Abstract
Choline kinase alpha is hyperactivated in many solid tumours and regulates malignant progression, making it a promising cancer drug target. The successful design and synthesis of novel inhibitors with high cellular activity are described.
Aberrant cell membrane phospholipid metabolism is a common feature of many cancers. A major contributor to this deregulated phenotype is overexpression of choline kinase α (CHKA), an enzyme that catalyses the conversion of choline (Cho) to phosphocholine (PCho). PCho is further metabolised in the CDP-choline pathway to form the major membrane phospholipid, phosphatidylcholine. Choline kinase exists in at least 3 isoforms, choline kinase α (comprising CHKA1 and CHKA2) and choline kinase β (CHKB), of which the α, but not the β isoforms, have been implicated in cancer.1 Overexpression of CHKA has been reported in a wide array of solid tumours and correlates with poor clinical outcome and reduced survival in breast and non-small-cell lung cancer patients.2,3 Although accelerated turnaround of membrane lipids can be expected in highly proliferating tissues, increased activation of the CDP-choline pathway through CHKA overexpression occurs independent of cell proliferation and correlates with increasing malignant transformation of cancer cells, suggesting its active role in cancer progression.4 Activated CHKA facilitates progression through the cell cycle and the cognate metabolites of the CDP-choline pathway serve as precursors for mitogenic lipid second messengers.5,6 Deregulated choline metabolism therefore constitutes a malignant characteristic and can be utilised for diagnosis and treatment surveillance using positron emission tomography (PET) or magnetic resonance spectroscopy (MRS).7–10
The active involvement of CHKA in cancer progression and interaction with oncogenic signalling pathways make it a putative drug target. Several CHK inhibitors have been developed to date and usually comprise of two cationic moieties (usually quaternary pyridinium salts) connected by a lipophilic linker. Despite good antiproliferative properties in vitro with half-maximal growth inhibitory concentrations (GI50) around 1 μM after continuous exposure to live cells for 72 hours, these compounds are not able to completely inhibit the CHK reaction at physiologically relevant doses and are challenging to synthesise.11,12 Here, we describe the development of highly effective and synthetically available CHK inhibitors.
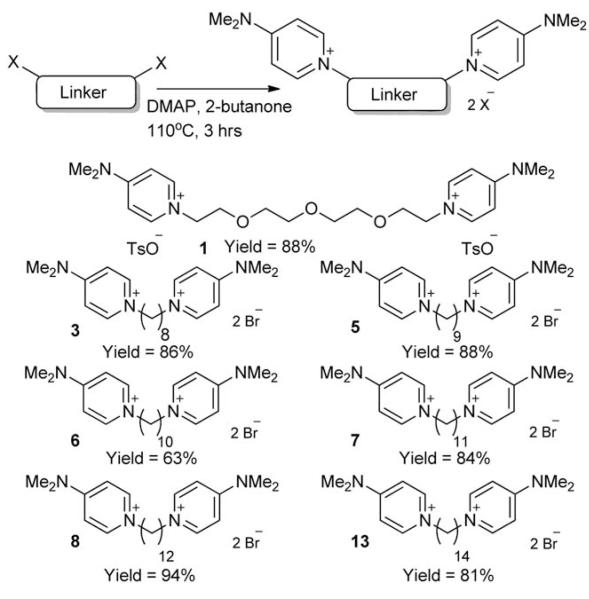
The synthetic approach used for the formation of symmetrical compounds 1, 3, 5–8 and 13 is summarised in Scheme 1 below. N,N-dimethylaminopyridine (DMAP) was employed as cationic choline-mimicking moiety, as this had been shown in previous work to be a highly effective pharmacophore.13 Seven different linker groups (six alkyl chains and one PEG chain) were used to test whether the distance between the two polar end groups would affect choline kinase inhibition and, in the case of 1, whether it was possible to synthesise a more water soluble compound whilst retaining any inhibition properties demonstrated within the remaining examples. The PEG-containing compounds had improved solubility in water but unfortunately proved to be relatively unstable in solution. Each linker was dissolved in 2-butanone with DMAP and subsequently heated to 110 °C for three hours. Precipitation began to occur within ten minutes of the reaction starting, due to the insolubility of the desired doubly charged bis-DMAP products in 2-butanone, allowing straight-forward purification by filtration. Yields were good in each example (~80% in all but one case) and the reactions could be easily carried out routinely on a large scale.
Scheme 1.
Synthesis of symmetrical compounds 1, 3, 5–8 and 13.
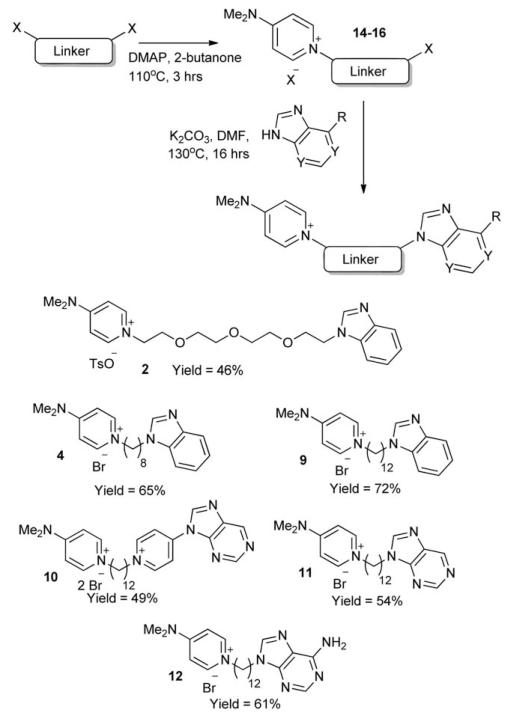
The crystal structure of CHKA has been shown previously,14 and reveals the proximity of an ATP pocket to the choline pocket. Our bis-DMAP compounds were thought to be targeting the choline pocket due to the polar nature of the end groups. We hypothesised that by substituting one of the DMAP groups with an ATP-mimicking head group, we may be able to target both choline and ATP pockets within the CHKA active site, and increase the affinity of our structures for CHKA. The route used to synthesise nonsymmetric compounds is shown in Scheme 2. A dodecyl linker was chosen, as a distance of ~ 14Å is required to bridge both the choline and ATP active sites. Initially, nonsymmetric ‘half’-linker compounds 14–16 (where 14 = C12 linker, 15 = C8 linker, 16 = PEG4 linker) were synthesised using a modified procedure based upon our route for the symmetrical compounds. When a 1 : 1 ratio of DMAP to linker was used, in 2-butanone at 110 °C for three hours, yields of the desired compound containing only one DMAP end group were ~70%. However, under these reaction conditions, up to 15% of the analogous symmetrical compound was also formed, and due to the similar nature of these compounds, separation proved difficult. A revised chemical procedure was developed, using only ½ an equivalent of DMAP (four times less than the initial procedure for the symmetrical synthesis), which gave lower yields of the desired compounds (~50%), but importantly, afforded none of the unwanted symmetrical analogue.
Scheme 2.
Synthesis of nonsymmetrical compounds 2, 4, and 9–12.
With compounds 14–16 in hand, subsequent coupling of a number of ATP-mimicking groups was attempted. Benzimidazole was used as an initial test compound, due to its symmetry preventing any possibly regioisomers, to ascertain conditions required for coupling. After optimisation, it was found that by using DMF as a solvent instead of 2-butanone, and adding 1.2 equivalents of K2CO3, coupling occurred in moderate to good isolated yields (46–72%) to give compounds 2, 4, and 9–12. One drawback which became apparent upon using adenine or purine, was the formation of an N-9 substituted product, as well as the N-3 substituted product, in approximately a 2 : 1 ratio of N-3 to N-9, which has been noted in previous work. Separation of these compounds could be carried out efficiently by column chromatography, giving the pure nonsymmetrical N-3 substituted compounds for biological testing.
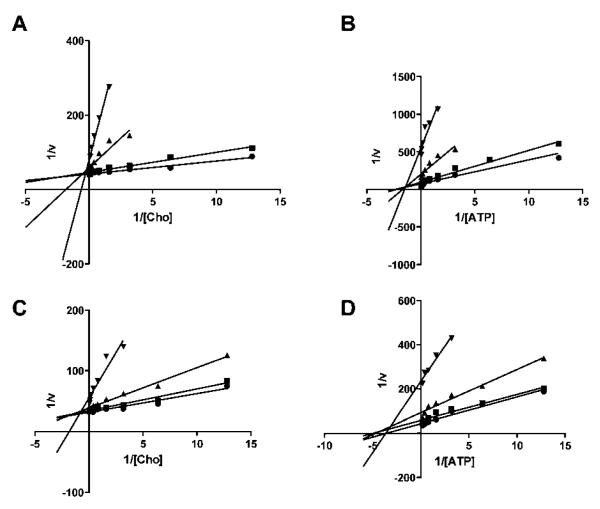
Elongation of the alkyl chain in the symmetrical bis-DMAP compounds successively increased potency against recombinant CHKA2. The C8 linker compound 3 had an IC50 of 2.7 μM, which decreased to 150 nM when the two pharmacophores were separated by an additional 6 carbons (Table 1 and Fig. S1). As expected, these compounds were found to be competitive with choline, but not with ATP as exemplified by Lineweaver–Burk plots of 8 (Fig. 1A and B). In the series of nonsymmetrical inhibitors, substitution of one DMAP resulted in loss of activity, although this was less severe when both charges were retained (10). Compound 11 was found to be unstable and therefore not investigated. While the benzimidazole derivative 9 had only low activity, introduction of an adenine (12) was associated with a significant increase in inhibitory activity (IC50 = 803 nM). As expected, 12 displayed a mixed type of inhibition, as shown by Lineweaver–Burk plots, where the lines intersect at the second quadrant above the x-axis (Fig. 1C and D).
Table 1.
Inhibitory activity against recombinant Δ49N CHKA2 (IC50) or human HCT116 or A549 cancer cell lines (GI50)
| Δ49N CHKA2 |
HCT-116 |
A549 |
|||||
|---|---|---|---|---|---|---|---|
| Compound | Linker | IC50 (μM) | SD | GI50 (μM) | SD | GI50 (μM) | SD |
| 1 | PEG | n/d | >1 mM | n/d | |||
| 2 | PEG | n/d | >1 mM | n/d | |||
| 3 | C8 | 2.7 | 0.6 | 2.5 | 0.3 | 3.3 | 2 |
| 5 | C9 | 1.3 | 0.1 | 2.6 | 0.5 | 4.0 | 0.3 |
| 6 | C10 | 0.67 | 0.08 | 2.8 | 0.2 | 4.2 | 0.6 |
| 7 | C11 | 0.29 | 0.02 | 2.0 | 0.05 | 1.6 | 0.3 |
| 8 | C12 | 0.27 | 0.06 | 0.64 | 0.05 | 0.38 | 0.04 |
| 9 | C12 | 5.1 | 1 | 0.69 | 0.03 | 1.3 | 0.5 |
| 10 | C12 | 0.34 | 0.009 | 1.1 | 0.05 | 0.75 | 0.07 |
| 12 | C12 | 0.80 | 0.08 | 4.1 | 1 | 5.3 | 3 |
| 13 | C14 | 0.15 | 0.04 | 0.29 | 0.006 | 0.33 | 0.009 |
Fig. 1.
Lineweaver–Burk plots of compounds 8 (A and B) and 12 (C and D) against Δ49N CHKA2. Reaction kinetics were measured without inhibitor (●) or in presence of 0.1 (∎), 1 (▴) or 10 μM (▾) 8 or 12. In A and C, choline concentrations were varied and ATP kept constant at KM, while in B and D choline was kept constant at KM and ATP concentrations altered.
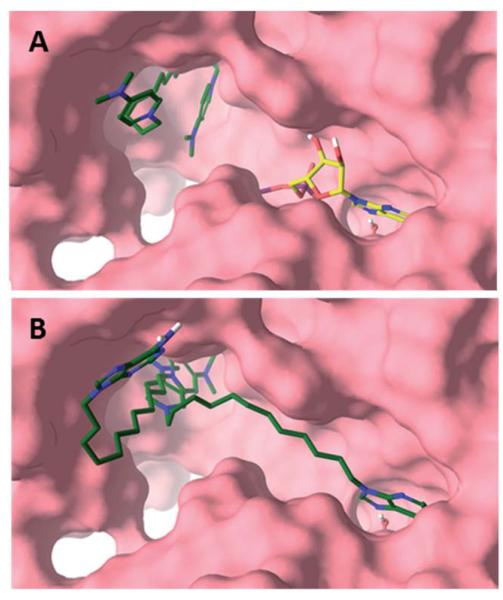
We employed molecular modelling to further characterise possible binding modes. When the bis-DMAP compounds 3, 5–8 and 13 were docked, none of these interacted with the ATP cassette in their most stable poses and instead had a binding resembling the co-crystallised inhibitor hemicholinium-3. The inner cationic DMAP interacted with the positively charged Asp306 in the choline pocket while the carbon linker interacted with the hydrophobic side chains of Tyr333, Trp423, Trp420, Tyr354 and Tyr437 (Fig. 2A and S4). The second DMAP was more solvent exposed and interacted predominately with the hydrophobic residues at the rim of the pocket. Ranking of the bis-DMAP compounds by an estimation of the free energy of binding (MM-GBSA) showed excellent correlation with the experimental IC50 values (Fig. S2). Altogether the biochemical and modelling data suggest that 8 and most likely also the other bis-DMAP compounds are choline-competitive inhibitors and solely interact with the choline active site.
Fig. 2.
Proposed binding modes of 8 (A) and 12 (B) with CHKA2. 8 solely interacted with the choline pocket in its most stable pose. ADP (green) is shown as reference. While the DMAP of 12 interacted with the choline active site, the adenine moiety could interact with the ATP cassette (purple) or the rim (yellow).
Compounds 9–12 on the other hand were designed with an adenine mimic specifically to interact with the ATP pocket. For example, when docked in the absence of ADP, 12 showed two predominant conformations. In its most stable pose, it simultaneously interacted with the ATP and choline binding sites. The adenine moiety of 12 almost completely superimposed with the co-crystallised adenine moiety of ADP and interacted with Glu206 via a water bridge in addition to hydrogen bonding to Ile209 and Gln207. Alternatively, in a lower ranked binding mode the adenine moiety of 12 pointed towards the solvent and hydrogen bonded to the backbone carbonyl of Ser355 at the rim of the enzyme without any interaction with the ATP cassette (Fig. 2B and S5). Compound 12 can interact with both cavities, however, when docked in the presence of ATP, resembling physiological ATP concentrations, the adenine moiety is forced out of the ATP cassette and instead binds to the pocket rim in a fashion resembling hemicholinium-3 (Fig. S3) and compounds 3, 5–8 and 13. Therefore, docking studies together with the kinetic experimental data suggested a mixed type of binding.
Anti-CHKA activity of symmetrical and nonsymmetrical compounds translated well into antiproliferative activity against HCT-116 colorectal adenocarcinoma and A549 lung cancer cell lines, which express CHKA at high levels (Table 1, Fig. S6). In accordance with the cell-free assay, linker elongation in the symmetrical compounds strongly increased cellular efficacy and resulted in compounds with submicromolar growth inhibitory concentrations. The nonsymmetrical compound 9 showed higher cellular than enzymatic activity, which suggests that growth inhibition is mediated by effect on targets other than CHKA.
We then assessed the ability of the most active compounds 8 and 13 to inhibit the CHK reaction in whole cells. To this end, HCT-116 cells were incubated with [3H]Cho in the presence of varying concentrations of inhibitors and a dose-dependent reduction in [3H]PCho formation was measured. The highly effective compounds 8 and 13 were associated with EC50 of 1.3~ 0.7 μM and 0.52~ 0.1 μM and inhibited [3H]PCho formation >75% at concentrations above 6.5 ± 4 and 2.4 ± 1 μM, respectively.
Conclusions
The increased expression and activity of CHKA in many different solid tumours together with the growth-promoting role of choline metabolites make CHKA an attractive target for anticancer therapy. Here, we present the synthesis and evaluation of highly effective CHKA inhibitors, which are synthetically readily available. The symmetrical inhibitors 8 and 13 potently inhibited CHKA, which translated well into high cellular efficacy in different cancer cell lines. These compounds were able to fully inhibit the CHK reaction in whole cells, which is a key requirement for further development, as CHKA inhibitors need to compete with high endogenous Cho concentrations.15
We further aimed to develop novel scaffolds of CHKA inhibitors, which simultaneously inhibit the choline and ATP pockets. A similar approach was published by another group while this manuscript was in preparation.16 Although structurally related to the nonsymmetrical inhibitors presented here, these molecules were relatively inactive (GI50 > 50 μM, IC50 6–10 μM). With our more potent compounds we could demonstrate through direct biochemical evaluation backed by computational modelling that such a simultaneous inhibition may exist but the inability of these compounds to elicit ATP-competitiveness at higher ATP concentrations suggest that symmetrical inhibitors may be more favourable candidates.
The structures presented here could serve as new leads for further optimisation to enhance efficacy, safety and ADMET properties with the view of developing new clinical candidates.
Supplementary Material
NMR spectra and synthetic methods of relevant compounds, biological procedures and molecular modelling
Acknowledgements
We are grateful to Arnon Lavie (University of Illinois) and Manfred Konrad (Max Planck Institute for Biophysical Chemistry) for provision of Δ49N CHKA2. We thank Mr Matt Harvey at Imperial College Computing Service and Prof. Anders Karlén at Uppsala University for computation related services. This work was funded by Cancer Research UK-Engineering and Physical Sciences Research Council grant (in association with the Medical Research Council and Department of Health, England, grant C2536/A10337. E.O.A.’s laboratory receives core funding from the UK Medical Research Council (MC_A652_5PY80).
Notes and references
- 1.Gruber J, Too WC, Wong MT, Lavie A, McSorley T, Konrad M. FEBS J. 2012;279:1915–1928. doi: 10.1111/j.1742-4658.2012.08573.x. [DOI] [PubMed] [Google Scholar]
- 2.Ramirez de Molina A, Gutierrez R, Ramos MA, Silva JM, Silva J, Bonilla F, Sanchez JJ, Lacal JC. Oncogene. 2002;21:4317–4322. doi: 10.1038/sj.onc.1205556. [DOI] [PubMed] [Google Scholar]
- 3.Ramirez de Molina A, Sarmentero-Estrada J, Belda-Iniesta C, Taron M, Ramirez de Molina V, Cejas P, Skrzypski M, Gallego-Ortega D, de Castro J, Casado E, Garcia-Cabezas MA, Sanchez JJ, Nistal M, Rosell R, Gonzalez-Baron M, Lacal JC. Lancet Oncol. 2007;8:889–897. doi: 10.1016/S1470-2045(07)70279-6. [DOI] [PubMed] [Google Scholar]
- 4.Aboagye EO, Bhujwalla ZM. Cancer Res. 1999;59:80–84. [PubMed] [Google Scholar]
- 5.Ramirez de Molina A, Gallego-Ortega D, Sarmentero-Estrada J, Lagares D, Gomez Del Pulgar T, Bandres E, Garcia-Foncillas J, Lacal JC. Int. J. Biochem. Cell Biol. 2008;40:1753–1763. doi: 10.1016/j.biocel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Griner EM, Kazanietz MG. Nat. Rev. Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 7.Contractor KB, Kenny LM, Stebbing J, Al-Nahhas A, Palmieri C, Sinnett D, Lewis JS, Hogben K, Osman S, Shousha S, Lowdell C, Coombes RC, Aboagye EO. Clin. Cancer Res. 2009;15:5503–5510. doi: 10.1158/1078-0432.CCR-09-0666. [DOI] [PubMed] [Google Scholar]
- 8.Leyton J, Smith G, Zhao Y, Perumal M, Nguyen Q-D, Robins E, Årstad E, Aboagye EO. Cancer Res. 2009;69:7721–7728. doi: 10.1158/0008-5472.CAN-09-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witney TH, Alam IS, Turton DR, Smith G, Carroll L, Brickute D, Twyman FJ, Nguyen QD, Tomasi G, Awais RO, Aboagye EO. Clin. Cancer Res. 2012;18:1063–1072. doi: 10.1158/1078-0432.CCR-11-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glunde K, Bhujwalla ZM. Semin. Oncol. 2011;38:26–41. doi: 10.1053/j.seminoncol.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Saffar NM, Troy H, Ramirez de Molina A, Jackson LE, Madhu B, Griffiths JR, Leach MO, Workman P, Lacal JC, Judson IR, Chung YL. Cancer Res. 2006;66:427–434. doi: 10.1158/0008-5472.CAN-05-1338. [DOI] [PubMed] [Google Scholar]
- 12.Chua BT, Gallego-Ortega D, Ramirez de Molina A, Ullrich A, Lacal JC, Downward J. Mol. Cancer. 2009;8:131. doi: 10.1186/1476-4598-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campos J, Nunez MC, Conejo-Garcia A, Sanchez-Martin RM, Hernandez-Alcoceba R, Rodriguez-Gonzalez A, Lacal JC, Gallo MA, Espinosa A. Curr. Med. Chem. 2003;10:1095–1112. doi: 10.2174/0929867033457539. [DOI] [PubMed] [Google Scholar]
- 14.Malito E, Sekulic N, Too WC, Konrad M, Lavie A. J. Mol. Biol. 2006;364:136–151. doi: 10.1016/j.jmb.2006.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeisel SH. Nutr. J. 2000;16:669–671. doi: 10.1016/s0899-9007(00)00349-x. [DOI] [PubMed] [Google Scholar]
- 16.Rubio-Ruiz B, Conejo-Garcia A, Rios-Marco P, Carrasco-Jimenez MP, Segovia J, Marco C, Gallo MA, Espinosa A, Entrena A. Eur. J. Med. Chem. 2012;50:154–162. doi: 10.1016/j.ejmech.2012.01.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NMR spectra and synthetic methods of relevant compounds, biological procedures and molecular modelling