule was reached and a stability criterion of ≤ 15% variance of the mean number of reinforcers across 3 days had been met, after which training progressed to the cocaine-only phase. In the cocaine-only phase, rats were trained with cocaine (0.75 mg/kg/0.1 ml, IV) as the reinforcer in 2-hr daily sessions that were identical to the sucrose-only sessions except that the positions of the active and inactive lever (left/right) were reversed. Thus, upon schedule completion on the active (left) lever, a) cocaine was delivered over a 6 sec period, b) both levers retracted, c) the cue light above the active lever turned off, d) a tone played for 6 sec, and e) the house light turned on after 6 sec and remained on for the next 24 sec. Once the same stability criterion used in the sucrose-only phase was met, training progressed to the multiple-schedule phase. In the multiple-schedule phase, rats were trained on a multiple schedule consisting of eight 15-min components. The reinforcer available during each component alternated between sucrose and cocaine. The schedule contingency used in each component was the same as that used during the first two phases for each respective reinforcer type. That is, the right lever was the active lever during a sucrose component and the left lever was the active lever during a cocaine component. The cue light above the right (active) lever was turned on during a sucrose component whereas during a cocaine component the cue light above the left (active) lever was turned on. Reinforcement was delivered using a VI60 schedule for all components. The cocaine dose used for the multiple schedule was reduced to 0.375 mg/kg/0.1 ml. The reinforcer available in the initial component of each session was varied randomly between sessions. A 1 min time-out was implemented between components during which both levers were retracted and all lights were turned off. The total session length was therefore 2 hr and 8 min.
Testing of the WC compounds started once a rat achieved the 3-day stability criterion for both reinforcers simultaneously on the multiple schedule. Test sessions were identical to the multiple schedule training sessions described above, with the exception that 1) test sessions were shortened to four 15-min components with alternating reinforcers, resulting in a total test session length of 1 hr 4 min, in order to match the approximate window during which the behavioral effects of the WC compounds would be the strongest (Kumar et al. 2009), and 2) sucrose was always the reinforcer available during the initial component. For each WC compound, five doses (vehicle, 1.0, 3.0, 5.6, 10.0 mg/kg, IP) were tested across separate test sessions using a within-subject design, with the dose order counterbalanced between subjects. The WC compound was injected 5 min before the start of the test session based on our previous study showing that all three WC compounds attenuated L-DOPA-induced dyskinesia within this timeframe (Kumar et al. 2009). We verified that the WC compounds were behaviorally effective 5 min post-treatment based on locomotor activity data (see below). Between test sessions, rats were maintained under the eight-component multiple schedule used during training, and the 3-day stability criterion described above had to be met for both reinforcers before a rat was tested on the next dose of the WC compound.
2.5 Effects of WC compounds on the cocaine dose-effect function
For these experiments, we chose to compare the weak partial agonist/antagonist WC10 and the agonist WC44 at the 10.0 mg/kg doses. A lower dose of WC10 (5.6 mg/kg) was also tested. WC26 was not tested because we chose to focus on the compounds with the lowest and the highest intrinsic activities. Separate cohorts of experimentally naïve rats were used to examine the effects of WC10 and WC44 on the cocaine dose-effect function (WC10 5.6 mg/kg, n=9; WC10 10.0 mg/kg, n=7; WC44 10.0 mg/kg, n=8). Rats were trained to self-administer cocaine (0.75 mg/kg/0.1ml, IV) in 2-hr daily sessions, under the same contingency as that used in the cocaine-only phase described above. Once the 3-day stability criterion had been met and a minimum of 10 sessions had occurred, a series of test days was initiated. The dose of cocaine available during the test session was increased across test days in the following order: 0, 0.094, 0.188, 0.375, 0.75, and 1.5 mg/kg/0.1 ml IV. For each test dose of cocaine, animals were tested twice: once following pretreatment with vehicle and once following pretreatment with the WC compound, with the treatment order counterbalanced between rats. Pretreatments were given IP 5 min before the start of the test sessions. Each test session lasted 1 hr. Between test days, rats were maintained under the same daily 2-hr sessions used during training (with a cocaine dose of 0.75 mg/kg/0.1 ml), and the 3-day stability criterion was implemented before a rat was tested again.
2.6 Effects of WC compounds on spontaneous and cocaine-induced locomotion
Between one to nine days after completing the above self-administration experiments, rats were tested for the effects of WC10 (n=8), WC26 (n=8) and WC44 (n=6) on spontaneous and cocaine-induced locomotion. All eight rats used to test WC10 were from the cocaine dose-effect experiment testing the effect of 10 mg/kg WC10, including one rat whose catheter became non-patent and was excluded from that self-administration experiment. All eight rats used to test WC26 were from the multiple schedule experiment testing the dose-effect of WC26. Two of the six rats used to test WC44 were from the multiple schedule experiment testing the dose-effect of WC44. The remaining four rats were from the multiple schedule experiment testing the dose-effect of WC26 and after they had been tested for the locomotor effect of WC26. For these rats, 4 days separated the end of the WC26 locomotor experiment and the start of the WC44 locomotor experiment. Locomotor activity tests were conducted in Plexiglas cages that were 25 cm by 46 cm and 20 cm deep with a wire bar lid. A video tracking system (TopScan Realtime Option Version 2.00, Clever Sys., Reston, VA, USA) produced a continuous record of the rats’ movement, reported as total cm traveled. Each rat received the following four treatments across four non-consecutive test days in a within-subject 2 × 2 factorial design: vehicle, cocaine (10 mg/kg, IP), the assigned WC compound (10 mg/kg, IP), and both cocaine and the assigned WC compound. The order of treatments was counterbalanced between rats, and treatments were given 5 minutes before a test session. Each test session was 1 hr long and rats were given 1 day off between test sessions.
2.7 Data Analyses
The following dependent variables were analyzed: reinforcement rates, total cocaine intake (cocaine dose-effect experiments only), active and inactive lever response rates, and response latencies. In addition, for the multiple schedule experiment, the change from vehicle (0 mg/kg WC compound) was calculated and analyzed for each reinforcer type in order to take into account potential baseline differences for sucrose vs. cocaine after vehicle pretreatments. These analyses are included in the supplementary materials because they largely corroborated with the analyses of the raw data. Response latency was calculated as the latency from the insertion of levers to the first response on the active lever. For the cocaine dose-effect experiments, if a rat failed to respond during a session, the latency was capped at the duration of the session (60 min). Response latency data were averaged across cocaine doses because the animals had no way of knowing the upcoming cocaine dose at the beginning of the test sessions, and therefore response latency should not change as a function of tested cocaine dose. For the experiments investigating the dose-effects of the WC compounds on sucrose and cocaine reinforcement (i.e., the multiple schedule experiments), if a rat failed to respond during a component, the latency was capped at the duration of a component (15 min). With the exception of response latencies, data for each reinforcer type in the multiple schedule experiments were averaged across its two components during test sessions. Therefore, data for sucrose was averaged across the 1st and 3rd components, while data for cocaine was averaged across the 2nd and 4th components. Response latencies were not averaged because they may differ in the first session half (1st and 2nd components) compared to the last session half (3rd and 4th components). This is because rats were under the influence of cocaine at the beginning of the 3rd and 4th components, but not at the beginning of the 1st and 2nd components.
Two separate approaches were used to analyze the dependent measures. In the first approach, two-factor repeated measures ANOVAs were performed. Unless otherwise specified in the results section, the two factors in the multiple schedule experiments were reinforcer type (2 levels: sucrose and cocaine) × WC compound dose (5 levels: 0, 1, 3, 5.6 and 10 mg/kg). The two factors in the cocaine dose-effect experiments were drug pretreatment (2 levels: vehicle and WC compounds) × cocaine dose (6 levels: 0, 0.094, 0.188, 0.375, 0.75 and 1.5 mg/kg). In addition, response latencies in the multiple schedule experiments were analyzed using linear trend analysis, with the dose of the WC compounds as the linear contrast factor. Evidence that a WC compound affected responding for cocaine differently from sucrose in the multiple schedule experiment would be revealed by a significant reinforcer type × WC compound dose interaction. Evidence that a WC compound affected the shape of the cocaine dose-effect function would be revealed by a significant WC compound × cocaine dose interaction. If a main effect was found for factors with ≥ 3 levels (i.e., WC compound dose or cocaine dose), the other factor was collapsed, and post-hoc comparisons were made to verify that the effect was due to a difference between control and one of the higher doses. Post-hoc comparisons were made using two-tailed paired-sample t-tests, with significance level of each test (α) adjusted using the Šidák correction such that the family-wise Type I error rate was 0.05 (Howell 2007; Keppel and Wickens 2004). Therefore, for post-hoc comparisons of the effect of WC compound dose, 4 comparisons were made, and the Šidák-corrected α (αcorr) was 0.013. For post-hoc comparisons of the effect of cocaine dose, 5 comparisons were made, and αcorr = 0.010. In the case when a significant interaction between the two factors was found, tests of simple main effects were performed to determine the source of the interaction as follows. For each level of the two-level factor (e.g., reinforcer type in the multiple schedule experiments), separate one way ANOVAs were performed on the factor with ≥ 3 levels (e.g., WC compound dose), and in the case of a significant effect, post-hoc multiple t-tests using the Šidák correction was performed (Howell 2007; Keppel and Wickens 2004). Because Mauchly’s test of sphericity (1940) confirmed that homogeneity of variance was violated for much of the response latency data in the multiple schedule experiments, response latencies were log10-transformed prior to statistical analysis. Log10-transformation reduced the cases of variance homogeneity violations from 10 (pretransformation) to just 1 (the main effect of WC26 dose, which was non-significant in any case).
In the second approach, planned comparisons were used to test specific hypotheses that the WC compounds, being ligands at D3Rs, are able to alter responding for cocaine (Heidbreder and Newman 2010) and sucrose (Peng et al. 2009). For the multiple schedule experiments, planned comparisons between vehicle (0 mg/kg dose) versus each dose of the WC compound were performed for each reinforcer type. For the cocaine dose-effect experiments, planned comparisons between vehicle and WC compound pretreatments were performed at each dose of cocaine. All planned comparisons were conducted using 2-tailed t-tests with a significance level (α) of 0.05. Planned comparisons were not performed on inactive lever response rates as we had no a priori hypotheses regarding this measure.
Locomotor activity for each WC compound was analyzed using a 2 × 2 (WC compound × cocaine) repeated ANOVA. In case of a significant interaction, simple effects were assessed using 2-tailed t-tests. Planned t-tests were also used to verify that cocaine pretreatment increased locomotor activity. In addition, in order to verify that the WC compounds were behaviorally active at the start of the session for all experiments in this study (i.e., 5 minutes post-treatment), locomotor activity during the first minute of the session (i.e., between the 5th and 6th minutes post-treatment) after pretreatment with vehicle pretreatment vs. with the WC compounds was compared using t-tests.
3. Results
3.1 Dose-effects of WC compounds on sucrose and cocaine reinforcement
The pharmacological properties of the three WC-series phenylpiperazine derivatives and their structures have been reported previously (Chu et al. 2005; Kumar et al. 2009). All three compounds have nanomolar affinity at human D3 dopamine receptors, exhibit 23- to 51-fold binding selectivity at D3 compared to D2 dopamine receptor subtype, and are weak partial agonists (30% to 35% intrinsic activity of the full agonist quinpirole) at D2 receptors using an adenylyl cyclase inhibition assay (Kumar et al. 2009). The pharmacological property that differentiates these compounds is their intrinsic activity at D3 receptors, with WC10 being a weak partial agonist/antagonist (20% intrinsic activity of the full agonist quinpirole), WC26 being a partial agonist (69% intrinsic activity), and WC44 being a full agonist (96% intrinsic activity, Chu et al. 2005). We selected these compounds for our initial studies because they each have partition coefficients which predict their ability to cross the blood brain barrier (log P values = 2.9 to 3.5, Chu et al. 2005).
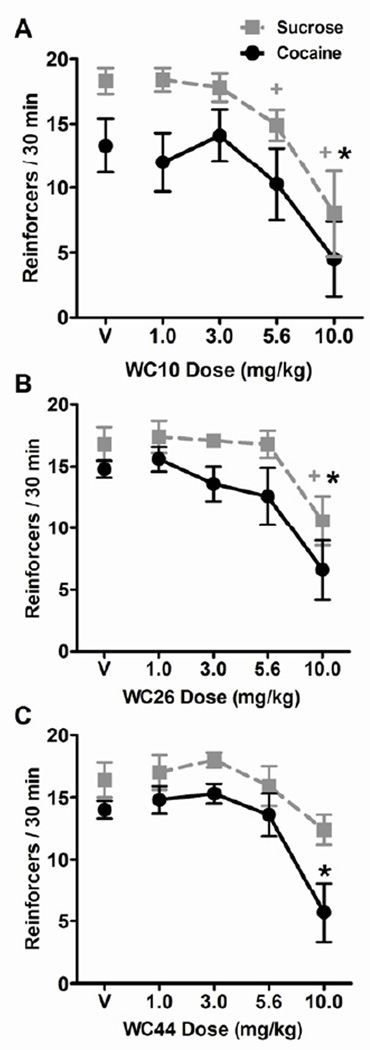
The dose-dependent effects of the WC compounds on the number of reinforcers delivered across the two 15-min test components for sucrose and cocaine are shown in Figure 1. The repeated measures ANOVA (2 reinforcer types × 5 WC compound doses) revealed a main effect of reinforcer type on reinforcement rates in all three experiments: WC10 (F(1,7) = 9.44), WC26 (F(1,7) = 17.2), and WC44 (F(1,8) = 5.84, p < 0.05 for all), suggesting that sucrose intake was higher in general than cocaine intake. There was also a main effect of dose of WC compound in each case: WC10 (F(4,28) = 9.68), WC26 (F(4,28) = 6.96), and WC44 (F(4,32) =10.3, p < 0.05 for all), but no interaction between reinforcer type and dose. These results indicate that all three WC compounds dose-dependently reduced the reinforcement rate regardless of whether cocaine or sucrose was available. For all three WC compounds, post-hoc tests of the main effect of dose, collapsed across reinforcer types, revealed a significant decrease in reinforcement rate relative to vehicle at the 10.0 mg/kg dose (αcorr for 4 comparisons = 0.013). In addition, planned comparisons of each dose relative to vehicle revealed that WC10 significantly decreased cocaine reinforcement rate at the 10.0 mg/kg dose (t(7) = 3.08, p = 0.018), and decreased sucrose reinforcement rate at both the 5.6 mg/kg (t(7) = 3.07, p = 0.018) and the 10.0 mg/kg doses (t(7) = 3.50, p = 0.010, Figure 1A). For WC26 these planned comparisons revealed that 10.0 mg/kg dose decreased reinforcement rates for cocaine (t(7) = 3.85, p = 0.006) and sucrose (t(7) = 2.98, p = 0.021, Figure 1B). For WC44, planned comparisons revealed that the 10.0 mg/kg dose decreased cocaine reinforcement rate (t(8) = 3.60, p = 0.007) but there was no significant effect on sucrose reinforcement rates at any dose (Figure 1C).
Figure 1.
Effect of WC compounds on sucrose and cocaine reinforcement rates. Graphs show the mean number of reinforcers/30 min (± SEM) totaled across the two 15-min sucrose (grey squares) or cocaine (black circles) components of the multiple VI60 schedule following pretreatment with vehicle (V) or varying doses of WC10 (A), WC26 (B), or WC44 (C). Separate cohorts of rats were used to test each compound (n = 8, 8, and 9 respectively). Animals received all doses of their assigned compound across repeated tests with additional self-administration sessions between tests to re-establish stable intake rates. The pretreatments were given 5 min prior to the beginning of the test sessions. There was a main effect of reinforcer type in all three WC experiments, indicating higher sucrose reinforcement rates relative to cocaine. There was also a main effect of dose in all experiments, but no dose by reinforcer type interactions. Planned comparisons of each dose of WC compounds to their respective vehicle treatments revealed differences both when cocaine was available (*) and when sucrose was available (+), planned t-test, p < 0.05.
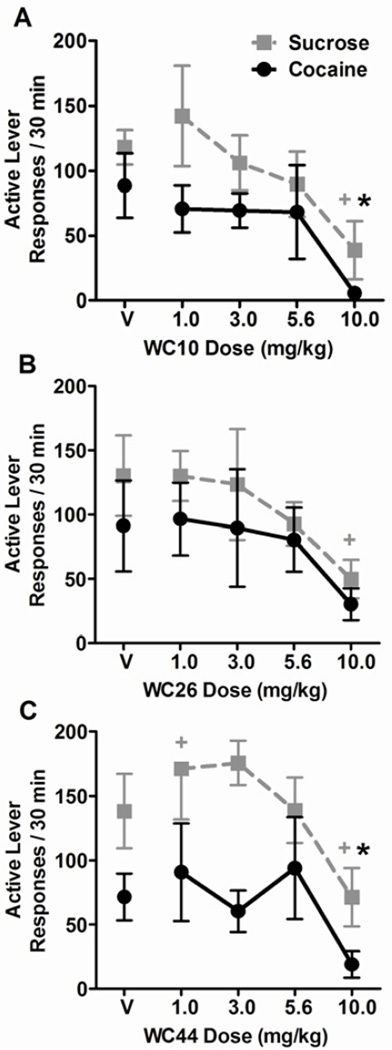
The effects of the WC compounds on the number of active lever responses totaled across the two 15-min test components for each reinforcer type are shown in Figure 2. In the WC10 experiment, one rat was an outlier for both active and inactive response rates under both cocaine and sucrose schedule components (> 7 standard deviations above group means). Therefore, we omitted this subject from the analyses and graphs involving response rates. Overall repeated measures ANOVAs (2 levers × 2 reinforcer types × 5 WC compound doses) of lever response rates following pretreatment with all three WC compounds revealed a main effect of lever: WC10 (F(1,6) = 13.8), WC26 (F(1,7) = 24.0), and WC44 (F(1,8) = 34.2, p < 0.05 for all), indicating higher response rates on the active lever versus the inactive lever and confirming that the rats were sensitive to the change of active lever contingency between sucrose and cocaine components. We subsequently analyzed active and inactive lever response rates separately. The ANOVAs (2 reinforcer types × 5 WC compound doses) of active lever response rates revealed a main effect of reinforcer type in the WC44 experiment only (F(1,8) = 7.48, p = 0.026), indicating lower active lever response rates during the cocaine components relative to the sucrose components regardless of WC44 dose. With all three WC compounds tested, there were main effects of dose: WC10 (F(4,24) = 7.12), WC26 (F(4,28) = 4.05), and WC44 (F(4,32) = 5.13, p < 0.05 for all), but no dose × reinforcer type interactions. Post-hoc tests of the main effect of dose, collapsed across reinforcer type, revealed that 10.0 mg/kg of WC10 significantly reduced active lever response rate, whereas none of the post-hoc tests comparing WC26 and WC44 against their vehicles were significant (Figure 2). However, differences relative to vehicle were revealed by planned comparison t-tests. For all three WC compounds the 10.0 mg/kg dose reduced active lever response rates relative to vehicle during the sucrose components: WC10 (t(6) = 5.01, p = 0.002, Figure 2A), WC26 (t(7) = 2.84, p = 0.025, Figure 2B), and WC44 (t(8) = 2.67, p = 0.028, Figure 2C). The 1.0 mg/kg dose of WC44 actually increased active lever response rates relative to vehicle (t(8) = −2.45, p = 0.04, Figure 2C). During the cocaine components, the 10.0 mg/kg dose decreased active lever response rates relative to vehicle for WC10 (t(6) = 3.21, p = 0.018) and WC44 (t(8) = 2.34, p = 0.047), but not for WC26 (t(7) = 1.51, p = 0.17, Figure 2).
Figure 2.
Effect of WC compounds on active lever response rates for sucrose and cocaine. Graphs show the mean active lever responses/30 min (± SEM) totaled across the two 15-min sucrose (grey squares) or cocaine (black circles) components of the multiple schedule following pretreatment with vehicle (V) or varying doses of WC10 (A), WC26 (B), and WC44 (C). For all three WC compounds there was a main effect of dose. For the WC44 experiment, there was also a main effect of reinforcer type, indicating higher response rates overall during sucrose components relative to cocaine components. Planned comparisons of each dose to the respective vehicle treatments revealed differences both when cocaine was available (*) and when sucrose was available (+), planned t-test, p < 0.05.
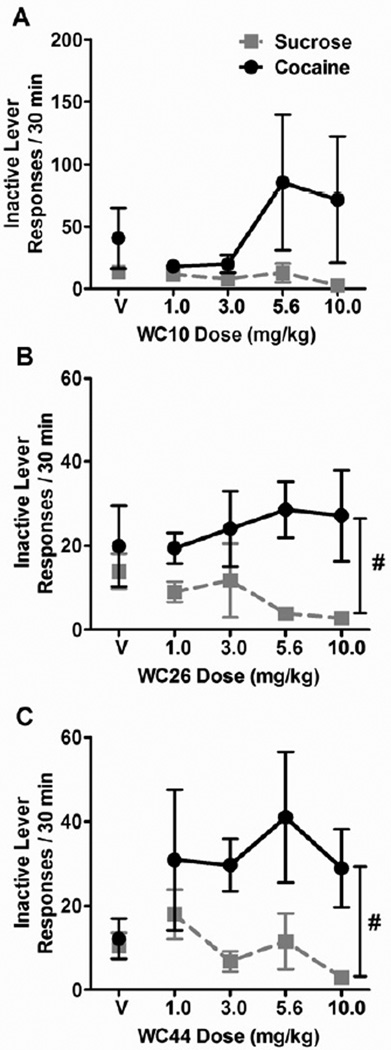
The ANOVAs (2 reinforcer types × 5 WC compound doses) of inactive lever response rates (Figure 3) revealed main effects of reinforcer type for WC26 (F(1,7) = 16.1) and WC44 (F(1,8) = 11.1, p < 0.05 for all), but not for WC10. There was no effect of WC compound dose or reinforcer type × WC compound dose interaction for any of the compounds. These findings indicate higher inactive lever response rates during cocaine components relative to sucrose components in the WC26 and WC 44 experiments, regardless of the pretreatment dose. These observations may reflect a bias for pressing the lever associated with sucrose, since overall response rates on the sucrose lever were higher in general.
Figure 3.
Effect of WC compounds on inactive lever responding for sucrose and cocaine. Graphs show the mean inactive lever responses/30 min (± SEM) totaled across the two 15-min sucrose (grey squares) or cocaine (black circles) components of the multiple schedule following pretreatment with vehicle (V) or varying doses of WC10 (A), WC26 (B), and WC44 (C). For WC26 and WC44 there was a main effect of reinforcer type (#), indicating higher inactive lever response rates during cocaine components relative to sucrose components, regardless of WC compound dose.
Response latency for components 1 (sucrose) and 2 (cocaine) was analyzed separately from components 3 (sucrose) and 4 (cocaine), because rats were under the influence of cocaine at the beginning of components 3 and 4 but not 1 and 2, and there is a possibility that the effect of the WC compounds may depend on whether the rats are under the influence of cocaine.
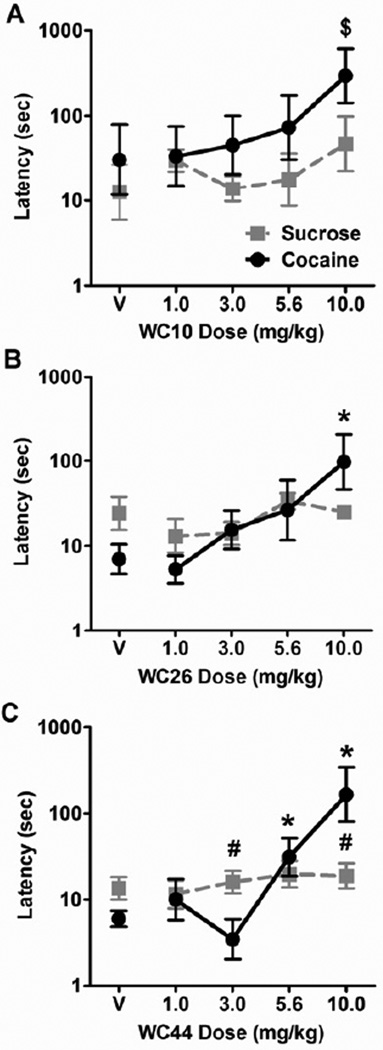
The effects of the WC compounds on response latencies during components 1 (sucrose) and 2 (cocaine), when rats were not under the influence of cocaine, are shown in Figure 4. For WC10, there was a main effect of dose (F(4,28) = 2.79, p = 0.045), but no reinforcer type × dose interaction (Figure 4A). Trend analysis also revealed a significant linear contrast of dose (F(1,7) = 13.59, p = 0.008), but no significant reinforcer type × dose interaction of the linear contrast. This suggests that WC10 increased response latency linearly as a function of dose, regardless of the reinforcer available. Post-hoc analysis with reinforcer type collapsed revealed that 10.0 mg/kg of WC10 increased response latency compared to vehicle regardless of the reinforcer available. However, planned comparisons against vehicle separately for each reinforcer type were unable to detect any significant differences, likely due to a lack of power.
Figure 4.
Effect of WC compounds on latencies to respond for sucrose and cocaine during components 1 and 2. Graphs show the geometric mean latency in sec (± SEM) from the beginning of the respective components to the first active lever response for sucrose (component 1, grey squares) and cocaine (component 2, black circles) following pretreatment with vehicle (V) or varying doses of WC10 (A), WC26 (B), and WC44 (C). Note that the ordinates are on log scales. There was a significant main linear contrast effect of dose for WC10. Post-hoc comparisons averaged across reinforcer typed revealed that 10 mg/kg of WC10 increased response latency ($). There were significant dose × reinforcer type interactions of linear contrast in the WC26 and WC44 experiments. Tests of simple effects found differences in latencies between reinforcer types for WC44 (#). Planned comparisons of each dose to the respective vehicle treatments revealed differences during the cocaine component (*) but not during the sucrose component, planned t-test, p < 0.05.
For WC26, there was a trend towards significance for the reinforcer type × dose interaction (F(4,28) = 2.60, p = 0.058), while linear contrast of the reinforcer type × dose interaction was significant (F(1,7) = 8.84, p = 0.021, Figure 4B). For WC44, significant reinforcer type × dose interactions were found for both ANOVA (F(4,32) = 6.43, p = 0.001) and linear contrast (F(1,8) = 12.10, p = 0.008, Figure 4C). This suggests that WC26 and WC44 affected response latency differently for cocaine vs. sucrose. This was supported by analyses of the simple effects of WC26 and WC44 on cocaine vs. sucrose separately. When sucrose was the reinforcer, neither WC26 nor WC44 had a significant linear contrast effect on response latency. However, when cocaine was the reinforcer, the linear contrast effects were significant for WC26 (F(1,7) = 25.62) and WC44 (F(1,8) = 21.73, p < 0.05 for all). Post-hoc comparisons of vehicle against higher doses of the WC compounds for the cocaine component revealed that 10.0 mg/kg of both WC26 (t(7) = 3.39, p = 0.011) and WC44 (t(8) = 4.02, p = 0.004) significantly increased response latency. In addition, planned comparisons found that 5.6 mg/kg of WC44 also increased response latency for cocaine (t(8) = 2.76, p = 0.025), while none of the doses of WC26 and WC44 affected response latency for sucrose. Tests of the simple effects of reinforcer type at each dose of the WC compound found that response latency was higher for cocaine than sucrose at 10 mg/kg of WC44 (t(8) = 2.60, p = 0.032). Surprisingly, the opposite was found at 3 mg/kg of WC44 (t(8) = 3.35, p = 0.010).
Analyses of the effects of the WC compounds on response latency during components 3 (sucrose) and 4 (cocaine), after rats had been exposed to cocaine during component 2, found that despite differences in baseline response latency for sucrose compared with cocaine after vehicle pretreatment, the effects of the three WC compounds were similar to those observed during components 1 and 2. That is, WC10 caused a non-selective linear increase in response latency for both cocaine and sucrose, whereas WC26 and WC44 caused a selective linear increase in response latency only for cocaine but not sucrose. Details of the analyses are provided in the supplementary materials (section S1).
It is possible that increased response latencies on the active lever caused by the WC compounds were associated with changes in responding on the inactive lever prior to the first active lever press. However, ANOVAs (2 reinforcer type × 5 WC compound doses) failed to detect any main effects or interactions involving WC compound dose for inactive lever response rate or the total number of inactive lever responses prior to first active lever press, suggesting that inactive lever responding during the active lever response latency period was not affected by the WC compounds. Detailed analyses and figures are included in the supplementary materials.
3.2 Effects of WC compounds on the cocaine dose-effect function
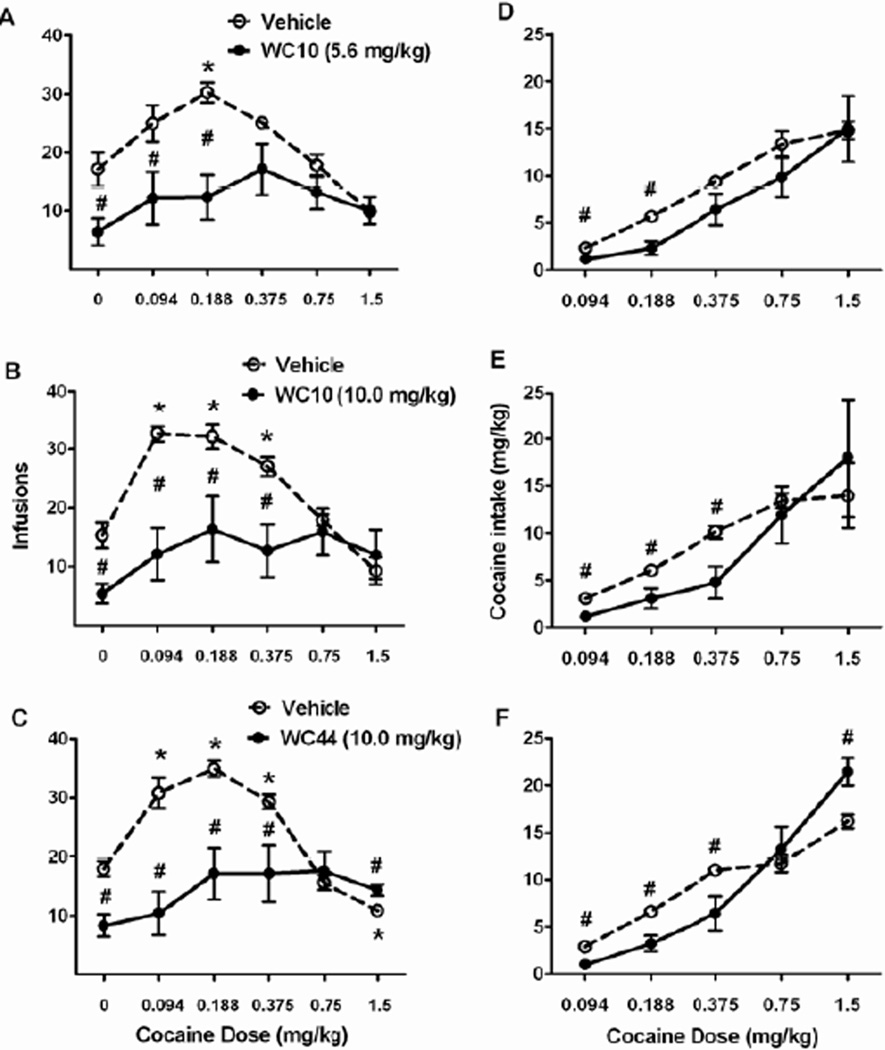
The effects of 5.6 and 10.0 mg/kg of WC10 and 10.0 mg/kg of WC40 on the cocaine dose-effect function were examined. Note that WC26 was not tested in these experiments because we chose to focus on the compounds with the lowest and the highest intrinsic activity. In the WC10 experiments, the catheters of 2 out of 9 subjects pretreated with the 5.6 mg/kg dose and 3 out of 7 subjects pretreated with the 10.0 mg/kg dose became non-patent prior to the test at the highest cocaine dose (1.5 mg/kg). Therefore, the highest cocaine dose was omitted from the ANOVAs for these experiments, but the means for the subjects that were tested at this dose are shown in the graphs. The effects of WC10 on these subjects at this dose were analyzed using planned comparisons only.
Both WC10 (5.6 and 10.0 mg/kg) and WC44 (10.0 mg/kg) flattened the inverted U-shaped cocaine dose-effect function of cocaine reinforcement rates (Figure 5A–C). In all three experiments the overall ANOVAs revealed significant main effects of a) WC compound: WC10 5.6 mg/kg (F(1,8) = 32.53), WC10 10.0 mg/kg (F(1,6) = 19.37), WC44 (F(1,7) = 42.47, p < 0.05 for all), and b) cocaine dose: WC10 5.6 mg/kg (F(4,32) = 7.92), WC10 10.0 mg/kg (F(4,24) = 8.41), WC44 (F(5,35) = 7.48, p < 0.005 for all). There were also significant WC compound × cocaine dose interactions in all three experiments: WC10 5.6 mg/kg (F(4,32) = 3.32), WC10 10.0 mg/kg (F(4,24) = 2.90), WC44 (F(5,35) = 8.60, p < 0.05 for all).
Figure 5.
Effect of WC10 and WC44 on cocaine reinforcement rates and intake. Graphs show the mean number of reinforcers/60 min (± SEM) after pretreatment with either 5.6 (A) or 10.0 mg/kg WC10 (B), or 10.0 mg/kg WC44 (C, black circles), versus vehicle (open circles) under a VI60 schedule with varying doses of cocaine available, and the mean cocaine intake in mg/kg (± SEM) in each of these experiments (D, E, and F, respectively). Separate cohorts of rats were used to test each compound (n = 9, 7, and 8 respectively). Animals were tested with each dose of cocaine available after pretreatment with either vehicle or WC compound across test days. The different cocaine doses were tested in an ascending order of dose, while the order of vehicle versus WC compound pretreatment at each cocaine dose was counterbalanced between rats. Pretreatment occurred 5 min prior to the beginning of the test session. Additional self-administration sessions were given between tests to re-establish stable intake rates. ANOVAs of cocaine reinforcement rates found significant cocaine dose × WC compound pretreatment interactions in all three experiments. ANOVAs of total cocaine intake found a significant main effect of 5.6 mg/kg of WC10, a trend towards significance in the case of 10 mg/kg of WC10 (p = 0.08), and a significant cocaine dose × WC44 pretreatment interaction. Asterisks (*) indicate a significant difference relative to 0 mg/kg dose of cocaine after vehicle pretreatment, p < 0.01. Pound signs (#) indicate a significant difference between pretreatment with vehicle versus with WC compounds, p ≤ 0.05.
To further analyze the interactions, separate ANOVAs of the effect of cocaine dose were conducted for pretreatment with vehicle and WC compounds. In all three experiments, main effects of cocaine dose on reinforcement rates were observed when animals were pretreated with vehicle: WC10 5.6 mg/kg (F(4,32) = 9.34), WC10 10.0 mg/kg (F(4,24) = 21.48), WC44 (F(5,35) = 35.67, p < 0.05 for all). There were also significant quadratic trends as a function of cocaine dose (p < 0.05 for all), in agreement with the inverted U-shaped dose-effect functions typically observed with cocaine. Post-hoc analyses of the cocaine dose main effect in vehicle-pretreated animals revealed that, relative to when saline was available (0 mg/kg cocaine dose), reinforcement rate increased at the 0.188 mg/kg cocaine dose in all 3 experiments (αcorr for 5 comparisons = 0.010; Figure 5A–C). Post-hoc analyses found that reinforcement rates also increased relative to 0 mg/kg dose at the 0.094 and 0.375 mg/kg cocaine doses in the 10.0 mg/kg WC10 and WC44 experiments. In the WC44 experiment, there was also a significant decrease in cocaine reinforcement rate at the 1.5 mg/kg cocaine dose relative to 0 mg/kg dose in the vehicle-pretreated condition (p = 0.001, Figure 5C).
In contrast to pretreatments with vehicle, when animals were pretreated with the WC compounds, ANOVAs found a significant main effect of cocaine dose only in the 5.6 mg/kg WC10 experiment (F(4,32) = 3.15, p = 0.027, Figure 5A). However, post-hoc analyses failed to detect differences in reinforcement rates at any of the cocaine doses relative to 0 mg/kg dose (αcorr = 0.010; minimum p = 0.013 at 0.375 mg/kg cocaine). The main effects of cocaine dose in animals pretreated with either 10.0 mg/kg WC10 or WC44 were not significant (Figure 5B–C). Furthermore, there were no significant quadratic trends typical of the inverted U-shaped cocaine dose-effect function after pretreatments with any of the WC compounds.
Tests of simple effects of each WC compound on cocaine reinforcement rate at each cocaine dose were also performed. In all 3 experiments (Figure 5A – C), pretreatment with the WC compound reduced the reinforcement rates relative to vehicle pretreatment when animals had access to a) 0 mg/kg of cocaine: WC10 5.6 mg/kg (t(8) = 5.37, p = 0.001), WC10 10.0 mg/kg (t(6) = 3.68, p = 0.010), WC44 (t(7) = 4.13, p = 0.004), b) 0.094 mg/kg of cocaine: WC10 5.6 mg/kg (t(8) = 4.17, p = 0.003), WC10 10.0 mg/kg (t(6) = 5.53, p = 0.001), WC44 (t(7) = 6.14, p = 0.001), and c) 0.188 mg/kg of cocaine: WC10 5.6 mg/kg (t(8) = 5.11, p = 0.001), WC10 10.0 mg/kg (t(6) = 2.44, p = 0.050), WC44 (t(7) = 4.24, p = 0.004). WC10 and WC44 (10.0 mg/kg) also decreased reinforcement rate for 0.375 mg/kg cocaine dose: WC10 10.0 mg/kg (t(6) = 2.69, p = 0.036), WC44 (t(7) = 2.72, p = 0.030). Surprisingly, WC44 pretreatment also produced a slight increase in reinforcement rate at the high 1.5 mg/kg cocaine dose (t(7) = 4.04, p = 0.005).
Both WC10 (5.6 mg/kg and 10.0 mg/kg) and WC44 (10.0 mg/kg) also altered total cocaine intake (mg/kg, Figure 5D–F). In all three experiments, the overall ANOVAs revealed significant main effects of cocaine dose: WC10 5.6 mg/kg (F(3,24) = 49.3), WC10 10.0 mg/kg (F(3,18) = 36.1), and WC44 (F(4,28) = 96.97, p < 0.05 for all). In each case, there was also a significant linear trend for cocaine dose, indicating that as the cocaine dose increased, total cocaine intake increased regardless of pretreatment. For the 5.6 mg/kg WC10 experiment (Figure 5D), there was also a main effect of pretreatment (F(1,8)= 11.3, p = 0.010), indicating that cocaine intake was lower following pretreatment with 5.6 mg/kg WC10 versus vehicle regardless of cocaine dose. In the 10.0 mg/kg WC10 experiment (Figure 5E) there was only a trend towards a main effect of WC10 pretreatment (F(1,6) = 4.50, p = 0.078). There was no significant interaction at 5.6 and 10.0 mg/kg of WC10. Planned comparisons between vehicle and WC10 pretreatments at each dose of cocaine further revealed that both 5.6 and 10.0 mg/kg of WC10 reduced cocaine intake at a) 0.094 mg/kg cocaine dose: WC10 5.6 mg/kg (t(8) = 4.17, p = 0.003), WC10 10.0 mg/kg (t(6) = 5.53, p = 0.001), and b) 0.188 mg/kg cocaine dose: WC10 5.6 mg/kg (t(8) = 5.11, p = 0.001), WC10 10.0 mg/kg (t(6) = 2.44, p = 0.050). WC10 (10.0 mg/kg) also reduced cocaine intake when 0.375 mg/kg cocaine dose was available (t(6) = 2.68, p = 0.037). In the WC44 experiment (Figure 5F), there was a significant interaction between WC44 pretreatment and cocaine dose (F(4,28) = 6.66, p = 0.001). Tests of simple effects of WC44 pretreatment at each dose of cocaine revealed significant decreases in cocaine intake following WC44 pretreatment at the three lowest doses of cocaine: 0.094 mg/kg (t(7) = 6.14, p = 0.001), 0.188 mg/kg (t(7) = 3.69, p = 0.008), and 0.375 mg/kg (t(7) = 2.74, p = 0.029), and an increase at the highest dose of cocaine (t(7) = 4.04, p = 0.005).
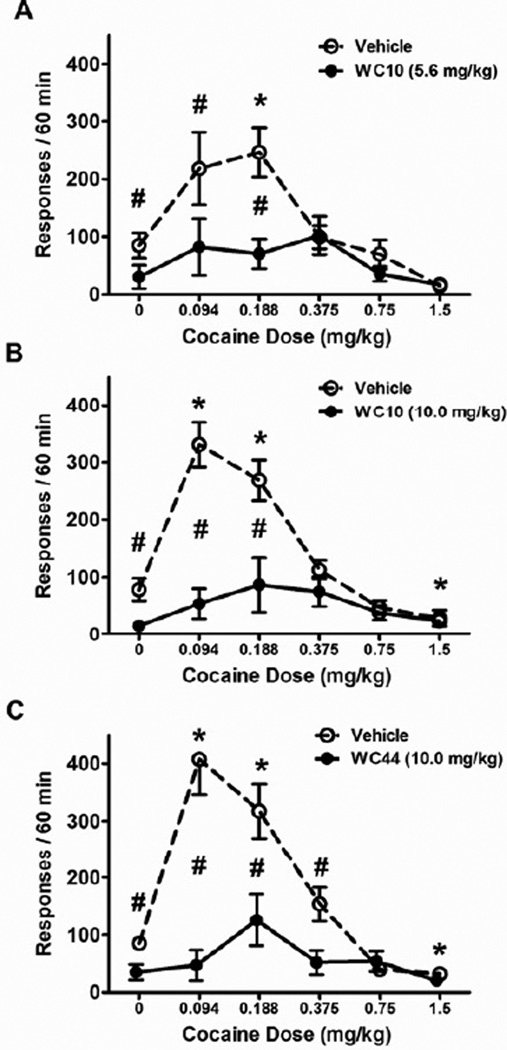
In regard to response rates, in the WC44 experiment one subject was an outlier. Both its active and inactive lever response rates (averaged across cocaine doses) were > 3 standard deviations from group means for both vehicle and WC44 pretreatment conditions. Therefore, we omitted this subject from analyses and graphs depicting response rates. Similar to reinforcement rates, both WC10 (5.6 mg/kg and 10.0 mg/kg) and WC44 (10.0 mg/kg) produced a flattening of the inverted U-shaped cocaine dose-effect function of active lever response rates (Figure 6). In all three experiments the overall ANOVAs revealed significant main effects of a) WC compound: WC10 5.6 mg/kg (F(1,8) = 76.4), WC10 10.0 mg/kg (F(1,6) = 98.24), WC44 (F(1,6) = 120.64, p < 0.05 for all), b) cocaine dose: WC10 5.6 mg/kg (F(4,32) = 5.18), WC10 10.0 mg/kg (F(4,24) = 11.68), WC44 (F(5,30) = 22.82, p < 0.05 for all), and c) WC compound × cocaine dose interactions: WC10 5.6 mg/kg (F(4,32) = 8.23), WC10 10.0 mg/kg (F(4,24) = 10.41), WC44 (F(5,30) = 12.34, p < 0.05 for all). To further analyze the interactions, separate ANOVAs of the effect of cocaine dose were conducted for pretreatment with vehicle and WC compounds. There were main effects of cocaine dose when animals were pretreated with vehicle in all three experiments: WC10 5.6 mg/kg (F(4,32) = 9.21), WC10 10.0 mg/kg (F(4,24) = 19.3), and WC44 (F(5,30) = 25.8, p < 0.05 for all). Post-hoc comparisons revealed that active lever response rates increased at the 0.188 mg/kg cocaine dose in all three experiments relative to when 0 mg/kg cocaine dose was available. Active lever response rates also increased at the 0.094 mg/kg cocaine dose in the WC10 (10.0 mg/kg) and WC44 experiments (Figure 6B–C). In addition, the highest dose of cocaine (1.5 mg/kg) reduced active lever response rates relative to when 0 mg/kg of cocaine was available in vehicle-pretreated rats in the 10.0 mg/kg WC10 and WC44 experiments (Figure 6B–C). In contrast to vehicle pretreatment, post-hoc comparisons revealed that after pretreatments with WC10 and WC44, active lever response rates did not significantly differ across cocaine doses relative to when saline (0 mg/kg cocaine) was available.
Figure 6.
Effect of WC10 and WC44 on responding for cocaine. Graphs show the mean number of active lever responses/60 min (± SEM) after pretreatment with either 5.6 (A) or 10.0 mg/kg WC10 (B), or 10.0 mg/kg WC44 (C, black circles) versus vehicle (open circles) under a VI60 schedule with varying doses of cocaine available. ANOVAs found significant cocaine dose × WC compound pretreatment interactions in all three experiments. Asterisks (*) indicate a significant difference relative to 0 mg/kg dose of cocaine after vehicle pretreatment, p < 0.01. Pound signs (#) indicate a significant difference between pretreatment with vehicle versus with WC compound, p < 0.05.
Tests of simple effects of WC compounds on active lever response rates at each dose of cocaine indicated that all three WC compounds reduced active lever response rates when 0 mg/kg or the next 2 lowest doses of cocaine were available (Figure 6): a) at 0 mg/kg cocaine dose: WC10 5.6 mg/kg (t(7) = 6.47, p = 0.001), WC10 10.0 mg/kg (t(6) = 2.93, p = 0.026), WC44 (t(6) = 3.24, p = 0.009), b) at 0.094 mg/kg cocaine dose: WC10 5.6 mg/kg (t(7) = 3.98, p = 0.004), WC10 10.0 mg/kg (t(6) = 10.68, p = 0.001), WC44 (t(6) = 5.32, p = 0.001), and c) at 0.188 mg/kg cocaine dose: WC10 5.6 mg/kg (t(7) = 5.27, p = 0.001), WC10 10.0 mg/kg (t(6) = 3.62, p = 0.011), WC44 (t(6) = 3.88, p = 0.001). WC44 also reduced response rates relative to vehicle at the 0.375 mg/kg cocaine dose (t(6) = 3.27, p = 0.017).
The ANOVAs of inactive lever response rates revealed a main effect of WC compound for both WC10 experiments: WC10 5.6 mg/kg (F(1,8)=8.26), WC10 10.0 mg/kg (F(1,6)=12.27, p < 0.05 for all). However there was no effect of cocaine dose or WC compound × cocaine dose interactions. The main effects indicate that inactive lever response rates were higher following vehicle pretreatment than following WC10 pretreatments regardless of the available cocaine dose (Table 1). There were no significant effects in the ANOVA of inactive lever response rates in the WC44 experiment.
Table 1.
Mean inactive lever responses (± SEM) during the cocaine dose-effect experiment.a
| Cocaine Dose-effect Experiment (responses/60 min) | ||
|---|---|---|
| Vehicle | WC Compound | |
| WC10 (5.6 mg/kg) | 3.93 ± 1.84 | 2.69 ± 1.48* |
| WC10 (10.0 mg/kg) | 12.54 ± 3.40 | 2.34 ± 1.17* |
| WC44 (10.0 mg/kg) | 16.93 ± 11.81 | 7.81 ± 5.85 |
There was no effect of cocaine dose in any of the cocaine dose-effect experiments. Inactive lever responses were therefore collapsed across doses for presentation.
Difference from vehicle pretreatment, ANOVA main effect, p < 0.05.
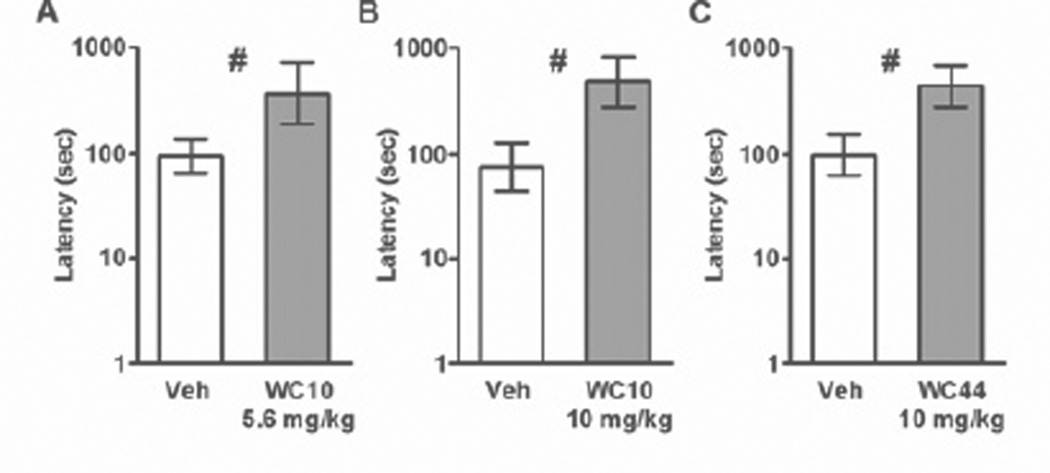
The latencies to first response on the active lever are shown in Figure 7. Response latency data were averaged across cocaine doses because the animals had no way of knowing the upcoming cocaine dose at the beginning of the test sessions. For all three experiments, paired sample t-tests revealed that response latencies were higher after WC compound pretreatment: WC10 5.6 mg/kg (t(8) = 3.18, p = 0.013), WC10 10.0 mg/kg (t(6) = 7.21, p = 0.001), WC44 (t(7) = 3.39, p = 0.012).
Figure 7.
Effect of WC10 and WC44 on latencies to respond for cocaine. Response latencies were averaged across cocaine doses because the animals had no way of knowing the upcoming cocaine dose at the beginning of the test sessions. Graphs show the geometric mean latency to active lever press (± SEM) for pretreatment with either 5.6 (A) or 10.0 mg/kg WC10 (B), or 10.0 mg/kg WC44 (C) versus vehicle pooled across does of cocaine obtained under a VI60 schedule. T-tests found a significant effect of WC compound in all three experiments (#), suggesting that response latencies were increased by WC10 and WC44, p <0.05.
3.3 Effects of WC compounds on spontaneous and cocaine-induced locomotion
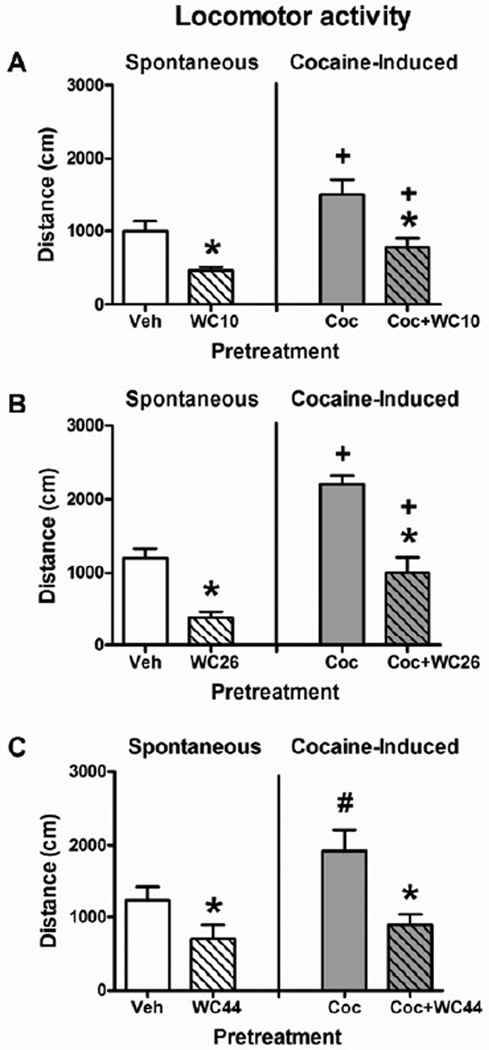
Figure 8 shows the effects of the three WC compounds on spontaneous and cocaine-induced locomotion. ANOVAs with WC pretreatment (vehicle or 10.0 mg/kg WC compound) and cocaine pretreatment (vehicle or 10.0 mg/kg cocaine) as repeated-measure factors revealed main effects of WC pretreatment for all three WC compounds: WC10 (F(1,7) = 16.36, Figure 8A), WC26 (F(1,7) = 113.87, Figure 8B), and WC44 (F(1,5) = 8.22, Figure 8C; p < 0.05 for all). There were also main effects of cocaine in the WC10 (F(1,7) = 14.83, p = 0.006) and WC26 experiments (F(1,7) = 93.85, p = 0.001), reflecting an increase in locomotor activity after cocaine pretreatment. Although the main effect of cocaine was not significant in the WC44 experiment (F(1,5) = 3.77, p = 0.11), the planned t-test of locomotor activity after cocaine pretreatment alone vs. vehicle alone revealed that cocaine increased locomotor activity in this experiment (t(5) = 3.37, p = 0.020). Although inspection of Figure 8C may suggest that pretreatment with WC44 attenuated cocaine-induced locomotor activity, there were no significant cocaine × WC compound interactions in any of the experiments, although the interaction between WC44 and cocaine did approach significance (F(1,5) = 6.10, p = 0.057). The lack of cocaine × WC compound interactions suggests that the magnitude of locomotion decrease caused by the WC compounds was the same regardless of whether the rat was pretreated with cocaine or not, and that the magnitude of locomotion increase caused by cocaine was the same regardless of whether the rat was pretreated with the WC compounds or their vehicles.
Figure 8.
Effects of WC10 (A), WC26 (B), and WC44 (C) on spontaneous and cocaine-induced (Coc) locomotion. Rats were tested 4 times, receiving one of each of the following treatments 5 minutes before the 1-hr test session: vehicle, cocaine (10 mg/kg, IP), their assigned WC compound (10 mg/kg, IP), or both cocaine and their assigned WC compound. The order of these tests was randomized and rats were given 1 day off between test sessions. Graphs show the distance traveled (cm + SEM) in 1 hr. Asterisks (*) indicate a significant main effect of WC compound pretreatment, regardless of cocaine pretreatment. Plus signs (+) indicate a significant main effect of cocaine, regardless of WC compound pretreatment. Planned t-test also indicates a difference between pretreatment with cocaine and pretreatment with vehicle in the WC44 experiment (#).
In order to verify that the WC compounds were behaviorally active at the start of the session for all experiments in this study (i.e., 5 minutes post-treatment), locomotor activity during the first minute of the session (i.e., between the 5th and 6th minute post-treatment) after pretreatments with vehicle vs. with the WC compounds was compared using t-tests. All three WC compounds significantly reduced locomotor activity during the first minute of the session: WC10 (t(7) = 2.38, p = 0.049), WC26 (t(7) = 2.79, p = 0.027), WC44 (t(5) = 4.58, p = 0.006), suggesting that all three WC compounds were behaviorally active between the 5th and 6th minutes post-treatment.
4. Discussion
The present data contribute to a body of evidence suggesting that the D3R modulates cocaine abuse-related behaviors (Blaylock and Nader 2012; Luedtke and Mach 2003). These lines of evidence include 1) high levels of D3R expression in ventral striatum (Lévesque et al. 1992; Sokoloff et al. 1990), a system that has been implicated in both addiction-related behavior and motivated behavior in general (Kalivas and Volkow 2005; Wise 2004), 2) elevated D3R expression in the ventral striatum in rodents upon withdrawal from cocaine self-administration (Conrad et al. 2010; Neisewander et al. 2004), 3) up-regulated D3R expression in the ventral striatum of human cocaine-overdose fatalities (Segal et al. 1997; Staley and Mash 1996), and 4) inhibited motivation for cocaine after treatments with D3R-preferring antagonists (Heidbreder and Newman 2010). The present findings further demonstrate that the D3R-preferring compounds WC26 and WC44 increased response latency when cocaine, but not when sucrose, was available, suggesting a selective decrease in motivation for cocaine. All three WC compounds also non-selectively reduced response rate and reinforcement rate under both the cocaine and sucrose schedule components.
WC10, WC26 and WC44 are three structurally similar arylamide phenylpiperazines with varying degrees of intrinsic activity (Chu et al. 2005). Based on in vitro forskolin-stimulated adenylyl cyclase assays (Chu et al. 2005), WC10, WC26 and WC44 have been characterized as a D3R weak partial agonist/antagonist, partial agonist, and full agonist, having intrinsic activities of 20%, 69% and 96% respectively. Their wide range of intrinsic activity and their predicted ability to cross the blood brain barrier (based on calculated log P values from Chu et al. 2005) suggest that, despite their moderate selectivity for the D3 vs. D2R (23- to 51-fold), the WC compounds are useful tools that can provide insight into the role of the D3R in the reinforcing and motivating effects of cocaine.
Interestingly, the present study found that despite differences in intrinsic activity, the three WC compounds had similar effects on operant responding for cocaine in the multiple schedule experiment. All three compounds dose-dependently reduced cocaine reinforcement rates (Figure 1), and WC10 and WC44 also reduced response rates for cocaine (Figure 2). WC10 and WC44 also produced similar effect on the cocaine dose-effect function, flattening the inverted U-shaped cocaine dose-effect functions of reinforcement rates and response rates mainly by reducing reinforcement rates and response rates at low doses of cocaine (Figures 5 and 6). The ability of WC10 and WC44 to reduce the reinforcement rates at the 0.375 mg/kg cocaine dose replicated the results of the multiple schedule experiments. Pretreatments with WC10 and WC44 also reduced cocaine intake at the lower doses of cocaine (Figure 5). However, WC44 increased reinforcement rates and cocaine intake for the highest dose of cocaine (1.5 mg/kg; Figure 5F), suggesting that the inhibitory effect of WC44 at low cocaine doses is surmountable.
Results of the multiple schedule experiments suggest that all three WC compounds dose-dependently reduced operant responding for sucrose (Figure 2), while WC10 and WC26 also reduced sucrose reinforcement rates (Figure 1). All three WC compounds (10.0 mg/kg) also reduced spontaneous locomotor activity and attenuated cocaine-induced locomotor activity (Figure 8).
The finding that all three WC compounds reduced responding for sucrose and decreased spontaneous locomotor activity raises the possibility that the reduction of cocaine self-administration might be completely explained by a general behavioral suppressant effect, possibly due to motor impairment. However, the response latency data in the multiple schedule experiments suggest that the effects of WC26 and WC44 on responding for cocaine are dissociable from sucrose. Both WC26 and WC44 dose-dependently increased response latency for cocaine in component 2 (Figure 4B–C). However neither compound affected response latency for sucrose in component 1. Surprisingly, 3.0 mg/kg of WC44 reduced response latency for cocaine. The reason for this is unclear. Nonetheless, WC26 and WC44’s ability to dose-dependently increase response latency for cocaine but not sucrose suggests a decrease in motivation to seek cocaine apart from the general (non-reinforcer specific) suppressant effects of these two drugs. In contrast, we cannot rule out a general behavioral suppressant effect for WC10 since it caused a dose-related increase in response latency during cocaine and sucrose components (Figure 4A). Finally, the increases in response latency by WC10 and WC44 were replicated in the cocaine dose-effect experiments (Figure 7).
One drawback of the design of the multiple schedule experiments is that the reinforcer was always sucrose in components 1 and 3, and cocaine in components 2 and 4. Thus, the post-injection time for response latencies shown in Figure 4 was 5 minutes for sucrose but 21 minutes for cocaine. It is therefore possible that WC26 and WC44 had a larger effect during the cocaine component simply because the blood/brain concentrations of the WC compounds were higher. Although we cannot completely rule out this possibility, results from the locomotor activity experiments suggest that all three WC compounds were at least behaviorally active between the 5th and the 6th minute post-treatment (Table 2). Furthermore, analyses of response latency in components 3 and 4 found that, despite a higher baseline response latency for sucrose than for cocaine, the effects of WC26 and WC44 were qualitatively similar to those found in components 1 and 2. That is, both WC compounds dose-dependently and selectively increased latency to respond for sucrose but not cocaine (Figure S1 in the supplementary materials). This suggests that the effects of WC26 and WC44 on response latency persisted throughout the session, despite the possible variations in blood/brain drug concentration during the session.
Table 2.
Mean distance travelled (± SEM) during the first minute since the start of the locomotor sessions (i.e., between the 5th and the 6th minute post-treatment). Only the vehicle and the WC compound-only conditions are compared.
| Distance travelled (cm) | ||
|---|---|---|
| Vehicle | WC Compound | |
| WC10 | 44.61 ± 6.22 | 20.88 ± 6.68* |
| WC26 | 57.32 ± 4.60 | 34.14 ± 5.39* |
| WC44 | 56.07 ± 8.52 | 32.28 ± 7.34* |
Difference from vehicle pretreatment, t-test, p < 0.05.
Interestingly, the multiple schedule experiment found that 1.0 mg/kg of WC44 increased responding during the sucrose components (Figure 2C). This finding was unexpected, since larger doses of WC44 reduced responding, but it may be worth noting that Weber et al. (2009) found that, over a range of doses of WC44 similar to those used in the present experiment, 1.0 mg/kg of WC44 increased the magnitude of the startle response in the rat. It is unclear whether the two observations are related, but one possibility is that this dose of WC44 enhanced reactivity to the experimental environment and tone cues used in these two studies. These findings provide evidence that WC44 is pharmacologically active at 1.0 mg/kg, but had the opposite effect on responding compared to the larger dose of 10 mg/kg. One reason why the two doses had opposite effects may be that WC44 was acting at two different receptors, for example D3Rs vs. serotonin-1A (5-HT1A) receptors, or was acting at D3Rs with different second messenger systems (see below). Alternatively, the lower dose of WC44 may have predominantly affected dopamine autoreceptors while the higher dose may have predominately affected postsynaptic dopamine receptors (Bouthenet et al. 1991; Diaz et al. 1995), resulting in opposite behavioral effects.
Based on their ability to inhibit forskolin-dependent stimulation of adenylyl cyclase via cells expressing human D3Rs, WC10 is weak partial agonist/ antagonist, WC26 is a partial D3R agonist and WC44 is a full D3R agonist, while all three WC compounds are partial agonists at D2R (Chu et al. 2005; Kumar et al. 2009). It is therefore important to consider whether the observed effects of the WC compounds are mediated via the D2R, D3R, or both.
Although adenylyl cyclase assays suggest that WC44 acts as a D3R agonist, several of its behavioral effects seem contradictory. First, previous studies found that D2/D3R agonists such as quinpirole can reinstate cocaine seeking behavior, suggesting that motivation for cocaine is increased (De Vries et al. 2002; De Vries et al. 1999; Khroyan et al. 2000; Self et al. 1996), while the D3R-preferring agonist PD-128,907 also reinstated cocaine seeking (Achat-Mendes et al. 2010; although see Khroyan et al. 2000). In contrast, WC44 selectively increased response latency for cocaine in the multiple schedule experiment (Figure 4C), in agreement with a decrease in motivation for cocaine. However it should be noted that the effect of D2/D3R agonist on cocaine seeking can be dose dependent. For example, Fuchs et al. (2002) found that, while cocaine seeking was increased by acute treatments with a high dose of the D2/D3R agonist 7-OH-DPAT, cocaine seeking could also be reduced by 7-OH-DPAT at low doses that also reduced locomotor activity (Khroyan et al. 1995) and increased yawning (Collins et al. 2005), possibly by activating the D3R (Svensson et al. 1994). Second, D3R-preferring agonists with comparable selectivity for D3Rs over D2Rs have been found to shift the cocaine dose-effect function to the left (Barrett et al. 2004; Caine and Koob 1995; Caine et al. 1997; Caine et al. 1999). In contrast, WC44 appeared to shift the dose-effect function of cocaine reinforcement rates to the right (Figure 5C), typically indicative of a competitive antagonism of receptor-mediated effects (Caine et al. 2002), although it should be noted that the present study used a variable interval schedule instead of a fixed ratio schedule. Third, Weber et al. (2009) found that unlike D3R-prefering agonists such as pramipexole, WC44 did not disrupt prepulse inhibition (PPI). Instead, 10 mg/kg of WC44 attenuated PPI disruption induced by pramipexole, presumably by blocking the D3R and not the D2R because the D2R-selective antagonist L-741,626 failed to attenuate pramipexole’s effect. The latter study suggests that WC44 might in fact act as a D3R antagonist in vivo.
One possible reason why WC44’s behavioral effects do not resemble those of D2/D3R agonists may be because WC44 is a partial agonist, not a full agonist, at the D2R (Kumar et al. 2009). Indeed, all three WC compounds used in the present study are partial agonists at the D2R (Kumar et al. 2009), which may explain why they had similar effects on locomotor activity and responding for cocaine and sucrose, with the exception that WC10 also increased response latency for sucrose. D2R partial agonists have been proposed to have therapeutic utility for treating psychostimulant addiction (Pulvirenti and Koob 2002). Previous studies with D2R partial agonists terguride and aripiprazole, which also have affinities at other receptors (including dopamine and serotonin receptors; Millan et al. 2002; Shapiro et al. 2003), found that they caused a rightward/downward shift of the cocaine dose-effect function (Pulvirenti et al. 1998; Sørensen et al. 2008; although see Feltenstein et al. 2007), similar to the effects of WC10 and WC44. In agreement with WC26 and WC44 increasing response latency only for cocaine but not sucrose, acute aripiprazole also shifted preference from cocaine towards food in a concurrent choice paradigm (Thomsen et al. 2008). However, terguride reduced responding for food at a lower dose than for cocaine (Platt et al. 2003). Terguride and aripiprazole also reduced cue- and cocaine-primed cocaine seeking (Feltenstein et al. 2007; Feltenstein et al. 2009; Khroyan et al. 2000). It is currently unclear to what extent D2R partial activation contributed to the present effects (and the effects of other non-selective D2R partial agonists). However, considering that all three WC compounds have similar intrinsic activities at the D2R but differ in several behavioral effects in the present study (see Figure 4 and Table 1) and previous studies (Kumar et al. 2009; Weber et al. 2009), it is unlikely that partial activation at the D2R alone accounted for all of the behavioral effects of the three WC compounds. It is possible that the behavioral effects of the WC compounds result from an interaction between D2R partial activation and binding at the D3Rs.
Another reason why WC44’s effects do not resemble those of D2/D3R agonists may be because its behavioral effects were mediated via an intracellular signaling cascade downstream of the receptor that is independent of adenylyl cyclase. The concept that a drug can cause the differential activation of second-messenger signaling pathways mediated via a single G-proteincoupled receptor is referred to as functional selectivity of a drug (Kilts et al. 2002; Mailman 2007; Mottola et al. 2002; Urban et al. 2007). For example, a recent study found that several D3R-selective phenylpiperazines have high intrinsic activity for the adenylyl cyclase inhibition pathway but moderate to low intrinsic activity in the mitogenic activation signally pathway (Taylor et al. 2010). However, it is unclear whether an inhibition of the mitogenic activation signally pathway can explain the present results. Although there is a correlation between mitogenic stimulation via the D3R and potency for shifting the cocaine dose-effect function to the left (Caine et al. 1997), NGB 2904 and PG619 failed to inhibit cocaine self-administration despite acting as D3R antagonists in mitogenic assays (Blaylock et al. 2011; Martelle et al. 2007; Xi et al. 2006).
Finally, it should be noted that all three of the WC compounds evaluated in the present experiments also display high affinities for the 5-HT1A receptors (Chu et al. 2005), although it is currently not known if these compounds have functional activity at these receptors. The 5-HT1A receptor agonist 8-OH-DPAT has previously been found to have inconsistent effects on cocainerelated behavior (Czoty et al. 2005; Peltier and Schenk 1993) and food-related behavior (Dourish et al. 1985; Ebenezer 1992; Ho et al. 2003; Zhang et al. 2005), whereas the 5-HT1A receptor antagonist WAY-100635 attenuated cocaine-primed reinstatement of extinguished cocaine seeking (Burmeister et al. 2004; Schenk 2000) and decreased breakpoints for food on progressive ratio schedules (Ho et al. 2003). If the effects observed in this study were really due to 5-HT1A receptor activation, they should be attenuated by WAY-100635, whereas if the effects were really due to 5-HT1A receptor antagonism, they should be attenuated by a 5-HT1A receptor agonist. A further possibility that the observed effects reflect the interaction between D2Rs or D3Rs and 5-HT1A receptors cannot be ruled out, especially since WC44 increased response rate at a low dose but decrease response rate at a high dose during the sucrose components in the multiple schedule experiment. Further experiments will be required to establish the extent to which the WC compounds’ effects in the present study are mediated via the 5-HT1A receptors.
In conclusion, three high affinity D3 dopamine receptor-selective arylamide phenylpiperazines with varying intrinsic activities at D3 receptors were found to effectively reduce self-administration of low to moderate doses of cocaine in rats. Responding for sucrose and spontaneous locomotor activity were also reduced in a dose-dependent manner. Although WC10 increased response latency for both sucrose and cocaine, WC26 and WC44 selectively increased response latency for cocaine but not for sucrose. The latter observation suggests that motivation for cocaine may have been reduced as a consequence of D3 receptor occupancy.
Supplementary Material
HIGHLIGHTS.
-
-
We tested the effect of three D3R compounds on responding for cocaine and sucrose.
-
-
WC10, WC26 and WC44 reduced responding for cocaine and sucrose.
-
-
WC26 and WC44 increased latency to respond for cocaine but not sucrose.
-
-
WC10 and WC44 reduced responding for several doses of cocaine.
-
-
Results suggest that motivation for cocaine is reduced by D3R compounds.
Acknowledgements
The authors thank Ms. Maria Barajas and Mr. Jeffrey Pang for their expert technical assistance with this project. We also thank Michelle Taylor for proofreading the manuscript. This work was supported by the National Institute on Drug Abuse (DA023957).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achat-Mendes C, Grundt P, Cao JJ, Platt DM, Newman AH, Spealman RD. Dopamine D3 and D2 Receptor Mechanisms in the Abuse-Related Behavioral Effects of Cocaine: Studies with Preferential Antagonists in Squirrel Monkeys. J Pharmacol Exp Ther. 2010;334:556–565. doi: 10.1124/jpet.110.167619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology. 2004;47:256–273. doi: 10.1016/j.neuropharm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Blaylock BL, Gould RW, Banala A, Grundt P, Luedtke RR, Newman AH, Nader MA. Influence of Cocaine History on the Behavioral Effects of Dopamine D-3 Receptor-Selective Compounds in Monkeys. Neuropsychopharmacology. 2011;36:1104–1113. doi: 10.1038/npp.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaylock BL, Nader MA. Dopamine D3 Receptor Function and Cocaine Exposure. Neuropsychopharmacology. 2012;37:297–298. doi: 10.1038/npp.2011.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Lungren EM, Kirschner KF, Neisewander JL. Differential roles of 5-HT receptor subtypes in cue and cocaine reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology. 2004;29:660–668. doi: 10.1038/sj.npp.1300346. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Pretreatment with the dopamine agonist 7-OH-DPAT shifts the cocaine self-administration dose-effect function to the left under different schedules in the rat. Behavioural pharmacology. 1995;6:333–347. [PubMed] [Google Scholar]
- Caine SB, Koob GF, Parsons LH, Everitt BJ, Schwartz JC, Sokoloff P. D3 receptor test in vitro predicts decreased cocaine self-administration in rats. Neuroreport. 1997;8:2373–2377. doi: 10.1097/00001756-199707070-00054. [DOI] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Bergman J. Effects of dopamine D1-like and D2-like agonists in rats that self-administer cocaine. J Pharmacol Exp Ther. 1999;291:353–360. [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J, Vallone D, Saiardi A, Borrelli E. Role of dopamine D2-like receptors in cocaine self-administration: Studies with D2 receptor mutant mice and novel D2 receptor antagonists. J Neurosci. 2002;22:2977–2988. doi: 10.1523/JNEUROSCI.22-07-02977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervo L, Burbassi S, Colovic M, Caccia S. Selective antagonist at D3 receptors, but not non-selective partial agonists, influences the expression of cocaine-induced conditioned place preference in free-feeding rats. Pharmacol Biochem Behav. 2005;82:727–734. doi: 10.1016/j.pbb.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Chu WH, Tu Z, McElveen E, Xu JB, Taylor M, Luedtke RR, Mach RH. Synthesis and in vitro binding of N-phenyl piperazine analogs as potential dopamine D-3 receptor ligands. Bioorg Med Chem. 2005;13:77–87. doi: 10.1016/j.bmc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao JJ, Woods JH. Dopamine agonist-induced yawning in rats: A dopamine D3 receptor-mediated behavior. J Pharmacol Exp Ther. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Ford K, Marinelli M, Wolf ME. Dopamine receptor expression and distribution dynamically change in the rat nucleus accumbens after withdrawal from cocaine self-administration. Neuroscience. 2010;169:182–194. doi: 10.1016/j.neuroscience.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, McCabe C, Nader MA. Effects of the 5-HT1A agonist (+/−)-8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) on cocaine choice in cynomolgus monkeys. Behavioural pharmacology. 2005;16:187–191. doi: 10.1097/00008877-200505000-00008. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer ANM, Binnekade R, Raaso H, Vanderschuren L. Relapse to cocaine- and heroin-seeking behavior mediated by dopamine D2 receptors is time-dependent and associated with behavioral sensitization. Neuropsychopharmacology. 2002;26:18–26. doi: 10.1016/S0893-133X(01)00293-7. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer ANM, Binnekade R, Vanderschuren L. Dopaminergic mechanisms mediating the incentive to seek cocaine and heroin following long-term withdrawal of IV drug self-administration. Psychopharmacology. 1999;143:254–260. doi: 10.1007/s002130050944. [DOI] [PubMed] [Google Scholar]
- Diaz J, Levesque D, Lammers CH, Griffon N, Martres MP, Schwartz JC, Sokoloff P. Phenotypical characterization of neurons expressing the dopamine D3 receptor in the rat brain. Neuroscience. 1995;65:731–745. doi: 10.1016/0306-4522(94)00527-c. [DOI] [PubMed] [Google Scholar]
- Dourish CT, Hutson PH, Curzon G. Low doses of the putative serotonin agonist 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT) elicit feeding in the rat. Psychopharmacology. 1985;86:197–204. doi: 10.1007/BF00431709. [DOI] [PubMed] [Google Scholar]
- Duarte C, Lefebvre C, Chaperon F, Hamon M, Thiebot MH. Effects of a dopamine D3 receptor ligand, BP 897, on acquisition and expression of food-, morphine-, and cocaine-induced conditioned place preference, and food-seeking behavior in rats. Neuropsychopharmacology. 2003;28:1903–1915. doi: 10.1038/sj.npp.1300276. [DOI] [PubMed] [Google Scholar]
- Ebenezer IS. Effects of the 5-HT1A agonist 8-OH-DPAT on food intake in food-deprived rats. Neuroreport. 1992;3:1019–1022. doi: 10.1097/00001756-199211000-00019. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Altar CA, See RE. Aripiprazole blocks reinstatement of cocaine seeking in an animal model of relapse. Biol Psychiatry. 2007;61:582–590. doi: 10.1016/j.biopsych.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Do PH, See RE. Repeated aripiprazole administration attenuates cocaine seeking in a rat model of relapse. Psychopharmacology. 2009;207:401–411. doi: 10.1007/s00213-009-1671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Tran-Nguyen LTL, Weber SM, Khroyan TV, Neisewander JL. Effects of 7-OH-DPAT on cocaine-seeking behavior and on re-establishment of cocaine self-administration. Pharmacol Biochem Behav. 2002;72:623–632. doi: 10.1016/s0091-3057(02)00731-1. [DOI] [PubMed] [Google Scholar]
- Gal K, Gyertyan I. Dopamine D3 as well as D2 receptor ligands attenuate the cue-induced cocaine-seeking in a relapse model in rats. Drug Alcohol Depend. 2006;81:63–70. doi: 10.1016/j.drugalcdep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Gilbert JG, Newman AH, Gardner EL, Ashby CR, Jr, Heidbreder CA, Pak AC, Peng XQ, Xi ZX. Acute administration of SB-277011A, NGB 2904, or BP 897 inhibits cocaine cue-induced reinstatement of drug-seeking behavior in rats: role of dopamine D3 receptors. Synapse. 2005;57:17–28. doi: 10.1002/syn.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Newman AH. Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. J Med Chem. 2005;48:839–848. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH. Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann N Y Acad Sci. 2010;1187:4–34. doi: 10.1111/j.1749-6632.2009.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley AE, Spiller K, Grundt P, Newman AH, Kiefer SW, Xi ZX, Gardner EL. PG01037, a novel dopamine D3 receptor antagonist, inhibits the effects of methamphetamine in rats. J Psychopharmacol. 2011;25:263–273. doi: 10.1177/0269881109358201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MY, Body S, Kheramin S, Bradshaw CM, Szabadi E. Effects of 8-OH-DPAT and WAY-100635 on performance on a time-constrained progressive-ratio schedule. Psychopharmacology. 2003;167:137–144. doi: 10.1007/s00213-002-1375-9. [DOI] [PubMed] [Google Scholar]
- Howell DC. Statistical Methods for Psychology. Fourth edn. Belmont, California: Wadsworth; 2007. [Google Scholar]
- Joyce JN, Millan MJ. Dopamine D3 receptor antagonists as therapeutic agents. Drug discovery today. 2005;10:917–925. doi: 10.1016/S1359-6446(05)03491-4. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Keppel G, Wickens TD. Design and analysis: a researcher’s handbook. Fourth edn. London: Prentice-Hall; 2004. [Google Scholar]
- Khroyan TV, Baker DA, Neisewander JL. Dose-dependent effects of the D-3-preferring agonist 7-OH-DPAT on motor behaviors and place conditioning. Psychopharmacology. 1995;122:351–357. doi: 10.1007/BF02246265. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Barrett-Larimore RL, Rowlett JK, Spealman RD. Dopamine D1-and D2-like receptor mechanisms in relapse to cocaine-seeking behavior: effects of selective antagonists and agonists. J Pharmacol Exp Ther. 2000;294:680–687. [PubMed] [Google Scholar]
- Kilts JD, Connery HS, Arrington EG, Lewis MM, Lawler CP, Oxford GS, O’Malley KL, Todd RD, Blake BL, Nichols DE, Mailman RB. Functional selectivity of dopamine receptor agonists. II. Actions of dihydrexidine in D(2L) receptor-transfected MN9D cells and pituitary lactotrophs. J Pharmacol Exp Ther. 2002;301:1179–1189. doi: 10.1124/jpet.301.3.1179. [DOI] [PubMed] [Google Scholar]
- Kumar R, Riddle LR, Griffin SA, Chu WH, Vangveravong S, Neisewander J, Mach RH, Luedtke RR. Evaluation of D2 and D3 dopamine receptor selective compounds on L-dopa-dependent abnormal involuntary movements in rats. Neuropharmacology. 2009;56:956–969. doi: 10.1016/j.neuropharm.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P. Increased dopamine D3 receptor expression accompanying behavioral sensitization to nicotine in rats. Synapse. 2003;47:176–183. doi: 10.1002/syn.10170. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Frances H, Diaz J, Schwartz JC, Sokoloff P. Role of the dopamine D3 receptor in reactivity to cocaine-associated cues in mice. Eur J Neurosci. 2002;15:2016–2026. doi: 10.1046/j.1460-9568.2002.02049.x. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR, Sokoloff P. The dopamine D3 receptor and drug dependence: effects on reward or beyond? Neuropharmacology. 2005;49:525–541. doi: 10.1016/j.neuropharm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Levant B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol Rev. 1997;49:231–252. [PubMed] [Google Scholar]
- Lévesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, Schott D, Morgat JL, Schwartz JC, Sokoloff P. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci U S A. 1992;89:8155–8159. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limpert E, Stahel WA, Abbt M. Log-normal distributions across the sciences: Keys and clues. Bioscience. 2001;51:341–352. [Google Scholar]
- Luedtke RR, Mach RH. Progress in Developing D3 Dopamine Receptor Ligands as Potential Therapeutic Agents for Neurological and Neuropsychiatric Disorders. Current Pharmaceutical Design. 2003;9:643–671. doi: 10.2174/1381612033391199. [DOI] [PubMed] [Google Scholar]
- Mach RH, Huang Y, Freeman RA, Wu L, Blair S, Luedtke RR. Synthesis of 2-(5-bromo-2,3-dimethoxyphenyl)-5-(aminomethyl)-1H-pyrrole analogues and their binding affinities for dopamine D2, D3, and D4 receptors. Bioorg Med Chem. 2003;11:225–233. doi: 10.1016/s0968-0896(02)00341-3. [DOI] [PubMed] [Google Scholar]
- Mailman RB. GPCR functional selectivity has therapeutic impact. Trends Pharmacol Sci. 2007;28:390–396. doi: 10.1016/j.tips.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelle JL, Claytor R, Ross JT, Reboussin BA, Newman AH, Nader MA. Effects of two novel D-3-selective compounds, NGB 2904 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxa mide] and CJB 090 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benza mide], on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 2007;321:573–582. doi: 10.1124/jpet.106.113571. [DOI] [PubMed] [Google Scholar]
- Mauchly JW. Significance test for sphericity of a normal n-variate distribution. Annals of Mathematical Statistics. 1940;11:204–209. [Google Scholar]
- Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J Pharmacol Exp Ther. 2002;303:791–804. doi: 10.1124/jpet.102.039867. [DOI] [PubMed] [Google Scholar]
- Mottola DM, Kilts JD, Lewis MM, Connery HS, Walker QD, Jones SR, Booth RG, Hyslop DK, Piercey M, Wightman RM, Lawler CP, Nichols DE, Mailman RB. Functional selectivity of dopamine receptor agonists. I. Selective activation of postsynaptic dopamine D-2 receptors linked to adenylate cyclase. J Pharmacol Exp Ther. 2002;301:1166–1178. doi: 10.1124/jpet.301.3.1166. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Fuchs RA, Tran-Nguyen LT, Weber SM, Coffey GP, Joyce JN. Increases in dopamine D3 receptor binding in rats receiving a cocaine challenge at various time points after cocaine self-administration: implications for cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1479–1487. doi: 10.1038/sj.npp.1300456. [DOI] [PubMed] [Google Scholar]
- Newman AH, Grundt P, Nader MA. Dopamine D3 receptor partial agonists and antagonists as potential drug abuse therapeutic agents. J Med Chem. 2005;48:3663–3679. doi: 10.1021/jm040190e. [DOI] [PubMed] [Google Scholar]
- Peltier R, Schenk S. Effects of serotonergic manipulations on cocaine self-administration in rats. Psychopharmacology. 1993;110:390–394. doi: 10.1007/BF02244643. [DOI] [PubMed] [Google Scholar]
- Peng XQ, Ashby CR, Spiller K, Li X, Li J, Thomasson N, Millan MJ, Mocaer E, Munoz C, Gardner EL, Xi ZX. The preferential dopamine D3 receptor antagonist S33138 inhibits cocaine reward and cocaine-triggered relapse to drug-seeking behavior in rats. Neuropharmacology. 2009;56:752–760. doi: 10.1016/j.neuropharm.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt DM, Rodefer JS, Rowlett JK, Spealman RD. Suppression of cocaine- and food-maintained behavior by the D2-like receptor partial agonist terguride in squirrel monkeys. Psychopharmacology. 2003;166:298–305. doi: 10.1007/s00213-002-1347-0. [DOI] [PubMed] [Google Scholar]
- Pulvirenti L, Balducci C, Piercy M, Koob GF. Characterization of the effects of the partial dopamine agonist terguride on cocaine self-administration in the rat. J Pharmacol Exp Ther. 1998;286:1231–1238. [PubMed] [Google Scholar]
- Pulvirenti L, Koob GF. Being partial to psychostimulant addiction therapy. Trends Pharmacol Sci. 2002;23:151–153. doi: 10.1016/s0165-6147(00)01991-x. [DOI] [PubMed] [Google Scholar]
- Schenk S. Effects of the serotonin 5-HT2 antagonist, ritanserin, and the serotonin 5-HT1A antagonist, WAY 100635, on cocaine-seeking in rats. Pharmacol Biochem Behav. 2000;67:363–369. doi: 10.1016/s0091-3057(00)00377-4. [DOI] [PubMed] [Google Scholar]
- Segal DM, Moraes CT, Mash DC. Up-regulation of D3 dopamine receptor mRNA in the nucleus accumbens of human cocaine fatalities. Mol Brain Res. 1997;45:335–339. doi: 10.1016/s0169-328x(97)00025-9. [DOI] [PubMed] [Google Scholar]
- Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D-1- and D-2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, Roth BL, Mailman R. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28:1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Song R, Yang RF, Wu N, Su RB, Li J, Peng XQ, Li X, Gaal J, Xi ZX, Gardner EL. YQA14: a novel dopamine D(3) receptor antagonist that inhibits cocaine self-administration in rats and mice, but not in D(3) receptor-knockout mice. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2011.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen G, Sager TN, Petersen JH, Brennum LT, Thøgersen P, Bengtsen CH, Thomsen M, Wörtwein G, Fink-Jensen A, Woldbye DPD. Aripiprazole blocks acute self-administration of cocaine and is not self-administered in mice. Psychopharmacology. 2008;199:37–46. doi: 10.1007/s00213-008-1069-z. [DOI] [PubMed] [Google Scholar]
- Spangler R, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Elevated D3 dopamine receptor mRNA in dopaminergic and dopaminoceptive regions of the rat brain in response to morphine. Brain Res Mol Brain Res. 2003;111:74–83. doi: 10.1016/s0169-328x(02)00671-x. [DOI] [PubMed] [Google Scholar]
- Spiller K, Xi ZX, Peng XQ, Newman AH, Ashby CR, Jr, Heidbreder C, Gaal J, Gardner EL. The selective dopamine D3 receptor antagonists SB-277011A and NGB 2904 and the putative partial D3 receptor agonist BP-897 attenuate methamphetamine-enhanced brain stimulation reward in rats. Psychopharmacology (Berl) 2008;196:533–542. doi: 10.1007/s00213-007-0986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson K, Carlsson A, Waters N. Locomotor inhibition by the D3 ligand R-(+)-7-OH-DPAT is independent of changes in dopamine release. J Neural Transm-Gen Sect. 1994;95:71–74. doi: 10.1007/BF01283032. [DOI] [PubMed] [Google Scholar]
- Taylor M, Grundt P, Griffin SA, Newman AH, Luedtke RR. Dopamine D3 Receptor Selective Ligands With Varying Intrinsic Efficacies at Adenylyl Cyclase Inhibition and Mitogenic Signaling Pathways. Synapse. 2010;64:251–266. doi: 10.1002/syn.20725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Fink-Jensen A, Woldbye DPD, Wortwein G, Sager TN, Holm R, Pepe LM, Caine SB. Effects of acute and chronic aripiprazole treatment on choice between cocaine self-administration and food under a concurrent schedule of reinforcement in rats. Psychopharmacology. 2008;201:43–53. doi: 10.1007/s00213-008-1245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Leonardi-Essmann F, Perreau-Lenz S, Gebicke-Haerter P, Drescher K, Gross G, Spanagel R. The dopamine D3 receptor plays an essential role in alcohol-seeking and relapse. FASEB J. 2006;20:2223–2233. doi: 10.1096/fj.06-6110com. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Ashby CR, Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Mach RH, Luedtke RR, Reichert DE. Subtype selectivity of dopamine receptor ligands: insights from structure and ligand-based methods. J Chem Inf Model. 2010;50:1970–1985. doi: 10.1021/ci1002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Chang WL, Durbin JP, Park PE, Luedtke RR, Mach RH, Swerdlow NR. Using prepulse inhibition to detect functional D3 receptor antagonism: Effects of WC10 and WC44. Pharmacol Biochem Behav. 2009;93:141–147. doi: 10.1016/j.pbb.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gardner EL. Pharmacological actions of NGB 2904, a selective dopamine D3 receptor antagonist, in animal models of drug addiction. CNS drug reviews. 2007;13:240–259. doi: 10.1111/j.1527-3458.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Pak AC, Ashby CR, Jr, Heidbreder CA, Gardner EL. Selective dopamine D-3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. Eur J Neurosci. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR, Jr, Gitajn L, Gardner EL. The novel dopamine D-3 receptor antagonist NGB 2904 inhibits cocaine's rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology. 2006;31:1393–1405. doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]
- Xu J, Chu W, Tu Z, Jones LA, Luedtke RR, Perlmutter JS, Mintun MA, Mach RH. [(3)H]4-(Dimethylamino)-N-[4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl]benzamide, a selective radioligand for dopamine D(3) receptors. I. In vitro characterization. Synapse. 2009;63:717–728. doi: 10.1002/syn.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Rickard JF, Body S, Asgari K, Bradshaw CM, Szabadi E. Comparison of the effects of clozapine and 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) on progressive ratio schedule performance: evidence against the involvement of 5-HT1A receptors in the behavioural effects of clozapine. Psychopharmacology. 2005;181:381–391. doi: 10.1007/s00213-005-2258-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.